Abstract
The close proximity of arteries and veins is a well-known anatomical finding documented in the extremities of all vertebrates. However, the physiological consequences of this arrangement are rarely given proper consideration nor are they covered in the textbook list of mechanisms that aid blood flow. We hypothesized that arterial pressure pulsations can significantly increase blood flow in the adjacent valve-containing vein segments. To demonstrate the existence of this mechanism, 10- to 15-cm sections of the bovine forelimb neurovascular bundle were isolated. The proximal and distal ends of the median artery and adjacent veins were cannulated, their tributaries were tied off, and the dissected bundle was then inserted into an airtight enclosure to mimic in vivo encasement by surrounding muscle. Pulsatile pressure was subsequently applied to the arterial segment while recording venous flow. At pressure settings mimicking physiological scenarios, arterial pulsations caused a highly significant increase in venous return. The amplitude of this effect was dependent on the arterial pulsation rate, stroke volume, and pressure gradient across the vein segment.
Keywords: neurovascular bundle, pulse pressure, venous return
INTRODUCTION
At least four physiological mechanisms are known to help the beating heart circulate blood through the body (1, 2). First, the passive recoil of large arteries helps to redistribute the systolic surge in blood pressure during diastole, known as the Windkessel effect (3). The second mechanism is the so-called skeletal muscle pump, which involves the compression of valve-containing vein segments by surrounding muscle during walking, running, and other nonsedentary activities (4). The third mechanism is the respiratory pump, which involves an inspiration-related decrease in thoracic pressure that in turn stretches the walls of the inferior vena cava, helping to drive venous blood back to the heart (5). An even stronger suctioning effect occurs during rapid cardiac ejection periods following the downward movement of the atrioventricular (AV) septum, a phenomenon called AV plane displacement. The latter functions as a piston to draw venous blood from the lower extremities (6, 7).
Herein, we explore yet another mechanism that can aid blood circulation. It is based on physiological consequences of the anatomical arrangement of veins and arteries in the lower extremities. It is well known that these two types of vessels run side by side, often enclosed in a common sheath also containing a nerve. These structures are called neurovascular bundles (NVBs) and can be readily seen using MRI, high-resolution computer tomography scans, or ultrasonography (8, 9). The immediate proximity of the veins to a pulsating artery and the fact that NVBs are surrounded by muscle and other tissues suggest that transient increases in arterial pressure also cause concurrent compressions of the adjacent veins. Given that veins contain multiple one-way valves, these periodic compressions may help increase venous return toward the heart. This overall concept is somewhat similar to the skeletal pump mechanism, albeit on a smaller scale. This putative mechanism appears intuitively obvious, and occasionally, a line or two in the literature may mention a pulsating artery near a vein. However, we were unable to locate research articles or textbook sections explicitly describing this phenomenon. Therefore, we conducted proof-of-principle experiments to directly demonstrate how arterial pulse can impact flow through an adjacent vein and to evaluate the extent to which this can happen at physiologically relevant ranges of pressures and heart rates.
METHODS
Isolation of the Neurovascular Bundle
Bovine forelimbs were purchased from a local butcher shop (Fig. 1A). The George Washington University Institutional Animal Care and Use Committee guidelines (based on the 2015 US Animal Welfare Act, Title 7, Chapter 54, Section 2132 g) do not require committee approval for studies involving postmortem tissues from farm animals. The vessel openings were clearly visible near the proximal end of the cross-sectioned radius (Fig. 1B). The subcutaneous tissue and fascia between the flexor carpi radialis and the superficial digital flexor, along with the deep digital flexor, were distally dissected, with care taken to keep the vessels intact. Retracting the flexor carpi radialis revealed the full course of the median nerve, artery, and vein. The bundle was carefully dissected from the surrounding tissue using a sharp scalpel blade, and the side tributaries of the artery and its satellite veins were tied using surgical thread. Custom-made cannulas were inserted into the distal and proximal ends of the vessels, labeled, and secured using surgical thread, creating leak-free connections between the vessels and the attached tubing (Fig. 1C).
Figure 1.
Bovine forelimb neurovascular bundle (NVB). A: bovine forelimb. Flexor carpi radialis and superficial digital flexor muscles distally to proximally dissected, along with the deep digital flexor muscle. Neuromuscular bundle (NVB) visualized and isolated from surrounding tissue. Six bovine NVBs were used to derive the quantitative and qualitative conclusions discussed in this report. B: blood vessel openings as seen after dissection. Center, artery; sides, veins. C: dissected NVB with marked cannulas (scale bar: 1 cm). D: longitudinal view of crosscut NVB demonstrating multiple valves inside the veins (scale bar: 1 cm).
Patency of the Venous Valves
To ensure that the tissue storage and dissection processes had not damaged the fragile leaflets of the venous valves and to confirm that all tributaries were tied off, the vessels were examined for leakage and valve patency using gravity-driven flow. For the latter purpose, the cannulated ends of the vein were connected to a vertical column of saline, creating a gravitational pressure of ∼90 mmHg. The saline readily flowed in a distal-to-proximal direction (i.e., toward the heart), while no flow was observed in the opposite direction, confirming that the valves remained intact. Upon completion of the flow experiments, the presence of multiple valves in both veins was confirmed by taking images of the longitudinally cut vessels (Fig. 1D).
Arterial Pressure Pulsations
A schematic of the experimental setup is illustrated in Fig. 2A. To create pulse-like transient increases in pressure within the arterial segment, the arterial cannula was connected to a piston pump (Harvard Apparatus Rodent Ventilator Model No. 683) with adjustable volume and rate settings, whereas the other end of the cannula was sealed off. The pump was turned on and off to create multiple pulse/no pulse episode sequences, with each episode lasting 1 min (Fig. 2B). Pressure recordings were acquired using an in-line pressure transducer connected to a LabChart station from ADInstruments.
Figure 2.
Schematics of the experimental setup. A: distal end of the cannulated vein within a tightly wrapped neuromuscular bundle (NVB) was connected to a saline reservoir positioned at different heights. Flow rate was measured by timing the saline weight exiting the proximal end of the vein. A piston pump was used to create rhythmic pulsations inside the artery. B: pressure recordings from an in-line transducer connected to an ADInstruments bridge amplifier were displayed using ADI LabChart software. Impacts of various variables, including pump rate, pump stroke volume, and height of the saline reservoir, were tested using three sequential on/off pump sequences, each lasting 1 min.
Flow Recordings
Once the integrity of the vessels and the venous valve patency had been checked, the bundle was tightly wrapped to create an airtight enclosure mimicking surrounding muscle tissue. The distal end of the vein was then connected to a wide flask that was later elevated to create different values of gravitational pressure. To convert the gravitational pressure of the saline column from cmH2O to mmHg, the height of the column in mm was divided by the ratio of mercury to saline density. The flowrate was calculated by dividing the weight of the exiting saline by the corresponding time. Notably, as the saline flowrate in our experiments was influenced by external resistances (i.e., custom-made cannulas), its absolute value has little physiological relevance. Instead, attention should be paid to the relative changes in flowrate in the presence of arterial pulsations.
Statistical Analysis
Six bovine NVBs were used to derive the quantitative and qualitative conclusions discussed in this report. The experiments included the dissection and visualization of NVB, examining the observed phenomenon under different settings including positioning of the bundle (vertical vs. horizontal), pulse rate, lack/presence of the enclosure, and changes in venous backpressure. Data are presented as means ± SDs. A two-sided unpaired heteroscedastic t test was used to derive P values for differences in the mean flowrates during pulse/no pulse conditions. Values of P < 0.5 were considered significant (*), values of P < 0.01 (**), and P < 0.001 (***) were considered highly significant. The data underlying our conclusions are available in the article and in its figures.
RESULTS
Impact of Arterial Pulse on Venous Flowrate
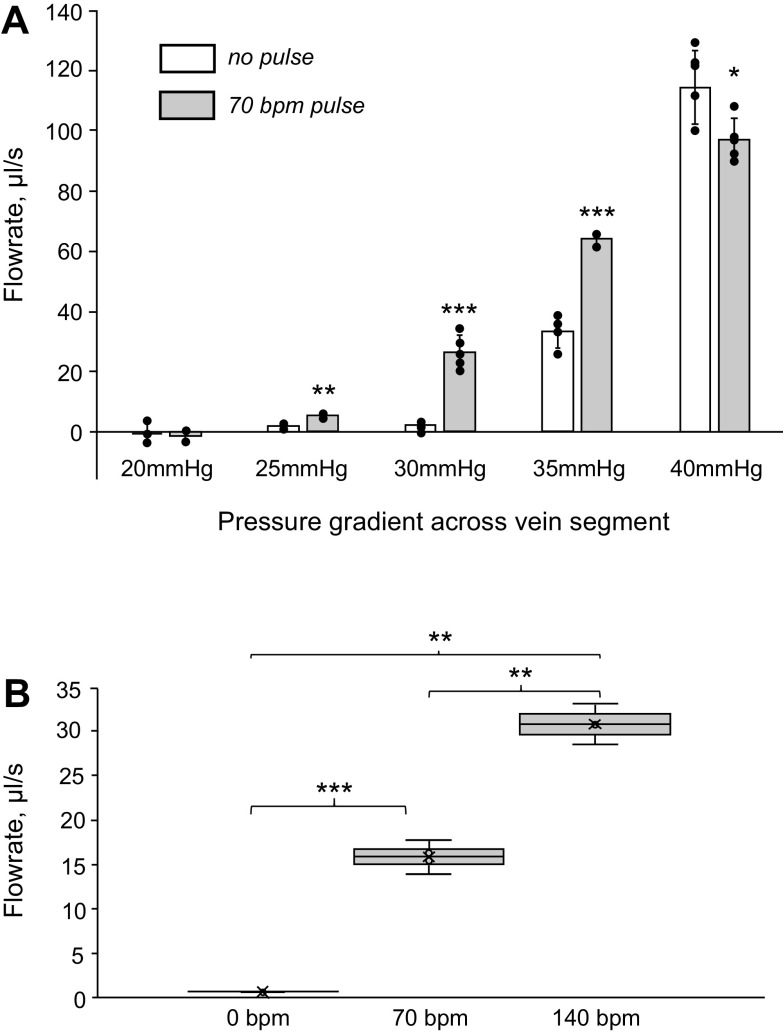
To mimic physiological values typical for healthy individuals, the piston pump was set to create transient ∼50-mmHg pressure increases in a 1- to 2.5-Hz frequency range. These values correspond to heart rates of 60–150 beats/min and pulse pressure of ∼50 mmHg, typical for healthy adults (10). Hydrostatic pressure across the distal and proximal ends of the vein was varied from 0 to 40 mmHg (Fig. 3).
Figure 3.
Impact of arterial pulsations on flowrate through the adjacent vein segment. A: open bars correspond to the flowrate in the absence of pulsations. Gray bars indicate flowrate when the arterial pulse was present. The x-axis shows the pressure gradient values across a vertically positioned NVB. The gradient corresponds to the height difference between the saline reservoir and the point where saline exited the vein. The individual experimental values are shown using black dots. B: whisk plot illustrating the impact of pulsation rate on the saline outflow through the vein. The experiment was conducted three times at a 28-mmHg pressure gradient across a vertically positioned NVB. *P < 0.5; **P < 0.01; ***P < 0.001.
At the lowest values of hydrostatic pressure across the vein segment, no flow through a vertically positioned vein was observed due to the combined resistance imposed by the valves, the cannulas, and the vein segment itself. In these conditions, compression of the vein by a pulsating arterial wall did not yield any measurable flow. Once the pressure gradient across the vein was increased to ∼25 mmHg, the arterial pulse-dependent flowrate effect became highly significant. When the pressure difference across the vein segment reached 40 mmHg, the effect reversed its direction, with arterial pulsations having a negative impact on venous flow values (Fig. 3A).
When the venous pressure gradient was set within the 25- to 35-mmHg range, doubling the pulse rate from 70 beats/min to 140 beats/min led to a proportional increase in flowrate values (Fig. 3B).
Figure 3 shows quantitative data from an individual bundle to which each setting was applied at least three different times. The tight range of outflow values then enabled to reveal the bell-shaped relationship between the hydrostatic pressure across the vein versus change in venous flowrate when arterial pulse was present (Fig. 4). Such relationship would have been hidden by averaging the absolute values of saline outflow from different bundle preparations. The variability in absolute outflow values can be attributed to hemodynamic differences introduced by the insertion of cannulas and ligation of multiple tributaries, multiplied by cross-animal variations in NVB anatomy.
Figure 4.
Physiological implications of the observed effect. The graph shows the difference in flowrates through the vein in the absence and presence of the arterial pulse. Individual value points represent the difference between the flowrate in the presence of 70 beats/min pulsations and the average flowrate in absence of pulsations. Top: physiological meaning of the presented quantitative data (see explanation in DISCUSSION).
Role of the Enclosure
For the effect of arterial pulsations on venous flow to take place, the artery and adjacent veins must be enclosed in an airtight environment or tightly bound together. To confirm that, the cannulated bundle was tested without the enclosure and the flowrates were recorded with and without the pump providing pulsations. In the absence of an enclosure, the pressure rises inside the artery led to visible outward bulging of the arterial wall, which did not push on the walls of the veins next to it. As a result, without the enclosure, arterial pulsations did not yield any measurable increase in saline outflow through the vein.
Beyond Physiological Values
When the frequency or stroke volume of the pump was increased above physiologically relevant values, it led to pressure buildup within the enclosure. This was due to insufficient time for the artery to dissipate the pressure, causing the artery to balloon inside the enclosure. This dramatic increase in arterial volume then compressed the adjacent veins, narrowing their inner diameters and increasing their resistance to flow. In this scenario, turning arterial pulsations off actually increased the venous flowrate as the veins returned to their initial, uncompressed sizes. Although this scenario is unlikely to occur physiologically, it seems important to mention these observations to help others avoid artifacts or getting conflicting data.
DISCUSSION
This report presents direct evidence of an auxiliary, yet fundamental physiological mechanism aiding blood circulation on a beat-by-beat basis. This mechanism relies on the anatomical proximity of arteries and valve-containing veins in the lower extremities. Although this mechanism may appear intuitively obvious, we could not find any published studies that provided quantitative measurements of its impact.
The cartoon in Fig. 4 attempts to explain the physiological meaning of our quantitative observations. The graph below the cartoon was derived from the data shown in Fig. 3A. At very low backend pressure values, the leaflets of the venous valves stick together, closing the valves. In these conditions, a pulsating artery next to the vein has a negligible impact on venous flow (Fig. 4, case a). When the pressure gradient becomes sufficient to partially open the venous valves, an extra push from the adjacent artery can increase the flow by further opening the valves (Fig. 4, case b). When the pressure gradient across the vein becomes sufficiently large to keep the valves fully open, the arterial pulse effect disappears. Moreover, as compression episodes reduce vein diameter, the flow may begin to decrease (Fig. 4, case c).
Conceptually, the effect of the arterial pulse is similar to the effect of the skeletal muscle pump, external compression devices, or massage therapy procedures commonly used to treat venous insufficiency. Each involves the periodic compression of the veins, which contain multiple one-way valves. The main difference is that the arterial pulse is constantly present. This constitutes a continuous impact on venous return and highlights yet another ingenious way in which evolution has coupled anatomical structures with their physiological function.
It is important to underscore that the described mechanism does not involve peristaltic waves of pressure caused by the coordinated contraction of smooth muscles, which are known to drive intraluminal content of the digestive system, esophagus, or ureter (1). In the case of a peristaltic wave, the speed of compression is almost identical to the speed of flow it creates. In contrast, compression of the adjacent vein by a pulsating artery constitutes a nearly instantaneous event, estimated to be several orders of magnitude faster than the venous flow velocity.
The described hypothetical mechanism also did not involve changes in tissue pressure. A gradual increase in tissue pressure can occur when there is leakage of blood from acutely damaged vessels into the surrounding tissue, clinically known as a “compartment syndrome.” In our experiments, there was no leakage of the fluid into the enclosure as all the tributaries of the vessels were thoroughly ligated preventing fluid escape from these vessels. The enclosure then brought the vessels together to model the physiological scenario of the neurovascular bundle being surrounded on all sides by skeletal muscles.
One might also wonder about the opposite directions of blood flow in the vein versus artery. Here, it is important to reemphasize that here we focus on the arterial pressure wave and not on the movement of arterial blood. In medium-sized arteries, such as the ones considered in this paper, the velocity of pulse wave propagation is many times higher than the velocity of blood flow (11). For example, published values for the human radial artery are ∼10 cm/s for blood flow velocity (12) and ∼8 m/s for pulse pressure wave velocity (10). Therefore, a passing pressure wave expands the walls of a 15 cm long arterial segment almost instantaneously (∼20 ms from one end to another, based on the abovementioned numbers). Consequently, for a putative mechanism depicted in Fig. 4, neither direction of blood flow nor the direction of pulse pressure wave plays any role.
One can suggest that in vivo measurements of pressure-flow patterns using vascular ultrasound may serve as a better way to demonstrate the existence of the described mechanism. Indeed, published Doppler recordings of flow patterns in the lower extremities have shown that each systole causes a sharp burst in arterial flow velocity and an increase in antegrade venous flow in the adjacent vein (13, 14). As convincing as these observations may appear, they cannot be used as direct evidence of the described mechanism. This is because, during systole, the AV septum is swiftly pulled down by the contracting ventricle, essentially acting as a piston to withdraw blood from the venous compartment (7). Therefore, transient increases in venous flow observed during systole in large and medium veins are most likely due to a combination of AV plane movement and arterial pulse impact. Consequently, the only way to clearly separate these two processes is to take the movement of the AV plane out of the picture by conducting ex vivo bench experiments, such as those described in this report.
Perspectives and Significance
The described phenomenon raises several clinically relevant questions. For example, does the impact of arterial pulse on venous return decrease with age-related changes in arterial stiffness (15)? How is this impact affected by a decrease in the number of intact venous valves? It is well known that a sedentary lifestyle and prolonged periods of immobility are associated with the development of small thrombi in the pockets formed by venous valve leaflets (16). The resolution of these clots by the coordinated efforts of neutrophils and macrophages (17) also damages the leaflets, leading to valve incompetency (18). These processes are well-established and commonly used to describe the pathophysiology of deep venous insufficiency. Experiments presented in this report suggest that a lack of competent valves would negate the effect of the arterial pulse on venous return, leading to further expansion of the veins, increased venous pressure, and subsequent edema. These and other questions warrant further investigation into the largely overlooked role of arterial pulse in venous return.
GRANTS
This work was supported by the US National Science Foundation EAGER Award No. 1927694 (to N. Sarvazyan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S. conceived and designed research; N.M.L. and N.S. performed experiments; N.S. analyzed data; N.S. interpreted results of experiments; N.S. prepared figures; N.S. drafted manuscript; N.S. edited and revised manuscript; N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Drs. Ara Arutunyan, Nguyen Bao-Ngoc, and Patricia Latham are gratefully acknowledged for helpful discussions, Dr. Christian LeFevre for critically reading the manuscript, and Hovhannes Arestakesyan for help with tissue dissection.
REFERENCES
- 1. Hall JE, Hall ME. Guyton and Hall: Textbook of Medical Physiology (14th ed). Philadelphia, PA: Elsevier; 2020. [Google Scholar]
- 2. Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 146: 977–982, 1986. [PubMed] [Google Scholar]
- 3. Dart AM, Kingwell BA. Pulse pressure - a review of mechanisms and clinical relevance. J Am Coll Cardiol 37: 975–984, 2001. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 4. Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 563: 925–943, 2005. doi: 10.1113/jphysiol.2004.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abel FL, Waldhausen JA. Respiratory and cardiac effects on venous return. Am Heart J 78: 266–275, 1969. doi: 10.1016/0002-8703(69)90019-2. [DOI] [PubMed] [Google Scholar]
- 6. Carlsson M, Ugander M, Mosén H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 292: H1452–H1459, 2007. doi: 10.1152/ajpheart.01148.2006. [DOI] [PubMed] [Google Scholar]
- 7. Arutunyan AH. Atrioventricular plane displacement is the sole mechanism of atrial and ventricular refill. Am J Physiol Heart Circ Physiol 308: H1317–H1320, 2015. doi: 10.1152/ajpheart.00058.2015. [DOI] [PubMed] [Google Scholar]
- 8. Felisaz PF, Chang EY, Carne I, Montagna S, Balducci F, Maugeri G, Pichiecchio A, Calliada F, Baldi M, Bastianello S. In vivo MR microneurography of the tibial and common peroneal nerves. Radiol Res Pract 2014: 780964, 2014. doi: 10.1155/2014/780964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Netter FH. Atlas of Human Anatomy, Professional Edition. Philadelphia, PA: Elsevier, 2022. [Google Scholar]
- 10. McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity - The Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46: 1753–1760, 2005. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 11. Mynard JP, Kondiboyina A, Kowalski R, Cheung MMH, Smolich JJ. Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front Physiol 11: 1085, 2020. doi: 10.3389/fphys.2020.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krullaards RL, Pel JJM, Snijders CJ, Kleinrensink GJ. The potential effects of a biofeedback writing exercise on radial artery blood flow and neck mobility. Int J Biomed Sci 5: 192–197, 2009. [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang JY. Doppler ultrasonography of the lower extremity arteries: anatomy and scanning guidelines. Ultrasonography 36: 111–119, 2017. doi: 10.14366/usg.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu-Yousef MM, Mufid M, Woods KT, Brown BP, Barloon TJ. Normal lower limb venous Doppler flow phasicity: is it cardiac or respiratory? AJR Am J Roentgenol 169: 1721–1725, 1997. doi: 10.2214/ajr.169.6.9393197. [DOI] [PubMed] [Google Scholar]
- 15. O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15: 426–444, 2002. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 16. Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 15: 175–184, 2005. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17. Nicklas JM, Gordon AE, Henke PK. Resolution of deep venous thrombosis: proposed immune paradigms. Int J Mol Sci 21: 2080, 2020. doi: 10.3390/ijms21062080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 28: 387–391, 2008. doi: 10.1161/atvbaha.108.162289. [DOI] [PubMed] [Google Scholar]