Abstract
Higher γδ T cell counts in patients with malignancies are associated with better survival. However, γδ T cells are rare in the blood and functionally impaired in patients with malignancies. Promising results are reported on the treatment of various malignancies with in vivo expansion of autologous γδ T cells using zoledronic acid (zol) and interleukin-2 (IL-2). Here we demonstrated that zol and IL-2, in combination with a novel genetically engineered K-562 CD3scFv/CD137L/CD28scFv/IL15RA quadruplet artificial antigen-presenting cell (aAPC), efficiently expand allogeneic donor-derived γδ T cells using a Good Manufacturing Practice (GMP) compliant protocol sufficient to achieve cell doses for future clinical use. We achieved a 633-fold expansion of γδ T cells after day 10 of coculture with aAPC, which exhibited central (47%) and effector (43%) memory phenotypes. In addition, >90% of the expanded γδ T cells expressed NKG2D, although they have low cell surface expression of PD1 and LAG3 inhibitory checkpoint receptors. In vitro real-time cytotoxicity analysis showed that expanded γδ T cells were effective in killing target cells. Our results demonstrate that large-scale ex vivo expansion of donor-derived γδ T cells in a GMP-like setting can be achieved with the use of quadruplet aAPC and zol/IL-2 for clinical application.
Key Words: Quadruplet artificial antigen-presenting cell, Autologous gamma delta T cells, GMP-compliant protocol
γδ T cells are innate-like T lymphocytes that have a T cell receptor (TCR) from rearranged γ and δ genes and can quickly identify pathogens in a major histocompatibility complex-independent manner and act as the first line of defense for the immune system.1 In contrast to αβ T cells, γδ T cells can recognize a broad range of antigens in cancer cells with their innate cytotoxicity receptors, which reduces the chance of treatment failure by antigen loss.2 The ability to kill tumor cells and their lack of alloreactivity have made γδ T cells a focus of research for adoptive immunotherapy in recent years.
Intratumoral γδ T cells are found to be strongly predictive of survival in patients with solid organ malignancies.3 The presence of high levels of γδ T cells in the bone marrow or peripheral blood of patients is associated with improved survival in patients with leukemia and many other cancer types.4–7 The increased recovery of γδ T cells in patients with acute leukemia who receive αβ T cell depleted hematopoietic stem cell transplant (HCT) influences better disease-free survival.8 Preemptive or prophylactic immunotherapy with donor lymphocyte infusion after HCT can reduce the risk of relapse and prolong survival in patients with acute leukemia at a high risk of relapse after HCT.9,10 However, the aβ T cell content of donor lymphocytes can result in life-threatening graft versus host disease (GVHD).11 Selective removal of donor aβ T cells can eliminate the risk of GVHD while preserving the antileukemia effects of γδ T cells, which have potent cytotoxic activity against neoplastic cells in a major histocompatibility complex-independent pathway.12 The antineoplastic activity of γδ T cells is mainly through direct cytolysis, but also includes NKG2D-mediated cytolysis and antibody-dependent cell-mediated cytotoxicity.13–15 Antineoplastic cytotoxic activity of γδ T cells has also been demonstrated in a xenogenic leukemia model without increased risk of GVHD.16
The infusion of greater numbers of γδ T cells from donor lymphocytes may increase the curative outcome of patients with leukemia. However, γδ T cells are rare in the blood of healthy donors, comprising only 0.5%–5% of circulating total T cells, and even lower in numbers and functionally impaired or exhausted in patients with malignancies,17 resulting in an insufficient dose for effective antineoplastic activity.18,19 Promising early-phase clinical trial results have shown that γδ T cells circulating in the blood can be expanded in vivo with zoledronic acid (zol) and interleukin-2 (IL-2).5,6,16,20,21 Zol is an aminobisphosphonate that inhibits farnesyl pyrophosphate synthase, the enzyme acting downstream of isopentenyl pyrophosphate in the mevalonate pathway, leading to increased intracellular levels of isopentenyl pyrophosphate and subsequent activation of γδ T cells.22 However, there is little experience with donor-derived allogeneic γδ T cell adoptive immunotherapy.23
Ex vivo expansion of γδ T cells with the use of artificial antigen-presenting cells (aAPC) has previously been reported to be feasible, particularly when zol and IL-2 were used in the initial γδ T cell enrichment phase.24,25 Previous studies suggest that CD137 ligation induces proliferative signaling24,25 and CD28 mediates costimulation, which can lead to the activation of γδ T cells.26 We expanded upon these observations by incorporating a novel genetically engineered K-562 CD3scFv/CD137L/CD28scFv/IL15RA aAPC with zol and IL-2 in an ex vivo expansion protocol of donor-derived γδ T cells for the treatment of HCT recipients with high-risk acute leukemia.27 These quadruple aAPCs were originally developed for the expansion of αβ T cells.27 Herein, we conducted this study to develop a current Good Manufacturing Practice (cGMP)-like protocol for large-scale manufacturing of clinical-grade γδ T cells with the use of our engineered K-562 aAPC in combination with zol/IL-2.
MATERIALS AND METHODS
Cells
Healthy donor apheresis was purchased from All Cells. K-562 and Chinese hamster ovary (CHO) cells were purchased from ATCC. Cell lines were authenticated by using a cell line authentication kit (ATCC). Before coculture with γδ T cells CHO target cells and K-562 cells were cultured in their own unique media. CHO cells were grown in F-12K media supplemented with 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin. RPMI media supplemented with 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin was used to culture K-562 cells. Certified bovine spongiform encephalitis-free fetal bovine serum was purchased from Atlanta Biologicals, and all other media and reagents were obtained from ThermoFisher.
Genetic Constructs and Cell-based aAPCs
All constructs used the SFG retroviral backbone and were cloned by GENEWIZ. The SFG plasmid was modified to include an antihuman CD3 scFv, a P2A selfcleaving sequence, and human CD137L. The second SFG-based construct included an antihuman CD28 scFv, a P2A selfcleaving sequence, and human IL15RA. Full sequences can be found in Table S1 (Supplemental Digital Content 1, http://links.lww.com/JIT/A695). Both SFG constructs were transfected into H29 packaging cells using a Calcium Phosphate Transfection Kit (Prometa, Madison) as previously described.28,29 K-562 cells were transduced with H29 retroviral supernatant expressing CD3scFv/CD137L, and cultured in RPMI complete media for 4–5 days. K-562 CD137L positive cells were flow-sorted with a 5-laser FACSAria (BD Biosciences) and expanded in RPMI media. K-562 CD3scFv/137L cells were then transduced with H29 retroviral supernatant expressing CD28scFv/IL15RA. CD137L and IL15RA double-positive cells were flow-sorted with a 5-laser FACSAria. K-562 CD3scFv/CD137L/CD28scFv/IL15RA cells were expanded, collected, and cryopreserved.
Enrichment and Expansion of γδ T Cells
1×109 Peripheral blood mononuclear cells (PBMC) were obtained from apheresis products from each donor and used to initiate the expansion process in AIM-V supplemented with 10% human AB serum, 5 μM zol, and 300IU/mL IL-2. On day 0, 1×109 PBMC were seeded into GREX 100 flasks at a concentration of 1×106 cells/ml in a total of 1000 mL per flask. On day 7, cells were harvested and subjected to αβ T cell depletion. Seeding densities of γδ T cells post αβ T cell depletion and total viable counts of γδ T cells at harvest are listed in the supplementary Table S2 (Supplemental Digital Content 1, http://links.lww.com/JIT/A695). Media changes occurred if the Glucose level was ≤250 dL or the lactate level was ≥7 mmol/L. Total viable cell count was not held constant. The AIM-V supplemented media was used at all times with γδ T cells including when they were cocultured with K-562 aAPCs and CHO target cells.
Flow Cytometry
γδ T cells were defined by gating on live CD45+ CD3+ TCRγδ+ CD20− TCRαβ− cells. The percentage natural killer (NK) cells (live CD45+ CD16+ CD56+ CD3−) was also assessed. All the other biomarkers were gated on γδ T cells including γδ T cell memory subtypes: central memory (CM) defined as CD45RO+ CD45RA− CCR7+, effector memory (EM) as CD45RO+ CD45RA− CCR7−, terminally differentiated effector memory (EMRA) cells as CD45RO− CD45RA−, and naïve cells as CD45RO− CD45RA+.
γδ T cell Cytotoxicity
Cytotoxicity assays were performed on an xCelligence RTCA (real-time cell analysis) instrument (ACEA Biosciences) according to the manufacturer’s instructions. Briefly, γδ T cells were stimulated with CD3/CD28 Dynabeads (ThermoFisher) for 7 days. Target CHO cells were plated at 1×104 per well on an E-Plate 96. The next day γδ T cells were resuspended in a fresh complete medium without IL-2 and added onto target cells at various E/T ratios, and growth was monitored.
RESULTS
K-562 aAPCs Enhance γδ T cell Expansion
Cell-based aAPCs can be an economical way to generate a large number of antineoplastic T cells.30–33 We had previously generated aAPCs expressing CD3scFv, CD137L, and CD28scFv but these constructs included fluorescent proteins which are not suitable for clinical applications.27 To expand γδ T cells, we created a novel quadruple aAPC, K-562 CD3scFv/CD137L/CD28scFv/IL15RA, by transducing 2 vectors into K-562 cells (Fig. 1A). The first vector contained anti-human CD3 scFv, a P2A selfcleaving sequence, and human CD137L. The second was encoded for an anti-human CD28 scFv, a P2A self-cleaving sequence, and human IL15RA. After transduction, K-562 cells were FACS sorted, and only cells that were positive for both CD137L and IL15RA were collected and used for subsequent experiments (Fig. 1B).
FIGURE 1.
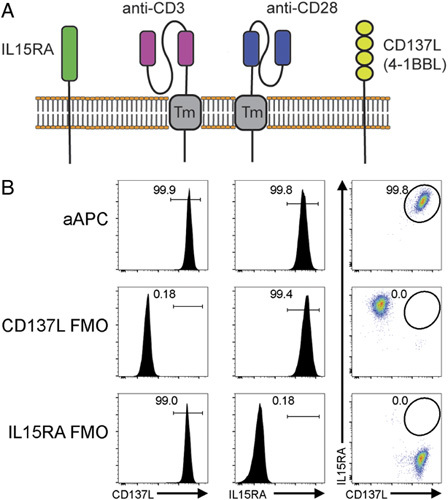
K-562 CD3scFv/CD137L/CD28scFv/IL15RA aAPC characterization. A, Schema of K-562 CD3scFv/CD137L/CD28scFv/IL15RA aAPC. B, Postsort analysis of aAPC. Flow cytometry plots and histograms of aAPCs and FMO controls. FMO indicates fluorescence minus one.
To investigate the ability of aAPCs to support γδ T cell expansion, we isolated γδ T cells from healthy donor PBMCs by αβ T cell depletion followed by CD3 positive selection. aAPCs were cultured with the enriched γδ T cells at a 100:1 aAPC:γδ T cell ratio for up to 14 days (Fig. 2A). At days 7, 10, and 14 after aAPC addition, cells were counted, and γδ T cell percentage was determined by flow cytometry (Fig. 2B). We found a 156-fold γδ T cell expansion at day 7 but by days 10 and 14, there was a 2612 and a 2429-fold increase in γδ T cells respectively from day 0 (Fig. 2C). We also observed an 820-fold expansion of CD16+ (Fig. 2D) and 1461-fold increase in CD56+ (Fig. 2E) γδ T cells after day 10 of aAPC coculture. The fold increase of these fell by day 14. These data are representative of 4 independent donors and demonstrate that γδ T cells can rapidly and significantly increase in numbers after aAPC coculture.
FIGURE 2.
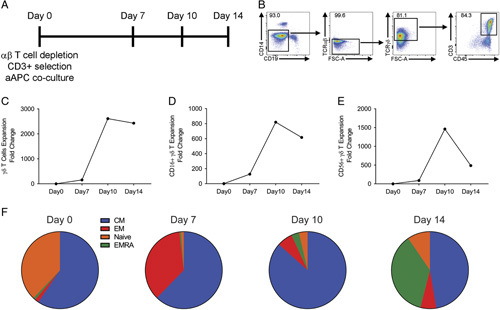
The coculture of K-562 aAPC enhances γδ T cell expansion and memory phenotype. A, Experimental timeline. At day 0, 1×106 γδ T cells were added to 1×108 irradiated aAPCs. At days 7, 10, and 14, a portion of cells was removed, counted, and phenotypic markers analyzed by flow cytometry. B, Flow cytometry gating strategy for γδ T cells. C, Coculture of γδ T cells with aAPCs results in 2429-fold expansion. D, CD16+ γδ T cell counts expand between days 0 and 10. E, CD56+ γδ T cells counts increase between days 7 and 10. F, Percentages of γδ T cell memory phenotypes at indicated days. Data representative of 4 independent, healthy donors.
To examine these γδ T cell phenotypes, we used flow cytometry and found that at all days examined the CM γδ T cells constituted the most abundant phenotype (Fig. 2F). At day 10, there were 86% CM cells, whereas at day 14, there was a reduction of CM cells to 47%, and effector γδ T cells had increased to 36%. In addition, we found <0.05% aAPC in the final expanded γδ T cell product, which is below our release criteria of 0.05%. Based on γδ T cell fold increase and memory phenotype, we determined that 10 days was the optimal coculture period.
γδ T cell Enrichment and Expansion by Zoledronic Acid and IL-2
Our data demonstrate that γδ T cell coculture with aAPCs enhances γδ T cell expansion and memory phenotypes. To further enhance γδ T cell expansion, we incorporated a preculture of PBMCs with 5μM zol and 300 IU/mL IL-2, as previously reported,25 before αβ T cell depletion and coculture with K-562 quadruplet aAPCs. By using this method, we were able to achieve enrichment of γδ T cells from 1.98%–54.58% while reducing the αβ T cell component from 67.40%–26.83% after 7 days of culture. γδ T cells were further enriched by αβ T cell depletion, which increased the average percentage of γδ T cells to 74.80% and decreased the αβ T cells to 0.05% (Table 1).
TABLE 1.
αβ T cell Depletion Enhances γδ T cell Purity
| γδ T cell average (%) | SD | |
|---|---|---|
| PBMC isolation (d-7) | 1.98 | 0.54 |
| Pre αβ depletion (d-0) | 54.58 | 58.80 |
| Post αβ depletion (d-0) | 74.80 | 26.80 |
| Harvest (d-10) | 75.23 | 32.43 |
Average γδ T cell percentage during GMP-like enrichment of 3 independent healthy donors.
PBMC indicates peripheral blood mononuclear cells.
10:1 is the Optimal aAPC:γδ T cell Ratio for Expansion
Our previous experiments (Fig. 2) were performed at aAPC:γδ T cell ratios of 100:1. To determine whether the total number of aAPCs could be reduced, thus reducing the culture volume and facilitating scale-up for clinical use, aAPC:γδ T cell ratios were examined. Enriched γδ T cells were cocultured with various numbers of aAPCs in fresh media containing the same concentration of zol and IL-2 that had been used from day −7–day 0 (Fig. 3A). We observed no substantial differences between 100:1, 50:1, and 10:1 aAPC:γδ T cell ratios in γδ T cell fold change or absolute count (Figure S1A, Supplemental Digital Content 1, http://links.lww.com/JIT/A695). Lower aAPC:γδ T cell ratios (0:1, 1:1, and 5:1) were evaluated in subsequent experiments and it was determined that γδ T cells had the greatest fold change and increased in the absolute count at a 10:1 ratio at both days 7 and 10 (Fig. 3B and Table 2). CD16+ γδ T cells (Fig. 3C and Figure S1B, Supplemental Digital Content 1, http://links.lww.com/JIT/A695) and CD56+ γδ T cells (Fig. 3D and Figure S1C, Supplemental Digital Content 1, http://links.lww.com/JIT/A695) were also optimally expanded at ratios of 10:1 and 50:1. Therefore, all subsequent experiments were performed at 10:1 aAPC:γδ T cell. Contamination of αβ T cells in postexpansion γδ T cell product was reproducibly <1%.
FIGURE 3.
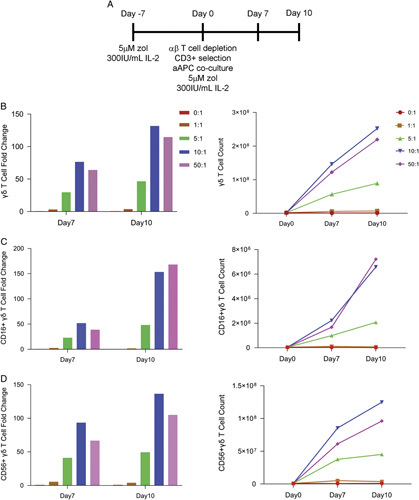
10:1 aAPC:γδ T cell ratio is optimal for expansion. A, Experimental timeline. PBMCs were cultured with zol and IL-2 at day -7. Zol enriched γδ T cells were cocultured with irradiated aAPCs at 1:0, 1:1, 5:1, 10:1, and 50:1 aAPC:γδ T cell ratios. A portion of cells was collected at days 7 and 10 for enumeration and phenotyping by flow cytometry. B, γδ T cell fold change and cell counts are highest at a 10:1 aAPC:γδ T cell ratio at days 7 and 10. C, CD16+ γδ T cell fold change and counts are similar at either a 10:1 or 50:1 aAPC:γδ T cell ratio. D, CD56+ γδ T cells have the highest fold change and count at a 10:1 aAPC:γδ T cell ratio at days 7 and 10. Data is from 1 healthy donor.
TABLE 2.
γδ T cell Expansion With Different aAPC Ratios
| aAPC:γδ T cell ratio | Total donors (N=) | γδ T cell fold change | SD |
|---|---|---|---|
| 0:1 | 2 | 5.73 | 6.73 |
| 1:1 | 1 | 4.9 | |
| 5:1 | 1 | 53.5 | |
| 10:1 | 3 | 491.3 | 296.0 |
| 50:1 | 2 | 451.0 | 409.1 |
| 100:1 | 1 | 778.7 |
Fold change and SD of γδ T cells at indicated aAPC:γδ T cell ratios.
Zol/IL-2 Enriched γδ T Cells Have Increased Expansion After aAPC Coculture
We performed flow cytometry to determine whether preculture with zol affects subsequent γδ T cell expansion with aAPC and their memory phenotype (Fig. 4A). We found γδ T cells cocultured with aAPCs resulted in a 730-fold increase for donor 1, a 132-fold increase for donor 2, and a 633-fold increase for donor 3 by day 10 (Fig. 4B). Absolute numbers of γδ T cells for donor 1 increased from 4.4×106 at day 0–3.2×109 at day 10. Donor 2 γδ T cells increased from 1.9×106–2.5×108 from day 0–10. γδ T cells also increased from 4.0×106 at day 0–2.5×109 by day 10 for donor 3 (Fig. 4C). We also observed a fold increase of 244, 153, and 259 in CD16+ (Fig. 4D) and 397, 136, and 2578 in CD56+ (Fig. 4E) γδ T cells on day 10 in the 3 donors respectively.
FIGURE 4.
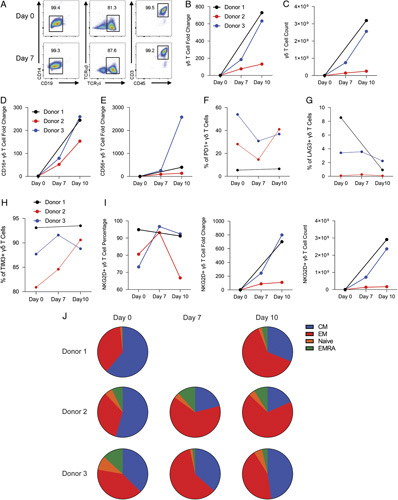
The coculture of zol enriched γδ T cells with K-562 aAPCs enhances expansion and memory phenotype. A, Flow cytometry gating strategy for γδ T cells. At days 7 and 10, a portion of cells was removed, counted, and phenotypic markers were analyzed by flow cytometry. B, Coculture of γδ T cells with aAPCs results in expansion. C, γδ T cell absolute numbers increase with aAPC coculture. D, CD16+ γδ T fold change increases after 10 days of aAPC coculture. E, CD56+ γδ T fold change expands with aAPC coculture. Percentage of γδ T cells that are PD1+ (F), LAG3+ (G), or TIM3+ (H). I, NKG2D percentage, fold change, and the number of γδ T cells expressing NKG2D increases after 10 days of aAPC coculture. J, Percentages of γδ T cell memory phenotypes at indicated days. At day 0 γδ T cells were added to irradiated aAPCs at a 10:1 aAPC:γδ T cell ratio. Data shows 3 independent, healthy donors.
The expression of inhibitory or cytotoxic markers on γδ T cells can affect function. After aAPC coculture, we found variability in PD1 expression between the donors (Fig. 4F), but a consistent decrease in the γδ T cell LAG3 expression level in all 3 donors (Fig. 4G). After aAPC coculture for 10 days we found increased TIM3 expression in donor 2, but stable levels in donors 1 and 3 (Fig. 4H). We also observed an increase in the percentage, fold change, and total count of γδ T cells expressing NKG2D for donors 1 and 3. In contrast, there was a decreased percentage and only a small increase in fold change and total count of NKG2D+ γδ T cells in donor 2 on day 10 (Fig. 4I).
To assess γδ T cell differentiation we analyzed naïve, CM, EM, and EMRA γδ T cells. We found that 32.3%±14.7 had a CM phenotype and 58.3%±13.1 expressed an EM phenotype at day 10 (Fig. 4J). We also observed a low percentage of 5.3%±2.9 of γδ T cells consistent with the EMRA cell phenotype at day 10.
γδ T Cells are Cytotoxic After aAPC Expansion
To demonstrate that culture with zol/IL-2 and quadruple aAPCs results in functional γδ T cells we examined their cytotoxicity in vitro using a real-time cell-killing assay. To better approximate use in a clinical setting, we used γδ T cells that were previously cryopreserved. When we examined the cytotoxic ability of these cells from 2 healthy donors we found they were able to effectively kill the target cells (Fig. 5). These results demonstrate that zol-enriched γδ T cells after 10 days of aAPC coculture retain their cytotoxic abilities.
FIGURE 5.
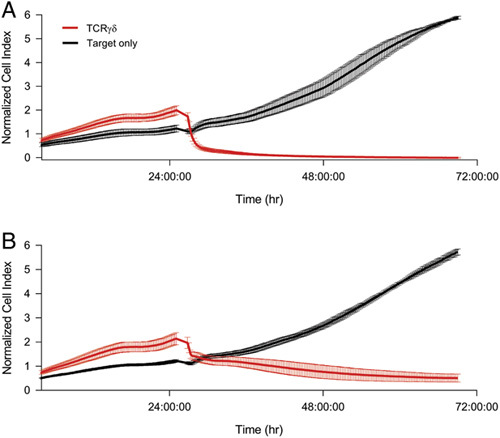
γδ T cells maintain cytotoxic function after expansion with aAPCs. A, Donor 1 γδ T cell cytotoxicity. B, Donor 2 γδ T cell cytotoxicity. Target CHO cells were cocultured with γδ T cells at a 10:1 E:T ratio in triplicate. Cytotoxicity was measured by an xCelligence RTCA assay.
DISCUSSION
Healthy donor γδ T cell infusion has antineoplastic therapeutic potential. However, low numbers of circulating peripheral blood γδ T cells limit their clinical use. Here we demonstrate that γδ T cells can significantly expand ex vivo in coculture with genetically engineered K-562 CD3scFv/CD137L/CD28scFv/IL15RA aAPC using a scaled-up production system suitable for clinical-grade cells. Thus, this methodology provides an opportunity to use ex vivo expanded healthy donor-derived γδ T cells for clinical application as antineoplastic immunotherapy.
Although our process builds upon the γδ T cell expansion protocol reported by Xiao et al,25 there are several critical differences between the methodologies used. After the initial step of zol and IL-2 treatment and subsequent αβ T cell depletion, we proceed with coculture using K-562 quadruplet aAPC and zol/IL-2 without the need to use human anti-CD3 monoclonal antibody OKT3 as our aAPCs already express anti-CD3. We originally generated aAPCs expressing anti-CD3, anti-CD28, and CD137L to use for the expansion of αβ T cells.27 The original genes published by Shrestha and colleagues included fluorescent proteins, which could not be used for GMP clinical production of cells. Therefore, we replaced the fluorescent reporters with CD137L and 41BBL to allow us to use them as surrogate markers for the scFv and to potentially provide benefits to ex vivo T cells in coculture. We did not examine how CD3scFv, CD137L, CD28scFv, or IL15RA individually affect γδ T cell expansion, or which may be the most important. However, CD137L is shown to be the dominant costimulatory proliferative signal on aAPCs for the expansion of γδ T cells.24 Xiao et al25 also reported using an aAPCs expressing CD137L in addition to CD64 and CD86 to expand Vγ9Vδ2 T cells in their study. In addition, CD28-mediated costimulation is necessary for the activation of γδ T cells,26 and IL-15 is important for in vivo expansion of γδ T cells in the absence of exogenous IL-2.34 This suggests that the expression of both anti-CD28scFv and IL15RA on aAPCs could further optimize our protocol for clinical application.
We determined that a 10:1 aAPC to γδ T cell ratio was optimal for expansion. This ratio is markedly <100:1 ratio used by Xiao et al25 which also included zol and IL-2 in their aAPC and γδ T cell coculture. The reduced ratio in our system can decrease costs by needing fewer aAPCs for a sufficient number of γδ T cell expansions to be used in a clinical trial setting.
Although several studies report the effective expansion of γδ T cells with in vivo use of zol in patients with malignancies,5,6,16,21,23 the experience of ex vivo γδ T cell expansion is still limited.25,35,36 We identified that the initial treatment of PBMCs with zol and IL-2 is an important phase that yields >90% γδ T cell enrichment. These γδ T cells preferentially express NKG2D that can further enhance the cytotoxicity of γδ T cells as previously reported.37,38 NKG2D is an activating receptor expressed on γδ T cells, CD8 T cells, and natural killer cells that can provide potent costimulatory and activation signals39,40 and mediate antineoplastic cytotoxicity.24,38,41 NKG2D expression with the use of quadruplet aAPCs in our protocol further increased to >90% after day 10 of expansion. These cells were found to exhibit potent cytotoxic activity against target cells. These findings suggest that our aAPC expanded γδ T cells can enhance tumor killing by NKG2D expression in addition to γδ T cell expansion. This is particularly important in acute myeloid leukemia (AML) therapy as NKG2D ligand expression in leukemic blasts is a determinant of susceptibility to γδ T cell cytotoxicity.15 We also observed a significant fold increase in CD16+ and CD56+ expressing γδ T cells following aAPC coculture, which can further enhance γδ T cell cytotoxicity by mechanisms that also include antibody-dependent cell-mediated cytotoxicity through CD16.42–45
Ex vivo stimulation and expansion of T cells can cause a transition through progressive stages of differentiation, which is characterized by a loss of effector function and therapeutic potential.46,47 We found that 10 days of coculture with aAPCs resulted in the optimal expansion of γδ T cell with less terminal differentiation as compared with expansion results at day 14. We also found an increase in EM but not in EMRA phenotype with the addition of zol into the coculture compared with our initial experiments without the use of zol. Thus, our observations demonstrate that incorporating zol in ex vivo expansion of γδ T cells with the use of aAPCs maintains their antineoplastic efficacy. This is an informative observation as T cells that maintain a less differentiated state are critical for therapeutic efficacy.25,47 Downregulation of immune checkpoint receptors can potentially promote effective antineoplastic activity.48 We could not determine the trend for checkpoint markers PD1, LAG3, and TIM3 because of high expression level variability between donors. This suggests that screening of donors for optimal phenotype after expansion may be beneficial.
Although the scarcity of γδ T cells circulating in patients with malignancies is a significant obstacle for γδ T cell adoptive transfer,17 our robust production system results in >600-fold increase in γδ T cells for donors 1 and 3, making ex vivo expanded γδ T cell immunotherapy feasible in patients with malignancies. Donor 2 had a modest 132-fold expansion that would likely not support use in the clinic. We hope to mitigate these occurrences in future clinical applications by screening healthy donors before expansion and only using high responders for allogeneic therapies. The effective reduction of aβ T cells to <1% in a final expansion product makes γδ T cells an attractive allogeneic donor-derived immunotherapy that is not associated with an increased risk of GVHD.11,25 Such therapy can potentially benefit patients with various cancer.49,50 Moreover, T cells in patients with malignancies can exhibit increased exhaustion phenotype,51 thus using allogeneic donor-derived γδ T cells can provide an additional advantage over the use of autologous cells as anticancer immunotherapy. We currently have an ongoing clinical trial that studies the safety and effectiveness of ex vivo aAPC expanded donor-derived γδ T cells for the treatment of patients with high-risk acute leukemia (ClinicalTrials.gov Identifier: NCT05015426).
Supplementary Material
Acknowledgments
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
None reported. All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
Grants from State of Florida Bankhead-Coley award, Moffitt Cancer Immunotherapy IIT award, PACT award-LLK NHLBI-ECB-HB-2016-09-JB, CCSG award-Cell Therapy Core Lab P30 CA076292.
A provisional patent has been filed over the CD3/CD137L/CD28/IL15RA aAPC and the production process.
J.C.B. and N.B.: wrote the manuscript; M.L.D. and N.B.: conceived the study and designed the experiments; B.Y., G.L., B.S., and A.M.L.: performed experiments; all authors interpreted the results, edited the manuscript, and approved the final manuscript.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Contributor Information
Justin C. Boucher, Email: justin.boucher@moffitt.org.
Bin Yu, Email: bin.yu@moffitt.org.
Gongbo Li, Email: gongbo.li@moffitt.org.
Bishwas Shrestha, Email: bishwas75@gmail.com.
David Sallman, Email: david.sallman@moffitt.org.
Ana Marie Landin, Email: ana.landin@moffitt.org.
Claudio Anasetti, Email: claudio.anasetti@moffitt.org.
Marco L. Davila, Email: marco.davila@roswellpark.org.
Nelli Bejanyan, Email: nelli.bejanyan@moffitt.org.
REFERENCES
- 1. Chien YH, Meyer C, Bonneville M. Gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. [DOI] [PubMed] [Google Scholar]
- 2. Capsomidis A, Benthall G, Van Acker HH, et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther. 2018;26:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gertner-Dardenne J, Castellano R, Mamessier E, et al. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immun. 2012;188:4701–4708. [DOI] [PubMed] [Google Scholar]
- 5. Tosolini M, Pont F, Poupot M, et al. Assessment of tumor-infiltrating TCRVgamma9Vdelta2 gammadelta lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology. 2017;6:e1284723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Godder KT, Henslee-Downey PJ, Mehta J, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–757. [DOI] [PubMed] [Google Scholar]
- 7. Perko R, Kang G, Sunkara A, et al. Gamma delta T-cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant. 2015;21:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamb LS, Jr, Henslee-Downey PJ, Parrish RS, et al. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother. 1996;5:503–509. [DOI] [PubMed] [Google Scholar]
- 9. Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–3262. [DOI] [PubMed] [Google Scholar]
- 10. Schmid C, Labopin M, Veelken H, et al. Efficacy, safety and long term results of prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia: a registry-based evaluation on 343 patients by the acute leukemia working party of EBMT. Blood. 2015;2015:863. [Google Scholar]
- 11. Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Airoldi I, Bertaina A, Prigione I, et al. Gammadelta T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minculescu L, Sengelov H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand J Immunol. 2015;81:459–468. [DOI] [PubMed] [Google Scholar]
- 14. Correia DV, d’Orey F, Cardoso BA, et al. Highly active microbial phosphoantigen induces rapid yet sustained MEK/Erk- and PI-3K/Akt-mediated signal transduction in anti-tumor human gammadelta T cells. PLoS One. 2009;4:e5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanca T, Correia DV, Moita CF, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–2411. [DOI] [PubMed] [Google Scholar]
- 16. Siegers GM, Felizardo TC, Mathieson AM, et al. Anti-leukemia activity of in vitro-expanded human gamma delta T cells in a xenogeneic Ph+ leukemia model. PLoS One. 2011;6:e16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribeiro ST, Ribot JC, Silva-Santos B. Five layers of receptor signaling in gammadelta T-cell differentiation and activation. Front immunol. 2015;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Rosa SC, Andrus JP, Perfetto SP, et al. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–1645. [DOI] [PubMed] [Google Scholar]
- 19. Acuto O, Hussey RE, Fitzgerald KA, et al. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983;34:717–726. [DOI] [PubMed] [Google Scholar]
- 20. Gertner-Dardenne J, Bonnafous C, Bezombes C, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. [DOI] [PubMed] [Google Scholar]
- 21. Kunzmann V, Smetak M, Kimmel B, et al. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–213. [DOI] [PubMed] [Google Scholar]
- 22. Nada MH, Wang H, Workalemahu G, et al. Enhancing adoptive cancer immunotherapy with Vgamma2Vdelta2 T cells through pulse zoledronate stimulation. J Immunother Cancer. 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilhelm M, Smetak M, Schaefer-Eckart K, et al. Successful adoptive transfer and in vivo expansion of haploidentical gammadelta T cells. J Transl Med. 2014;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deniger DC, Maiti SN, Mi T, et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res. 2014;20:5708–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao L, Chen C, Li Z, et al. Large-scale expansion of Vgamma9Vdelta2 T cells with engineered K562 feeder cells in G-Rex vessels and their use as chimeric antigen receptor-modified effector cells. Cytotherapy. 2018;20:420–435. [DOI] [PubMed] [Google Scholar]
- 26. Sperling AI, Linsley PS, Barrett TA, et al. CD28-mediated costimulation is necessary for the activation of T cell receptor-gamma delta+ T lymphocytes. J Immunol. 1993;151:6043–6050. [PubMed] [Google Scholar]
- 27. Shrestha B, Zhang Y, Yu B, et al. Generation of antitumor T cells for adoptive cell therapy with artificial antigen presenting cells. J Immunother. 2020;43:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li G, Park K, Davila ML. Gammaretroviral production and T cell transduction to genetically retarget primary T cells against cancer. Methods Mol Biol. 2017;1514:111–118. [DOI] [PubMed] [Google Scholar]
- 29. Gallardo HF, Tan C, Ory D, et al. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 30. Maus MV, Riley JL, Kwok WW, et al. HLA tetramer-based artificial antigen-presenting cells for stimulation of CD4+ T cells. Clin Immunol. 2003;106:16–22. [DOI] [PubMed] [Google Scholar]
- 31. Butler MO, Lee JS, Ansen S, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;13:1857–1867. [DOI] [PubMed] [Google Scholar]
- 32. Hasan AN, Kollen WJ, Trivedi D, et al. A panel of artificial APCs expressing prevalent HLA alleles permits generation of cytotoxic T cells specific for both dominant and subdominant viral epitopes for adoptive therapy. J Immunol. 2009;183:2837–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. [DOI] [PubMed] [Google Scholar]
- 34. Izumi T, Kondo M, Takahashi T, et al. Ex vivo characterization of gammadelta T-cell repertoire in patients after adoptive transfer of Vgamma9Vdelta2 T cells expressing the interleukin-2 receptor beta-chain and the common gamma-chain. Cytotherapy. 2013;15:481–491. [DOI] [PubMed] [Google Scholar]
- 35. Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol. 2015;15:683–691. [DOI] [PubMed] [Google Scholar]
- 36. Legut M, Cole DK, Sewell AK. The promise of gammadelta T cells and the gammadelta T cell receptor for cancer immunotherapy. Cell Mol Immunol. 2015;12:656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Niu C, Jin H, Li M, et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and gammadelta T cell-mediated lysis in multiple myeloma. Oncotarget. 2017;8:5954–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ang WX, Ng YY, Xiao L, et al. Electroporation of NKG2D RNA CAR improves Vgamma9Vdelta2 T cell responses against human solid tumor xenografts. Molecular Ther Oncolytics. 2020;17:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J, Basher F, Wu JD. NKG2D ligands in tumor immunity: two sides of a coin. Front Immunol. 2015;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rincon-Orozco B, Kunzmann V, Wrobel P, et al. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. [DOI] [PubMed] [Google Scholar]
- 41. Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [DOI] [PubMed] [Google Scholar]
- 42. Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tokuyama H, Hagi T, Mattarollo SR, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab. Int J Cancer. 2008;122:2526–2534. [DOI] [PubMed] [Google Scholar]
- 44. Seidel UJ, Vogt F, Grosse-Hovest L, et al. Gammadelta T cell-mediated antibody-dependent cellular cytotoxicity with CD19 antibodies assessed by an impedance-based label-free real-time cytotoxicity assay. Front Immunol. 2014;5:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fisher JP, Heuijerjans J, Yan M, et al. Gammadelta T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology. 2014;3:e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Witte MA, Sarhan D, Davis Z, et al. Early reconstitution of NK and gammadelta T Cells and its implication for the design of post-transplant immunotherapy. Biol Blood Marrow Transplant. 2018;24:1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abate G, Eslick J, Newman FK, et al. Flow-cytometric detection of vaccinia-induced memory effector CD4(+), CD8(+), and gamma delta TCR(+) T cells capable of antigen-specific expansion and effector functions. J Infect Dis. 2005;192:1362–1371. [DOI] [PubMed] [Google Scholar]
- 48. Lopez RD. Inhibiting inhibitory pathways in human gammadelta T cells. Blood. 2013;122:857–858. [DOI] [PubMed] [Google Scholar]
- 49. Bejanyan N, Oran B, Shanley R, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant. 2014;49:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Catakovic K, Klieser E, Neureiter D, et al. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal. 2017;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


