Précis:
The iStent Infinite Trabecular Micro-Bypass System implanted in patients with open angle glaucoma (OAG) (uncontrolled by prior surgical or medical therapy) was effective in reducing mean diurnal intraocular pressure with a favorable safety profile.
Purpose:
The purpose of this study is to evaluate safety and effectiveness of the iStent infinite Trabecular Micro-Bypass System in patients with OAG uncontrolled by prior surgical or medical therapy.
Design:
Prospective, multicenter, single-arm, open-label clinical trial.
Methods:
Implantation of iStent infinite (3 iStent inject W stents) was performed as a stand-alone surgical procedure in eyes with OAG uncontrolled by prior incisional or cilioablative surgeries or maximum tolerated medical therapy (MTMT). Prospectively declared effectiveness endpoints were proportion of eyes achieving ≥20% mean diurnal intraocular pressure (MDIOP) reduction from baseline at month 12 on the same or fewer intraocular pressure (IOP)-lowering medication classes (responder endpoint) and mean change in MDIOP from baseline at month 12. Safety parameters included visual acuity, slit-lamp and fundus examinations, gonioscopy, perimetry, surgical complications, and adverse events.
Results:
Seventy-two eyes of 72 patients (mean age 71.9 y) with preoperative mean medicated MDIOP of 23.4±2.8 mm Hg on a mean of 3.1±0.9 IOP-lowering medication classes were enrolled: 61 eyes with failed prior surgery/ies (Failed-Surgery subgroup) and 11 eyes uncontrolled on MTMT (MTMT subgroup). A total of 76.1% of all enrolled patients met the responder endpoint (73.4% Failed-Surgery, 90.9% MTMT), with mean reduction (SE) in MDIOP at month 12 of 5.9(0.6) mm Hg [5.5(0.7) mm Hg Failed-Surgery subgroup, 8.1(0.9) mm Hg MTMT subgroup]. For patients on the same or fewer medication(s) as baseline, 53.0% achieved ≥30% MDIOP reduction without surgical interventions/other events. Safety was favorable, with no explants, infection, or device-related interventions or hypotony.
Conclusions:
iStent infinite stand-alone surgery achieved clinically significant IOP reduction and favorable safety in patients with OAG uncontrolled by prior therapy.
Key Words: microinvasive glaucoma surgery, MIGS, glaucoma, iStent inject, trabecular micro-bypass, IOP
Glaucoma is the leading cause of permanent blindness worldwide, with prevalence projected to increase over time.1,2 Currently available therapies for open angle glaucoma (OAG) focus on reducing intraocular pressure (IOP), established as the only proven modifiable risk factor in disease progression.3–5 To achieve IOP reduction, 1 or a combination of different treatments may be used, from early intervention with topical IOP-lowering medications and laser treatment to traditional filtration procedures such as trabeculectomy or tube shunt implantation for patients not well controlled on IOP-lowering medications or laser procedures. As disease progresses, escalation toward the more invasive end of the treatment spectrum is often necessary.
Although various types of IOP-lowering medications have been proven safe and efficacious for lowering IOP, their effectiveness and utility in the long-term management of OAG may be limited by poor patient compliance, ocular surface disease in part because of preservative exposure, local and systemic side effects, and diminished quality of life.6–10 Selective laser trabeculoplasty (SLT) is also frequently used as an early intervention; however, the IOP-lowering effect of SLT may wane over time, and additional treatment is often required to attain the IOP target.11,12 For cases with inadequate response to topical IOP-lowering medication and laser-based therapies, traditional bleb-forming or newer subconjunctival filtration surgeries can dramatically reduce IOP. However, these surgeries have both early and lifelong, rare but serious, risks of sight-threatening adverse events (AEs) such as endophthalmitis, hypotony maculopathy, massive choroidal effusion, suprachoroidal hemorrhage, corneal decompensation, double vision, rapid cataract progression, and bleb-related complications.13
Microinvasive glaucoma surgery (MIGS) has emerged as a class of procedures enabling earlier surgical intervention in the treatment of OAG. In addition to effective IOP lowering, this class of procedures aims to address challenges associated with medication adherence and ocular surface disease by reducing medication burden, while avoiding many of the risks of more invasive filtration procedures.14,15 A recent real-world head-to-head comparison of trabeculectomy versus MIGS with the placement of 2 or 3 trabecular micro-bypass stents highlighted this benefit-to-risk balance, with significantly higher safety-adjusted treatment success in multistent eyes than in trabeculectomy eyes.16
The MIGS category covers a relatively wide range of procedures and devices that have varying levels of invasiveness and consequent risks. Goniotomies have been performed in a variety of ways (eg, Kahook dual blade, trabectome, OMNI, and gonioscopy-assisted transluminal trabeculotomy) that remove or incise the trabecular meshwork and inner wall of Schlemm canal to reduce the resistance to aqueous outflow. Trabecular bypass devices retain virtually all of the inner wall of Schlemm canal, but still are able to reduce the resistance to aqueous outflow. To date, the MIGS devices with the largest evidence base (commercial longevity and number of peer-reviewed publications) are the iStent Trabecular Micro-Bypass System (first-generation iStent) and iStent inject Trabecular Micro-Bypass System (second generation iStent) (Glaukos Corp., Aliso Viejo). A third trabecular bypass device approved in the United States is the Hydrus Microstent (Alcon). In the United States, all 3 devices are indicated for use in mild to moderate OAG in combination with cataract surgery, supported by randomized controlled pivotal trials demonstrating effective IOP lowering and substantial medication reduction;17–19 in the case of iStent and iStent inject, overall safety parameters in these trials were shown to be comparable to cataract surgery alone.17,18 Beyond the pivotal trials evaluating combined procedures for mild to moderate primary OAG, multiple additional studies have shown that implantation of iStent and iStent inject can effectively and safely reduce IOP and medication burden in both combined (with phacoemulsification) and stand-alone (without phacoemulsification) surgery.20–32 The evidence also demonstrates an incremental increase in IOP-reducing and medication-reducing effect with a higher number of iStents (1 vs. 2 vs. 3)20,33–39 and suggests a specific incremental benefit of 3 over 2 stents.16
Subconjunctival bleb-associated surgeries, which bypass the eye’s natural drainage system and create a filtering bleb (eg, trabeculectomy, tube shunt implantation, XEN Gel Stent implantation, Preserflo), may be considered when greater IOP lowering is required. Trabeculectomy and tube shunt implantation, frequently referred to as the traditional filtration procedures, can produce substantial IOP reduction, but they also carry rare but significant safety risks, which have been shown to increase even further over time.13,40–42
The XEN Glaucoma Treatment System (AbbVie Inc.) was introduced as a less invasive ab interno bleb-forming treatment option. The pivotal trial for the XEN Gel Stent-enrolled OAG eyes inadequately controlled on maximum tolerated medical therapy (MTMT) or by prior surgical procedures,43 with 76.3% of eyes achieving ≥20% IOP reduction and an average of 6.4 mm Hg of IOP reduction from baseline at 12 months. As XEN implantation requires bleb formation, associated complications (eg, bleb needling in 32.3% of eyes, hypotony (defined as IOP <6 mm Hg) in 24.6%, and IOP spikes in 21.5% in the XEN pivotal trial) are similar to traditional bleb-associated filtering glaucoma surgeries.43 In addition, 11% of the Xen stents in the pivotal trial needed to be explanted. Therefore, there remains a need for microinvasive, bleb-free devices that limit such safety concerns while also maintaining substantial IOP-lowering effect and reduction of medication-based treatment burden.
The iStent infinite Trabecular Micro-Bypass System (Glaukos Corp.) aims to extend the benefits of the safety profile and effective IOP lowering of trabecular micro-bypass stenting to achieve effective IOP lowering when implanted in a stand-alone surgical procedure in OAG patients inadequately controlled by prior surgical procedures or MTMT. iStent infinite consists of 3 microscale wide-flange (iStent inject W) stents on a single preloaded injector. The stents are designed to be implanted ab interno in 3 separate areas (each ~2 clock-hours apart) over the nasal 4 clock-hours of the trabecular meshwork, creating a patent bypass through the trabecular meshwork into Schlemm canal to increase physiological aqueous outflow and reduce IOP. Trabecular bypass has been shown angiographically to reactivate formerly dormant outflow networks, thus demonstrating the utility of trabecular bypass even in eyes thought to have compromised downstream function.44 It also preserves the native angle architecture, preventing disruption of the peristaltic function of Schlemm canal in aiding aqueous outflow.45 Furthermore, each additional stent incrementally increases the treatment effect.16,33–39 By targeting physiological trabecular outflow, the procedure aims to avoid the risks associated with bleb-forming, subconjunctival, or suprachoroidal procedures; and does not require tissue or angle destruction, disruption of the blood-aqueous barrier, or conjunctival manipulation that could hinder future successful glaucoma interventions if needed.
The present pivotal trial was a 12-month multicenter prospective study evaluating the safety and effectiveness of the iStent infinite Trabecular Micro-Bypass System administered via a stand-alone procedure to OAG patients who had failed prior intervention(s) with filtering or cilioablative surgery and/or who were on MTMT.
METHODS
Trial Design and Endpoints
This prospective, multicenter, single-arm, open-label pivotal trial evaluated the 12-month safety and effectiveness of iStent infinite implanted as a stand-alone procedure in OAG patients whose glaucoma was inadequately controlled by prior surgical procedures or MTMT. Key elements of this clinical trial design were based on the Food and Drug Administration (FDA) Guidance on Premarket Studies of Implantable Minimally Invasive Glaucoma Devices (December 2015) and the ANSI Z80.27-2014 (American National Standard for Ophthalmics-Implantable Glaucoma Devices) Standard for Implantable Glaucoma Devices. The trial was conducted under Investigational Device Exemption G180001 and with Institutional Review Board approval; and it followed the tenets of the Declaration of Helsinki, including written informed consent of all patients. The trial was registered with the National Library of Medicine (clinicaltrials.gov, NCT03639870).
The trial had 2 effectiveness endpoints: the proportion of patients achieving a ≥20% reduction from baseline in mean diurnal IOP (MDIOP) at 12 months while remaining on the same or fewer medication classes and without safety events (responder effectiveness endpoint); and the mean change from baseline in MDIOP at 12 months (IOP change from baseline endpoint). Safety events that resulted in nonresponder classification included (1) hypotony (IOP <6 mm Hg) associated with clinically significant findings, (2) loss of light perception, (3) IOP-related secondary surgical interventions (SSIs), (4) cyclodialysis cleft, and/or (5) no stents visible.
IOP measurements were taken by Goldmann applanation using a standard 2-person method common in glaucoma studies;4 MDIOP was calculated as the mean of 3 individual IOP measurements on the same day (at ~8:00 am, 12:00 pm, and 4:00 pm), and was completed at the baseline and month 12 visits.
Safety parameters included intraoperative complications, postoperative AEs, best spectacle-corrected visual acuity (BSCVA), gonioscopy, central corneal pachymetry, visual field testing, and slit-lamp and fundus examinations. Trial visits consisted of preoperative Screening and Baseline examinations, and postoperative day 1, week 1, month 1, month 3, month 6, month 9, and month 12 examinations. Patients were not washed-out of medications at preoperative or postoperative examinations.
Trial Participants
The trial was designed to enroll patients across ~19 investigational sites to ensure a minimum of 50 evaluable eyes at 12 months postoperatively. Inclusion criteria were a diagnosis of OAG (primary OAG, pseudoexfoliative glaucoma, or pigmentary glaucoma); and failed or inadequate response to prior OAG therapy. Inadequate response to prior OAG therapy was defined as glaucoma that was (1) uncontrolled by medical therapy and one or more conventional incisional or cilioablative procedures (eg, trabeculectomy, tube implantation, cryotherapy, and cyclodiode therapy) (these patients comprised the Failed-Surgery subgroup) and/or (2) unresponsive to MTMT (≥4 classes of topical IOP-lowering medications, or fewer in cases of intolerance or contraindications) (these patients comprised the MTMT subgroup). The study design was predicated on the prior FDA-approved trial of the Xen stent in a similar study population and in accordance with ANSI guidelines for refractory glaucoma.
Additional inclusion criteria included open angle configuration (Shaffer grade ≥3), normal angle anatomy, and absence of ocular inflammation, corneal opacities or edema, peripheral anterior synechia, or any other angle abnormalities that could impair stent visualization and placement. Patients could be either phakic (with no expectation of cataract surgery within 1 year) or pseudophakic (with posterior chamber intraocular lens). Preoperative-medicated IOP at the Screening and Baseline visits was required to be ≥20 and ≤35 mm Hg. Patients were required to have visual field defects and optic nerve abnormalities consistent with glaucoma, and visual acuity of light perception or better in the study eye at screening. Key exclusion criteria included traumatic, uveitic, neovascular, or angle closure glaucoma; glaucoma associated with vascular disorders; active corneal dystrophy, inflammation, or surgery that could alter IOP measurement; and use of systemic medications that could cause an increase in IOP. Of note, enrollment into the trial was not restricted by disease severity (mild, moderate, or severe) nor by level of visual field loss. To date, it has not been determined whether eyes with different stages of glaucoma severity respond differently to trabecular bypass microstent surgery.
Study Device
The iStent infinite Trabecular Micro-Bypass System is a sterile, single-use injector system preloaded with 3 heparin-coated, implant-grade titanium wide-flange stents (iStent inject W) (Figs. 1A, B). The length of each microscale stent is 360 microns, and the width of the rear flange is 360 microns. Despite its microscopic size, the stent design has been designed and proven to allow the same outflow rate as aqueous humor production by the human body (average 2.5 µL/min).35,36 The thorax, located midway along the device, retains the stent within the trabecular meshwork, whereas 4 lateral outlet lumens on the head of the device facilitate multidirectional aqueous outflow from the anterior chamber into Schlemm canal. Once implanted, the 3 stents occupy ~3% of the angle, leaving the remaining 97% of angle tissue untouched. Three stents are placed over at least 4 clock hours of the angle, providing access to up to 240 degrees of collector channels for aqueous outflow.46
FIGURE 1.
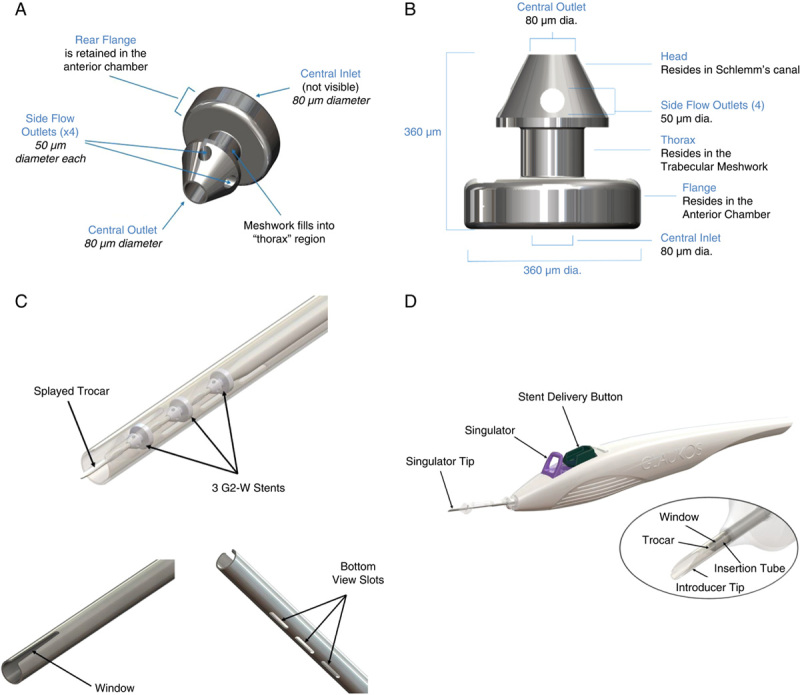
A, iStent infinite Stent (iStent inject W model). B, iStent infinite Stent dimensions. C, iStent infinite injector distal end. D, iStent infinite injector design.
Surgical Technique
The iStent infinite implantation procedure followed the same approach as for iStent inject, which was described in detail previously.18 iStent infinite implantation was composed of the following steps: through a single temporal peripheral clear corneal incision, after filling the anterior chamber with viscoelastic, the iStent infinite injector was advanced under direct gonioscopy to the nasal trabecular meshwork, where the first stent was implanted through the meshwork into Schlemm canal. The injector tip was then repositioned to implant the second stent ~2 clock-hours away from the first stent. The third stent was then implanted using the same procedure as the previous stents, placing the implant ~2 clock-hours away from either of the first 2 stents. To ensure optimal positioning and angle of approach, the surgeon may choose to exit the eye and reposition after the first or second stent implant. After insertion of the 3 stents, proper placement and seating in the trabecular meshwork were confirmed under intraoperative gonioscopy. Viscoelastic was then removed and sealing of the corneal incision was ensured. After surgery in this clinical trial, patients were prescribed topical antibiotic and topical anti-inflammatory medication (nonsteroidal anti-inflammatory drug) for 1 week. Topical steroids were not used routinely because (1) there was minimal surgical trauma, with only mild and transient postoperative inflammation; and (2) to avoid a steroid response with IOP elevation. All the investigators were experienced MIGS surgeons familiar with intraoperative gonioscopy and placement of MIGS devices.
Statistical Analysis
Outcomes were analyzed according to 3 separate population cohorts. First, the intent-to-treat (ITT) population consisted of all patients where an attempt was made to implant 1 or more stents; this population was used for the primary effectiveness analyses (responder effectiveness endpoint, IOP change from baseline endpoint) and the associated sensitivity analyses. The responder endpoint was summarized using frequency counts and percentages, and the IOP change from baseline endpoint was summarized using mean, SD, median, and range. Both endpoints utilized multiple imputation methodology for missing 12-month data and presented 2-sided 95% CIs (about the proportion of responders and the mean IOP change from baseline). For both effectiveness endpoints, the sensitivity analyses imputed missing 12-month data using: worst postoperative IOP and last available medication classes, assumption of treatment failure, and exclusion from cohort.
Second, the outcomes were assessed in the per protocol population, defined as the subset of the ITT population that excludes those patients without the month 12 examination and patients with a major protocol deviation expected to impact the effectiveness endpoints. Third, safety parameters were assessed in the safety population, which was defined as patients implanted with at least 1 stent.
Sample Size Calculation
Sample size was determined in accordance with the ANSI Z80.27-2014 and the FDA Guidance Document entitled “Aqueous Shunts—510(k) Submissions,” issued November 16, 1998, which recommended inclusion of a minimum of 50 evaluable investigational device patients at 12 months.
RESULTS
Trial Population and Accountability
A total of 72 patients were enrolled from 15 sites in this trial. All patients were implanted with the iStent infinite System and received 3 stents. At the 12-month postoperative visit, 71 patients were available for analysis (1 patient had died because of respiratory failure before the month 12 visit). As noted previously, outcomes were analyzed according to the 3 separate population cohorts. The ITT population included 72 patients (100%). The per protocol population consisted of 69 patients, as 1 patient died because of respiratory failure before month 12, and 2 major protocol deviations were reported (glaucoma secondary to elevated episcleral venous pressure in 1 patient, and impaired visualization due to arcus senilis, corneal striae, and external marker location-dye during surgery in another patient). The safety population consisted of 72 patients (100%) and matched the ITT population (n=72, 100%), as all patients received 3 stents in this trial.
Demographics and Preoperative Ocular Characteristics
Demographics and preoperative characteristics are provided in Tables 1 and 2, respectively. There were 6 phakic and 66 pseudophakic eyes enrolled. Patients had a mean IOP-lowering medication burden of 3.1 medications, a mean of 2.3 prior glaucoma surgeries, and average visual field mean deviation (VF MD) of −14.4 dB. Mild, moderate, and severe patients were enrolled in the trial, representing a range of baseline disease severity: 31 patients (43.1%) had VF MD of −12–0 dB, 16 patients (22.2%) had VF MD of −20 to >−12 dB, and 25 patients (34.7%) had VF MD scores worse than −20 dB, including 5 subjects with visual fields unable to be measured because of severity of glaucomatous damage (Table 2).
TABLE 1.
Preoperative Demographics: Overall Trial Population, Failed-Surgery Subgroup, MTMT Subgroup*
| Parameter | Overall Trial Population N=72 | Failed-Surgery Subgroup N=61 | MTMT Subgroup N=11 |
|---|---|---|---|
| Age (y) | |||
| Mean (SD) | 71.9 (8.42) | 71.7 (8.14) | 72.9 (10.19) |
| Median (range) | 72.0 (49–89) | 71.0 (49–88) | 75.0 (54–89) |
| Sex, n (%) | |||
| Male | 35 (48.6) | 28 (45.9) | 7 (63.6) |
| Female | 37 (51.4) | 33 (54.1) | 4 (36.4) |
| Race, n (%) | |||
| White | 45 (62.5) | 37 (60.7) | 8 (72.7) |
| Black | 15 (20.8) | 15 (24.6) | 0 (0.0) |
| Asian | 9 (12.5) | 6 (9.8) | 3 (27.3) |
| Not reported | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 13 (18.1) | 11 (18.0) | 2 (18.2) |
| Not Hispanic or Latino | 59 (81.9) | 50 (82.0) | 9 (81.8) |
| Study eye, n (%) | |||
| Oculus dextra (right eye) | 38 (52.8) | 35 (57.4) | 3 (27.3) |
| Oculus sinistra (left eye) | 34 (47.2) | 26 (42.6) | 8 (72.7) |
Data from Intent-to-Treat population for all groups.
MTMT indicates maximum tolerated medical therapy.
TABLE 2.
Preoperative Ocular Characteristics: Overall Trial Population, Failed-Surgery Subgroup, MTMT Subgroup*
| Parameter | Overall Trial Population N=72 | Failed-Surgery Subgroup N=61 | MTMT Subgroup N=11 |
|---|---|---|---|
| Visual field mean deviation (MD) at screening (dB) | |||
| Mean | −14.44 | −15.10 | −10.67 |
| SD | 8.64 | 8.56 | 8.56 |
| Visual field MD at screening†, n (%) | |||
| VF MD −12 to 0 dB | 31 (43.1) | 24 (39.3) | 7 (63.6) |
| VF MD −20 to worse than −12 dB | 16 (22.2) | 15 (24.6) | 1 (9.1) |
| VF MD worse than −20 dB | 25 (34.7) | 18 (29.5) | 2 (18.2) |
| Shaffer angle grade at screening, n (%) | |||
| III (≥25 and <35) | 24 (33.3) | 21 (34.4) | 3 (27.3) |
| IV (≥35) | 48 (66.7) | 40 (65.6) | 8 (72.7) |
| Glaucoma treatment history at screening, n (%) | |||
| Primary open angle glaucoma | 55 (76.4) | 55 (90.2) | 10 (90.9) |
| Pseudoexfoliative glaucoma | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Pigmentary glaucoma | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Glaucoma secondary to elevated episcleral venous pressure | 1 (1.4) | 0 | 1 (9.1) |
| Lens status, n (%) | |||
| Phakic | 6 (8.3) | 3 (4.9) | 3 (27.3) |
| Pseudophakic | 66 (91.7) | 58 (95.1) | 8 (72.7) |
| Diurnal medicated IOP at baseline (mm Hg) | |||
| Mean (SD) | 23.4 (2.8) | 23.5 (2.8) | 22.8 (2.6) |
| Range | 20–35 | 20–35 | 20–29 |
| No. ocular hypotensive medication classes at baseline, n (%) | |||
| 1–2 | 19 (26.4) | 19 (31.1) | 0 (0.0) |
| 3 | 26 (36.1) | 26 (42.6) | 0 (0.0) |
| ≥4 | 27 (37.5) | 16 (26.2) | 11 (100.0) |
| Mean (SD) | 3.1 (0.9) | 3.0 (0.9) | 4.1 (0.3) |
| Ocular hypotensive medication class at baseline, n (%) | |||
| Alpha agonist | 40 (55.6) | 30 (49.2) | 10 (90.9) |
| Beta blocker | 59 (81.9) | 49 (80.3) | 11 (100.0) |
| Carbonic anhydrase inhibitor | 53 (73.6) | 43 (70.5) | 11 (100.0) |
| Carbonic anhydrase inhibitor (oral) | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Prostaglandin analogue | 62 (86.1) | 50 (82.0) | 11 (100.0) |
| Rho kinase inhibitor | 6 (8.3) | 5 (8.2) | 2 (18.2) |
| Miotics | 1 (1.4) | 1 (1.6) | 0 (0.0) |
| BSCVA at baseline, n (%) | |||
| 20/20 or better | 15 (20.8) | 13 (21.3) | 2 (18.2) |
| 20/25 or better | 31 (43.1) | 25 (41.0) | 6 (54.5) |
| 20/32 or better | 43 (59.7) | 37 (60.7) | 6 (54.5) |
| 20/40 or better | 55 (76.4) | 46 (75.4) | 9 (81.8) |
| Worse than 20/40 | 17 (23.6) | 15 (24.6) | 2 (18.2) |
Oral medications count as 1 medication. Combination medications count as 2 medications. For medication class, subjects are counted once for each class of medication taken.
Data from intent-to-treat population for all groups.
There were 5 subjects with visual fields not measured at screening (not required if BSCVA 20/100 or worse).
A high percentage of patients (61/72 or 84.7%) had failed 1 or more incisional glaucoma surgeries or cilioablative procedures. At the time of trial enrollment, these patients had undergone a collective total of 169 glaucoma procedures, predominantly trabeculectomy procedures (n=43), SLT (n=44), XEN stent procedures (n=15), tube shunt procedures (n=14), bleb revisions (n=18), and endocyclophotocoagulation (n=6). The remaining patients (11/72 or 15.3%) were on MTMT.
Effectiveness
Responder Effectiveness Endpoint
In the overall trial population (n=72), the proportion of patients with ≥20% reduction in MDIOP at month 12 versus baseline on the same or fewer IOP-lowering medication classes as baseline was 76.1% (95% CI, 66.2%, 86.1%) (Fig. 2). In the failed-surgery subgroup, this proportion was 73.4% (95% CI, 62.2%, 84.6%); and in the MTMT subgroup, this proportion was 90.9% (95% CI, 73.9%, 100.0%).
FIGURE 2.
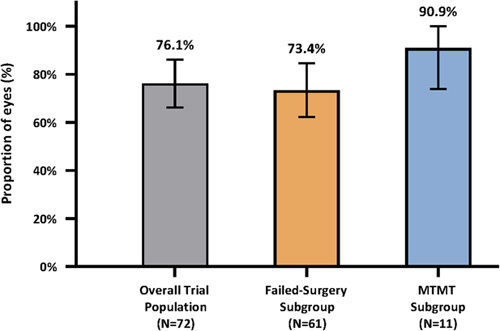
Responder effectiveness endpoint: proportion of responders at month 12. Data from intent-to-treat population in all groups. Multiple imputation was used for 1 patient with missing data at month 12. Responders: patients with ≥20% reduction in mean diurnal intraocular pressure at month 12 versus baseline on the same or fewer ocular hypotensive medication classes as baseline were responders; Nonresponders: patients with (1) hypotony (intraocular pressure <6 mm Hg) associated with clinically significant findings, (2) loss of light perception, (3) intraocular pressure -related SSIs, (4) cyclodialysis cleft, and/or (5) no stents visible were treated as nonresponders. Vertical lines represent 95% CI. MTMT indicates maximum tolerated medical therapy.
In the failed-surgery subgroup (n=61), the majority of patients (44) were responders; there were a total of 17 nonresponders. The primary reasons for nonresponse were month 12 MDIOP <20% reduction from baseline (n=12), on more IOP-lowering medication classes at 12 months versus baseline (n=2), and SSI affecting IOP (n=2). In addition, 1 patient had missing 12-month MDIOP data because of death. In the MTMT subgroup (n=11), 10 patients were responders. One patient was a nonresponder as MDIOP reduction was <20% at month 12 versus baseline (MDIOP was 20.3 mm Hg on 5 medications at baseline, and 19.3 mm Hg on 5 medications at month 12).
The sensitivity analyses involving the responder effectiveness endpoint showed similar outcomes. Regardless of the analysis population or imputation method, the proportion of patients with ≥20% reduction in MDIOP at month 12 versus baseline on the same or fewer IOP-lowering medication classes as baseline ranged from 75.0% to 76.8% in the overall trial population, 72.1% to 73.4% in the failed-surgery subgroup, and 90.9% to 100% in the MTMT subgroup. Subgroup analyses of the effect of lens status (phakic vs. pseudophakic) showed the outcomes to be poolable and supported effectiveness of the iStent infinite treatment regardless of lens status.
IOP Change From Baseline Endpoint
In the overall trial population (n=72), the mean reduction in MDIOP from baseline at 12 months was 5.9 mm Hg (SE: 0.6, 95% CI, 4.8, 7.1) (Fig. 3). The mean reduction in MDIOP from baseline at 12 months was 5.5 mm Hg (SE: 0.7, 95% CI, 4.2, 6.9) in the failed-surgery subgroup and 8.1 mm Hg (SE 0.9, 95% CI, 6.0, 10.1) in the MTMT subgroup. The sensitivity analyses involving the IOP change from baseline endpoint produced similar outcomes. Regardless of the analysis population or imputation method, the mean reduction in MDIOP from baseline at 12 months was either 5.9 or 6.0 mm Hg in the overall trial population, 5.5 or 5.6 mm Hg in the failed-surgery subgroup, and 8.8 or 8.1 mm Hg in the MTMT subgroup, underscoring the strength of this effectiveness endpoint.
FIGURE 3.
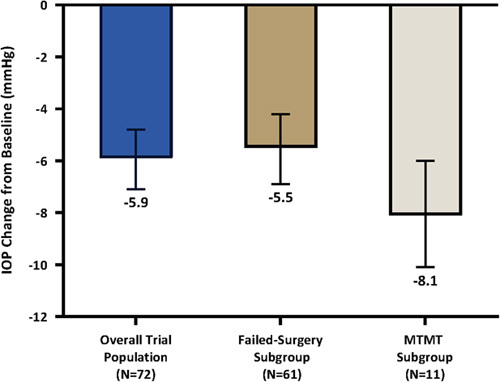
IOP change from baseline effectiveness endpoint: 12-month diurnal IOP change from baseline. Data from intent-to-treat population in all groups. Multiple imputation was used for 1 patient with missing data at month 12. Vertical lines represent 95% CI. The following imputation methods were used for the 12-month mean diurnal intraocular pressure: (1) For patients with hypotony associated with clinically significant findings, cyclodialysis cleft, and/or no stents visible, the worst postoperative IOP on the same or greater number of OHT medication classes as baseline was used. If a patient was on fewer OHT medication classes than baseline, then the baseline IOP was used. (2) For patients with loss of light perception, the observed 12-month MDIOP was used. (3) For patients with IOP-related secondary surgical interventions, the worst postoperative IOP before secondary surgical intervention on the same or greater number of OHT medication classes as baseline was used. If a patient was on fewer OHT medication classes than baseline, then the baseline IOP was used. (4) For patients on more OHT medication classes than at baseline, the worst postoperative IOP on the same or greater number of OHT medication classes as baseline was used. IOP indicates intraocular pressure; MTMT, maximum tolerated medical therapy; OHT, ocular hypotensive.
In addition to the aforementioned formal trial endpoints, 2 additional responder analyses were carried out: the proportion of eyes on the same or fewer medication classes with month 12 MDIOP reduction of ≥20%, ≥30%, and ≥40% is shown in Figure 4A; and the proportion of eyes on the same or fewer medication classes and with month 12 MDIOP of ≥6 and ≤21, ≤18, and ≤15 mm Hg is shown in Figure 4B. In the overall trial population, 53.0% (n=35) achieved MDIOP reduction ≥30%, and 74.2% (n=49) achieved M12 MDIOP of ≤18 mm Hg.
FIGURE 4.
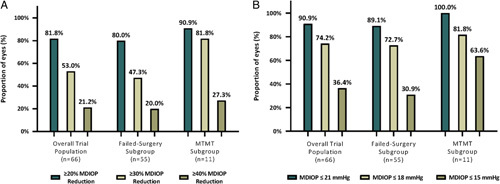
A, Distribution of 12-month diurnal intraocular pressure (IOP) percent change categories. Data from intent-to-treat population in all groups. B, Distribution of diurnal IOP categories at month 12. Data from intent-to-treat population in all groups. MDIOP was ≥6 mm Hg for all IOP categories. N=number of patients with month 12 diurnal IOP; %=100 × (n ÷ N); not reported = number of available patients without month 12 diurnal IOP and with no IOP-related secondary surgical interventions or other events. Other events include hypotony (IOP <6 mm Hg) associated with clinically significant findings, loss of light perception, cyclodialysis cleft, and/or no stents visible. MDIOP indicates mean diurnal intraocular pressure; MTMT, maximum tolerated medical therapy.
Medications
The vast majority of patients (93.0%) in the overall trial population with month 12 data reduced (n=31) or maintained (n=35) their medication burden; only 5 patients (all in failed-surgery subgroup) increased medication burden. The mean number of medications reduced from 3.10±0.89 medications at baseline to 2.70±1.03 medications at 12 months postoperative.
For the failed-surgery subgroup, 55/60 eyes (91.7%) used the same or fewer number of IOP-lowering medication classes at 12 months than at baseline. Specifically, 26 eyes (43.3%) used fewer, 29 eyes (48.3%) used the same, and 5 eyes (8.3%) used more medication classes than at baseline. For the MTMT subgroup, 5 eyes (45.5%) used fewer medication classes at 12 months than at baseline, and 6 eyes (54.5%) used the same number of medication classes as baseline.
Safety
Intraoperative Ocular AEs
All 72 eyes (100%) in the trial were successfully implanted with 3 iStent infinite stents, with no failed implantation attempts. No intraoperative AEs were reported. In most procedures, a single injector was used (93.1%); a second injector was required in 5 eyes (6.9%). In all cases, there was a backup injector available in the event a second injector was needed to complete implantation of 3 stents.
Postoperative Ocular AEs and Secondary Surgeries
Over half (52.8%; n=38) of patients did not experience any postoperative study eye AEs. There were no unanticipated adverse device effects and no serious ocular AEs. No corneal decompensation, choroidal effusion, choroidal hemorrhage, hypotony maculopathy, deep stents (“buried” in the trabecular meshwork) that were not visible at the last 3 scheduled visits of the trial, stent explantation, stent dislocation, or stent repositioning were reported. The vast majority of patients (93%) maintained their preoperative BSCVA or had a decrease of <2 lines at month 12. None of the 5 cases of ≥2-line BSCVA decrease at month 12 were considered device related; all were considered related to their preexisting ocular disease mainly associated with severe preoperative visual field abnormalities, complicated glaucoma management history, glaucoma progression, and/or postoperative sequelae unrelated to the iStent infinite implants. A list of the most common AEs (occurring at ≥2%) and associated percentages are provided in Table 3.
TABLE 3.
Postoperative Ocular Adverse Events Occurring at 2% or Greater in the Study Eye, Overall Trial Population, Failed-Surgery Subgroup, MTMT Subgroup*
| Overall Trial Population N=72 | Failed-Surgery Subgroup N=61 | MTMT Subgroup N=11 | |
|---|---|---|---|
| Adverse Event† | No. Subjects (%) | No. Subjects (%) | No. Subjects (%) |
| Age-related macular degeneration | 1 (1.4) | 0 (0.0) | 1 (9.1) |
| Blepharitis | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Hyperemia | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| IOP increase ≥10 mm Hg vs. baseline IOP at ≥month 1 | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| IOP increase requiring oral medication | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| at >day 1 to ≤week 1 | 1 (1.4) | 1 (1.6) | 0 (0.0) |
| at ≥ month 1 | 1 (1.4) | 1 (1.6) | 0 (0.0) |
| IOP increase requiring surgical intervention | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| at >week 1 to <month 1 | 1 (1.4) | 1 (1.6) | 0 (0.0) |
| at ≥month 1 | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| Intraocular inflammation after tube shunt surgery | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| Loss of best spectacle corrected visual acuity (BSCVA) of 2 lines or more at >30 d‡ | 6 (8.3) | 6 (9.8) | 0 (0.0) |
| Macular edema | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| Ocular hypotensive medication intolerance | 3 (4.2) | 3 (4.9) | 0 (0.0) |
| Ocular surface disease | 7 (9.7) | 7 (11.5) | 0 (0.0) |
| Perioperative inflammation | 5 (6.9) | 4 (6.6) | 1 (9.1) |
| Significant hyphema (ie, ≥10% of anterior chamber) | 3 (4.2) | 2 (3.3) | 1 (9.1) |
| Stent migration§ | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| Stent obstruction∥ | 2 (2.8) | 2 (3.3) | 0 (0.0) |
| Visual field loss ≥2.5 dB | 5 (6.9) | 4 (6.6) | 1 (9.1) |
Data from safety population for all groups.
In addition to the adverse events reported in Table 3, events that occurred at a rate of <2% included 1 case (1.4%) each of conjunctival erosion due to tube shunt, conjunctivitis, disc hemorrhage, hypotony (IOP <6 mm Hg) associated with clinically significant findings that occurred 1 day after tube shunt surgery, intraocular inflammation arising after the protocol’s specified medication regimen is complete, loss of BSCVA of 2 lines or more ≤30 days postoperative, ocular pain, posterior vitreous detachment, stye, subconjunctival hemorrhage, and transient hypotony.
Five AEs of BSCVA loss of 2 lines or more were ongoing at month 12.
One subject was reported with 2 events of stent migration. The PI acknowledged that the visualization was impaired during implantation of the 1:00 and 4:30 stents due to corneal arcus, striae, and external location-marking dye. The stent reported as implanted at 1:00 was identified in the 1:00 position via UBM (“imbedded deep beyond iris insertion”), and the stent reported as implanted at 4:30 was identified in the 7:30 position via both gonioscopy and UBM.
The 2 AEs of stent obstruction involved complete obstruction of 2 stents each. The investigators reported associated findings of significant hyphema in 1 case and preexisting and postoperative focal goniosynechiae in both cases. One case of stent obstruction resolved after treatment with pilocarpine, and 1 case was not treated and was ongoing at month 12. Both subjects experienced month 12 MDIOP reduction on the same medication regimen as preoperative.
In addition to the AEs described above, events that occurred at a rate of <2% included 1 case each of age-related macular degeneration, conjunctival erosion due to tube shunt, conjunctivitis, disc hemorrhage, hypotony (IOP <6 mm Hg) associated with clinically significant findings that occurred 1 day after tube shunt surgery in the postoperative period, intraocular inflammation arising after the protocol’s specified medication regimen was complete, loss of BSCVA of 2 lines or more ≤30 days postoperative, ocular pain, posterior vitreous detachment, stye, subconjunctival hemorrhage, and transient hypotony.
No serious device-related AEs were recorded. Device-related AEs that were considered nonserious (n=13 events in 9 eyes) were largely transient in nature and without long-term clinical sequelae. The events included 14 postoperative AEs consisting of the following: IOP increase requiring oral medication within 1 week postoperative (n=1), transient BSCVA loss of ≥2 lines within 30 days (n=1), perioperative inflammation at 1 day postoperative (n=5), significant hyphema at 1 day postoperative (n=3), stent migration (n=2 events in 1 eye), and stent obstruction (n=2). Of the 13 reported device-related AEs, 8 AEs involved 8 eyes, and 5 AEs involved 1 eye that experienced significant hyphema, ocular inflammation, and IOP increase requiring oral medication within 3 days postoperative, followed by transient BSCVA loss within 30 days and stent obstruction. Hyphema is a recognized postoperative finding after angle surgery with retrograde blood flow through collector channels. In eyes with prior surgery, there may be greater risk of bleeding. With the exception of 1 case of stent obstruction (not treated), device-related postoperative AEs resolved completely. A total of 3 eyes underwent SSI (tube shunt surgery); all 3 were because of AEs that were not device related. No other SSIs were performed. Of the 6 phakic eyes, none had progressive cataracts and no cataract surgery was required. Additional safety findings included 8 eyes treated with topical steroids postoperatively to supplement topical nonsteroidal anti-inflammatory drugs.
DISCUSSION
In this 12-month prospective multicenter pivotal trial for the iStent infinite Trabecular Micro-Bypass System, 76.1% of patients met the responder endpoint, with significant MDIOP reduction of nearly 6 mm Hg from baseline. In the MTMT subgroup in particular, 90.9% of patients met the responder endpoint and MDIOP reduced by 8.1 mm Hg. Safety parameters were favorable and notably did not include any of the short-term and long-term complications frequently seen with filtration surgeries or cilioablative procedures, such as bleb-related complications, endophthalmitis, hypotony maculopathy, persistent corneal edema or decompensation, flat anterior chamber, significant inflammation, or choroidal effusion or hemorrhage.13 Outcomes were achieved in eyes with OAG that had failed prior surgery and/or MTMT, and for whom such higher-risk, bleb-forming, or tissue-destructive procedures often would have been the next step. In contrast to the lifelong risks of these filtering/cilioablative procedures, the ab interno implantation of iStent trabecular micro-bypass technology is conjunctival-sparing and by design does not jeopardize the success or increase the complexity of future filtering surgery.
Effectiveness outcomes were highly favorable in this trial. The majority of patients (76.1%) implanted with iStent infinite met the responder endpoint of MDIOP reduction ≥20% without the use of additional medication or secondary surgical procedures. Over half of patients (53.0%) had ≥30% MDIOP reduction from baseline and 21.2% had ≥40% reduction. These rates are particularly noteworthy considering the challenges of successful IOP reduction and limited long-term control of glaucoma after repeat filtration surgery.47 From the baseline visit to the 12-month visit, a clinically important mean reduction in MDIOP of 5.9 mm Hg was achieved. At month 12, 74.2% of patients had MDIOP ≤18 mm Hg on the same or fewer medication classes, a threshold of particular clinical interest since the subgroup of eyes in the Advanced Glaucoma Intervention Study observed to have IOP <18 mm Hg at all visits had little to no mean change in visual field score.48 Furthermore, excellent patient retention and high trial completion rate (98.6% at 12 months) enabled high consistency of outcomes across analysis populations and imputation methods, underscoring the veracity of the findings.
Alongside effectiveness, the safety profile also was highly favorable. There were no unanticipated adverse device effects nor serious AEs. Consistent with the iStent inject pivotal trial data, AEs specifically related to the iStent infinite were largely transient in nature, mild to moderate in severity, and without long-term clinical sequelae. No eyes necessitated a procedure to reposition or explant the stents during follow-up. Although the majority of patients had failed a prior filtration or cilioablative surgery and likely would have needed additional invasive procedures in the near future, only 3 eyes (4.2%) required such surgery. The nature and number of these AEs and secondary glaucoma surgeries were not unexpected given this patient population’s prior history of failed treatment(s). Furthermore, visual acuity outcomes remained stable throughout the trial, with no device-related cases of confirmed ≥2-line BSCVA loss. Lens injury with subsequent cataract development is unlikely with maintenance of the anterior chamber with viscoelastic and precise microstent delivery under gonioscopic guidance.
The study was designed to demonstrate the ability of the iStent infinite to lower IOP in a difficult-to-treat population. As such, the study endpoint was in accord with FDA guidance and with the protocol definition of a reduction in IOP of at least 20% and not based on stable visual field outcomes. The study met the treatment target as established by FDA and the protocol with regard to the ability to lower IOP, despite the patients entering the study on a mean of 3.1 medications and a mean of 2 prior failed glaucoma procedures.
In many trials, a ≥20% IOP reduction below baseline has been agreed upon as a target for the study population. However, it is always at the surgeon’s discretion to decide whether that is adequate for each patient and, if not, then suggest a more aggressive IOP reduction.
This favorable risk-benefit profile of trabecular bypass procedure was corroborated in a recent comparative study of 2–3 trabecular bypass stents versus trabeculectomy, which showed substantial reductions in IOP and medications for both procedures, but significantly higher percentage of safety-adjusted treatment success in the trabecular micro-bypass versus trabeculectomy group.16
The iStent infinite also has a favorable microscopic size-versus-outflow ratio, with 3 stents occupying ~3% of the trabecular meshwork angle but designed to enable up to 240 degrees of aqueous outflow with minimal tissue disruption.46 Despite this microscale tissue footprint, each stent can sustain outflow equal to the entire rate of aqueous humor production by the human body.35,36 Furthermore, the incremental benefits of additional stents (eg, 3 vs. 2 vs. 1 stent), such as additional IOP reduction and reduced medication burden, have been validated in both preclinical and clinical studies, with favorable safety in all treatment groups.16,33–39
The trial was not without limitations. This was a single-arm, open-label trial, consistent with FDA standards for a 510(k) device registration submission. Although the follow-up period of 12 months is of relatively short duration, the iStent infinite has the same mechanism of action and treatment approach as the first-generation iStent and second-generation iStent inject devices, which have shown consistent durability in maintaining IOP and medication reductions for up to 8 years postoperative.16–18,20–32,37–39 Hence, long-term studies with iStent infinite would be expected to demonstrate similarly consistent effectiveness and safety over time. No medication washout was done, as this might have placed the enrolled glaucoma patients at undue risk for optic nerve damage. However, this trial design is consistent in this regard with similar studies on patients at high risk for glaucoma progression.
The findings in this prospective pivotal trial support the addition of the iStent infinite Trabecular Micro-Bypass System to the ophthalmologist’s treatment algorithm as a stand-alone procedure for medically or surgically uncontrolled OAG patients. These patients traditionally have been managed with more invasive alternatives that carry lifelong risks. An effective surgical option that reduces IOP and/or medication dependence and is relatively low risk, bleb-free, does not require permanent tissue destruction, and does not preclude other later interventions is highly desirable. The data in this trial demonstrate that iStent infinite can successfully and safely fill this important clinical need.
Footnotes
Glaukos Corporation (Aliso Viejo, CA) participated in the design and conduct of the trial; the collection, management, and analysis of data; and the preparation of the manuscript.
Disclosure: The authors declare no conflict of interest.
Contributor Information
Steven R. Sarkisian, Jr, Email: submissiondrsarkisian@gmail.com.
Davinder S. Grover, Email: dgrover@glaucomaassociates.com.
Mark J. Gallardo, Email: gallardomark@hotmail.com.
Jacob W. Brubaker, Email: jbrubaker@SacEye.com.
Jane Ellen Giamporcaro, Email: jegiamporcaro@glaukos.com.
Dana M. Hornbeak, Email: dhornbeak@glaukos.com.
L. Jay Katz, Email: ljkatz@willseye.org.
Tomas Navratil, Email: janeelleng2@gmail.com.
REFERENCES
- 1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 2. Tham YCC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 3. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 4. Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720; discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 5. Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian glaucoma study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126:1030–1036. [DOI] [PubMed] [Google Scholar]
- 6. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11 suppl):S30–S36. [DOI] [PubMed] [Google Scholar]
- 8. Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. [DOI] [PubMed] [Google Scholar]
- 9. Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol. 2014;8:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9.e2. [DOI] [PubMed] [Google Scholar]
- 11. The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol. 1995;120:718–731. [DOI] [PubMed] [Google Scholar]
- 12. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (LiGHT) trial. A multicentre, randomised controlled trial: design and methodology. Br J Ophthalmol. 2018;102:593–598. [DOI] [PubMed] [Google Scholar]
- 13. Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boland MV, Corcoran KJ, Lee AY. Changes in performance of glaucoma surgeries 1994 through 2017 based on claims and payment data for United States Medicare Beneficiaries. Ophthalmol Glaucoma. 2021;4:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. [DOI] [PubMed] [Google Scholar]
- 16. Paletta Guedes RA, Gravina DM, Paletta Guedes VM. Standalone implantation of 2-3 trabecular micro-bypass Stents (iStent inject ± iStent) as an alternative to trabeculectomy for moderate-to-severe glaucoma. Ophthalmol Ther. 2022;11:271–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467. [DOI] [PubMed] [Google Scholar]
- 18. Samuelson TW, Sarkisian SRJ, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126:811–821. [DOI] [PubMed] [Google Scholar]
- 19. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126:29–37. [DOI] [PubMed] [Google Scholar]
- 20. Healey PR, Clement CI, Kerr NM, et al. Standalone iStent trabecular micro-bypass glaucoma surgery: a systematic review and meta-analysis. J Glaucoma. 2021;30:606–620. [DOI] [PubMed] [Google Scholar]
- 21. Hengerer FH, Auffarth GU, Conrad-Hengerer I. iStent inject trabecular micro-bypass with or without cataract surgery yields sustained 5-year glaucoma control. Adv Ther. 2022;39:1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindstrom R, Sarkisian SR, Lewis R, et al. Four-year outcomes of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication. Clin Ophthalmol. 2020;14:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berdahl J, Voskanyan L, Myers JS, et al. iStent inject trabecular micro-bypass stents with topical prostaglandin as standalone treatment for open-angle glaucoma: 4-year outcomes. Clin Exp Ophthalmol. 2020;48:767–774. [DOI] [PubMed] [Google Scholar]
- 24. Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis (Lond). 2020;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neuhann TH, Hornbeak DM, Neuhann RT, et al. Long-term effectiveness and safety of trabecular microbypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension: five-year outcomes. J Cataract Refract Surg. 2019;45:312–320. [DOI] [PubMed] [Google Scholar]
- 26. Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis (Lond). 2021;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salimi A, Watt H, Harasymowycz P. Three-year outcomes of second-generation trabecular micro-bypass stents (iStent inject) with phacoemulsification in various glaucoma subtypes and severities. J Glaucoma. 2021;30:266–275. [DOI] [PubMed] [Google Scholar]
- 28. Clement C, Howes F, Ioannidis AS, et al. Two-year multicenter outcomes of iStent inject trabecular micro-bypass stents combined with phacoemulsification in various types of glaucoma and ocular hypertension. Clin Ophthalmol. 2020;14:3507–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferguson TJ, Dockter Z, Bleeker A, et al. iStent inject trabecular microbypass stent implantation with cataract extraction in open-angle glaucoma: early clinical experience. Eye Vis (Lond). 2020;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferguson TJ, Ibach M, Schweitzer J, et al. Trabecular microbypass stent implantation in pseudophakic eyes with open-angle glaucoma: long-term results. J Cataract Refract Surg. 2019;45:414–420. [DOI] [PubMed] [Google Scholar]
- 31. Ferguson TJ, Mechels KB, Dockter Z, et al. iStent trabecular microbypass stent implantation with phacoemulsification in patients with open-angle glaucoma: 6-year outcomes. Clin Ophthalmol. 2020;14:1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferguson T, Swan R, Ibach M, et al. Evaluation of a trabecular microbypass stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 2018;27:71–76. [DOI] [PubMed] [Google Scholar]
- 33. Belovay GW, Naqi A, Chan BJ, et al. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38:1911–1917. [DOI] [PubMed] [Google Scholar]
- 34. el Wardani M, Bergin C, Achache F, et al. Evaluating the trabecular micro-bypass stent combined with phacoemulsification compared to phacoemulsification alone. Klin Monbl Augenheilkd. 2015;232:442–445. [DOI] [PubMed] [Google Scholar]
- 35. Hunter KS, Fjield T, Heitzmann H, et al. Characterization of micro-invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014;8:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bahler CK, Hann CR, Fjield T, et al. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012;153:1206–1213. [DOI] [PubMed] [Google Scholar]
- 37. Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paletta Guedes RA, Gravina DM, Paletta Guedes VM, et al. Two-year comparative outcomes of first- and second-generation trabecular micro-bypass stents with cataract surgery. Clin Ophthalmol. 2021;15:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manning D. Real-world case series of iStent or iStent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol Ther. 2019;8:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131:1573–1582. [DOI] [PubMed] [Google Scholar]
- 41. Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005;140:16–22. [DOI] [PubMed] [Google Scholar]
- 42. Jampel HD, Solus JF, Tracey PA, et al. Outcomes and bleb-related complications of trabeculectomy. Ophthalmology. 2012;119:712–722. [DOI] [PubMed] [Google Scholar]
- 43. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new Ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. [DOI] [PubMed] [Google Scholar]
- 44. Huang AS, Penteado RC, Papoyan V, et al. Aqueous angiographic outflow improvement after trabecular microbypass in glaucoma patients. Ophthalmol Glaucoma, 2:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–438. [DOI] [PubMed] [Google Scholar]
- 46. Glaukos data on file. Accessed February 2, 2022.
- 47. Law SK, Shih K, Tran DH, et al. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol. 2009;148:685–695.e1. [DOI] [PubMed] [Google Scholar]
- 48. Gaasterland DE, Ederer F, Beck A, et al. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. [DOI] [PubMed] [Google Scholar]


