Abstract
Background
Emerging data and case reports have found coagulation abnormalities and thrombosis as sequelae of infection with SARS-CoV-2 (COVID-19). Case reports have reported thrombotic complications caused by COVID-19-related coagulopathy leading to limb loss. Alarmingly, many of these patients had no underlying vascular disease prior to being infected with COVID-19. Many of these case reports discuss patients developing gangrene in the intensive care unit (ICU). Our study compares the incidence of gangrene in the ICU in COVID-19 patients to baseline inpatient levels prior to the pandemic.
Methods
This retrospective analysis investigates two subsets of patients from a single institution. The first was from 2020 during the COVID-19 pandemic; the second subset was from 2019 before the pandemic. Demographic data and medication history were ascertained for both groups. Primary outcomes measures included extremity gangrene that developed in the ICU, mortality, and major amputation.
Results
There were 249 COVID-19 positive patients admitted to the ICU in 2020. In 2019, 1,846 admissions to the ICU took place, of which 249 patients were randomized to chart review. There were 13 cases of gangrene that developed in the ICU, 12 of which took place in 2020. In-hospital mortality was 11.6% in nonCOVID-19 patients in 2019 vs. 41.4% in 2021 (P < 0.001). Only 16.7% of the COVID-19 gangrene patients had previously known arterial disease. Also, patients in the COVID-19 group with gangrene were four times more likely to be smokers (P = 0.004). When the data were stratified to compare between gangrene development and no gangrene development, the combined total gangrene group had longer hospital stays, higher need for blood transfusions, required major amputations, and revascularization. A multivariate logistic regression from the total study similarly demonstrated that COVID-19 infection is associated with an 18.23 times increased risk of gangrene.
Conclusions
COVID-19 has resulted in an incomprehensible societal impact that will linger for years to come. The last 2 years have reinforced that COVID-19 will be a part of our clinical practice indefinitely. This study emphasizes the importance of clinician awareness of COVID-19 induced critical limb ischemia in those without underlying arterial disease and few medical comorbidities. More research efforts toward preventing limb loss and COVID-19 coagulopathy must be performed expeditiously to achieve a better understanding.
Introduction
The SARS-CoV-2 (COVID-19) infection has led to rather unexpected complications. While many individuals do not develop significant complications, those with moderate to severe cases of COVID-19 infection have been known to suffer atypical acute respiratory distress syndrome with maintained compliance, multiorgan failure as a result of sepsis, acute kidney injury, and strokes as a result of coagulation abnormalities.1 Multiple case reports have shown serious coagulation abnormalities and thrombosis as a sequelae of severe infection COVID-19.2, 3, 4 Upwards of 55% of patients with COVID-19 can develop mild to severe coagulation abnormalities, with more severe abnormalities leading to a higher incidence of mortality.4 In many cases of COVID-19 coagulopathy, there is development of both arterial and venous thrombotic events.5 Currently, there is no consensus on the pathophysiology behind the COVID-19 coagulopathy, leaving variation in the management of these patients.6
Some have hypothesized that the pathophysiology is similar to coagulopathic mechanisms found in severe systemic inflammatory response and disseminated intravascular infections.2 , 5 , 7 Multiple case reports during the pandemic have also reported acute limb ischemic as a complication in this coagulopathy.8, 9, 10 Alarmingly, many of these patients had no underlying vascular disease prior to getting infected with COVID-19. Bellosta et al. reported an increased incidence of acute limb ischemic increased during the COVID-19 pandemic in a twenty patient cohort. Limb salvage is complicated by the inherent coagulopathy, but therapeutic intravenous heparin postoperatively improves outcomes.6 Incidentally, many of the case reports have illustrated patients developing gangrene during intensive care unit (ICU) admission early, within four to 6 weeks.11, 12, 13 The purpose of this study is to compare the incidence of gangrene in the ICU associated the limb loss–in COVID-19 patients specifically–to baseline inpatient levels before the pandemic. Secondary outcomes report favorable and unfavorable predictors associated with ICU limb gangrene.
Methods
A retrospective observational case-control review of patients with COVID-19 admitted to intensive care over a 2-year period at a single community high-capacity Level 1 trauma center was performed to assess for gangrene development during their hospitalization. Arrowhead Regional Medical Center (ARMC) is a Level 1 trauma center, the county facility for San Bernardino County in California, and a teaching hospital seeing more than 120,000 patients in the emergency department annually. For this study, 498 patients were identified with 249 being COVID-19 positive patients and 249 COVID-19 negative patients. Patients in the year 2019 were considered as pre-COVID-19, and all COVID-19 diagnosed patients admitted to the ICU are sampled from the year 2020. All patients with a diagnosis of COVID-19 were gathered and using a randomization generator a sample of 249 was considered. Similarly, we randomly sampled 249 patients from all ICU admissions in the year 2019 that were admitted to the ICU. All patients (aged 18 and above) admitted to the ICU between January 2019 to December 2020 were included and screened for the development of gangrene. Data gathering and analysis was conducted according to the ARMC Institutional Review Board.
The retrospective chart review was performed for all patients’ records and included admission records, inpatient charts, and emergency department records. Infection with COVID-19 was confirmed in all patients with a nasal PCR analysis. The data collected included demographics, comorbidities, medication use, mortality, gangrene development in extremity digits, and therapeutic anticoagulation use. Medication use was focused on hematologic medications such as aspirin, clopidogrel, other antiplatelet agents, coumadin, direct oral anticoagulants, and statins. The rates of hospital acquired gangrene were gathered for ICU patients in 2019 (pre-COVID); this served as the control. Anticoagulation administration in the COVID-19 group consisted of an unfractured Heparin continues intravenous administration with a partial thromboplastin time goal of 90–120 sec. Additional outcome measures such as major limb loss (below knee amputation or above knee amputation), revascularization in form of open surgical embolectomy and percutaneous thrombectomy and/or thrombolysis, and prehospital vascular status (e.g., whether the patient had underlying “peripheral arterial disease” to start) were investigated, and history of hypercoagulable state were both acquired and genetic.
The data were collected using Excel and analyzed using descriptive statistics and univariate analysis. Statistical analysis was performed using the Statistical Package for the Social Sciences version 27.0 (SPSS Inc, Chicago, IL) software. Univariate analyses were performed using chi-squared test for categorical data. Continuous data were analyzed using nonparametric Mann-Whitney U tests. Continuous data were presented according to the medians with interquartile ranges (IQR), and categorical data are presented with percentages. A binary logistic regression analysis was performed to determine if the odds of gangrene are impacted by COVID-19 while adjusting for other risk factors. Unless otherwise indicated, a P-value less than 0.05 was considered to be statistically significant.
Results
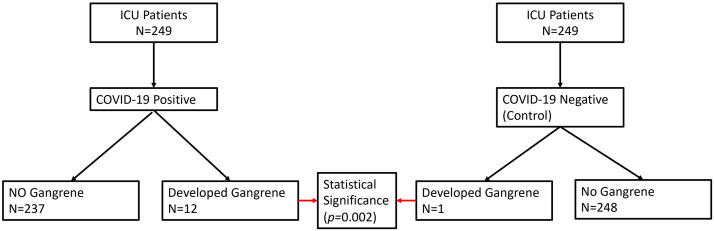
249 patients were identified as having COVID-19 requiring intensive care admission at ARMC in 2020. In 2019, 249 patients were identified as controls pre COVID-19. From the COVID-19 group, 12 patients were found to have developed gangrene, and only 1 patient developed gangrene in the nonCOVID-19 group (Fig. 1 ).
Fig. 1.
Consort Diagram detailing the patient selection in the COVID-19-positive group and COVID-19-negative group.
In the study the median age preCOVID was 60 (IQR 48–73) and the median age of patients that were diagnosed with COVID-19 and admitted to the ICU was 60 (IQR 49–69) (P = 0.643). The percentage of male patients in the pre-COVID group was 54.6% and the percentage of males in the COVID-19 group was 58.2% (P = 0.416). Compared to non-COVID-19 patients, patients with COVID-19 had multiple significant comorbidities. Notably, COVID-19 patients compared to pre-COVID patients had a higher body mass index (BMI) (30.8 (IQR 27, 36) vs. 27.5 (IQR 23.5, 32.2), P < 0.001), higher percentage of diabetes mellitus (56.6%, 36.9%, P = 0.001), lower percentage of hypertension (48.9%, 59%, P = 0.025), lower percentage of cerebrovascular disease (5.2%, 34.5%, P < 0.001), higher percentage of arterial disease (20.8%, 5.2%, P < 0.001), higher percentage of having a hypercoagulable state (16%, 2.8%), and lower percentage of having a smoking history (9.6%, 37.3%, P < 0.001). The reminder of the patient demographics is outlined in Table I .
Table I.
Patient characteristics between pre-COVID ICU patients and COVID-19 ICU patients. Categorical variables are presented as total number with percentages; nonnormally distributed continuous variables are expressed as mean with IQR
| Pre-Covid 2019 N = 249 | COVID-19 N = 249 | P-value | |
|---|---|---|---|
| Demographics | |||
| Agea | 60 (48, 73) | 60 (49, 69) | 0.643 |
| Male (%) | 136 (54.6%) | 145 (58.2%) | 0.416 |
| BMI | 27.5 (23.5, 32.2) | 30.8 (27, 36) | <0.001 |
| Ethnicity | - | - | <0.001 |
| White | 73 | 14 | - |
| African American | 38 | 11 | - |
| Hispanic | 130 | 201 | - |
| Asian | 5 | 21 | - |
| Native American | 3 | 2 | - |
| Comorbid Conditions | |||
| Diabetes Mellitus (%) | 92 (36.9%) | 141 (56.6%) | <0.001 |
| Hypertension (%) | 147 (59%) | 122 (48.9%) | 0.025 |
| Hyperlipidemia (%) | 67 (26.9%) | 75 (30.1%) | 0.427 |
| Chronic Kidney Disease (%) | 32 (12.9%) | 37 (14.9%) | 0.517 |
| End Stage Renal Disease (%) | 18 (7.2%) | 19 (7.6%) | 0.864 |
| Congestive Heart Failure (%) | 26 (10.4%) | 30 (12%) | 0.570 |
| Coronary Artery Disease (%) | 41 (16.5%) | 58 (23.3%) | 0.056 |
| Chronic Obstructive Pulmonary Disease (%) | 16 (6.4%) | 21 (8.4%) | 0.568 |
| Asthma (%) | 10 (4%) | 10 (4%) | 1.000 |
| Cerebrovascular Disease (%) | 86 (34.5%) | 13 (5.2%) | <0.001 |
| Cirrhosis (%) | 9 (3.6%) | 5 (2%) | 0.278 |
| Dementia (%) | 9 (3.6%) | 4 (1.6%) | 0.160 |
| Arterial Disease (%) | 13 (5.2%) | 52 (20.8%) | <0.001 |
| Venous Disease (%) | 8 (3.2%) | 4 (1.6%) | 0.242 |
| Hypercoagulable State (%) | 7 (2.8%) | 40 (16%) | <0.001 |
| Smoking (%) | 93 (37.3%) | 24 (9.6%) | <0.001 |
| Medication Use | |||
| Aspirin (%) | 75 (30%) | 71 (28.5%) | 0.694 |
| Clopidogrel (%) | 31 (12.4%) | 19 (7.6%) | 0.074 |
| Other Antiplatelet (%) | 2 (0.8%) | 0 | 0.156 |
| Coumadin (%) | 4 (1.6%) | 8 (3.2%) | 0.242 |
| Direct Oral Anticoagulant (%) | 17 (6.8%) | 12 (4.8%) | 0.339 |
| Statin (%) | 86 (34.5%) | 62 (24.9%) | 0.019 |
Significant differences are bolded.
Analyzed with nonparametric Mann Whitney U test. All other comparisons were analyzed with chi-squared test.
Patient outcomes and hospital anticoagulation information between preCOVID-19 patients and COVID-19 patients can be found in Table II . We can find that patients diagnosed with COVID-19 had a statistically significant longer (P < 0.001) median hospitalization of 9 days (IQR 4,14) compared with pre-COVID median hospitalization of 3 days (IQR 2,6). In the COVID-19 population there were 4 patients who developed extremity gangrene in the left lower extremity, 3 patients who developed gangrene in the right lower extremity, 5 patients who developed gangrene in bilateral lower extremities, and 2 patients who developed extremity digit gangrene in the upper extremity compared to 1 patient in the pre-COVID population (P = 0.002). The percentage mortality in the COVID-19 population was 41.4% compared to 11.6% in the pre-COVID-19 population (P < 0.001). Overall, patients with COVID-19 had longer hospitalization stay, more frequently developed lower extremity gangrene, had increased mortality, and had an increased need for major amputations and revascularization (open surgical embolectomy, percutaneous thrombectomy, and/or thrombolysis) surgeries.
Table II.
Patient outcomes between nonCOVID-19 ICU patients and COVID-19 ICU patients. Categorical variables are presented as total number with percentages; nonnormally distributed continuous variables are expressed as median with IQR
| Pre-COVID 2019 N = 249 | COVID-19 N = 249 | P-value | |
|---|---|---|---|
| Patient Outcomes | |||
| Hospital Length of Staya | 3 (2, 6) | 9 (4,14) | <0.001 |
| Blood Transfusion (%) | 40 (16%) | 56 (22.5%) | 0.069 |
| Gangrene (%) | 1 (0.4%) | 12 (4.8%) | 0.002 |
| Gangrene Lower (%) | - | - | 0.017 |
| Gangrene Lower - None | 248 | 237 | - |
| Gangrene Lower – Left Lower Extremity | 0 | 4 | - |
| Gangrene Lower – Right Lower Extremity | 1 | 3 | - |
| Gangrene Lower – Bilateral Lower Extremities | 0 | 5 | - |
| Gangrene Upper (%) | 0 | 2 | 0.249 |
| Mortality (%) | 29 (11.6%) | 103 (41.4%) | <0.001 |
| Major Amputation (%) | 0 | 4 (1.6%) | 0.045 |
| Minor Amputation (%) | 0 | 0 | 0 |
| Revascularization (%) | 2 (0.8%) | 5 (2%) | 0.253 |
| Hospital Anticoagulation | |||
| Timing of Anticoagulation | - | - | 0.212 |
| Not Started (%) | 228 (91.6%) | 227 (91.2%) | - |
| Admission (%) | 21 | 19 | - |
| Hospital Day Two and Onward (%) | 0 | 3 | - |
| Contraindications to Anticoagulation (%) | 8 (3.2%) | 12 (4.8%) | 0.361 |
Significant differences are bolded.
Analyzed with nonparametric Mann Whitney U test. All other comparisons were analyzed with chi-squared test.
Table III demonstrates the difference in patients that developed gangrene in the COVID-19-only group and, separately, the combined control and COVID-19 group. Within the cohort, 485 patients did not develop gangrene and 13 developed lower extremity gangrene. BMI was higher in the gangrene group, as was the prevalence of COVID-19. There was a statistically significant increased number of individuals with diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease, and dementia. Patients with gangrene, regardless of COVID-19 status, had longer hospital stays, increased need for blood transfusions, requirement for major amputations, and need for revascularization. Similarly, the COVID-19 cohort, without gangrene, was significant for more diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease, dementia, and smoking. Additionally, there were longer hospital stays, a greater requirement for major amputations, and revascularization in the COVID-19 gangrene group.
Table III.
Patient development of gangrene. Categorical variables are presented as total number with percentages; nonnormally distributed continuous variables are expressed as median with IQR
| COVID-19 |
Total |
P-value (COVID-19) | P-Value (total) | |||
|---|---|---|---|---|---|---|
| No-gangrene N = 237 | Gangrene N = 12 | No-gangrene N = 485 | Gangrene N = 13 | |||
| Demographics | ||||||
| Agea | 60 (19.5) | 59.5 (14) | 60 (23) | 60 (15.5) | 0.689 | 0.918 |
| Male (%) | 137 (57.8%) | 8 (66.7%) | 273 (54.8%) | 8 (61.5%) | 0.706 | |
| BMIa | 30 (9.15) | 33 (10.48) | 28.7 (8.7) | 35 (8.95) | 0.144 | 0.013 |
| Ethnicity | - | - | - | - | 0.722 | 0.585 |
| White | 13 | 1 | 85 | 2 | - | - |
| African American | 11 | 0 | 49 | 0 | - | - |
| Hispanic | 190 | 11 | 320 | 11 | - | - |
| Asian | 21 | 0 | 26 | 0 | - | - |
| Native American | 2 | 0 | 5 | 0 | ||
| Comorbid Conditions | ||||||
| COVID-19 (%) | - | - | 237 (48.9%) | 12 (92.3%) | - | 0.002 |
| Diabetes Mellitus (%) | 130 (54.8%) | 11 (91.7%) | 221 (45.5%) | 12 (92.3%) | 0.012 | <0.001 |
| Hypertension (%) | 114 (48%) | 8 (66.7%) | 260 (53.6%) | 9 (69.2%) | 0.209 | 0.265 |
| Hyperlipidemia | 67 (28.3%) | 8 (66.7%) | 133 (27.4%) | 9 (69.2%) | 0.005 | <0.001 |
| Chronic Kidney Disease | 34 (14%) | 3 (25%) | 65 (13.4%) | 4 (30.7%) | 0.311 | 0.074 |
| End Stage Renal | 17 (7.2%) | 2 (16.7%) | 35 (7.2%) | 2 (15.4%) | 0.227 | 0.268 |
| Disease | ||||||
| Congestive Heart | 27 (11.4%) | 3 (25%) | 53 (11%) | 3 (23%) | 0.158 | 0.171 |
| Failure | ||||||
| Coronary Artery | 54 (24.1%) | 4 (34%) | 94 (19.4%) | 5 (38%) | 0.399 | 0.089 |
| Disease | ||||||
| Chronic Obstructive | 18 (7.6%) | 3 (25%) | 34 (7.8%) | 3 (23%) | 0.034 | 0.029 |
| Pulmonary Disease | ||||||
| Asthma | 9 (3.7) | 1 (8.3%) | 19 (3.9%) | 1 (7.7%) | 0.435 | 0.494 |
| Cerebrovascular | 11 (4.6%) | 2 (16.7%) | 96 (19.8%) | 3 (23%) | 0.068 | 0.770 |
| Disease | ||||||
| Cirrhosis | 5 (2.1%) | 0 | 14 (2.9%) | 0 | 0.611 | 0.534 |
| Dementia | 2 (0.8%) | 2 (16.7%) | 11 (2.3%) | 2 (15%) | <0.001 | 0.003 |
| Arterial Disease | 50 (21.1%) | 2 (16.7%) | 62 (12.8%) | 3 (23%) | 0.713 | 0.277 |
| Venous Disease | 4 (1.7%) | 0 | 12 (2.5%) | 0 | 0.650 | 0.566 |
| Hypercoagulable State | 39 (16.5%) | 1 (8.3%) | 46 (9.5%) | 1 (7.7%) | 0.455 | 0.827 |
| Smoking | 20 (8.4%) | 4 (34%) | 112 (23.1%) | 5 (38.5%) | 0.004 | 0.197 |
| Medication Use | ||||||
| Aspirin | 69 (29%) | 2 (16.7%) | 143 (29.5%) | 3 (23.1%) | 0.351 | 0.616 |
| Clopidogrel | 19 (8%) | 0 | 50 (10.3%) | 0 | 0.307 | 0.222 |
| Other Antiplatelet | - | - | 2 (0.4%) | 0 | - | 0.817 |
| Coumadin | 8 (3.34%) | 0 | 12 (2.5%) | 0 | 0.518 | 0.566 |
| Direct Oral | 11 (4.6%) | 1 (8.3%) | 28 (5.8%) | 1 (7.7%) | 0.560 | 0.771 |
| Anticoagulant | ||||||
| Statin | 59 (25%) | 3 (25%) | 145 (30%) | 3 (23.1%) | 0.993 | 0.595 |
| Patient Outcomes | ||||||
| Hospital Length of Staya | 8 (9) | 22.5 (27) | 5 (82) | 20 (65) | <0.001 | <0.001 |
| Blood Transfusion (%) | 51 (22%) | 5 (42%) | 90 (18.6%) | 6 (46.2%) | 0.103 | 0.013 |
| Mortality (%) | 98 (41.2%) | 5 (42%) | 127 (26.2%) | 5 (38.5%) | 0.983 | 0.322 |
| Major Amputation (%) | 0 | 4 (34%) | 0 | 4 (31%) | <0.001 | <0.001 |
| Minor Amputation (%) | - | - | - | - | - | - |
| Revascularization (%) | 3 (1.2%) | 2 (16.7%) | 5 (1%) | 2 (15.4%) | <0.001 | <0.001 |
| Hospital Anticoagulation | ||||||
| Therapeutic | 28 (12%) | 7 (58%) | 49 (10%) | 7 (54%) | <0.001 | <0.001 |
| Anticoagulation Started | ||||||
| (%) | ||||||
| Timing of | - | - | - | - | <0.001 | <0.001 |
| Anticoagulation | ||||||
| Not Started | 221 (93.2%) | 6 (50%) | 448 (92.3%) | 7 (54%) | - | |
| Admission | 16 (6.8%) | 3 (25%) | 37 (7.6%) | 3 (23.1%) | - | |
| Hospital Day 2 | 0 | 3 (25%) | 0 | 3 (23.1%) | - | |
| Contraindications (%) | 7 (3%) | 5 (42%) | 15 (3.1%) | 5 (38.5%) | <0.001 | <0.001 |
Significant differences are bolded.
Analyzed with nonparametric Mann Whitney U test. All other comparisons were analyzed with chi-squared test.
To determine whether COVID-19 infection affects the development of gangrene, we analyzed the data utilizing binary logistic regression. Logistic regression was performed for the total set of patients (N = 498) and the COVID-19-only patients (N = 249). In the COVID-19-only group, patients with gangrene were more likely to smoke and experienced longer hospital stays. In the total patient population, those patients with gangrene were more likely to have COVID-19 infection, smoke, and experienced increased hospital length of stay. A multivariate logistic regression from the total patient population demonstrated that COVID-19 infection is associated with an 18.23 times increased risk of gangrene after adjusting for BMI, diabetes mellitus, hyperlipidemia, smoking, length of hospital stay, therapeutic anticoagulation initiation, timing of anticoagulation, history of arterial disease, and history of hypercoagulable state (Table IV ).
Table IV.
Logistic regression predicting patient development of gangrene for COVID-19-only patients and COVID-19 and nonCOVID-19 patients
| COVID-19-onlya |
COVID-19- onlya |
P | Total patientsb |
Total patientsb |
Pc | |
|---|---|---|---|---|---|---|
| OR N = 249 | 95% CI N = 249 | OR N = 249 | 95% CI N = 249 | |||
| BMI | 0.970 | 0.877–1.073 | 0.558 | 0.966 | 0.879–1.061 | 0.466 |
| COVID-19 | - | - | - | 18.237 | 1.187–280.2 | 0.037 |
| Diabetes Mellitus (%) | 13.3 | 0.677–260.993 | 0.088 | 13.214 | 0.752–232.27 | 0.078 |
| Hyperlipidemia | 3.775 | 0.488–29.21 | 0.203 | 6.088 | 0.863–42.97 | 0.070 |
| Smoking | 22.51 | 2.021–250.73 | 0.011 | 35.40 | 3.164–396.05 | 0.004 |
| Hospital Length of Stay | 0.897 | 0.847–0.950 | <0.001 | 0.893 | 0.841–0.950 | <0.001 |
| Therapeutic Anticoagulation Started (%) | 1.259 | 0.081–19.572 | 0.869 | 0.772 | 0.046–13.06 | 0.858 |
| Timing of Anticoagulation | 0.082 | 0.006–1.119 | 0.061 | 0.095 | 0.008–1.136 | 0.063 |
| Arterial Disease | 0.321 | 0.022–4.577 | 0.402 | 1.191 | 0.143–9.909 | 0.871 |
| Hypercoagulable State | 0.086 | 0.001–36.585 | 0.426 | 0.041 | 0.001–4.126 | 0.175 |
Significant values are bolded.
Total cases analyzed = 249. A Hosmer-Lemeshow test indicated that model fit was good (chi-square with 8 df = 6.077, P = 0.639).
Total cases analyzed = 489. A Hosmer-Lemeshow test indicated that model fit was good (chi-square with 8 df = 3.771, P = 0.877).
Discussion
COVID-19 infection produces a wide range of clinical symptoms from mild to severe cases. Mild symptoms can include fever, myalgia, cough, dyspnea, diarrhea, and nausea. More severe symptoms include multiorgan dysfunction, acute respiratory distress syndrome, and thrombosis.14 Additionally, it has been noted that COVID-19 leads to more severe infection in patients with advanced age and multiple medical comorbidities.6 From our results in Table I, we found that patients with COVID-19 admitted to the ICU had a statistically higher BMI and larger percentage of comorbidities, such as diabetes mellitus, hypertension, cerebrovascular disease, arterial disease, hypercoagulable state, and smoking. This mirrors results found in other published reports, which found similar significant medical comorbidities in patients infected with more severe cases of COVID-19.14 While there have been clinical case reports that have showed an association between COVID-19 and acute limb ischemia resulting in limb gangrene, the exact pathophysiology and specific cause has been difficult to determine.
The hypercoagulable state as a result of the cytokine storm with severe cases of infection has been debated.6 , 8 , 9 , 15 Regardless of the etiology, published data demonstrates increased thromboembolic events in patients, including both acute limb ischemia and venous thromboembolism.15 Tang et al. determined that over 74% of patients who died with a diagnosis of COVID-19 also suffered a disseminated intravascular coagulopathy,16 Additionally, Cui et al. showed in a study of 81 COVID-19 patients in the ICU that the incidence of venous thromboembolism was 25% and was related to a higher rate of mortality.17 A study by Klok et al. also found that there was a large percentage of patients with venous thromboembolisms and, out of 184 analyzed patients, 3.7% had arterial thrombotic events that lead to limb gangrene,18 We found that there was a 4.8% incidence of gangrene from arterial thrombotic events in COVID-19 patients admitted to the ICU. Table III highlights the difference in patients who developed gangrene in the COVID-19 group and, separately, the total combined control and COVID-19 group. In the total group, there were 485 patients who did not develop gangrene compared to 13 that did. Like other previously published studies, the combined total risk factors that predisposed patients to the development of gangrene included elevated BMI, diabetes mellitus, hyperlipemia, chronic obstructive pulmonary disease, smoking, and COVID-19 infection. Interestingly, we also noted that patients who developed gangrene in the COVID-19 or combined total group had a history of arterial, venous, or hypercoagulable pathologies was statistically similar and nonsignificant. This suggests that a history of vascular pathologies does not lead to extremity gangrene when infected with COVID-19. Acute limb ischemia resulting in gangrene can have drastic implications and result in severe disability in patients. While a prompt diagnosis is needed for successful treatment, determining potential causes of gangrene is similarly important.
When we analyzed our patient population for clinical outcomes, we noted several factors including longer hospital stay, need for major amputations, and revascularization. We found our median hospital stay in the group that developed gangrene to be 15 days longer. Additionally, the need for major amputation was 31% greater and revascularization was 14% greater. Unlike other studies, we noted that the start of anticoagulation prior to the development of gangrene was not protective.6 Over 50% of patients that developed gangrene had started therapeutic anticoagulation. The American Society of Hematology has recommended that all patients with COVID-19 receive some form of pharmacologic thromboprophylaxis and, unless contraindicated, full therapeutic intensity anticoagulation,19 Our data and patient population suggest that starting anticoagulation and the timing of anticoagulation was not protective in patients that developed extremity gangrene. Patients on anticoagulation without a history of arterial and venous pathologies can develop acute limb ischemia and gangrene from the intense infectious burden of COVID-19.
When we addressed the relationship between causes of gangrene and COVID-19 infection, we demonstrated an 18.2 times increased risk of gangrene when adjusting for BMI, diabetes mellitus, hyperlipidemia, smoking, length of hospital stay, therapeutic anticoagulation initiation, timing of anticoagulation, history of arterial disease, and history of hypercoagulable state. This suggests that regardless of medical and vascular comorbidities during a COVID-19 infection there is a strong associated risk of extremity gangrene. Additionally, it was noted that smoking also carried a 35.4 times increased risk of gangrene. A combination of COVID-19 and history of smoking can explain the increased disease burden and increased hospital length of stay requiring major amputations and revascularization of the femoropopliteal arterial level.
Our current study has several limitations. First, as a retrospective review of data from a single institution, it is possible that our findings may represent a cohort of patients unique to a particular region. We focused on outcomes such as gangrene and considered patients risk factors. Unlike other studies that measured patients d-dimmer levels and correlated it with COVID-19 disease severity, this study is unable to determine whether there were any secondary causes leading to extremity gangrene. Additionally, the hypercoagulable panel included acquired and genetic causes. Our study did not differentiate between the two causes, but this could be addressed in a follow up study. Additionally, we did not account for the degree of vasopressor requirement in these critically ill patients. Regardless, we feel that these limitations are relatively minor and likely do not change the overall findings presented in this study.
Conclusion
COVID-19 has resulted in an incomprehensible societal impact that will linger for years to come. Our study has shown that COVID-19 infection is associated with extremity gangrene development in the absence of arterial and venous medical history. We noted in patients with gangrene there was a strong association with BMI, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease, and smoking. It is imperative providers at every level are aware of the vascular complications associated with COVID-19 infection.
Footnotes
Submission Type: Retrospective Review.
Conflict of interest: The authors declare there is no conflict of interest.
Funding: The research presented in this manuscript had no specific funding from any agency in the public, commercial, or not-for-profit sectors.
Author Contributions: Aldin Malkoc assisted with drafting, editing, and data analysis of the manuscript. Raja GnanaDev assisted with data collection, review, and editing of the manuscript. Lev Botea assisted with data collection and writing of the initial manuscript. Ashtin Jeney assisted with data collection and writing of the initial manuscript. Keith Glover assisted with patient care, review, and editing of the manuscript. Milton Retamozo assisted with patient care, review, and editing of the manuscript. Dev GnanaDev assisted with patient care, review, and editing of the manuscript. Samuel Schwartz assisted with patient care, review, data collection, writing, and editing of the manuscript.
References
- 1.Novara E., Molinaro E., Benedetti I., et al. Severe acute dried gangrene in COVID-19 infection: a case report. Eur Rev Med Pharmacol Sci. 2020;24:5769–5771. doi: 10.26355/eurrev_202005_21369. [DOI] [PubMed] [Google Scholar]
- 2.Galanis N., Stavraka C., Agathangelidis F., et al. Coagulopathy in COVID-19 infection: a case of acute upper limb ischemia. J Surg Case Rep. 2020;2020:rjaa204. doi: 10.1093/jscr/rjaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kipshidze N., Dangas G., White C.J., et al. Clin Appl Thromb SAGE Publications Sage Ca; Los Angeles, CA: 2020. Viral Coagulopathy in Patients With Covid-19: Treatment and Care; p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S.G., Fralick M., Sholzberg M. Coagulopathy associated with COVID-19. Can Med Assoc. 2020;192:E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med Mass Med Soc. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B. Anticoagulation in COVID-19: randomized trials should set the balance between excitement and evidence. Thromb Res Elsevier. 2020;196:638–640. doi: 10.1016/j.thromres.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran R., Pillai A.V., Raja S., et al. Axillary artery thrombosis resulting in upper limb amputation as a COVID-19 sequela. BMJ Case Rep CP. BMJ. 2021;14:e240981. doi: 10.1136/bcr-2020-240981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanif M., Ali M.J., Haider M.A., et al. Acute upper limb ischemia Due to arterial thrombosis in a mild COVID-19 patient: a case report. Cureus Cureus Inc. 2020;12:e10349. doi: 10.7759/cureus.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamgboje A., Hong J., Mushiyev S., et al. A 61-year-Old man with SARS-CoV-2 infection and venous thrombosis presenting with painful swelling and gangrene of the lower limb consistent with phlegmasia cerulea Dolens. Am J Case Rep. 2020;21:e928342. doi: 10.12659/AJCR.928342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasinathan G., Sathar J. Haematological manifestations, mechanisms of thrombosis and anti-coagulation in COVID-19 disease: A review. Ann Med Surg. 2020;56:173–177. doi: 10.1016/j.amsu.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung J., Anjum S. Coronavirus Disease 2019 (COVID-19) infection associated with antiphospholipid antibodies and four-extremity deep vein thrombosis in a previously healthy female. Cureus Cureus Inc. 2020;12:e8408. doi: 10.7759/cureus.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury Y.S., Mitre C.A., Rotella V.E., et al. Extensive peripheral arterial thrombosis in a patient with SARS-CoV-2 infection. Am J Med Case Rep. 2020;8:486. [Google Scholar]
- 14.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med Mass Med Soc. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melissano G., Mascia D., Baccellieri D., et al. Pattern of vascular disease in Lombardy, Italy, during the first month of the COVID-19 outbreak. J Vasc Surg. 2020;72:4–5. doi: 10.1016/j.jvs.2020.04.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost Wiley Online Libr. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui S., Chen S., Li X., et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok F., Kruip M., Van der Meer N., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensaid A., Melhaoui I., Oujidi Y., et al. Acute limb ischemia in patients with COVID-19 pneumonia. Ann Med Surg. 2021;69:102747. doi: 10.1016/j.amsu.2021.102747. [DOI] [PMC free article] [PubMed] [Google Scholar]