Keywords: e-cigarette, fibrosis, in utero, pulmonary, vaping
Abstract
The in utero environment is sensitive to toxicant exposure, altering the health and growth of the fetus, and thus sensitive to contaminant exposure. Though recent clinical data suggest that e-cigarette use does no further harm to birth outcomes than a nicotine patch, this does not account for the effects of vaping during pregnancy on the long-term health of offspring. Pregnant mice were exposed to: 1) e-cigarette vapor with nicotine (PV + Nic; 2% Nic in 50:50 propylene glycol: vegetable glycerin), 2) e-cigarette vapor without nicotine [PV; (50:50 propylene glycol:vegetable glycerin)], or 3) HEPA filtered air (FA). Dams were removed from exposure upon giving birth. At 5 mo of age, pulmonary function tests on the offspring revealed female and male mice from the PV group had greater lung stiffness (Ers) and alveolar stiffness (H) compared with the FA group. Furthermore, baseline compliance (Crs) was reduced in female mice from the PV group and in male mice from the PV and PV + Nic groups. Lastly, female mice had decreased forced expiratory volume (FEV0.1) in the PV group, but not in the male groups, compared with the FA group. Lung histology revealed increased collagen deposition around the vessels/airways and in alveolar tissue in PV and PV + Nic groups. Furthermore, goblet hyperplasia was observed in PV male and PV/PV + Nic female mice. Our work shows that in utero exposure to e-cigarette vapor, regardless of nicotine presence, causes lung dysfunction and structural impairments that persist in the offspring to adulthood.
INTRODUCTION
Electronic cigarettes (e-cigs) are devices that use a heated coil to produce an inhalable aerosol from a solution usually consisting of nicotine, flavorings, and a carrier commonly composed of propylene glycol (PG) and vegetable glycerin (VG) (1). Often advertised as a safer alternative to combustible cigarettes, e-cigarettes started their rise as a cessation device in 2006 (2). The e-cigarette market has continued to grow exponentially and is projected to exceed $60 billion globally by 2025, surpassing sales of conventional cigarettes (3). Furthermore, ∼7% of pregnant mothers reported the use of e-cigarette devices throughout their pregnancy (4), despite guidelines from the Centers for Disease Control and Prevention (CDC) stating that e-cigarettes are not safe to use during pregnancy.
The Barker hypothesis describes how the in uterine environment can have lasting implications on health and disease. Barker’s proposal suggests that exposure to environmental risk factors during sensitive periods of fetal development can lead to the development of specific diseases later in life (5). Studies have shown that smoking conventional cigarettes whereas pregnant results in sudden infant death syndrome (SIDS) (6), low birth weight (7), and asthma (8), but the effects of e-cigarette vapor exposure during development are not clear. A recent randomized controlled trial of birth outcomes demonstrated that e-cigarette use is just as safe for birth outcomes as nicotine patches for cessation in pregnant women who previously smoked combustible cigarettes (9); however, the long-term implications for offspring of mothers who use e-cigarette devices is a work in progress, and emerging evidence suggests that e-cigarette use during pregnancy leads to offspring cerebrovascular dysfunction (10), increased pulmonary inflammatory markers, epigenetic remodeling, impaired fetal lung structure, and altered pulmonary gene expression (3, 11, 12). Thus, in the present study, we aimed to determine how pulmonary function and structure are altered in the adult offspring of e-cigarette-exposed mice.
MATERIALS AND METHODS
Animals and Exposure
All animal work was performed with prior approval from the Institutional Animal Care and Use Committee at The Ohio State University. Pregnant FVB mice (Jackson Labs, Bar Harbor, ME) were exposed in Scireq inExpose 5 L chambers (inExpose, SCIREQ Scientific Respiratory Equipment Inc., Montreal, Canada) to HEPA filtered air (FA), e-cigarette carrier (50:50 propylene glycol: vegetable glycerin, PV), or e-cigarette carrier with 2% nicotine (PV + Nic) at a flow rate of 2 L/min. The nicotine level used was mid-range as common usage in humans ranges from 0.3% to 5.0% (13). E-cig vapor was generated with the ECX-JoyeTech E-Vic Mini device with an output of 70 Watts. Airflow to FA chambers passed through three HEPA filters with a 0.3 µm pore size before chamber entry. Female mice were paired with males and the following day began exposure. Female mice were exposed to e-cigarette vapor five days a week, four hours a day (PV and PV + NIC groups were exposed at a rate of 1-puff/minute, with a 3 s pump duration) throughout the entire length of gestation. At least one mouse each from 4 to 6 litters per experimental group was used for each experiment. Offspring of the exposed mothers were assessed at 5 mo of age to model the time at which age-induced lung function may begin (14).
Pulmonary Function Measurements
Mice were anesthetized with isoflurane (Covetrus Inc., Portland, ME) and underwent tracheostomies with an 18 G cannula. Mice were mechanically ventilated with a FlexiVent FX2 system (SCIREQ Inc., Montreal, Quebec, Canada) with 150 breathes/min, tidal volume of 10 mL/kg, and positive end-expiratory pressure (PEEP) of 3 cmH2O before baseline measurements. To eliminate breathing efforts and measurement errors, pancuronium bromide (1 mg/kg, Sigma Aldrich, St. Louis, MO) was administered intraperitoneally. Using a “Snapshot-150 perturbation,” respiratory stiffness (Ers) and compliance (Crs) were measured from the forced oscillation technique (FOT) fitted to a single-compartment model. In addition, a broadband FOT maneuver (“Quick Prime-3 perturbation”) was completed to capture the alveolar tissue stiffness (H). The negative pressure forced expiration (NPFE) extension of the FlexiVent then recorded forced expiratory volume by inflating the lungs to a pressure of +30 cmH2O over 1.2 s and then rapidly decreased to a negative pressure of 55 cmH2O. The forced expiratory volume was calculated from the flow-volume loop over 0.1 s (FEV0.1).
Lung Tissue Histology and Immunoblotting
The left lung was harvested and inflation-fixed with 10% neutral buffer formalin (ThermoFisher Scientific, Waltham, MA) for histology. Tissue was paraffin-embedded and sliced into 5 μm sections. Sections were then stained using Masson’s trichrome (Abcam, Cambridge, UK), Periodic Acid Schiff (PAS) Stain Kit (Abcam), or Hematoxylin and Eosin (H&E) stain (Merck KGaA, Darmstadt, Germany). Goblet cell hyperplasia was calculated as previously described (15). Images of each stain were captured using an Olympus IX73 microscope; ten fields per mouse were imaged at ×40 magnification using CellSense software and averaged. Immunoblotting was performed using SDS-PAGE as described previously (16). Antibodies for collagen III alpha 1 (Novus Bio NBP1-05119) or β-actin (Sigma-Aldridge A1978) were used at 1:5000 dilution.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA). Data are presented as means ± standard deviation (SD) or standard error of the mean (SE). Differences were considered statistically significant when P < 0.05 via one-way ANOVA, followed by Tukey’s test for multiple comparisons.
RESULTS
Female Mice Exposed to E-Cigarette Vapor In Utero Had Significantly Higher Body Weight at Adulthood, but Not at Birth
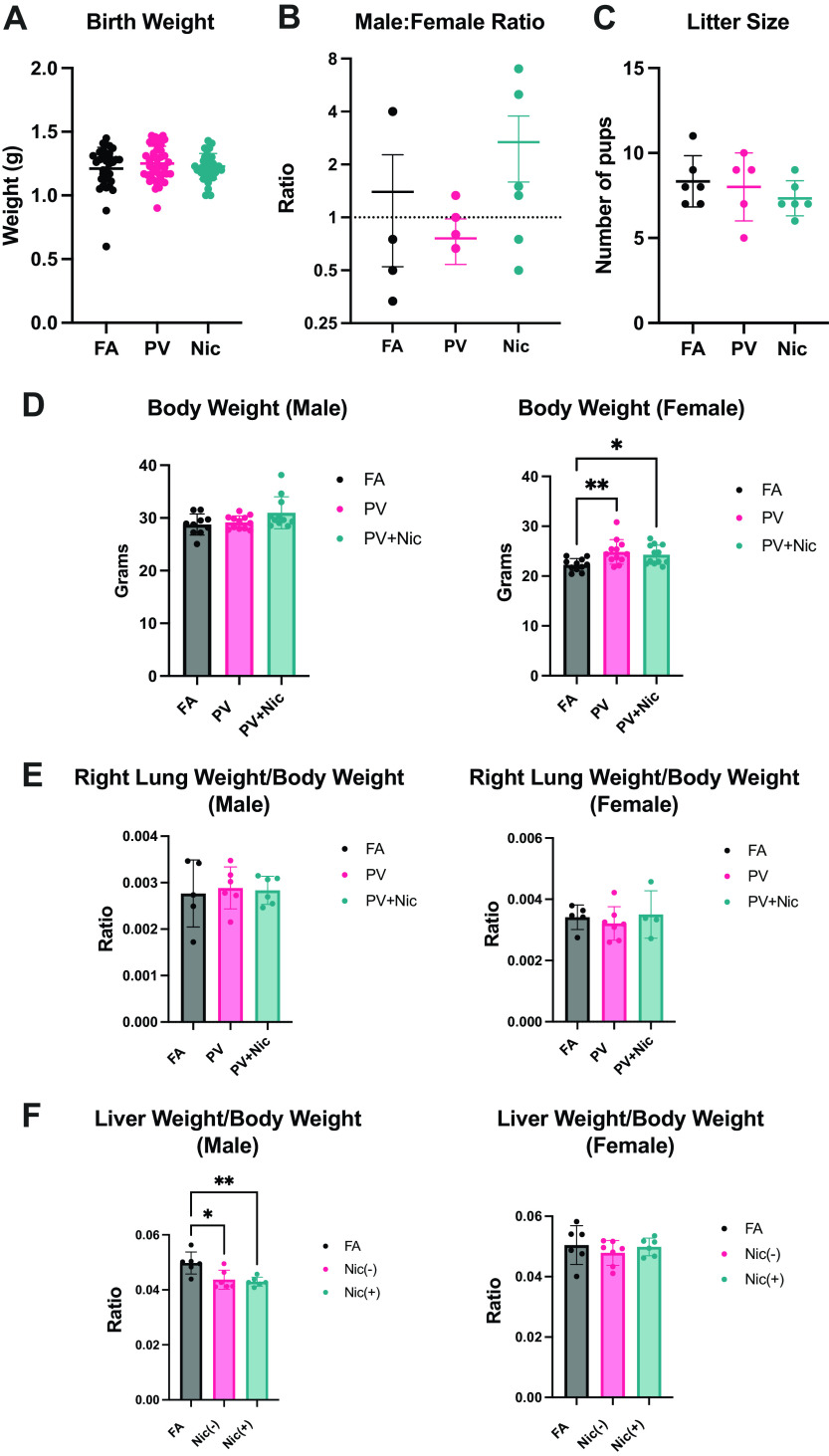
Mean body weight of pups was not significantly different at postnatal day 1 (Fig. 1A). Male:female ratio (Fig. 1B) and litter size (Fig. 1C) were also not changed with e-cigarette exposure in utero compared with FA. At 5 mo of age, male mice had no significant changes in body weight, whereas female mice exposed to the PV and PV + Nic had significantly increased body weight compared with FA control (Fig. 1D). Thus, though birth outcomes were unchanged, e-cigarette vapor exposure in utero caused increased weight in female offspring. We found no pulmonary edema (lung weight/body weight, Fig. 1E) or hepatic congestion (liver weight/body weight, reduced in males, Fig. 1F) in these mice at adulthood.
Figure 1.
A–C: litter characterization from mice exposed to e-cigarette vapor in utero revealed no changes in pup weight (A), male:female ratio (B), or litter size (C). D: When pups grew to adulthood (5 mo), body weight was significantly increased in PV and PV + Nic groups compared with FA in female, but not male mice. E and F: adult mice exposed to e-cigarette vapor in utero had no changes in lung weight/body weight (E), but male mice had reduced liver weight (F) compared with FA control (*P < 0.05, **P < 0.01 via one way ANOVA. N = 10–12; error bars indicate means ± SE). FA, filtered air; PV, e-cigarette vapor; PV + Nic, e-cigarette vapor with nicotine.
Mice Exposed to E-Cigarette Vapor In Utero Have Impaired Pulmonary Function
Mice exposed to e-cigarette vapor in utero were subject to pulmonary function tests using the FlexiVent system. Male and female mice exposed to PV had increased lung and alveolar stiffness (Ers, H) relative to FA. FOT measurements demonstrated significantly decreased lung compliance (Crs) in PV female, PV male, and PV + Nic male offspring when compared with their respective FA controls (Fig. 2A). In addition, PV females also had a significantly lower FEV0.1 and increased tissue stiffness (H) compared with FA (Fig. 2B). Thus, these in vivo pulmonary measurements indicate that both male and female mice have impaired pulmonary function when exposed to e-cigarette vapor in utero, with or without nicotine in the carrier vapor.
Figure 2.
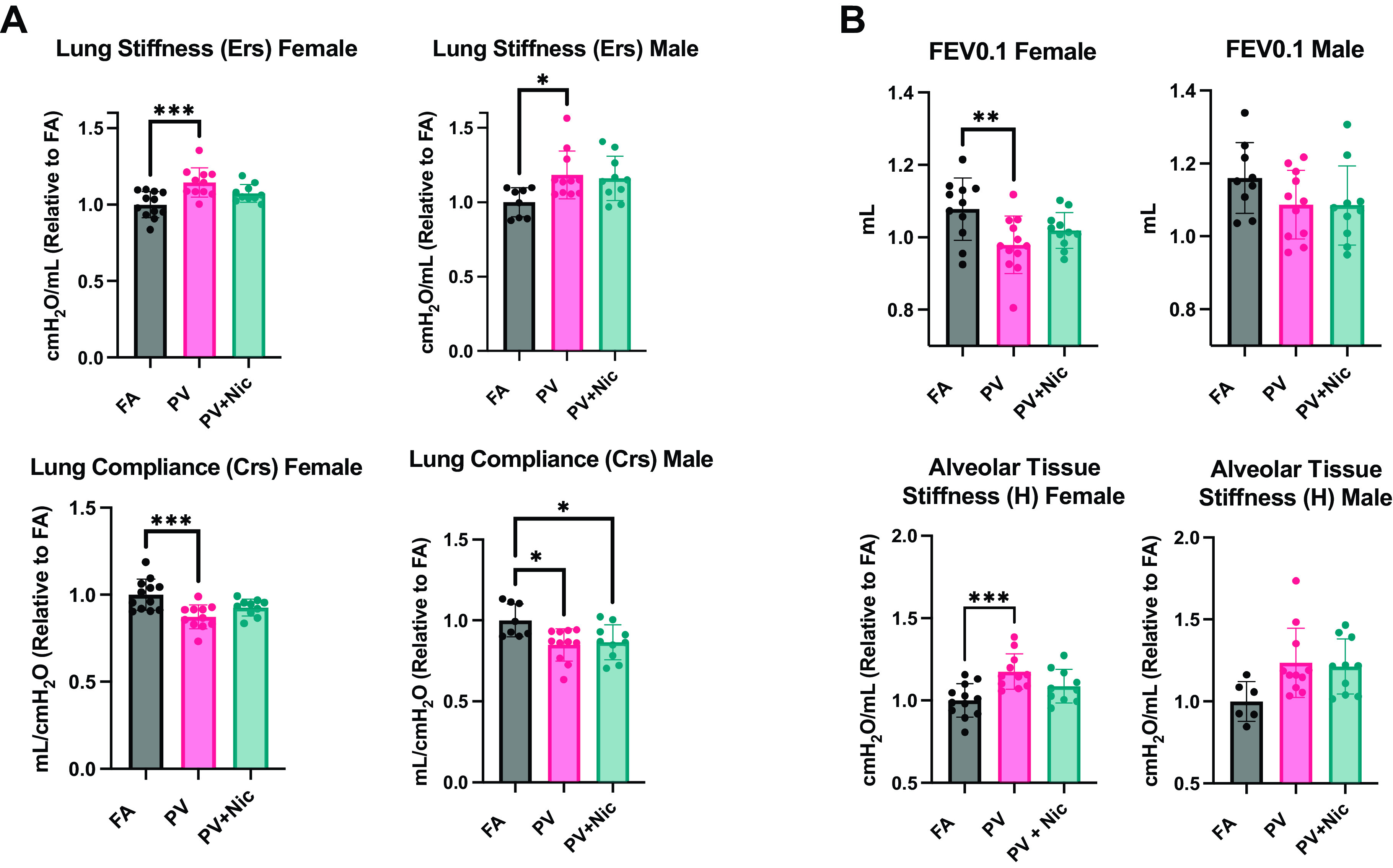
FOT measurements from pulmonary function demonstrated (A) in utero e-cigarette exposure increased stiffness (Ers) and decreased compliance (Crs) in PV and PV + Nic lungs compared with FA (filtered air). B: NPFE measured a FEV0.1 decrease in PV females and Quick-Prime measurements show an increase of alveolar tissue stiffness (H) in PV males and females compared with FA control (*P < 0.05, **<0.01, ***P < 0.001 via one way ANOVA. N = 8–12; error bars indicate means ± SE). FEV0.1, decreased forced expiratory volume; FOT, forced oscillation technique; PV, e-cigarette vapor; PV + Nic, e-cigarette vapor with nicotine.
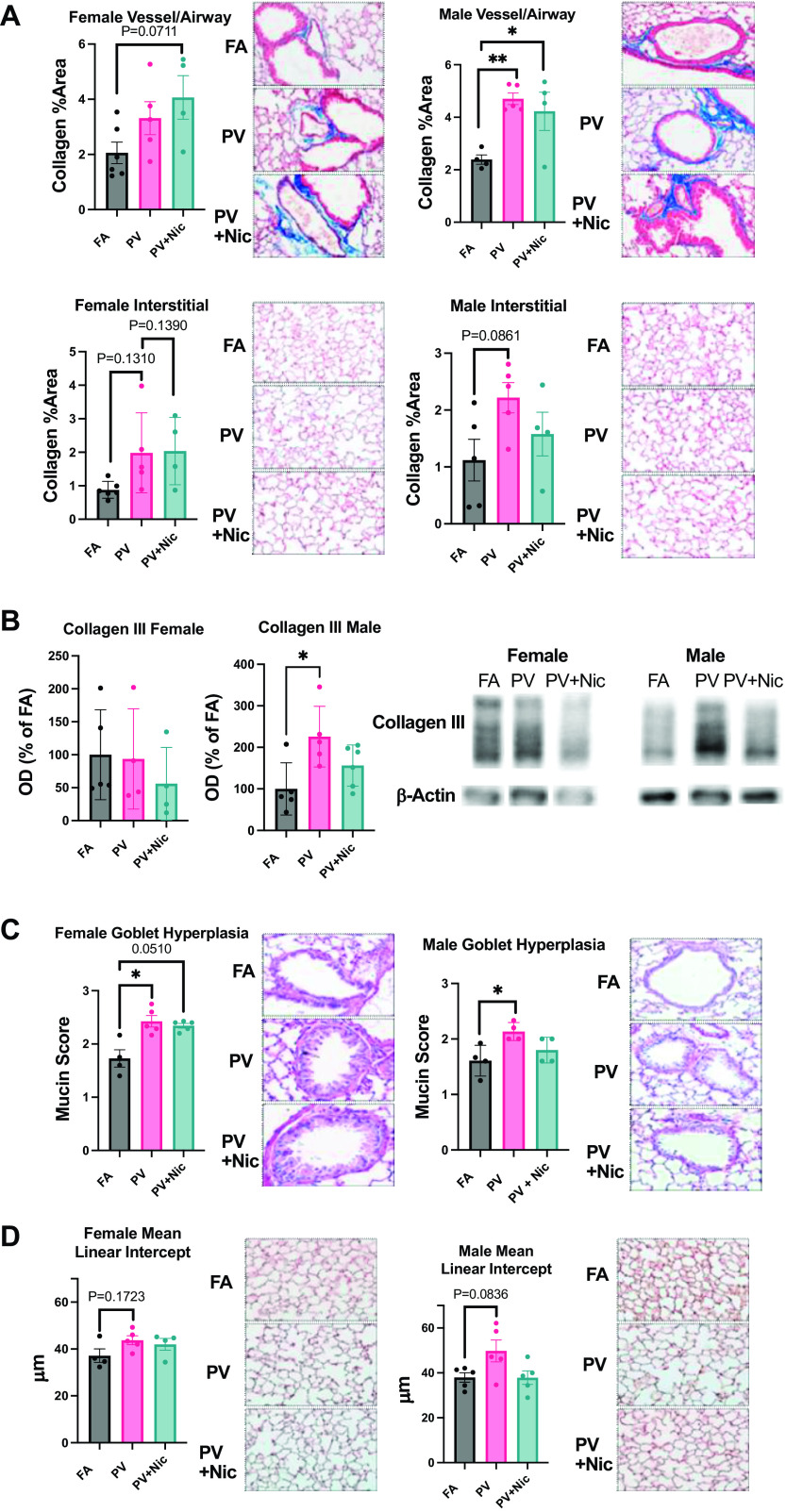
Mice Exposed to E-Cigarette Vapor In Utero Have Increased Pulmonary Fibrosis
Masson’s Trichrome staining showed increased levels of fibrotic tissue (blue) around the airways and blood vessels in PV and PV + Nic groups when compared with FA in male offspring, with a similar effect trending (P = 0.0711) in female mice (Fig. 3A). Furthermore, immunoblotting revealed male mice exposed to PV in utero had significantly increased collagen III compared with FA, but this was unchanged in female mice (Fig. 3B).
Figure 3.
A: Masson’s Trichrome staining indicates increased levels of fibrotic tissue (blue) around the airways and blood vessels between FA, PV, and PV + Nic, for both females and males. (*P < 0.05, **P < 0.01 via one way ANOVA, N = 4–6); error bars show avg ± SE. In utero e-cigarette exposure also increases alveolar interstitial fibrosis. B: collagen III immunoblot of lung tissue indicates increased fibrosis in the male PV group compared to FA (*P < 0.05 via one way ANOVA, N = 4–6); error bars show avg ± SE. C: an increased amount of goblet cells is observed in PV and PV + Nic compared with FA. Goblet cells in the airway epithelium were quantified based on percent area using a five-point system: 0, no goblet cells; 1, <25% of the epithelium; 2, 25%–50% of the epithelium; 3, 50%–75% of the epithelium; and 4, >75% of the epithelium. (*P < 0.05 via Kruskal–Wallis Test, N = 4–5); error bars show avg ± SD. D: measurement of the distance-free space between alveoli (MLI). PV females and males are observed to have a larger MLI, or decreased amount of alveolar attachments, than the FA group (via one way ANOVA, N = 4–5); error bars show avg ± SE. PV, e-cigarette vapor; PV + Nic, e-cigarette vapor with nicotine.
Mice Exposed to E-Cigarette Vapor In Utero Have Increased Goblet Cell Hyperplasia
Assessment of goblet cell hyperplasia (Fig. 3C) revealed that mice exposed to PV in utero had increased mucus secretion (mucin scores) compared with FA in male and female mice. A trending increase in mean linear intercept (MLI) was found for both male and female mice, indicating a loss of alveolar attachments, though this was not statistically significant (Fig. 3D).
DISCUSSION
It is known that an adverse intrauterine environment may predispose the fetus to the development of disease during adulthood (17). Emerging animal studies have examined the effects of e-cigarette vapor exposure in utero and have demonstrated pulmonary consequences in the offspring of e-cigarette-exposed mice. Mice examined at 6 wk of age had altered extracellular matrix proteins and lipogenic markers, and lung histology was found to be altered in male offspring (11). A recent study exposing pregnant mice to e-cigarette vapor before and during pregnancy demonstrated altered lung structure in offspring at postnatal day 0 and postnatal day 28, as well as dysregulated Wnt signaling (3). A similar study found DNA methylation changes and cytokine expression in the lungs of offspring exposed before and during pregnancy to both nicotine and nicotine-free e-cigarette vapor (12), suggesting a potential epigenetic mechanism. It is critical to examine the mechanism(s) involved and the physiological consequences of in utero e-cigarette exposure, especially when combined with pulmonary disease.
Pups that were exposed to e-cigarette vapor during gestation had no changes in birth weight, litter size, or male: female ratio (Fig. 1). This contrasts with other similar studies reporting a reduced pup weight from dams exposed to e-cigarette vapor with various flavors (3, 18). In humans, an increased prevalence of low birth weight compared with those born from non-smokers has been documented in human e-cigarette users (9, 19), but another study found no difference (20). This could be because many e-cigarette users also smoke traditional cigarettes (21). However, our study emphasizes a long-term negative impact on the respiratory system of adult mice exposed in utero to e-cigarette vapor both with and without nicotine.
In restrictive diseases, such as fibrotic and other interstitial lung diseases, lung compliance (Crs) is decreased (22). From the FlexiVent measurements in PV mice relative to FA, the e-cigarette carrier alone caused a loss of lung compliance and increased stiffness (Fig. 2). To understand the physiological consequences of the significant impairment in lung function, we analyzed the structural features of the airways histologically (Fig. 3). Increased fibrosis was demonstrated by enhanced collagen deposition in both the interstitial alveolar tissue and the airways/vessels the PV and PV + Nic groups compared with the FA control group. The increased fibrosis was only observed in males (i.e., airways/vessels), which could suggest a sex-dependent effect. But, we would note, a similar tendency (albeit not statistically significant) was also noted in females. This merits further study, including if these mice have a greater propensity for fibrotic disease in the presence of additional insults.
We measured morphometric changes in lung structure and found a trend towards increased space between gas exchange surfaces, indicating a loss of alveolar attachments. A reduction in the number of bronchial-alveolar attachment points indicates reduced alveolarization, affecting gas exchange, which may indicate an emphysema-like injury. Thus, the components of e-cigarette vapor are important for the resultant pathology of offspring and are worth further consideration.
Nicotine is thought to play a crucial role in altered lung development processes by inducing epigenetic alterations (23). As nicotine crosses the human placenta, it gathers in the fetal respiratory tract (3) and activates nAChRs that are present in the underdeveloped fetal lungs, leading to alterations in development (3), which we have seen in the overall structure and molecular observations of the PV + Nic mice. We have previously reported serum cotinine levels in female mice exposed with the same parameters to be 180.5 ± 53.6 μM (24), which is in the range of other mouse studies (25–27), and less than that of human e-cigarette users (28). However, the changes observed in our study demonstrate that the carrier has similar detrimental effects.
The heating of e-cigarettes causes the thermal decomposition of propylene glycol generating acetone, acetaldehyde, and formaldehyde. The thermal decomposition of glycerin results in more acrolein and formaldehyde, and many other chemical constituents (29). These chemicals have reproductive and developmental toxicity by possibly crossing the placenta (30–32). In utero inhalation of formaldehyde demonstrated an increase in the number and size of trophoblastic giant cells and an enlargement of spongiotrophoblastic cells in the basal zone of the placenta. This study may suggest that exposure to formaldehyde during the organogenesis period induces toxic changes in the placental structure (31). Thus, further work in determining the dosage of these compounds in e-cigarette vapor is merited as these may explain our observed pulmonary effects.
We describe clear changes that demonstrate vaping e-cigarettes with and without nicotine during pregnancy is detrimental to the lung structure and function of adult offspring. As e-cigarettes can generate many toxic chemicals, it is plausible that these and other features of e-cigarette vapor could be responsible for our findings, strongly suggesting in utero exposure to e-cigarettes may predispose male offspring to a greater risk of developing lung airway disease in adulthood. It is important to note this study was conducted in a small cohort of mice with one specific exposure paradigm, and thus more work is necessary to examine the molecular alterations of these mice in response to various e-cigarette components, as well as determine how these mice respond to various pulmonary insults. There may be implications of our study to clinical diseases suggesting that developmental exposure to e-cigarette vapor may alter lung disease development in humans, highlighting the importance of future clinical studies on this topic.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by the National Institutes of Health (R01 HL139348, R01 AG057046) and the American Heart Association (20YVNR35490079) to L.E.W.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M.A., O.A., R.A.M., L.E.W., and M.W.G. conceived and designed research; D.M.A., O.A., T.A.S., K.G.E., M.D.Y., D.M.M., R.A.M., and M.W.G. performed experiments; D.M.A., O.A., T.A.S., K.G.E., D.M.M., R.A.M., and M.W.G. analyzed data; D.M.A., O.A., T.A.S., M.D.Y., R.A.M., L.E.W., and M.W.G. interpreted results of experiments; D.M.A., O.A., T.A.S., M.D.Y., D.M.M., and M.W.G. prepared figures; D.M.A., O.A., T.A.S., K.G.E., and M.W.G. drafted manuscript; D.M.A., O.A., T.A.S., K.G.E., L.E.W., and M.W.G. edited and revised manuscript; D.M.A., O.A., T.A.S., K.G.E., M.D.Y., D.M.M., R.A.M., L.E.W., and M.W.G. approved final version of manuscript.
REFERENCES
- 1. Canistro D, Vivarelli F, Cirillo S, Babot Marquillas C, Buschini A, Lazzaretti M, Marchi L, Cardenia V, Rodriguez-Estrada MT, Lodovici M, Cipriani C, Lorenzini A, Croco E, Marchionni S, Franchi P, Lucarini M, Longo V, della Croce CM, Vornoli A, Colacci A, Vaccari M, Sapone A, Paolini M. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep 7: 2028, 2017. doi: 10.1038/s41598-017-02317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy 32: 16–31, 2011. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 3. Noël A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, Xiao R, Penn A. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol 318: L705–L722, 2020. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- 4. Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest 149: 552–561, 2016. doi: 10.1378/chest.15-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker DJP. The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 6. DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract 40: 385–394, 1995. [PubMed] [Google Scholar]
- 7. Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health 15: 239, 2015. doi: 10.1186/s12889-015-1552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 129: 735–744, 2012. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 9. Hajek P, Przulj D, Pesola F, Griffiths C, Walton R, McRobbie H, Coleman T, Lewis S, Whitemore R, Clark M, Ussher M, Sinclair L, Seager E, Cooper S, Bauld L, Naughton F, Sasieni P, Manyonda I, Myers Smith K. Electronic cigarettes versus nicotine patches for smoking cessation in pregnancy: a randomized controlled trial. Nat Med 28: 958–964, 2022. doi: 10.1038/s41591-022-01808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burrage EN, Aboaziza E, Hare L, Reppert S, Moore J, Goldsmith WT, Kelley EE, Mills A, Dakhlallah D, Chantler PD, Olfert IM. Long-term cerebrovascular dysfunction in the offspring from maternal electronic cigarette use during pregnancy. Am J Physiol Heart Circ Physiol 321: H339–H352, 2021. doi: 10.1152/ajpheart.00206.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Q, Sundar IK, Blum JL, Ratner JR, Lucas JH, Chuang T-D, Wang Y, Liu J, Rehan VK, Zelikoff JT, Rahman I. Prenatal exposure to electronic-cigarette aerosols leads to sex-dependent pulmonary extracellular-matrix remodeling and myogenesis in offspring mice. Am J Respir Cell Mol Biol 63: 794–805, 2020. doi: 10.1165/rcmb.2020-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, Oliver BG. Maternal E-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 58: 366–377, 2018. doi: 10.1165/rcmb.2017-0206RC. [DOI] [PubMed] [Google Scholar]
- 13. Stratton K, Kwan LY, Eaton DL (Editors). Public Health Consequences of E-Cigarettes. Washington, D.C.: National Academies Press, 2018, p. 14. [PubMed] [Google Scholar]
- 14. Schulte H, Mühlfeld C, Brandenberger C. Age-related structural and functional changes in the mouse Lung. Front Physiol 10: 1466, 2019. doi: 10.3389/fphys.2019.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee CD, Choi WS, Choi YG, Kang HS, Lee WT, Kim HJ, Lee J-Y. Inhibition of phosphodiesterase suppresses allergic lung inflammation by regulating MCP-1 in an OVA-induced asthma murine model with co-exposure to lipopolysaccharide. J Int Med Res 48: 300060520903663, 2020. doi: 10.1177/0300060520903663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulas JA, Hettwer J. V, Sohrabi M, Melvin JE, Manocha GD, Puig KL, Gorr MW, Tanwar V, McDonald MP, Wold LE, Combs CK. In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environ Pollut 241: 279–288, 2018. doi: 10.1016/j.envpol.2018.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sooranna SR, Oteng-Ntim E, Meah R, Ryder TA, Bajoria R. Characterization of human placental explants: morphological, biochemical and physiological studies using first and third trimester placenta. Hum Reprod 14: 536–541, 1999. doi: 10.1093/humrep/14.2.536. [DOI] [PubMed] [Google Scholar]
- 18. Cahill KM, Johnson TK, Perveen Z, Schexnayder M, Xiao R, Heffernan LM, Langohr IM, Paulsen DB, Penn AL, Noël A. In utero exposures to mint-flavored JUUL aerosol impair lung development and aggravate house dust mite-induced asthma in adult offspring mice. Toxicology 477: 153272, 2022. doi: 10.1016/j.tox.2022.153272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regan AK, Bombard JM, O'Hegarty MM, Smith RA, Tong VT. Adverse birth outcomes associated with prepregnancy and prenatal electronic cigarette use. Obstet Gynecol 138: 85–94, 2021. doi: 10.1097/AOG.0000000000004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonnell B, Dicker P, Regan C. Electronic cigarettes and obstetric outcomes: a prospective observational study. BJOG 127: 750–756, 2020. doi: 10.1111/1471-0528.16110. [DOI] [PubMed] [Google Scholar]
- 21. Cardenas V, Fischbach L, Chowdhury P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: a systematic review of the literature. Tob Induc Dis 17, 2019. doi: 10.18332/tid/104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards Z, Annamaraju P. Physiology, Lung Compliance. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 23. Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 10: 129, 2012. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neczypor EW, Saldaña TA, Mears MJ, Aslaner DM, Escobar YNH, Gorr MW, Wold LE. E-Cigarette aerosol reduces left ventricular function in adolescent mice. Circulation 145: 868–870, 2022. doi: 10.1161/CIRCULATIONAHA.121.057613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eden MJ, Farra YM, Matz J, Bellini C, Oakes JM. Pharmacological and physiological response in Apoe−/− mice exposed to cigarette smoke or e-cigarette aerosols. Inhal Toxicol 34: 260–274, 2022. doi: 10.1080/08958378.2022.2086948. [DOI] [PubMed] [Google Scholar]
- 26. Zhu M, Echeveste Sanchez M, Douglass EA, Jahad J. V, Hanback TD, Guhr Lee TN, Esther CR, Cole M, Roberts AJ, Herman MA. Electronic nicotine vapor exposure produces differential changes in central amygdala neuronal activity, thermoregulation and locomotor behavior in male mice. eNeuro 8: ENEURO.0189-21.2021, 2021. doi: 10.1523/ENEURO.0189-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laube BL, Afshar-Mohajer N, Koehler K, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol 29: 197–205, 2017. doi: 10.1080/08958378.2017.1336585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rapp JL, Alpert N, Flores RM, Taioli E. Serum cotinine levels and nicotine addiction potential of e-cigarettes: an NHANES analysis. Carcinogenesis 41: 1454–1459, 2020. doi: 10.1093/carcin/bgaa015. [DOI] [PubMed] [Google Scholar]
- 29. Ogunwale MA, Li M, Ramakrishnam Raju M. V, Chen Y, Nantz MH, Conklin DJ, Fu X-A. Aldehyde detection in electronic cigarette aerosols. ACS Omega 2: 1207–1214, 2017. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L. Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat Res 728: 118–138, 2011. doi: 10.1016/j.mrrev.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monfared AL. Histomorphological and ultrastructural changes of the placenta in mice exposed to formaldehyde. Toxicol Ind Health 30: 174–181, 2014. doi: 10.1177/0748233712452603. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Zhang Z, Zhang H, Hong K, Tang W, Zhao L, Lin H, Liu D, Mao J, Wu H, Jiang H. Effects of maternal acrolein exposure during pregnancy on testicular testosterone production in fetal rats. Mol Med Rep 16: 491–498, 2017. doi: 10.3892/mmr.2017.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.