Abstract
Background
Hydroxychloroquine is often used as a first-line treatment of rheumatoid arthritis despite limited evidence on its cardiovascular risk.
Objectives
We conducted a cardiovascular safety evaluation comparing hydroxychloroquine to methotrexate among patients with rheumatoid arthritis.
Methods
Using Medicare data (2008–2016), we identified 54,462 propensity score-matched patients with rheumatoid arthritis, aged ≥ 65 years, who initiated hydroxychloroquine or methotrexate. Primary outcomes were sudden cardiac arrest or ventricular arrythmia (SCA/VA), and major adverse cardiovascular event (MACE). Secondary outcomes were cardiovascular mortality, all-cause mortality, myocardial infarction, stroke, and hospitalized heart failure (HF). We also examined treatment effect modification by history of HF.
Results
Hydroxychloroquine was not associated with risk of SCA/VA (HR 1.03, 95% CI 0.79–1.35) or MACE (HR 1.07, 95% CI 0.97–1.18) compared to methotrexate. In patients with history of HF, hydroxychloroquine initiators had a higher risk of MACE (HR 1.30, 95% CI 1.08–1.56), cardiovascular mortality (HR 1.34, 95% CI 1.06–1.70), all-cause mortality (HR 1.22, 95% CI 1.04–1.43), myocardial infarction (HR 1.74, 95% CI 1.25–2.42), and hospitalized HF (HR 1.29, 95% CI 1.07–1.54) methotrexate initiators. Cardiovascular risks were not different in patients without history of HF except for an increased hospitalized HF risk (HR 1.57, 95% CI 1.30–1.90) among hydroxychloroquine initiators.
Conclusions
In older patients with rheumatoid arthritis, hydroxychloroquine and methotrexate showed similar SCA/VA and MACE risks. However, hydroxychloroquine initiators with history of HF had higher risks of MACE, cardiovascular mortality, all-cause mortality, and myocardial infarction. An increased hospitalized HF risk was observed among hydroxychloroquine initiators regardless of a HF history.
Keywords: Administrative Data, Cardiovascular, Rheumatoid Arthritis, Disease-Modifying Antirheumatic Drugs (Dmards), Pharmacoepidemiology
Condensed Abstract
Hydroxychloroquine has been used as a first-line treatment of rheumatoid arthritis for decades despite limited evidence on its cardiovascular risk. We conducted an active comparator, new user cohort study using Medicare fee-for-service to evaluate the cardiovascular safety of hydroxychloroquine compared to methotrexate among 54,462 older patients with rheumatoid arthritis. Hydroxychloroquine did not confer excess in SCA/VA or MACE risks compared to methotrexate. However, among individuals with history of HF, hydroxychloroquine appears to increase the risks of MACE, cardiovascular mortality, all-cause mortality, and myocardial infarction. An increased risk of hospitalized HF was observed among hydroxychloroquine initiators regardless of HF history.
Hydroxychloroquine is a commonly used treatment for rheumatoid arthritis, particularly recommended in patients with low disease activity.1 In 2019, it accounted for almost one third of all the disease-modifying anti-rheumatic drugs (DMARD) prescribed in the U.S., second only to methotrexate, which is the recommended first-line treatment for DMARD-naïve patients with a moderate to high disease activity.1–3 Although hydroxychloroquine has been used for over 60 years to treat rheumatic conditions, there are limited data on its cardiovascular safety in patients with rheumatoid arthritis, mostly coming from case reports, case series or exploratory studies.1,4,5
On one hand, prior research suggests that hydroxychloroquine may reduce underlying cardiovascular risk by improving lipid profile, glucose tolerance and by modulating platelet activation.6–8 On the other hand, cardiovascular events have been described over the years as possible effect of functional and structural interactions of hydroxychloroquine with cardiomyocytes.4,9–15 Clinical manifestations of potential cardiotoxicity were often reported in the form of cardiac conduction disturbances, such as prolongation of the QT interval or various forms of conduction blocks (e.g., atrioventricular and bundle-branch blocks) and cardiomyopathy, which may progress to congestive heart failure (HF). 4,10–15 Although conduction anomalies appear before any structural changes, they can also be favored by a preexisting cardiomyopathy or HF. In 2016, the American Heart Association added hydroxychloroquine to the list of drugs that increase the risk of HF exacerbation by direct myocardial toxicity,16 albeit this indication was not supported by compelling evidence.
We aimed to provide a comprehensive cardiovascular safety evaluation for hydroxychloroquine by studying the risk of several cardiovascular outcomes among a large group of DMARD-naïve patients with rheumatoid arthritis initiating hydroxychloroquine or methotrexate. Further, we explored whether a preexisting diagnosis of HF modifies the association between hydroxychloroquine initiation and the risk of cardiovascular events.
Methods
Data sources and study design
We performed an active comparator, incident user cohort study using Medicare Fee-for-Service Parts A (inpatient coverage), B (outpatient coverage), and D (prescription benefits), a U.S. federal health insurance program that provides health care to legal residents with at least 65 years of age or disabilities. The database covers about 50 million people and contains demographic information, health plan enrollment status, longitudinal patient-level information on all reimbursed medical services, and both inpatient and outpatient diagnoses and procedures-along with pharmacy-dispensing records. Information on the exact date and cause of death was available for the entire study period through linkage with the National Death Index (NDI) file. The study was approved by the Mass General Brigham Institutional Review Board.
Study population and exposure
The study population included DMARD-naïve patients with rheumatoid arthritis, aged 65 years or older, who initiated hydroxychloroquine or methotrexate between January 1st, 2008, and December 31st, 2016. Cohort entry was the day of the first filled prescription of the exposure drugs, defined as no use of either hydroxychloroquine or methotrexate in the previous year among patients who had at least one year of continuous enrollment prior to cohort entry. To-capture only DMARD-naïve patients, we excluded all users of any other conventional, biologic, or targeted synthetic DMARD any time before and on cohort entry. To select-patients with rheumatoid arthritis, we applied a validated algorithm (positive predictive value, PPV = 86%) which required at least one inpatient or two outpatient diagnoses within the year before and including cohort entry.17 We excluded patients with a diagnosis of systemic lupus erythematosus any time before and on cohort entry, and a diagnosis of malignancy (except non-melanoma skin cancer), or a nursing home admission during the year before and on cohort entry.
Outcome and Follow-up
This study had two primary outcomes: (1) sudden cardiac arrest or ventricular arrythmia (SCA/VA), and (2) 3-point major adverse cardiovascular event (MACE), i.e., a hospitalization for acute myocardial infarction, ischemic or hemorrhagic stroke, or cardiovascular mortality. In prior studies, the PPV of claims-based algorithms was 85% for SCA/VA,18 and at least 87% for myocardial infarction, and stroke.19–21 Causes of death were determined through NDI linkage and cardiovascular deaths were identified through recorded International Classification of Diseases 10th, ICD-10 codes (I00–I99). To increase the specificity of this outcome, cardiovascular death events were considered only when a cardiovascular death from the NDI file was recorded along with a death event from the Master Beneficiary Summary Files of Medicare.22 Secondary outcomes were cardiovascular mortality, all-cause mortality, myocardial infarction, stroke, and hospitalized HF. The hospitalized HF outcome was based on a HF discharge diagnosis in the primary position (PPV = 84–100%).23 Definitions are validated against medical records19–23 and provided in Supplementary Table 1.
Follow-up for study outcomes began on the day after cohort entry and continued, using an “on-treatment” approach, until treatment-discontinuation, switch to the other index drug, initiation of any other conventional, biologic, or targeted synthetic DMARD to specifically isolate the effect of the exposure drugs (See Supplementary Table 2), first occurrence of the outcome of interest, death, end of continuous health plan enrollment, or end of the study period. We allowed a 60-day grace period between prescriptions and a 60-day exposure effect window after the termination of the last prescription’s supply.
Baseline characteristics
Observed patient characteristics were measured during the year before and on the date of cohort entry. Covariates of interest included demographics (age, sex, race, and region of residency), calendar time (year of cohort entry), cardiovascular and metabolic comorbidities (e.g., coronary artery disease, cerebrovascular infarction, HF, atrial fibrillation, hypertension, peripheral vascular disease, diabetes, hyperlipidemia), other comorbidities, indexes of the overall health state such as combined comorbidity score and claims-based frailty index,24,25 use of medications related to rheumatoid arthritis (i.e., glucocorticoids, NSAIDs and selective COX-2 inhibitors), use of-other medications, indicators of health care use as proxy for overall disease state, surveillance, and intensity of care. Baseline characteristics were defined through ICD-9 or ICD-10 diagnosis or procedure codes, Current Procedural Terminology 4th Edition procedure codes, and National Drug Code (pharmacy). A complete list of the baseline covariates is shown in Supplementary Table 3.
Statistical Analysis
We used a multivariable logistic regression model to estimate an exposure propensity score (PS), defined as the probability of receiving hydroxychloroquine compared to-methotrexate conditional on 59 baseline covariates. Covariates were-chosen a priori as potential confounders and included-in the model without selection. To mitigate the risk of confounding, initiators of hydroxychloroquine were 1:1 matched to initiators of methotrexate on their estimated PS using nearest neighbor matching with a caliper width of 0.01 on the PS. Overlap in the PS distributions of the treatment groups was evaluated before and after matching. The PS matching performance in confounding control was assessed by the calculation of standardized differences for each covariate, with meaningful imbalances set at values higher than 10%.26 For all-outcomes, we tabulated numbers of events, person-years, incidence rates and rate differences (RD) in the PS-matched cohort. Cox proportional hazard models, with the exposure as independent variable, were used to estimate the hazard ratios (HR) and 95% confidence interval (CI). Kaplan-Meier curves-were generated to visualize the cumulative incidence of the primary outcomes over time and log-rank tests were used to compare hazard rates between treatment-groups. All analyses were implemented using Aetion Evidence Platform® and STATA 15.1 statistical software (StataCorp LLC, College Station, TX).
Subgroup analyses
We conducted subgroup analyses for all primary and secondary outcomes stratified by history of HF and history of atherosclerotic cardiovascular disease (ASCVD). We also conducted a subgroup analysis by history of ASCVD within a subcohort of PS-matched patients without history of HF. The PS was recalculated, and 1:1 PS-matching was reperformed within each subgroup category. To determine whether the relative and absolute effects of the study treatments varied between subgroups, we performed a Wald test for homogeneity. A p-value for homogeneity < 0.05 was considered indicative of treatment heterogeneity, suggesting that the effect of the study treatments on the outcome differs between subgroup categories. The subgroups of interest were pre-specified and selected at baseline using the definitions reported in the Supplementary Table 4.
Sensitivity analyses
To explore whether the findings could have been specific to the comparator group (methotrexate), we further compared DMARD-naïve patients who initiated hydroxychloroquine with those who initiated-sulfasalazine for all study outcomes. We also explored potential informative censoring conducting a-sensitivity analysis with a time-limited “intent-to-treat” approach (i.e., within one year from cohort entry), thus without considering drug discontinuation or switching. We also considered the competing risk analysis for the primary outcomes using Fine and Gray’s proportional subhazards model.27
Results
Study population and baseline characteristics
We identified a total of 70,062 DMARD-naïve patients with rheumatoid arthritis, who initiated either hydroxychloroquine (n = 28,167) or methotrexate (n = 41,895) after applying eligibility criteria. Of those, we included a total of 27,231 PS-matched pairs of hydroxychloroquine and methotrexate initiators-in the primary analysis (See flowchart selection in Supplementary Figure 1).
In the unmatched cohort, hydroxychloroquine users were more likely to be female (78.9% vs 73.9%), and less likely to use oral glucocorticoids (65% vs 70.1%) compared to methotrexate users (Supplementary Table 3). All other baseline characteristics were similar between the two treatment groups and their PS distributions largely overlapped (Supplementary Figure 2A). After PS matching, the two treatment groups were well balanced on all observed confounders and their PS distributions overlapped completely (Supplementary Table 3 and Supplementary Figure 2B).
Table 1 shows selected baseline characteristics of patients initiating hydroxychloroquine versus methotrexate after PS matching. Overall, the mean age was 74 years, 79% of the patients were female, 84% were white, 86% had hypertension, 12% had history of HF, 72% had hyperlipidemia, 32% had diabetes, 53% had recent use of glucocorticoids, and 19% had at least one hospitalization during the baseline period.
Table 1.
Selected baseline* characteristics of patients initiating hydroxychloroquine versus methotrexate after 1:1 propensity-score matching
| Variables | Hydroxychloroquine (n = 27,231) | Methotrexate (n = 27,231) | Standardized Difference |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age, years, mean (SD) | 74.27 (6.52) | 74.27 (6.46) | 0.00 |
| Female; n (%) | 21,344 (78.4%) | 21,412 (78.6%) | 0.01 |
| Race | |||
| White; n (%) | 22,833 (83.9%) | 22,871 (84.0%) | 0.01 |
| Black; n (%) | 2,540 (9.3%) | 2,499 (9.2%) | 0.00 |
| Others or Unknown; n (%) | 1,858 (6.8%) | 1,861 (6.8%) | 0.00 |
|
| |||
| Comorbidities | |||
|
| |||
| Obesity; n (%) | 3,074 (11.3%) | 3,054 (11.2%) | 0.00 |
| Hypertension; n (%) | 23,382 (85.9%) | 23,444 (86.1%) | 0.01 |
| Hyperlipidemia; n (%) | 19,724 (72.4%) | 19,686 (72.3%) | 0.00 |
| Coronary Artery Disease; n (%) | 6,585 (24.2%) | 6,571 (24.1%) | 0.00 |
| Stroke or Transient Ischemic Attack; n (%) | 1,707 (6.3%) | 1,729 (6.3%) | 0.00 |
| Heart Failure; n (%) | 3,417 (12.5%) | 3,381 (12.4%) | 0.00 |
| Atrial Fibrillation; n (%) | 3,236 (11.9%) | 3,197 (11.7%) | −0.01 |
| Diabetes; n (%) | 8,799 (32.3%) | 8,847 (32.5%) | 0.00 |
| Renal dysfunction; n (%) | 4,087 (15.0%) | 4,123 (15.1%) | 0.00 |
|
| |||
| Medications | |||
|
| |||
| Oral glucocorticoids; n (%) | 17,818 (65.4%) | 17,800 (65.4%) | 0.00 |
| Cumulative prednisone-equivalent mg, mean (SD) | 487.09 (1,153.75) | 492.01 (1,001.94) | 0.01 |
| NSAIDs or coxibs; n (%) | 12,521 (46.0%) | 12,498 (45.9%) | 0.00 |
| Angiotensin II Receptor Blockers; n (%) | 6,336 (23.3%) | 6,395 (23.5%) | 0.01 |
| ACE inhibitors; n (%) | 8,870 (32.6%) | 8,868 (32.6%) | 0.00 |
| Beta Blockers; n (%) | 11,211 (41.2%) | 11,172 (41.0%) | 0.00 |
| Calcium channel blockers; n (%) | 8,499 (31.2%) | 8,514 (31.3%) | 0.00 |
| Diuretics; n (%) | 13,480 (49.5%) | 13,482 (49.5%) | 0.00 |
| Statins; n (%) | 13,611 (50.0%) | 13,602 (50.0%) | 0.00 |
| Antiplatelets; n (%) | 2,646 (9.7%) | 2,730 (10.0%) | 0.01 |
| Insulin; n (%) | 1,700 (6.2%) | 1,705 (6.3%) | 0.00 |
| Other anti-diabetic medications; n (%) | 4,893 (18.0%) | 4,911 (18.0%) | 0.00 |
|
| |||
| Surgical procedures | |||
|
| |||
| Coronary Revascularization †; n (%) | 230 (0.8%) | 228 (0.8%) | 0.00 |
| Other cardiovascular surgery; n (%) | 972 (3.6%) | 958 (3.5%) | −0.01 |
|
| |||
| Healthcare utilization | |||
|
| |||
| Emergency Department Visits; n (%) | 9,335 (34.3%) | 9,271 (34.0%) | −0.01 |
| Hospitalizations; n (%) | 5,161 (19.0%) | 5,098 (18.7%) | −0.01 |
| Number of Rheumatologist Visits, mean (SD) | 2.11 (2.02) | 2.11 (2.26) | 0.00 |
| Number of Cardiologist Visits, mean (SD) | 1.11 (2.79) | 1.12 (2.80) | 0.00 |
Baseline characteristics were measured during the year before and on the date of cohort entry. Additional baseline characteristics are reported in the Supplementary Table 3.
Coronary revascularization includes surgical procedures such as coronary artery bypass grafting (CABG), percutaneous transluminal angioplasty (PTA) and stenting.
SD, standard deviation; NSAIDs, nonsteroidal anti-inflammatory drugs; Coxibs, selective cyclooxygenase 2 inhibitors; ACE, Angiotensin Converting Enzyme.
Median duration of follow-up on treatment was overall 209 days, 228 days in the methotrexate group and 180 days in the hydroxychloroquine group. About 32% (n = 17,466) of the patients had follow-up time >1 year, and 15% (n = 8,041) had follow-up time >2 years. Most patients were censored due to treatment discontinuation (60%). Details on follow-up and censoring reasons are reported in the Supplementary Table 5.
Primary outcome analyses
In the PS-matched cohort, the incidence rates per 1,000 person-years in hydroxychloroquine versus methotrexate initiators were 3.7 vs. 3.6 for SCA and/or VA, and 29.9 vs. 27.8 for MACE, respectively (Table 2). The-initiation of hydroxychloroquine was not associated with a risk of SCA/VA (HR 1.03, 95% CI 0.79–1.35) or MACE (HR 1.07, 95% CI 0.97–1.18) compared to methotrexate. Kaplan-Meier curves comparing the cumulative incidence of SCA/VA and MACE among initiators of hydroxychloroquine versus methotrexate were consistent with these findings (Figure 2A,2B).
Table 2.
Primary and secondary outcomes in 1:1 PS-matched patients initiating hydroxychloroquine versus methotrexate
|
|
||||||
|---|---|---|---|---|---|---|
| Hydroxychloroquine (n = 27,231) | Methotrexate (n = 27,231) | Hydroxychloroquine vs Methotrexate | ||||
|
|
||||||
| N of events | IR/1,000 PY | N of events | IR/1,000 PY | RD/1,000 PY (95% CI) | HR (95% CI) | |
|
| ||||||
| Primary Outcomes | ||||||
|
| ||||||
| Sudden Cardiac Arrest or Ventricular Arrythmia | 102 | 3.67 | 109 | 3.57 | 0.11 (−0.87, 1.08) | 1.03 (0.79, 1.35) |
| MACE | 820 | 29.91 | 839 | 27.81 | 2.10 (−0.68, 4.88) | 1.07 (0.97, 1.18) |
|
| ||||||
| Secondary Outcomes | ||||||
|
| ||||||
| Cardiovascular Mortality | 389 | 13.99 | 366 | 11.96 | 2.03 (0.18, 3.89) | 1.17 (1.02, 1.35) |
| All-cause Mortality | 972 | 34.96 | 972 | 31.76 | 3.21 (0.24, 6.18) | 1.10 (1.01, 1.21) |
| Myocardial Infarction | 316 | 11.46 | 315 | 10.38 | 1.08 (−0.62, 2.79) | 1.10 (0.94, 1.28) |
| Stroke | 223 | 8.07 | 257 | 8.45 | −0.38 (−1.86, 1.10) | 0.95 (0.79, 1.14) |
| Hospitalized Heart Failure | 523 | 19.06 | 407 | 13.42 | 5.64 (3.55, 7.73) | 1.41 (1.24, 1.61) |
IR, incidence rate; PY, person-years; RD, rate difference; HR, hazard ratio; CI, confidence interval; MACE, 3-point major adverse cardiac events (i.e., myocardial infarction, stroke, and cardiovascular mortality).
Figure 2. Cumulative incidence for SCA/VA and MACE, overall and by HF history.



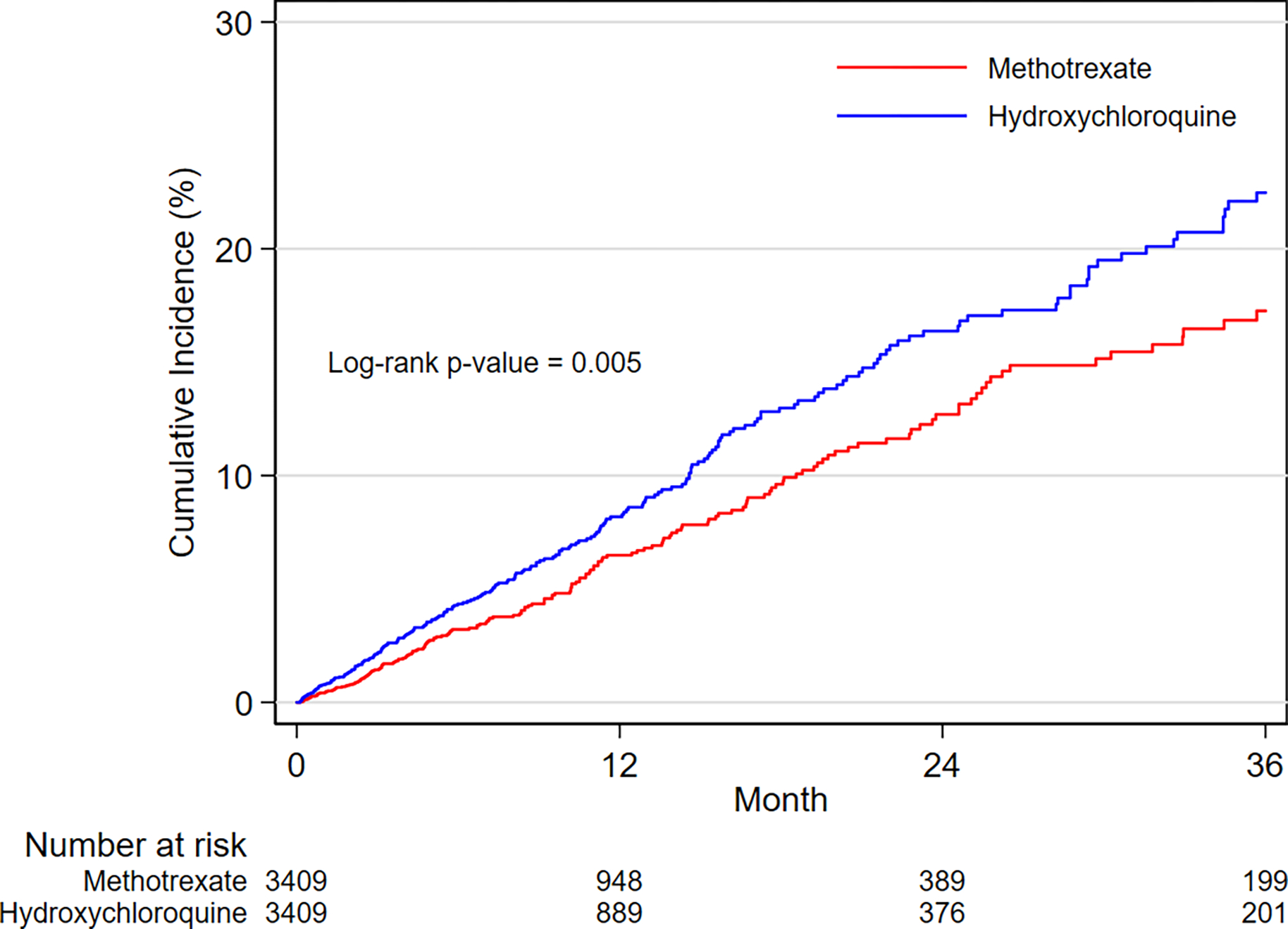
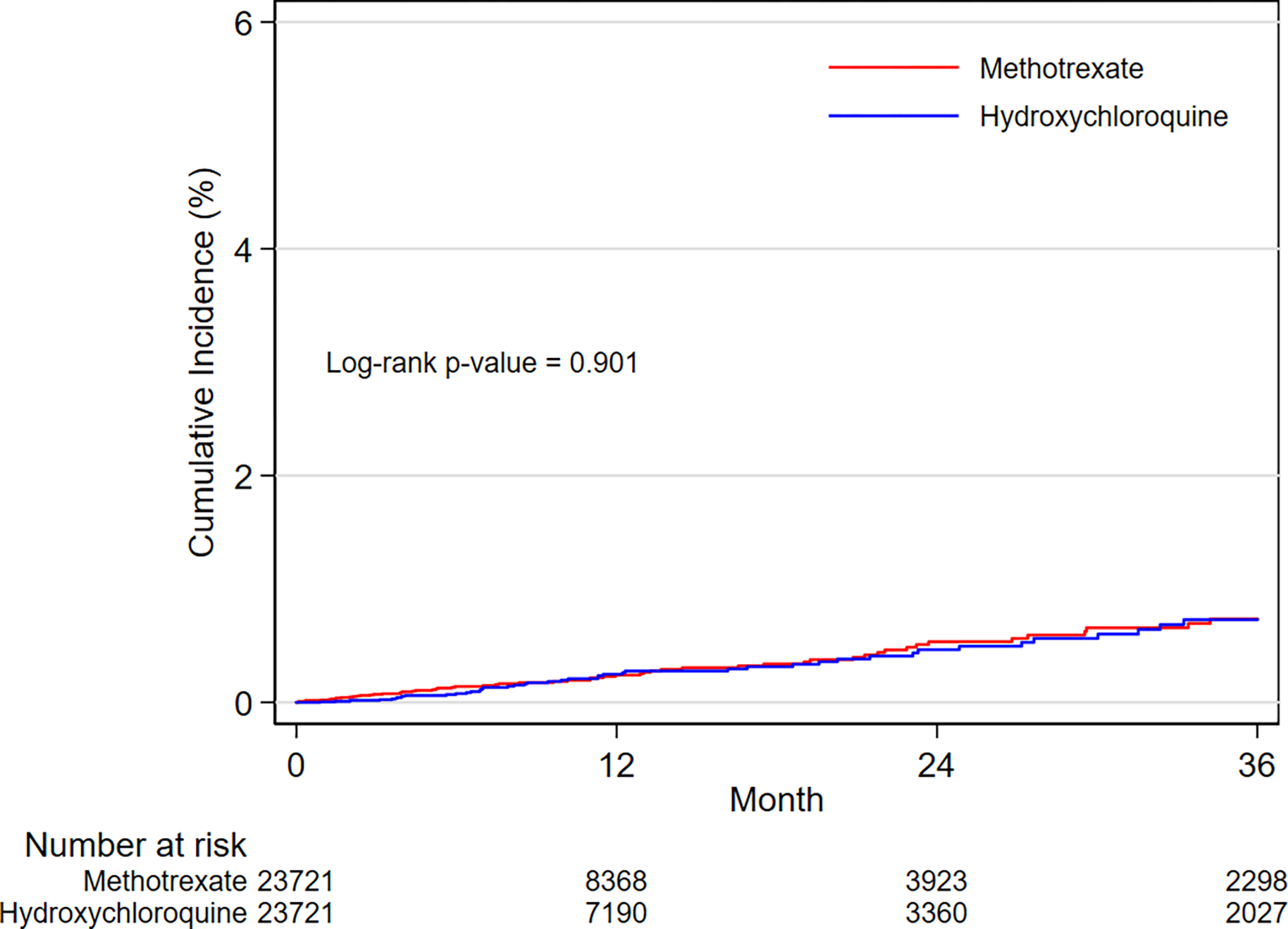

A. SCA/VA outcome in the overall population
B. MACE outcome in the overall population
C. SCA/VA outcome in the subgroup with history of HF
D. MACE outcome in the subgroup with history of HF
E. SCA/VA outcome in the subgroup without history of HF
F. MACE outcome in the subgroup without history of HF
Kaplan-Meier plots of cumulative incidence for SCA/VA and MACE comparing 1:1 PS-matched hydroxychloroquine vs. methotrexate initiators overall and by history of HF.
Incidence rates between treatment groups are compared with log-rank tests.
ABBREVIATIONS: SCA/VA, sudden cardiac arrest and ventricular arrythmia; MACE, 3-point major adverse cardiac events (i.e., myocardial infarction, stroke, and cardiovascular mortality); HF, heart failure.
Secondary outcome analyses
Among the individual components of the MACE outcome, a 17% increased risk of cardiovascular mortality (HR 1.17, 95% CI 1.02–1.35), corresponding to 2.03 additional events (RD 2.03, 95% CI 0.18–3.89) per 1,000 person-years, was observed among initiators of hydroxychloroquine compared to methotrexate; however, we found no differences in the risks of myocardial infarction (HR 1.10, 95% CI 0.94, 1.28) or stroke (HR 0.95, 95% CI 0.79, 1.14) between the two groups. The initiation of hydroxychloroquine vs. methotrexate was also associated with a 10% increased risk of all-cause mortality (HR 1.10, 95% CI 1.01–1.20), corresponding to 3.21 additional all-cause death events (RD 3.21, 95% CI 0.24–6.18) per 1,000 person-years (Table 2).
A 41% increased risk of hospitalized HF (HR 1.41, 95% CI 1.24–1.61) was recorded in patients treated with hydroxychloroquine versus methotrexate, equivalent to 5.64 additional hospitalizations for HF (RD 5.64, 95% CI 3.55–7.73) per 1,000 person-years (Table 2).
Subgroup analyses
After subgroup-specific PS matching, the treatment groups were balanced on all observed confounders within each subgroup category (Supplementary Figure 3). The Central Illustration displays the relative risk from the subgroup analyses by history of HF. The stratified absolute risk is reported in the Supplementary Figure 4. In assessment of the primary outcomes, no differences in the relative or absolute risks of SCA/VA were noted between hydroxychloroquine and methotrexate initiators with or without history of HF (p-value for homogeneity > 0.10). Evidence of effect heterogeneity on both the relative and the absolute scales was observed for the risk of MACE (p-value for homogeneity < 0.05). Specifically, we found a 30% increased risk of MACE (HR 1.30, 95% CI 1.08–1.56), corresponding to 19.66 additional cases per 1,000 person-years (RD 19.66, 95% CI 5.98–33.35), among hydroxychloroquine versus methotrexate initiators with history of HF; whilst similar risks of MACE (HR 1.00, 95% CI 0.88–1.11; RD −0.13, 95% CI −2.74–2.47) were observed among initiators with no history of HF (Figure 2C–2F, Central Illustration and Supplementary Figure 4).
Central Illustration. Cardiovascular outcomes across subgroups stratified by history of HF.

Cardiovascular safety outcomes in subgroups by history of HF of 1:1 PS-matched hydroxychloroquine vs. methotrexate initiators. Patients with history of HF included in the subgroup analyses were 6,818 while patients without history of HF were 47,442. Relative effect estimates for individual outcomes are indicated by the squares and 95% CI by horizontal lines. The null value equal to 1 is indicated by a dashed vertical line. A p-value for homogeneity < 0.05 is suggestive of treatment effect modification. ABBREVIATIONS: MACE, 3-point major adverse cardiac events (i.e., myocardial infarction, stroke, and cardiovascular mortality); HR, hazard ratio; CI, confidence interval; HF, heart failure; HCQ, hydroxychloroquine; MTX, methotrexate.
In assessment of secondary outcomes, evidence of treatment effect heterogeneity was found on relative and absolute scales of all outcomes except for stroke (p-values for homogeneity are reported in the Central Illustration). Specifically, among patients with HF, hydroxychloroquine initiation was found to increase the risk of cardiovascular mortality (HR 1.34, 95% CI 1.06–1.70; RD 12.56, 95% CI 2.30–22.82), all-cause mortality (HR 1.22, 95% CI 1.04–1.43; RD 18.44, 95% CI 3.38–33.51), myocardial infarction (HR 1.74, 95% CI 1.25–2.42; RD 12.80, 95% CI 5.09–20.51) and hospitalized HF (HR, 1.29, 95% CI 1.07–1.54; RD 19.53 95% CI 5.44–33.61) compared to methotrexate initiation. Among patients with no history of HF, no differences in risks were noted between hydroxychloroquine and methotrexate initiators except for an increased risk of hospitalized HF (HR 1.57, 95% CI 1.30–1.90; RD 3.73 95% CI 2.13–5.33) (Central Illustration and Supplementary Figure 4).
Subgroup analyses stratified by history of ASCVD showed findings similar to the subgroup analyses by HF (Supplementary Table 6A). However, when we stratified by ASCVD within the subcohort of patients without history of HF, no evidence of effect heterogeneity between subgroup categories of ASCVD was observed (Supplementary Table 6B).
Sensitivity analyses
Findings remained consistent with the primary and secondary analyses when we compared hydroxychloroquine to sulfasalazine initiation as first-line DMARD, when we adopted an intent-to-treat approach, or when we performed competing-risk regression analyses (Supplementary Tables 7–9). In the subgroup analysis by history of HF, the effect estimates of the comparison hydroxychloroquine versus sulfasalazine followed a trend similar to the effect estimates of the main comparison except for a risk for hospitalized HF toward the null in patients without a history of HF (Supplementary Table 7).
Discussion
In this large population-based cohort of 54,462 older adults with rheumatoid arthritis, mean age of 74 years and mostly women, we found overall similar risks of SCA/VA and MACE between hydroxychloroquine and methotrexate initiators. Among the secondary outcomes, the risk of hospitalized HF in individuals initiating hydroxychloroquine appears to be increased regardless of a preexisting-diagnosis of HF or ASCVD (41% increased risk, corresponding to ~6 additional cases per 1,000 person-years). When stratified by HF, individuals with a diagnosis of the disease who initiated hydroxychloroquine had a 30% increased risk of MACE (~20 additional cases per 1,000 person-years), 34% higher risk of cardiovascular mortality (~13 additional cases per 1,000 person-years), 22% greater risk of all-cause mortality (~18 additional cases per 1,000 person-years), 74% higher risk of myocardial infarction (~13 additional cases per 1,000 person-years) and 29% increased risk of hospitalized HF (~20 additional cases per 1,000 person-years) compared to methotrexate. Among initiators without history of HF, no difference in risks was seen between hydroxychloroquine and methotrexate, except for a 57% increased risk of hospitalization for HF (~4 additional cases per 1,000 person-years).
Almost half of all SCA/VA events identified in our study were recorded within less than one year from the first prescription of the index drugs and occurred similarly across the two treatment groups indicating no difference in SCA/VA risk between hydroxychloroquine and methotrexate initiators. These results suggest that, despite the many effects of hydroxychloroquine on potassium channels,28,29 initiation of hydroxychloroquine at conventional doses used to treat rheumatoid arthritis does not appear to be associated with life-threatening ventricular arrhythmias and attendant sudden cardiac death. The findings-are in line with several exploratory studies suggesting that QT syndrome and arrhythmias, occurring within a short period of time after hydroxychloroquine initiation, are not a substantial safety concern among rheumatic disease patients.29,30 These events appeared also to be quite rare, as confirmed by a recent analysis of the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) database on adverse drug event reports received between 1969 and 2019 which concluded that short-term treatment with hydroxychloroquine was not associated with a safety signal related to ventricular arrhythmia due to prolonged QT interval.31
In this study, hydroxychloroquine was associated with an increased risk of hospital admission for HF regardless of a preexisting diagnosis for cardiovascular comorbidity (i.e., HF or ASCVD). Several case reports discussed the development of cardiomyopathy and subsequent congestive HF as possible drug-induced effect following long-term treatments.23,28 A pharmacovigilance analysis on the U.S. FAERS database showed that the most frequent pre-COVID-19 reports for cardiovascular adverse events-of hydroxychloroquine were those for cardiomyopathy and HF.32 Another analysis of spontaneous reports of suspected cardiovascular adverse drug reactions on the World Health Organization pharmacovigilance database found an association between hydroxychloroquine and functional disorders (e.g., atrioventricular and bundle-branch blocks) and HF, the only two cardiovascular adverse reactions associated with the drug.33 Given its long half-life, accumulation over time of hydroxychloroquine in lysosomes of cardiac myocytes may occur even at repeated administration of low doses, producing structural myocardial changes and leading to cardiomyopathy.28,29 Cardiomyopathy, often with evidence of hypertrophy and restrictive physiology, may ultimately present with congestive HF and conduction abnormalities.23,28,29
We observed some evidence of heterogeneous treatment effects across HF subgroups for all the outcomes evaluated, except for SCA/VA and stroke. In DMARD-naïve patients with a history of HF, the risks of developing MACE, cardiovascular mortality, all-cause mortality, myocardial infarction, and hospitalized HF were significantly increased compared to the subgroup of patients without a history of HF. The larger number of MACE, deaths, or HF exacerbations in patients with compensated or decompensated HF may be related to hydroxychloroquine-induced cardiotoxicity, particularly cardiomyopathy, which can function as a precipitating factor in individuals with an already deteriorated cardiac tissue contributing to the cardiovascular burden and increased mortality.23,28,29,33 In patients with a history of HF, incident cardiac events or HF relapses are more likely to emerge due to the underlying cardiac condition.34 Hydroxychloroquine initiation was also associated with an increased risk of hospital admissions for HF in subjects without a previous diagnosed HF, albeit the absolute risk was much smaller in patients without HF. As before, this finding might be in part linked to the lysosomal dysfunction of cardiomyocytes due to the long-term use of hydroxychloroquine which causes imbalances in homeostatic processes and is implicated in the development of cardiomyopathy and HF. 23,28,29,33
Since we used methotrexate as an active comparator, an alternative hypothesis is that at least part of the increased cardiovascular risk noted in hydroxychloroquine initiators among patients with history of HF would be due to a potential cardio-protective activity of methotrexate.35 However, recent results from the Cardiovascular Inflammation Reduction Trial (CIRT), the only trial that investigated the use of low-dose methotrexate for the secondary prevention of atherosclerotic events, showed that methotrexate was not associated with fewer cardiovascular events (i.e., combined major adverse cardiovascular events, myocardial infarction, stroke, cardiovascular mortality, all-cause mortality, or hospitalization for congestive HF) than placebo in patients with either type 2 diabetes or metabolic syndrome and no chronic inflammatory conditions.36 To further investigate whether the comparison with methotrexate could have affected our main findings, we conducted an exploratory analysis comparing hydroxychloroquine versus sulfasalazine initiation. The exploratory results for primary and secondary outcomes did not contradict our main findings, although statistical power to detect a difference was much lower in this comparison because of the small number of patients treated with sulfasalazine in clinical practice. In the subgroup analysis, hydroxychloroquine and sulfasalazine initiators without a HF history appeared to have similar hospitalized HF risk. However, this result should be interpreted with caution due to the low number of cases in this comparison. Moreover, only 18% of the hydroxychloroquine initiators included in the main comparative analysis with methotrexate were included in the exploratory analysis with sulfasalazine, limiting the generalizability of these findings. Finally, it is unknown whether sulfasalazine may increase the risk of HF. We could not compare hydroxychloroquine treatment versus no treatment because, contrary to the placebo arm of randomized control trials, the non-user group of non-randomized studies is fundamentally different from the treated group. Thus, such comparison would have been susceptible to-many methodological issues including confounding by indication, healthy user bias, and immortal time bias.37
This study has several strengths. First, it has a large cohort size which allowed us to calculate comparative risks of rare events such as SCA/VA and to perform pre-specified subgroup analyses testing the heterogeneity between groups. Second, it provided a more comprehensive cardiovascular safety evaluation of the long-term use of hydroxychloroquine, and it was the first to explore effect treatment modification due to history of HF. Third, because of the linkage with the NDI database, we have been able to identify cardiovascular cause of deaths and to detect more accurately all-cause deceases. Further, we adopted an active comparator, new user design, which mitigates numerous biases (e.g., confounding by indication, depletion of susceptibles, immortal time bias) and seeks to emulate the design of a head-to-head randomized controlled trial.37,38 Lastly, to select only DMARD-naïve patients, we used all available data before cohort entry to exclude individuals with a previous use of any DMARDs.
Study limitations
Our study has also limitations. First, the severity or disease activity of rheumatoid arthritis is not measured in claims; thus, confounding by indication might exist due to different prescription recommendations (i.e., methotrexate is recommended in patients with moderate or high disease activity while hydroxychloroquine is mainly prescribed in individuals with low disease activity).1,39 Whilst residual confounding for unmeasured characteristics, such as disease severity, cannot be entirely ruled out, we balanced 59 baseline covariates between treatment groups through PS matching. Additionally, the directionality of our estimates could not be explained by residual confounding for disease activity. Since disease activity is a negative confounder (i.e., milder disease activity in hydroxychloroquine users), our observed associations are potentially biased toward the null. Second, our results on SCA/VA did not consider drug interactions, such as a concomitant administration of medications prolonging QT interval or other arrhythmogenic drugs. Third, misspecification of the outcome SCA/VA cannot be excluded since the current definition does not capture diagnosis of out-of-hospital sudden cardiac death. However, there is no reason to suspect that the number of missed outcome events would differ between treatment groups. Fourth, since our study cohort includes Medicare enrollees aged ≥ 65, it remains clinically important to investigate the cardiovascular safety of hydroxychloroquine in a younger population with rheumatic conditions. Although a randomized controlled trial would be needed to answer this question, it is unlikely that such option would be pursued, and if were, high risk, older individuals would be studied to have adequate statistical power. Lastly, concern might be raised by our choice of selecting methotrexate as an active comparator. Given that in the U.S. hydroxychloroquine is commonly used as first-line therapy for rheumatoid arthritis, second only to methotrexate, selecting methotrexate as referent drug reduces potential for confounding by indication which could have been larger in new user comparative safety analyses with other DMARDs. Moreover, findings from the sensitivity analysis comparing hydroxychloroquine versus sulfasalazine were consistent with those from the main analysis.
Conclusions
In conclusion, in this large cohort of older DMARD-naïve patients with rheumatoid arthritis, hydroxychloroquine treatment is not associated with-an increased risk of SCA/VA compared with methotrexate. Within the subgroup-with a history of HF, hydroxychloroquine versus methotrexate initiation appears to be associated with increased risks of MACE, cardiovascular mortality, all-cause mortality, and myocardial infarction. An increased risk of hospitalization for HF was observed in initiators of hydroxychloroquine regardless of a prior history of HF. Based on our findings, health care providers should be more cautious in prescribing hydroxychloroquine to older patients with baseline HF or at high risk for developing HF, paying careful attention to cardiac manifestations.
Supplementary Material
Figure 1. Study flowchart.
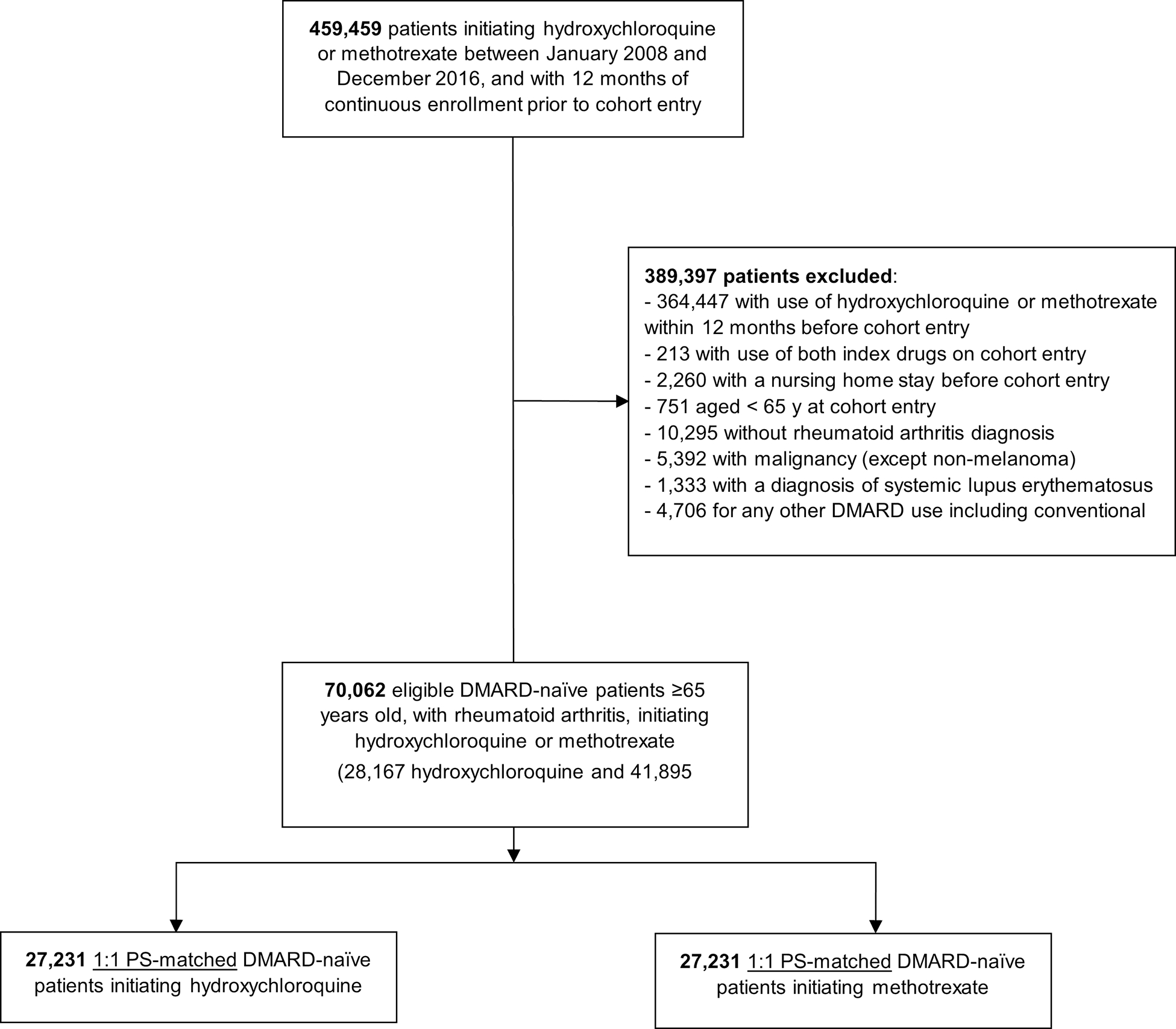
A total of 459,459 hydroxychloroquine or methotrexate users were assessed for eligibility; 389,397 patients were excluded based on the inclusion (incident users, age ≥ 65, rheumatoid arthritis) or exclusion (nursing home admission, malignancy, systemic lupus erythematosus, or use of any other DMARD) criteria. Overall, 54,462 were included in the analysis.
ABBREVIATIONS: DMARD, disease-modifying antirheumatic drug; PS, propensity score.
Clinical Perspectives.
Competency in Patient Care and Procedural Skills:
In older patients with rheumatoid arthritis, treatment with hydroxychloroquine is associated with a greater risk of hospitalization for heart failure (HF) than methotrexate, and those with a history of HF face a higher risk of cardiovascular complications and death when treated with hydroxychloroquine compared to methotrexate.
Translational Outlook:
Further research is needed to clarify the mechanisms responsible for the higher risk of cardiovascular complications and HF during hydroxychloroquine treatment.
Funding:
This work was supported by the NIH K24-AR078959 as well as internal resources in the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School.
Abbreviations and Acronyms
- DMARD
disease-modifying anti-rheumatic drugs
- HF
heart failure
- SCA/VA
sudden cardiac arrest or ventricular arrythmia
- MACE
3-point major adverse cardiovascular event
- ASCVD
atherosclerotic cardiovascular disease
- PPV
positive predictive value
- HR
hazard ratio
- RD
rate difference
- CI
confidence interval
- PS
propensity score
Footnotes
Disclosures: ED, MH, HL have nothing to disclose. RJD reports research grants from Vertex, Bayer, and Novartis for unrelated studies. RJG reports research grants from AstraZeneca, Kowa, Novartis, and Pfizer for unrelated studies. MEW reports research grants from Amgen, Bristol Myers Squibb, Eli Lilly and Sanofi for unrelated studies, stock options from Canfite, Scipher, Vorso, and Inmedix and consultanting for AbbVie, Amgen, Aclaris, Arena, Bristol Myers Squibb, CorEvitas, EqRx, Genosco, GlaxoSmithKline, Gilead, Eli Lilly, Merck, Novartis, Pfizer, Roche, Scipher, SetPoint, Tremeau. SCK reports research grants from Roche, Pfizer, AbbVie, and Bristol-Myers Squibb for unrelated studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline-for the Treatment of Rheumatoid Arthritis. Arthritis-Rheumatol. 2021;73:1108–1123. [DOI] [PubMed] [Google Scholar]
- 2.Medical Expenditure Panel Survey 2013–2019. Agency for Healthcare Research and Quality, - Rockville, MD. ClinCalc-DrugStats-Database-version 2021.10. Available at: https://clincalc.com/DrugStats/TC/Antirheumatics_Accessed-3/30/2022 [PubMed] [Google Scholar]
- 3.Jin Y, Desai RJ, Liu J, et al. -Factors associated with initial or subsequent-choice of biologic-disease-modifying antirheumatic drugs for-treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatre C, Roubille F, Vernhet H, et al. Cardiac Complications-Attributed to Chloroquine and-Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018;41:919–931. [DOI] [PubMed] [Google Scholar]
- 5.Sepriano A, Kerschbaumer A, Smolen JS, et al. Safety of synthetic and biological DMARDs: a-systematic literature review informing the 2019-update of the EULAR recommendations for the-management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo JF, Del Rincon I, Molina E, et al. Use of Hydroxychloroquine Is Associated With-Improved Lipid Profile in-Rheumatoid-Arthritis-Patients. J Clin Rheumatol. 2017;23:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic-drugs and diabetes risk in patients-with-rheumatoid-arthritis-and-psoriasis. JAMA. 2011;305:2525–31 [DOI] [PubMed] [Google Scholar]
- 8.He M, Pawar A, Desai RJ, et al. Risk of Venous Thromboembolism Associated With-Methotrexate Versus Hydroxychloroquine-for Rheumatoid Arthritis: A Cohort Study. Semin-Arthritis Rheum. 2021. Oct 19;51(6):1242–1250. [DOI] [PubMed] [Google Scholar]
- 9.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:155–66. [DOI] [PubMed] [Google Scholar]
- 10.Haeusler IL, Chan XHS, Guerin PJ, White NJ. The arrhythmogenic cardiotoxicity of the-quinoline and structurally related antimalarial drugs: a systematic review. BMC Med 2018;16:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costedoat-Chalumeau N, Hulot JS, Amoura Z et al. Heart conduction disorders related to-antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with-hydroxychloroquine for connective tissue diseases. Rheumatology 2007;46:808–10. [DOI] [PubMed] [Google Scholar]
- 12.Touze JE, Heno P, Fourcade L et al. The effects of antimalarial-drugs on ventricular-repolarization. Am J Trop Med Hyg 2002;67:54–60. [DOI] [PubMed] [Google Scholar]
- 13.Traebert M, Dumotier B. Antimalarial drugs: QT prolongation and cardiac arrhythmias. Expert-Opin Drug Saf. 2005;4(3):421–31. [DOI] [PubMed] [Google Scholar]
- 14.Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart. - 1999;81:221–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy - a review of the literature. -Immunopharmacol Immunotoxicol. 2013;35:434–42. [DOI] [PubMed] [Google Scholar]
- 16.Page RL, O’Bryant CL, Cheng D, et al. Drugs That May Cause or Exacerbate Heart Failure: A-Scientific Statement From the American Heart Association. Circulation. 2016;134:e32–69. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health careutilization data. Arthritis Res Ther. 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennessy S, Leonard CE, Freeman CP, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims-data. Pharmacoepidemiol Drug Saf. 2010;19:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value based on review of hospital records. - Am Heart J 2004;148:99–104 [DOI] [PubMed] [Google Scholar]
- 20.Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured-population. Pharmacoepidemiol Drug Saf 2010;19:596–603 [DOI] [PubMed] [Google Scholar]
- 21.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 22.Olubowale OT, Safford MM, Brown TM, et al. Comparison of Expert Adjudicated Coronary-Heart Disease and Cardiovascular Disease Mortality With the National Death Index: Results-From the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Am-Heart Assoc. 2017. May 3;6(5):e004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for-identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf 2012;21(Suppl. -1):129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly-patients better than existing scores. Journal of clinical epidemiology. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and-validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between-treatment groups in propensity-score matched samples. Stat*Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of-Competing Risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mubagwa K Cardiac effects and toxicity of chloroquine: a short update. Int J Antimicrob Agents. −2020;56:106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desmarais J, Rosenbaum JT, Costenbader KH, et al. American College of Rheumatology White-Paper-on-Antimalarial-Cardiac-Toxicity. Arthritis Rheumatol. 2021;73:2151–2160. [DOI] [PubMed] [Google Scholar]
- 30.Faselis C, Zeng-Treitler Q, Cheng Y, et al. Cardiovascular-Safety-of-Hydroxychloroquine in US-Veterans-With-Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73(9):1589–1600. [DOI] [PubMed] [Google Scholar]
- 31.Sarayani A, Cicali B, Henriksen CH, Brown JD. Safety signals for QT prolongation or Torsades-de Pointes associated with azithromycin with or without chloroquine or hydroxychloroquine. Res-Social Adm Pharm. 2021;17:483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman A, Bomze D, Dankner R, et al. Cardiovascular adverse events associated with-hydroxychloroquine and chloroquine: A comprehensive pharmacovigilance analysis of pre-COVID-19 reports. Br J Clin Pharmacol. 2021;87:1432–1442. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen LS, Dolladille C, Drici MD, et al. Cardiovascular Toxicities Associated With-Hydroxychloroquine and-Azithromycin: An-Analysis of the-World-Health-Organization-Pharmacovigilance Database. Circulation. 2020;142:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feenstra J, Grobbee DE, Jonkman FA, et al. Prevention of relapse in patients with congestive-heart failure: the role of precipitating factors. Heart. 1998;80:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micha R, Imamura F, Wyler-von-Ballmoos M, et al. Systematic review-and-meta-analysis of-methotrexate-use and risk of cardiovascular-disease. Am J Cardiol 2011;108:1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM, Everett BM, Pradhan A, et al. ; CIRT Investigators. Low-Dose Methotrexate for the-Prevention of Atherosclerotic-Events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Andrea E, Vinals L, Patorno E, et al. How well can we assess the validity of non-randomised-studies of medications? A systematic review of assessment tools. BMJ Open. 2021;11(3):e043961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol-Rep. 2015;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Food and Drug-Administration. Label-Plaquenil®-hydroxychloroquine-sulfate tablets. - Available-at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/009768s053lbl.pdf Accessed 11/15/2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


