INTRODUCTION:
Patients with Crohn's disease (CD) experience a variety of symptoms that significantly affect their lives. In this study, we (i) ascertain the most prevalent and impactful symptoms in CD and (ii) identify modifying factors that are associated with a higher disease burden in CD.
METHODS:
We conducted semistructured interviews with adult participants with CD to determine what issues have the greatest impact on their lives. Next, we conducted a large cross-sectional study of individuals with CD to determine the prevalence and relative importance of those symptoms and themes and to identify the demographic features that are associated with a higher disease burden.
RESULTS:
Sixteen individuals with CD provided 792 direct quotes regarding their symptomatic burden. Four hundred three people with CD participated in our cross-sectional study. The symptomatic themes with the highest prevalence in CD were gastrointestinal issues (93.0%), fatigue (86.4%), dietary restrictions (77.9%), and impaired sleep or daytime sleepiness (75.6%). The symptomatic themes that had the greatest impact on patients' lives (0–4 scale) related to fatigue (1.82), impaired sleep or daytime sleepiness (1.71), gastrointestinal issues (1.66), and dietary restrictions (1.61). Symptomatic theme prevalence was strongly associated with a higher number of soft stools per day, greater number of bowel movements per day, missed work, employment and disability status, and having perianal disease.
DISCUSSION:
Patients with CD experience numerous symptoms that affect their daily life. These symptoms, some underrecognized, vary based on disease and demographic characteristics and represent potential targets for future therapeutic interventions.
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) with a rising global incidence that affects over 500,000 adults in the United States (1–4). Abdominal pain, diarrhea, fatigue, weight loss, and malnutrition occur commonly in CD and can dramatically affect patient quality of life (QoL) (5). Patients with CD also experience a variety of symptoms and issues not related to the luminal gastrointestinal tract including inflammation of the skin, eyes, and joints; anemia; functional limitations; emotional distress; body image dissatisfaction; and problems with sexual relationships (6,7).
Previous studies have investigated the wide spectrum of symptoms, such as lack of energy and poor relationships with food, that affect the physical, emotional, and social realms of CD patient QoL (8,9). A large online cohort study in 2011–2012 conducted through the Crohn's & Colitis Foundation of America reported more depression, anxiety, fatigue, sleep disturbance, pain interference, and social dissatisfaction in a cohort of patients with IBD (∼63% with CD) compared with the general population (10). A meta-analysis of 29 studies showed that factors such as disability level, disease/remission status, steroid treatment, and hospitalization rate were prime indicators of QoL in those with CD (11). Studies like these have also hypothesized that a deeper understanding of the determinants of CD patient health may facilitate interventions that improve patient outcomes (12–15).
An understanding of the relative importance of symptoms to a select group of patients is necessary to develop outcome measures that can quantify changes in disease burden over time. Additional data regarding symptomatic disease burden of CD will be useful to understand the content validity of the numerous outcome measures (e.g., Short Form Health Survey-36 [SF-36], Individualized Neuromuscular Quality of Life [INQoL], European Quality of Life [EuroQoL], Crohn's Disease Activity Index [CDAI], Inflammatory Bowel Disease Questionnaire [IBDQ], IMPACT, Rating Form of IBD Patient Concerns [RFIPC], Crohn's and Ulcerative Colitis Questionnaire-32 [CUCQ-32], Symptoms and Impacts Questionnaire for CD [SIQ-CD], Short Inflammatory Bowel Disease Questionnaire [SIBDQ]) that have been historically used in CD clinical trials (14–21).
In this study, we take a large-scale, patient-centric approach to comprehensively assess the physical, emotional, and social symptoms of CD. We collect data from semistructured interviews with individuals with CD and conduct a large cross-sectional study to determine the most prevalent and important symptoms from the perspective of patients in this disease population. This knowledge is potentially useful to clinicians who care for patients with CD and regulatory agencies, such as the Food and Drug Administration and European Medicines Agency, who evaluate the significance of clinical trial results and relevance of clinical trial outcome measures (22–24). The information gained from this study may also assist in determining clinically relevant targets, facilitating clinical decision making and therapeutic goal setting, and improving outcomes for patients with CD, according to the Selecting Therapeutic Targets in Inflammatory Bowel Disease-II study that encompasses evidence-based and consensus-based recommendations for treatment strategies in patients with CD (25).
METHODS
Study participants
Interviewed participants for this study were recruited from the University of Rochester gastrointestinal clinics. Participants for our cross-sectional study were recruited through the IBD Partners patient registry sponsored by the Crohn's & Colitis Foundation. Eligible participants were those who (i) had a current diagnosis of CD and (ii) were 18 years or older.
The University of Rochester Institutional Review Board approved all study activities. We conducted interviews between February 9 and December 20, 2018, and we conducted the subsequent cross-sectional study between March 12 and April 27, 2020.
Study design
Phase 1: CD qualitative interviews.
We conducted 30–60-minute semistructured, qualitative interviews with people with CD to identify the symptoms that have the greatest impact on their lives. Before beginning the research study, potential participants did not have any relationship or involvement with the study team. They were approached, with the permission of the attending physician, during clinic visits to the University of Rochester. They were informed of the purpose, risks, and benefits of the research and the names and contact information of the research team before they consented to participating in this study.
During the interviews, clinical research coordinators on the research team (C.Z. and E.L.) asked open-ended questions to participants regarding various physical, mental, social, and disease-specific areas of patient health. Interviews and participant quotes were recorded using Zoom (a Health Insurance Portability and Accountability Act-compliant conferencing software), transcribed, and coded by the research team (C.Z., E.L., and C.H.) to identify individual symptoms and symptomatic themes (groups of related symptoms). Coding of symptoms followed a qualitative framework technique and a 3-investigator consensus approach (26–31). We conducted patient interviews until data saturation was reached (i.e., when there was a minimal number of new symptoms being identified through the final interviews) (32).
Phase 2: Cross-sectional study of individuals with CD.
We conducted a large online cross-sectional study of people with CD from several countries, mainly the United States, to determine the prevalence and relative importance of the symptoms identified during qualitative interviews. Participants completed an online consent form and answered demographic questions before taking the symptom survey that consisted of questions representing 148 individual symptoms and 17 symptomatic themes. Individuals were asked to rate each symptom on a 6-point Likert-type scale, consisting of the following options: (i) I do not experience this; (ii) I experience this but it does not affect my life; (iii) It affects my life a little; (iv) It affects my life moderately; (v) It affects my life very much; and (vi) It affects my life severely. At the end, individuals were asked to list and rate any additional symptoms that were not included in the survey. Participants were required to complete at least 1 demographic question and 1 symptom question for their data to be included in the analysis.
Statistical analysis
We calculated the prevalence and impact of each symptom and symptomatic theme from phase 2 data. Prevalence was characterized as the number of participants who experienced a symptom (options 2–6 on the Likert scale) normalized by the total number of participants who responded to the symptom question. The average life impact of a symptom was calculated using all participants who reported having the symptom (options 2–6 on the Likert scale). Average life impact (0–4) was determined by assigning numerical values to each of the rating options on the Likert scale: 0 = I experience this but it does not affect my life; 1 = It affects my life a little; 2 = It affects my life moderately; 3 = It affects my life very much; and 4 = It affects my life severely. This methodology is similar to that used in existing health-related quality-of-life questionnaires in CD and more broadly in IBD (33).
Population impact (PIP) was calculated on a 0–4 scale by multiplying the prevalence of a symptom by its average life impact. A score of 0 corresponded to no impact on the population, whereas a score of 4 was linked to the highest possible impact on all patients. The methods performed here have been described and used previously for other diseases (26–30,34).
In addition to determining the prevalence and relative importance of symptoms in the overall population, we compared the prevalence of the symptomatic themes in predetermined subcategories based on (i) age (above mean vs below mean), (ii) sex (male vs female), (iii) education level (grade school, high school, technical degree, or none vs college, master's, or doctorate), (iv) employment standing (working full time, working part time, or stay-at-home parent vs on disability or not working/not on disability, excluding students, retired individuals, others), (v) disability status (on disability vs all other employment categories not on disability), and (vi) instances of missing work because of disease (missed work vs no missed work). Responses were also divided based on the experience of various symptoms or treatments, specifically (i) having a fistula (yes vs no), (ii) having a stricture (yes vs no), (iii) having an ostomy (yes vs no), (iv) having perianal disease (yes vs no), (v) symptom duration as the number of years since the onset of symptoms (above mean vs below mean), (vi) having bowel surgery (yes vs no), (vii) number of bowel movements per day (0, 1, or 2 = below median vs 3 or more = above median), (viii) number of liquid/soft stools per day (0 or 1 = below median vs 2 or more = above median), (ix) taking a steroidal drug (yes vs no), and (x) taking an immunomodulatory drug (yes vs no). Fisher exact tests were used to compare the prevalence of each symptomatic theme between groups. To correct for multiple comparisons, the Benjamini-Hochberg procedure was used with a false discovery rate of 0.05 and 272 test statistics. The 272 p-values were sorted from smallest to largest, and the largest value of i such that P(i) ≤ 0.05, i/272, was determined. The null hypotheses associated with the p-values P(1), …, P(i) were rejected, resulting in 116 “discoveries.”
Data availability
Anonymized data will be shared with qualified investigators on request.
RESULTS
Phase 1: CD qualitative interviews
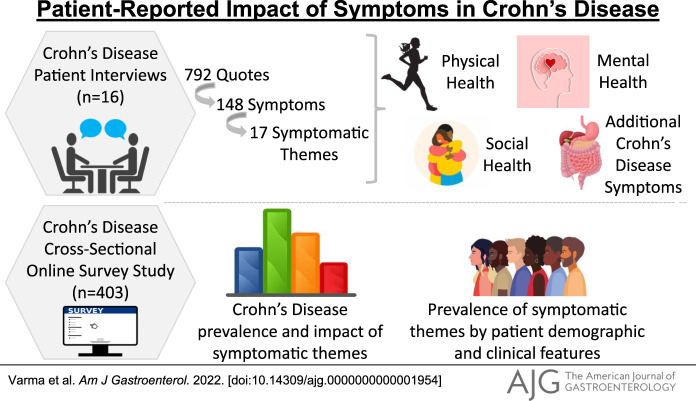
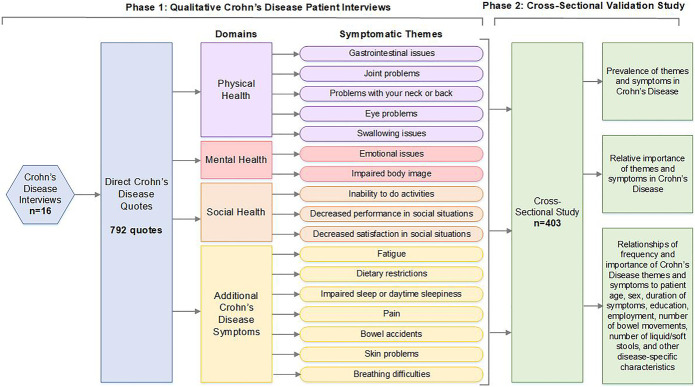
We conducted interviews with 16 participants with CD and obtained 792 patient quotes regarding the issues of greatest importance to participants. From these quotes, 148 unique symptoms were identified. These symptoms represented 17 symptomatic themes related to gastrointestinal issues, inability to do activities, problems with neck or back, fatigue, bowel accidents, emotional issues, swallowing issues, decreased performance in social situations, decreased satisfaction in social situations, impaired sleep or daytime sleepiness, impaired body image, pain, joint problems, eye problems, breathing difficulties, skin problems, and dietary restrictions.
Phase 2: Cross-sectional study of individuals with CD
Our cross-sectional study involved 403 participants from a geographically diverse population. This sample was 26.6% male and 73.4% female and represented an age range from 20 to 88 years with a mean age of 49.6 ± 15.9 years. Most of the participants (96.4%) identified as White, and 83.2% had a college degree or higher. Employed individuals made up 64.3% of the sample population. Participants were from 11 countries, with most of them (97%) being from the United States and Canada. Participants from the United States represented 45 of the 50 states. Table 1 provides additional details regarding the participants in this cross-sectional study, with percentages normalized for the number of people who answered each demographic question. Figure 1 presents a complete outline of our study activities.
Table 1.
CD demographic information from this cross-sectional study
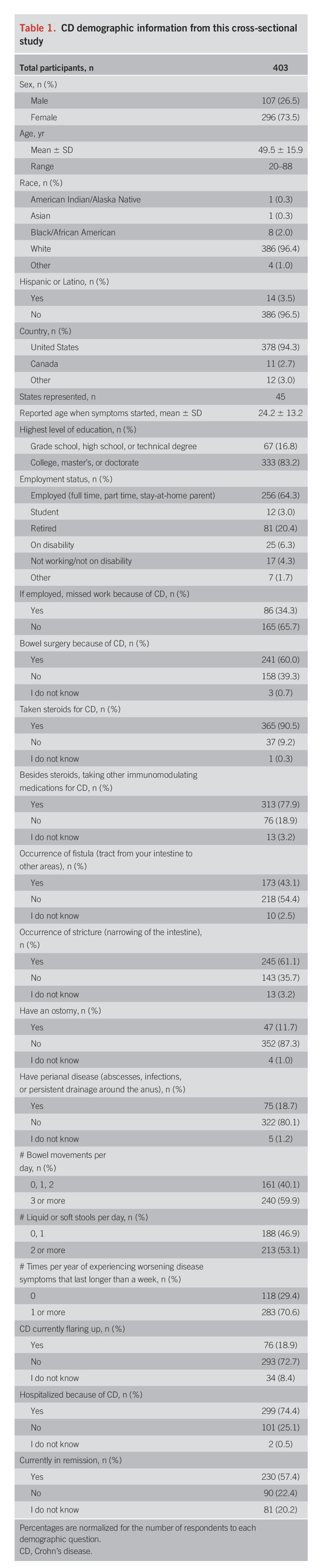
Figure 1.
Overview of study activities to identify symptoms of importance to individuals with Crohn's disease.
Prevalence of symptomatic themes and symptoms
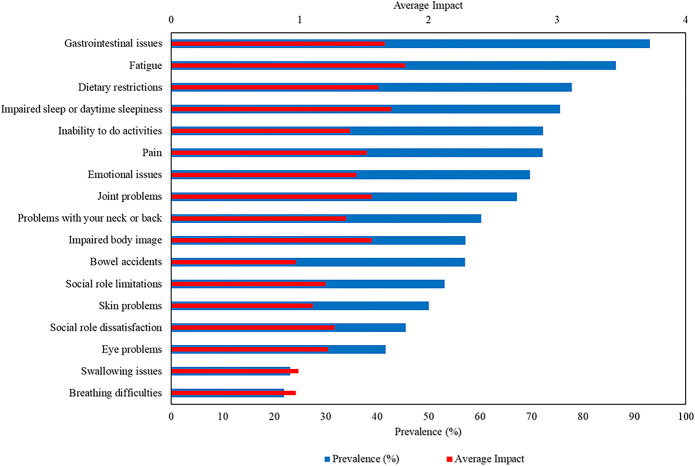
Among the 17 symptomatic themes included in the cross-sectional survey, the 4 symptomatic themes that occurred in more than three-quarters of participants with CD were gastrointestinal issues (93.0%), fatigue (86.4%), dietary restrictions (77.9%), and impaired sleep or daytime sleepiness (75.6%). Of the 148 symptoms evaluated, not including the symptomatic themes, the 5 most prevalent were tiredness (88.9%), decreased energy (87.6%), passing gas/farting (84.7%), frequent bathroom visits (82.0%), and having to modify diet because of disease (82.3%) (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C644).
Average life impact of symptomatic themes and symptoms
The symptomatic themes with the highest average life impact (on a scale of 0–4), as reported by those with CD from the cross-sectional survey, were fatigue (1.82), impaired sleep or daytime sleepiness (1.71), gastrointestinal issues (1.66), and dietary restrictions (1.61). The individual symptoms with the highest average life impact were worry about triggering symptoms with specific foods (1.86), having to modify diet because of disease (1.84), problems associated with the ostomy bag (1.82), exhaustion (1.77), and fatigue interfering with activities (1.77) (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C644). Prevalence and average life impact for the 17 symptomatic themes in CD are shown in Figure 2.
Figure 2.
Prevalence and average life impact of symptomatic themes, with prevalence values (blue bars) on the lower x-axis (ranging from 0% to 100%) and average life impact values (red bars) on the upper x-axis (ranging from 0 to 4).
PIP of symptomatic themes and symptoms
The symptomatic themes with the largest PIP were fatigue (1.57), gastrointestinal issues (1.54), impaired sleep or daytime sleepiness (1.30), and dietary restrictions (1.26). The individual symptoms with the highest PIP were tiredness (1.52), decreased energy (1.52), having to modify diet because of disease (1.52), worry about triggering symptoms with specific foods (1.45), and having to constantly be aware of where the nearest bathroom is (1.33) (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C644).
Demographic and clinical subgroup analysis of symptomatic theme prevalence
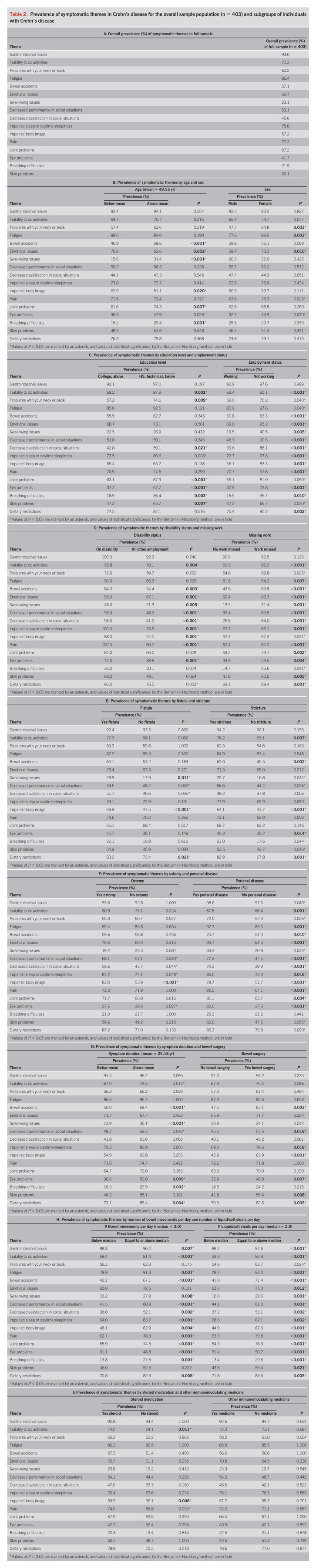
Symptomatic themes significantly differed in prevalence among subgroups, as listed in Table 2. The clinical feature that had the strongest association with the prevalence of symptomatic themes was the number of liquid/soft stools per day. Participants who reported equal to or more than the median number of liquid/soft stools per day (median = 2) had a higher prevalence in 16 of the 17 symptomatic themes (all categories, except problems with neck or back).
Table 2.
Prevalence of symptomatic themes in Crohn's disease for the overall sample population (n = 403) and subgroups of individuals with Crohn's disease
Other characteristics that were robustly associated with the prevalence of symptomatic themes were as follows:
Having equal to or more than the median number of bowel movements per day (median = 3) was connected with a higher frequency of 14 of the 17 symptomatic themes (all themes, except skin problems, problems with neck or back, and emotional issues).
Missed work because of CD in employed participants was associated with a higher prevalence of 13 symptomatic themes (all themes, except gastrointestinal issues, problems with neck or back, impaired sleep or daytime sleepiness, and breathing difficulties).
Those who were either on disability or not working compared with those who were working full time or part time or who were stay-at-home parents showed a higher occurrence of 12 themes: inability to do activities, bowel accidents, emotional issues, swallowing issues, decreased performance in social situations, decreased satisfaction in social situations, impaired sleep or daytime sleepiness, impaired body image, pain, eye problems, breathing difficulties, and dietary restrictions.
Having perianal disease was associated with a higher frequency of 11 themes: inability to do activities, fatigue, bowel accidents, emotional issues, decreased performance in social situations, decreased satisfaction in social situations, impaired sleep or daytime sleepiness, impaired body image, pain, joint problems, and eye problems.
Those on disability experienced 10 symptomatic themes at a higher frequency: pain, decreased performance in social situations, decreased satisfaction in social situations, emotional issues, impaired sleep or daytime sleepiness, impaired body image, eye problems, bowel accidents, inability to do activities, and swallowing issues.
Participants younger than the mean age of 49.6 years were more likely to report emotional issues and impaired body image while those who were older were more likely to report swallowing issues, bowel accidents, breathing difficulties, and joint problems. Female participants were more likely to have symptoms related to problems with neck or back, fatigue, and emotional issues compared with male participants.
The prevalence of several symptomatic themes was associated with participant education level. Participants with higher levels of education were less likely to report an inability to do activities, problems with their neck or back, a decreased satisfaction in social situations, joint problems, eye problems, breathing difficulties, and skin problems compared with individuals who did not obtain higher levels of education.
The presence of an ostomy was only associated with an increase in impaired body image, whereas the presence of strictures and presence of fistulas were associated with a higher prevalence of multiple symptomatic themes. The use of steroid medications was associated with an impaired body image and an inability to do activities. The use of other immunomodulation medications was not associated with a higher prevalence of any symptomatic themes.
DISCUSSION
This study explores the prevalence and relative importance of 148 symptoms comprising 17 symptomatic themes in a large population of individuals with CD recruited from the IBD Partners patient registry. This research reveals that people with CD are affected by a variety of symptoms that span physical, emotional, social, and disease-specific domains of health.
Prevalence and average life impact of patient-reported symptoms in CD
Although CD is frequently recognized and diagnosed because of its gastrointestinal symptoms, this research highlights the effects that many nongastrointestinal issues have on those diagnosed with CD. Indeed, fatigue was identified by respondents as the symptomatic theme that has the greatest impact on their lives. Other nongastrointestinal themes, such as those related to sleep, pain, and emotional issues, also had a high prevalence and relative impact in the study population and represent issues that are potentially amenable to therapeutic interventions in the clinical setting.
The symptoms in CD that had the greatest prevalence were not always the symptoms that had the greatest impact on participants' lives. We have found this to be true in multiple other disease populations studied through similar methods (26–30,35–39). Of the 148 symptoms evaluated, worry about triggering symptoms with specific foods had the greatest relative impact on patients' lives (1.86); however, this issue, which was experienced by 78.2% of the participants, was less prevalent than numerous other symptoms. Conversely, tiredness was the most common symptom reported by patients (88.9%), yet this symptom had a lower life impact (1.71) compared with many less prevalent symptoms. Understanding the distinction between prevalence of certain symptoms vs their impact on patients' lives is crucial for clinicians to be able to successfully treat their individual patients.
Symptomatic theme prevalence in CD patient subgroups
We discovered that the prevalence of many symptomatic themes varied based on both the clinical and demographic features of the population. Specifically, we found that a higher frequency of liquid/soft stools per day or bowel movements per day was the clinical feature most robustly associated with a higher prevalence of symptomatic themes in CD.
Our results showed differences in the prevalence of CD symptoms based on sex. CD is known to have a higher prevalence in women (40), and certain symptomatic issues, such as fatigue, problems with neck or back, and emotional issues, were also reported at a higher frequency by women than men. Additional studies are needed to better understand the potential pathomechanisms behind sex-associated symptoms in CD.
This study demonstrated the impact that CD symptom burden has on workforce participation. Participants with a higher prevalence of CD symptoms were more likely to miss work and be on disability as opposed to those with a lower prevalence of symptoms. As effective therapies are developed in CD, it is possible that these therapies will not only lower individual patient burden but also allow for more productive and meaningful employment.
A higher prevalence of select symptoms was associated with a lower education level. Of all the symptomatic themes, joint problems and eye problems were most heavily associated with a lower education status. Although the presence of joint or eye problems may reflect a more severe autoimmune process in CD (40–42), additional studies are needed to investigate the relationship between these symptoms and educational achievement.
Relation of this study to current literature
This research adds to the existing body of literature that explores the unique symptoms, QoL, and comorbidities of patients living with CD. The symptoms and themes established in our study that contribute most to patient-reported disease burden parallel, for the most part, those seen in patients with CD from other studies (10,20,21). However, unlike many previous studies, this research used extensive patient input from the very beginning to develop a comprehensive survey of patient-relevant symptoms, which was then further explored in a large population of adults with CD.
Limitations of this study
There are limitations associated with this research. We acknowledge that the large sample of people with CD who participated in this cross-sectional study likely does not represent the greater population of those with CD. Although our sample size was more than 400, participants were limited to those who were enrolled in the IBD registry. Thus, most of the participants were highly educated (83.2% possessing a college degree or higher) and were willing to engage in medical research. Although higher education level is common and well known in survey research (43), this potentially introduces a source of bias into our results. In addition, members of the IBD registry may differ from the broader patient population in the types and severity of some symptoms. For example, individuals with mild or no symptoms may not seek out research participation through the Crohn's & Colitis Foundation; conversely, those with very severe symptoms may not have the capacity to engage in research. Thus, our cross-sectional study sample was likely based on a stabilizing selection, in which patients on either the mild or severe ends of the disease spectrum were underrepresented. As a result, the variance of the reported relative impact of symptoms may have been artificially lowered.
In our cross-sectional study, the mean age of individuals with CD was older than that of the typical population of those with CD. Furthermore, 73.5% of our participants were female and 96.4% were White. While CD is reportedly more common in these populations (40,44), interpretation of the data must account for the demographic features of these respondents. In particular, the prevalence,average impact, and PIP of symptoms reflect what was reported by patients in our study cohort, which may not be generalizable to the entire international CD community.
Because our survey was conducted online, it is possible that individuals with limited internet access because of technical or socioeconomic factors were underrepresented. Nevertheless, the results from our study likely do reflect the responses for the section of the CD population that is likely to participate in research and clinical trials in the future.
Patient-Reported Impact of Symptoms in CD adds to existing knowledge regarding the multifactorial disease burden faced by individuals with CD. These data have the potential to better equip clinicians who care for patients with CD to recognize common, important, and potentially treatable symptoms associated with this disease. This work also highlights a unique symptomatic profile in CD, supports the merit of multidisciplinary clinics that can address these symptoms, and identifies clinically relevant and patient meaningful symptomatic targets for future therapeutic research.
CONFLICTS OF INTEREST
Guarantor of the article: Chad Heatwole, MD, MS-CI.
Specific author contributions: A.V. and J.H.: major role in analysis and interpretation of data and drafting and revising the manuscript for intellectual content. J.W., J.S., and S.R.: major role in drafting and revising the manuscript for intellectual content. E.W., C.Z., and E.L.: major role in conducting study activities (patient interviews and cross-sectional survey). N.D. and M.M.: major role in formal analysis of data. L.S. and L.T.: major role in participant recruitment for study activities and drafting and revising the manuscript for intellectual content. S.R.: patient advocate for participant recruitment from the Crohn's & Colitis Foundation patient registry. C.H.: design and conceptualization of the study, analysis and interpretation of data, and drafting and revising the manuscript for intellectual content. All authors have approved the final draft submitted.
Financial support: This study was supported by a research grant from UR Ventures at the University of Rochester, Rochester, New York.
Potential competing interests: A.V., J.W., J.S., S.R., and E.W. have no conflicts of interest. C.Z. has provided consultation to Recursion Pharmaceuticals. E.L., N.D., M.M., J.H., L.S., L.T., and S.R. have no conflicts of interest. C.H. receives royalties for the use of multiple disease-specific instruments; he has provided consultation to Biogen Idec, Ionis Pharmaceuticals, aTyr Pharma, AMO Pharma, Acceleron Pharma, Cytokinetics, Expansion Therapeutics, Harmony Biosciences, Regeneron Pharmaceuticals, Astellas Pharmaceuticals, AveXis, Recursion Pharmaceuticals, IRIS Medicine, Takeda Pharmaceutical Company, Scholar Rock, Avidity Biosciences, Novartis Pharmaceuticals Corporation, SwanBio Therapeutics, and the Marigold Foundation; and he received grant support from the Department of Defense, Duchenne UK, Parent Project Muscular Dystrophy, Recursion Pharmaceuticals, SwanBio Therapeutics, the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, the Friedreich's Ataxia Research Alliance, Cure Spinal Muscular Atrophy, and the Amyotrophic Lateral Sclerosis Association.
Study Highlights.
WHAT IS KNOWN
✓ Studies on quality of life in Crohn's disease (CD) have identified areas of physical, mental, emotional, social, and disease-specific health that affect individuals with the disease.
✓ Patient-centric research and a thorough understanding of the multifactorial disease burden experienced by patients with CD, including patient-reported impact, is currently limited.
✓ Additional information is needed regarding how the symptomatic burden experienced in CD varies based on demographic and clinical subgroups.
WHAT IS NEW HERE
✓ Using a large cross-sectional study, patients with CD reported which symptomatic themes were the most common and had the greatest impact on their lives. The issues with the highest prevalence in CD related to gastrointestinal issues, fatigue, dietary restrictions, and impaired sleep or daytime sleepiness.
✓ Participants who had a higher number of liquid/soft stools per day, had a higher number of bowel movements per day, were unemployed, or were disabled had a higher disease burden across multiple symptomatic categories.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C644
Contributor Information
Anika Varma, Email: anika.varma@chet.rochester.edu.
Jennifer Weinstein, Email: jennifer.weinstein@chet.rochester.edu.
Jamison Seabury, Email: jamison.seabury@chet.rochester.edu.
Spencer Rosero, Email: spencer.rosero@chet.rochester.edu.
Ellen Wagner, Email: ellen.wagner@chet.rochester.edu.
Christine Zizzi, Email: christine.zizzi@chet.rochester.edu.
Elizabeth Luebbe, Email: Elizabeth_Luebbe@URMC.Rochester.edu.
Nuran Dilek, Email: Nuran_Dilek@URMC.Rochester.edu.
Michael McDermott, Email: Michael_McDermott@URMC.Rochester.edu.
John Heatwole, Email: jmheatwole@gmail.com.
Lawrence Saubermann, Email: Lawrence_Saubermann@URMC.Rochester.edu.
Larissa Temple, Email: Larissa_Temple@URMC.Rochester.edu.
Scott Rogoff, Email: srogoff@barclaydamon.com.
REFERENCES
- 1.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 2012;58(2):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dresden D, Weatherspoon D. Crohn's disease facts: Causes, risks factors, and prevalence. (https://www.medicalnewstoday.com/articles/324622). 2019. Accessed August 5, 2021.
- 3.Inflammatory bowel disease (IBD) | CDC. (https://www.cdc.gov/ibd/index.htm). 2019. Accessed August 5, 2021. [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46–54. [DOI] [PubMed] [Google Scholar]
- 5.The facts about inflammatory bowel diseases. Crohn's & Colitis Foundation of America: New York, 2014. [Google Scholar]
- 6.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis 2007;1(1):10–20. [DOI] [PubMed] [Google Scholar]
- 7.Matos R, Lencastre L, Rocha V, et al. Quality of life in patients with inflammatory bowel disease: The role of positive psychological factors. Health Psychol Behav Med 2021;9(1):989–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varbobitis I, Kokkotis G, Gizis M, et al. The IBD-F patient self-assessment scale accurately depicts the level of fatigue and predicts a negative effect on the quality of life of patients with IBD in clinical remission. Inflamm Bowel Dis 2021;27(6):826–35. [DOI] [PubMed] [Google Scholar]
- 9.Whelan K, Murrells T, Morgan M, et al. Food-related quality of life is impaired in inflammatory bowel disease and associated with reduced intake of key nutrients. Am J Clin Nutr 2021;113(4):832–44. [DOI] [PubMed] [Google Scholar]
- 10.Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12(8):1315–23.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Have M, van der Aalst, Karen S, et al. Determinants of health-related quality of life in Crohn's disease: A systematic review and meta-analysis. J Crohns Colitis 2014;8(2):93–106. [DOI] [PubMed] [Google Scholar]
- 12.Mahalli AAE, Alharthi HMA. Assessment of health-related quality of life of patients with inflammatory bowel diseases in Eastern Province, Saudi Arabia. J Infect Public Health 2017;10(1):93–101. [DOI] [PubMed] [Google Scholar]
- 13.Canavan C, Abrams KR, Hawthorne B, et al. Long-term prognosis in Crohn's disease: Factors that affect quality of life. Aliment Pharmacol Ther 2006;23(3):377–85. [DOI] [PubMed] [Google Scholar]
- 14.Ho PYM, Hu W, Lee YY, et al. Health-related quality of life of patients with inflammatory bowel disease in Singapore. Intest Res 2018;17(1):107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallis AG, Vlachonikolis IG, Mouzas IA. Assessing health-related quality of life in patients with inflammatory bowel disease, in Crete, Greece. BMC Gastroenterol 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhong L, Wen Y, et al. Inflammatory bowel disease-specific health-related quality of life instruments: A systematic review of measurement properties. Health Qual Life Outcomes 2017;15(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70(3):439–44. [PubMed] [Google Scholar]
- 18.Drossman DA, Leserman J, Li Z, et al. The rating form of IBD patient concerns: A new measure of health status. Psychosom Med 1991;53(6):701–12. [DOI] [PubMed] [Google Scholar]
- 19.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: A valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2002;35(4):557–63. [DOI] [PubMed] [Google Scholar]
- 20.Alrubaiy L, Cheung W, Dodds P, et al. Development of a short questionnaire to assess the quality of life in Crohn's disease and ulcerative colitis. J Crohns Colitis 2015;9(1):66–76. [DOI] [PubMed] [Google Scholar]
- 21.Dulai PS, Jairath V, Khanna R, et al. Development of the symptoms and impacts questionnaire for Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther 2020;51(11):1047–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labelling claims. U.S. Department of Health and Human Services, Food and Drug Administration: Washington, DC, 2009. [Google Scholar]
- 23.Qualification process for drug development tools: Guidance for industry and FDA staff. U.S. Department of Health and Human Services, Food and Drug Administration: Washington, DC, 2020. [Google Scholar]
- 24.Committee for Medicinal Products for Human Use (CHMP). Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency: Amsterdam, the Netherlands, 2016. [Google Scholar]
- 25.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 26.Glidden AM, Luebbe EA, Elson MJ, et al. Patient-reported impact of symptoms in Huntington disease: PRISM-HD. Neurology 2020;94(19):e2045–53. [DOI] [PubMed] [Google Scholar]
- 27.Hamel J, Johnson N, Tawil R, et al. Patient-reported symptoms in facioscapulohumeral muscular dystrophy (PRISM-FSHD). Neurology 2019;93(12):e1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heatwole C, Bode R, Johnson N, et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology 2012;79(4):348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heatwole C, Johnson N, Bode R, et al. Patient-reported impact of symptoms in myotonic dystrophy type 2 (PRISM-2). Neurology 2015;85(24):2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongiovi P, Dilek N, Garland C, et al. Patient reported impact of symptoms in spinal muscular atrophy (PRISM-SMA). Neurology 2018;91(13):e1206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasileiou K, Barnett J, Thorpe S, et al. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol 2018;18(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green J, Thorogood N. Qualitative methods for health research. 4th edn. Sage: London, 2004. [Google Scholar]
- 33.Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol 1996;91(8):1571–8. [PubMed] [Google Scholar]
- 34.Johnson NE, Heatwole CR, Dilek N, et al. Quality-of-life in Charcot–Marie–Tooth disease: The patient's perspective. Neuromuscul Disord 2014;24(11):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson NE, Quinn C, Eastwood E, et al. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve 2012;46(6):951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guber RD, Kokkinis AD, Schindler AB, et al. Patient-identified impact of symptoms in spinal and bulbar muscular atrophy. Muscle Nerve 2018;57(1):40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson NE, Heatwole CR, Ferguson M, et al. Patient identification of the symptomatic impact of Charcot-Marie-Tooth disease type 1A. J Clin Neuromuscul Dis 2013;15(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter M, Ekstrom A, Campbell C, et al. Patient-reported study of the impact of pediatric-onset myotonic dystrophy. Muscle Nerve 2019;60(4):392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson NE, Ekstrom A, Campbell C, et al. Parent-reported multi-national study of the impact of congenital and childhood onset myotonic dystrophy. Dev Med Child Neurol 2016;58(7):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greuter T, Manser C, Pittet V, et al. Gender differences in inflammatory bowel disease. Digestion 2020;101(Suppl 1):98–104. [DOI] [PubMed] [Google Scholar]
- 41.Mady R, Grover W, Butrus S. Ocular complications of inflammatory bowel disease. ScientificWorldJournal 2015;2015:438402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troncoso LL, Biancardi AL, de Moraes HV, Jr, et al. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J Gastroenterol 2017;23(32):5836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spitzer S. Biases in health expectancies due to educational differences in survey participation of older Europeans: It's worth weighting for. Eur J Health Econ 2020;21(4):573–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aniwan S, Harmsen WS, Tremaine WJ, et al. Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010. Therap Adv Gastroenterol 2019;12:1756284819827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared with qualified investigators on request.