Abstract
Considerable evidence has implicated Streptococcus pneumoniae neuraminidase in the pathogenesis of otitis media (OM); however, its exact role has not been conclusively established. Recently, an S. pneumoniae neuraminidase-deficient mutant, ΔNA1, has been constructed by insertion-duplication mutagenesis of the nanA gene of S. pneumoniae strain D39. The relative ability of ΔNA1 and the D39 parent strain to colonize the nasopharynx and to induce OM subsequent to intranasal inoculation and to survive in the middle ear cleft after direct challenge of the middle ear were evaluated in the chinchilla model. Nasopharyngeal colonization data indicate a significant difference in the ability of the ΔNA1 mutant to colonize as well as to persist in the nasopharynx. The neuraminidase-deficient mutant was eliminated from the nasopharynx 2 weeks earlier than the D39 parent strain. Both the parent and the mutant exhibited similar virulence levels and kinetics during the first week after direct inoculation of the middle ear. The ΔNA1 neuraminidase-deficient mutant, however, was then completely eliminated from the middle ear by day 10 postchallenge, 11 days before the D39 parent strain. Data from this study indicate that products of the nanA gene have an impact on the ability of S. pneumoniae to colonize and persist in the nasopharynx as well as the middle ear.
Streptococcus pneumoniae is one of the primary bacterial pathogens associated with otitis media (OM) and accounts for approximately 30% of all cases of this disease (11).
Neuraminidase is an enzyme which cleaves N-acetylneuraminic acid from mucin, glycolipids, glycoproteins, and oligosaccharides on host cell surfaces. Although a precise role for S. pneumoniae neuraminidase in the pathogenesis of S. pneumoniae-caused diseases has not been established, it has been proposed that neuraminidase could enhance colonization by decreasing the viscosity of mucus (13) or by exposing cell surface receptors for S. pneumoniae (1, 7, 8). All clinical S. pneumoniae isolates examined to date have been shown to produce neuraminidase (6). Production of neuraminidase is believed to contribute to the poor prognosis for pneumococcal meningitis (12). Moreover, S. pneumoniae neuraminidase has been detected in 78% of culture-positive middle ear effusions from patients with acute OM and in 96% of S. pneumoniae-positive middle ear effusions from patients with chronic OM (4). Two recent reports from our laboratory indicate that during S. pneumoniae-induced OM in the chinchilla model, terminal sialic acid residues are removed from the epithelial surface lining the lumen of the eustachian tube, presumably as a result of S. pneumoniae neuraminidase production (7, 8). Similar data have been derived clinically from adenoidal tissue obtained from children with chronic OM with effusion. Adenoids colonized with S. pneumoniae demonstrated removal of sialic acid and exposure of N-acetylglycosamine (9). The result is exposure of GlcNAcβ1-4Gal, which is part of one of the eukaryotic receptors of S. pneumoniae (1). Moreover, we have recently demonstrated that the treatment of chinchilla tracheas with neuraminidase in vitro increases S. pneumoniae adherence and reverses the inhibitory effects of lacto-N-neotetraose (LNnT), suggesting that neuraminidase treatment results in an increase in the number of available receptors for S. pneumoniae (16).
Two S. pneumoniae neuraminidase genes, nanA and nanB, have been cloned and sequenced previously (2, 3), and a neuraminidase-deficient isogenic mutant has been constructed by insertion-duplication mutagenesis of the nanA gene (17). This neuraminidase-deficient mutant is a valuable tool for studying the definitive role of S. pneumoniae neuraminidase in the pathogenesis of S. pneumoniae infections. The purpose of this study was to compare the virulence of the parent strain with that of the neuraminidase-deficient mutant in the chinchilla model of OM in order to better define the role of S. pneumoniae neuraminidase in the entire spectrum of pathogenesis of this disease.
Bacterial strains and preparation of inocula.
S. pneumoniae serotype 2 strain D39 (NCTC 7466) and strain ΔNA1, an isogenic derivative of D39 deficient in neuraminidase, kindly provided by T. J. Mitchell, Division of Infection and Immunity, University of Glasgow, Glasgow, United Kingdom, were used for these experiments. ΔNA1 is a new derivative of D39, deficient in neuraminidase and constructed by insertion-duplication mutagenesis of the nanA gene. It makes no detectable neuraminidase in vitro (17). Both strains have been described in detail previously (17). In order to maintain selection pressure, erythromycin (1 μg/ml) was added to the blood agar when ΔNA1 was cultured. Following growth on plates, colonies were transferred to Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) containing erythromycin (5 μg/ml). Prior to inoculation of the chinchillas, both strains were passaged once in Swiss mice as previously described (17). Approximately four colonies from the mouse-passaged culture were grown on blood agar and transferred to Todd-Hewitt broth. After an overnight incubation at 37°C, the cultures were centrifuged at 3,500 × g for 20 min, washed twice with and resuspended in Dulbecco's phosphate-buffered saline, and used for the various inoculations described below. The S. pneumoniae concentration in the inoculum was confirmed by standard colony plate count.
Intranasal (i.n.) challenge, assessment of nasopharyngeal (NP) colonization, and invasion of the middle ear.
Chinchillas (Chinchilla lanigera) (400 to 600 g), free of middle ear diseases as determined by otoscopy and tympanometry, were used in these studies. Two cohorts of 27 and 21 chinchillas each were inoculated i.n. with 0.5 ml of a suspension containing approximately 107 CFU of S. pneumoniae D39 or ΔNA1 bacteria per ml. Three chinchillas from each cohort, preselected and randomized, were evaluated by tympanocentesis and NP lavage at 4.5 h and on days 1, 3, 5, 7, 10, and 14 postinoculation with D39 or ΔNA1 as previously described (15). Additional chinchillas were evaluated on days 21 and 28 postinoculation with D39. Tympanocentesis was performed on both ears of the chinchillas by aspiration with a tuberculin syringe fitted with a 25-gauge needle. If no middle ear fluid (MEF) was present, the bullas were lavaged with 0.5 ml of sterile saline. Subsequent to tympanocentesis, NP lavage was performed on each chinchilla as described previously (15). Chinchillas were not subjected to repeat tympanocentesis or nasal lavage. Tympanocentesis and bulla lavage were always performed before NP lavage to prevent contamination of the middle ear. The MEF or lavage sample and nasal lavage sample were cultured on blood agar and incubated overnight at 37°C in a humidified atmosphere with 5% CO2, and the number of CFU per milliliter was determined by standard dilution and plate counting. All assessments were made blindly by the same observer throughout this study. The experiment was repeated once.
Transbullar (TB) middle ear challenge and induction of OM.
Injection of an inoculum through the superior aspect of the cephalid bulla directly into the middle ear of the chinchilla, referred to as TB inoculation, is the method most widely used to induce S. pneumoniae OM. The TB route allows for the collection of sufficient volumes of effusions and bulla washes and leaves the tympanic membrane intact. i.n. inoculation of the chinchilla with S. pneumoniae strains alone typically induces only inconsistent tympanic membrane inflammation and culture-positive OM with MEF (5). To specifically assess the role of neuraminidase in the multiplication of, persistence of, induction of disease by, and clearance of S. pneumoniae from the middle ear cleft, a comparison of the survival of D39 and ΔNA1 in the middle ear was performed. The inoculum was prepared as described above, and approximately 5 × 103 CFU of either D39 or ΔNA1 bacteria per ear was injected into the left middle ear of 30 chinchillas. Three chinchillas were preselected randomly from the D39- and ΔNA1-inoculated cohorts, and MEFs were aspirated by means of epitympanic taps of the inferior bulla on days 4, 7, 10, 14, and 21 postchallenge. If no MEFs were present, the bullas were lavaged with sterile saline. Standard dilution and plate counting were used to determine the CFU per milliliter of MEF or bulla lavage sample. All assessments were made blindly by the same observers throughout this study. The experiment was repeated twice.
Phenotypic analysis.
Organisms recovered from NP lavage samples and MEF or middle ear lavage samples of chinchillas were tested for erythromycin resistance by the standard macrodilution broth method. For neuraminidase activity assays, representative colonies were inoculated into 10 ml of brain heart infusion with or without erythromycin (5 μg/ml) and incubated in a humidified atmosphere with 5% CO2 overnight at 37°C. The bacterial cells were harvested and measured for neuraminidase activity as described by Winter et al. (17). Assays were performed in duplicate on two separate occasions.
Statistical analysis.
Data are expressed as means ± standard errors from at least duplicate experiments. Differences of S. pneumoniae concentration in nasal lavage samples between the D39- and the ΔNA1-inoculated cohorts were analyzed by the Mann-Whitney rank sum test. The Student t test was used to compare the differences in middle ear pressure between these two cohorts. A P value of <0.05 was accepted as the minimal level of significance.
Effect of nanA gene disruption on NP colonization.
The relative ability of the parent and the neuraminidase-deficient mutant to colonize and persist in the nasopharynx for up to 28 days post-i.n. challenge is shown in Fig. 1. There was no significant difference in the concentration of the parent or mutant in the first lavage sample obtained at 4.5 h postinoculation. However, by 24 h a statistically significant reduction in the concentration of the ΔNA1 mutant, compared with the D39 parent, was evident and persisted up to day 10, at which time the ΔNA1 S. pneumoniae bacteria were eliminated from five of the six chinchillas sampled at this time point. By day 14, no ΔNA1 S. pneumoniae bacteria were present in the lavage samples. Throughout the duration of the experiment, the D39 parent persisted in the nasopharynx at a significantly higher concentration than did the mutant, exhibited a gradual but steady decline, and colonized the nasopharynx for approximately 14 days beyond the time when the neuraminidase-deficient mutant was eliminated. A total of five chinchillas from the D39-inoculated cohort and two from the ΔNA1-inoculated cohort developed OM with culture-positive MEFs.
FIG. 1.
NP colonization dynamics in chinchillas challenged i.n. with parent strain S. pneumoniae D39 (●) or the ΔNA1 neuraminidase-deficient mutant (■). Each data point represents the geometric mean number of CFU of S. pneumoniae bacteria ± the standard error of the mean per milliliter of nasal lavage fluid from a total of six animals combined from two separate experiments. ∗, P < 0.05, and ∗∗, P < 0.01, compared to the D39-inoculated group.
Effect of nanA gene disruption on induction of OM subsequent to TB inoculation.
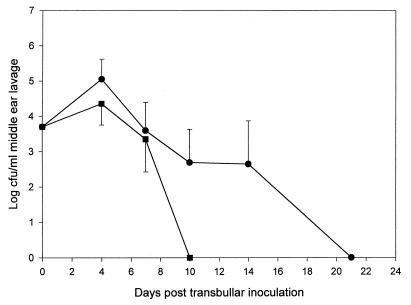
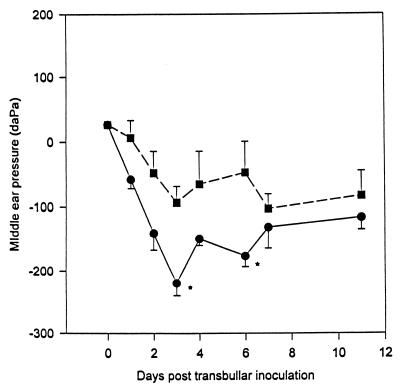
A marked difference in the virulence and survival of the ΔNA1 mutant compared to those of the D39 parent was also evident subsequent to TB inoculation but after a lag period of over 7 days. The dose required to induce disease was the same for both strains. Moreover, multiplication and persistence of the parent and mutant in the middle ear were almost identical for up to 7 days postinoculation (Fig. 2). However, by day 10 ΔNA1 was completely eliminated from the middle ear, whereas the D39 parent routinely persisted in the middle ear of the TB-inoculated cohort for an additional 11 days. This experiment was repeated twice, and each time the ΔNA1 mutant was not recovered in the middle ears beyond 10 days. The ΔNA1 neuraminidase-deficient mutant also demonstrated a reduced ability to induce negative middle ear pressure changes compared with that of the D39 parent subsequent to TB challenge (Fig. 3). Normal chinchilla middle ear pressure was considered to be between −60 and +40 daPa (13). Both the parent and mutant exhibited declines in middle ear pressure, which peaked by day 3 postinoculation. However, the D39 parent induced a statistically significant decline in middle ear pressure on day 3 and day 6 post-TB challenge compared to that for the ΔNA1 mutant.
FIG. 2.
Survival of parent strain D39 (●) and the ΔNA1 mutant (■) in the middle ear of chinchillas inoculated TB with approximately 3 × 105 CFU of S. pneumoniae bacteria. Each data point represents the geometric mean number of CFU of S. pneumoniae bacteria ± standard error of the mean per milliliter of MEF from a total of nine animals combined from three separate experiments.
FIG. 3.
Comparison of mean middle ear pressures (± standard errors of the means) between the D39 parent (●) and the ΔNA1 neuraminidase-deficient mutant (■) post-TB challenge as determined by tympanometry over an 11-day observation period. Values below −60 daPa are considered abnormal for the chinchilla (17). ∗, P < 0.05, compared to the ΔNA1-inoculated group.
Phenotypes of D39 and ΔNA1.
ΔNA1 recovered from the NP lavage samples and MEFs was erythromycin resistant (MIC > 32 μg/ml), while D39 from those fluids was erythromycin sensitive (MIC < 1 μg/ml). Neuraminidase activity for ΔNA1 was below the lower limit of detection of the assay (0.5 mU/μg of cell protein), while D39 expressed 12.9 mU/μg of cell protein.
The data presented here demonstrate an alteration in the virulence of S. pneumoniae subsequent to mutation of the nanA gene.
A prior investigation into the role of neuraminidase in the guinea pig model for meningitis conducted by Winter and colleagues, who developed the ΔNA1 isogenic derivative of D39, indicated no clear pathogenic role of neuraminidase in the guinea pig model of meningitis (17). In the present study, however, the extent and duration of NP colonization and the induction of negative middle ear pressure, as well as the survival and persistence of S. pneumoniae in the middle ear, were altered significantly by disruption of the nanA gene. It is noteworthy that the impact of the disruption on virulence appears variable depending on the anatomical niche involved. Whereas differences in NP colonization between the parent and mutant were apparent at 24 h, differences in survival in the middle ear manifested themselves after a lag period of 7 days before the mutant was rapidly eliminated from the middle ear. In both instances, however, the altered virulence of the neuraminidase-deficient mutant was evident by its elimination by a minimum of 2 weeks from NP colonization and 11 days from the middle ear before the parent strain.
The underlying mechanisms responsible for the induction of middle ear negative pressure have not been defined; however, data from the present study indicate a significant difference in middle ear pressure at a time when the concentration of each strain in the MEF is indistinguishable. S. pneumoniae neuraminidase may affect middle ear pressure by disrupting the eustachian tube function, a key determinant in the maintenance of normal middle ear pressure. Previous data from our laboratory indicate that sialic acid residues are removed from the eustachian tube during S. pneumoniae OM in the chinchilla (7, 8). The extent of this disruption of the carbohydrate surface structure and the surfactant-like substances known to be required for normal eustachian tube function has not been elucidated.
Although we could not detect any neuraminidase activity from ΔNA1 NP or middle ear isolates in vitro, one could speculate that residual neuraminidase activity, derived from the nanB gene, may have been produced in vivo to compensate for the lack of nanA gene products. Berry et al. cite unpublished data which indicate that NanA-deficient mutants have residual enzyme activity because of the production of NanB (2). The fact that we could not detect ΔNA1 neuraminidase activity in our in vitro assay, from either the inoculum or the ΔNA1 isolated from the chinchilla middle ears and nasal lavage samples, suggests that this might not be the case but does not rule out the possibility of nanB neuraminidase production in vivo. Our data confirm those of Winter et al., who developed ΔNA1 and also did not detect any residual neuraminidase activity from this strain in vitro. The contribution of nanB to the pathogenesis of OM requires the development of appropriate mutants. It is intriguing, however, that Berry and coworkers have suggested that the production of two distinct neuraminidases may assist exploitation and invasion of distinct anatomical niches by S. pneumoniae (2).
It is not clear what sequence of events triggers the abrupt elimination of ΔNA1 from the middle ear. The immune response induced by each strain needs to be evaluated and might also provide an explanation for the abrupt elimination of the ΔNA1 mutant by day 10, a time when specific, induced humoral responses against invading pathogens typically begin to manifest themselves. Lock et al. determined that a significant portion of NanA, unlike NanB, remains cell associated as a surface-expressed protein antigen readily available for interaction with the host's immune defense system (10). Neuraminidase may be capable of interfering with immune responses and may delay seroconversion in chinchillas challenged with the D39 parent strain.
In conclusion, the data from the present study indicate that disruption of nanA, which renders S. pneumoniae ΔNA1 neuraminidase deficient, diminishes the ability of S. pneumoniae to colonize and persist in the chinchilla nasopharynx and middle ear.
Acknowledgments
This study was supported, in part, by a grant from the NIDCD/NIH (R01 DC03105-03).
We gratefully acknowledge Timothy Mitchell for providing S. pneumoniae D39 and ΔNA1. We thank Lisa Routt for manuscript preparation.
REFERENCES
- 1.Andersson B, Dahmen J, Frejd T, Leffler H, Magnusson G, Noori G, Eden C S. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983;158:559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry A M, Lock R A, Paton J C. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J Bacteriol. 1996;178:4854–4860. doi: 10.1128/jb.178.16.4854-4860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camara M, Boulnois G J, Andrew P W, Mitchell T J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diven W F, Doyle W J, Vietmeier B. Hydrolytic enzymes in otitis media pathogenesis. Ann Otol Rhinol Laryngol Suppl. 1988;132:6–9. doi: 10.1177/00034894880970s303. [DOI] [PubMed] [Google Scholar]
- 5.Giebink G S. Studies of Streptococcus pneumoniae and influenza virus vaccines in the chinchilla otitis media model. Pediatr Infect Dis J. 1989;8:S42–S44. [PubMed] [Google Scholar]
- 6.Kelly R T, Farmer S, Greiff D. Neuraminidase activities of clinical isolates of Diplococcus pneumoniae. J Bacteriol. 1967;94:272–273. doi: 10.1128/jb.94.1.272-273.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder T E, Daniels R L, Lim D J, DeMaria T F. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb Pathog. 1994;16:435–441. doi: 10.1006/mpat.1994.1043. [DOI] [PubMed] [Google Scholar]
- 8.Linder T E, Lim D J, DeMaria T F. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb Pathog. 1992;13:293–303. doi: 10.1016/0882-4010(92)90039-q. [DOI] [PubMed] [Google Scholar]
- 9.Linder T E, Marder H P, Munzinger J. Role of adenoids in the pathogenesis of otitis media: a bacteriologic and immunohistochemical analysis. Ann Otol Rhinol Laryngol. 1997;106:619–623. doi: 10.1177/000348949710600801. [DOI] [PubMed] [Google Scholar]
- 10.Lock R A, Paton J C, Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumonia. Microb Pathog. 1988;5:461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 11.Luotonen J, Herva E, Karma P, Timonen M, Leinonen M, Makela P H. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13:177–183. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- 12.O'Toole R D, Goode L, Howe C. Neuraminidase activity in bacterial meningitis. J Clin Investig. 1971;50:979–985. doi: 10.1172/JCI106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenfeld R M, Doyle W J, Swarts J D, Seroky J, Pinero B P. Third-generation cephalosporins in the treatment of acute pneumococcal otitis media. An animal study. Arch Otolaryngol Head Neck Surg. 1992;118:49–52. doi: 10.1001/archotol.1992.01880010053015. [DOI] [PubMed] [Google Scholar]
- 14.Scanlon K L, Diven W F, Glew R H. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme. 1989;41:143–150. doi: 10.1159/000469069. [DOI] [PubMed] [Google Scholar]
- 15.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong H H, McIver M A, Fisher L M, DeMaria T F. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb Pathog. 1999;26:111–119. doi: 10.1006/mpat.1998.0257. [DOI] [PubMed] [Google Scholar]
- 17.Winter A J, Comis S D, Osborne M P, Tarlow M J, Stephen J, Andrew P W, Hill J, Mitchell T J. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect Immun. 1997;65:4411–4418. doi: 10.1128/iai.65.11.4411-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]