Abstract
Background
Severe alcohol-associated hepatitis (SAH) patients with infections have a high short-term mortality rate. Gut microbiota dysbiosis plays an important role in the pathogenesis of SAH. Preliminary studies have demonstrated long-term benefits with healthy donor fecal microbiota transplantation (FMT). Data on FMT compared with pentoxifylline for SAH and relevant gut microbial changes are lacking in literature.
Methods
From January 2019 to February 2021, retrospective analysis of a single hospital’s records revealed 47 SAH patients undergoing FMT (100 mL/day via nasoduodenal tube for 7 days) and 25 matched patients receiving pentoxifylline (400 mg/8 h for 28 days). The primary end point was a 6-month survival rate. Secondary end points included incidence of ascites, hepatic encephalopathy, infections, acute kidney injury, and gut microbiota changes between post-therapy groups. Biomarker discovery and network analysis were also performed to identify significant taxa of gut microbiota in post-treatment groups in retrospectively stored stool samples.
Results
All were males. The 6-month survival rate was higher in the patients undergoing FMT than in patients receiving pentoxifylline (83.0% vs 56.0%, P = 0.012). At the end of 6-month follow-up, the incidences of clinically significant ascites (56.0% vs 25.5%, P = 0.011), hepatic encephalopathy (40.0% vs 10.6%, P = 0.003), and critical infections (52.0% vs 14.9%, P < 0.001) in patients administered pentoxifylline were significantly higher than those in patients treated with FMT. At 3 months, biomarker analysis revealed a significant abundance of Bifidobacterium and Eggerthella in the FMT group and the pentoxifylline group, respectively. At 6 months, Bifidobacterium in the FMT group and pathogenic Aerococcaceae in the pentoxifylline group were notable. Network analysis showed beneficial taxa (Bifidobacterium) as a central influencer in those undergoing FMT at 6 months.
Conclusions
Healthy donor FMT improved survival rate and reduced liver-related complications compared with pentoxifylline. These clinical benefits were associated with favorable modulation of intestinal bacterial communities. Difficult-to-treat SAH patients may be safely bridged to transplantation using FMT. Controlled trials evaluating long-term outcomes are an unmet need.
Keywords: fecal microbiota, stool transplant, alcoholic hepatitis, liver transplant, dysbiosis
Introduction
Severe alcohol-associated hepatitis (SAH) is a severe form of alcohol-associated liver disease with high morbidity and mortality [1]. Untreated SAH patients with a ≥32 Maddrey’s discriminant function score or hepatic encephalopathy have a 28-day survival rate of 50%–60% [2]. Corticosteroid therapy is the only recommended therapy for SAH patients at present, though ∼40% of patients fail to respond [2, 3]. The short-term mortality rate of patients with SAH and infections is ∼67% [3]. The Lille score helps in determining non-responders to corticosteroids therapy. Discontinuation of corticosteroids therapy is warranted if the Lille score is >0.45 at the end of 7 days [4]. Steroid therapy may predispose SAH patients to infections and is associated with poor clinical outcomes [5]. Pentoxifylline, which was found to be ineffective in the short term, was no less effective than corticosteroids beyond a month and could be utilized in patients who have contraindications to steroid use [6]. A recent systematic review and meta-analysis showed that dual therapy with pentoxifylline and corticosteroids was not inferior to corticosteroid monotherapy and reduced the incidence of acute kidney injury and risk of infection [7].
Liver transplant is the curative option for patients with SAH and improves survival rates compared with those receiving conservative care [8]. However, the paucity of organs, contraindications for immediate transplant such as uncontrolled infections, rapid-onset extrahepatic organ failures associated with SAH, and high risk of alcohol relapse are concerning. Recently, the role of the gut–liver axis and its modulation for improving clinical outcomes in alcohol-associated liver disease was shown [9, 10]. Multiple studies demonstrated that the manipulation of gut microbiota could favorably influence the course and outcome of SAH [11, 12].
Clinical outcomes and gut microbiota changes associated with healthy donor fecal microbiota transplantation (FMT) or pentoxifylline therapy in the long term remain unknown. In this study, we aimed to analyse 6-month outcomes of FMT vs pentoxifylline treatment for patients with SAH and to describe pertinent gut microbiota before and after treatment in both groups.
Methods
Patients
We analysed hospital records of Rajagiri Hospital, Aluva, Kerala, India to include SAH patients undergoing FMT or pentoxifylline therapy from January 2019 to February 2021. Patients with SAH who underwent FMT (100 mL of freshly processed stool samples once a day via nasoduodenal tube for 7 days) or pentoxifylline (400 mg thrice daily for 28 days) and completed treatment were retrospectively included in this study. Patients were matched for age, gender, and liver disease severity scores at baseline, which included Maddrey’s discriminant function, Child Pugh, and model for end-stage liver disease scores. These patients had absolute and relative contraindications for corticosteroid treatment and could not be considered for liver transplant due to alcohol-use disorder and active alcohol use within the past 30 days. All the patients had histologically proven evidence of SAH. Patients with upper gastrointestinal bleed within the past month; multiple organ failure requiring support; concomitant complementary and alternative medicine use; uncontrolled sepsis on inotropes, requiring hemodialysis at admission; associated viral hepatitis, dengue, and leptospirosis; intestinal paralysis; hepatic or extrahepatic malignancy; and disseminated intravascular coagulation were excluded.
The study protocol was approved by the Institutional Review Board of Rajagiri Hospital and was performed in conformance with the Helsinki declaration of 1975 and its pertinent revisions. Only per-protocol analysis was undertaken within this retrospective design. All patients were initiated on maximally tolerated beta-blocker therapy in the presence of clinically significant portal hypertension. For those with ascites, the lowest effective dose of diuretics was continued. In those who developed hepatic encephalopathy after completion of treatment, rifaximin was added as secondary prophylaxis. Additionally, in those who developed infections or acute kidney injury on follow-up and required admission, intravenous antimicrobial therapy or albumin infusions with or without terlipressin were initiated.
Stool samples were collected within 24 h of admission before the start of FMT or pentoxifylline treatment. Post-treatment follow-up samples were collected in each outpatient or admission at 3–4 and 6–7 months. Only the latest stored samples within the above specified follow-up periods were sent for sequencing and further analysis. All participants (or their immediate family members) provided informed consent for the procedure and for the use of their de-identified stored fecal samples for future research. Stored stool samples were analysed for significant changes in bacterial genera at 3 and 6 months after completion of therapy among survivors in both groups. The study confirms with the principles outlined in the Declaration of Helsinki and its amendments.
Stool donors
Family members were interviewed and screened for eligibility for being a FMT donor since a pooled stool donor facility was not available at our institute. The potential stool donors were screened for routine laboratory tests such as hemogram, fasting blood sugar and glycated hemoglobin, liver biochemistries, fasting lipid profile, stool routine, and microscopy for cysts, parasites, ova, and for occult blood and stool cultures. Donors were also screened for novel coronavirus, chronic viral hepatitis, HIV 1 and 2, Clostridium difficile, and common venereal diseases. A written informed consent was taken from the donor for the purpose of daily stool donation. Donors were excluded if they were <18 or >50 years old, had primary or secondary immunosuppressed state, documented enteric infections within the last 3 months, or had a family history of inflammatory bowel disease, prior abdominal surgery, pancreatic disorders, gastrointestinal neoplasms, metabolic syndrome, systemic autoimmunity, atopic diseases, food and respiratory allergies, chronic neurologic disorders, neuro-developmental disorders, or use of antibiotics within the last 3 months. Donors were also excluded if they had features suggesting functional gastrointestinal disorders, travel history within the past 3 months, or predisposing factors for potentially transmittable diseases, occasional alcohol intake, history of substance abuse, or failed to provide consent. Donors were advised to follow a normal diet plan for the duration of the protocol in consultation with the hospital dietician who closely monitored the donor diet plan during the study period. Donors were advised to eat food based on the recommended daily allowance as calculated by a dietician and not permitted to eat street foods or attend public functions or eat at family functions or parties. Daily monitoring of donors was carried out to assess deviation from the diet plan.
Stool collection and preparation
Donors were advised to collect and submit a fresh stool sample daily after arriving at the hospital in sterile plastic collection containers. All daily stool samples were obtained 6 h before the procedure. A stool specimen with a weight of ∼30 g (∼2 cm3 with 3 × 1010 microbial loads) was considered adequate. A total of 100 mL of sterile normal saline was added to the stool sample and homogenized using a blender (HR1363/04; Philips®; Amsterdam, Netherlands) for 2 min, in 30-s pulses, with a 5-s wait between each pulse. The homogenous suspension was then filtered three times through sterile gauze pieces until the filtrate was devoid of solid matter. Personnel involved in stool specimen preparation were required to wear eye shields, masks, and fluid-resistant gowns.
Stool administration procedure
Briefly, 100 mL of manually filtered stool was delivered through a nasoduodenal tube that was placed under fluoroscopy guidance 1 day prior to the FMT. The recipient was kept nil per oral for at least 4 h prior to the stool instillation. A total of 100 mL of freshly prepared stool suspension were given daily for 7 days. Non-absorbable antibiotics were avoided from the time of enrollment into the study and therapy initiation. All patients received a third-generation cephalosporin as part of the SAH protocol at admission and antibiotics were upgraded as per the treating physician’s decision based on culture and sensitivity. Stool instillation was done with the patient positioned supine on a bed at an angle of 45° and this position was maintained for at least 30 min after the procedure. The nasoduodenal tube was flushed with 30 mL of normal saline after the stool instillation. The patient was allowed to resume food intake 2 h after FMT. All patients were continued on salt restriction, taking diuretics and 1–1.5 g/(kg·day) of protein, and keeping weight-based recommended calorie intake as prescribed by the nutritionist in charge. Intravenous albumin was continued as indicated during the hospitalized period. While rifaximin and other non-absorbable antibiotics were withheld, disaccharides were permitted for patients to have soft stools two or three times per day.
Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software (Ostend, Belgium). Data are given as mean and standard deviation or as median and range between brackets as applicable. The Fischer’s exact or chi-square test was used for evaluation of categorical data. The Wilcoxon rank test was used for pairwise comparison between baseline and post-interventional data. If the data were normally distributed, the means were used for assessing statistical significance between parameters and if the distribution were not (log-normal or a similar distribution), then the medians of parameters analysed were used. One-way analysis of variance (ANOVA) was used to test for differences at baseline between the means of investigational variables of groups. P < 0.05 was considered statistically significant. Before the ANOVA test, Levene’s test for equality of variances was performed. If the Levene’s test was positive (P < 0.05), logarithmic transformation was applied to the data. The probability of patients surviving up to the study end points was calculated using the Kaplan–Meier method and graphically represented by the survival curves. Survival curves were compared using the log-rank test and P < 0.05 was considered significant.
Microbiota analysis post-therapy
The stool samples were aliquoted into 500-µg samples. All the samples were stored at −80°C until processing and DNA isolation. Using an Illumina MiSeq next-generation sequencer (Illumina; San Diego, CA, USA), we performed fecal 16S rRNA amplicon sequencing at the V3–V4 region of bacterial DNA extracted from ∼200 mg of collected stool samples. Bacterial DNA was extracted using a validated protocol modification of the commercially available QIAmp DNA Stool Mini Kit1 (Qiagen; Venlo, Netherlands) to identify bacterial communities, which were classified taxonomically according to the Greengenes Database (version 13.8). The Quantitative Insights into Microbial Ecology (version 1.9.1) was used to ascertain quantitative microbial communities. The multivariable biomarker discovery method known as the linear discriminant analysis effect size, which combines the Kruskal–Wallis and pairwise Wilcoxon tests, was used to identify significantly different microbial communities between groups post-therapy among survivors between groups. We used default significance (i.e. an alpha value of 0.05) and a linear discriminant analysis threshold of 2.0 at all taxonomic levels between time points and between groups. Output data are demonstrated in the form of bar charts portraying significant bacterial taxa.
Network analysis of bacterial taxa of FMT recipients
Networks were inferred by CoNet® (version 1.1.1-beta) applications within Cytoscape™ (version 3.7.2). For computing, the significance of the association between each pair of nodes and bootstrapping with the ReBoot feature of CoNet was used. Edges (associations) with a P-value of <0.05 were retained and considered significant. The following network measures were calculated using NetworkX® (version 2.2): degree centrality, betweenness centrality, and closeness centrality. These were all added to the node attributes in the graph. Network topology was assessed using the radial analysis feature within Cytoscape™ to identify central bacterial taxa influencing the core pathways and output illustrated in the form of radial-networked interactions.
Results
Patient characteristics
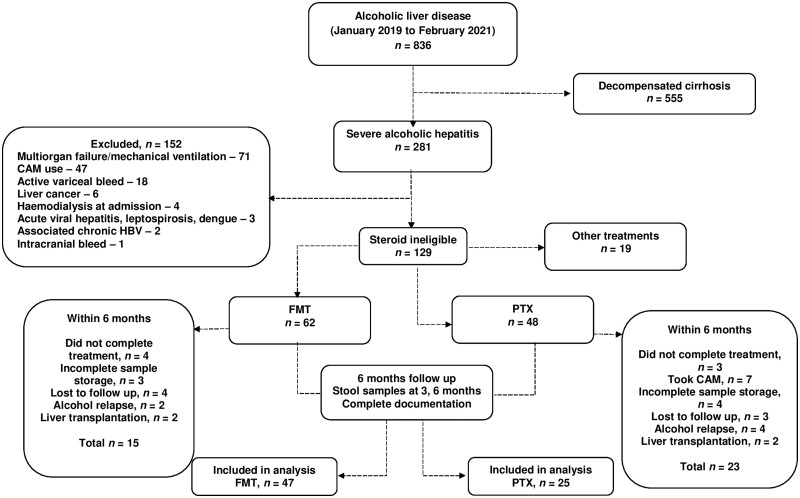
From January 2019 to February 2021, a total of 281 patients with SAH were included in this retrospective study and 129 (45.9%) of them had relative or absolute contraindications for steroid treatment. After excluding patients on other treatments, those without complete follow-up, with interrupted follow-up, alcohol relapse, and liver transplantation within 6 months after treatment, 47 patients undergoing FMT and 25 patients undergoing pentoxifylline therapy were identified for final analysis inclusion (Figure 1).
Figure 1.
Study methods, inclusion and exclusion of patients and analysis pathway flow diagram. CAM, alternative medicine; FMT, fecal microbiota transplantation; PTX, pentoxyfilline.
Baseline infections were notable among 11 patients (44.0%) in the pentoxifylline group and 16 patients (34.0%) in the FMT group (P = 0.407). Pulmonary infection (n = 14; 51.9%) was the commonest in these 27 patients followed by spontaneous bacterial peritonitis (n = 3; 11.1%) and urinary tract infection (n = 3; 11.1%). The commonest organism cultured was Klebsiella pneumoniae (n = 9; 33.3%) followed by Escherichia coli (n = 7; 25.9%). The two study groups were comparable concerning demographic data and disease severity scores. The patient characteristics, clinical parameters, and investigational details between both groups are shown in Table 1.
Table 1.
Laboratory parameters and disease severity between both groups at baseline
| Parameter | PTX (n = 25) | FMT (n = 47) | P-value |
|---|---|---|---|
| Age (years) | 47.7 ± 9.9 | 45.2 ± 10.2 | 0.321 |
| Ascites Grade 2 and above | 18 (72.0%) | 31 (65.9%) | 0.059 |
| Encephalopathy Grade 2 and above | 15 (60.0%) | 30 (63.8%) | 0.753 |
| Hemoglobin (g/L) | 10.4 ± 2.2 | 10.1 ± 1.6 | 0.509 |
| Total leuacocyte count (× 109/L) | 18.3 ± 8.4 | 15.6 ± 9.3 | 0.229 |
| Platelet counta (× 109/L) | 52 (43–516) | 104 (29–282) | 0.134 |
| Total bilirubin (mg/dL) | 21.9 ± 11.6 | 21.2 ± 11.5 | 0.807 |
| AST (U/L) | 162.6 ± 92.5 | 145.3 ± 63.8 | 0.353 |
| ALT (U/L) | 52.5 ± 29.1 | 51.8 ± 30.1 | 0.924 |
| Albumin (g/dL) | 2.4 ± 0.4 | 2.3 ± 0.4 | 0.316 |
| INR | 2.2 ± 0.7 | 2.3 ± 0.6 | 0.527 |
| Blood urea nitrogena (mg/dL) | 35.5 (14–122) | 34 (10–130) | 0.158 |
| Serum creatinine (mg/dL) | 1.1 ± 0.7 | 1.2 ± 0.5 | 0.485 |
| Serum sodium (mmol/L) | 129.3 ± 5.3 | 130.6 ± 4.6 | 0.282 |
| CTPa | 13 (11–14) | 12 (10–15) | 0.068 |
| MELD | 28.2 ± 6.3 | 28.1 ± 4.7 | 0.939 |
| MDFa | 79.8 (45.1–166.9) | 90.7 (45.6–199.2) | 0.269 |
PTX, pentoxifylline; FMT, fecal microbiota transplantation; AST, aspartate transaminase; ALT, alanine transaminase; INR, international normalized ratio; CTP, Child Turcotte Pugh score; MELD, model for end-stage liver disease; MDF, Maddrey’s discriminant function.
Values given in median (range).
Clinical outcomes
A total of 72.0% (n = 18) and 65.9% (n = 31) of patients in the pentoxifylline and FMT groups, respectively, had ascites of grade ≥2 at the start of therapy. At the end of 6 months of follow-up, any grade of ascites (≥1) was evident in 56.0% (n = 14) and 25.5% (n = 12) in the pentoxifylline and FMT groups, respectively (P = 0.011). Similarly, 60.0% (n = 15) and 63.8% (n = 30) of patients in the pentoxifylline and FMT groups, respectively, had overt hepatic encephalopathy at the start of therapy. At the end of 6 months of follow-up, overt hepatic encephalopathy was evident in 40.0% (n = 10) and 10.6% (n = 5) in the pentoxifylline and FMT groups, respectively (P = 0.003). Patients who underwent FMT had better improvement in ascites mobilization and control of hepatic encephalopathy at 6 months post-therapy. The incidence of acute kidney injury before and after therapy between both groups was not significantly different. At the end of 6 months of follow-up, proportions of patients who experienced non-critical infections requiring outpatient management (40.0% [10/25] vs 17.0% [8/47], P = 0.033) and critical infections requiring hospital admissions (52.0% [13/25] vs 14.9% [7/47], P < 0.001) were significantly higher in the pentoxifylline group than in the FMT group. At the 6-month follow-up of those hospitalized for infection, the incidence of Gram-negative bacterial infections (K. pneumoniae, Pseudomonas aeruginosa) and fungal infections (Candida spp.) were slightly higher in the pentoxifylline group (61.5% [8/13] vs 33.3% [3/9], P = 0.204) than in the FMT group. Overall, 11 (44.0%) patients in the pentoxifylline group and 8 (17.0%) patients in the FMT group died within 6 months of follow-up. The mean survival time was longer in patients treated with FMT (159.8 days) than those treated with pentoxifylline (131.0 days). The percentage of patients surviving up to the end of 6 months was lower in the pentoxifylline group than in the FMT group (56.0% vs 83.0%; hazard ratio 3.4 [1.31–9.18]; P = 0.012). Healthy donor fecal transplant improved survival in SAH patients, beyond 6 months—an aspect not seen with the current standards of care (Figure 2).
Figure 2.
(A) Baseline and follow-up details of pertinent variables between groups. (B) Survival analysis demonstrates better clinical outcomes in patients treated with FMT. FMT, fecal microbiota transplantation; PTX, pentoxyfilline.
Significant gut microbiome changes between groups on follow-up
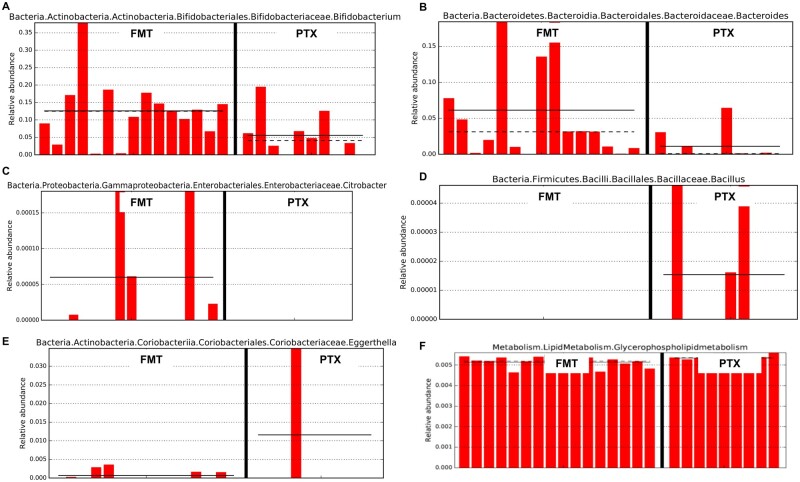
We utilized the multivariable biomarker discovery method known as the linear discriminant analysis effect size to identify significantly different microbial communities between groups post-therapy among survivors. At 3 months, we found that the relative abundance of Bifidobacterium, Bacteroides, and Citrobacter was significantly higher among FMT-treated patients while Bacillus and Eggerthella were abundant in those treated with pentoxifylline (Figure 3).
Figure 3.
Bar charts of most significant results using the LDA Effect Size method (LEfSe). (A)–(E) LEfSe identifies those bacterial groups that showed statistical significance effect size and associate them between treatment groups (at end of 3 months of follow-up) in the context of the highest median. Dotted lines represent medians; straight lines represent averages; (F) LEfSe demonstrating significant predictive functional metabolic pathway that was significant between treatment groups at end of 6 months of follow-up. FMT, fecal microbiota transplantation; PTX, pentoxyfilline.
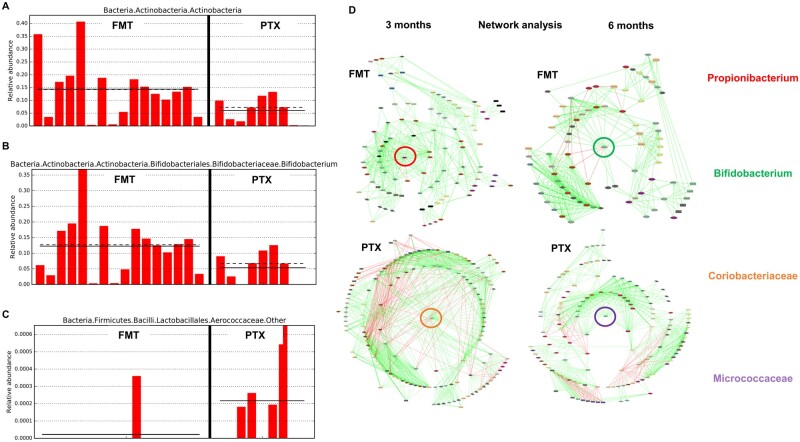
At 6 months, the genus Bifidobacterium was significantly higher in patients on FMT compared with those on pentoxifylline, where Aerococcaceae were significantly higher. Biomarker discovery on predictive functional metabolic pathway analysis revealed that glycerophospholipid metabolism, which is associated with the maintenance of cell membrane stability to negate hypoxic stress aimed at relieving cell injury, was significantly upregulated at 6 months in patients undergoing FMT. Interestingly, the network analysis showed beneficial bacterial taxa in the FMT group were Propionibacterium and Bifidobacterium as a central influencer at 3- and 6-month follow-up, respectively. In the pentoxifylline group, Coriobacteriaceae and Micrococcaceae worked as a central influencer at 3 and 6 months post-therapy, respectively (Figures 3 and 4).
Figure 4.
Bar charts of most significant results using the LDA Effect Size method (LEfSe; at end of 6 months of follow-up). (A)–(C) Those bacterial groups that showed statistical significance effect size and associate them between treatment groups in the context of the highest median. Dotted lines represent medians; straight lines represent averages; (D) Topography of network analysis represented by radial construct showing specific central bacterial taxa predominating between treatment groups at end of 3 and 6 months of follow-up. FMT, fecal microbiota transplantation; PTX, pentoxyfilline.
Adverse effects
The commonest adverse event noted in patients on pentoxifylline was abdominal pain (32.0%, n = 8) followed by vomiting (16.0%, n = 4) and these patients did not require dose modification. The most common adverse events noted in patients on FMT included flatulence (25.5%, n = 12), self-limiting diarrhea (8.5%, n = 4), and mild nasal bleeding (4.3%, n = 2), and they required a change of nasoduodenal tube on Days 3 and 4 of therapy. No other major or life-threatening adverse events were noted as directly related to treatment in both groups.
Discussion
The early evidence-based use of FMT in humans was for pseudomembranous colitis caused by Micrococcus pyogenes via fecal enema in 1958 in a series of four patients by Eiseman and colleagues [13]. In patients with severe alcohol-associated liver disease, intestinal dysbiosis was strongly associated with the initiation and progression of this type of liver disease and linked with specific clinical events such as hepatic encephalopathy, acute kidney injury, and sepsis [14]. Intestinal microbiota as the potential target for the management of severe alcohol-induced liver injury was put forth by Llopis et al. [14]. Ferrere and coworkers [15] demonstrated that gut microbiota modulation was associated with the prevention of dysbiosis and amelioration of alcohol-induced liver injury in mice models. The pilot human study on FMT in subjects with steroid-ineligible SAH demonstrated an improvement in 1-year survival rates compared with those in historical controls (87.5% vs 33.3%) [11]. In the steroids or pentoxifylline for alcoholic hepatitis (STOPAH) trial, a non-significant mortality benefit was shown in the prednisolone-treated group at 28 days, which was not seen at the end of 3 months and 1 year, while pentoxifylline did not improve survival in patients with SAH at any time [16].
In our study, we analysed liver disease severity, clinical events, and survival outcomes between SAH patients who either underwent FMT or pentoxifylline at 3 months. We found significant improvement in ascites and a reduction in overt hepatic encephalopathy in patients on FMT. Furthermore, a significantly higher proportion of patients with SAH survived at the end of 3 months in the FMT group than in the pentoxifylline group. This is noteworthy as none of the currently recommended conventional medical treatments is associated with improved survival in patients with SAH beyond 28 days. Supportive of the clinical improvement, we also found consequential changes in bacterial communities before and after FMT that were associated with the beneficial modulation of functional metabolic pathways in the gut microenvironment. In the study by Philips et al. [11], FMT in SAH patients was associated with significant changes in the intestinal microbiome. Firmicutes, the dominant phylum among healthy donors, was preferentially abundant in recipients with FMT at 1-year follow-up [11] whereas the reduction in pathogenic Proteobacteria as well as an increase in beneficial short-chain fatty acid producing Actinobacteria showed after FMT [11]. The authors also found that the relative abundance of K. pneumonia reduced, and that beneficial taxa such as Megasphaera elsdenii, Bifidobacterium longum, and Enterococcus villorum increased at 6 months and 1 year after FMT [11]. In another open-label study, researchers compared clinical outcomes of SAH patients on FMT, pentoxifylline, corticosteroid, or nutritional therapy at 3 months and found that the highest survival proportion showed in the FMT group among them [17]. The relative abundance of pathogenic taxa such as Bilophila, Enterobacter, and Klebsiella decreased and that of beneficial genera such as Bacteroides, Parabacteroides, and Porphyromonas increased at the end of a week as well as the relative abundance of Roseburia and Micrococcus increased beyond 30 days after FMT [17]. Interestingly, the functional metabolism of the bacterial communities also changed post-FMT. The beneficial peroxisome proliferator-activated receptor signaling pathways were significantly upregulated while deleterious lipopolysaccharide signaling pathways were downregulated [17].
The first study on FMT in patients with alcoholic hepatitis-related acute-on-chronic liver failure (ACLF) demonstrated an overall survival rate of 66% at 548 days of follow-up [18]. The survival rates in ACLF patients with higher grades at the end of 548 days was 58.3%, demonstrating proof of concept on the benefits of FMT in this very sick group with SAH-related ACLF [18]. Similarly, a recent study from India by researchers at the Post Graduate Institute, Chandigarh also validated findings that FMT was safe, improved short-term and medium-term survival, and led to improvement in clinical severity scores in patients [19]. Bajaj et al. [20] showed that FMT from a rational stool donor improved hepatic encephalopathy in a randomized clinical trial that is in accordance with our findings. Recently, the same group demonstrated that antibiotic-associated disruption of microbiota composition and function in cirrhosis was restored by healthy donor FMT [21]. In this study, we found that the incidence of Gram-negative bacterial infections and Candida-related infections was higher in those treated with pentoxifylline than in those on FMT. In a similar vein, a study analysing 3-year outcomes in patients with SAH found that FMT was associated with significantly lesser ascites, infections, encephalopathy, and alcohol relapse (with a trend toward higher survival rates) compared with corticosteroid therapy, which was strikingly associated with beneficial gut microbiota modulation even in the long term [22]. In our previous study on FMT in alcohol-associated liver disease treatment, we demonstrated a significant increase in the abundance of beneficial bacterial taxa producing short-chain fatty acids, such as Propionibacterium and Bifidobacterium, which were related to good clinical outcomes on follow-up [11, 17].
We also affirm beneficial modulation of microbial metabolic pathways that possibly correlate with clinical improvement in our patients. The functional metabolic pathway analysis in the current study revealed that glycerophospholipid metabolism signaling was upregulated at 6 months in those receiving FMT. Ethanol-induced hypoxia and related alterations in cytokine production, which lead to hepatocyte oxidant stress, is a well-known factor that promotes hepatitis and disease progression in alcohol-associated liver disease. The metabolites of the glycerophospholipid pathway have been shown to maintain the stability of cell membranes against hypoxic stress and relieve cell injury. The studies show early proof of concept that microbial modulation via FMT could improve local as well as systemic inflammatory signaling to benefit host and improve clinical outcomes [23, 24]. In the present study, the topographical evaluation of network analysis on bacterial communities from baseline to post-treatment revealed that beneficial taxa Propionibacterium and Bifidobacterium were central influencers in the FMT group, and Coriobacteriaceae and Micrococcaceae were central influencers in the the pentoxifylline group. Despite its ability to behave as an opportunistic pathogen, Propionibacterium, as a part of the normal human microbiota, consequently also plays a role in human health via occupation of niches that could be colonized by other, more pathogenic, microorganisms. The significant immunomodulatory properties of Propionibacterium via its functional metabolism also make it able to protect the host against Th-2-mediated diseases [25].
Bifidobacterium-mediated health benefits result due to the complex dynamic interplay between other members of the gut microbiota and the human host. Bifidobacteria can inhibit pathogens by producing organic acids, antibacterial peptides, quorum-sensing inhibitors, and immune stimulation [26]. The ability of Bifidobacteria to expand the T-regulatory response via production of short-chain fatty acids makes it a potent gut microbiota member that maintains local and systemic immune functions and intestinal barrier function [26]. Bifidobacteria were reported to prevent gastrointestinal infections by competitive exclusion of pathogens based on common binding sites on epithelial cells. Thus, it is possible that post-FMT expansion of Bifidobacterium was an important aspect that played a key role in the clinical outcome and survival benefit in patients with SAH [26, 27]. Coriobacteriaceae are usually non-motile, non-spore-forming, non-hemolytic, and strictly anaerobic bacteria that include 30 species belonging to 14 genera, e.g. Eggerthella. They are considered pathobionts because their occurrence has been associated with various pathologies, such as bacteremia, periodontitis, and vaginosis. In pentoxifylline-treated patients, Coriobacteriaceae were found to be central influencers on follow-up with significant elevation of Eggerthella. In a large study analysing the correlation of health and disease markers with gut microbiome composition across thousands of people, Eggerthella was been described as an opportunistic pathogen associated with disease causation and a putative opportunistic pathogen [28]. The finding from a group of patients in the present study possibly incriminates pentoxifylline use with poor clinical outcomes compared with FMT. Besides, Citrobacter species, which are known to be associated with enhanced host energy metabolism as well as potent sources of inflammatory cytokines and promoting robust innate and adaptive responses to infection, were upregulated after FMT [29, 30].
The current study was a single-center retrospective study with a small number of patients. Our findings need to be confirmed in larger prospective controlled trials. Long-term outcomes of FMT in a randomized–controlled trial (RCT) setting are lacking and remain an unmet need. We did not consider the role of environmental factors and regional dietary practices that could have altered gut microbiota after FMT in our patients and also in the donors during the study period. However, the microbiota changes that were demonstrated correlated well with studied clinical events and were also in line with the current literature. Thus, our proof-of-concept paper on the comparison of FMT to pentoxifylline for SAH demonstrates improvement in the severity of SAH with better transplant-free survival beyond a month (≤6 months) that is not seen with conventional guideline-based medical management. Larger prospective multicentre RCTs on FMT and further metagenomic insights into beneficial bacterial communities and functional pathways remain highly anticipated.
Authors’ Contributions
C.A.P. and R.A. conceived and designed the project. C.A.P., R.A., A.T., and J.K.A. collected the data. A.T., C.A.P., and R.A. analysed and interpreted the data. C.A.P., R.A., and A.T. drafted the manuscript. S.R., S.S., and P.A. revised and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
None.
Contributor Information
Cyriac Abby Philips, Clinical and Translational Hepatology, The Liver Institute, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India; Monarch Liver Laboratory, The Liver Institute, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Rizwan Ahamed, Department of Gastroenterology and Advanced GI Endoscopy, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Sasidharan Rajesh, Diagnostic and Interventional Radiology, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Shobhit Singh, Diagnostic and Interventional Radiology, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Ajit Tharakan, Department of Gastroenterology and Advanced GI Endoscopy, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Jinsha K Abduljaleel, Department of Gastroenterology and Advanced GI Endoscopy, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Philip Augustine, Monarch Liver Laboratory, The Liver Institute, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India; Department of Gastroenterology and Advanced GI Endoscopy, Center of Excellence in GI Sciences, Rajagiri Hospital, Chunangamvely, Aluva, Ernakulam, Kerala, India.
Funding
None.
Conflict of Interest
None declared.
References
- 1. Abenavoli L, Milic N, Rouabhia S. et al. Pharmacotherapy of acute alcoholic hepatitis in clinical practice. World J Gastroenterol 2014;20:2159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casanova J, Bataller R.. Alcoholic hepatitis: prognosis and treatment. Gastroenterol Hepatol 2014;37:262–8. [DOI] [PubMed] [Google Scholar]
- 3. Forrest E, Mellor J, Stanton L. et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials 2013;14:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spengler EKJ, Dunkelberg J, Schey R.. Alcoholic hepatitis: current management. Dig Dis Sci 2014;59:2357–66. [DOI] [PubMed] [Google Scholar]
- 5. Altamirano J, Miquel R, Katoonizadeh A. et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papastergiou V, Burroughs AK, Tsochatzis EA.. Prognosis and treatment of patients with acute alcoholic hepatitis. Expert Rev Gastroenterol Hepatol 2014;8:471–86. [DOI] [PubMed] [Google Scholar]
- 7. Lee YS, Kim HJ, Kim JH. et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017;51:364–77. [DOI] [PubMed] [Google Scholar]
- 8. Singal AK, Duchini A.. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011;3:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015;148:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parker R, Armstrong MJ, Corbett C. et al. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther 2013;37:845–54. [DOI] [PubMed] [Google Scholar]
- 11. Philips CA, Pande A, Shasthry SM. et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600–2. [DOI] [PubMed] [Google Scholar]
- 12. Philips CA, Augustine P, Yerol PK. et al. Modulating the intestinal microbiota: therapeutic opportunities in liver disease. J Clin Transl Hepatol 2020;8:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eiseman B, Silen W, Bascom GS. et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958;44:854–9. [PubMed] [Google Scholar]
- 14. Llopis M, Cassard AM, Wrzosek L. et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–9. [DOI] [PubMed] [Google Scholar]
- 15. Ferrere G, Wrzosek L, Cailleux F. et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 2017;66:806–15. [DOI] [PubMed] [Google Scholar]
- 16. Thursz MR, Richardson P, Allison M. et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 17. Philips CA, Phadke N, Ganesan K. et al. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol 2018;37:215–25. [DOI] [PubMed] [Google Scholar]
- 18. Philips CA, Augustine P, Padsalgi G. et al. Only in the darkness can you see the stars: severe alcoholic hepatitis and higher grades of acute-on-chronic liver failure. J Hepatol 2019;70: 550–1. [DOI] [PubMed] [Google Scholar]
- 19. Sharma A, Roy A, Premkumar M. et al. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int 2022;16:433–46. [DOI] [PubMed] [Google Scholar]
- 20. Bajaj JS, Kassam Z, Fagan A. et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajaj JS, Kakiyama G, Savidge T. et al. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018;68:1549–58. [DOI] [PubMed] [Google Scholar]
- 22. Philips CA, Ahamed R, Rajesh S. et al. Long-term outcomes of stool transplant in alcohol-associated hepatitis: analysis of clinical outcomes, relapse, gut microbiota and comparisons with standard care. J Clin Exp Hepatol 2022;12:1124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tilg H, Moschen AR, Kaneider NC.. Pathways of liver injury in alcoholic liver disease. J Hepatol 2011;55:1159–61. [DOI] [PubMed] [Google Scholar]
- 24. Xia Z, Zhou X, Li J. et al. Multiple-omics techniques reveal the role of glycerophospholipid metabolic pathway in the response of Saccharomyces cerevisiae against hypoxic stress. Front Microbiol 2019;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDowell A, Patrick S, Eishi Y. et al. Propionibacterium acnes in human health and disease. Biomed Res Int 2013;2013:493564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hidalgo-Cantabrana C, Delgado S, Ruiz L. et al. Bifidobacteria and their health-promoting effects. Microbiol Spectr 2017;5. https://doi.org/10.1128/microbiolspec.BAD-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Callaghan A, van Sinderen D.. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 2016;7:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manor O, Dai CL, Kornilov SA. et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun 2020;11:5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffmann C, Hill DA, Minkah N. et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect Immun 2009;77:4668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang ML, Li M, Sheng Y. et al. Citrobacter species increase energy harvest by modulating intestinal microbiota in fish: nondominant species play important functions. mSystems 2020;5:e00303-20. [DOI] [PMC free article] [PubMed] [Google Scholar]