Abstract
Craving and impulsivity are addiction components which explain why heroin-dependant individuals (HDI), continue using heroin despite not wanting to do so. Opioid maintenance treatment (OMT), such as slow-release oral morphine (SROM), is the most effective treatment for opioid dependence. However, the impact of SROM on craving and impulsivity remains unclear. In this observational study, 23 HDI receiving SROM, their usual OMT, took part in the experiment. Each of the participants filled in the perceived level of craving with a visual analog scale. Their impulsivity was assessed via three laboratory tasks, the stop-signal reaction time, the Balloon Analogue Risk Task and delay discounting. Each evaluation was performed before and after SROM administration. Craving was significantly reduced after administration of SROM (difference 2.83; P = 0.0010), whereas there were no significant differences in performance in the three laboratory tasks. In the long term, we observed an improvement on delay discounting correlated with the duration and dosage of SROM. The acute impact of SROM appears to significantly reduce craving, without impacting impulsivity. Observation of the correlation between delay discounting and the duration and dosage of OMT is of great interest and should be studied further.
Keyswords: Balloon Analogue Risk Task, craving, delay discounting, impulsivity, slow-release oral morphine, stop-signal reaction time
Introduction
Heroin addiction, like other addictions, is a chronic condition characterized by remissions and relapses (Daglish et al., 2001) and a compulsion to seek and use heroin despite negative consequences (Ferri et al., 2011). Indeed, as in other addictions, Heroin dependant individuals (HDI), even if they are aware of the negative consequences and choose to abstain from heroin use, eventually relapse. This loss of control which is one of the main elements of all addictions could be explained by craving and impulsivity (Moshier et al., 2013).
Impulsivity has been described in all addictions and potentially plays a role in all phases of the addiction cycle (Dissabandara et al., 2014; Vassileva et al., 2014). However, impulsivity is a complex construct with several components related to risk-taking and lack of reflection that impact behavior in different ways (Xu et al., 2013; Antons and Brand, 2018). By extension, the term ‘impulsive’ is often used indiscriminately to define a series of maladaptive behaviors that reflect these different components, including the inability to suppress inappropriate behavior (motor impulsivity), risk-taking (risky behaviors) and the inability to defer gratification (impulsive choice) (Bari and Robbins, 2013). Craving, on the other hand, has been identified to occur once an addiction is present and could influence its trajectory. Craving is an irrepressible compulsion to use an addictive substance despite not wanting to do so. Craving could explain persistent substance use (Bernheim and Rangel, 2004) and relapses (Daglish et al., 2001). Thus, craving is a key symptom of substance use disorders (American Psychiatric Association, 2013). Craving can be triggered by cues that, in the context of addiction, may become salient after a process of associative learning. Craving indicates a higher likelihood of an automatic impulsive response to cues rather than a controlled and reflected response (Antons and Brand, 2018). Through this definition, we understand how craving and compulsive behavior are two components that interact and lead to addictive behavior.
HDIs are particularly interesting for studies on impulsivity and craving because they are the only group of patients receiving a single valid treatment, Opioid maintenance treatment (OMT). In addition, they are a specific subpopulation of opioid use disorder (OUD), where the opiate (heroin) is obtained outside a clinical context. The OMT strategy is to substitute an illicit opioid that is particularly addictive due to its shorter action with a licit opioid which can be administered once a day due to its pharmacokinetics (Amato et al., 2013). Indeed, OMT is the most effective treatment for opioid dependence (Carlsen et al., 2020) allowing the reduction or disappearance of withdrawal symptoms and cravings (Fareed et al., 2011). However, its impact on impulsivity and the expression of impulsive behaviors is still unclear. Existing literature showed contradictory data potentially explained by different research methodologies. Indeed, some studies have identified that opioid use, even OMT, leads to higher impulsivity (Odum et al., 2000; Lee and Pau, 2002; Verdejo et al., 2005; Fishbein et al., 2007; Dissabandara et al., 2014; Yang et al., 2015) while others conclude that there is a decrease (Liao et al., 2014; Li et al., 2020 or simply no impact (Zacny and de Wit, 2009; Harty et al., 2011). In view of the impact of impulsivity, on the course of addictive disorders, it seems necessary to better understand the impact of OMT on impulsivity. All these data lead us to question whether the long-term prescription of OMT has a favorable or unfavorable impact on the different components of impulsivity and on the disorder itself. To answer this question, we have studied a group of patients undergoing treatment for heroin dependence, all of whom were receiving the same OMT, slow release oral morphine (SROM). SROM has been suggested as an alternative treatment for HDI, especially for those with intolerance for other OMTs (Socias et al., 2020). Although its utilization for opioid dependence is still not authorized in many countries (Koller et al., 2019), it has been available for several years in Switzerland. In this context, the purpose of our study is to determine the impact on craving and impulsivity of a single opioid administration in a therapeutic setting on HDI under OMT with SROM.
Method
Study sample
Twenty-three patients were recruited from the Division of Addictology (Service d’Addictologie) of the University Hospital of Geneva (Switzerland) with a diagnostic and statistical manual of mental disorder fifth edition (DSM-5)diagnosis of opioid Dependence. Participants were eligible if they fulfilled all of the following inclusion criteria: informed consent as documented by signature; able to communicate in French; age over 18 years old; on a stable dose of SROM not modified at least 14 days before inclusion. Non-inclusion/exclusion-criteria included unstable psychiatric disorder and acute withdrawal syndrome. Participants received 50CHF-vouchers for their participation. The study was approved by the Ethical Committee of the Canton of Geneva and the Swiss Agency for Therapeutic Products (Swissmedic), and was carried out in accordance to the protocol and according to the principles enunciated in the Declaration of Helsinki and the guidelines of Good Clinical Practice (GCP) issued by International conference on harmonization.
Assessment measures
Craving evaluation
Usually, self-reported general craving for heroin is assessed with three-item Likert-type rating or visual analog scale (VAS). This self-reported measure assesses the global sense of craving. It is easy to use and especially suitable for frequent and repeated measures, it is also sensitive to rapid changes (Yen et al., 2016). In this study, self-reported general craving for heroin was assessed pre- and post-SROM administration using a VAS with three questions: (1) “How would you rate your desire to use in the last 30 min?”; (2) “Imagine yourself in a situation associated with your past substance use; if you were in that place now, how likely would you be to actually use?” and three (3) “Confronted with a triggering situation without the possibility to use immediately, evaluate the intensity of your cravings?”. For every question, subjects could give a score from 0 (not intense at all’) to 10 (‘very intense’) (Falcato et al., 2015). The total score was computed as the sum of the scores for each item.
Alcohol, Smoking and Substance Involvement Screening Test
The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) is a short screening questionnaire developed by the WHO to assess the use of different substances (tobacco products, alcohol, cannabis, cocaine, amphetamine-type stimulants, sedatives and sleeping pills, hallucinogens, inhalants, opiates and ‘other drugs’) and the associated consequences (Humeniuk et al., 2008; WHO ASSIST Working Group, 2002). The ASSIST in its current French version (ASSIST V3.0) [28] is composed of eight questions that determine a risk score for each substance, which allows to conclude the most appropriate intervention for that level of use. The score for each substance is categorized as low risk (occasional or nonharmful use), moderate risk (more regular or harmful use) or high risk (frequent risky use or suggestive of dependence). Each substance has a threshold between each risk category. Scores above 3 for all products (tobacco, cannabis, cocaine, psychostimulants, inhalants, tranquilizers and hallucinogens), with the exception of alcohol (score above 10), are considered moderate or high risk. The ASSIST is therefore a well-validated screening test for substance overuse and dependence in an adult population (Khan et al., 2011).
Experimental tasks
Stop-signal reaction task
The stop-signal reaction task (SSRT) is the prototypical task used to assess the capacity of inhibitory mechanisms, measuring the ability to inhibit an automatic motor response. The task consists in responding to a visual signal (go signal) as fast as possible (go task), but to refrain this answer (stop task) when an auditory signal (stop signal) is heard. The frequency of this stop signal is set on one trial out of four (25%) but the delay between the go signal and this stop signal varies and is successively adjusted to make it tend towards the median reaction time. The latency of the response to the stop signal (stop-signal reaction time) is calculated as a quantitative measure of inhibitory control. Longer stop-signal reaction times are associated with higher impulsivity (Logan et al., 1997).
Delay discounting task
The delay discounting task is designed to assess impulsive decision-making (Rachlin et al., 1991). This task uses a computerized adjusting-amount procedure to measure how a delay impinging a granted reward decreases the attractiveness of this reward, hence the term ‘discount’. Hypothetically monetary rewards are adopted to quantify this effect. In a series of choice trials, participants have to decide repeatedly between two options: a smaller amount of money (hypothetically) available immediately or a larger amount of money available after a delay (e.g. $100 immediately or $1000 in 1 year). There are three blocks of trials (1 month, 6 months and 1 year). On each block of trials, the large delayed amount of money is constant across trials, while the immediately available amount of money is changed on each trial. Each of these blocks is presented twice: in one series the immediate smaller amounts of money are presented in descending order and in the other, they are presented in ascending order.
On successive trials, manipulation of parameters allows estimation of the rate of discount, which allows us to find the delay at which the large and the smaller amount of the reward would be valued equally, namely the ‘equivalence point’. The ‘equivalence point’ is calculated by averaging the ascending and descending values for each time period. The ‘equivalence point’ is the value of the last immediate amount when a participant ceases to prefer the immediate amount and chooses the deferred amount, that is the point at which the immediate and deferred amounts have the same subjective value for the participant. In this task, the dependent variable is the area under the curve (AUC) defined by the three equivalence points (1 month, 6 months and 1 year). A lower AUC indicates a stronger preference for smaller sums, but immediate rewards, and is associated with higher impulsivity (Lynam and Miller, 2004).
Balloon analogue risk task
The balloon analogue risk task (BART) is a computerized laboratory-based assessment of risk-taking tendencies (Lejuez et al., 2002). In this task, a small, simulated balloon with a balloon pump is displayed on the computer screen.
Participants may inflate the balloon by clicking on the pump in exchange of a monetary reward for each pump. With each click, the balloon inflates and 10 points are added to the participant’s temporary bank. At any point, the participant may decide to stop to inflate the balloon and collect the sum garnered on this balloon. The sum is banked in the permanent bank.
However, each balloon is set to explode at random with the result of the loss of all money accumulated for that balloon. Each balloon has a different explosion point and is programmed to pop between 1 and 64 pumps (maximum number of clicks per balloon). The participants are only informed that the balloon can explode anywhere from the first pump all the way to the point where it fills the whole screen. After each balloon explosion or money collection, a further balloon appears until a total of 30 balloons have spawned.
The main dependent measure on the BART is quantified by the average number of pumps delivered in balloons that did not explode (Lejuez et al., 2002). Higher scores imply a higher risk-taking predilection (Lejuez et al., 2002; White et al., 2008).
Procedure
This study is an observational study, with a single dose in an open-label design: all subjects received their usual SROM treatment with a stabilized dosage. In the first part of the study, participants had to complete questionnaires about their socio-demographic status and give information about other substances used with the ASSIST self-rating questionnaire. Patients were informed that they would receive their regular dose of SROM during the experiment. In the pre-SROM assessment patients’ craving was evaluated with the instrument described above, and the three experimental tasks (SSRT, delay discounting and BART) were performed. After the pre-SROM assessment patients received their regular dose of SROM. The post-SROM evaluation was performed between 60 and 120 min after drug administration, using the exact same assessments as for the pre-SROM assessment.
Data analysis
Statistical analyses were conducted using R (R Core Team (2020). Variables were described as frequencies or mean values and SD. To evaluate the acute impact of SROM, the pre/postanalysis of the scores of craving, delay discounting, SSRT and BART for the entire population was performed using the Wilcoxon test for repeated measures. To assess whether the long-term effect of SROM treatment could affect clinical parameters, the relationship between various variables such as craving, positive DSM-5 criteria or number of substances used and overused, and performance obtained in the laboratory task before SROM administration and treatment parameters (SROM duration and dosage) was assessed using nonparametric Spearman rank correlation. All statistical tests were considered significant if P < 0.05.
Results
Sociodemographics, substance use patterns, the severity of the OUD defined by the number of positive DSM-5 criteria, and SROM treatment parameters (duration and dosage) are presented in Table 1. In addition, other substance use was taken into consideration with the ASSIST scores (Table 1).
Table 1.
Socio-demographic and diagnostic characteristics of the study sample
| Experimental group (n: 23) Mean (SD) |
||
|---|---|---|
| Age | 43.8 (8.6) | |
| Gender male (%) | 20 (87) | |
| Positive DSM-5 criteria | 9.87 (3.9) | |
| Doses of SROM (mg/day) | 528.6 (289.26) | |
| Duration of SROM treatment (weeks) | 128.20 (66.48) | |
| Total substance used | 3.91 (1.38) | |
| Total substance abused | 2.43 (1.04) | |
| Substance use | Users (%) | Score ASSIST |
| Tobacco | 100 | 17.65 (5.93) |
| Alcohol | 78 | 7.56 (8.39) |
| Cannabis | 74 | 8 (7.66) |
| Cocaine | 65 | 6.91 (8.66) |
| Psychostimulant | 13 | 0.39 (1.03) |
| Hallucinogen | 17 | 0.65 (1.55) |
| Tranquilizer | 43 | 2.82 (5.37) |
DSM-5, diagnostic and statistical manual of mental disorder fifth edition; SROM, slow-release oral morphine.
There was a significant decrease in craving, pre- vs. post-SROM administration with a difference of 2.83 (SD: 1.69), [5.22 (SD: 5.74) vs. 2.39 (SD: 4.05); P = 0.0010].
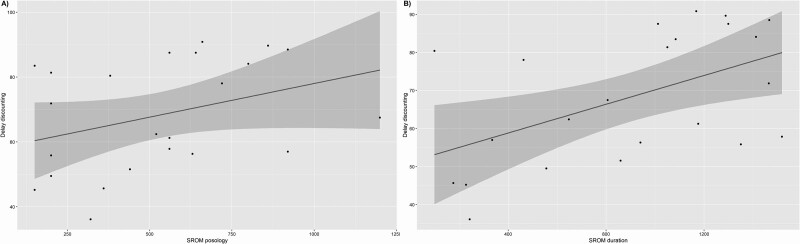
Further analysis showed a positive correlation between delay discounting and duration of SROM treatment (S = 983.74; P value = 0.01212; rho = 0.513961), as well as SROM dosage (S = 1078; P-value = 0.02453; rho = 0.4673811) (see Fig. 1). Finally, we found no correlation between SROM treatment parameters (dosage and duration) and perceived craving, other laboratory tasks (SSRT and BART) or even OUD severity.
Fig. 1.
Correlation between the delay discounting task and slow-release oral morphine treatment parameters, A/ dosage in mg/day and B/ duration in weeks.
With respect to the laboratory tasks, we found no correlation between each of them and the number of substances used or overused. However, we found a trend between delay discounting and the number of substances overused (S = 2796.2; P value = 0.07243; rho = −0.3815313). This trend is also maintained between delay discounting and the total ASSIST score (S = 2761.7; P value = 0.08727; rho = −0.3644906).
In addition, we found no correlation between the number of DSM-5 criteria and treatment parameters or laboratory tasks or the number of substances used/overused.
Discussion
The analysis showed a positive acute effect of SROM on craving but not on impulsivity, while it’s long-term effect appeared to be inverse with no effect on craving and a positive correlation between SROM treatment parameters and delay discounting.
Indeed, we observed a significant decrease in craving after SROM administration even though SROM dosages were stable for at least 3 months. Concerning the impact on craving, it has been proven for all OMTs that higher dosages are more effective (Fareed et al., 2011). However, higher dosages risk precipitating adverse effects and generating patient resistance in treatment adhesion (Coffa and Snyder, 2019). Thus, SROM appears to be an attractive OMT for patients with cardiac disease or intolerance to other OMTs because of the lesser physical side effects and the lack of impact on the QT interval (Klimas et al., 2019; Verthein et al., 2015). Besides, SROM appears to have similar efficacy on the illicit drug consumption as methadone (Beck et al., 2014) and even superior efficacy for craving (Klimas et al., 2019). Concerning this last point, our data provided some nuances regarding the impact of SROM on craving. Indeed, our data suggest that although SROM is effective on the perception of craving just after administration, it appears that this effect does not persist beyond 24 h as shown by the reappearance of significant craving just before the next treatment intake. Other studies which suggest the superiority of SROM on craving over methadone give no information about when craving was evaluated. These findings on craving appear to be a general perception (Falcato et al., 2015; Klimas et al., 2019). To our knowledge, the pharmacokinetic characteristics of the two molecules could explain why the perception of craving may be different. Methadone, the reference treatment always compared to SROM, is well known for its long elimination half-life of about 24 h (Grissinger, 2011), whereas SROM has a long action due to extended-release capsules with a shorter elimination half-life of about 11–13 h (Gourlay, 1998). From a pharmacokinetic point of view, this SROM characteristic could induce an end-of-dose effect with the re-emergence of more important craving than for methadone. Despite this aspect, it appears that patients report sufficient relief of their craving with SROM (Falcato et al., 2015; Klimas et al., 2019), and a majority even prefer it to methadone (Mitchell et al., 2003). At this stage of our reflection, we can confirm the favorable therapeutic impact of SROM on craving and exclude the risk of dosage escalation.
Regarding acute SROM impact on impulsivity, we did not observe any significate difference in the three tasks before and after SROM administration. This observation is in line with certain previous studies that conclude a lack of impact of opioids on impulsivity (Harty et al., 2011; Zacny and de Wit, 2009). However, these studies were difficult to transpose to alternative clinical settings because their results were derived from animal studies (Harty et al., 2011) or studies on healthy volunteers (Zacny and de Wit, 2009). Studies with opposite results on the impact of opioids on impulsivity found them to either increase or decrease it. These contradictory data seem to be explained by their methodological frameworks that differed significantly. Observation of an increased impulsivity effect of opiates is obtained either by self-report measures (Kirby et al., 1999; Madden et al., 1997; Giordano et al., 2002) or by clinical observation of risky behavior (Odum et al., 2000; Giordano et al., 2002; Dissabandara et al., 2014). Evidence from laboratory tasks that showed a deficit in inhibition and impulse control (Lee and Pau, 2002; Yang et al., 2015; Li et al., 2020) and response shifting (Fishbein et al., 2007) induced by opioids, are conducted through population comparison. The methodology applied cannot differentiate the effects of the substance and what may be an inherent component of the addictive disorder. Thus, the increase in impulsivity observed in these studies is much more likely to reflect the addictive disorder than a true pharmacological impact. In fact, in Yang et al. (2015), methadone was identified as altering the control of inhibition. However, it seems in this study that the HDI subjects receiving OMT tend to present better performances than the HDI without OMT (Yang et al., 2015). Besides, in a therapeutic context lower impulsivity has been observed in patients who received methadone than in abstinent individuals with a history of heroin dependence (Liao et al., 2014). This observation leads to the hypothesis that opioids used in a therapeutic context could in the long term correct the deficit in inhibitory control observed in HDI (Liao et al., 2014; Yang et al., 2015).
To determine whether the therapeutic context could have a long-term impact on performance we realized a correlation analysis of scores obtained before administration of SROM and the duration of SROM and its dosage. We found a positive correlation between the SROM treatment parameters, such as duration and posology and results obtained in the delay discounting task. These results led us to conclude that the longer the patients have been in treatment and the higher the dosage, the less impulsive they are. This observation between treatment parameters and delay discounting has been made in previous studies with two main possible explanations for this phenomenon. The first explanation was that patients with lower impulsivity are more likely to engage in a long-term care protocol, and show a higher level of adherence to treatment. Indeed, higher impulsivity has been associated with low retention and weak outcomes in psychotherapy (Hershberger et al., 2017). The second explanation is that therapy could have a positive influence on impulsivity and therefore the longer people are treated the less impulsive they would become (Liao et al., 2014; Yang et al., 2015). Only the correlation made on the dosage could lead us to conclude that the molecule may impact on the performances. However, the reality seems more complex. Clinical experiences teach us that subjects who wish to pursue illicit consumption alongside OMT are often reluctant to take higher doses for fear of losing the positive reinforcement of secondary consumption. Thus, a higher dosage could also reflect a higher level of treatment adherence. In our study setting, it is not possible to determine whether less impulsivity in delay discounting is a favorable marker of good adherence to care or whether the quality of treatment improves this aspect.
Our study has several limitations, the main ones being the small sample size and its heterogeneity. Our sample consisted mainly of poly-consumers and only included a very small proportion of women. In fact, among HDI poly-substance use appears to be the rule rather than the exception (Carlsen et al., 2020) and epidemiological studies highlight the under-representation of women (Observatoire européen des drogues et des toxicomanies, 2013; Pierce et al., 2018). Even if these aspects confer certain representativeness of the HDI population, we do not deny the impact on the laboratory performance. Notably, as higher impulsivity in delay discounting has been associated in the previous literature with the severity of addiction (Amlung et al., 2017; Kluwe-Schiavon et al., 2020) and with the number of substances overused (Moody et al., 2016), we performed additional analyses. However, in our study, we failed to identify any correlation between the severity evaluated by the DSM-5 and delay discounting. And in terms of the number of substances overused, we only found a trend with delay discounting, which could be explained by a two-substance threshold effect described previously (Moody et al., 2016), whereas our participants used a mean of 3.91 substances and overused 2.43 substances. Another limitation is our lack of knowledge of the last substance used, whose acute effect may affect the performance in the tasks. Finally, the last limitations were caused by the open-label observational design. Thus, to conclude on the acute and long-term effect, respectively, two different designs should be considered. Regarding the acute effect of SROM, it will be interesting to continue the investigation with a double-blind randomized controlled trial to compare each opioid. Indeed, the current methodology does not allow concluding on a specific action of SROM. Considering the result of the delay discounting, it will be very interesting for future research to explore this aspect in a longitudinal protocol, to clarify whether the better performance at delay discounting reflects a better prognostic or a positive impact of the therapeutic.
In conclusion, we were able to demonstrate the acute therapeutic impact of SROM on craving, with no impact on impulsivity. Finally, the observation of the correlation between delay discounting and the duration and dosage of OMT is of great interest and should be studied further.
Table 2.
Effect of slow-release oral morphine administration
| Measurements | Before SROM administration | After SROM administration | P value | Effect size |
|---|---|---|---|---|
| Craving | 5.22 (SD: 5.74) | 2.39 (SD: 4.05) | 0.0010 | 0.759 |
| Impulsivity measures | ||||
| BARTa | 16.28 (SD: 6.57) | 17.49 (SD: 8.18) | 0.211 | 0.266 |
| SSRTb | 323.53 (SD: 43.66) | 330.56 (SD: 77.79) | 0.823 | 0.050 |
| Delay discountingc | 68.24 (SD: 16.98) | 69.10 (SD: 18.15) | 0.961 | 0.006 |
BART, Balloon Analogue Risk Task; SROM, slow-release oral morphine; SSRT,stop-signal reaction time.
Mean adjusted pumps.
Mean reaction time in ms.
AUC.
Acknowledgements
The authors thank all volunteers for participating in the study.
This study was supported by a grant from Mundipharma Medical Company and CARIGEST SA: www.carigest.ch (24, rue de l’Athénée, CH - 1206 Genève, +41 (0)22 839 72 90 Fax: +41 (0)22 839 72 99, carigest@carigest.ch.
Conceived and designed the experiments: D.Z., G.T., S.R., G.C. Performed the experiments: G.C. Analyzed the data: L.C. and J.G. Contributed reagents/materials/analysis tools: L.C., S.T. and J.G. Contributed to the writing of the manuscript: J.G., G.T., S.R., G.C., L.P., D.Z.
Conflicts of interest
G.T. has received reimbursement for attending congresses from the following companies: Eli Lilly; D.Z. has received research support from Eli Lilly, Organon, Wyeth, Sanofi-Synthelabo, Aventis and Janssen-Cilag; He is/has been a member of advisory boards for Eli Lilly, Wyeth, Astra Zeneca, Pfizer and Lundbeck; He has received speakers fees from Astra Zeneca, Eli Lilly, Janssen-Cilag, GlaxoSmithKline, Novartis, Pfizer, Organon, Wyeth, Lundbeck. He has received reimbursement for attending congresses from the following companies: Eli Lilly, Wyeth, Astra Zeneca, GlaxoSmithKline, Organon, Janssen-Cilag, GlaxoSmithKline, Lundbeck and Mundipharma. For the remaining authors, there are no conflicts of interest.
References
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. (2013). Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev 2013:CD003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). American Psychiatric Association. [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. (2017). Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction 112:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antons S, Brand M. (2018). Trait and state impulsivity in males with tendency towards Internet-pornography-use disorder. Addict Behav 79:171–177. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Beck T, Haasen C, Verthein U, Walcher S, Schuler C, Backmund M, et al. (2014). Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone. Addiction 109:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim BD, Rangel A. (2004). Addiction and cue-triggered decision processes. Am Econ Rev 94:1558–1590. [DOI] [PubMed] [Google Scholar]
- Carlsen SL, Lunde LH, Torsheim T. (2020). Opioid and polydrug use among patients in opioid maintenance treatment. Subst Abuse Rehabil 11:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffa D, Snyder H. (2019). Opioid use disorder: medical treatment options. Am Fam Physician 100:416–425. [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, et al. (2001). Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry 158:1680–1686. [DOI] [PubMed] [Google Scholar]
- Dissabandara LO, Loxton NJ, Dias SR, Dodd PR, Daglish M, Stadlin A. (2014). Dependent heroin use and associated risky behaviour: the role of rash impulsiveness and reward sensitivity. Addict Behav 39:71–76. [DOI] [PubMed] [Google Scholar]
- Falcato L, Beck T, Reimer J, Verthein U. (2015). Self-reported cravings for heroin and cocaine during maintenance treatment with slow-release oral morphine compared with methadone: a randomized, crossover clinical trial. J Clin Psychopharmacol 35:150–157. [DOI] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. (2011). Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis 30:27–38. [DOI] [PubMed] [Google Scholar]
- Ferri M, Davoli M, Perucci CA. (2011). Heroin maintenance for chronic heroin-dependent individuals. Cochrane Database Sys Rev 12:CD003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, et al. (2007). Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend 90:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. (2002). Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Berl) 163:174–182. [DOI] [PubMed] [Google Scholar]
- Gourlay GK. (1998). Sustained relief of chronic pain. Pharmacokinetics of sustained release morphine. Clin Pharmacokinet 35:173–190. [DOI] [PubMed] [Google Scholar]
- Grissinger M. (2011). Keeping patients safe from methadone overdoses. P T 36:462–466. [PMC free article] [PubMed] [Google Scholar]
- Harty SC, Whaley JE, Halperin JM, Ranaldi R. (2011). Impulsive choice, as measured in a delay discounting paradigm, remains stable after chronic heroin administration. Pharmacol Biochem Behav 98:337–340. [DOI] [PubMed] [Google Scholar]
- Hershberger AR, Um M, Cyders MA. (2017). The relationship between the UPPS-P impulsive personality traits and substance use psychotherapy outcomes: a meta-analysis. Drug Alcohol Depend 178:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. (2008). Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 103:1039–1047. [DOI] [PubMed] [Google Scholar]
- Khan R, Chatton A, Nallet A, Broers B, Thorens G, Achab-Arigo S, et al. (2011). Validation of the French version of the alcohol, smoking and substance involvement screening test (ASSIST). Eur Addict Res 17:190–197. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87. [DOI] [PubMed] [Google Scholar]
- Klimas J, Gorfinkel L, Giacomuzzi SM, Ruckes C, Socías ME, Fairbairn N, Wood E. (2019). Slow release oral morphine versus methadone for the treatment of opioid use disorder. BMJ Open 9:e025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe-Schiavon B, Viola TW, Sanvicente-Vieira B, Lumertz FS, Salum GA, Grassi-Oliveira R, Quednow BB. (2020). Substance related disorders are associated with impaired valuation of delayed gratification and feedback processing: a multilevel meta-analysis and meta-regression. Neurosci Biobehav Rev 108:295–307. [DOI] [PubMed] [Google Scholar]
- Koller G, Schwarzer A, Halfter K, Soyka M. (2019). Pain management in opioid maintenance treatment. Expert Opin Pharmacother 20:1993–2005. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Pau CWH. (2002). Impulse control differences between abstinent heroin users and matched controls. Brain Inj 16:885–889. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. (2002). Evaluation of a behavioral measure of risk taking: the balloon analogue risk task (BART). J Exp Psychol Appl 8:75–84. [DOI] [PubMed] [Google Scholar]
- Li J, Weidacker K, Mandali A, Zhang Y, Whiteford S, Ren Q, et al. (2020). Impulsivity and craving in subjects with opioid use disorder on methadone maintenance treatment. Drug Alcohol Depend 219:108483. [DOI] [PubMed] [Google Scholar]
- Liao DL, Huang CY, Hu S, Fang SC, Wu CS, Chen WT, et al. (2014). Cognitive control in opioid dependence and methadone maintenance treatment. PLoS One 9:e94589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. (1997). Impulsivity and inhibitory control. Psychol Sci 8:60–64. [Google Scholar]
- Lynam DR, Miller JD. (2004). Personality pathways to impulsive behavior and their relations to deviance: results from three samples. J Quant Criminol 20:319–341. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. (1997). Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256–262. [DOI] [PubMed] [Google Scholar]
- Mitchell TB, White JM, Somogyi AA, Bochner F. (2003). Comparative pharmacodynamics and pharmacokinetics of methadone and slow-release oral morphine for maintenance treatment of opioid dependence. Drug Alcohol Depend 72:85–94. [DOI] [PubMed] [Google Scholar]
- Moody L, Franck C, Hatz L, Bickel WK. (2016). Impulsivity and polysubstance use: a systematic comparison of delay discounting in mono-, dual-, and trisubstance use. Exp Clin Psychopharmacol 24:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshier SJ, Ewen M, Otto MW. (2013). Impulsivity as a moderator of the intention-behavior relationship for illicit drug use in patients undergoing treatment. Addict Behav 38:1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Observatoire européen des drogues et des toxicomanies (2013). Rapport européen sur les drogues: Tendances et évolutions. Office des publications officielles des Communautés européennes. [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. (2000). Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend 60:259–266. [DOI] [PubMed] [Google Scholar]
- Pierce M, Millar T, Robertson JR, Bird SM. (2018). Ageing opioid users’ increased risk of methadone-specific death in the UK. Int J Drug Policy 55:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. (1991). Subjective probability and delay. J Exp Anal Behav 55:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socias ME, Wood E, Dong H, Brar R, Bach P, Murphy SM, Fairbairn N. (2020). Slow release oral morphine versus methadone for opioid use disorder in the fentanyl era (pRESTO): protocol for a non-inferiority randomized clinical trial. Contemp Clin Trials 91:105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Paxton J, Moeller FG, Wilson MJ, Bozgunov K, Martin EM, et al. (2014). Heroin and amphetamine users display opposite relationships between trait and neurobehavioral dimensions of impulsivity. Addict Behav 39:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo A, Toribio I, Orozco C, Puente KL, Pérez-García M. (2005). Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug Alcohol Depend 78:283–288. [DOI] [PubMed] [Google Scholar]
- Verthein U, Beck T, Haasen C, Reimer J. (2015). Mental symptoms and drug use in maintenance treatment with slow-release oral morphine compared to methadone: results of a randomized crossover study. Eur Addict Res 21:97–104. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. (2008). Test-retest characteristics of the balloon analogue risk task (BART). Exp Clin Psychopharmacol 16:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ASSIST Working Group (2002). The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction (Abingdon, England) 97:1183–1194. [DOI] [PubMed] [Google Scholar]
- Xu S, Korczykowski M, Zhu S, Rao H. (2013). Assessment of risk-taking and impulsive behaviors: a comparison between three tasks. Soc Behav Pers 41:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Xu Q, Li S, Zhao X, Ma L, Zheng Y, et al. (2015). The effects of methadone maintenance treatment on heroin addicts with response inhibition function impairments: evidence from event-related potentials. J Food Drug Anal 23:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Lin HC, Wang PW, Ko CH, Lee KH, Hsu CY, et al. (2016). Heroin craving and its correlations with clinical outcome indicators in people with heroin dependence receiving methadone maintenance treatment. Compr Psychiatry 65:50–56. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. (2009). The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav 94:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]