To the Editor:
Patients with COVID-19 are at increased risk for developing new or recurrent psychosis (1,2). Viral infections—including SARS-CoV-2 (3, 4, 5)—can cause psychosis in the context of autoimmune encephalitis (6). However, some individuals with parainfectious psychosis do not meet criteria for autoimmune encephalitis, yet they respond to immunotherapy (7,8). We identified anti-SARS-CoV-2 and candidate autoantibodies in the serum and cerebrospinal fluid (CSF) of a case of COVID-19–associated subacute psychosis that did not meet criteria for autoimmune or infectious encephalitis yet remitted after treatment with intravenous immunoglobulin (IVIg).
A 30-year-old man without medical, psychiatric, or substance use history developed fever and malaise. The following day, he developed a delusion that the rapture was imminent. On day 2, a nasopharyngeal swab was positive for SARS-CoV-2 by real-time reverse transcription–polymerase chain reaction. He began a 14-day isolation but maintained daily contact with family. He did not have anosmia, ageusia, or respiratory symptoms, nor did he receive treatment for COVID-19. He initially suffered from hypersomnia and slept 22 hours/day. He then developed insomnia, sleeping only 3 to 4 hours/day. During this time, he paced, rambled, and believed that he was dying and communicating with deceased relatives and God.
On day 22, he kicked through a door and pushed his mother, prompting an emergency department evaluation. In the emergency department, he falsely claimed to be a veteran, and worried about being experimented on with radiation. He did not have suicidal ideation, homicidal ideation, or hallucinations. Noncontrast head computed tomography was normal, and urine toxicology was negative. He was started on haloperidol 5 mg by mouth twice daily with significant improvement of his agitation and delusions. After 48 hours, he was discharged to outpatient follow-up. Outpatient magnetic resonance imaging of the brain with and without gadolinium was unremarkable.
After discharge, his restlessness, insomnia, and cognitive slowing recurred, as did his fears that he would be experimented on “like a guinea pig.” On day 34, he punched through a wall and was hospitalized and evaluated for autoimmune encephalitis. A detailed neurological exam was unremarkable. He had a flat affect, slowed speech, and akathisia, which resolved after decreasing haloperidol and starting benztropine and lorazepam. A 12-hour video electroencephalogram was normal. CSF studies, including a clinical autoimmune encephalitis autoantibody panel, were notable only for an elevated IgG of 4.8 mg/dL (reference 1.0–3.0 mg/dL) with a normal IgG index (see Table 1 ).
Table 1.
Clinical Studies
| Source | Test | Result (Reference) |
|---|---|---|
| Nasopharyngeal Swab | SARS-CoV-2 RNA PCR | Day 2: positive |
| Day 34: negative | ||
| Urine | 9-drug toxicology screen | Negative |
| Serum | Basic metabolic panel | Within acceptable limits:
|
| Prothrombin time | 11.5 seconds (9.6–12.3 seconds) | |
| International normalized ratio | 1.07 | |
| Complete blood count | Day 24 WBC: 6.9 × 1000/μL (4.0–10.0 × 1000/μL) | |
| Day 34 WBC: 5.4 × 1000/μL (4.0–10.0 × 1000/μL) | ||
| MPV 11.6 fL (6.0–11.0 fL) | ||
| Thyroid-stimulating hormone | 2.520 μIU/mL (0.270–4.200 μIU/mL) | |
| D-dimer | 1.89 mg/L (≤0.50 mg/L) | |
| Liver enzymes | AST 156 U/L (<35 U/L) | |
| ALT 372 U/L (<59 U/L) | ||
| C-reactive protein | 1.7 mg/L (<1.0 mg/L) | |
| Ferritin | 1124 ng/mL (30–400 mg/mL) | |
| Ammonia | 27 μmol/L (11–35 μmol/L) | |
| Albumin | 4.2 g/dL (3.6–4.9 g/dL) | |
| IgG | 1230 mg/dL (700–1600 mg/dL) | |
| CSF | Cell count | 0 nucleated cells |
| Protein | 41.2 mg/dL (15–45 mg/dL) | |
| Glucose | 60 mg/dL (40–70 mg/dL) | |
| Culture | No growth | |
| Oligoclonal banding | None | |
| Albumin | 25.8 mg/dL (10–30 mg/dL) | |
| IgG | 4.8 mg/dL (1.0–3.0 mg/dL) | |
| IgG index | 0.67 (<0.7) | |
| Autoimmune encephalopathy panel | Negative for AMPA Ab, amphiphysin Ab, anti-glial nuclear Ab, neuronal nuclear Ab (types 1, 2, and 3), CASPR2, CRMP–5, DPPX, GABA-B receptor, GAD65, GFAP, IgLON5, LGI1-IgG, MGLUR1, NIF, NMDA receptor, Purkinje cell cytoplasmic Ab (types Tr, 1, and 2) | |
| Imaging | CT head without contrast | No acute intracranial findings |
| MRI brain with contrast | No acute intracranial abnormality or definitive structural abnormality identified; specifically, no imaging findings suggestive of encephalitis or acute demyelination | |
| Electroencephalography | Normal prolonged (>12 hours) awake and asleep inpatient video EEG |
Ab, antibody; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalogram; MPV, mean platelet volume; MRI, magnetic resonance imaging; PCR, polymerase chain reaction.
Lacking focal neurologic symptoms, seizures, magnetic resonance imaging abnormalities, or CSF pleocytosis, his presentation did not meet consensus criteria for autoimmune encephalitis (8). Nevertheless, his subacute psychosis, cognitive slowing, and recent SARS-CoV-2 infection raised concern for autoimmune-mediated psychosis. Therefore, starting on day 35, he received a total of 2 g/kg of IVIg over 3 days. His cognitive slowing and psychotic symptoms remitted after the first day of treatment. His sleep cycle normalized, and he was discharged without scheduled antipsychotics. He returned to work immediately after discharge and remained symptom-free 3 months later.
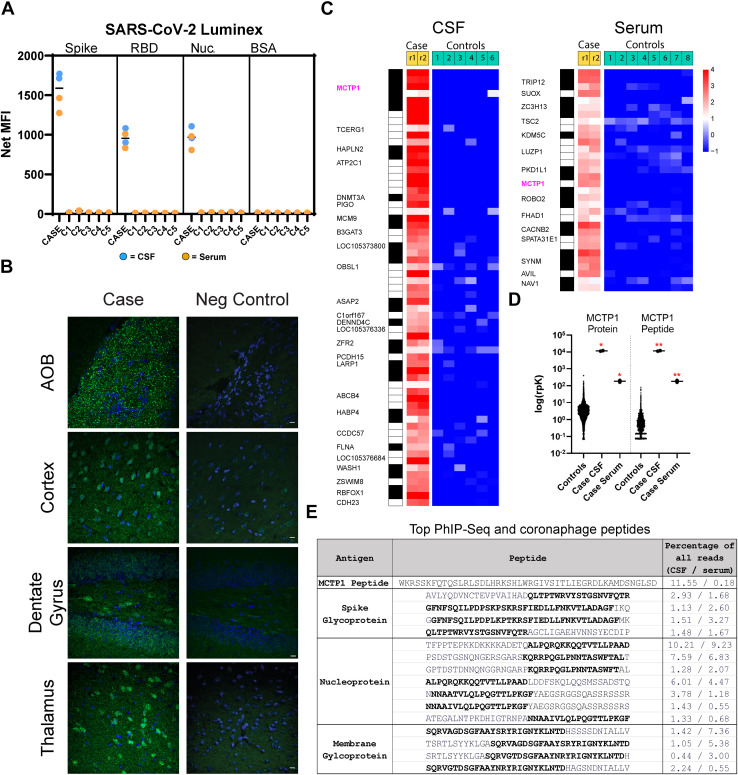
Because his robust response to IVIg suggested an underlying neuroinflammatory process, we tested for anti-SARS-CoV-2 and anti-neural autoantibodies. Using a Luminex SARS-CoV-2 antigen panel (9,10), we detected anti-spike, anti-receptor binding domain, and anti-nucleocapsid protein antibodies in his serum and CSF (Figure 1A ) (9,10).
Figure 1.
Characterization of anti-neuronal antibody staining. (A) Case and control (n = 5, C1–C5) CSF and serum were screened in technical replicate for anti-SARS-CoV-2 antibodies by Luminex antigen assay. Both replicates are shown. BSA was used as a negative control. Horizontal bars = median of technical replicates. C1 through C5 refer to control individuals 1 through 5. (B) Mice were perfused with 4% paraformaldehyde. Twelve-micrometer frozen sagittal brain sections were immunostained with CSF at a 1:4 dilution and counterstained with an anti-human IgG secondary antibody (green) (Jackson #709-545-149 at 2 μg/mL). Neg control = secondary only staining. Nuclei were labeled with DAPI (blue). (C) Heatmap of log(fold change) PhIP-Seq human peptide enrichments relative to the mean of controls. Both technical replicates for case CSF and serum are plotted (r1 and r2), while the means of technical replicates are plotted for controls. Each row represents 1 peptide and peptides mapping to the same protein are grouped together according to the black-and-white vertical runner. (D) Dot plot of MCTP1 PhIP-Seq enrichment compared with a database of 4216 control samples. The y-axis is log(rpK) (rpK = sequencing reads per 100,000 reads). Total MCTP1 and the top MCTP1 peptide enrichments were tested for statistical significance by Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparisons testing using GraphPad Prism 9.4.1. Note, the top MCTP1 peptide represented 99.98% of all MCTP1 reads. ∗p < .05, ∗∗p < .01. PhIP-Seq and other data are available upon request. (E) Table of the most enriched MCTP1 and coronaphage peptides. Bolded regions represent minimal sequences present in 2 or more coronaphage peptides. Neither SARS-CoV-2 peptides nor epitopes mapped to any of the enriched PhIP-Seq peptides according to the blastp suite of the National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST). ANOVA, analysis of variance; AOB, accessory olfactory bulb; BSA, bovine serum albumin; CSF, cerebrospinal fluid; MFI, mean fluorescence intensity; Nuc., nucleocapsid protein; PhIP-Seq, peptidome phage display immunoprecipitation sequencing; RBD, receptor binding domain.
We then screened for anti-neural autoantibodies using anatomic mouse brain tissue staining (11), a validated and standard method performed by incubating rodent brain sections with CSF. At a 1:4 dilution, his CSF IgG produced prominent punctate immunostaining of the accessory olfactory bulb, cytoplasmic and neuropil staining in upper layers of the cortex and thalamus, and cytoplasmic staining of hilar and granule neurons in the hippocampus (Figure 1B).
We next used whole human peptidome phage display immunoprecipitation sequencing (PhIP-Seq) (12) to screen for candidate autoantigens. Similar to COVID-19 patients with neurological symptoms (13), the patient’s CSF enriched a diverse set of candidate autoantigens (n = 27), including multiple peptides mapping to MCTP1, a protein implicated in neurotransmitter release (Figure 1C) (14,15). The top PhIP-Seq–enriched peptide is encoded by 11 MCTP1 isoforms—but not the canonical isoform MCTP1L (National Center for Biotechnology Information RefSeq [https://www.ncbi.nlm.nih.gov/refseq/]). Surprisingly, MCTP1 autoantibodies did not validate by overexpression cell-based assay or immunoprecipitation using a representative isoform (isoform 3). However, an expanded PhIP-Seq comparison revealed that the patient enriched MCTP1 significantly more than a combined 3408 healthy CSF and sera and 808 negative control samples (Figure 1D).
Finally, we evaluated whether PhIP-Seq candidate antigen enrichment was due to sequence similarity with SARS-CoV-2. We mapped our patient’s anti-SARS-CoV-2 target epitopes by SARS-CoV-1/2 phage display (9) and compared viral epitopes with PhIP-Seq–identified candidate autoantigens using National Center for Biotechnology Information BlastP (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Among the top 10 CSF- and serum-enriched SARS-CoV-2 peptides, we identified 15 unique peptides, none of which aligned to PhIP-Seq candidate autoantigens (Figure 1E).
In this correspondence, we have profiled the antibody response of a COVID-19 patient with antipsychotic-refractory subacute psychosis whose symptoms rapidly and completely remitted after treatment with IVIg. We identified and mapped the epitope specificity of anti-SARS-CoV-2 antibodies in the patient’s CSF and characterized autoantibodies by rodent brain tissue staining and PhIP-Seq. Although anti-neural autoantibodies have been described in neurologically impaired COVID-19 patients (16, 17, 18), autoantibody screening is rarely performed in COVID-19–associated psychosis (19, 20, 21, 22, 23, 24, 25, 26, 27, 28).
The need for autoantigen discovery in psychotic spectrum disorders is well recognized (29,30). By PhIP-Seq, our patient’s CSF and serum significantly enriched MCTP1. MCTP1 enrichment was not explained by sequence similarity with SARS-CoV-2 proteins, suggesting a distinct antibody response, rather than molecular mimicry. Although anti-MCTP1 autoantibodies did not validate by cell-based assay or immunoprecipitation, neither method is dispositive (11), and only 1 of 11 candidate MCTP1 isoforms was tested. Given the patient’s extreme PhIP-Seq enrichment of MCTP1, it remains a candidate autoantigen.
Importantly, early initiation of immunotherapy for autoimmune disorders of the central nervous system significantly improves outcomes (31). Although autoimmune encephalitis can be established on clinical grounds, the diagnosis requires neurologic, magnetic resonance imaging, and/or CSF abnormalities (8). To identify individuals with potentially immune-responsive acute psychosis without neurological impairment, Pollak et al. (32) proposed criteria for autoimmune psychosis. While “possible” autoimmune psychosis relies solely on clinical factors, “probable” and “definite” autoimmune psychosis require abnormal imaging or laboratory studies.
Our patient’s subacute psychosis and cognitive dysfunction qualified him for possible autoimmune psychosis. However, he had several red flags for autoimmune psychosis: infectious prodrome, rapid progression, and insufficient response to antipsychotics (32). Moreover, his mood dysregulation, cognitive slowing, and hypersomnia were evocative of the mixed symptomatology more typical of autoimmune encephalitis (33,34). Given his overall clinical picture, we administered IVIg with apparent clinical response. Only by relying on ancillary criteria were we able to justify immunotherapy for our patient, suggesting that re-evaluating the criteria for autoimmune psychosis may improve its sensitivity (35).
Even so, this case should be interpreted with caution. Psychotic disorders are protean by nature, mixed symptomatology does occur, and most psychotic presentations are unlikely to be immune mediated. However, given the scale of the COVID-19 pandemic, psychiatric practitioners should consider autoimmune psychosis in patients with COVID-19–associated psychosis.
Acknowledgments and Disclosures
This work was supported by National Institute of Mental Health Grant Nos. R01MH122471 (to SJP, MRW), R01MH125396 (to SSS), K23MH118999 (to SFF), and R21MH118109 (to SS); National Institute of Neurological Disorders and Stroke Grant No. R01NS118995-14S (to SJP); the Brain Research Foundation (to SJP); the National Intitute of Allergy and Infectious Diseases Grant No. R01AI157488 (to SFF); the Hanna H. Gray Fellowship of the Howard Hughes Medical Institute (to CMB); the President’s Postdoctoral Fellowship Program of the University of California (to CMB); the John A. Watson Scholar Program of the University of California, San Francisco (to CMB); and the Deeda Blair Research Initiative for Disorders of the Brain of the Foundation for the National Institutes of Health (to CMB). Sequencing was performed at the University of California, San Francisco (UCSF) Center for Advanced Technology, supported by UCSF Sandler Program for Breakthrough Biomedical Research, Research Resource Program Institutional Matching Instrumentation Awards, and National Institutes of Health (NIH Office of the Director) Grant Nos. 1S10OD028511-01.
We thank Trung Huynh and Anne Wapniarski for laboratory assistance. We thank Andrew Kung and Joseph Derisi for use of the PhIP-Seq database.
During the course of treatment, we obtained surrogate consent to use surplus cerebrospinal fluid for research. After regaining capacity, the patient provided written informed consent for this case report. This work has not previously been published in any form.
MRW received a research grant from Roche/Genentech. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
LSM and BL contributed equally to this work.
References
- 1.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taquet M., Sillett R., Zhu L., Mendel J., Camplisson I., Dercon Q., Harrison P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9:815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panariello A., Bassetti R., Radice A., Rossotti R., Puoti M., Corradin M., et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: A case report. Brain Behav Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monti G., Giovannini G., Marudi A., Bedin R., Melegari A., Simone A.M., et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez Bravo G., Ramió i Torrentà L. Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurología (Engl Ed) 2020;35:699–700. doi: 10.1016/j.nrleng.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linnoila J.J., Binnicker M.J., Majed M., Klein C.J., McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e245. doi: 10.1212/NXI.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gungor İ., Derin S., Tekturk P., Tüzün E., Bilgiç B., Çakır S. First-episode psychotic disorder improving after immunotherapy. Acta Neurol Belg. 2016;116:113–114. doi: 10.1007/s13760-015-0519-8. [DOI] [PubMed] [Google Scholar]
- 8.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T., et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamecnik C.R., Rajan J.V., Yamauchi K.A., Mann S.A., Loudermilk R.P., Sowa G.M., et al. ReScan, a multiplex diagnostic pipeline, pans human sera for SARS-CoV-2 antigens. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabatino J.J., Jr., Mittl K., Rowles W.M., McPolin K., Rajan J.V., Laurie M.T., et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight. 2022;7 doi: 10.1172/jci.insight.156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricken G., Schwaiger C., De Simoni D., Pichler V., Lang J., Glatter S., et al. Detection methods for autoantibodies in suspected autoimmune encephalitis. Front Neurol. 2018;9:841. doi: 10.3389/fneur.2018.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Donovan B., Mandel-Brehm C., Vazquez S.E., Liu J., Parent A.V., Anderson M.S., et al. High-resolution epitope mapping of anti-Hu and anti-Yo autoimmunity by programmable phage display. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa059. fcaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song E., Bartley C.M., Chow R.D., Ngo T.T., Jiang R., Zamecnik C.R., et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genç Ö., Dickman D.K., Ma W., Tong A., Fetter R.D., Davis G.W. MCTP is an ER-resident calcium sensor that stabilizes synaptic transmission and homeostatic plasticity. Elife. 2017;6 doi: 10.7554/eLife.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Téllez-Arreola J.L., Silva M., Martínez-Torres A. MCTP-1 modulates neurotransmitter release in C. elegans. Mol Cell Neurosci. 2020;107 doi: 10.1016/j.mcn.2020.103528. [DOI] [PubMed] [Google Scholar]
- 16.Song E., Bartley C.M., Chow R.D., Ngo T., Jiang R., Zamecnik C.R., et al. Exploratory neuroimmune profiling identifies CNS-specific alterations in COVID-19 patients with neurological involvement. bioRxiv. 2020 doi: 10.1101/2020.2009.2011.293464. [DOI] [Google Scholar]
- 17.Franke C., Ferse C., Kreye J., Momsen Reincke S., Sanchez-Sendin E., Rocco A., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severance E.G., Dickerson F.B., Viscidi R.P., Bossis I., Stallings C.R., Origoni A.E., et al. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. 2011;37:101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra A., Juanes A., Losada C.P., Álvarez-Sesmero S., Santana V.D., Martí I., et al. Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C.M., Komisar J.R., Mourad A., Kincaid B.R. COVID-19-associated brief psychotic disorder. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrando S.J., Klepacz L., Lynch S., Tavakkoli M., Dornbush R., Baharani R., et al. COVID-19 psychosis: A potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61:551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLisi L.E. A commentary revisiting the viral hypothesis of schizophrenia: Onset of a schizophreniform disorder subsequent to SARS CoV-2 infection. Psychiatry Res. 2021;295 doi: 10.1016/j.psychres.2020.113573. [DOI] [PubMed] [Google Scholar]
- 23.Lanier C.G., Lewis S.A., Patel P.D., Ahmed A.M., Lewis P.O. An unusual case of COVID-19 presenting as acute psychosis. J Pharm Pract. 2022;35:488–491. doi: 10.1177/0897190020977721. [DOI] [PubMed] [Google Scholar]
- 24.Majadas S., Pérez J., Casado-Espada N.M., Zambrana A., Bullón A., Roncero C. Case with psychotic disorder as a clinical presentation of COVID-19. Psychiatry Clin Neurosci. 2020;74:551–552. doi: 10.1111/pcn.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clouden T.A. Persistent hallucinations in a 46-year-old woman after COVID-19 infection: A case report. Cureus. 2020;12 doi: 10.7759/cureus.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chacko M., Job A., Caston F., 3rd, George P., Yacoub A., Cáceda R. COVID-19-induced psychosis and suicidal behavior: Case report. SN Compr Clin Med. 2020;2:2391–2395. doi: 10.1007/s42399-020-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillett G., Jordan I. Severe psychiatric disturbance and attempted suicide in a patient with COVID-19 and no psychiatric history. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-239191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartley C.M., Johns C., Ngo T.T., Dandekar R., Loudermilk R.L., Alvarenga B.D., et al. Anti-SARS-CoV-2 and autoantibody profiles in the cerebrospinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. 2021;78:1503–1509. doi: 10.1001/jamaneurol.2021.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endres D., von Zedtwitz K., Matteit I., Bünger I., Foverskov-Rasmussen H., Runge K., et al. Spectrum of novel anti-central nervous system autoantibodies in the cerebrospinal fluid of 119 patients with schizophreniform and affective disorders. Biol Psychiatry. 2022;92:261–274. doi: 10.1016/j.biopsych.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenreich H., Gastaldi V.D., Wilke J.B.H. Quo vaditis anti-brain autoantibodies: Causes, consequences, or epiphenomena? Biol Psychiatry. 2022;92:254–255. doi: 10.1016/j.biopsych.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Nosadini M., Mohammad S.S., Ramanathan S., Brilot F., Dale R.C. Immune therapy in autoimmune encephalitis: A systematic review. Expert Rev Neurother. 2015;15:1391–1419. doi: 10.1586/14737175.2015.1115720. [DOI] [PubMed] [Google Scholar]
- 32.Pollak T.A., Lennox B.R., Müller S., Benros M.E., Prüss H., Tebartz van Elst L., et al. Autoimmune psychosis: An international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7:93–108. doi: 10.1016/S2215-0366(19)30290-1. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz-Lopetegi A., Graus F., Dalmau J., Santamaria J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. 2020;19:1010–1022. doi: 10.1016/S1474-4422(20)30341-0. [DOI] [PubMed] [Google Scholar]
- 34.Al-Diwani A., Handel A., Townsend L., Pollak T., Leite M.I., Harrison P.J., et al. The psychopathology of NMDAR-antibody encephalitis in adults: A systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry. 2019;6:235–246. doi: 10.1016/S2215-0366(19)30001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franke C., Prüss H. Letter to the Editor: Comment on Mulder J et al. (2021) Indirect Immunofluorescence for Detecting Anti-Neuronal Autoimmunity in CSF after COVID-19 - Possibilities and pitfalls. Brain Behav Immun. 2021;94:473–474. doi: 10.1016/j.bbi.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]