Abstract
Interleukin (IL)-11, a multifunctional cytokine, contributes to numerous biological processes, including adipogenesis, hematopoiesis, and inflammation. Asthma, a respiratory disease, is notably characterized by reversible airway obstruction, persistent lung inflammation, and airway hyperresponsiveness (AHR). Nasal insufflation of IL-11 causes AHR in wild-type mice while lung inflammation induced by antigen sensitization and challenge, which mimics features of atopic asthma in humans, is attenuated in mice genetically deficient in IL-11 receptor subunit α-1 (IL-11Rα1-deficient mice), a transmembrane receptor that is required conjointly with glycoprotein 130 to transduce IL-11 signaling. Nevertheless, the contribution of IL-11Rα1 to characteristics of nonatopic asthma is unknown. Thus, based on the aforementioned observations, we hypothesized that genetic deficiency of IL-11Rα1 attenuates lung inflammation and increases airway responsiveness after acute inhalation exposure to ozone (O3), a criteria pollutant and nonatopic asthma stimulus. Accordingly, 4 and/or 24 h after cessation of exposure to filtered room air or O3, we assessed lung inflammation and airway responsiveness in wild-type and IL-11Rα1-deficient mice. With the exception of bronchoalveolar lavage macrophages and adiponectin, which were significantly increased and decreased, respectively, in O3-exposed IL-11Rα1-deficient as compared with O3-exposed wild-type mice, no other genotype-related differences in lung inflammation indices that we quantified were observed in O3-exposed mice. However, airway responsiveness to acetyl-β-methylcholine chloride (methacholine) was significantly diminished in IL-11Rα1-deficient as compared with wild-type mice after O3 exposure. In conclusion, these results demonstrate that IL-11Rα1 minimally contributes to lung inflammation but is required for maximal airway responsiveness to methacholine in a mouse model of nonatopic asthma.
Keywords: adiponectin, airway hyperresponsiveness, asthma, coefficient of lung tissue elastance, interleukin-11
INTRODUCTION
Interleukin (IL)-11 is a pleiotropic, cationic cytokine that is expressed by a plethora of cells: chondrocytes, endothelial and epithelial cells, eosinophils, fibroblasts, neurons, osteoblasts, smooth muscle cells, spermatids, synoviocytes, and trophoblasts (1–10). As a multifunctional cytokine, IL-11 regulates a diverse number of biological processes, such as adipogenesis, fertility, fibrosis, hematopoiesis, inflammation, metastasis, neurogenesis, and osteoclastogenesis (11–16). To transduce classic signaling in humans, IL-11 requires, in part, the presence of IL-11 receptor subunit α (IL-11Rα), which is a transmembrane receptor that is part of the type I cytokine receptor family (12, 17, 18). Consistent with the pleiotropic effects of IL-11, IL-11Rα or its murine homolog [IL-11 receptor subunit α-1 (IL-11Rα1)] is expressed by several diverse cell populations, including adipocyte-like cells, decidual cells, epithelial cells, lymphocytes, megakaryocytes, oligodendrocytes, osteoblasts, osteoclasts, smooth muscle cells, stellate cells, and stromal cells (19–28).
IL-11 binds with low affinity to IL-11Rα on the plasma membrane, yet this interaction is inadequate to initiate classic signaling (12). However, in accordance with its membership in the IL-6 family of cytokines, IL-11 also requires glycoprotein (gp) 130 for effective signal transduction (29). Thus, after IL-11 binds to IL-11Rα, it creates a high-affinity complex with gp130, which results in gp130 dimerization and the formation of a heterohexameric complex that consists of two molecules of IL-11, IL-11Rα, and gp130 (2, 12, 18). Once this six-member receptor complex is created, IL-11 signal transduction commences with activation of the Janus kinase-signal transducer and activator of the transcription (STAT) pathway (12, 18). Finally, the ectodomain of IL-11Rα can be cleaved from the plasma membrane by ADAM10, a sheddase, to generate a soluble form of IL-11Rα that can elicit trans-signaling along with IL-11 and membrane-bound gp130 in cells that do not express IL-11Rα (30, 31).
A single gene encodes the IL-11Rα protein in humans [interleukin 11 receptor subunit α (IL11RA)] and the IL-11Rα1 protein in mice [interleukin 11 receptor, α chain 1 (Il11ra1)] (32, 33). Present in select strains of mice, however, is the interleukin 11 receptor, α chain 2 (Il11ra2) gene, which encodes a protein and second receptor for IL-11 [IL-11 receptor subunit α-2 (IL-11Rα2)] (34, 35). Despite impressive semblance between the coding sequences of the Il11ra1 and Il11ra2 genes, the expression pattern of these genes is quite dissimilar (36). Specifically, Il11ra1 messenger ribonucleic acid (mRNA) is expressed in numerous tissues and organs, such as bone marrow, brain, heart, kidney, and lung, whereas expression of Il11ra2 mRNA is more restricted to the lymph nodes, testes, and thymus (34, 36). Although IL-11 binds IL-11Rα1 and IL-11Rα2 with similar affinity (34, 37), no published data are in existence concerning the signaling capabilities of IL-11Rα2.
Asthma is a chronic respiratory disease that is characterized, in part, by cough, dyspnea, wheeze, airway remodeling, mucous metaplasia, persistent lung inflammation, variable expiratory airflow limitation, and airway hyperresponsiveness (AHR) to nonspecific bronchoconstrictors, including methacholine chloride, histamine acid phosphate, and leukotriene E4 (38–40). As a heterogeneous lung disease, asthma materializes as a diverse number of phenotypes such as atopic, nonatopic, obesity-related, and smoking-associated (39, 41). Minshall et al. (4) previously reported that expression of IL11 mRNA was greater in lung tissue of subjects with asthma as compared with nonasthmatic subjects, an observation that suggests that IL-11 may contribute to the pathogenesis of asthma. Indeed, in the absence of any inciting stimulus, administration of recombinant human IL-11 to BALB/c mice via nasal insufflation causes AHR, whereas overexpression of IL11 mRNA in a lung-specific fashion leads to airway wall thickening, peribronchiolar inflammation, subepithelial lung fibrosis, and AHR (11, 42). Furthermore, Lee et al. (16) demonstrated that eosinophilic lung inflammation, T-helper cell type-2 cytokine expression, and goblet cell hyperplasia are significantly reduced in antigen-sensitized and challenged mice that do not express IL-11Rα1. In many strains of mice, antigen sensitization and challenge leads to a phenotype that mimics many of the characteristic features of atopic asthma in humans (43).
Atopic asthma is often distinguished from other asthma phenotypes by its early onset, the presence of antigen-specific immunoglobulin E (IgE), allergenic provocation, and demonstrable, positive responsiveness to corticosteroids, whereas nonatopic asthma is uniquely identified by its late onset, the absence of antigen-specific IgE, female preponderance, and poor responsiveness to corticosteroids (44, 45). As described in the preceding paragraph, Lee et al. (16) reported the contribution of IL-11Rα1 to the development of atopic lung inflammation. However, the involvement of IL-11Rα1 in the manifestation of lung inflammation and AHR induced by a nonatopic asthma stimulus in mice has not been addressed.
Ozone (O3) is a common air pollutant, an impressively efficacious oxidant, and a nonatopic asthma stimulus. Globally, in 2015, a minimum of nine million emergency room visits for asthma were attributed to O3 (46). By reacting with unsaturated fatty acids in the lung lining fluid, O3 initiates a cascade of chemical reactions that generate lipid peroxides and protein adducts in the plasma membrane of lung epithelial cells (47). When structurally important plasma membrane lipids and proteins are altered via peroxidation and adduction, respectively, cell injury ensues by disruption of transmembrane ion gradients and by impairment of cellular metabolism (48). In response to this injury, lung inflammation emanates, which is notably characterized by the presence of proinflammatory cytokines [e.g., IL-6, keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-2, and osteopontin] and soluble tumor necrosis factor receptor (sTNFR) 1 and 2 in bronchoalveolar lavage (BAL) fluid (49–51). Furthermore, many of the aforementioned cytokines are required for the migration of neutrophils to the lungs and for AHR, which are the principal pathological features induced by acute exposure to O3 (49–54).
Because IL-11Rα1 is necessary for maximal lung inflammation induced by atopic asthma stimuli and because IL-11 causes AHR, we hypothesized that IL-11Rα1 is also required for the manifestation of lung inflammation and AHR induced by O3, a nonatopic asthma stimulus. To that end, we exposed wild-type mice and mice homozygous for a null mutation in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) to either filtered room air (air) or O3 [2 parts/million (ppm)] for 3 h, and subsequent to the cessation of exposure, we assessed lung inflammation and airway responsiveness in the experimental animals.
MATERIALS AND METHODS
Animals
IL-11Rα1-deficient mice were generated via homologous recombination by replacing exons 9 through 12 and parts of exons 8 and 13 of the Il11ra1 gene with a phosphoglycerate kinase 1 (PGK1) promoter-aminoglycoside 3'-phosphotransferase (neo) gene construct (55). IL-11Rα1-deficient mice are viable and demonstrate no abnormalities in hematopoietic and nonhematopoietic organs or in complete blood counts when compared with wild-type mice and mice heterozygous for a null mutation in the gene encoding Il11ra1 (IL-11Rα1+/− mice) (55).
To generate IL-11Rα1-deficient mice for this study, two breeding phases were executed. First, two breeding pairs of IL-11Rα1+/− mice (Strain No. 004621) were purchased from The Jackson Laboratory (Bar Harbor, ME). Among the offspring of these mice, we identified, at the Il11ra1 allele, wild-type, IL-11Rα1+/−, and IL-11Rα1-deficient mice via genotyping by polymerase chain reaction (PCR). Second, because IL-11Rα1-deficient female mice are infertile (27), all subsequent breeding pairs consisted of IL-11Rα1-deficient male and IL-11Rα1+/− female mice to produce IL-11Rα1-deficient mice for our experiments.
Progeny of breeding pairs was genotyped for Il11ra1 alleles as follows. First, genomic DNA was extracted from a tail snip using the HotSHOT method (56). Second, an aliquot of genomic DNA was added to a solution containing KAPA2G Fast Genotyping Mix (Kapa Biosystems, Inc.; Wilmington, MA) and a common sense primer (5′- GGCTCCCGTCATTACCTACA-3′) and specific antisense primers for the wild-type (5′- AGCAGTCCTACCCGCTACAA-3′) and mutant (PGK1-neo-containing; 5′- ACTTGTGTAGCGCCAAGTGC-3′) Il11ra1 alleles. Following amplification of genomic DNA via PCR using the aforementioned primers, the PCR amplicons were visualized on a 1.8% ethidium bromide-stained agarose gel using a GelVue ultraviolet transilluminator (Model Number GV2M30; Syngene, Cambridge, UK). The primers produced a wild-type amplicon of 766 base pairs (bp) and a mutant amplicon of 593 bp. Figure 1 illustrates a representative ethidium-bromide-stained agarose gel containing Il11ra1 amplicons generated from wild-type, IL-11Rα1+/−, and IL-11Rα1-deficient mice.
Figure 1.
Representative interleukin 11 receptor, α chain 1 (Il11ra1) amplicons generated from the progeny of IL-11Rα1+/− breeding pairs. As described in materials and methods, genomic DNA was extracted from tails snips obtained from wild-type mice, mice heterozygous for a null mutation in Il11ra1 (IL-11Rα1+/− mice), and mice homozygous for a null mutation in Il11ra1 (IL-11Rα1-deficient mice) and amplified via PCR using specific primers for the Il11ra1 wild-type and mutant alleles. PCR products were resolved on a 1.8% ethidium bromide-stained agarose gel. Amplification of genomic DNA via PCR from 1) wild-type mice generated one amplicon of 766 base pairs (bp), 2) IL-11Rα1+/− mice generated two amplicons of 766 and 593 bp, and 3) IL-11Rα1-deficient mice generated one amplicon of 593 bp.
Because IL-11Rα1-deficient mice were backcrossed into a C57BL/6J background for at least 12 generations, age- and sex-matched C57BL/6J mice (Strain No. 000664) were used as wild-type controls and purchased from The Jackson Laboratory. All breeding pairs and their progeny as well as wild-type mice were housed in the same room within a larger multispecies, modified barrier animal care facility at McGovern Medical School at The University of Texas Health Science Center at Houston (Houston, TX) under conditions we previously described (57). For all experiments, male C57BL/6J and IL-11Rα1-deficient mice between 8 and 20 wk of age were used. The care and use of all animals in this study adhered to the guidelines of the National Institutes of Health (Bethesda, MD), and each of the experimental protocols used in this study was approved by the Animal Welfare Committee of The University of Texas Health Science Center at Houston (Houston, TX).
Protocol
Two separate cohorts of mice were used in this study. In the first cohort, mice were euthanized 4 or 24 h after cessation of a 3-h exposure to either air or O3 (2 ppm), and blood, BAL fluid, and lungs were subsequently obtained from these animals. Data from air‐exposed, genotype‐matched mice in this cohort were pooled for each outcome indicator that was assessed at 4 or 24 h after cessation of exposure. In the second cohort, mice were anesthetized 24 h after cessation of a 3-h exposure to either air or O3 (2 ppm), and quasistatic respiratory system pressure-volume (PV) curves were then generated from each mouse before measurement of airway responsiveness to acetyl-β-methylcholine chloride (methacholine).
Air and O3 Exposures
Each mouse was removed from its microisolator cage, weighed, and placed in its own cell that was part of a larger stainless steel wire mesh cage, which was then positioned inside a powder-coated aluminum and Plexiglas exposure chamber. Without access to food or water, mice were subsequently exposed to either air or O3 (2 ppm) for 3 h. After the exposure ceased, mice were returned to the microisolator cage they occupied before the exposure until either 4 or 24 h after cessation of exposure. Mice were weighed again immediately before euthanasia or anesthesia. A detailed description of the air- and O3-exposure system has been previously described by us (49).
Finally, when the sensitivity to inhaled O3 was assessed in nine genetically diverse inbred strains of mice, C57BL/6J and 129S1/SvlmJ mice were categorized as “highly sensitive” since both strains exhibited profound lung inflammation and increases in airway responsiveness as compared with seven other inbred strains after acute exposure to O3 (58). Thus, C57BL/6J mice and genetically altered mice backcrossed into a C57BL/6J background, including IL-11Rα1-deficient mice, are invaluable tools to assess O3-induced lung pathophysiology.
Blood Collection, BAL, and Lung Harvest
Mice in the first cohort were euthanized with an intraperitoneal injection of pentobarbital sodium (200 mg/kg; Vortech Pharmaceuticals, Ltd., Dearborn, MI) 4 or 24 h after cessation of exposure to either air or O3. Once the animal failed to elicit ocular and pedal withdrawal reflexes, a median thoracotomy was performed, and blood was collected from the right ventricle of the heart. Serum was subsequently isolated from the blood and stored at −20°C until needed. After blood was collected, the lungs were lavaged a total of four times with ice-cold lavage buffer [1× phosphate-buffered saline (PBS) containing 0.6 mM ethylenediaminetetraacetic acid]. The lavagates were pooled, the liquid and cellular components of the lavagate separated by centrifugation, and the supernatant stored at −80°C until needed. The cell pellet that remained after centrifugation was resuspended in 1 mL of Hanks’ balanced salt solution (HyClone Laboratories, Logan, UT), and the total number of cells in this suspension was enumerated with a hemacytometer (Hausser Scientific; Horsham, PA). For differential cell analysis, BAL cells were first deposited onto microscope slides using a CytoZEN cytology centrifuge (Hettich Instruments; Beverly, MA). Subsequently, the slides were air-dried and stained with the Hema 3 stain set (Fisher Diagnostics; Middletown, VA). The cells that were deposited onto the slides were examined under a light microscope at a total magnification of ×400 for differential counts. Finally, when lavages were complete, the animal’s circulation was flushed with 10 mL of ice-cold 1× PBS, the left main bronchus severed, and the left lung removed from the animal and snap-frozen in liquid nitrogen and stored at −80°C until needed for ribonucleic acid (RNA) extraction. These experimental methods have been previously described by us in extensive detail (57).
RNA Extraction, cDNA Synthesis, and Reverse Transcription-Quantitative Real-Time Polymerase Chain Reaction
Using previously described methods (57, 59), total RNA was extracted from frozen lung tissue, complementary cDNA was synthesized from mRNA, quantitative polymerase chain reaction (qPCR) was performed, and data were analyzed using the comparative threshold cycle method so that the relative abundance of Il11ra1 mRNA 4 or 24 h after cessation of exposure to O3 was expressed relative to the abundance of Il11ra1 mRNA after cessation of exposure to air. All data were normalized to the abundance of hypoxanthine-guanine phosphoribosyl transferase (Hprt) mRNA, a reference gene (60). Primers for murine Il11ra1 and Hprt were purchased from Qiagen (Germantown, MD) and Bio-Rad Laboratories, Inc. (Hercules, CA), respectively.
Cytokine and Protein Quantification
BAL supernatant and/or serum IL-11, adiponectin, IL-6, KC, macrophage inflammatory protein (MIP)-3α, hyaluronan, osteopontin, and sTNFR 1 and 2 were quantified by either Quantikine or DuoSet ELISA kits according to the manufacturer’s instructions (R&D Systems, Inc.; Minneapolis, MN). Total BAL protein was quantified using the Bio-Rad protein assay (Bio-Rad Laboratories, Inc.) as previously described (57).
Quasi-Static Respiratory System PV Curves and Airway Responsiveness to Methacholine
Twenty-four hours after cessation of exposure to either air or O3, mice were 1) anesthetized with pentobarbital sodium (50 mg/kg; Oak Pharmaceuticals, Inc.; Lake Forest, IL) and xylazine hydrochloride (7 mg/mL; Vedco Inc.; St. Joseph, MO), 2) tracheostomized with an 18-gauge tubing adaptor (Becton, Dickinson and Company; Franklin Lakes, NJ), and 3) ventilated at a frequency of 2.5 Hz, a tidal volume of 0.3 mL, and a positive end-expiratory pressure of 3 cmH2O for the generation of quasi-static respiratory system PV curves and the measurement of airway responsiveness to methacholine (Sigma Aldrich, Inc.; St. Louis, MO) as we previously described (49, 61). From each PV curve, A, an estimate of inspiratory capacity; K, curvature of the upper portion of the expiratory limb of the PV curve; Cstat, quasi-static respiratory system compliance; and area, respiratory system hysteresis, were calculated. A, K, and Cstat were determined using the Salazar–Knowles equation (62, 63). After the generation of PV curves was complete, respiratory system impedance (ZRS) was determined using the forced oscillation technique as we and others have previously described (49, 64–66). The constant-phase model was used to partition ZRS into components representing airway resistance (Raw), the coefficient of lung tissue damping (G), and the coefficient of lung tissue elastance (H) after administration of 1× PBS (Sigma Aldrich, Inc.) alone and after administration of increasing concentrations of methacholine dissolved in 1× PBS (0.1–100 mg/mL) (49, 64). The flexiVent (SCIREQ Scientific Respiratory Equipment; Montréal, Québec, Canada) was used to ventilate the lungs, deliver stepwise inspiratory and expiratory volume increments for the generation of PV curves, and superimpose sinusoidal forcing functions of multiple frequencies on the breathing frequency of the animal (2.5 Hz) for the determination of ZRS.
Statistical Analyses of Data
The effect of genotype (wild‐type or IL-11Rα1‐deficient) and exposure (air or O3) on body mass, PV curve parameters, and the majority of BAL analytes was assessed by a two-way analysis of variance (ANOVA) or by a Kruskal–Wallis one‐way ANOVA. Depending on the ANOVA utilized, the Fisher’s least significant difference (LSD) test or Wilcoxon rank-sum test were used for post hoc analyses. BAL IL-11 and the relative abundance of Il11ra1 mRNA were analyzed using a one‐way ANOVA. Pre- and postexposure body masses were compared using a Student’s t test for paired samples, whereas serum sTNFR 1 and sTNFR 2 were compared using a Student’s t test for npaired samples and a Wilcoxon rank-sum test, respectively. Statistical analyses of methacholine dose-response curves were performed using a mixed-model repeated measures three-way ANOVA (genotype × exposure × concentration of methacholine), whereas pairwise post hoc comparisons were calculated using Fisher’s LSD test. When necessary, data were transformed using the natural logarithm to meet the assumptions of the aforementioned statistical tests. Methacholine dose-response curves were analyzed using PROC MIXED in SAS (Version 9.4; SAS Institute, Inc.; Cary, NC), whereas all other data were analyzed using JMP 15 (SAS Institute, Inc.). Results are expressed as the means ± SE. A P < 0.05 was considered significant. Finally, different sample sizes for the various experiments in this study were due to power requirements.
RESULTS
Effect of O3 on the Relative Abundance of Lung Il11ra1 mRNA in Wild-Type Mice
To ascertain whether O3 altered the relative abundance of lung Il11ra1 mRNA in wild-type C57BL/6J mice 4 and/or 24 h after cessation of exposure, we employed RT-qPCR. As illustrated in Fig. 2A, O3 had no effect on the abundance of lung Il11ra1 mRNA when expressed relative to lung Il11ra1 mRNA from air‐exposed wild‐type mice.
Figure 2.
Relative abundance of interleukin 11 receptor, α chain 1 (Il11ra1) mRNA in the left lung lobe (A) or concentration of bronchoalveolar lavage interleukin (IL)-11 (B) obtained from wild-type C57BL/6J mice 4 or 24 h after cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). The abundance of Il11ra1 mRNA in O3-exposed mice was expressed relative to Il11ra1 mRNA in the left lung lobe of wild-type C57BL/6J mice 4 or 24 h after cessation of a 3-h exposure to air. In A, all data were normalized to the abundance of hypoxanthine guanine phosphoribosyl transferase mRNA. The data in A and B were analyzed via a one-way analysis of variance. Each value is expressed as the means ± SE. n = 4 or 5 mice in each group for A and n = 8 mice in each group for B.
Effect of O3 on BAL and Serum IL-11 in Wild-Type Mice
IL-11 was detectable in BAL obtained from air- or O3-exposed wild-type mice (Fig. 2B). However, regardless of the time interval examined, there was no statistically significant difference in BAL IL-11 between air- or O3-exposed wild-type mice. Finally, consistent with results from healthy adult humans (67), IL-11 was undetectable in serum of wild-type mice irrespective of exposure (data not shown).
Effect of IL-11Rα1 Deficiency and O3 on Inspiratory Capacity and Quasi-Static PV Relationships of the Respiratory System
In the absence of any inciting stimulus, overexpression of IL11 mRNA in a lung-specific fashion in mice increases lung volume and alters the quasi-static PV relationships of the lungs (68). Thus, it is plausible that deficiency of Il11ra1 may impact lung volumes or capacities in addition to the quasi-static PV relationships of the respiratory system in IL-11Rα1-deficient mice.
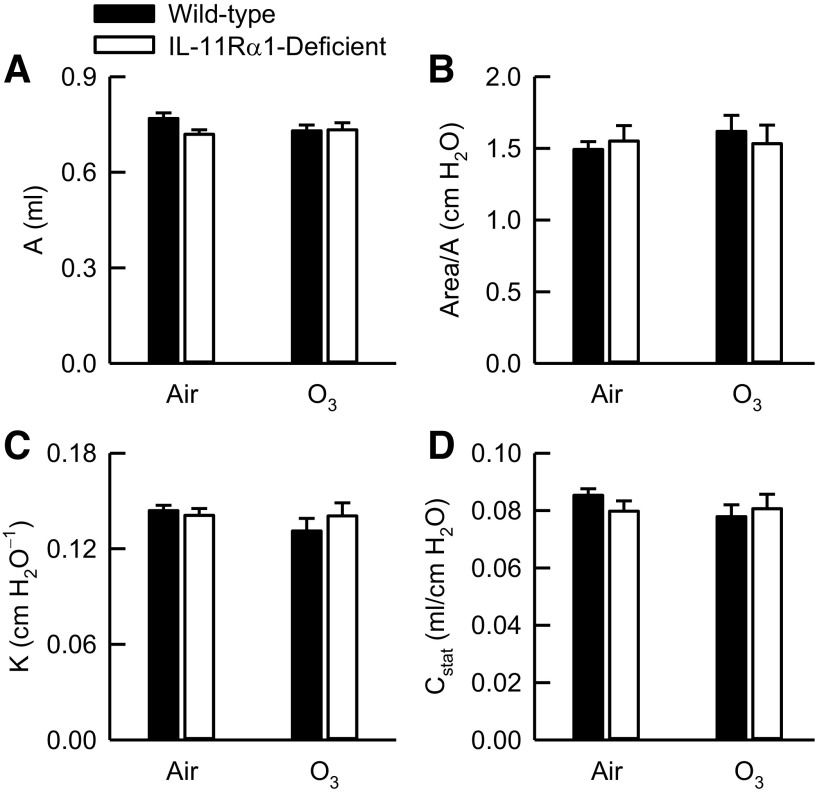
To that end, we measured A, an estimate of inspiratory capacity, and parameters describing the quasi-static PV relationships of the respiratory system (Area/A, K, and Cstat). A, Area/A, K, and Cstat were not different between air-exposed wild-type and IL-11Rα1-deficient mice (Fig. 3). In addition, O3 had no effect on any of these indices in either genotype. Finally, there were no genotype-related differences in A, Area/A, K, or Cstat after O3 exposure.
Figure 3.
A, an estimate of inspiratory capacity (A), area (hysteresis) of pressure-volume (PV) curves normalized for A (B), K, the parameter from the Salazar–Knowles equation reflecting the curvature of the upper portion of the expiratory limb of the PV curve (C), and Cstat, static compliance of the respiratory system (D). These indices were obtained from PV curves generated from wild-type C57BL/6J mice and mice genetically deficient in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) 24 h after cessation of a 3‐h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). Data were analyzed via a two-way analysis of variance, and pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. Each value is expressed as the means ± SE. For air-exposed mice, n = 8–10, whereas for O3-exposed mice, n = 4.
Effect of IL-11Rα1 Deficiency and O3 on Airway Responsiveness to Methacholine
Before and after exposure to air, wild-type mice weighed significantly more than IL-11Rα1-deficient mice (Table 1). Furthermore, when including the time from when the O3 exposure commenced until the time the mice were anesthetized 24 h after cessation of exposure, body masses of wild-type and IL-11Rα1-deficient mice decreased, on average, by 12.7% (Table 1).
Table 1.
Pre- and postexposure body masses and baseline airway and lung parenchymal oscillation mechanics for wild-type and IL-11Rα1-deficient mice exposed to filtered room air or ozone
| Genotype (Exposure) | Body Mass, g |
Raw (cmH2O/mL/s) | G (cmH2O/mL) | H (cmH2O/mL) | |
|---|---|---|---|---|---|
| Preexposure | Postexposure | ||||
| Wild-type (Air) | 29.5 ± 0.8† | 29.5 ± 0.8† | 0.34 ± 0.01 | 3.71 ± 0.09 | 24.22 ± 0.71 |
| IL-11Rα1-deficient (Air) | 26.8 ± 0.7 | 26.4 ± 0.8‡ | 0.33 ± 0.01 | 3.61 ± 0.12 | 23.59 ± 1.04 |
| Wild-type (O3) | 28.3 ± 0.4 | 24.9 ± 0.4‡§ | 0.35 ± 0.01 | 4.51 ± 0.20* | 26.32 ± 0.85 |
| IL-11Rα1-deficient (O3) | 28.4 ± 0.7 | 24.6 ± 0.8‡ | 0.32 ± 0.01 | 4.41 ± 0.44& | 23.29 ± 1.52 |
The results are expressed as the means ± SE. n = 11 or 12 mice in each group. Measurements of preexposure body mass were made immediately before exposure to air or O3 (2 parts/million) for 3 h, whereas measurements of postexposure body mass were obtained for the same animals 24 h after cessation of a 3-h exposure to air or O3. Measurements of baseline airway (Raw) and lung parenchymal (G and H) oscillation mechanics were made after administration of 1× phosphate-buffered saline 24 h after cessation of a 3-h exposure to either air or O3. The effect of genotype and exposure on body mass were analyzed via a two-way ANOVA, and pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. Pre- and postexposure body mass comparisons of mice with the same genotype and exposure were calculated using a Student’s t test for paired samples. †P < 0.05 compared with IL-11Rα1-deficient mice with an identical exposure within respective pre- or postexposure column. ‡P < 0.05 compared with preexposure body mass. §P < 0.05 compared with wild-type mice exposed to air within postexposure column. *P < 0.05 compared with wild-type mice exposed to air. &P < 0.05 compared with IL-11Rα1-deficient mice exposed to air. air, filtered room air; G, coefficient of lung tissue damping; H, coefficient of lung tissue elastance; IL-11Rα1, interleukin-11 receptor subunit α-1; O3, ozone; Raw, airway resistance.
No differences in baseline Raw, G, or H existed between air-exposed wild-type and IL-11Rα1-deficient mice (Table 1). Compared with genotype-matched, air-exposed controls, exposure to O3 significantly increased baseline G but had no effect on baseline Raw or H. Furthermore, no genotype-related differences in Raw, G, or H existed after O3 exposure.
Administration of aerosolized methacholine to wild-type and IL-11Rα1-deficient mice significantly increased Raw, G, and H regardless of exposure (Fig. 4). There were no genotype-related differences in responses to methacholine for Raw, G, or H after air exposure. As compared with genotype-matched, air-exposed controls, responses to methacholine for Raw, G, and H were significantly increased by O3 at several doses of methacholine. However, after O3 exposure, responses to methacholine for Raw and H were significantly attenuated in IL-11Rα1-deficient as compared with wild-type mice (Fig. 4, A and C). Specifically, responses to methacholine for Raw and H were lower in O3-exposed IL-11Rα1-deficient as compared with wild-type mice after administration of 10 and 30 mg/mL and 0.1, 1, 10, 30, and 100 mg/mL of methacholine, respectively.
Figure 4.
Responses to aerosolized acetyl-β-methylcholine chloride (methacholine) for (A) airway resistance (Raw), (B) the coefficient of lung tissue damping (G), and (C) the coefficient of lung tissue elastance (H) in wild-type C57BL/6J mice and mice genetically deficient in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) 24 h after cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). Dose-response curves were analyzed via a mixed-model repeated measures three-way analysis of variance, whereas pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. Each value is expressed as the means ± SE. n = 11 or 12 mice for each group. *P < 0.05 compared with wild-type mice exposed to air. &Significant difference (P < 0.05) in responses to methacholine at the designated concentrations between air- and O3-exposed IL-11Rα1-deficient mice. #P < 0.05 compared with IL-11Rα1-deficient mice exposed to O3.
Effect of IL-11Rα1 Deficiency and O3 on Lung Injury and Lung Inflammation
O3-induced AHR is mechanistically coupled to select phenomena emblematic of O3-induced lung injury and lung inflammation (49, 50, 54, 69–71), and IL-11 or IL-11Rα1 influences the severity of lung injury and lung inflammation in response to a number of insalubrious stimuli (16, 72–74). Thus, to determine if differences in airway responsiveness between O3-exposed wild-type and IL-11Rα1-deficient mice are a result of genotype-related differences in the severity of O3-induced lung injury and lung inflammation, we measured representative indices of these phenomena.
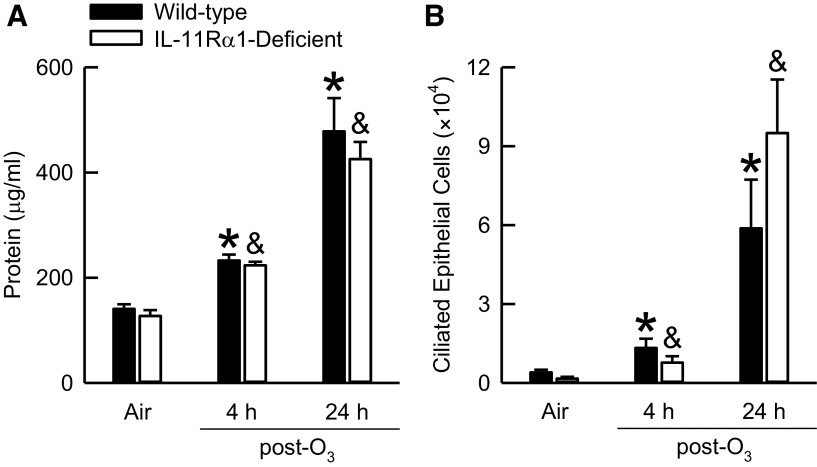
O3-induced lung injury was assessed by surveying 1) lung hyperpermeability via quantification of BAL protein and 2) airway epithelial cell desquamation via enumeration of BAL ciliated epithelial cells (75, 76). After exposure to air, no genotype-related differences in BAL protein existed (Fig. 5A). As compared with genotype-matched, air-exposed controls, O3 significantly increased BAL protein in wild-type and IL-11Rα1-deficient mice regardless of whether BAL was performed on the animals 4 or 24 h after cessation of exposure. Nevertheless, no genotype-related differences in BAL protein were extant between O3-exposed wild-type and IL-11Rα1-deficient mice at either time interval. Similar results were obtained for BAL ciliated epithelial cells (Fig. 5B).
Figure 5.
Concentration of bronchoalveolar lavage (BAL) protein (A) and number of BAL ciliated epithelial cells (B) obtained from wild-type C57BL/6J and mice genetically deficient in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) 4 or 24 h after cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). BAL protein data were analyzed via a Kruskal–Wallis one-way analysis of variance (ANOVA), whereas pairwise post hoc comparisons were calculated using a Wilcoxon rank-sum test. BAL ciliated epithelial cells data were analyzed via a two-way analysis of variance, whereas pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. Each value is expressed as the means ± SE. n = 8 mice for each group. *P < 0.05 compared with wild-type mice exposed to air. &P < 0.05 compared with IL-11Rα1-deficient mice exposed to air.
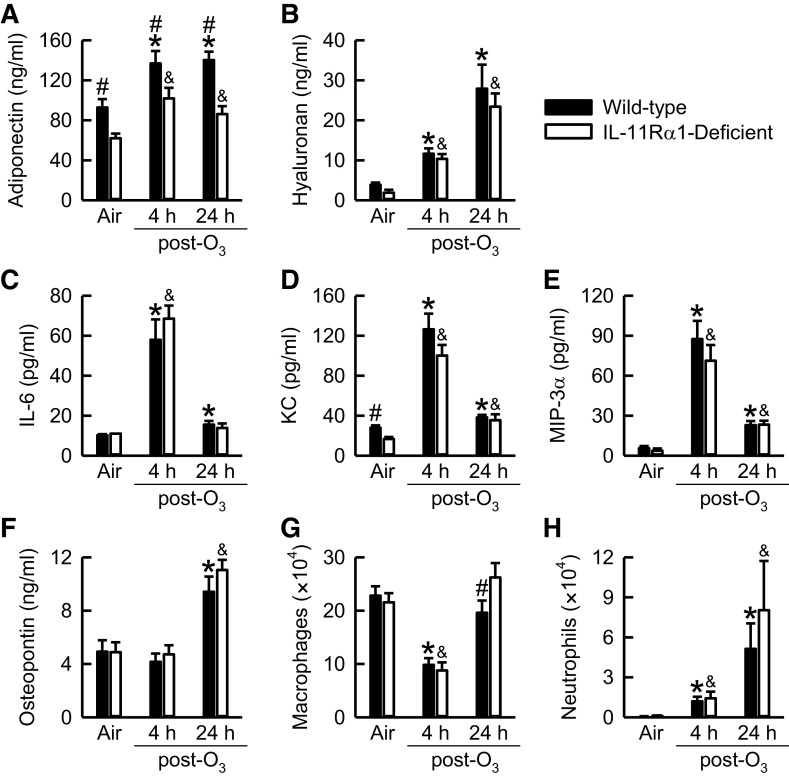
To assess O3-induced lung inflammation, we quantified select indices [BAL cytokines (adiponectin, hyaluronan, IL-6, KC, MIP-3α, and osteopontin) and BAL leukocytes (macrophages and neutrophils)] that contribute to O3-induced lung pathology (49–54, 70, 77–79). Except for adiponectin and KC, which were significantly lower in IL-11Rα1-deficient compared with wild-type mice, no other genotype-related differences in any indices existed after exposure to air (Fig. 6). In wild-type and IL-11Rα1-deficient mice, BAL adiponectin, hyaluronan, IL-6, KC, MIP-3α, and neutrophils were significantly greater, whereas BAL macrophages were significantly lower compared with genotype-matched air-exposed controls 4 h after cessation of exposure to O3. Twenty-four hours after the O3 exposure ceased, BAL adiponectin, hyaluronan, KC, MIP-3α, osteopontin, and neutrophils were significantly greater in O3-exposed wild-type and IL-11Rα-deficient mice compared with genotype-matched air-exposed controls. However, at 4 or 24 h after cessation of exposure to O3, BAL adiponectin was significantly decreased in IL-11Rα1-deficient compared with wild-type mice, whereas BAL macrophages were significantly greater in O3-exposed IL-11Rα1-deficient as compared with wild-type mice 24 h after cessation of exposure (Fig. 6, A and G). Finally, regardless of genotype or exposure, eosinophils and lymphocytes were rarely observed in BAL fluid (data not shown).
Figure 6.
Concentration of bronchoalveolar lavage (BAL; A) adiponectin, (B) hyaluronan, (C) interleukin (IL)-6, (D) keratinocyte chemoattractant (KC), (E) macrophage inflammatory protein (MIP)-3α, and (F) osteopontin in addition to the number of BAL (G) macrophages and (H) neutrophils obtained from wild-type C57BL/6J mice and mice genetically deficient in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) 4 or 24 h after cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). BAL adiponectin, KC, osteopontin, and macrophage data were analyzed via a two-way analysis of variance, whereas pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. BAL hyaluronan, IL-6, MIP-3α, and neutrophil data were analyzed via a Kruskal–Wallis one-way ANOVA, whereas pairwise post hoc comparisons were calculated using a Wilcoxon rank-sum test. Each value is expressed as the means ± SE. n = 7 or 8 mice for each group. *P < 0.05 compared with wild-type mice exposed to air. &P < 0.05 compared with IL-11Rα1-deficient mice exposed to air. #P < 0.05 compared with IL-11Rα1-deficient mice exposed to O3.
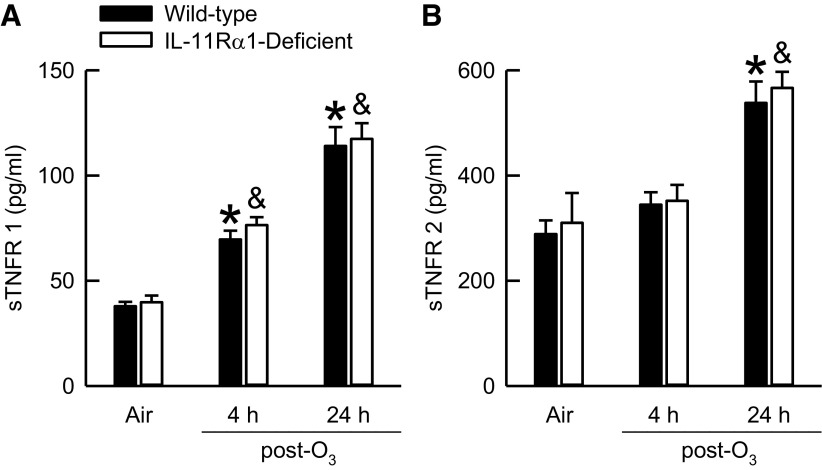
Engagement of TNF-α with either of its single-pass transmembrane receptors, TNF receptor (TNFR) 1 or 2, initiates a myriad of intracellular signaling cascades (80). In addition, both TNFR 1 and 2 are essential for the manifestation of O3-induced AHR (81, 82), and the soluble forms of TNFR 1 and 2, sTNFR 1 and sTNFR 2, respectively, can interfere with the binding of TNF to TNFR 1 or TNFR 2 on the cell surface (80). Furthermore, the administration of recombinant human IL-11 to patients with hematological malignancies increases serum sTNFR 1 (83). Consequently, if IL-11 alters sTNFR 1 or 2 expression, this could potentially impact the development of O3-induced AHR. To that end, we quantified BAL sTNFR 1 and 2. There were no genotype-related differences in BAL sTNFR 1 or 2 in air-exposed mice (Fig. 7). As compared with genotype-matched, air-exposed controls, exposure to O3 significantly increased BAL sTNFR 1 in wild-type and IL-11Rα1-deficient mice 4 or 24 h after cessation of exposure, whereas BAL sTNFR 2 was only increased at the 24-h time interval. In addition, there were no differences in serum sTNFR 1 (1.7 ± 0.2 vs. 1.9 ± 0.2 ng/mL) or sTNFR 2 (5.9 ± 0.5 vs. 6.4 ± 0.6 ng/mL) in air-exposed wild-type and IL-11Rα1-deficient mice, respectively. Thus, it is improbable that sTNFR 1 or 2 contribute to genotype-related differences in O3-induced AHR (Fig. 4).
Figure 7.
Concentration of bronchoalveolar lavage (BAL; A) soluble tumor necrosis factor receptor (sTNFR) 1 and (B) sTNFR 2 obtained from wild-type C57BL/6J mice and mice genetically deficient in the gene encoding Il11ra1 (IL-11Rα1-deficient mice) 4 or 24 h after cessation of a 3-h exposure to either filtered room air (air) or ozone (O3; 2 parts/million). BAL sTNFR 1 data were analyzed via a two-way analysis of variance (ANOVA), whereas pairwise post hoc comparisons were calculated using Fisher’s least significance difference test. BAL sTNFR 2 data were analyzed via a Kruskal–Wallis one-way ANOVA, whereas pairwise post hoc comparisons were calculated using a Wilcoxon rank-sum test. Each value is expressed as the means ± SE. n = 8 mice for each group. *P < 0.05 compared with wild-type mice exposed to air. &P < 0.05 compared with IL-11Rα1-deficient mice exposed to air.
DISCUSSION
Administration of recombinant human IL-11 to wild-type mice via nasal insufflation causes AHR (42). Consistent with this observation, we demonstrate that IL-11Rα1, an indispensable receptor for IL-11 signal transduction (12), is required for maximal airway responsiveness to methacholine after acute inhalation exposure to O3 (Fig. 4). Decreases in airway responsiveness in IL-11Rα1-deficient mice after O3 exposure were not associated with concomitant decreases in BAL hyaluronan, KC, osteopontin, or neutrophils (Fig. 6), each of which contributes to the development of O3-induced AHR (49, 50, 53, 70, 77).
IL-11Rα1 is expressed in multitudinous organs, including the brain, heart, liver, lung, kidney, spleen, and thymus (34), and we confirm this observation, in part, by demonstrating Il11ra1 mRNA is present in the lungs of wild-type C57BL/6J mice exposed to either air or O3 (Fig. 2A). However, O3 did not alter the relative abundance of Il11ra1 mRNA in wild-type mice at either 4 or 24 h after cessation of exposure. Il11ra1 mRNA expression has rarely been quantified in animal models of lung disease, and when it has been, the results are conflicting. For example, Cardó-Vila et al. (84) reported that Il11ra1 mRNA expression is upregulated in cancerous lung tissue of mice, whereas Traber et al. (72) demonstrated that Il11ra1 mRNA is unchanged in mouse lung tissue in the 24-h period after intratracheal instillation of Escherichia coli. In contrast, IL-11 protein levels or Il11 mRNA have been quantified in animal lungs by numerous investigators in the presence or absence of experimental lung disease. Lung IL-11 protein and/or Il11 mRNA expression is increased in rodent models of asthma, idiopathic pulmonary fibrosis, immune complex disease, and infection with Mycobacterium tuberculosis (85–88). However, BAL IL-11 was unaffected by exposure to 100% oxygen for 72 h (89). Consistent with this latter observation, we report that BAL IL-11 is unchanged by inhalation of O3 (Fig. 2B), which analogously causes oxidant-induced lung injury. Nevertheless, we cannot rule out the possibility that IL-11 levels in the lung lining fluid were affected by O3 exposure at time points other than 4 or 24 h after cessation of exposure. We can conclude, however, that the ability of IL-11Rα1 to contribute to AHR in wild-type mice 24 h after cessation of O3 exposure does not require lung Il11ra1 mRNA expression or BAL IL-11 protein levels to be concomitantly altered.
In wild-type and IL-11Rα1-deficient mice, O3 significantly increased responsiveness to methacholine for Raw, G, and H (Fig. 4). However, O3-induced increases in these indices were significantly attenuated in IL-11Rα1-deficient as compared with wild-type mice. Among responses to methacholine for Raw, G, and H, differences in H were the most pronounced between O3-exposed wild-type and IL-11Rα1-deficient mice (Fig. 4C). Increases in H after O3 exposure result from enhanced closure of small airways, which may be a consequence of decreased surfactant activity and/or greater airway constriction (90, 91). O3 deactivates lung surfactant, a phenomenon that causes small airway closure, and thus, increases H (90, 92). Because IL-11 increases surfactant expression (93), the loss of IL-11 signaling in IL-11Rα1-deficient mice would theoretically decrease surfactant expression, increase surface tension in the lung, enhance small airway closure, and subsequently cause greater increases in H in IL-11Rα1-deficient as compared with wild-type mice. However, we observed the exact opposite: IL-11Rα1-deficient mice exhibited decreased responsiveness to methacholine for H. Nevertheless, airway constriction was significantly decreased in IL-11Rα1-deficient as compared with wild-type mice after O3 exposure (Fig. 4A). Therefore, diminished airway constriction in IL-11Rα1-deficient mice may be the reason responsiveness to methacholine for H was attenuated in these animals.
The lung epithelium is a physical barrier that limits diffusion of inhaled, injurious entities (e.g., antigens, microbes, and ultrafine particles) to the subepithelium (94). O3 causes desquamation of the airway epithelium and lung hyperpermeability (61), phenomena that would theoretically increase access of inhaled methacholine to the airway smooth muscle and increase airway responsiveness. However, we found no genotype-related differences in O3-induced increases in BAL protein or in the number of BAL ciliated epithelial cells (Fig. 5), which are indices of lung permeability and epithelial desquamation, respectively. Thus, it is improbable that differences in the availability of methacholine to the airway smooth muscle account for genotype-related differences in airway responsiveness, especially for Raw.
Two signaling elements downstream of IL-11Rα1 could influence the development of O3-induced AHR. First, classic IL-11 signaling requires formation of a heterohexameric complex that consists of two molecules of IL-11, IL-11Rα1, and gp130, and once formed, this complex results in the activation of STAT proteins, including STAT3 (2, 95, 96). STAT3 expressed in airway epithelial cells is required for the development of antigen-induced AHR (97). Therefore, it is conceivable that diminished airway responsiveness in O3-exposed IL-11Rα1-deficient mice results from the loss of STAT3 activation initiated via engagement of IL-11 with IL-11Rα1. However, if abatement of STAT3 activation is the cause of decreased airway responsiveness in O3-exposed IL-11Rα1-deficient mice, insufficient STAT3 activation in airway smooth muscle is an improbable cause for this phenomenon since IL-11-induced STAT3 activation is virtually nonexistent in airway smooth muscle cells (28). Second, IL-11 elicits phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 (96, 98), and impeding ERK 1/2 activation via U0126, a mitogen-activated protein kinase kinase (MEK) inhibitor, reduces antigen-induced AHR (99). Thus, diminished activation of these aforementioned elements within the IL-11, IL-11Rα1, and gp130 signal transduction cascade (i.e., STAT3 AND ERK 1/2) could potentially reduce airway responsiveness in IL-11Rα1-deficient mice after exposure to O3. However, we have also considered that differences in airway responsiveness between O3-exposed IL-11Rα1-deficient and wild-type mice are a result of differences in expression of proinflammatory cytokines.
We and others have demonstrated that O3-induced AHR is mechanistically coupled to several moieties associated with O3-induced lung inflammation, including adiponectin, hyaluronan, KC, neutrophils, and osteopontin (49, 50, 52–54, 70). We found no differences in BAL hyaluronan, KC, osteopontin, or neutrophils between IL-11Rα1-deficient and wild-type mice after O3 exposure, (Fig. 6, B, D, F, and H), which suggests that neither these cytokines nor neutrophils can account for the genotype-related differences in O3-induced AHR. O3 increased BAL adiponectin in both wild-type and IL-11Rα-deficient mice (Fig. 6A). However, BAL adiponectin was significantly decreased in IL-11Rα1-deficient as compared with wild-type mice after O3 exposure. We previously reported that O3-induced AHR was attenuated in mice genetically deficient in adiponectin, a hormone exclusively produced by adipocytes (100), and the locus of this attenuation was the lung parenchyma, which is represented by G and H, and not the central airways, which is represented by Raw (54). Thus, in IL-11Rα1-deficient mice, it is conceivable that reductions in BAL adiponectin, in addition to diminished airway constriction, could account for the significant reduction in methacholine responsiveness for H in these animals after O3 exposure.
IL-11 was originally designated as adipogenesis inhibitory factor because of its capacity to inhibit the differentiation of preadipocytes to adipocytes. However, adiponectin promotes adipogenesis (101, 102) and is produced exclusively in adipocytes and secreted into the circulation (100). Thus, adiponectin found in the epithelial lining fluid of the lung is derived from blood, and our previous data demonstrate that transport of adiponectin from blood to the lungs is facilitated by T-cadherin, an adiponectin binding protein (54). As demonstrated in Fig. 6A, BAL adiponectin was significantly lower in air- or O3-exposed IL-11Rα1-deficient as compared with wild-type mice, and there are two scenarios we have considered to explain this observation. First, in adipocyte-like cells, IL-11 induces phosphorylation of ribosomal protein S6 kinase β-1, also known as p70 S6K (96), which is required for induction of adiponectin expression in white adipose tissue (103). Thus, the loss of p70 S6K phosphorylation in IL-11Rα1-deficient mice could decrease adiponectin expression in adipose tissue, and consequently reduce the amount of adiponectin released into the circulation. Second, it is conceivable that BAL adiponectin levels are decreased in IL-11Rα1-deficient mice because IL-11Rα1 contributes to the transport of adiponectin from blood to the epithelial lining fluid of the lung via regulating T-cadherin expression. Nevertheless, our data do not allow us to distinguish if either of these scenarios is true. Regardless of the precise mechanism, this is the first study to demonstrate that IL-11Rα1 regulates in vivo expression of adiponectin and/or its transit from blood to the epithelial lining fluid of the lung. Finally, because 1) IL-11Rα1 and IL-11 influence expression of adiponectin and leptin, respectively [Fig. 6A and (104)], 2) expression of these hormones in visceral adipose tissue is significantly different between obese asthmatics and obese nonasthmatics (105), and 3) visceral adipose tissue leptin expression is significantly related to AHR (105), it is reasonable to speculate that IL-11 could contribute in some manner to the natural history of obesity-induced asthma.
We previously reported that BAL protein, IL-6, KC, and neutrophils were significantly lower in adiponectin-deficient as compared with wild-type mice after cessation of a 3-h exposure to O3 (2 ppm) (54). However, in this study, despite observing significantly less BAL adiponectin in IL-11Rα1-deficient mice, we observed no genotype-related differences in any of the aforementioned indices 4 or 24 h after cessation of O3 exposure (Figs. 5A and 6, A, D, and H). These data suggest that BAL adiponectin must be decreased to a greater extent or completely absent to significantly impact the development of O3-induced lung inflammation.
Since 1) overexpression of IL11 mRNA in a lung-specific fashion in mice decreases BAL neutrophils in response to oxygen toxicity, 2) administration of a neutralizing antibody against IL-11 reduces neutrophil migration to the air spaces in a mouse model of Escherichia coli pneumonia, and 3) deficiency of IL-11Rα1 decreases BAL eosinophils and IL-13 in antigen sensitized and challenged mice (16, 72, 73), we were surprised that IL-11Rα1 deficiency had only minimal effects on O3-induced lung inflammation (i.e., BAL adiponectin and macrophages). The three aforementioned observations suggest that the contribution of IL-11 and/or IL-11Rα1 to inflammatory responses is stimulus-specific, analogous to IL-6 (51, 106, 107). Alternatively, Kleeberger et al. (108) reported that separate genetic loci control the appearance of polymorphonuclear leukocytes in BAL fluid after acute (2 ppm for 3 h) and subacute (0.3 ppm for 48 h) exposure to O3, and in support of this work, we previously demonstrated that IL-6 and the type I IL-1 receptor minimally contribute to O3-induced lung inflammation after acute O3 exposure (2 ppm for 3 h) but profoundly influence the development of lung inflammation after subacute O3 exposure (0.3 ppm for 72 h) (51, 109). Thus, because IL-6 and IL-11 belong to the same cytokine family (29), it is plausible that IL-11 and/or IL-11Rα1 contribute more extensively to O3-induced lung inflammation after subacute exposure. Furthermore, Smith et al. (110) recently demonstrated that mice from the Collaborative Cross exposed to 0.8 ppm O3 for 4 h per day for 9 days exhibited eosinophilic lung inflammation, airway mucous cell metaplasia, and AHR. Given that IL-11Rα1 deficiency lessens the severity of O3-induced AHR (Fig. 4) and given that IL-11Rα1 deficiency reduces BAL eosinophils and mucus in antigen-sensitized and challenged mice (16), it is not unreasonable to speculate that IL-11Rα1 deficiency could reduce all of the aforementioned pathological features induced by repeated exposure to O3 described by Smith et al. (110).
As a criteria pollutant, the United States Environmental Protection Agency is required to establish primary and secondary National Ambient Air Quality Standards for O3, and as of 2020, both standards are 0.070 ppm (111, 112). However, in this study, mice were exposed to a concentration of O3 (2 ppm) whose environmental or occupational existence is quite inconceivable. In laboratory experiments assessing the respiratory effects of O3 on human subjects, participants are moderately or strenuously exercising while exposed to O3 concentrations significantly less (0.12−0.4 ppm) than 2 ppm (113–115). Since exercise increases minute ventilation (V̇e) and since the dose of O3 delivered to the lungs is the product of O3 concentration, exposure time, and V̇e (116), exercising humans inhale a greater dose of O3 than their resting counterparts. In contrast to humans, wild-type C57BL/6J mice exhibit prolonged sedentariness and profound decreases in V̇e during exposure to O3 (82). Consequently, based on differences in V̇e among these species during O3 exposure, the dose of O3 delivered to the lungs is presumably different when humans and mice are exposed to the same O3 concentration for the same length of time. Consistent with this hypothesis, Hatch et al. (115) reported that the dose of O3 delivered to the lungs of exercising humans and sedentary rodents is dissimilar when exposed to O3 (0.4 ppm) for 2 h. In fact, for humans and rodents to exhibit equivalent degrees of O3-induced lung pathology, Hatch et al. (115) demonstrated that rodents must be exposed to an O3 concentration five times greater than humans (2 ppm as compared with 0.4 ppm, respectively). Therefore, in this study, we exposed mice to 2 ppm O3 because it causes a similar degree of lung injury and lung inflammation in human subjects exposed to lower and more environmentally relevant concentrations of O3.
PERSPECTIVES AND SIGNIFICANCE
In conclusion, we demonstrate that BAL adiponectin and increases in airway responsiveness to methacholine are significantly attenuated in IL-11Rα1-deficient as compared with wild-type mice after acute inhalation exposure to O3. Since adiponectin contributes to the development of O3-induced AHR (54), decreases in BAL adiponectin in IL-11Rα1-deficient mice could be mechanistically coupled to the attenuation of O3-induced AHR in these animals. Regardless of the role of adiponectin, this is the first study to demonstrate that IL-11Rα1 is essential for maximal increases in airway responsiveness to methacholine after exposure to O3, a nonatopic asthma stimulus.
DATA AVAILABILITY
Raw data used to calculate means and standard errors of the means presented in Table 1, Figs. 2–7, and the text are publicly available on the NIOSH Data and Statistics Gateway under Data set Number RD-1047–2022-0.
GRANTS
The research reported in this publication was supported, in part, by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Nos. R03ES022378 (to R.A.J.) and R03ES024883 (to R.A.J.) and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award No. R03AI107432 (to R.A.J.).
DISCLOSURES
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Furthermore, the content contained within this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
R.A.J. and I.U.H. conceived and designed research; R.A.J., C.L.A., S.R.S., W.T.J., N.C.M., C.Y.S., and A.W.P.4th. performed experiments; R.A.J., S.R.S., W.T.J., N.C.M., A.W.P.4th., and M.L.K. analyzed data; R.A.J. and M.L.K. interpreted results of experiments; R.A.J. and A.W.P.4th. prepared figures; R.A.J. drafted manuscript; R.A.J., C.L.A., S.R.S., W.T.J., N.C.M., C.Y.S., A.W.P.4th., M.L.K., and I.U.H. edited and revised manuscript; R.A.J., C.L.A., S.R.S., W.T.J., N.C.M., C.Y.S., A.W.P.4th., M.L.K., and I.U.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Brendan J. Jenkins (Hudson Institute of Medical Research; Clayton, Victoria, Australia) and Dr. Trevelyan R. Menheniott (Murdoch Children’s Research Institute; Parkville, Victoria, Australia) for providing us with genotyping protocols for IL-11Rα1-deficient mice.
REFERENCES
- 1. Paul SR, Bennett F, Calvetti JA, Kelleher K, Wood CR, O'Hara RM Jr, Leary AC, Sibley B, Clark SC, Williams DA. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA 87: 7512–7516, 1990. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton VA, Hall MA, Hudson KR, Heath JK. Interleukin-11 signals through the formation of a hexameric receptor complex. J Biol Chem 275: 36197–36203, 2000. doi: 10.1074/jbc.M004648200. [DOI] [PubMed] [Google Scholar]
- 3. Zheng T, Nathanson MH, Elias JA. Histamine augments cytokine-stimulated IL-11 production by human lung fibroblasts. J Immunol 153: 4742–4752, 1994. [PubMed] [Google Scholar]
- 4. Minshall E, Chakir J, Laviolette M, Molet S, Zhu Z, Olivenstein R, Elias JA, Hamid Q. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol 105: 232–238, 2000. doi: 10.1016/s0091-6749(00)90070-8. [DOI] [PubMed] [Google Scholar]
- 5. Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol Lung Cell Mol Physiol 273: L648–L655, 1997. doi: 10.1152/ajplung.1997.273.3.L648. [DOI] [PubMed] [Google Scholar]
- 6. Maier R, Ganu V, Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem 268: 21527–21532, 1993. [PubMed] [Google Scholar]
- 7. Elias JA, Tang W, Horowitz MC. Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology 136: 489–498, 1995. doi: 10.1210/endo.136.2.7835281. [DOI] [PubMed] [Google Scholar]
- 8. Suen Y, Chang M, Lee SM, Buzby JS, Cairo MS. Regulation of interleukin-11 protein and mRNA expression in neonatal and adult fibroblasts and endothelial cells. Blood 84: 4125–4134, 1994. doi: 10.1182/blood.V84.12.4125.bloodjournal84124125. [DOI] [PubMed] [Google Scholar]
- 9. Chen HF, Lin CY, Chao KH, Wu MY, Yang YS, Ho HN. Defective production of interleukin-11 by decidua and chorionic villi in human anembryonic pregnancy. J Clin Endocrinol Metab 87: 2320–2328, 2002. doi: 10.1210/jcem.87.5.8478. [DOI] [PubMed] [Google Scholar]
- 10. Du X, Everett ET, Wang G, Lee WH, Yang Z, Williams DA. Murine interleukin-11 (IL-11) is expressed at high levels in the hippocampus and expression is developmentally regulated in the testis. J Cell Physiol 168: 362–372, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 11. Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C 3rd, Flavell RA, Elias JA. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest 98: 2845–2853, 1996. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood 89: 3897–3908, 1997. doi: 10.1182/blood.V89.11.3897. [DOI] [PubMed] [Google Scholar]
- 13. Winship AL, Van Sinderen M, Donoghue J, Rainczuk K, Dimitriadis E. Targeting Interleukin-11 receptor-α impairs human endometrial cancer cell proliferation and invasion in vitro and reduces tumor growth and metastasis in vivo. Mol Cancer Ther 15: 720–730, 2016. doi: 10.1158/1535-7163.MCT-15-0677. [DOI] [PubMed] [Google Scholar]
- 14. Keller DC, Du XX, Srour EF, Hoffman R, Williams DA. Interleukin-11 inhibits adipogenesis and stimulates myelopoiesis in human long-term marrow cultures. Blood 82: 1428–1435, 1993. doi: 10.1182/blood.V82.5.1428.1428. [DOI] [PubMed] [Google Scholar]
- 15. Marwood M, Visser K, Salamonsen LA, Dimitriadis E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology 150: 2915–2923, 2009. doi: 10.1210/en.2008-1538. [DOI] [PubMed] [Google Scholar]
- 16. Lee CG, Hartl D, Matsuura H, Dunlop FM, Scotney PD, Fabri LJ, Nash AD, Chen NY, Tang CY, Chen Q, Homer RJ, Baca M, Elias JA. Endogenous IL-11 signaling is essential in Th2- and IL-13-induced inflammation and mucus production. Am J Respir Cell Mol Biol 39: 739–746, 2008. doi: 10.1165/rcmb.2008-0053OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liongue C, Ward AC. Evolution of Class I cytokine receptors. BMC Evol Biol 7: 120, 2007. doi: 10.1186/1471-2148-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putoczki T, Ernst M. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J Leukoc Biol 88: 1109–1117, 2010. doi: 10.1189/jlb.0410226. [DOI] [PubMed] [Google Scholar]
- 19. Tenney R, Turnbull JR, Stansfield KA, Pekala PH. The regulation of adipocyte metabolism and gene expression by interleukin-11. Adv Enzyme Regul 43: 153–166, 2003. doi: 10.1016/s0065-2571(02)00036-5. [DOI] [PubMed] [Google Scholar]
- 20. Linjawi S, Li TC, Tuckerman EM, Blakemore AI, Laird SM. Expression of interleukin-11 receptor α and interleukin-11 protein in the endometrium of normal fertile women and women with recurrent miscarriage. J Reprod Immunol 64: 145–155, 2004. doi: 10.1016/j.jri.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci 26: 12174–12185, 2006. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weich NS, Wang A, Fitzgerald M, Neben TY, Donaldson D, Giannotti J, Yetz-Aldape J, Leven RM, Turner KJ. Recombinant human interleukin-11 directly promotes megakaryocytopoiesis in vitro. Blood 90: 3893–3902, 1997. doi: 10.1182/blood.V90.10.3893. [DOI] [PubMed] [Google Scholar]
- 23. Bozza M, Bliss JL, Dorner AJ, Trepicchio WL. Interleukin-11 modulates Th1/Th2 cytokine production from activated CD4+ T cells. J Interferon Cytokine Res 21: 21–30, 2001. doi: 10.1089/107999001459123. [DOI] [PubMed] [Google Scholar]
- 24. Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T, Hilton DJ, Suda T, Ng KW, Martin TJ. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med 183: 2581–2591, 1996. doi: 10.1084/jem.183.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D'Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, Tripathi M, Lim SY, Shekeran SG, Chothani SP, Rabes A, Sombetzki M, Bruinstroop E, Min LP, Sinha RA, Albani S, Yen PM, Schafer S, Cook SA. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of non-alcoholic steatohepatitis. Gastroenterology 157: 777–792.e14, 2019. doi: 10.1053/j.gastro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 26. Kiessling S, Muller-Newen G, Leeb SN, Hausmann M, Rath HC, Strater J, Spottl T, Schlottmann K, Grossmann J, Montero-Julian FA, Scholmerich J, Andus T, Buschauer A, Heinrich PC, Rogler G. Functional expression of the interleukin-11 receptor α-chain and evidence of antiapoptotic effects in human colonic epithelial cells. J Biol Chem 279: 10304–10315, 2004. doi: 10.1074/jbc.M312757200. [DOI] [PubMed] [Google Scholar]
- 27. Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med 4: 303–308, 1998. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 28. Lahiri T, Laporte JD, Moore PE, Panettieri RA Jr, Shore SA. Interleukin-6 family cytokines: signaling and effects in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280: L1225–L1232, 2001. doi: 10.1152/ajplung.2001.280.6.L1225. [DOI] [PubMed] [Google Scholar]
- 29. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15: 797–819, 1997. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 30. Lokau J, Nitz R, Agthe M, Monhasery N, Aparicio-Siegmund S, Schumacher N, Wolf J, Möller-Hackbarth K, Waetzig GH, Grötzinger J, Müller-Newen G, Rose-John S, Scheller J, Garbers C. Proteolytic cleavage governs interleukin-11 trans-signaling. Cell Rep 14: 1761–1773, 2016. doi: 10.1016/j.celrep.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 31. Lokau J, Agthe M, Garbers C. Generation of soluble interleukin-11 and interleukin-6 receptors: a crucial function for proteases during inflammation. Mediators Inflamm 2016: 1785021, 2016. doi: 10.1155/2016/1785021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hilton DJ, Hilton AA, Raicevic A, Rakar S, Harrison-Smith M, Gough NM, Begley CG, Metcalf D, Nicola NA, Willson TA. Cloning of a murine IL-11 receptor alpha-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J 13: 4765–4775, 1994. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nandurkar HH, Hilton DJ, Nathan P, Willson T, Nicola N, Begley CG. The human IL-11 receptor requires gp130 for signalling: demonstration by molecular cloning of the receptor. Oncogene 12: 585–593, 1996. [PubMed] [Google Scholar]
- 34. Bilinski P, Hall MA, Neuhaus H, Gissel C, Heath JK, Gossler A. Two differentially expressed interleukin-11 receptor genes in the mouse genome. Biochem J 320: 359–363, 1996. doi: 10.1042/bj3200359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robb L, Hilton DJ, Willson TA, Begley CG. Structural analysis of the gene encoding the murine interleukin-11 receptor alpha-chain and a related locus. J Biol Chem 271: 13754–13761, 1996. doi: 10.1074/jbc.271.23.13754. [DOI] [PubMed] [Google Scholar]
- 36. Robb L, Hilton DJ, Brook-Carter PT, Begley CG. Identification of a second murine interleukin-11 receptor α-chain gene (IL11Ra2) with a restricted pattern of expression. Genomics 40: 387–394, 1997. doi: 10.1006/geno.1996.4579. [DOI] [PubMed] [Google Scholar]
- 37. Karow J, Hudson KR, Hall MA, Vernallis AB, Taylor JA, Gossler A, Heath JK. Mediation of interleukin-11-dependent biological responses by a soluble form of the interleukin-11 receptor. Biochem J 318: 489–495, 1996. doi: 10.1042/bj3180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arm JP, O'Hickey SP, Hawksworth RJ, Fong CY, Crea AE, Spur BW, Lee TH. Asthmatic airways have a disproportionate hyperresponsiveness to LTE4, as compared with normal airways, but not to LTC4, LTD4, methacholine, and histamine. Am Rev Respir Dis 142: 1112–1118, 1990. doi: 10.1164/ajrccm/142.5.1112. [DOI] [PubMed] [Google Scholar]
- 39.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (Online). https://ginasthma.org/. [Accessed 26 August 2022]
- 40. Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164: S28–S38, 2001. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 41. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 56: 219–233, 2019. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Einarsson O, Geba GP, Panuska JR, Zhu Z, Landry M, Elias JA. Asthma-associated viruses specifically induce lung stromal cells to produce interleukin-11, a mediator of airways hyperreactivity. Chest 107: 132S–133S, 1995. doi: 10.1378/chest.107.3_supplement.132s. [DOI] [PubMed] [Google Scholar]
- 43. Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets 9: 485–494, 2008. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 44. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716–725, 2012. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 45. Loureiro CC, Amaral L, Ferreira JA, Lima R, Pardal C, Fernandes I, Semedo L, Arrobas A. Omalizumab for severe asthma: beyond allergic asthma. Biomed Res Int 2018: 3254094, 2018. doi: 10.1155/2018/3254094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anenberg SC, Henze DK, Tinney V, Kinney PL, Raich W, Fann N, Malley CS, Roman H, Lamsal L, Duncan B, Martin RV, van Donkelaar A, Brauer M, Doherty R, Jonson JE, Davila Y, Sudo K, Kuylenstierna JCI. Estimates of the global burden of ambient PM2.5, Ozone, and NO2 on asthma incidence and emergency room visits. Environ Health Perspect 126: 107004, 2018. doi: 10.1289/EHP3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Croze ML, Zimmer L. Ozone atmospheric pollution and alzheimer's disease: from epidemiological facts to molecular mechanisms. J Alzheimers Dis 62: 503–522, 2018. doi: 10.3233/JAD-170857. [DOI] [PubMed] [Google Scholar]
- 48. Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest 47: 412–426, 1982. [PubMed] [Google Scholar]
- 49. Barreno RX, Richards JB, Schneider DJ, Cromar KR, Nadas AJ, Hernandez CB, Hallberg LM, Price RE, Hashmi SS, Blackburn MR, Haque IU, Johnston RA. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol 305: L118–L129, 2013. doi: 10.1152/ajplung.00080.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 288: L61–L67, 2005. doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- 51. Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 288: L390–L397, 2005. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- 52. Garantziotis S, Hollingsworth JW. Comment on expression of concern: TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 196: 249–250, 2017. doi: 10.1164/rccm.1962comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [Erratum in J Biol Chem 291: 19257–19258, 2016]. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 54. Zhu M, Hug C, Kasahara DI, Johnston RA, Williams AS, Verbout NG, Si H, Jastrab J, Srivastava A, Williams ES, Ranscht B, Shore SA. Impact of adiponectin deficiency on pulmonary responses to acute ozone exposure in mice. Am J Respir Cell Mol Biol 43: 487–497, 2010. doi: 10.1165/rcmb.2009-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nandurkar HH, Robb L, Tarlinton D, Barnett L, Köntgen F, Begley CG. Adult mice with targeted mutation of the interleukin-11 receptor (IL11Ra) display normal hematopoiesis. Blood 90: 2148–2159, 1997. [PubMed] [Google Scholar]
- 56. Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29: 52, 54, 2000. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 57. Razvi SS, Richards JB, Malik F, Cromar KR, Price RE, Bell CS, Weng T, Atkins CL, Spencer CY, Cockerill KJ, Alexander AL, Blackburn MR, Alcorn JL, Haque IU, Johnston RA. Resistin deficiency in mice has no effect on pulmonary responses induced by acute ozone exposure. Am J Physiol Lung Cell Mol Physiol 309: L1174–L1185, 2015. doi: 10.1152/ajplung.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol 31: 69–77, 2004. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- 59. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60. Kraemer N, Neubert G, Issa L, Ninnemann O, Seiler AE, Kaindl AM. Reference genes in the developing murine brain and in differentiating embryonic stem cells. Neurol Res 34: 664–668, 2012. doi: 10.1179/1743132812Y.0000000060. [DOI] [PubMed] [Google Scholar]
- 61. Malik F, Cromar KR, Atkins CL, Price RE, Jackson WT, Siddiqui SR, Spencer CY, Mitchell NC, Haque IU, Johnston RA. Chemokine (C-C Motif) Receptor-Like 2 is not essential for lung injury, lung inflammation, or airway hyperresponsiveness induced by acute exposure to ozone. Physiol Rep 5: e13545, 2017. doi: 10.14814/phy2.13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hartney JM, Robichaud A. Assessment of airway hyperresponsiveness in mouse models of allergic lung disease using detailed measurements of respiratory mechanics. Methods Mol Biol 1032: 205–217, 2013. doi: 10.1007/978-1-62703-496-8_16. [DOI] [PubMed] [Google Scholar]
- 63. Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964. doi: 10.1152/jappl.1964.19.1.97. [DOI] [PubMed] [Google Scholar]
- 64. Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 72: 168–178, 1992. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 65. Hirai T, McKeown KA, Gomes RF, Bates JH. Effects of lung volume on lung and chest wall mechanics in rats. J Appl Physiol (1985) 86: 16–21, 1999. doi: 10.1152/jappl.1999.86.1.16. [DOI] [PubMed] [Google Scholar]
- 66. Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-α increases lung tissue hysteresivity in transgenic mice. J Appl Physiol (1985) 91: 2730–2734, 2001. doi: 10.1152/jappl.2001.91.6.2730. [DOI] [PubMed] [Google Scholar]
- 67. Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia 13: 1307–1315, 1999. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- 68. Kuhn C 3rd, Homer RJ, Zhu Z, Ward N, Flavell RA, Geba GP, Elias JA. Airway hyperresponsiveness and airway obstruction in transgenic mice. Morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J Respir Cell Mol Biol 22: 289–295, 2000. doi: 10.1165/ajrcmb.22.3.3690. [DOI] [PubMed] [Google Scholar]
- 69. Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis 121: 389–413, 1980. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- 70. DeLorme MP, Yang H, Elbon-Copp C, Gao X, Barraclough-Mitchell H, Bassett DJ. Hyperresponsive airways correlate with lung tissue inflammatory cell changes in ozone-exposed rats. J Toxicol Environ Health A 65: 1453–1470, 2002. doi: 10.1080/00984100290071432. [DOI] [PubMed] [Google Scholar]
- 71. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med 205: 385–393, 2008. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Traber KE, Dimbo EL, Symer EM, Korkmaz FT, Jones MR, Mizgerd JP, Quinton LJ. Roles of interleukin-11 during acute bacterial pneumonia. PLoS One 14: e0221029, 2019. doi: 10.1371/journal.pone.0221029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 101: 1970–1982, 1998. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ng B, Widjaja AA, Viswanathan S, Dong J, Chothani SP, Lim S, Shekeran SG, Tan J, McGregor NE, Walker EC, Sims NA, Schafer S, Cook SA. Similarities and differences between IL11 and IL11RA1 knockout mice for lung fibro-inflammation, fertility and craniosynostosis. Sci Rep 11: 14088, 2021. doi: 10.1038/s41598-021-93623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bhalla DK, Mannix RC, Kleinman MT, Crocker TT. Relative permeability of nasal, tracheal, and bronchoalveolar mucosa to macromolecules in rats exposed to ozone. J Toxicol Environ Health 17: 269–283, 1986. doi: 10.1080/15287398609530822. [DOI] [PubMed] [Google Scholar]
- 76. Scheel LD, Dobrogorski OJ, Mountain JT, Svirbely JL, Stokinger HE. Physiologic, biochemical, immunologic and pathologic changes following ozone exposure. J Appl Physiol 14: 67–80, 1959. doi: 10.1152/jappl.1959.14.1.67. [DOI] [PubMed] [Google Scholar]
- 77. Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 291: 19257–19258, 2016. doi: 10.1074/jbc.A116.802400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mathews JA, Williams AS, Brand JD, Wurmbrand AP, Chen L, Ninin FM, Si H, Kasahara DI, Shore SA. γδ T cells are required for pulmonary IL-17A expression after ozone exposure in mice: role of TNFα. PLoS One 9: e97707, 2014. doi: 10.1371/journal.pone.0097707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am J Respir Cell Mol Biol 13: 125–132, 1995. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- 80. Wajant H, Siegmund D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front Cell Dev Biol 7: 91, 2019. doi: 10.3389/fcell.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-α receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- 82. Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001. doi: 10.1164/ajrccm.164.4.2001016. [DOI] [PubMed] [Google Scholar]
- 83. Ellis M, Hedstrom U, Frampton C, Alizadeh H, Kristensen J, Shammas FV, Al-Ramadi BK. Modulation of the systemic inflammatory response by recombinant human interleukin-11: a prospective randomized placebo controlled clinical study in patients with hematological malignancy. Clin Immunol 120: 129–137, 2006. doi: 10.1016/j.clim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 84. Cardó-Vila M, Marchiò S, Sato M, Staquicini FI, Smith TL, Bronk JK, Yin G, Zurita AJ, Sun M, Behrens C, Sidman RL, Lee JJ, Hong WK, Wistuba II, Arap W, Pasqualini R. Interleukin-11 receptor is a candidate target for ligand-directed therapy in lung cancer: analysis of clinical samples and BMTP-11 preclinical activity. Am J Pathol 186: 2162–2170, 2016. doi: 10.1016/j.ajpath.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kapina MA, Shepelkova GS, Avdeenko VG, Guseva AN, Kondratieva TK, Evstifeev VV, Apt AS. Interleukin-11 drives early lung inflammation during Mycobacterium tuberculosis infection in genetically susceptible mice. PLoS One 6: e21878, 2011. doi: 10.1371/journal.pone.0021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Jin SM, Lee YC. Cysteinyl leukotriene upregulates IL-11 expression in allergic airway disease of mice. J Allergy Clin Immunol 119: 141–149, 2007. doi: 10.1016/j.jaci.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 87. Lentsch AB, Crouch LD, Jordan JA, Czermak BJ, Yun EC, Guo R, Sarma V, Diehl KM, Ward PA. Regulatory effects of interleukin-11 during acute lung inflammatory injury. J Leukoc Biol 66: 151–157, 1999. [PubMed] [Google Scholar]
- 88. Ng B, Dong J, D'Agostino G, Viswanathan S, Widjaja AA, Lim WW, Ko NSJ, Tan J, Chothani SP, Huang B, Xie C, Pua CJ, Chacko AM, Guimaraes-Camboa N, Evans SM, Byrne AJ, Maher TM, Liang J, Jiang D, Noble PW, Schafer S, Cook SA. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med 11: eaaw1237, 2019. doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 89. Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol 178: 4993–5000, 2007. doi: 10.4049/jimmunol.178.8.4993. [DOI] [PubMed] [Google Scholar]
- 90. Heil M, Hazel AL, Smith JA. The mechanics of airway closure. Respir Physiol Neurobiol 163: 214–221, 2008. doi: 10.1016/j.resp.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 91. Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol (1985) 102: 221–230, 2007. doi: 10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]
- 92. Podgórski A, Sosnowski TR, Gradoń L. Deactivation of the pulmonary surfactant dynamics by toxic aerosols and gases. J Aerosol Med 14: 455–466, 2001. doi: 10.1089/08942680152744668. [DOI] [PubMed] [Google Scholar]
- 93. Yan C, Naltner A, Martin M, Naltner M, Fangman JM, Gurel O. Transcriptional stimulation of the surfactant protein B gene by STAT3 in respiratory epithelial cells. J Biol Chem 277: 10967–10972, 2002. doi: 10.1074/jbc.M109986200. [DOI] [PubMed] [Google Scholar]
- 94. Brune K, Frank J, Schwingshackl A, Finigan J, Sidhaye VK. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol 308: L731–L745, 2015. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Putoczki TL, Ernst M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy 7: 441–453, 2015. doi: 10.2217/imt.15.17. [DOI] [PubMed] [Google Scholar]
- 96. Tenney R, Stansfield K, Pekala PH. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol 202: 160–166, 2005. doi: 10.1002/jcp.20100. [DOI] [PubMed] [Google Scholar]
- 97. Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol 178: 6191–6199, 2007. doi: 10.4049/jimmunol.178.10.6191. [DOI] [PubMed] [Google Scholar]
- 98. Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, Fujio Y, Azuma J. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 38: 107–115, 2007. doi: 10.1016/j.cyto.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 99. Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol 172: 7053–7059, 2004. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 100. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 101. Ohsumi J, Miyadai K, Kawashima I, Ishikawa-Ohsumi H, Sakakibara S, Mita-Honjo K, Takiguchi Y. Adipogenesis inhibitory factor. A novel inhibitory regulator of adipose conversion in bone marrow. FEBS Lett 288: 13–16, 1991. doi: 10.1016/0014-5793(91)80991-b. [DOI] [PubMed] [Google Scholar]
- 102. Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46: 1369–1379, 2005. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 103. Yi SA, Jeon YJ, Lee MG, Nam KH, Ann S, Lee J, Han JW. S6K1 controls adiponectin expression by inducing a transcriptional switch: BMAL1-to-EZH2. Exp Mol Med 54: 324–333, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Granowitz EV. Transforming growth factor-beta enhances and pro-inflammatory cytokines inhibit ob gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 240: 382–385, 1997. doi: 10.1006/bbrc.1997.7663. [DOI] [PubMed] [Google Scholar]
- 105. Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 186: 598–605, 2012. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang J, Homer RJ, Chen Q, Elias JA. Endogenous and exogenous IL-6 inhibit aeroallergen-induced Th2 inflammation. J Immunol 165: 4051–4061, 2000. doi: 10.4049/jimmunol.165.7.4051. [DOI] [PubMed] [Google Scholar]
- 107. Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 22: 535–542, 2000. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 108. Kleeberger SR, Levitt RC, Zhang LY. Susceptibility to ozone-induced inflammation. II. Separate loci control responses to acute and subacute exposures. Am J Physiol Lung Cell Mol Physiol 264: L21–26, 1993. doi: 10.1152/ajplung.1993.264.1.L21. [DOI] [PubMed] [Google Scholar]
- 109. Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 37: 477–484, 2007. doi: 10.1165/rcmb.2006-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Smith GJ, Tovar A, McFadden K, Moran TP, Wagner JG, Harkema JR, Kelada SNP. A murine model of ozone-induced nonatopic asthma from the collaborative cross. Am J Respir Cell Mol Biol 65: 672–674, 2021. doi: 10.1165/rcmb.2020-0577LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.United States Environmental Protection Agency. Criteria Air Pollutants (Online). https://www.epa.gov/criteria-air-pollutants. [Accessed 26 August 2022]
- 112.Environmental Protection Agency. Review of the ozone national ambient air quality standards. Fed Reg 85: 87256–87351, 2020. [Google Scholar]
- 113. Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol (1985) 111: 679–687, 2011. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J Appl Physiol (1985) 89: 1804–1810, 2000. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- 115. Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med 150: 676–683, 1994. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 116. Wiester MJ, Williams TB, King ME, Ménache MG, Miller FJ. Ozone uptake in awake Sprague-Dawley rats. Toxicol Appl Pharmacol 89: 429–437, 1987. doi: 10.1016/0041-008x(87)90162-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data used to calculate means and standard errors of the means presented in Table 1, Figs. 2–7, and the text are publicly available on the NIOSH Data and Statistics Gateway under Data set Number RD-1047–2022-0.