Abstract
Irregularities in nuclear shape and/or alterations to nuclear size are a hallmark of malignancy in a broad range of cancer types. Though these abnormalities are commonly used for diagnostic purposes and are often used to assess cancer progression in the clinic, the mechanisms through which they occur are not well understood. Nuclear size alterations in cancer could potentially arise from aneuploidy, changes in osmotic coupling with the cytoplasm, and perturbations to nucleocytoplasmic transport. Nuclear shape changes may occur due to alterations to cell-generated mechanical stresses and/or alterations to nuclear structural components, which balance those stresses, such as the nuclear lamina and chromatin. A better understanding of the mechanisms underlying abnormal nuclear morphology and size may allow the development of new therapeutics to target nuclear aberrations in cancer.
Keywords: nuclear lamina, nuclear envelope, cytoskeleton, heterochromatin, euchromatin, chromatin compaction, LINC complex, pathology
Introduction
Regular diagnostic screening and histopathological analysis of patient samples are instrumental in cancer detection. These analyses are also crucial for informing prognosis and, ultimately, developing treatment plans. For pathologists to formulate holistic diagnosis and prognosis, many criteria need to be considered, including biomarker status, cell structure, tissue structure, tumor size, and lymph node status. In the context of cell and tissue structure, pathologists commonly use abnormalities in nuclear shape and size to grade different cancers (Zink et al. 2004).
Nuclear shape abnormalities have been observed since the early days of cytology. In the mid-nineteenth century, Beale was one of the first to observe unusual nuclear morphology in various cancer types and assign diagnostic value to differences in nuclear size and shape (Beale 1860; de las Heras and Schirmer 2014). Other early work includes Bennett’s observations of multinucleation and increased numbers of nucleoli (Bennett 1849) and Lebert’s discovery of increased ratio of nuclear size to cellular size (Lebert 1851), or karyoplasmic ratio (Wilson 1925), in cancer cells.
Nuclear metrics currently used in pathology to grade cancer tissue include abnormal nuclear size, nuclear contour irregularities, hyperchromasia (dark nuclear staining due to increased DNA content), and aberrant distribution of chromatin (Fischer 2020). Figure 1 shows a representative schematic of a nucleus from a healthy cell and a nucleus from a cancerous cell. Enlarged nuclei and variation in size have been observed in breast cancer (Abdalla et al. 2009; Kashyap et al. 2018), melanoma (Mijovic et al. 2013), prostate (Epstein et al. 1984; Diamond et al. 1982), cervical (Papanicolaou and Traut 1941), head and neck (Giardina et al. 1996; Speight 2007), bladder (Bhatia et al. 2006), and kidney cancers (Nativ et al. 1996). (Table 1) In some cases, increased nuclear size can be a signifier of poor prognoses and clinical outcomes. For instance, in breast cancer, enlarged nuclei correlated with higher histological grade, increased numbers of affected lymph nodes, and greater tumor size (Alhudiri et al. 2019). Similarly, in mucinous ovarian cancer, increased nuclear size is correlated with increased proliferation rates and reduced probability of survival (Zeimet et al. 2011). Conversely, in osteosarcoma, decreased nuclear size along with contour irregularities indicate malignancy (de Andrea et al. 2011). Nuclear size may potentially be used to assess treatment efficacy – tumor regression in breast cancer after anti-estrogen treatment correlated with decreased nuclear size in tumor cells (Samarnthai et al. 2012).
Figure 1.
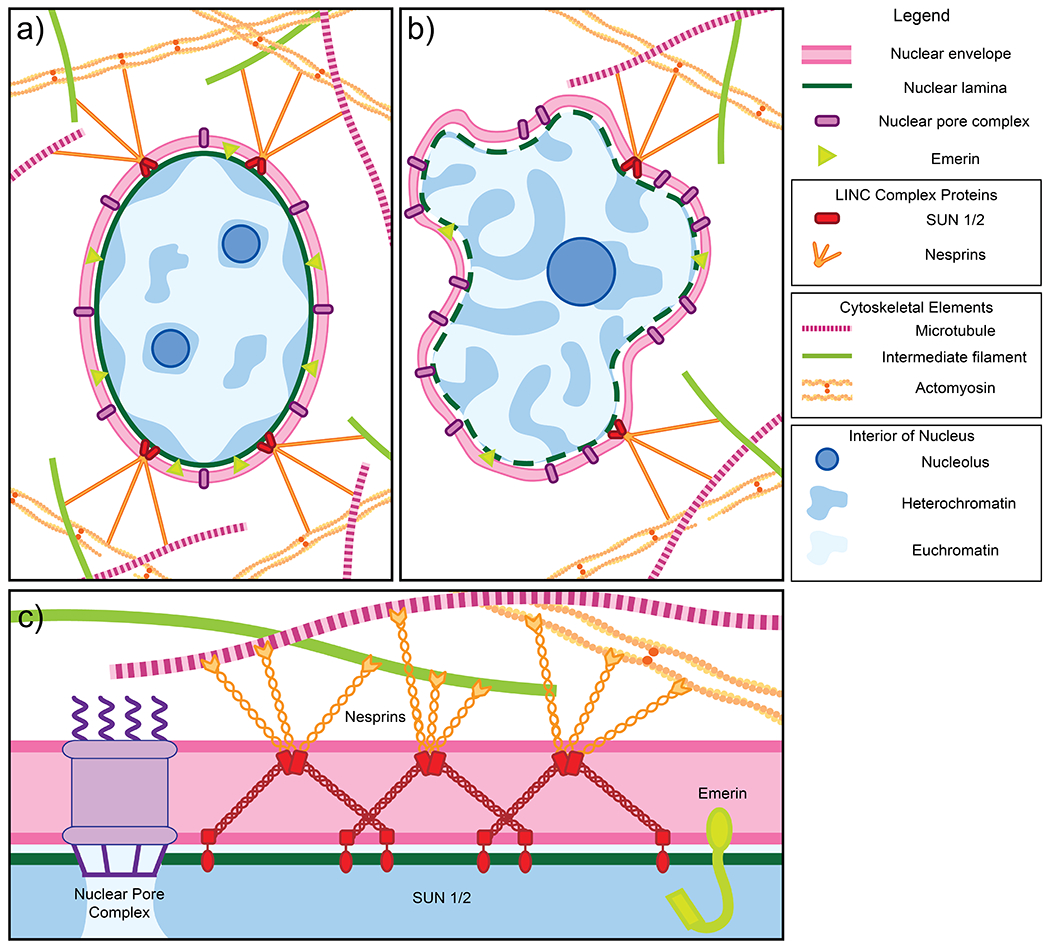
a) Schematic of a normal nucleus. The nucleus is enclosed by the nuclear envelope (pink), which consists of an inner and outer lipid membrane. The inner membrane is studded with numerous proteins including LEM domain proteins such as emerin (light green). Other protein complexes span both the inner and outer membranes, including nuclear pore complexes (purple), which facilitate nucleocytoplasmic transport, and the LINC complex, comprised of SUN1/2 (red) and nesprins/KASH proteins (orange), which transmits mechanical force from the cytoskeleton to the nucleus. The lamina (dark green), a fibrillar mesh network, provides structure and support to the nucleus. Peripheral heterochromatin (dark blue regions) that is compacted and less accessible for transcription is anchored to the nuclear lamina. Euchromatin (light blue regions) are less compacted and more accessible for transcription. Normal cells usually have two to three nucleoli (dark blue circles), providing sites for ribosome assembly. b) Schematic of a cancerous nucleus and examples of changes to nuclear structure and organization, as observed in human samples, which may contribute to abnormalities in size and shape. Cancerous nuclei in many tissue types exhibit downregulation of a number of proteins including emerin, SUN1/2, and nesprins. In contrast, nucleoporins may be upregulated. Different cancer types are characterized by abnormal expression of nuclear lamins. Finally, chromatin organization is also often disrupted in nuclei of cancerous cells. c) A schematic of a cross-section of the nuclear envelope. Shown in greater detail is the structure of the LINC complex. Two SUN trimers and three KASH dimers interact via SUN-KASH 6:6 complexes which serve as nodes for force transduction and distribution (Gurusaran and Davies 2021).
Table 1.
Changes in nuclear shape, nuclear size, chromatin texture, and nuclear protein expression across different cancer types.
| Cancer Type | Changes in nuclear shape | Changes in nuclear size | Changes in chromatin/nucleoli | Changes to nuclear proteins |
|---|---|---|---|---|
| Bladder cancer | Enlarged mean nuclear area (Krüger and Müller 1995) High nuclear to cytoplasmic size ratio (Bhatia et al. 2006) |
Variation in nuclear size (Bhatia et al. 2006) | Coarse chromatin (Bhatia et al. 2006) | |
| Breast cancer | Variation in nuclear shape (Abdalla et al. 2009; Kashyap et al. 2018) | Enlarged nuclear area, variation in nuclear area (Abdalla et al. 2009; Kashyap et al. 2018) | Reduced levels or loss of lamin A/C (Alhudiri et al. 2019; Capo-Chichi et al. 2011b; Matsumoto et al. 2015) Ratio of mRNA levels for lamin C:lamin A increased in breast cancer samples (Aljada et al. 2016) Loss of SUN1, SUN2, nesprins (Matsumoto et al. 2015) Mutated nesprins 1, 2 (Sjöblom et al. 2006; Stephens et al. 2012) Nup88 overexpression (Agudo et al. 2004; Gould et al. 2002) |
|
| Cervical cancer | Irregular nuclear shapes (Papanicolaou and Traut 1941) | Large nuclei (Papanicolaou and Traut 1941) | Speckled chromatin, one or more nucleoli (Papanicolaou and Traut 1941) | Nup88 overexpression (Brustmann and Hager 2009) |
| Colorectal cancer | Irregular nuclear contours with folds and dents (Fischer 2020) Abnormal morphology (Mulder et al. 1992) | Large nuclei (Fischer 2020) | Coarse chromatin (Fischer 2020; Mulder et al. 1992) | Low levels of lamin A/C expression (Belt et al. 2011; Moss et al. 1999) Abnormal levels of lamin B1/type B lamins (Moss et al. 1999; Alfonso et al. 2008) Aberrant staining of lamins in cytoplasm (Moss et al. 1999) Mutated nesprin 1 (Sjöblom et al. 2006) Nup88 overexpression (Emterling et al. 2003; Knoess et al. 2006) |
| Gastric cancer | Reduced levels or loss of lamin A/C (Moss et al. 1999; Wu et al. 2009) Reduced lamin B1 levels (Moss et al. 1999) Nup88 overexpression (Gould et al. 2002) |
|||
| Head and neck cancer | Nuclear contour irregularities and nuclear shape asymmetry(Giardina et al. 1996) | Abnormal variation in cell size (Speight 2007) | Hyperchromatic nuclei, increased number of nucleoli(Speight 2007) | |
| Renal cell carcinoma | Irregular contours, multilobed nuclei (Nativ et al. 1996) | Enlarged nuclei (Nativ et al. 1996) | Prominent and irregular nucleoli (Nativ et al. 1996) | Nup88 overexpression (Gould et al. 2002) |
| Liver cancer | Abnormal chromatin texture (Atupelage et al. 2014) | Lamin B1 is upregulated (Sun et al. 2010; Lim et al. 2002) Nup88 overexpression (Gould et al. 2002) |
||
| Lung cancer | Malleable nuclei with crush artifacts (DeMay 2012) Nuclear pleomorphisms (Petersen et al. 2009) |
Reduced expression or absence of B-type lamins in small-cell lung cancers (SCLCs)(Broers et al. 1993) Aberrant, cytoplasmic expression of A-type lamins in non-SCLCs (Broers et al. 1993) Nup88 overexpression (Gould et al. 2002; Zhang et al. 2007b) |
||
| Melanoma | Variation in nuclear circularity (Mijovic et al. 2013) | Increased nuclear size (Mijovic et al. 2013) | Reduced or complete loss of lamin A/C (Venables et al. 2001) High levels of lamin A/C (Tilli et al. 2003) Nup88 overexpression (Gould et al. 2002) |
|
| Ovarian cancer | Irregular nuclear membrane contours (Liu et al. 1999; Gurley et al. 1994) | Irregular nuclear size (Liu et al. 1999; Gurley et al. 1994) | Heterogeneous expression or total Increased levels of lamin B1 and B2 (Bengtsson et al. 2007) Loss of lamin A/C (Capo-chichi et al. 2011a) Loss of emerin (Capo-chichi et al. 2009) Mutations in nesprins (Doherty et al. 2010) Nup88 overexpression (Martínez et al. 1999; Gould et al. 2002) High levels of lamin A/C (Wang et al. 2009) |
|
| Pancreatic cancer | Irregular nuclear shape (Sato et al. 1993; Furuta et al. 1992; Barr Fritcher et al. 2007; Flores et al. 2021) | Overexpression of lamin B1 (Li et al. 2013) Overexpression of Nup88 (Gould et al. 2002) |
||
| Papillary thyroid carcinoma | Grooves and long clefts (Fische et al. 2003 ; Rosai et al. 1992) | Variability of nuclear size (Wright and Castles 1987) | Coarse heterochromatin (Fischer et al. 2003) | |
| Prostate cancer | Irregular contours and decreased roundness (Epstein et al. 1984; Diamond et al. 1982) | Enlarged nuclei (Veltri et al. 1994) | Lamin B upregulated (Coradeghini et al. 2006) Nup88 overexpression (Gould et al. 2002) |
In this chapter, we discuss mechanisms by which nuclear size and shape alterations occur in cancer.
Regulation of nuclear size
Aneuploidy, or an abnormal number of chromosomes, is highly prevalent in cancer, occurring in almost 90% of all solid tumors (Taylor et al. 2018), and is caused by chromosome instability or an increased rate of chromosome missegregation during mitosis (Ben-David and Amon 2020; Capo-Chichi et al. 2016). An increase in the number of chromosomes should increase the nuclear size and vice versa. As such, it is surprising that aneuploidy does not necessarily correlate with nuclear size changes in cancer. For example, an absence of correlation between nuclear size and aneuploidy has been reported for colon cancer (Dangou et al. 1993). In bladder cancer, there are opposing reports of a correlation and an absence of correlation (Van Velthoven et al. 1995; Helander and Tribukait 1988); the reasons for the differences between these reports are unclear. Nuclear size does correlate with aneuploidy in ovarian cancer (Zeimet et al. 2011) and in prostate cancer (Wang et al. 1992). Thus, aneuploidy may contribute to increased nuclear size in some but not all cancer types.
Alterations to nucleo-cytoplasmic transport through nuclear pores can also alter nuclear size. Nucleoporins, proteins that line nuclear pores, are upregulated in many different cancer types, including ovarian, colorectal, and breast cancer, as well as lymphomas and leukemia (Denais and Lammerding 2014). A high-throughput imaging RNAi screen identified nucleoporin ELYS as a determinant of nuclear size in mammalian cells (Jevtić et al. 2019). ELYS knockdown in breast cancer cells resulted in small nuclei and decreased nuclear import; in contrast, ELYS overexpression induced increased nuclear area and increased nuclear import (Jevtić et al. 2019). Similarly, knockdown of nuclear transport protein exportin 1 also increased nuclear size (Jevtić et al. 2019), demonstrating that nuclear transport may play a significant role in nuclear size maintenance and that its dysregulation can alter nuclear size in cancer.
Nuclear size alterations in cancer may alternatively occur because of alterations to the osmotic coupling between the nucleus and the cytoplasm (Finan and Guilak 2010; Katiyar et al. 2019). That the nucleus and the cytoplasm is osmotically coupled is evident from the fact that nuclear volume is correlated strongly with cytoplasmic volume (Finan and Guilak 2010; Katiyar et al. 2019; Jorgensen et al. 2007; Neumann and Nurse 2007). An increase in cytoplasmic volume is predicted to correlate with nuclear size. However, there are no systematic studies to our knowledge that have attempted to correlate these two parameters in human cancer tissues.
Perturbations to the nuclear lamins in cancers
The nuclear lamina is a mechanically stiff, mesh-like network located at the interface between the inner nuclear membrane and chromatin. This network is composed of nucleus-specific intermediate filaments: A-type lamins (lamins A and C) and B-type lamins (lamins B1 and B2). Alterations to proteins of the lamina have been associated with not only cancer but also with multiple degenerative disease states such as Hutchison-Gilford progeria, Emery-Dreifuss muscular dystrophy, and dilated cardiomyopathy (Davidson and Lammerding 2014).
Nuclear lamin expression is altered in numerous cancer types and is often associated with advanced disease state and poor survival. Reduced expression or complete absence of lamin A/C has been observed in breast cancer (Alhudiri et al. 2019; Matsumoto et al. 2015; Capo-Chichi et al. 2011b), melanoma (Venables et al. 2001), colorectal cancer (Belt et al. 2011; Moss et al. 1999) and gastric cancer (Moss et al. 1999; Wu et al. 2009). (Table 1)
In breast cancer, reduced lamin A/C mRNA and protein levels correlated with indicators of poor prognosis such as high histological grade, larger tumor size, poor Nottingham prognostic index, high invasiveness, and development of metastases in addition to decreased survival rates (Alhudiri et al. 2019; Wazir et al. 2013; Aljada et al. 2016). Similarly, reduced lamin A/C levels or complete absence correlated with increased chances of disease recurrence in patients diagnosed with stage II and stage III colorectal cancer (Belt et al. 2011). In ovarian cancer, there is heterogeneous expression and complete loss of lamin A/C expression (Wang et al. 2009; Capo-chichi et al. 2011a). Lamin A/C protein levels are downregulated in highly proliferative cells in basal cell carcinomas (BCCs) of the skin (Venables et al. 2001). In contrast, in skin, others have reported elevated lamin A/C in the basal cell layer of BCCs, squamous cell carcinomas, and actinic keratosis (Tilli et al. 2003).
Type B lamins also deviate from normal levels in breast cancer, lung cancer, and prostate cancer. Low lamin B1 mRNA levels corresponded to poor clinical outcomes in breast cancer patients (Wazir et al. 2013). Reduced expression or complete loss of B-type lamins occurs in small-cell lung cancers (Broers et al. 1993). Conversely, in prostate cancer, elevated lamin B expression correlated positively with tissues scored with higher Gleason scores which quantify poorly differentiated, more aggressive cancer cells. (Coradeghini et al. 2006)
Overall, alterations to nuclear lamin levels are well documented across a broad range of cancers and correlations with poor prognosis suggest a relation between the nuclear lamina and cancer progression.
Implications of nuclear lamin alterations for nuclear morphology
Nuclear morphological irregularities encompass a broad collection of alterations in the otherwise smooth surface of the nucleus, including grooves, dents, polylobulated nuclei (that is, nuclei with multiple lobes), a crushed appearance, budding, fragmentation, thickened contours, and folds (Fischer 2020; Dey 2010). Nuclear morphology in mammalian cells is determined primarily through a balance between mechanical forces generated by cells on the nuclear surface, and balancing mechanical stresses that develop in the nuclear lamina and chromatin (reviewed by us in Ref. (Lele et al. 2018)). Nuclear shape may be altered in cancer due to changes in cellular forces on the nucleus and/or changes in the mechanical stiffness of the nuclear lamina or chromatin.
There is contrasting evidence on whether lamin A/C downregulation causes abnormal nuclear morphologies. Lamin A/C knockdown did not result in abnormal nuclear contours in human mammary epithelial cells (Tamashunas et al. 2020). In contrast, lamin A/C knockdown in primary human ovarian surface epithelial (HOSE) cells results in abnormal nuclear shapes and aneuploidy (Capo-Chichi et al. 2016; Capo-chichi et al. 2011a). Overall, alterations to lamin A/C may cause nuclear morphological alterations in some cell types but not others.
Nuclear lamins are important determinants of the mechanical stiffness of the nucleus (Pajerowski et al. 2007; Neelam et al. 2015; Dahl et al. 2004; Stephens et al. 2018), which might contribute to alterations in nuclear morphology (Davidson and Lammerding 2014). Alternatively, reduced lamin A/C expression may promote mitotic failure and the consequent development of abnormally shaped aneuploid nuclei (Smith et al. 2018).
In cultured cells, depletion of lamin B1 is known to promote blebbing in the nuclear envelope (Lammerding et al. 2004; Hatch and Hetzer 2016). Compression of the nucleus in spread cells by the overlaying F-actin cortex results in a nuclear pressure. When the nuclear lamina is weakened, the envelope delaminates from the lamina and blebs, similar to how blebs develop in the cellular plasma membrane upon delamination from the F-actin cortex. Nuclear blebs can rupture, mixing cytoplasmic and nucleoplasmic contents, leading to DNA damage (Denais et al. 2016). Cancer cells with ruptured nuclei can migrate more invasively (de Freitas Nader et al. 2021); thus alterations to nuclear lamins may promote cancer progression by promoting nuclear ruptures.
Chromatin regulators and nuclear morphology in cancer
In general, chromatin has two types – tightly packed, compacted heterochromatin inaccessible for transcription, and loosely packed euchromatin, which is transcriptionally active. The main structural subunit of chromatin is the nucleosome, consisting of DNA wound around a histone. Chromatin structure can be altered through post-translational histone modifications, which affect histone-histone and histone-DNA interactions. The most well-studied post-translational histone modifications include acetylation, methylation, ubiquitination, and phosphorylation. Histone acetylation increases euchromatin content, while histone methylation increases heterochromatin.
In diagnosing malignant nuclei, pathologists look not only for nuclear shape irregularities but also for abnormalities in chromatin texture. Coarse chromatin texture has been observed in papillary thyroid carcinoma (Fischer 2020), cervical cancer (Papanicolaou and Traut 1941), and bladder cancer (Bhatia et al. 2006). This abnormal chromatin texture is likely caused by variation in heterochromatin distribution (Fischer 2020; Bhatia et al. 2006; Zink et al. 2004).
Histone modifications and nuclear morphology.
Increasing euchromatin or decreasing heterochromatin by modifying histones induces blebbing and nuclear contour irregularities (Stephens et al. 2018). The mechanism by which chromatin state alters nuclear shape may be due to a mechanical softening of the nucleus (Stephens et al. 2019). For example, treatment of cells with histone deacetylase inhibitors and histone methyltransferase inhibitors to increase euchromatin and decrease heterochromatin, respectively, in mouse embryonic fibroblasts (MEFs) and in human cancer cell lines caused nuclei to become softer (Stephens et al. 2018). The soft nuclei were prone to blebbing. The changes occurred independently of alterations to lamin A/C and lamin B1 in MEFs. Conversely, MEFs treated with histone demethylase inhibitors increased heterochromatin content, stiffened nuclei, and reduced blebbing. Likewise, inhibiting histone demethylases also normalized irregular nuclear shapes in Hutchinson-Gilford progeria syndrome (HGPS) patient-derived fibroblasts by increasing nuclear stiffness.
Dysregulation of histone-modifying enzymes may alter nuclear size and shape in human cancer tissue. For example, overexpression of histone acetyltransferase p300 in prostate cancer correlates with poor survival rates (Debes et al. 2003) and is implicated in cancer progression (Debes et al. 2002). Transfection of histone acetyltransferase p300 into prostate cancer cells grown in culture increases nuclear size (Debes et al. 2005).
Histones and histone-binding proteins.
Consistent with the above pharmacological studies, altered expression of histones or histone binding proteins promotes nuclear shape irregularities. Knockdown of H1F0, a gene that encodes linker histone H1.0, caused nuclear contour abnormalities in mammary epithelial cells (Tamashunas et al. 2020). H1.0 levels are correlated with tumor differentiation status, cancer cell sternness, and overall patient survival (Torres et al. 2016). Depletion of macroH2A1 and macroH2A2 histone variants induced increases in nuclear size and abnormal nuclear morphology in mouse liver cells (Fu et al. 2015) and in human hepatoma cells (Douet et al. 2017).
HMGN5, a nucleosome-binding protein that reduces interactions between histone H1 and chromatin, is involved in chromatin decompaction (Rochman et al. 2009). HMGN5 overexpression in MEFs and thyroid epithelial cells results in decreased heterochromatin content, lowered nuclear stiffness, and increased nuclear blebbing (Furusawa et al. 2015). Overexpression of HMGN5 in mice induced cardiac defects and cardiomyocytes featuring enlarged, decompacted, and deformed nuclei with disrupted lamina in vivo (Furusawa et al. 2015). Depletion of HMGN2, a member of the same protein family as HMGN5, also resulted in nuclear contour irregularities in mammary epithelial cells (Tamashunas et al. 2020).
Chromatin binding proteins.
Perturbations to chromatin remodeling proteins and enzymes other than histones have downstream effects which alter chromatin compaction and, subsequently, nuclear shape and size. The Polycomb complexes transfer ubiquitin residues and recruit methyltransferases to repress transcription (Richly et al. 2011). SUZ12, a Polycomb PRC2 complex subunit that can repress genes via trimethylation of lysine 27 on histone H3 (H3K27me3), is overexpressed in multiple cancer types (Lee et al. 2015; Li et al. 2012). SUZ12 knockdown in mammary epithelial cells promoted irregular nuclear shape (Tamashunas et al. 2020). Similarly, depletion of RING1B, a subunit of the Polycomb PRC1 complex, induces increased nuclear area and hyperploidy in mouse embryonic stem cells (Boyle et al. 2020).
BRG1 is a member of the SWI/SNF complex family, which includes ATP-dependent chromatin remodeling enzymes that destabilize histone-DNA contacts on the nucleosome. Knockdown of BRG1 in non-tumorigenic mammary epithelial cells promoted laminar grooves and multilobed nuclei (Imbalzano et al. 2013). Histone protein 1 (HP1) was not concentrated within these grooves, suggesting changes in chromatin compaction. Murine fibroblasts, which express ATPase-deficient versions of BRG1 increase cell volume and nuclear size (Hill et al. 2004).
DIDO1 knockdown in mammary epithelial cells caused irregular nuclear shapes (Tamashunas et al. 2020). DIDO1 has a role as an epigenetic reader and can recognize transcriptionally active H3K4me3 in a pH-dependent manner through the PHD finger motif, common to multiple chromatin-interacting proteins (Tencer et al. 2017). Furthermore, missense variants in DIDO1 have been implicated in hereditary colorectal cancer (Thutkawkorapin et al. 2019), and deletions frequently occur in myeloproliferative neoplasms on chromosome 20q where the encoding gene is located (Fütterer et al. 2005).
Oncogenes, cancer-related genes, and nuclear morphology.
Mutations in cancer-associated genes such as TP53 and RET/PTC are also associated with alterations to nuclear morphology.
TP53 is a well-studied cancer-associated gene that encodes the transcription factor p53, which serves a tumor suppressor function, protecting cells from unregulated proliferation. p53 mutations are observed in over 90% of uterine carcinomas and ovarian serous cystadenomas (Berchuck et al. 1994; Salani et al. 2008) as well as over 30% of breast cancers (Grossman et al. 2016). p53 positivity in breast cancer tissue correlated with the standard deviation of the nuclear shape factor used to assess nuclear shape irregularities (Friedrich et al. 1997). Mutations in p53 cause a loss of control over proliferation, which may lead to transmission of nuclear shape defects from mother to daughter cells as well (Tocco et al. 2018). In melanoma, p53 positive cells have increased nuclear size (Talve et al. 1996) and increased circularity (Mijovic et al. 2013), suggesting that p53 positivity may have mixed effects on nuclear shape in different tissue types. Increased p53 expression and decreased expression of p16INK4a, a regulator of tumor suppressor Rb, also correlated with increased nuclear size in lung cancer cells and adenocarcinomas (Okudela 2014).
p53 knockdown and expression in vitro also has varying effects on nuclear shape, depending on the cell culture system studied. p53 knockdown induced nuclear shape abnormalities in human breast epithelial cells (Tamashunas et al. 2020) and nuclear blebbing and nuclear envelope rupture in human retinal pigment epithelial cells due to nuclear enlargement (Yang et al. 2017). Conversely, transfection of p53 into p53-deficient colon cancer cells caused nuclei to become irregularly shaped (Yoon et al. 2019).
Proteins that interact with p53 may also cause abnormal nuclear shapes. For example, NOP53 ribosome biogenesis factor (NOP53) is a p53-binding protein that localizes to nucleoli and is involved in modulating DNA damage proteins using the MDM2-mediated polyubiquitination pathway (Lee et al. 2012). Downregulation of NOP53 in human cervical cancer cells led to chromosomal instabilities and multiple types of nuclear irregularities like enlarged nuclei, nuclear bud formation, micronucleation, and multinucleation (Lee et al. 2020). Also, depletion of tumor protein p63, a transcription factor belonging to the p53 family, decreased lamin A/C and lamin B1 expression in basal keratinocytes and caused irregular nuclear shape in mouse embryos. (Rapisarda et al. 2017)
Silencing of different cancer-associated genes can cause nuclear morphological changes in cultured cells in vitro. For example, knockdown of tumor suppressor Rb induced blebbing and nuclear envelope rupture in human retinal pigment epithelial cells (Yang et al. 2017). Additionally, knockdown of AT-rich interactive domain 1A, or ARID1A, a tumor suppressor gene reported in various cancers, led to increases in the nuclear area and the invasion capability and chemoresistance in canine kidney renal cells (Somsuan et al. 2019). AT-rich interactive domain 4A, or ARID4A, knockdown also induced nuclear shape irregularities in human mammary epithelial cells (Tamashunas et al. 2020). Loss of transcription factor GATA6 is commonly observed in ovarian cancer. GATA6 knockdown in HOSE cells increased nuclear size and caused nuclear shape abnormalities and aneuploidy (Capo-chichi et al. 2009). Mutation of the RET gene results in a chimeric oncogene commonly found in papillary thyroid carcinoma called RET/PTC (Nikiforov 2002). Microinjection of normal human thyroid epithelial cells with RET/PTC induced nuclear envelope irregularities (Fischer et al. 2003). These nuclear shape abnormalities developed within hours before cell division could occur. Therefore, RET/PTC-induced nuclear shape irregularities can arise without post-mitotic nuclear envelope assembly. The mechanism through which RET/PTC induces nuclear shape abnormalities remains unclear.
Nuclear membrane proteins
Inner nuclear membrane proteins.
Inner nuclear membrane (INM) proteins such as LAP1, LEM domain proteins (LAP2, emerin, and Man1), and lamin B receptor (LBR) bind to lamins and tether chromatin to the nuclear periphery (Schreiner et al. 2015; Castro-Obregon 2020). Dysregulation of INM proteins is associated with changes in chromatin compaction, nuclear shape abnormalities, and changes in nuclear size. Emerin is downregulated in some cancer tissues, such as ovarian cancer (Capo-chichi et al. 2009). Loss of emerin led to nuclear shape abnormalities in human embryonic kidney cells (Kituyi and Edkins 2018). Increased LBR expression, observed in aggressive breast cancer tissues (Wazir et al. 2013), induced invagination of the nuclear envelope (Ellenberg et al. 1997; Ma et al. 2007). LBR mutations are present in the Pelger-Huet anomaly, a genetic condition that causes the formation of multilobed, hypo-segmented nuclei in white blood cells (Gravemann et al. 2010). Disruption of other tethering proteins may also affect the nuclear shape. Depletion of PRR14, which tethers heterochromatin to the nuclear lamina, resulted in the loss of peripheral heterochromatin, multinucleation, and abnormal nuclear contours (Poleshko et al. 2013).
Nucleoporins and nucleocytoplasmic transport.
Changes to nucleocytoplasmic transport can also impact nuclear shape. Knockdown of nuclear import protein karyopherin alpha 7 (KPNA7) caused lobulation and elongation of nuclei in multiple cancer cell lines, illustrating how dysregulation of nuclear transport may also affect nuclear shape (Vuorinen et al. 2018). Depletion of nucleophosmin, a transport protein that shuttles between the nucleus and cytoplasm, induced nuclear shape defects in cervical cancer cells (Amin et al. 2008). Nuclear shape abnormalities were also induced in cervical cancer cells via depletion of NUP53 (Hawryluk-Gara et al. 2005), NUP98 (Fahrenkrog et al. 2016), and NUP153 (Zhou and Panté 2010). Overexpression of Nup136, a plant-specific nucleoporin, increased nuclear size in Arabidopsis thaliana (Tamura et al. 2010), and depletion of Nup199 increased nuclear size in Xenopus egg extract (Theerthagiri et al. 2010).
TMEM170A is a transmembrane protein that localizes to the endoplasmic reticulum and nuclear envelope membranes. Knockdown of TMEM170A in cervical cancer cells increases the nuclear size and nuclear shape irregularities, likely due to the involvement of this protein in nuclear envelope assembly and nucleoporin complex formation (Christodoulou et al. 2016).
Cytoskeletal forces
Stresses generated in actomyosin stress fibers that indent the apical nuclear surface in cultured cervical cancer cells indent the nucleus and fragment them, resulting in nuclear shape abnormalities (Takaki et al. 2017). Consistent with a role for actomyosin stresses, blocking contractility rescued abnormal nuclear shapes in xenografts in vivo.
Alterations to microtubules or microtubule-associated proteins can also impact nuclear shapes (Kaufmann et al. 2016). For example, depletion or overexpression of the microtubule-binding protein SIRT2 in osteosarcoma cells caused defects in nuclear envelope assembly, resulting in abnormal nuclei. Mislocalization of microtubules can promote nuclear lobulations due to defective post-mitotic nuclear envelope reassembly in Hela cells (Kawaguchi et al. 2015). Depletion of REEP3 and REEP4, endoplasmic reticulum proteins which bind microtubules to the nuclear membrane, cause nuclear envelope defects in Hela cells (Schlaitz et al. 2013). Depletion of chromatin-binding protein developmental pluripotency-associated 2 in Xenopus egg extract results in excess microtubules leading to abnormal morphologies post-mitosis (Xue et al. 2013).
Depolymerization of microtubules rescued nuclear shape abnormalities in lamin A/C-depleted human osteosarcoma cells and HGPS patient-derived cells but also caused noticeable cell rounding (Larrieu et al. 2014). Thus microtubule disruption may impact nuclear shape through indirect effects on cell shape. We have recently proposed the concept that the overall shape of the nucleus is a cumulative result of changes in the nuclear shape caused by dynamic changes to the shape of cells (Lele et al. 2018; Tocco et al. 2018; Li et al. 2015). In this mechanism, the resistance of cytoskeletal structures between the nuclear surface and the cell boundary to expansion or contraction results in stress on the nuclear surface (Lele et al. 2018; Li et al. 2015). The change in the space between the nuclear surface and the cell boundary can occur, for example, when cell protrusions form or the cell membrane retracts in motile cells. In support of this concept, nuclear contour abnormalities in cultured MDA-MB-231 breast cancer cells became amplified by the process of cell spreading (Tocco et al. 2018). Consistent with the notion that moving cell boundaries exert a force on the nucleus, cell membrane protrusions were observed to give rise to nuclear protrusions in MDA-MB-231 cells (Kent et al. 2019). Others have similarly observed that cytoskeletal remodeling modifies the stresses on the nuclear surface, as evident in dynamic fluctuations observed in the nuclear envelope in cultured cells (Schreiner et al. 2015; Chu et al. 2017).
Linker to nucleus and cytoskeleton complex.
The linker to nucleus and cytoskeleton (LINC) complex includes nesprin proteins, which are localized to the outer nuclear membrane and bind to SUN1 and SUN2 proteins localized in the inner nuclear membrane (Figure 1c). SUN proteins, in turn, bind to the nuclear lamina. Thus, the LINC (Linker to Nucleus and Cytoskeleton) complex provides a physical connection between the nucleus and the cytoskeleton, allowing the transmission of mechanical force to the nuclear surface (Lombardi and Lammerding 2011).
Mutations and abnormal expression of LINC complex proteins have been implicated in cancer. SUN1 and SUN2, and nesprins are downregulated in breast cancer tissue and cells lines (Matsumoto et al. 2015). Nesprins are also downregulated in colorectal cancer (Sjöblom et al. 2006), and nesprin mutations are associated with increased invasiveness in ovarian cancer (Doherty et al. 2010).
Knockdown of nesprins 1 and 2 induced irregular nuclear shape and increased nuclear area in murine myoblasts (Zhang et al. 2007a) and human endothelial cells (King et al. 2014). Both enlarged and misshapen nuclei in fibroblasts were observed in mice lacking the actin-binding domain of nesprin-2 (nesprin-2DeltaABD) (Lüke et al. 2008). Overexpression of nesprin-2 mini, a construct of ABD and KASH domain decreased nuclear size in human keratinocyte cells (Lu et al. 2012). In contrast, overexpression of Nesprin-2 ABD or Nesprin-2 C-terminal KASH domain increased nuclear size in the same cell type. Abnormal nuclear morphology in murine myoblasts was induced by the expression of mutant nesprin-1 (Zhou et al. 2017) and expression of a SUN1 mutant (Meinke et al. 2014). SUN1/2 depletion induced nuclear lobulations in rat mammary adenocarcinoma cells (Sharma et al. 2021) and human cervical cancer cells (Liu et al. 2007). Abnormal expression of torsinA, an ATPase present in the endoplasmic reticulum which interacts with the LINC complex, induces nuclear blebbing in cervical cancer cells (Laudermilch et al. 2016; Vander Heyden et al. 2009) and osteosarcoma cells. These findings suggest that alterations to LINC complex proteins may destabilize nuclear morphology and size, potentially through modulation of cytoskeletal stress on the nuclear surface.
Conclusions
Nuclear size and shape abnormalities are recognized as hallmarks of cancer. The mechanisms underlying these changes are complex, depend on cancer type, and are surprisingly understudied even though the first report of nuclear abnormalities in cancer was more than 150 years ago. Nuclear size changes in cancer have been attributed primarily to aneuploidy, but alterations to osmotic coupling with the cytoplasm and nucleo-cytoplasmic transport may contribute. Nuclear shape abnormalities can be caused by alterations to a large number of individual genes that are typically found in cancer. Mechanistically, these alterations may promote nuclear contour irregularities through a mechanical softening of the nucleus either due to a depletion of specific nuclear lamins, the decompaction of chromatin or the assembly of an abnormal nuclear envelope postmitosis. Increased cytoskeletal stress acting on the cancer nucleus due to contractile actomyosin stress fibers or due to the process of cell spreading can further magnify nuclear abnormalities. Thus, although mutations in or downregulation of a large number of genes can give rise to nuclear abnormalities, the number of distinct mechanisms by which the abnormalities arise is much smaller.
Virtually no studies exist on pharmacological targeting of nuclear shape and size abnormalities. Given the ubiquity of nuclear abnormalities in cancer, there is a high potential for developing new therapeutic strategies to target them and potentially regularize not only nuclear morphology but also associated cellular structural abnormalities and cellular dysfunction.
Acknowledgments
This work was supported by NIH U01 CA225566 (T.P.L.) and a CPRIT established investigator award grant # RR200043 (T.P.L.).
References
- Abdalla F, Boder J, Markus R, Hashmi H, Buhmeida A, Collan Y (2009) Correlation of nuclear morphometry of breast cancer in histological sections with clinicopathological features and prognosis. Anticancer research 29 (5):1771–1776 [PubMed] [Google Scholar]
- Agudo D, Gómez-Esquer F, Martínez-Arribas F, Núñez-Villar MJ, Pollán M, Schneider J (2004) Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. International journal of cancer 109 (5):717–720. doi: 10.1002/ijc.20034 [DOI] [PubMed] [Google Scholar]
- Alfonso P, Canamero M, Fernandez-Carbonie F, Núñez A, Casal Jl (2008) Proteome analysis of membrane fractions in colorectal carcinomas by using 2D-DIGE saturation labeling. Journal of proteome research 7 (10):4247–4255. doi: 10.1021/pr800152u [DOI] [PubMed] [Google Scholar]
- Alhudiri I, Nolan C, Ellis I, Elzagheid A, Rakha E, Green AR, Chapman C (2019) Expression of Lamin A/C in early-stage breast cancer and its prognostic value. Breast cancer research and treatment 174 (3):661–668. doi: 10.1007/sl0549-018-05092-w [DOI] [PubMed] [Google Scholar]
- Aljada A, Doria J, Saleh AM, Al-Matar SFI, AIGabbani S, Shamsa FIB, Al-Bawab A, Ahmed AA (2016) Altered Lamin A/C splice variant expression as a possible diagnostic marker in breast cancer. Cellular Oncology 39 (2):161–174. doi: 10.1007/sl3402-015-0265-1 [DOI] [PubMed] [Google Scholar]
- Amin MA, Matsunaga S, Uchiyama S, Fukui K (2008) Depletion of nucleophosmin leads to distortion of nucleolar and nuclear structures in HeLa cells. Biochemical Journal 415 (3):345–351. doi: 10.1042/BJ20081411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atupelage C, Nagahashi H, Kimura F, Yamaguchi M, Tokiya A, Hashiguchi A, Sakamoto M (2014) Computational hepatocellular carcinoma tumor grading based on cell nuclei classification. Journal of Medical Imaging 1 (3):034501. doi: 10.1117/l.JMI.1.3.034501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr Fritcher EG, Kipp BR, Slezak JM, Moreno-Luna LE, Gores GJ, Levy MJ, Roberts LR, Hailing KC, Sebo TJ (2007) Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. American journal of clinical pathology 128 (2):272–279. doi: 10.1309/BC6DY755Q3T5W9EE [DOI] [PubMed] [Google Scholar]
- Beale L (1860) Examination of sputum from a case of cancer of the pharynx and the adjacent parts. Arch Med 2 (44):1860–1861 [Google Scholar]
- Belt ET, Fijneman R, Van Den Berg E, Bril H, Delis-van Diemen P, Tijssen M, Van Essen H, De Lange-De Klerk E, Belien J, Stockmann H (2011) Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. European journal of cancer 47 (12):1837–1845. doi: 10.1016/j.ejca.2011.04.025 [DOI] [PubMed] [Google Scholar]
- Ben-David U, Amon A (2020) Context is everything: aneuploidy in cancer. Nature Reviews Genetics 21 (1):44–62. doi: 10.1038/s41576-019-0171-x [DOI] [PubMed] [Google Scholar]
- Bengtsson S, Krogh M, Szigyarto CA-K, Uhlen M, Schedvins K, Silfversward C, Linder S, Auer G, Alaiya A, James P (2007) Large-scale proteomics analysis of human ovarian cancer for biomarkers. Journal of proteome research 6 (4):1440–1450. doi: 10.1021/pr060593y [DOI] [PubMed] [Google Scholar]
- Bennett JH (1849) On cancerous and cancroid growths. Sutherland and Knox, [Google Scholar]
- Berchuck A, Kohler MF, Marks JR, Wiseman R, Boyd J, Bast RC Jr (1994) The p53 tumor suppressor gene frequently is altered in gynecologic cancers. American journal of obstetrics and gynecology 170 (1):246–252. doi: 10.1016/s0002-9378(94)70414-7 [DOI] [PubMed] [Google Scholar]
- Bhatia A, Dey P, Kakkar N, Srinivasan R, Nijhawan R (2006) Malignant atypical cell in urine cytology: a diagnostic dilemma. Cytojournal 3:28. doi: 10.1186/1742-6413-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S, Flyamer IM, Williamson I, Sengupta D, Bickmore WA, Illingworth RS (2020) A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes & development 34 (13–14):931–949. doi: 10.1101/gad.336487.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broers J, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers F (1993) Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. The American journal of pathology 143 (1):211. [PMC free article] [PubMed] [Google Scholar]
- Brustmann H, Hager M (2009) Nucleoporin 88 expression in normal and neoplastic squamous epithelia of the uterine cervix. Annals of diagnostic pathology 13 (5):303–307. doi: 10.1016/j.anndiagpath.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Capo-chichi CD, Cai KQ, Simpkins F, Ganjei-Azar P, Godwin AK, Xu X-X (2011a) Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer. BMC medicine 9 (1):1–12. doi: 10.1186/1741-7015-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, Xu X-X (2011b) Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chinese journal of cancer 30 (6):415. doi: 10.5732/cjc.010.10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-chichi CD, Cai KQ, Testa JR, Godwin AK, Xu X-X (2009) Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer. Molecular and cellular biology 29 (17):4766–4777. doi: 10.1128/MCB.00087-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Chichi CD, Yeasky TM, Smith ER, Xu X-X (2016) Nuclear envelope structural defect underlies the main cause of aneuploidy in ovarian carcinogenesis. BMC cell biology 17 (1):1–11. doi: 10.1186/sl2860-016-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Obregon S (2020) Lamin B receptor: role on chromatin structure, cellular senescence and possibly aging. Biochemical Journal 477 (14):2715–2720. doi: 10.1042/BCJ20200165 [DOI] [PubMed] [Google Scholar]
- Christodoulou A, Santarella-Mellwig R, Santama N, Mattaj IW (2016) Transmembrane protein TMEM170A is a newly discovered regulator of ER and nuclear envelope morphogenesis in human cells. Journal of cell science 129 (8):1552–1565. doi: 10.1242/jcs.175273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F-Y, Haley SC, Zidovska A (2017) On the origin of shape fluctuations of the cell nucleus. Proceedings of the National Academy of Sciences 114 (39):10338–10343. doi: 10.1073/pnas,1702226114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, Carmignani G, D’Arrigo C, Patrone E, Balbi C (2006) Differential expression of nuclear lamins in normal and cancerous prostate tissues. Oncology reports 15 (3):609–613 [PubMed] [Google Scholar]
- Dahl KN, Kahn SM, Wilson KL, Discher DE (2004) The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. Journal of cell science 117 (20):4779–4786. doi: 10.1242/jcs.01357 [DOI] [PubMed] [Google Scholar]
- Dangou JM, Kiss R, DePrez C, Jeannot M, Fastrez R, Pasteels JL, Verhest A (1993) Heterogeneity of DNA ploidy, proliferation index and nuclear size in human colorectal carcinomas. Analytical and quantitative cytology and histology 15 (1):23–31 [PubMed] [Google Scholar]
- Davidson PM, Lammerding J (2014) Broken nuclei-lamins, nuclear mechanics, and disease. Trends in cell biology 24 (4):247–256. doi: 10.1016/j.tcb.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrea CE, Petrilli AS, Jesus-Garcia R, Bleggi-Torres LF, Alves MTS (2011) Large and round tumor nuclei in osteosarcoma: good clinical outcome. International journal of clinical and experimental pathology 4 (2):169. [PMC free article] [PubMed] [Google Scholar]
- de Freitas Nader GP, AgUera-Gonzalez S, Routet F, Gratia M, Maurin M, Cancila V, Cadart C, Palamidessi A, Ramos RN, San Roman M (2021) Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell 184 (20):5230–5246. e5222. doi: 10.1016/j.cell.2021.08.035 [DOI] [PubMed] [Google Scholar]
- de las Heras JI, Schirmer EC (2014) The nuclear envelope and cancer: a diagnostic perspective and historical overview. Cancer Biology and the Nuclear Envelope:5–26. doi: 10.1007/978-1-4899-8032-8_1 [DOI] [PubMed] [Google Scholar]
- Debes JD, Schmidt LJ, Huang H, Tindall DJ (2002) p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer research 62 (20):5632–5636 [PubMed] [Google Scholar]
- Debes JD, Sebo TJ, Heemers HV, Kipp BR, De Anna LH, Lohse CM, Tindall DJ (2005) p300 modulates nuclear morphology in prostate cancer. Cancer research 65 (3):708–712 [PubMed] [Google Scholar]
- Debes JD, Sebo TJ, Lohse CM, Murphy LM, De Anna LH, Tindall DJ (2003) p300 in prostate cancer proliferation and progression. Cancer research 63 (22):7638–7640 [PubMed] [Google Scholar]
- DeMay RM (2012) The art & science of cytopathology. vol H682 MACa. doi: [DOI] [Google Scholar]
- Denais C, Lammerding J (2014) Nuclear mechanics in cancer. Cancer biology and the nuclear envelope:435–470. doi: 10.1016/j.semcancer.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, Te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352 (6283):353–358. doi: 10.1126/science.aad7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P (2010) Cancer nucleus: morphology and beyond. Diagnostic cytopathology 38 (5):382–390. doi: 10.1002/dc.21234 [DOI] [PubMed] [Google Scholar]
- Diamond DA, Berry SJ, Umbricht C, Jewett HJ, Coffey DS (1982) Computerized image analysis of nuclear shape as a prognostic factor for prostatic cancer. The Prostate 3 (4):321–332. doi: 10.1002/pros.2990030402 [DOI] [PubMed] [Google Scholar]
- Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, Pike MC, Ness RB, Moysich K, Chenevix-Trench G (2010) ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiology and Prevention Biomarkers 19 (l):245–250. doi: 10.1158/1055-9965.EPI-09-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douet J, Corujo D, Malinverni R, Renauld J, Sansoni V, Posavec Marjanovic M, Cantarifio N, Valero V, Mongelard F, Bouvet P (2017) MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. Journal of cell science 130 (9):1570–1582. doi: 10.1242/jcs,199216 [DOI] [PubMed] [Google Scholar]
- Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. Journal of Cell Biology 138 (6):1193–1206. doi: 10.1083/jcb,138.6.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emterling A, Skoglund J, Arbman G, Schneider J, Evertsson S, Carstensen J, Zhang H, Sun X-F (2003) Clinicopathological significance of Nup88 expression in patients with colorectal cancer. Oncology 64(4):361–369. doi: 10.1159/000070294 [DOI] [PubMed] [Google Scholar]
- Jl Epstein, Berry SJ, Eggleston JC (1984) Nuclear roundness factor. A predictor of progression in untreated stage A2 prostate cancer. Cancer 54 (8):1666–1671. doi: [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Martinelli V, Nilles N, Fruhmann G, Chatel G, Juge S, Sauder U, Di Giacomo D, Mecucci C, Schwaller J (2016) Expression of leukemia-associated Nup98 fusion proteins generates an aberrant nuclear envelope phenotype. PloS one 11 (3):e0152321. doi: 10.1371/journal.pone.0152321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan JD, Guilak F (2010) The effects of osmotic stress on the structure and function of the cell nucleus. Journal of cellular biochemistry 109 (3):460–467. doi: 10.1002/jcb.22437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Taysavang P, Jhiang SM (2003) Nuclear envelope irregularity is induced by RET/PTC during interphase. The American journal of pathology 163 (3):1091–1100. doi: 10.1016/S0002-9440(10)63468-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EG (2020) Nuclear Morphology and the Biology of Cancer Cells. Acta cytologica 64 (6):511–519. doi: 10.1159/000508780 [DOI] [PubMed] [Google Scholar]
- Flores LF, Tader BR, Tolosa EJ, Sigafoos AN, Marks DL, Fernandez-Zapico ME (2021) Nuclear Dynamics and Chromatin Structure: Implications for Pancreatic Cancer. Cells 10 (10):2624. doi: 10.3390/cellsl0102624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich K, Dimmer V, Flaroske G, Meyer W, Theissig F, Thieme B, Kunze KD (1997) Morphological heterogeneity of p53 positive and p53 negative nuclei in breast cancers stratified by clinicopathological variables. Analytical Cellular Pathology 14 (2):111–123. doi: 10.1155/1997/619309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Lv P, Yan G, Fan H, Cheng L, Zhang F, Dang Y, Wu H, Wen B (2015) MacroFI2Al associates with nuclear lamina and maintains chromatin architecture in mouse liver cells. Scientific reports 5 (1):1–12. doi: 10.1038/srepl7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa T, Rochman M, Taher L, Dimitriadis EK, Nagashima K, Anderson S, Bustin M (2015) Chromatin decompaction by the nucleosomal binding protein FIMGN5 impairs nuclear sturdiness. Nature communications 6 (1):1–10. doi: 10.1038/ncomms7138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta K, Watanabe H, Ikeda S (1992) Differences between solid and ductectatic types of pancreatic ductal carcinomas. Cancer 69 (6):1327–1333. doi: [DOI] [PubMed] [Google Scholar]
- Fütterer A, Campanero MR, Leonardo E, Criado LM, Flores JM, Hernández JM, San Miguel JF, Martínez-A C (2005) Dido gene expression alterations are implicated in the induction of hematological myeloid neoplasms. The Journal of clinical investigation 115 (9):2351–2362. doi: 10.1172/JCI24177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina C, Caniglia D, D’Aprile M, Lettini T, Serio G, Cipriani T, Ricco R, Delfino VP (1996) Nuclear morphometry in squamous cell carcinoma (SCC) of the tongue. European Journal of Cancer Part B: Oral Oncology 32 (2):91–96. doi: 10.1016/0964-1955(95)00062-3 [DOI] [PubMed] [Google Scholar]
- Gould VE, Orucevic A, Zentgraf H, Gattuso P, Martínez N, Alonso A (2002) Nup88 (karyoporin) in human malignant neoplasms and dysplasias: correlations of immunostaining of tissue sections, cytologic smears, and immunoblot analysis. Human pathology 33(5):536–544. doi: 10.1053/hupa.2002.124785 [DOI] [PubMed] [Google Scholar]
- Gravemann S, Schnipper N, Meyer H, Vaya A, Nowaczyk MJ, Rajab A, Hofmann W-K, Salewsky B, Tonnies H, Neitzel H (2010) Dosage effect of zero to three functional LBR-genes in vivo and in vitro. Nucleus 1 (2):179–189. doi: 10.4161/nucl.1.2.11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman RL, Heath AP, Ferretti V, Varmus HE, Lowy DR, Kibbe WA, Staudt LM (2016) Toward a shared vision for cancer genomic data. New England Journal of Medicine 375 (12):1109–1112. doi: 10.1056/NEJMpl607591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley AM, Hidvegi DF, Cajulis RS, Bacus S (1994) Morphologic and morphometric features of low grade serous tumours of the ovary. Diagnostic cytopathology 11 (3):220–225. doi: 10.1002/dc.2840110306 [DOI] [PubMed] [Google Scholar]
- Gurusaran M, Davies OR (2021) A molecular mechanism for LINC complex branching by structurally diverse SUN-KASH 6: 6 assemblies. Elife 10:e60175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EM, Hetzer MW (2016) Nuclear envelope rupture is induced by actin-based nucleus confinement. Journal of Cell Biology 215 (l):27–36. doi: 10.1083/jcb.201603053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk-Gara LA, Shibuya EK, Wozniak RW (2005) Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Molecular biology of the cell 16(5):2382–2394. doi: 10.1091/mbc.e04-10-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander K, Tribukait B (1988) Modal DNA values of normal and malignant urothelial cells of the bladder in relation to nuclear size. Analytical and quantitative cytology and histology 10 (2):127–133 [PubMed] [Google Scholar]
- Hill DA, Chiosea S, Jamaluddin S, Roy K, Fischer AH, Boyd DD, Nickerson JA, Imbalzano AN (2004) Inducible changes in cell size and attachment area due to expression of a mutant SWI/SNF chromatin remodeling enzyme. Journal of cell science 117 (24):5847–5854. doi: 10.1242/jcs.01502 [DOI] [PubMed] [Google Scholar]
- Imbalzano KM, Cohet N, Wu Q, Underwood JM, Imbalzano AN, Nickerson JA (2013) Nuclear shape changes are induced by knockdown of the SWI/SNF ATPase BRG1 and are independent of cytoskeletal connections. PloS one 8 (2):e55628. doi: 10.1371/journal.pone.0055628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtić P, Schibler AC, Wesley CC, Pegoraro G, Misteli T, Levy DL (2019) The nucleoporin ELYS regulates nuclear size by controlling NPC number and nuclear import capacity. EMBO reports 20(6):e47283. doi: 10.15252/embr.201847283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P, Edgington NP, Schneider BL, Rupes I, Tyers M, Futcher B (2007) The size of the nucleus increases as yeast cells grow. Molecular biology of the cell 18 (9):3523–3532. doi: 10.1091/mbc.e06-10-0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap A, Jain M, Shukla S, Andley M (2018) Role of nuclear morphometry in breast cancer and its correlation with cytomorphological grading of breast cancer: A study of 64 cases. Journal of cytology 35 (1):41. doi: 10.4103/JOC.JOC_237_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar A, Tocco V, Li Y, Aggarwal V, Tamashunas AC, Dickinson RB, Lele TP (2019) Nuclear size changes caused by local motion of cell boundaries unfold the nuclear lamina and dilate chromatin and intranuclear bodies. Soft matter 15 (45):9310–9317. doi: 10.1039/c9sm01666j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Kukolj E, Brachner A, Beltzung E, Bruno M, Kostrhon S, Opravil S, Hudecz O, Mechtler K, Warren G (2016) SIRT2 regulates nuclear envelope reassembly through ANKLE2 deacetylation. Journal of cell science 129 (24):4607–4621. doi: 10.1242/jcs.192633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A, Asaka MN, Matsumoto K, Nagata K (2015) Centrosome maturation requires YB-1 to regulate dynamic instability of microtubules for nucleus reassembly. Scientific reports 5 (l):1–8. doi: 10.1038/srep08768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent IA, Zhang Q, Katiyar A, Li Y, Pathak S, Dickinson RB, Lele TP (2019) Apical cell protrusions cause vertical deformation of the soft cancer nucleus. Journal of cellular physiology 234 (11):20675–20684. doi: 10.1002/jcp.28672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Nowak K, Suryavanshi N, Holt I, Shanahan CM, Ridley AJ (2014) Nesprin-1 and nesprin-2 regulate endothelial cell shape and migration. Cytoskeleton 71 (7):423–434. doi: 10.1002/cm.21182 [DOI] [PubMed] [Google Scholar]
- Kituyi SN, Edkins AL (2018) Hop/STIPl depletion alters nuclear structure via depletion of nuclear structural protein emerin. Biochemical and biophysical research communications 507 (l-4):503–509. doi: 10.1016/j.bbrc.2018.11.073 [DOI] [PubMed] [Google Scholar]
- Knoess M, Kurz AK, Goreva O, Bektas N, Breuhahn K, Odenthal M, Schirmacher P, Dienes HP, Bock CT, Zentgraf H (2006) Nucleoporin 88 expression in hepatitis B and C virus-related liver diseases. World journal of gastroenterology: WJG 12 (36):5870. doi: 10.3748/wjg.vl2.i36.5870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger S, Müller H (1995) Correlation of morphometry, nucleolar organizer regions, proliferating cell nuclear antigen and Ki67 antigen expression with grading and staging in urinary bladder carcinomas. British journal of urology 75 (4):480–484. doi: 10.1111/j.1464-410x.1995.tb07269.x [DOI] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT (2004) Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. The Journal of clinical investigation 113 (3):370–378. doi: 10.1172/JCI19670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP (2014) Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344 (6183):527–532. doi:doi: 10.1126/science.1252651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudermilch E, Tsai P-L, Graham M, Turner E, Zhao C, Schlieker C (2016) Dissecting Torsin/cofactor function at the nuclear envelope: a genetic study. Molecular biology of the cell 27 (25):3964–3971. doi: 10.1091/mbc.E16-07-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebert H (1851) Traité pratique des maladies cancéreuses et des affections curables confondues avec le cancer. Baillière, Paris [Google Scholar]
- Lee S-R, Roh Y-G, Kim S-K, Lee J-S, Seol S-Y, Lee H-H, Kim W-T, Kim W-J, Heo J, Cha H-J (2015) Activation of EZH2 and SUZ12 regulated by E2F1 predicts the disease progression and aggressive characteristics of bladder cancer. Clinical cancer research 21 (23):5391–5403. doi: 10.1158/1078-0432.CCR-14-2680 [DOI] [PubMed] [Google Scholar]
- Lee S, Ahn Y-M, Kim J-Y, Cho Y-E, Park J-H (2020) Downregulation of NOP53 ribosome biogenesis factor leads to abnormal nuclear division and chromosomal instability in human cervical cancer cells. Pathology & Oncology Research 26 (1):453–459. doi: 10.1007/s12253-018-0531-4 [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Kim Y, Seok K, Kim J, Chang Y, Kang H, Park J (2012) Nucleolar protein GLTSCR2 stabilizes p53 in response to ribosomal stresses. Cell Death & Differentiation 19 (10):1613–1622. doi: 10.1038/cdd.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele TP, Dickinson RB, Gundersen GG (2018) Mechanical principles of nuclear shaping and positioning. J Cell Biol 217 (10):3330–3342. doi: 10.1083/jcb.201804052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai Q, Wu H, Vathipadiekal V, Dobbin ZC, Li T, Hua X, Landen CN, Birrer MJ, Sanchez-Beato M (2012) SUZ12 promotes human epithelial ovarian cancer by suppressing apoptosis via silencing HRK. Molecular Cancer Research 10 (11):1462–1472. doi: 10.1158/1541-7786.MCR-12-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Du Y, Kong X, Li Z, Jia Z, Cui J, Gao J, Wang G, Xie K (2013) Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clinical Cancer Research 19 (17):4651–4661. doi: 10.1158/1078-0432.CCR-12-3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lovett D, Zhang Q, Neelam S, Kuchibhotla RA, Zhu R, Gundersen GG, Lele TP, Dickinson RB (2015) Moving cell boundaries drive nuclear shaping during cell spreading. Biophysical journal 109 (4):670–686. doi: 10.1016/j.bpj.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SO, Park S-J, Kim W, Park SG, Kim H-J, Kim Y-l, Sohn T-S, Noh J-H, Jung G (2002) Proteome analysis of hepatocellular carcinoma. Biochemical and biophysical research communications 291 (4):1031–1037. doi: 10.1006/bbrc.2002.6547 [DOI] [PubMed] [Google Scholar]
- Liu C, Sasaki H, Fahey M, Sakamoto A, Sato S, Tanaka T (1999) Prognostic value of nuclear morphometry in patients with TNM stage T1 ovarian clear cell adenocarcinoma. British journal of cancer 79 (11):1736–1741. doi:doi: 10.1038/sj.bjc.6690276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, Burke B, Roux KJ (2007) Functional association of Sunl with nuclear pore complexes. The Journal of cell biology 178 (5):785–798. doi: 10.1083/jcb.200704108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi ML, Lammerding J (2011) Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochemical Society Transactions 39 (6):1729–1734. doi: 10.1042/BST20110686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Schneider M, Neumann S, Jaeger V-M, Taranum S, Munck M, Cartwright S, Richardson C, Carthew J, Noh K (2012) Nesprin interchain associations control nuclear size. Cellular and Molecular Life Sciences 69 (20):3493–3509. doi: 10.1007/s00018-012-1034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüke Y, Zaim H, Karakesisoglou I, Jaeger VM, Sellin L, Lu W, Schneider M, Neumann S, Beijer A, Munck M (2008) Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. Journal of cell science 121 (11):1887–1898. doi: 10.1242/jcs.019075 [DOI] [PubMed] [Google Scholar]
- Ma Y, Cai S, Lv Q, Jiang Q, Zhang Q, Zhai Z, Zhang C (2007) Lamin B receptor plays a role in stimulating nuclear envelope production and targeting membrane vesicles to chromatin during nuclear envelope assembly through direct interaction with importin β. Journal of cell science 120 (3):520–530. doi: 10.1242/jcs.03355 [DOI] [PubMed] [Google Scholar]
- Martínez N, Alons A, Moragues MD, Pontón J, Schneider J (1999) The nuclear pore complex protein Nup88 is overexpressed in tumor cells. Cancer research 59 (21):5408–5411 [PubMed] [Google Scholar]
- Matsumoto A, Hieda M, Yokoyama Y, Nishioka Y, Yoshidome K, Tsujimoto M, Matsuura N (2015) Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN 1, SUN 2, and nesprin-2 in breast cancer. Cancer medicine 4 (10):1547–1557. doi: 10.1002/cam4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, Worman HJ, Gundersen GG, Lattanzi G, Wehnert M (2014) Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS genetics 10 (9):el004605. doi: 10.1371/journal.pgen.1004605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijovic Z, Kostov M, Mihailovic D, Zivkovic N, Stojanovic M, Zdravkovic M (2013) Correlation of nuclear morphometry of primary melanoma of the skin with clinicopathological parameters and expression of tumor suppressor proteins (p53 and pl6 (INK4a)) and bcl-2 oncoprotein. J buon 18 (2):471–476 [PubMed] [Google Scholar]
- Moss S, Krivosheyev V, De Souza A, Chin K, Gaetz H, Chaudhary N, Worman H, Holt P (1999) Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut 45 (5):723–729. doi: 10.1136/gut.45.5.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J, Offerhaus G, De Feyter E, Floyd J, Kern S, Vogelstein B, Hamilton S (1992) The relationship of quantitative nuclear morphology to molecular genetic alterations in the adenoma-carcinoma sequence of the large bowel. The American journal of pathology 141 (4):797. [PMC free article] [PubMed] [Google Scholar]
- Nativ O, Sabo E, Bejar J, Halachmi S, Moskovitz B, Miselevich I (1996) A comparison between histological grade and nuclear morphometry for predicting the clinical outcome of localized renal cell carcinoma. British journal of urology 78 (l):33–38. doi: 10.1046/j.l464-410x.1996.00447.x [DOI] [PubMed] [Google Scholar]
- Neelam S, Chancellor T, Li Y, Nickerson JA, Roux KJ, Dickinson RB, Lele TP (2015) Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proceedings of the National Academy of Sciences 112 (18):5720–5725. doi: 10.1073/pnas.1502111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann FR, Nurse P (2007) Nuclear size control in fission yeast. The Journal of cell biology 179 (4):593–600. doi: 10.1083/jcb.200708054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov YE (2002) RET/PTC rearrangement in thyroid tumors. Endocrine pathology 13 (1):3–16. doi: 10.1385/ep:13:1:03 [DOI] [PubMed] [Google Scholar]
- Okudela K (2014) An association between nuclear morphology and immunohistochemical expression of p53 and p16INK4A in lung cancer cells. Medical molecular morphology 47 (3):130–136. doi: 10.1007/s00795-013-0052-x [DOI] [PubMed] [Google Scholar]
- Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE (2007) Physical plasticity of the nucleus in stem cell differentiation. Proceedings of the National Academy of Sciences 104 (40):15619–15624. doi: 10.1073/pnas.0702576104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou GN, Traut HF (1941) The diagnostic value of vaginal smears in carcinoma of the uterus. American Journal of Obstetrics and Gynecology 42 (2):193–206 [Google Scholar]
- Petersen I, Kotb WFA, Friedrich K-H, SchlUns K, Bocking A, Dietel M (2009) Core classification of lung cancer: correlating nuclear size and mitoses with ploidy and clinicopathological paramaeters. Lung cancer 65 (3):312–318. doi: 10.1016/j.lungcan.2008.12.013 [DOI] [PubMed] [Google Scholar]
- Poleshko A, Mansfield KM, Burlingame CC, Andrake MD, Shah NR, Katz RA (2013) The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit. Cell reports 5 (2):292–301. doi: 10.1016/j.celrep.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda V, Malashchuk I, Asamaowei IE, Poterlowicz K, Fessing MY, Sharov AA, Karakesisoglou I, Botchkarev VA, Mardaryev A (2017) p63 transcription factor regulates nuclear shape and expression of nuclear envelope-associated genes in epidermal keratinocytes. Journal of Investigative Dermatology 137 (10):2157–2167. doi: 10.1016/j.jid.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richly H, Aloia L, Di Croce L (2011) Roles of the Polycomb group proteins in stem cells and cancer. Cell death & disease 2 (9):e204–e204. doi: 10.1038/cddis.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman M, Postnikov Y, Correll S, Malicet C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA, Grigoryev S (2009) The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Molecular cell 35 (5):642–656. doi: 10.1016/j.molcel.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosai J, Carcangiu M, Delellis R (1992) Tumors of the thyroid gland. Atlas of Tumor Pathology, vol 3. Armed Forces Institute of Pathology, Washington, D.C. [Google Scholar]
- Salani R, Kurman R, Giuntoli R, Gardner G, Bristow R, Wang T-L, Shih I-M (2008) Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. International Journal of Gynecologic Cancer 18 (3). doi: 10.1111/j.1525-1438.2007.01039.x [DOI] [PubMed] [Google Scholar]
- Samarnthai N, Elledge R, Prihoda TJ, Huang J, Massarweh S, Yeh IT (2012) Pathologic Changes in Breast Cancer After Anti-Estrogen Therapy. The breast journal 18 (4):362–366. doi: 10.1111/j.l524-4741.2012.01251.x [DOI] [PubMed] [Google Scholar]
- Sato M, Watanabe H, AJioka Y, Noda Y, Sakai Y (1993) Nucleolar and dispersed nucleolar organiser regions (NORs) in differentiating neoplastic from atypical non-neoplastic lesions of the pancreas. Gastroenterologia Japonica 28 (l):72–80. doi: 10.1007/BF02775006 [DOI] [PubMed] [Google Scholar]
- Schlaitz A-L, Thompson J, Wong CC, Yates III JR, Heald R (2013) REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Developmental cell 26 (3):315–323. doi: 10.1016/j.devcel.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner SM, Koo PK, Zhao Y, Mochrie SG, King MC (2015) The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nature communications 6 (1):1–13. doi: 10.1038/ncomms8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VP, Williams J, Leung E, Sanders J, Eddy R, Castracane J, Oktay MH, Entenberg D, Condeelis JS (2021) SUN-MKL1 Crosstalk Regulates Nuclear Deformation and Fast Motility of Breast Carcinoma Cells in Fibrillar ECM Microenvironment. Cells 10 (6):1549. doi: 10.3390/cellsl0061549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N (2006) The consensus coding sequences of human breast and colorectal cancers. science 314 (5797):268–274. doi: 10.1126/science.H33427 [DOI] [PubMed] [Google Scholar]
- Smith ER, Capo-Chichi CD, Xu X-X (2018) Defective nuclear lamina in aneuploidy and carcinogenesis. Frontiers in oncology 8:529. doi: 10.3389/fonc.2018.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsuan K, Peerapen P, Boonmark W, Plumworasawat i, Samol R, Sakulsak N, Thongboonkerd V (2019) ARID1A knockdown triggers epithelial-mesenchymal transition and carcinogenesis features of renal cells: role in renal cell carcinoma. The FASEB Journal 33 (11):12226–12239. doi: 10.1096/fj.201802720RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight PM (2007) Update on oral epithelial dysplasia and progression to cancer. Head and neck pathology 1 (l):61–66. doi: 10.1007/sl2105-007-0014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens AD, Banigan EJ, Marko JF (2019) Chromatin’s physical properties shape the nucleus and its functions. Current opinion in cell biology 58:76–84. doi: 10.1016/j.ceb.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens AD, Liu PZ, Banigan EJ, Almassalha LM, Backman V, Adam SA, Goldman RD, Marko JF (2018) Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Molecular biology of the cell 29 (2):220–233. doi: 10.1091/mbc.E17-06-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486 (7403):400–404. doi: 10.1038/naturell017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Xu MZ, Poon RT, Day PJ, Luk JM (2010) Circulating Lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. Journal of proteome research 9 (l):70–78. doi: 10.1021/pr9002118 [DOI] [PubMed] [Google Scholar]
- Takaki T, Montagner M, Serres MP, Le Berre M, Russell M, Collinson L, Szuhai K, Howell M, Boulton SJ, Sahai E (2017) Actomyosin drives cancer cell nuclear dysmorphia and threatens genome stability. Nature communications 8 (1):1–13. doi: 10.1038/ncommsl6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talve L, Kainu J, Collan Y, Ekfors T (1996) Immunohistochemical expression of p53 protein, mitotic index and nuclear morphometry in primary malignant melanoma of the skin. Pathology-Research and Practice 192 (8):825–833. doi: 10.1016/S0344-0338(96)80056-2 [DOI] [PubMed] [Google Scholar]
- Tamashunas AC, Tocco VJ, Matthews J, Zhang Q, Atanasova KR, Paschall L, Pathak S, Ratnayake R, Stephens AD, Luesch H (2020) High-throughput gene screen reveals modulators of nuclear shape. Molecular biology of the cell 31 (13):1392–1402. doi: 10.1091/mbc.E19-09-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I (2010) Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. The Plant Cell 22 (12):4084–4097. doi: 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, Schumacher SE, Wang C, Hu H, Liu J (2018) Genomic and functional approaches to understanding cancer aneuploidy. Cancer cell 33 (4):676–689. e673. doi: 10.1016/j.ccell.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencer AH, Gatchalian J, Klein BJ, Khan A, Zhang Y, Strahl BD, van Wely KH, Kutateladze TG (2017) A unique pH-dependent recognition of methylated histone H3K4 by PPS and DIDO. Structure 25 (10):1530–1539. el533. doi: 10.1016/j.str.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theerthagiri G, Eisenhardt N, Schwarz H, Antonin W (2010) The nucleoporin Nupl88 controls passage of membrane proteins across the nuclear pore complex. Journal of Cell Biology 189 (7):1129–1142. doi: 10.1083/jcb.200912045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thutkawkorapin J, Lindblom A, Tham E (2019) Exome sequencing in 51 early onset non-familial CRC cases. Molecular genetics & genomic medicine 7 (5):e605. doi: 10.1002/mgg3.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilli C, Ramaekers F, Broers J, Hutchison C, Neumann H (2003) Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. British Journal of Dermatology 148 (1):102–109. doi: 10.1046/j,1365-2133.2003.05026.x [DOI] [PubMed] [Google Scholar]
- Tocco VJ, Li Y, Christopher KG, Matthews JH, Aggarwal V, Paschall L, Luesch H, Licht JD, Dickinson RB, Lele TP (2018) The nucleus is irreversibly shaped by motion of cell boundaries in cancer and non-cancer cells. Journal of cellular physiology 233 (2):1446–1454. doi: 10.1002/jcp.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CM, Biran A, Burney MJ, Patel H, Henser-Brownhill T, Cohen A-HS, Li Y, Ben-Hamo R, Nye E, Spencer-Dene B (2016) The linker histone HI. 0 generates epigenetic and functional intratumor heterogeneity. Science 353 (6307). doi: 10.1126/science.aafl644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Velthoven R, Petein M, Oosterlinck WJ, Zandona C, Zlotta A, Van Der Meijden AP, Pasteels J-l, Roels H, Schulman C, Kiss R (1995) Image cytometry determination of ploidy level, proliferative activity, and nuclear size in a series of 314 transitional bladder cell carcinomas. Human pathology 26 (1):3–11. doi: 10.1016/0046-8177(95)90108-6. [DOI] [PubMed] [Google Scholar]
- Vander Heyden AB, Naismith TV, Snapp EL, Hodzic D, Hanson PI (2009) LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation. Molecular biology of the cell 20 (11):2661–2672. doi: 10.1091/mbc.e09-01-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri RW, Partin AW, Epstein JE, Marley GM, Miller CM, Singer DS, Patton KP, Criley SR, Coffey DS (1994) Quantitative nuclear morphometry, Markovian texture descriptors, and DNA content captured on a CAS-200 Image analysis system, combined with PCNA and HER-2/neu immunohistochemistry for prediction of prostate cancer progression. JOURNAL OF CELLULAR BIOCHEMISTRY-SUPPLEMENT- 19:249–249 [PubMed] [Google Scholar]
- Venables R, McLean S, Luny D, Moteleb E, Morley S, Quinlan R, Lane E, Hutchison C (2001) Expression of individual lamins in basal cell carcinomas of the skin. British journal of cancer 84 (4):512–519. doi: doi: 10.1054/bjoc.2000.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen EM, Rajala NK, Ihalainen TO, Kallioniemi A (2018) Depletion of nuclear import protein karyopherin alpha 7 (KPNA7) induces mitotic defects and deformation of nuclei in cancer cells. BMC cancer 18 (1):1–10. doi: 10.1186/s12885-018-4261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Stenkvist B, Tribukait B (1992) Morphometry of nuclei of the normal and malignant prostate in relation to DNA ploidy. Analytical and quantitative cytology and histology 14 (3):210–216 [PubMed] [Google Scholar]
- Wang Y, Wu R, Cho KR, Thomas DG, Gossner G, Liu JR, Giordano TJ, Shedden KA, Misek DE, Lubman DM (2009) Differential protein mapping of ovarian serous adenocarcinomas: identification of potential markers for distinct tumor stage. Journal of proteome research 8 (3):1452–1463. doi: 10.1021/pr800820z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazir U, Ahmed MH, Bridger JM, Harvey A, Jiang WG, Sharma AK, Mokbel K (2013) The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cellular & molecular biology letters 18 (4):595–611. doi: 10.2478/s11658-013-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E (1925) The karyoplasmic ratio. The cell in development and heredity, 3rd edn. The Macmillan Company, New York [Google Scholar]
- Wright R, Castles H (1987) Variability of thyroid cell nuclear size with Romanowsky stains. Acta cytologica 31 (4):526–527 [PubMed] [Google Scholar]
- Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F (2009) Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. Journal of Experimental & Clinical Cancer Research 28 (1):1–12. doi: 10.1186/1756-9966-28-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JZ, Woo EM, Postow L, Chait BT, Funabiki H (2013) Chromatin-bound Xenopus Dppa2 shapes the nucleus by locally inhibiting microtubule assembly. Developmental cell 27 (l):47–59. doi: 10.1016/j.devcel.2013.08.002. Epub 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Maciejowski J, de Lange T (2017) Nuclear envelope rupture is enhanced by loss of p53 or Rb. Molecular Cancer Research 15(11):1579–1586. doi: 10.1158/1541-7786.MCR-17-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M-H, Kang S-m, Lee S-J, Woo T-G, Oh A-Y, Park S, Ha N-C, Park B-J (2019) p53 induces senescence through Lamin A/C stabilization-mediated nuclear deformation. Cell death & disease 10 (2):1–18. doi: 10.1038/s41419-019-1378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimet AG, Fiegl H, Goebel G, Kopp F, Allasia C, Reimer D, Steppan I, Mueller-Holzner E, Ehrlich M, Marth C (2011) DNA ploidy, nuclear size, proliferation index and DNA-hypomethylation in ovarian cancer. Gynecologic oncology 121 (1):24–31. doi: 10.1016/j.ygyno.2010.12.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT (2007a) Nesprin-1 and-2 are involved in the pathogenesis of Emery-Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Human molecular genetics 16 (23):2816–2833. doi: 10.1093/hmg/ddm238 [DOI] [PubMed] [Google Scholar]
- Zhang Z-Y, Zhao Z-R, Jiang L, Li J-C, Gao Y-M, Cui D-S, Wang C-J, Schneider J, Wang M-W, Sun X-F (2007b) Nup88 expression in normal mucosa, adenoma, primary adenocarcinoma and lymph node metastasis in the colorectum. Tumor Biology 28 (2):93–99. doi: 10.1159/000099154 [DOI] [PubMed] [Google Scholar]
- Zhou C, Li C, Zhou B, Sun H, Koullourou V, Holt I, Puckelwartz MJ, Warren DT, Hayward R, Lin Z (2017) Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis. Human molecular genetics 26(12):2258–2276. doi: 10.1093/hmg/ddxll6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Panté N (2010) The nucleoporin Nupl53 maintains nuclear envelope architecture and is required for cell migration in tumor cells. FEBS letters 584 (14):3013–3020. doi: 10.1016/j.febslet.2010.05.038 [DOI] [PubMed] [Google Scholar]
- Zink D, Fischer AH, Nickerson JA (2004) Nuclear structure in cancer cells. Nature reviews cancer 4 (9):677–687. doi: 10.1038/nrcl430 [DOI] [PubMed] [Google Scholar]


