Abstract
The pathogenesis of irritable bowel syndrome (IBS)—a disorder of gut-brain interaction that affects up to 10% of the world's population—remains uncertain. It is puzzling that a disorder so prevalent and archetypal among humans can be explained by disparate theories, respond to treatments with vastly different mechanisms of action, and present with a dazzling array of comorbidities. It is reasonable to question whether there is a unifying factor that binds these divergent theories and observations, and if so, what that factor might be. This article offers a testable hypothesis that seeks to accommodate the manifold theories, clinical symptoms, somatic comorbidities, neuropsychological features, and treatment outcomes of IBS by describing the syndrome in relation to a principal force of human evolution: gravity. In short, the hypothesis proposed here is that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity management systems designed to optimize gastrointestinal form and function, protect somatic and visceral integrity, and maximize survival in a gravity-bound world. To explain this unconventional hypothesis of IBS pathogenesis, referred to herein as the gravity hypothesis, this article reviews the influence of gravity on human evolution; discusses how Homo sapiens imperfectly evolved to manage this universal force of attraction; and explores the mechanical, microbial, and neuropsychological consequences of gravity intolerance with a focus on explaining IBS. This article concludes by considering the diagnostic and therapeutic implications of this new hypothesis and proposes experiments to support or reject this line of inquiry. It is hoped that the ideas in this thought experiment may also help encourage new or different ways of thinking about this common disorder.
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic and often debilitating gastrointestinal (GI) disorder that presents with recurrent abdominal pain and altered stool frequency and form (1). It is among the most common GI disorders in the world and affects up to 10% of the population across nations, cultures, and demographic groups (2). Although the exact pathogenesis of IBS remains uncertain, a variety of evidence-based theories have been developed to explain its clinical features and comorbidities.
IBS is widely believed to be a disorder of gut-brain interaction as suggested by its strong overlap with psychological comorbidities and evidence that neuromodulators and brain-gut behavior therapies are effective (3–5). Another theory holds that IBS is driven by abnormalities in the gut microbiome as evidenced by a high prevalence of small intestinal bacterial overgrowth and clinical response to microbiome-targeted therapies, including antibiotics and low fermentable diets (6). Other theories posit that abnormalities in motility, visceral hypersensitivity, abnormal serotonin levels, or a dysregulated autonomic nervous system (ANS) cause IBS (2). Adding to the pathogenic complexity, IBS is comorbid with a wide variety of conditions including fibromyalgia, joint hypermobility syndromes, lower back pain, diverticulosis, postural tachycardia syndrome (POTS), headaches, and dizziness (7). It is puzzling that a disorder so prevalent and archetypal among humans can be explained by seemingly disparate theories, respond to treatments with vastly different mechanisms of action, and present with a dazzling array of comorbidities. It is reasonable to question whether there is a unifying factor that binds these divergent theories and observations, and if so, what that factor might be.
This article offers a testable hypothesis that seeks to accommodate the manifold theories, clinical symptoms, somatic comorbidities, neuropsychological features, and treatment outcomes of IBS by describing the syndrome in relation to a principal force of human evolution: gravity. In short, the hypothesis proposed here is that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity management systems designed to optimize GI form and function, protect somatic and visceral integrity, and maximize survival in a gravity-bound world.
To explain this hypothesis of IBS pathogenesis, referred to herein as the gravity hypothesis, this thought experiment begins by considering the influence of gravity on human evolution; discusses how Homo sapiens imperfectly evolved to manage this universal force; and explores the mechanical, microbial, and neuropsychological consequences of gravity intolerance with a focus on explaining IBS. The article concludes by considering the diagnostic and therapeutic implications of this new hypothesis and proposes specific experiments to help support or reject this line of inquiry. It is hoped that the ideas in this thought experiment may also help encourage new or different ways of thinking about this common disorder.
THE INEXORABLE INFLUENCE OF GRAVITY
As long as there has been life on Earth, from the earliest unicellular organisms to Homo sapiens, gravity has relentlessly shaped every object on the planet. Although the weakest of the 4 fundamental forces, gravitational force (g-force), nonetheless, exerts a profound impact on the form and function of the visible world, particularly when acting over an evolutionary timescale. In this article, we will refer to the g-force on Earth as 1 g (although this is a single-author article, the term “we” is used throughout to imply a dialog between the author and the reader in lieu of first or third-person constructions). From birds evolving to possess wings to glaciers carving fjords over massive landscapes, every observable object is deeply influenced by and precisely sensitive to 1 g gravity.
The human body is no different. No matter who we are, where we live, or what we look like, every fiber of every human is affected by gravity every day, all day, from the moment of our conception to the day we die. To survive and thrive on Earth, we must live successfully with and through gravity, a force so fundamental that we rarely note its presence despite its continual influence on our bodies. It is no wonder, then, that Homo sapiens evolved to manage gravity in manifold ways that optimize upright stability, structural support, locomotion, fluid dynamics, and neuropsychological integration in a 1 g world (8). It also follows that negative health consequences occur when form and function cannot manage gravity effectively (consider lower back pain, heart failure, or positional vertigo as prevalent models of gravity intolerance). Because our body spends most of its existence on terrestrial Earth, not only is our corporeal self deeply shaped by the inexorable pull of gravity but our neuropsychological self also emerges from possessing a 1 g body. In short, when our gravity management systems fail, our health fails.
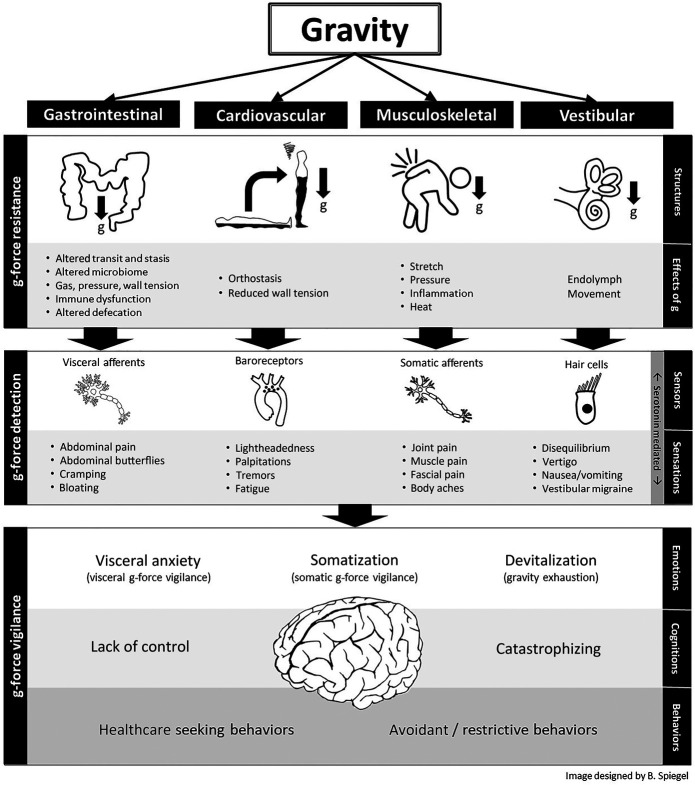
As bipedal organisms, we live two-thirds of our lives in an upright posture that exerts a downward pull on the body. Each body system evolved to resist and accommodate the caudal attraction of gravity in a way that optimizes anatomy and physiology. In this article, we will focus on the GI tract and describe 4 g-force resistance systems, all derived from the embryologic mesoderm, that jointly suspend and array the viscera within the peritoneal cavity. First, we will discuss these load-bearing mechanisms and consider their effects on GI topography in the upright stance. Then, we will assess how dysfunction in each mechanism leads to IBS symptoms, with brief consideration of other chronic GI and pelvic floor disorders. Later, we will expand our discussion beyond purely mechanical factors and explore neuropsychological modes of gravity tolerance arising from the embryological ectoderm, including g-force detection systems enabled by the peripheral nervous system (PNS) and mediated by the gut microbiome, and g-force vigilance systems emerging from the central nervous system (CNS), all sharing a common factor of serotonin signaling. We will review evidence that serotonin, a neurotransmitter strongly implicated in the pathogenesis of IBS and its disparate comorbidities (9,10), may have evolved in part to manage gravity. Then, we will discuss how abdominal butterflies, an archetypal gut feeling familiar to most everyone, universally occur in response to extreme g-force accelerations (imagine riding a rollercoaster). We will consider whether gut feelings operate as a g-force alarm calibrated to each person's unique set point for threat detection, itself determined by mechanical, microbial, and neuropsychological factors. Under this theory, pathologic gut feelings occur when the brain overpredicts g-force events that are not even occurring or may never occur, serving as a visceral analog of allodynia. Finally, we will contemplate how the mechanical and neuropsychological gravity management systems interact to maximize human survival on Earth and consider how IBS might result when these systems fail to manage 1 g, either individually or in tandem. Figure 1 presents an overview of the gravity hypothesis; we will refer to this image throughout the article as we explore the details of this thought experiment.
Figure 1.
Overview of the irritable bowel syndrome gravity hypothesis. Homo sapiens evolved a set of gravity management systems within the gastrointestinal, musculoskeletal, vestibular, and cardiovascular systems (among others not shown). When these systems are maladapted to resist gravity, a mismatch occurs between expected vs actual g-force strain on the body. This leads to physiological consequences, mediated in part by the gut microbiome, that trigger the peripheral nervous system. This culminates in visceral and somatic sensations in the central nervous system that are mediated, in part, by serotonin. If gravity intolerance persists over time, then afferent nerves become peripherally sensitized, ultimately leading to central sensitization and hypervigilance to protect the body against gravitational strain perceived to be outside the range for somatic and visceral integrity. In the extreme, the brain overpredicts g-force events that are not even occurring or may never occur, leading to a form a visceral allodynia marked by gut feelings. Uncorrected, this sequence results in maladaptive emotions, cognitions, and behaviors designed to maximize survival in a gravity-bound world. G-force, gravitational force.
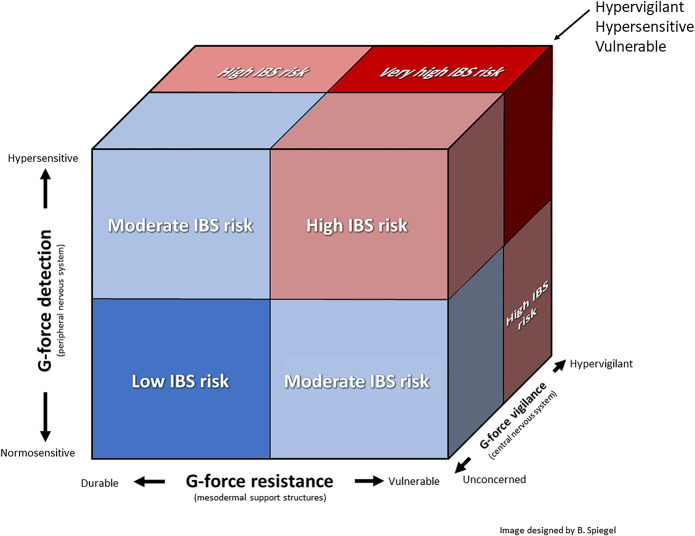
A FRAMEWORK FOR IBS SUSCEPTIBILITY: THE G-FORCE CUBE
The gravity hypothesis proposes that IBS susceptibility is determined by 3 factors: (i) g-force resistance defines the ability of the GI tract and its support structures to mechanically resist gravity, ranging from durable to vulnerable; (ii) g-force detection defines the ability of the PNS to detect gravity strain on visceral and somatic structures, ranging from normosensitive to hypersensitive; and (iii) g-force vigilance defines CNS readiness to predict and prevent threatening g-force events, ranging from unconcerned to hypervigilant. Figure 2 demonstrates how IBS susceptibility could be determined by the interplay among these factors, depicted as a g-force cube. For example, individuals with mechanically durable GI support structures, low sensitization, and low threat anxiety are unlikely to have IBS. By contrast, those with mechanical vulnerability, hypersensitivity, and hypervigilance are at very high risk of IBS. In the sections that follow, we will explore each of the factors, describe how they interrelate, and assess the potential explanatory power and research implications of this thought experiment.
Figure 2.
The IBS g-force cube. The gravity hypothesis proposes that IBS susceptibility may be determined by the interplay among 3 overarching factors: (i) g-force resistance, (ii) g-force detection, and (iii) g-force vigilance. Refer to the text for details about each factor and their interdependencies. G-force, gravitational force; IBS, irritable bowel syndrome.
FACTOR 1: G-FORCE RESISTANCE
The GI tract is not spooled haphazardly in the peritoneum like a coiled tube that settles at the bottom of a sack. Instead, the GI tract is arranged in a functional stack that both resists and accommodates downward traction in a manner that suspends, bolsters, and aligns the abdominal viscera to optimize form and function in a 1 g world. This is a key point: We evolved to both resist gravity so that peritoneal contents do not bunch up at the bottom of the abdominal cavity in a tangled pile and accommodate gravity so that we leverage its universal presence to support GI form and function, a fact that astronauts appreciate after experiencing digestive distress in a microgravity orbit (11). To help explain the concept of accommodating and resisting gravity, consider the image in Figure 3.
Figure 3.

A ceiling fixture in the Bayfront Hilton Hotel in San Diego, California. See text for how this installation helps visualize and elucidate gravity resistance mechanisms of the GI tract. Photograph courtesy of the author. GI, gastrointestinal.
The elegance of this fixture lies in its spiraling, 3-dimensional configuration that flows neatly under the influence of gravity. One can imagine the drapes as sheets of mesentery that suspend a downward-coiling intestinal tract from its trailing edge. Of course, the intestines do not flow in concentric spirals, nor is there empty space between sheets of mesentery, but the analogy is sufficient to inform an intuition about gravity and the gut.
As a thought experiment, imagine if the drapes were affixed to the ceiling of a spaceship. That would be an unwise design because the material would float aimlessly and lose its graceful order. This is analogous to the GI plight of astronauts who frequently report acid reflux, dyspepsia, bloating, diarrhea, constipation, and abdominal pain while in spaceflight (11). Because our GI tract is optimized for a 1 g world, astronauts experience abdominal distress from disrupted intestinal barrier function (12), changes in microbiome diversity (13,14), and altered GI morphology caused by the microgravity environment (11).
Next imagine if, back on Earth, the material in the drapes lost its integrity and began to stretch under the influence of gravity. In that case, the drapes might eventually drag along the ground and spread out in a jumbled pile. This is analogous to the weakening of connective tissue that occurs with aging, including the tissues that maintain integrity of the GI tract, contributing to constipation, bloating, hemorrhoids, diverticulosis, rectal prolapse, defecatory disorders, and other forms of GI distress common among the aging population.
Alternatively, the drapes might be composed of material that is overly elastic, causing them to lose their shape and droop from gravity. This is akin to what happens with Ehlers-Danlos syndrome (EDS), a condition that can lead to visceroptosis where the abdominal organs sink below their natural position and cause GI distress (15–17).
What if the ceiling began to sag or the walls buckled? In that case, the drapes would collapse because the superstructure maintaining its position would be caving in. This is what happens when the musculoskeletal structures that support the abdominal cavity are misshapen or break down. For example, GI symptoms are often reported in patients with kyphosis, lordosis, and vertebral compression fractures (18–20).
Having explained this analogy, we can now apply its principles to assess four g-force resistance mechanisms that jointly support the abdominal viscera. All 4 mechanisms arise from the embryological mesoderm, the germ layer which originates the structural components of the body including the skeleton, muscles, tendons, ligaments, and mesentery. Although anatomically distinct, these 4 mechanisms coordinate to resist gravity and optimize GI form and function. Figure 4 depicts these gravity resistance mechanisms of the GI tract, which we will call the suspension system, chassis, ceiling mount, and bolster. The next sections discuss each mechanism and consider how a breakdown causes IBS symptoms.
Figure 4.
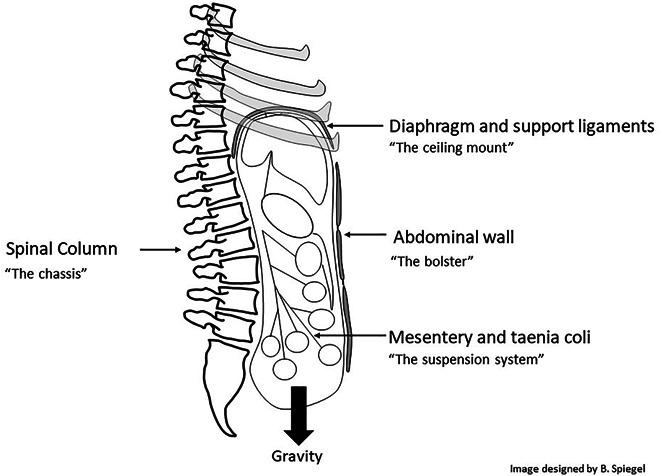
Four gravity resistance mechanisms of the GI tract. GI, gastrointestinal.
MESENTERY AND TAENIA COLI (THE SUSPENSION SYSTEM)
We begin our survey of gravity resistance mechanisms with the mesentery, an intricately plicated organ composed largely of connective and adipose tissues that adhere to and support the peritoneal organs. The mesentery originates at the level of the lumbar spine from its root along the posterior abdominal wall where it fans out to support and tether the abdominal viscera. The mesentery operates like a suspension system that prevents the intestines from collapsing into the pelvis and maintains the topographic order among the peritoneal viscera while preventing entanglement. Without the mesentery, intestinal transit would be severely undermined or altogether cease. It has been suggested that the mesenteric attachments were critical to support the upright posture of Homo sapiens through differential elongation of its segments to optimize GI function and prevent organ descent (21,22).
In addition to being suspended by the mesentery, the large intestine is bolstered and supported by 3 equidistant strips of smooth muscle that traverse the length of the colon, called the taenia coli (Figure 5). These structures maintain longitudinal integrity of the colon and serve as suspension cables that allow efficient contraction of the colonic circular muscles (23). When pressure builds in the large intestine, particularly in the sigmoid colon where gravity-bound stool is stored before evacuation, the taenia coli bolster and strengthen the colon.
Figure 5.
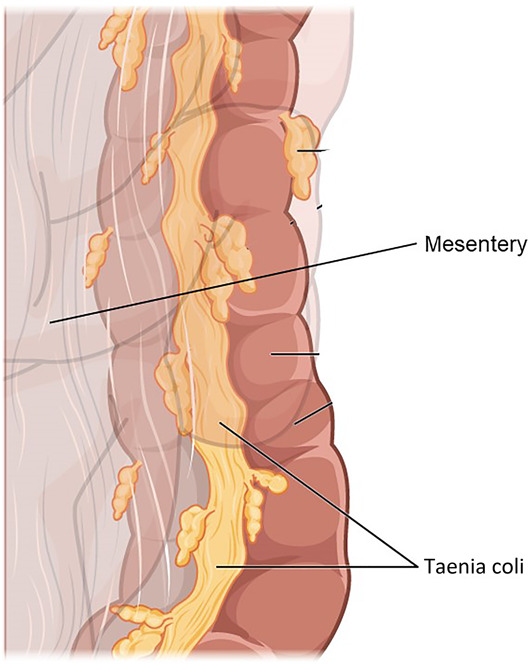
A segment of the colon demonstrating the mesentery intercalating with the bowel wall adjacent to a strip of taenia coli running the length of the large intestine. The mesentery and taenia coli jointly suspend and bolster the colon to optimize form and function under the influence of gravity (image used under Creative Commons license from Wikimedia Commons).
Taken together, the mesentery and taenia coli suspend, strengthen, protect, and bolster the GI tract so it can function in line with gravity. However, if these gravity resistance mechanisms are ineffective, then GI distress and IBS symptoms may result, as we will discuss next.
HOW ABNORMALITIES IN THE SUSPENSION SYSTEM CAUSE IBS SYMPTOMS
Having described the first of four g-force resistance mechanisms, we will now use the gravity hypothesis as a potential explanatory tool for IBS. We will continue applying the hypothesis to each of the gravity resistance mechanisms and later expand the discussion to encompass neuropsychological systems. Regarding dysfunction of the mesentery and taenia coli, the following clinical observations may be addressed.
Relationship between IBS and hypermobility disorders
In 2010, investigators discovered that nearly half of patients with unexplained chronic GI complaints, including IBS symptoms, have hypermobile joints (24). Further research found that two-thirds of people with EDS, an inherited connective tissue disorder marked by abnormal collagen deposition, meet criteria for IBS (25). These observations led investigators to determine that patients with EDS and other hypermobility disorders are at increased risk of abdominal organ collapse because of a lax mesentery (15–17). Furthermore, ineffective gravity management from stretchy support structures slows intestinal transit in 76% of patients with EDS (26), leading to luminal stasis and bacterial overgrowth—itself a key feature of IBS that we will discuss throughout this article. Although EDS is an uncommon disorder, joint hypermobility affects up to 20% of the general population (27) yet is rarely diagnosed (28). The high prevalence of IBS symptoms among patients with joint hypermobility offers a model for how lax mesentery can affect the structure and function of the GI tract and raises questions about whether some people with IBS may have suboptimally constructed suspension systems that remain undiagnosed. Separately, there is a high overlap between joint hypermobility and POTS, another gravity intolerance syndrome linked to IBS that we will discuss later.
High prevalence of defecatory disorders in the aging population
Although EDS is unusual, the process of aging is universal. Connective tissues become lax throughout the body under the relentless influence of gravity; the GI suspension systems are no exception. Although there is limited research examining mesenteric form and function across the lifespan, it is likely that loss of mesenteric integrity contributes to gravity-related defecatory disorders in the aged, including constipation and rectal prolapse, and could potentially underlie the small subset of IBS patients with late-onset symptoms. This is an area worthy of study. Hemorrhoids are also a common biomarker of ineffective gravity management that are more frequent with age. Beyond traditional medical treatments, use of a defecation posture modification device (e.g., Squatty Potty) is an effective therapy for constipation that works by aligning rectal evacuation with gravity (29). Although these examples are not specific to IBS, they illustrate how pressure gradients can underlie defecatory disorders as a model for gravity-related GI distress.
Link between diverticulosis and IBS
Diverticulosis occurs when high pressures within the large bowel cause outpouching of the colon wall at weak points between taenia coli (30). In essence, diverticula represent a visible biomarker of ineffective gravity management, where gravity-bound contents exert wall forces that overcome the intrinsic strength of GI suspension systems. This may help explain the considerable overlap between IBS and diverticulosis (31). Not only are diverticula associated with abdominal pain and constipation, but they have also been linked to diarrhea (32) and bacterial overgrowth (33). Separately, a condition called symptomatic uncomplicated diverticular disease (SUDD) describes patients with diverticulosis and IBS symptoms. The literature has struggled to disentangle diverticulosis and IBS, with some authors stating that SUDD is merely IBS with diverticulosis and others claiming that SUDD is a unique disorder (31). However, the gravity hypothesis suggests that gravity intolerance is the ab initio cause of IBS and that diverticulosis is a resulting biomarker of ineffective load management in patients with unusually high intraluminal forces and/or weak gravity support systems.
Colonic length and shape are associated with IBS symptoms
Many gastroenterologists report that patients with IBS often have a tortuous intestine during colonoscopy. It is also known that women are twice as likely to have a redundant bowel than men, consistently have longer colons, and are more likely to have a transverse colon that dips into the pelvis (34,35). These findings reveal natural variations among people not only in intestinal length but also necessarily in the suspension systems tethering the bowels within the abdominal cavity. It is likely that an elongated, redundant, and tortuous mesenteric-visceral complex is more likely to sag within the peritoneum under the influence of gravity, leading to a higher risk of dysmotility, luminal kinking, stasis, and altered microbiome. Combined with evidence that women have greater viscoelasticity of tendon structures than men (36), variations in GI suspension systems may partly explain (among other theories) why women are more likely to develop IBS and constipation than men. More broadly, measuring the length, shape, and topography of the mesentery may offer additional biomarkers of ineffective gravity management that could provide clinical value in a biogravitational approach to IBS. For example, some children with IBS may have inherited suspension systems that are suboptimized for gravity management, or perhaps the mesentery did not elongate sufficiently in lockstep with pubertal growth in the abdominal cavity for some teenagers with IBS. These are among 30 researchable questions proposed in Table 1 suggested by the gravity hypothesis.
Table 1.
Research questions suggested by the irritable bowel syndrome gravity hypothesis
SPINAL COLUMN, RIB CAGE, DIAPHRAGM, AND SUPPORT LIGAMENTS (THE CHASSIS AND CEILING MOUNT)
Next, we turn our attention from the GI suspension system to the coordinated set of structures that define and maintain the configuration of the abdominal cavity (Figure 4). The abdominal contents are heavy, like a sack of potatoes that we are destined to carry for our entire lives. To meet this demand, the body evolved to support the abdominal load using a set of mechanisms that hoist the viscera in an upright posture. Although these structures are anatomically distinct, they interlock to form a superstructure that supports the organs and bolsters the peritoneal suspension systems. As we will see, some people are better designed to carry the abdominal load than others and structural variations can influence GI health.
Musculoskeletal support of the GI tract begins with the spine. Recall that the mesentery attaches to the posterior abdominal wall at the level of the lumbar spine. By the Newton Third Law, the downward force of the mesentery must be met with an equal and opposite force of the lumbar spine to prevent the intraperitoneal organs from sagging. This engages the paraspinal extensor muscles to stiffen the backbone, allowing it to operate as an antigravity support chassis.
Next, we consider the ribcage that articulates off the thoracic spine. The ribs protect the heart and lung but also tack the diaphragm into place through a series of ligaments and tendons. Anteriorly, the diaphragm attaches to the xiphoid process and costal margin. Laterally, it connects to the eleventh and twelfth ribs. Posteriorly, it fastens directly to the spine by the crural tendons. Secured in place by the spine and ribcage, the diaphragm creates a ceiling mount for the upright abdominal cavity, on which organs suspend. The diaphragm supports the liver by the falciform ligament. The liver, in turn, helps to suspend other intra-abdominal organs including the stomach. Figure 6 demonstrates how the spinal column, ribcage, diaphragm, and support ligaments act like a crane to stabilize and suspend the peritoneal contents.
Figure 6.
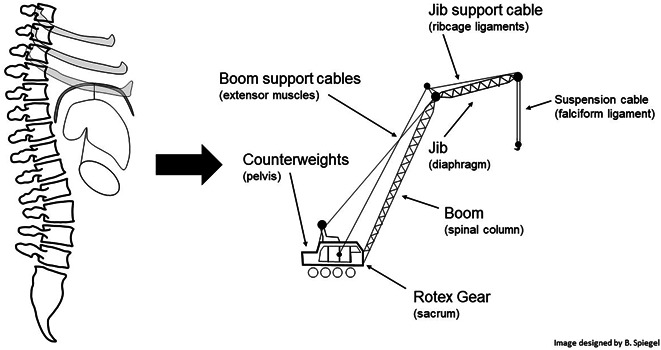
The “abdominal crane.” By analogy to a crane, the pelvis, sacrum, spinal column, extensor muscles, ribcage, diaphragm, and falciform ligament work together to hoist the intraperitoneal viscera within an upright abdominal cavity. GI, gastrointestinal.
HOW ABNORMALITIES IN THE ABDOMINAL CRANE CAUSE IBS SYMPTOMS
Figure 6 offers a framework to evaluate the musculoskeletal comorbidities of IBS. Patients can develop acute pain at each point along the abdominal crane if there is excess tension between the support scaffold and its gravity-bound load. Over time, acute pain can transform into chronic pain through a process of central sensitization (37). When the musculoskeletal system is well aligned with gravity, there is less tension, stretch, and nociceptive signaling. However, as alignment with gravity falters and there is a mismatch between expected vs actual g-force strain, pain grows in frequency and intensity. In essence, musculoskeletal pain can serve as a direct marker of gravity mismanagement. Musculoskeletal pain begins when gravity misalignment exceeds the perceived acceptable range for body integrity and protection and then worsens in lockstep with further deviation. Later, we will explore whether visceral pain also arises when g-force strain on the gut exceeds a safe range. For now, we will focus on musculoskeletal comorbidities of IBS that correspond with abdominal crane structures.
High prevalence of back pain in patients with IBS
Up to 80% of patients with IBS report back pain (38), and there is a disproportionate amount of unnecessary spine surgery in patients with IBS (39,40). The presence of back pain also helps differentiate IBS from other GI disorders (38). This raises a question of cause vs effect: Does visceral pain simply radiate or refer to the back, mimicking a musculoskeletal origin? Or is the reverse true: that musculoskeletal pain causes visceral pain? Or both? Although there are undoubtedly variations in cause and effect among different people with IBS, the gravity hypothesis reminds us that gravity was there from the start. Antigravity extensor muscles constantly strain to support the abdominal load under the inexorable pull of gravity—another example of the Newton Third Law. If the spine begins to fail for any reason independent of GI pathology, then the abdominal crane will sag and the mesentery will descend, leading to altered angulation of the mesenteric root, pressurization of the peritoneal organs, and disrupted visceral function. For example, patients with kyphosis—a model of spinal gravity mismanagement—often report GI complaints from compression of the intra-abdominal organs and resultant dysmotility (18). Lordosis can also push abdominal contents forward, reducing anterior-posterior space and causing a protuberant abdomen (19). Patients with a history of vertebral compression fractures also develop GI symptoms and a bulging abdomen from diminished vertical space (20). Finally, direct nerve root compression can secondarily affect GI motility. These observations raise questions that have not, as of this writing, been sufficiently addressed. For example, are there differences in lordosis, kyphosis, disk disease, or undiagnosed vertebral fractures between patients with IBS vs controls? How often do people develop severe back pain first and IBS second? These are among the questions listed in Table 1.
Costochondritis, sternum pain, and IBS
Although both GI and extraintestinal pain may arise from a central sensitization to all forms of pain (41–43), it is worth asking why certain pains occur in IBS. For example, in additional to back pain, many patients with IBS report costochondritis in the lower thorax or sternum. The gravity hypothesis suggests these symptoms may occur, in part, from dysfunction of the abdominal crane structures. Recall that the diaphragm attaches to the xiphoid process, costal margin, and 11th and 12th ribs. Tension in these junctions will cause focal pain under the relentless pull of gravity. Very little is published about the anatomic variations in diaphragm topography and support structures among patients with IBS vs controls—another area worthy of research.
Benefits of yoga, body awareness therapy, and aerobic exercise for IBS
If dysfunction in musculoskeletal support structures can lead to IBS symptoms, then it should follow that strengthening or realigning those structures will reduce IBS symptoms. In fact, there is considerable research demonstrating benefits of physical therapy, exercise, and osteopathic manipulative therapy for IBS (44–47). It is striking that exercise not only protects against GI symptoms but also promotes colonic transit and gas clearance, suggesting a cause-and-effect relationship between physical fitness and GI physiology (48–51). Research also indicates that yoga and body awareness therapy improve GI symptoms (51-53). Moreover, the clinical effect size of physical activity is often as large, or larger, than the benefits realized with standard pharmacotherapies, although further high-quality trials are warranted.
ANTERIOR ABDOMINAL WALL (THE BOLSTER)
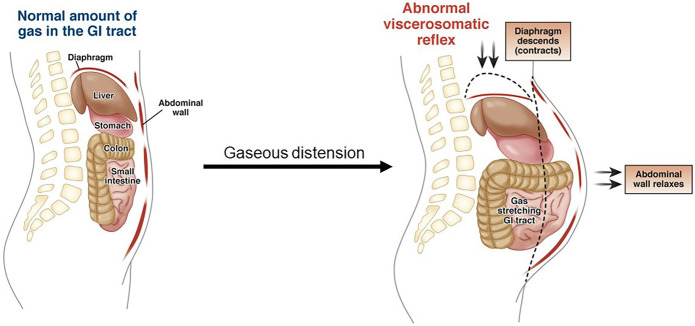
Whereas the spinal column offers posterior support of the abdominal viscera, the anterior abdominal wall bolsters the peritoneal cavity to prevent its contents from spilling forward in the upright stance. In addition, the abdominal wall contributes to gravity-dependent bloating and distension through a mechanism called abdominophrenic dyssynergia (54-55). Normally, when gas accumulates in the intestines, the diaphragm relaxes and the anterior abdominal wall contracts to maintain the upright visceral stack against gravity. However, in many people with bloating and distension, intraluminal gas causes paradoxical contraction of the diaphragm, leading to gravity-enabled descent of the peritoneal contents and dyssynergic relaxation of the abdominal wall. This sequence of events can lead to abdominal distension (Figure 7), a physical sign common among patients with IBS.
Figure 7.
Abdominophrenic dyssynergia. Normally, when the intestines fill with gas, the diaphragm relaxes and the anterior abdominal wall contracts to bolster the abdominal cavity. By contrast, abdominal distension results when the diaphragm contracts, the viscera fall with gravity, and the anterior abdominal wall relaxes, leading to outpouching in the upright position (Image from Lacy B et al. [56]). GI, gastrointestinal.
PHYSIOLOGICAL EFFECTS OF GRAVITY STRAIN
So far, we have discussed mesodermal structures that affect the form and function of the endodermal viscera. Some people possess mechanical systems that are better adapted to resist and accommodate gravity than others. In circumstances where the viscera become compressed, kinked, misshapen, or pressurized through gravity misalignment, the intestines may struggle to transit luminal contents efficiently (we might call this effect gut failure, analogous to heart failure where pump function also struggles against gravity). Dysmotility leads to stasis and microbiome overgrowth, which may cause increased permeability of the intestinal epithelium (57), mucosal inflammation, gas formation, and wall pressure within the intestines. In addition, diarrhea may result if bacterial overgrowth forms hydrogen sulfide gas or deconjugates bile salts (58), and constipation may occur if methane gas forms from archaea overgrowth (59). Simultaneously, if the abdominal crane is affected by gravity, then there may be stretch, pressure, inflammation, and heat generated within the musculoskeletal structures supporting the abdominal load (Figure 1). Taken together, the effects of gravity strain generate nociceptive mediators that activate sensory neurons, which brings us to our next discussion about g-force detection systems.
FACTOR 2: G-FORCE DETECTION
The peritoneal organs and their support structures are invested with visceral and somatic afferent nerves, respectively, which are derived from the embryological ectoderm. When gravity causes anatomic structures to deflect excessively, indicating a mismatch between expected vs actual g-force strain on the body, the PNS senses the deviation and transmits signals to the CNS where symptoms are perceived.
If the effects of gravity strain remain outside the safe range for body integrity, then the sensory neurons begin to discharge more frequently, with a larger magnitude, and at a lower threshold than they would in health—a process called peripheral sensitization (60). This leads to even more pain and discomfort, which alerts the brain to protect the injured tissue by finding ways to diminish g-force strain before the damage worsens. As the peripheral nerves become more sensitized, they begin to fire at lower thresholds and cause hyperalgesia or exaggerated symptoms beyond the level of injury. By now, if g-force strain remains uncorrected, the nerves trigger from innocuous stimuli—a phenomenon called allodynia—or even activate without any stimuli at all.
There is extensive evidence that patients with IBS develop peripheral sensitization. Viewed through the lens of the gravity hypothesis, unyielding g-force strain on GI support structures leads to overproduction of nociceptive mediators; persistent triggering of afferent neurons; and PNS sensitization resulting in hyperalgesia, allodynia, and chronic pain. This is illustrated in classic experiments showing that progressive rectal balloon inflation causes more intense pain at lower pressure thresholds in patients with IBS vs controls, a reproducible finding in up to 90% of patients (61). A detailed account of the mechanisms underlying peripheral sensitization in IBS is beyond our scope and summarized elsewhere (62–64).
EXTRAINTESTINAL G-FORCE DETECTION
There are 3 additional g-force management systems relevant to IBS: the cardiovascular system, the cerebrovascular system, and the vestibular system. Hypersensitive g-force detectors in these systems may help explain the following observations:
RELATIONSHIP BETWEEN IBS, POTS, EDS, AND INCREASED SYMPATHETIC TONE
In health, the cardiovascular system maintains a sufficient arterial pressure to perfuse the brain in an upright position. This feat of gravity management demands careful calibration of cardiac output, vascular resistance, and blood volume. Baroreceptors in the aortic arch and carotid artery monitor vascular pressure and maintain circulatory homeostasis. If blood pressure falls, then the baroreceptors detect a reduction in wall tension and generate increased sympathetic tone to raise blood pressure.
However, if the baroreceptors are hypersensitive to reductions in wall tension or if there is persistently low venous return or diminished cardiac output, then the system compensates through elevated sympathetic tone. This can lead to orthostatic intolerance, lightheadedness, palpitations, tremors, fatigue, and anxiety. Notably, all these extraintestinal symptoms are described in IBS and occur in overlapping neurovisceral phenotypes, including EDS and POTS (65). In EDS and other joint hypermobility syndromes, impaired collagen in blood vessels causes lax vascular tone and low venous return in the upright stance—another example of gravity intolerance. A predominant theory of POTS also implicates impaired vascular collagen, and 96% of patients with POTS have joint hypermobility (66). In reverse, 3-quarters of patients with EDS experience orthostatic intolerance while 40% also have POTS (67). These gravity-intolerance syndromes are also strongly associated with chronic GI symptoms, including IBS (67). Finally, baroreceptor sensitivity (66,68), autonomic dysfunction, and joint hypermobility have also been well-documented in IBS, completing a pathophysiologic triangle between EDS, POTS, and IBS—3 neurovisceral syndromes marked by gravity intolerance, dysregulated autonomic function, abnormal respiratory sinus arrhythmia (itself a gravity-dependent phenomenon [67]), and shared physical and neuropsychological symptom profiles (69).
RELATIONSHIP BETWEEN IBS, DISORDERED SLEEP, AND HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
Nearly 40% of patients with IBS report sleep disturbances (70). Disordered sleep is believed to dysregulate the ANS and hypothalamic-pituitary-adrenal (HPA) axis, leading to disrupted GI physiology. However, the gravity hypothesis offers an additional mechanism linking IBS, sleep, HPA function, and gravity. The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) explains this speculated mechanism.
RELATIONSHIP BETWEEN IBS AND DIZZINESS
Dizziness, vertigo, and disequilibrium are frequent IBS comorbidities (71–73). It is notable that disorders of the vestibular system and cerebellum—both of which are involved in gravity management—are commonly associated with these disabling symptoms. The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) explains how abnormalities in these systems may cause patients with IBS to misinterpret g-forces, leading to dizziness, mental distress, and high sympathetic tone.
SEROTONIN AS A GRAVITY MANAGEMENT SUBSTANCE
Up to this point, we have discussed how the gravity hypothesis emerges from observations linking IBS symptoms and comorbidities to dysfunction in gravity management systems. The hypothesis is further suggested by the overlap between IBS and other neurovisceral gravity intolerance syndromes, including EDS and POTS. Each of these syndromes is associated with GI dysmotility, altered microbiome, neural sensitization, and autonomic dysfunction (67). Furthermore, fibromyalgia is another syndrome associated with IBS (74) that is also characterized by pain sensitization (75), microbiome abnormalities (76), dysautonomia (77), and gravity intolerance (considering that the skeletal musculature is the body's principal antigravity organ [78]). It is reasonable to ask why these forms of gravity intolerance overlap, why the consequences of g-force strain affect multiple body systems, and why these conditions frequently present with seemingly disparate neuropsychological comorbidities.
One common factor is serotonin, a highly pleiotropic neurotransmitter with diverse effects throughout the body (79). Abnormalities in serotonin have been strongly implicated in IBS pathogenesis but also play a role in its comorbidities including chronic pain, migraine headache, sleep disturbances, dizziness, anxiety, fibromyalgia, and depression (80). Thus, variations in serotonin biology, whether from genetics or pharmacotherapy, can cause effects across the body. A full discussion regarding serotonin is beyond our scope and may be reviewed elsewhere (81). It is also recognized that other neurotransmitters and mediators contribute to IBS pathogenesis. In this article, it is sufficient to acknowledge the common thread of serotonin abnormalities among IBS and its comorbid conditions.
It is possible that serotonin evolved, in part, to manage gravity. Without it, we would not be able to stand up, stay up, maintain balance, circulate blood, or pump intestinal contents in a 1 g world. The word serotonin means to increase tone or cause contraction, indicating that without it, we lose tone, contractility, and pump function—a recipe for gravity intolerance.
It is curious that despite the widespread effects of serotonin, 95% of this gravity management substance is produced in the GI tract (81). There, serotonin plays a major role in regulating intestinal peristalsis and colonic tone (81). Without serotonin, we could not efficiently transit luminal contents against gravity, rendering our intestines a flaccid sac. In addition, intestinal bacteria play a key role in producing serotonin (81), suggesting a coevolution between the microbiome and antigravity pump functions. Because serotonin is also a potent pain sensitizer, excess serotonin from dysbiosis can promote peripheral pain sensitization (82,83). Thus, gravity mismanagement can lead to an altered gut microbiome, increased production of serotonin and other bacterial metabolites (among other mechanisms [84,85]), and pain sensitization of afferent nerves that alert the brain to the very g-force strain that triggered the homeostatic feedback loop in the first place. In this manner, the microbiome plays a critical role in g-force detection and supports communication about gravity intolerance between the gut and the brain (Figure 8).
Figure 8.
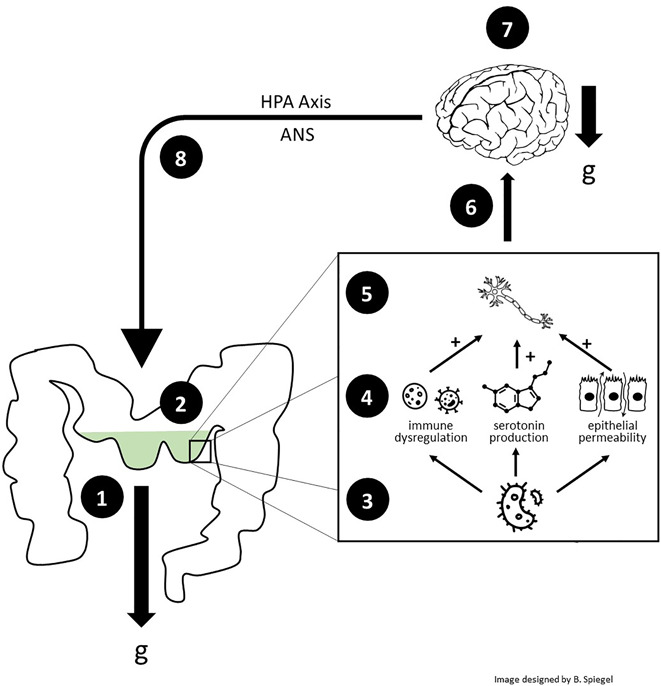
The gastrointestinal (GI) tract as serotonin-mediated home base for gravity management. (i) In those who are mechanically susceptible, gravity strain may cause compression, kinking, deformation, or pressurization of the intestines; (ii) dysmotility and stasis occur; (iii) microbiome alterations result; (iv) dysbiosis produces excess luminal serotonin (among other metabolites) and contributes to epithelial permeability and local immune dysregulation; (v) visceral afferent nerves become sensitized; (vi) nociceptive signals are transmitted to the brain with higher frequency and intensity; (v) alerted to the gravitational misalignment, the brain attempts to compensate by modifying physiological, psychological, and behavioral states in an effort to reduce g-force strain; (viii) disrupted sleep and stress from physical and psychosocial burden alter HPA axis and ANS function in a gravity-dependent manner (see Supplementary Digital Content, Appendix, http://links.lww.com/AJG/C771 for this mechanism), thus further dysregulating GI physiology and microbiology in a vicious cycle. A hypothesized alternative sequence of reverse causation begins with the brain rather than the gut, where primary abnormalities in structural and fluidic g-force support of the brain cause sleep disruptions, HPA axis and ANS dysregulation, and altered GI physiology, culminating in visceral sensitization and IBS symptoms. See the Supplementary Digital Content (Appendix, http://links.lww.com/AJG/C771) for this alternative sequence. ANS, autonomic nervous system; IBS, irritable bowel syndrome; GI, gastrointestinal; HPA, hypothalamic-pituitary-adrenal.
Beyond the GI tract, serotonin supports gravity management across body systems, including the cardiovascular and baroreceptor systems, vestibular system, and others (80). There is also evidence that changes in g-force can alter serotonin expression in mice (86), suggesting that serotonin levels change in relation to the gravitation field experienced by the organism. The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) provides further details.
In short, dysregulated serotonin may be a form of gravity failure, unto itself. If serotonin evolved in part to manage gravity across the body, then the GI tract—where serotonin is primarily manufactured—is the body's home base for gravity management. When the gut becomes intolerant of gravity strain through serotonin dysregulation, then the rest of the body suffers.
FACTOR 3: G-FORCE VIGILANCE
We now consider the third factor of the gravity hypothesis: g-force vigilance. Before exploring this CNS-derived factor, it is useful to acknowledge how the brain and body work as an integrated whole—not as distinct supratentorial vs infratentorial components. This insight will allow us to consider how all 3 factors may jointly explain IBS. The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) summarizes the gut-brain theory of IBS as an example of the more general embodied cognitions theory (87), which posits that our emotional and cognitive lives emerge not only because we have a brain but also because the brain integrates with the rest of the body. Because we spend most of our life on terrestrial Earth, our consciousness and its contents arise from possessing a 1 g body.
GUT FEELINGS AS A G-FORCE ACCELEROMETER
Most everyone has experienced abdominal butterflies, a sensation that ranges from a flutter to a kick in the gut. Butterflies are so universal that they may be considered an archetypal gut feeling with ancient origins. People often describe this sensation when experiencing emotions, such as falling in love or, conversely, feeling anxious. However, there is one stimulus that causes butterflies in most everyone: an abrupt deviation from 1 g. Just imagine riding a rollercoaster or dropping suddenly in an elevator; these visceral experiences are tied to feelings of thrill or, conversely, terror. These are gut-brain gravitational events.
It is curious that abrupt departures from 1 g trigger a primitive neurovisceral program. This observation suggests that gut feelings can operate like a g-force accelerometer. Normal g-forces, such as those experienced while walking or exercising, are typically far below the threshold for provoking gut feelings. Nonetheless, when g-forces exceed the natural dynamics of everyday life, as occurs on a rollercoaster or in a turbulent airplane, we may experience gut feelings. These examples can be classified as extreme g-force events, meaning they are marked by sudden g-force accelerations that exceed the typical and tolerable forces of normal 1 g living.
Homo sapiens did not evolve to fly on airplanes, ride rollercoasters, or bungee jump; these are examples of recent, purposeful, and controlled gravity challenges. For most of human evolution, extreme g-forces were not thrilling; they were serious threats to life and limb. Outside of thrill rides or extreme sports, corporeal deviations from 1 g often pose an existential threat: Consider falling from a ladder, tumbling down stairs, or being forcibly shoved to the ground.
Some people are more resilient to g-forces than others. For example, one person may raise their hands and grin while dropping on a rollercoaster, whereas another contracts their abdomen in a defensive posture, grits their teeth, and groans until they reapproach 1 g. The first person is amused and unconcerned while the second is threatened and vulnerable. In both cases, riding the rollercoaster is a way of rehearsing death in a controlled environment; the gut expects that death is imminent, but then the body defies expectations and survives. It is the predictability of the rollercoaster that converts terror into thrill. The first person in this example is confident that they cannot be harmed on the ride. The second person may understand that the ride is safe, but their ancient instincts are hypervigilant and unwilling to surrender control to the moment, so they gird themselves in fight-or-flight terror.
Moreover, if gut feelings arise before dangerous g-force events occur, then imminent threats can be predicted with enough warning to prevent or deflect harm. There is a survival advantage to having a sensitive gut; it alerts to the possibility of danger. This is why few people climb El Capitan in Yosemite National Park and why Alex Honnold, who accomplished the feat without ropes, is revered for his ability to defy gravity without paralyzing fear (88). By contrast, people with acrophobia develop gut feelings by the mere thought of dangling from a cliff. These examples reveal a natural spectrum: On one extreme, a rock climber may lack gut feelings despite risking a catastrophic fall. On the other, a person with acrophobia may have disabling gut feelings by imagining a fall that cannot occur because it is a figment of consciousness. These extremes represent 2 poles of the g-force vigilance factor. Gut feelings become pathologic along this spectrum when they create a feeling as if departing perilously from 1 g when, in fact, the chances are improbable—or even impossible—that will occur. In essence, pathologic gut feelings might be a form of visceral allodynia that result from overpredicting g-force events that are not even occurring or may never occur.
OUR INTERNAL MODEL OF GRAVITY
Homo sapiens evolved an internal model of gravity that allows us to anticipate, calculate, and compensate for g-forces without conscious effort (89). This can be observed in infants who are hesitant to crawl onto a glass surface where they view the ground beneath them (90). The same occurs with people who attempt to walk an elevated plank in virtual reality, of whom many experience overwhelming fear despite no risk of falling (91). We are born to protect against dangerous g-force accelerations.
The neural correlates of this instinct have been well-characterized. Functional imaging of Honnold's brain, for example, reveals that his amygdala is quiescent in the face of salient threats (89). By contrast, patients with IBS, EDS, POTS, and fibromyalgia, all have heightened amygdala reactivity and threat sensitivity (92). The amygdala is part of both the salience and emotional arousal circuits in the brain, which appraise and respond to perceived threats. These networks are dysregulated in IBS, in part because of serotonin-related gene polymorphisms (93), which results in overestimating the severity of potential threats and triggering fight-or-flight arousal.
Taken together, the gravity hypothesis suggests that everyone possesses an internal model of gravity from birth; that gut feelings arise when our internal gravity computer detects or predicts extreme g-force accelerations; that patients with IBS systematically overestimate the risk of dangerous g-force events; and therefore, that patients with IBS experience frequent and neurobiologically inappropriate ANS arousal and HPA dysregulation, leading to worse GI symptoms, heightened anxiety, and if unchecked, a vicious cycle of gut feelings and vigilance.
EMOTIONAL, COGNITIVE, AND BEHAVIORAL CONSEQUENCES OF G-FORCE HYPERVIGILANCE
We have discussed how gut feelings operate like a primitive g-force accelerometer. Now we can be more specific: Downward g-forces most consistently provoke gut feelings. Falling rapidly toward Earth—less so rising away from it—causes gut feelings in most people, possibly because negative g-forces cause the abdominal viscera to briefly float within the abdominal cavity, creating a strong visceral sensation. The theory of embodied cognitions helps explain our existential drive to avoid falling.
The first clues can be found in our use of language. Directional metaphors are strongly associated with an emotional valence (94). For example, language about looking up or rising against gravity implies positivity, happiness, and divinity (e.g., “wish upon a star,” “things are looking up,” and “up in heaven”). By contrast, downward language typically invokes negativity, fear, and suffering (e.g., “down in the dumps,” “have a sinking feeling,” and “down in hell”). Similarly, the word grave shares the same etymology as the word gravity (Latin: gravis, heavy or serious). The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) lists additional vertical metaphors. As an aside, it is notable that the phrase “falling in love”—an exception to this idiomatic rule—also describes a state of falling that can cause abdominal butterflies. Linguists explain that “falling in love” is like a metaphorical fall that is sudden, is uncontrollable, and creates vulnerability. It seems that both literal and metaphorical falls trigger gut feelings.
These examples are more than turns of phrase; they represent embodied concepts that are deeply encoded in our neurobiology. Gottwald et al. (95) explained that the twin concepts “up is good” and “down is bad” are “inseparable from their experiential basis” and “enable us to orient within our world.” Although reviewing the evidence of spatial embodiment is beyond our scope, key experiments are summarized in the Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771). In short, “up is good” and “down is bad” are not merely colloquialisms but are spatial cognitions that evolved from millennia of physical experiences in a 1 g world; they are grounded in our bodies from birth and can manifest as gut feelings.
However, gut feelings can also occur from events that are not always associated with falling. For example, losing a loved one, experiencing war, or sexual assault can provoke gut feelings and even trigger visceral anguish when they are recalled from memory—a process underlying posttraumatic stress disorder, which is comorbid in many patients with IBS (96). It is possible that as Homo sapiens evolved a larger and more complex brain capable of processing both physical and psychosocial threats, we co-opted our g-force accelerometer to alert for any serious threat rather than evolve a new program. Because “down” is neurobiologically bad, it is efficient that our time-tested down alarm would step in for any potentially unsafe threat, even those that do not involve literal g-forces. Still, it is notable that many causes of posttraumatic stress disorder involve extreme and uncontrolled g-forces (e.g., experiencing a car accident, sustaining a war injury, and being forcibly pushed down against one's will).
Persistent gravity intolerance can lead to emotional consequences (97) that include visceral anxiety—a form of distress marked by excessive worry about GI symptoms and their impact (Figure 1) (98). Viewed through the lens of the gravity hypothesis, visceral anxiety may result from hypervigilant surveillance of g-force events—in essence, a neurovisceral fear of falling. Many patients with IBS also exhibit somatization which is systematic overreporting of body pain that is disproportionate to the level of tissue injury (99)—a musculoskeletal analog of g-force vigilance. If gravity strain persists and g-force vigilance escalates without finding relief, patients may experience allostatic overload—a point beyond which the cumulative weight of chronic stress overwhelms the ability to cope (100). If allostatic overload is not corrected, then it can lead to vital exhaustion, a form of mental gravity where IBS sufferers can no longer tolerate the biopsychosocial toll of ineffective load management, as if they need to succumb to gravity.
Both visceral anxiety and vital exhaustion may generate 2 cognitions common among patients with IBS: lack of control and catastrophizing (Figure 1) (98). When patients believe they lack control over their illness, they may compensate by amplifying g-force vigilance. They may even believe their illness is a disaster, which literally means “the stars are against” them (Italian: dis, against; astro, star), as if universal forces (i.e., gravity) have conspired to cause disease. The second cognition, catastrophizing, is the belief that perceived threats are more severe than they de facto are. These cognitions lead to compensatory healthcare-seeking behaviors (e.g., high-volume physician visits, requesting potentially inappropriate tests) and avoidant/restrictive behaviors (e.g., minimizing social engagements, sex, exercise, or certain foods). If these behaviors do not provide relief and gravity failure seems inescapable, then patients with IBS may develop depression or even express suicidal ideations (101) resulting from a sort of gravity surrender. In the tragic extreme, some may commit suicide (101) to end their metaphorical and literal struggle against the relentless pull of gravity, as if feeling they were not born for this Earth.
CLINICAL, THERAPEUTIC, AND RESEARCH IMPLICATIONS OF THE IBS GRAVITY HYPOTHESIS
We can now reconsider the IBS g-force cube in Figure 2. According to this proposed model, we all inherit a set point for each g-force factor; the combination of those set points defines baseline IBS susceptibility. Life events and behaviors further shift an individual from their native set point, causing them to move up or down the range for each factor; these shifts reset an individual's IBS risk. A hypothesized biogravitational approach to IBS would determine the combination of current set points for each patient and attempt to shift all set points toward the bottom, left, and front of the g-force cube.
Table 2 summarizes a framework for this approach, including the determinants of native set points, factors that cause a shift from the set point, clinical assessments, and treatments. For example, strategies to shift patients away from the vulnerable end of g-force resistance might include aerobic exercise, tai chi, osteopathic therapies, tilt table therapy, experimenting with different sleep positions, or weight loss, among others. If these strategies are incapable of relieving g-force strain on the gut and the intestines continue to struggle against gravity, then supplemental gut failure medications are warranted to address dysmotility and/or dysbiosis. Treatments to shift patients away from the hypersensitive end of g-force detection might include modifying the gut microbiome through antibiotics or low fermentable diets, altering gut serotonin, PNS neuromodulation, vestibular treatments, and increasing hydration and salt intake (if safe) to maintain circulating pressure and lessen baroreceptor-mediated sympathetic tone. Treatments to shift patients away from the hypervigilant end of g-force vigilance might include cognitive behavioral therapy, gut-directed hypnotherapy, CNS neuromodulation, vagal nerve stimulation, sleep therapy, and possibly even experimenting with living at different altitudes or distances from the equator (one of many researchable questions in Table 1). Patients should also understand that even if they inherited a body that cannot thrive on 1 g Earth, they still possess neuroplasticity, a remarkable capacity that enables the brain to modify how it experiences the body. The Supplementary Digital Content (see Appendix, http://links.lww.com/AJG/C771) provides further details regarding the g-force cube, describes a clinical approach to each of the 8 profiles defined by the cube, and addresses how the hypothesis attempts to explain other important issues, such as the role of postinfectious IBS and the circular problem of whether physical distress causes mental distress, vice versa, or both together.
Table 2.
A biogravitational approach to management of irritable bowel syndrome
CONCLUSION
Our relationship to gravity is not unlike the relationship of fish to water: We live our entire life in it, are shaped by it, yet hardly notice its ever present influence on the nature of our existence. This article reviews how we live with and through gravity with a focus on the GI tract. It explores a proposed hypothesis that seeks to explain the manifold theories, symptoms, comorbidities, and treatment outcomes of IBS by describing the syndrome in relation to gravity, a force that was present before we arrived and will exist long after we are gone. The gravity hypothesis is not intended to usurp existing theories of IBS, but rather to accommodate multiple explanations and observations as the possible consequence of a fundamental and universal force that shapes reality as we know it. Further research, such as presented in Table 1, can help support, reject, expand, or contract the gravity hypothesis as proposed in this article.
We are the product of a gravity-bound environment. Our health and wellbeing depend on living successfully with and through gravity, and we may suffer when we succumb to it. This article suggests that IBS may result from gravity.
CONFLICTS OF INTEREST
Guarantor of the article: Brennan Spiegel, MD, MSHS.
Specific author contributions: Conceived and wrote the entire article.
Financial support: None to report.
Potential competing interests: None to report.
ACKNOWLEDGEMENT
The author acknowledges Kaelen Spiegel for digital design assistance for several of the figures.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C771
REFERENCES
- 1.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterol 2016;18:S0016. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313(9):949–58. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Tack J, Ford AC, et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome foundation working team report. Gastroenterology 2018;154(4):1140–71.e1. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Quigley EMM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: Systematic review and meta-analysis. Am J Gastroenterol 2014;109(9):1350–65. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: Management of irritable bowel syndrome. Am J Gastroenterol 2021;116(1):17–44. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel M, Lembo A. Microbiome and its role in irritable bowel syndrome. Dig Dis Sci 2020;65(3):829–39. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122(4):1140–56. [DOI] [PubMed] [Google Scholar]
- 8.Adamopoulos K, Koutsouris D, Zaravinos A, et al. Gravitational influence on human living systems and the evolution of species on Earth. Molecules 2021;26(9):2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershon MD. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharm Ther 1999;13:15–30. [PubMed] [Google Scholar]
- 10.Jenkins T, Nguyen JC, Polglaze KE, et al. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016;8(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JQ, Jiang N, Li ZP, et al. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci Space Res 2020;27:74–82. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez R, Stork CA, sayoc-Becerra A, et al. A simulated microgravity environment causes a sustained defect in epithelial barrier function. Sci Rep 2019;9(1):17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang P, Green SJ, Chlipala GE, et al. Reproducible changes in the gut microbiome suggest a shift in microbial and host metabolism during spaceflight. Microbiome 2019;7(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alauzet C, Cunat L, Wack M, et al. Hypergravity disrupts murine intestinal microbiota. Sci Rep 2019;9(1):9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikree A, Chelimsky G, Collins H, et al. Gastrointestinal involvement in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017;175(1):181–7. [DOI] [PubMed] [Google Scholar]
- 16.Rezai A, Raphael Y, Sukov R, et al. Ehlers-Danlos syndrome type III (EDS) and visceroptosis: Getting to the bottom of this diagnosis. Am J Gastroenterol;113(Suppl):S270–1. [Google Scholar]
- 17.Reinstein E, Pimentel M, Pariani M, et al. Visceroptosis of the bowel in the hypermobility type of Ehlers-Danlos syndrome: Presentation of a rare manifestation and review of the literature. Eur J Med Genet 2012;55(10):548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchie H, Miyakoshi N, Masutani N, et al. Impact of spinal kyphosis on gastric myoelectrical activity in elderly patients with osteoporosis. Biomed Res 2019;40(6):215–23. [DOI] [PubMed] [Google Scholar]
- 19.Filippiadis DK, Marcia S, Ryan A, et al. New implant-based technologies in the spine. Cardiovasc Intervent Radiol 2018;41(10):1463–73. [DOI] [PubMed] [Google Scholar]
- 20.Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992;13:S27–31. [DOI] [PubMed] [Google Scholar]
- 21.Coffey JC, O'Leary DP. The mesentery: Structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1(3):238–47. [DOI] [PubMed] [Google Scholar]
- 22.Byrnes KG, Walsh D, Walsh LG, et al. The development and structure of the mesentery. Commun Biol 2021;4(1):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharucha AE, Brroke SJ. Taenia coli. In Johnson LR. (ed). Physiology of the Gastrointestinal Tract. 5th edn, 2012. [Google Scholar]
- 24.Zarate N, farmer AD, Grahame R, et al. Unexplained gastrointestinal symptoms and joint hypermobility: Is connective tissue the missing link? Neurogastro Motil 2010;22(3):252.e78. [DOI] [PubMed] [Google Scholar]
- 25.Choudhary A, Fikree A, Aziz Q. Overlap between irritable bowel syndrome and hypermobile Ehlers-Danlos syndrome: An unexplored clinical phenotype? Am J Med Genet C Semin Med Genet 2021;187(4):561–9. [DOI] [PubMed] [Google Scholar]
- 26.Alomari M, Hitawala A, Chadalavada P, et al. Prevalence and predictors of gastrointestinal dysmotility in patients with hypermobile Ehlers-Danlos syndrome: A tertiary care center experience. Cureus 2020;12(4):e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvey MR, Macfarlane GJ, Beasley M, et al. Modest association of joint hypermobility with disabling and limiting musculoskeletal pain: Results from a large-scale general population-based survey. Arthritis Care Res 2013;65(8):1325–33. [DOI] [PubMed] [Google Scholar]
- 28.Castori M. Ehlers-danlos syndrome, hypermobility type: An underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol 2012;2012:751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modi RM, Hinton A, Pinkhas D, et al. Implementation of a defecation posture modification device. J Clin Gastroenterol 2019;53(3):216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel B, Guo X, Noblet J, et al. Computational analysis of mechanical stress in colonic diverticulosis. Sci Rep 2020;10(1):6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: Evolving epidemiologic and clinical insights. Am J Gastroenterol 2012;107(10):1486–93. [DOI] [PubMed] [Google Scholar]
- 32.Järbrink-Sehgal ME, Rassam L, Jasim A, et al. Diverticulosis, symptoms and colonic inflammation: A population-based colonoscopy study. Am J Gastroenterol 2019;114(3):500–10. [DOI] [PubMed] [Google Scholar]
- 33.Tursi A, Brandimarte G, Giorgetti GM, et al. Assessment of small intestinal bacterial overgrowth in uncomplicated acute diverticulitis of the colon. World J Gastroenterol 2005;11(18):2773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuda T, Gunnarsson R, de Costa A. The correlation between diverticulosis and redundant colon. Int J Colorectal Dis 2017;32(11):1603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders BP, Fukumoto M, Halligan S, et al. Why is colonoscopy more difficult in women? Gastrointest Endosc 1996;43(2):124–6. [DOI] [PubMed] [Google Scholar]
- 36.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 2003;88(6):520–6. [DOI] [PubMed] [Google Scholar]
- 37.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maxton DG, Morris J, Whorwell PJ. More accurate diagnosis of irritable bowel syndrome by the use of “non-colonic” symptomatology. Gut 1991;32(7):784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talley NJ. Unnecessary abdominal and back surgery in irritable bowel syndrome. Gastronterology 2004;126(7):1899–903. [DOI] [PubMed] [Google Scholar]
- 40.Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: A multivariable analysis☆. Gastroenterology 2004;126(7):1665–73. [DOI] [PubMed] [Google Scholar]
- 41.Drewes AM, Olesen AE, Farmer AD, et al. Gastrointestinal pain. Nat Rev Dis Prim 2020;6(1):1. [DOI] [PubMed] [Google Scholar]
- 42.Midenfjord I, Grinsvall C, Koj P, et al. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol Motil 2021;33(12):e14156. [DOI] [PubMed] [Google Scholar]
- 43.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep 2002;4:322–8. [DOI] [PubMed] [Google Scholar]
- 44.Daley AJ, Grimmett C, Roberts L, et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: A randomised controlled trial. Int J Sports Med 2008;29(09):778–82. [DOI] [PubMed] [Google Scholar]
- 45.Johannesson E, Simren M, Strid H, et al. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. Am J Gastroenterol 2011;106(5):915–22. [DOI] [PubMed] [Google Scholar]
- 46.Johannesson E, Ringstrom G, Abrahamsson H, et al. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol 2015;21(2):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller A, Franke H, Resch KL, et al. Effectiveness of osteopathic manipulative therapy for managing symptoms of irritable bowel syndrome: A systematic review. J Am Osteopath Assoc 2014;114(6):470–9. [DOI] [PubMed] [Google Scholar]
- 48.Ohlsson B, Manjer J. Physical inactivity during leisure time and irregular meals are associated with functional gastrointestinal complaints in middle-aged and elder subjects. Scand J Gastroenterol 2016;51(11):1299–307. [DOI] [PubMed] [Google Scholar]
- 49.Song BK, Cho KO, Jo Y, et al. Colon transit time according to physical activity level in adults. J Neurogastroenterol Motil 2012;18(1):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villoria A, Serra J, Azpiroz F, et al. Physical activity and intestinal gas clearance in patients with bloating. Am J Gastroenterol 2006;101(11):2552–7. [DOI] [PubMed] [Google Scholar]
- 51.D'Silva A, MacQueen G, Nasser Y, et al. Yoga as a therapy for irritable bowel syndrome. Dig Dis Sci 2020;65(9):2503–14. [DOI] [PubMed] [Google Scholar]
- 52.Schumann D, Anheyer D, Lauche R, et al. Effect of yoga in the therapy of irritable bowel syndrome: A systematic review. Clin Gastrenterol Hepatol 2016;14(12):1720–31. [DOI] [PubMed] [Google Scholar]
- 53.Eriksson EM, Möller IE, Söderberg RH, et al. Body awareness therapy: A new strategy for relief of symptoms in irritable bowel syndrome patients. World J Gastroenterol 2007;13(23):3206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villoria A, Azpiroz F, Burri E, et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol 2011;106(5):815–9. [DOI] [PubMed] [Google Scholar]
- 55.Tremolaterra F, Villoria A, Azpiroz F, et al. Impaired viscerosomatic reflexes and abdominal-wall dystony associated with bloating. Gastroenterology 2006;130(4):1062–8. [DOI] [PubMed] [Google Scholar]
- 56.Lacy BE, Cangemi DJ. A pragmatic approach to the evaluation and treatment of abdominal bloating. Am J Gastroenterol 2022;117:701–705. [DOI] [PubMed] [Google Scholar]
- 57.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009;136(6):2003–14. [DOI] [PubMed] [Google Scholar]
- 58.Quigley EMM. Bacterial flora in irritable bowel syndrome: Role in pathophysiology, implications for management. J Dig Dis 2007;8(1):2–7. [DOI] [PubMed] [Google Scholar]
- 59.Pimentel M, Saad RJ, Long MD, et al. ACG clinical guideline: Small intestinal bacterial overgrowth. Am J Gastroenterol 2020;115(2):165–78. [DOI] [PubMed] [Google Scholar]
- 60.Wei Si-Qi, Tao ZY, Xue Y, et al. Peripheral sensitization. In Turker H, García-Benavides L, Ramos-Gallardo G, et al. (eds). Peripheral Nerve Disorders and Treatment. IntechOpen: London, 2019. [Google Scholar]
- 61.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 2002;122(7):1771–7. [DOI] [PubMed] [Google Scholar]
- 62.Deiteren A, de Wit A, van der Linden L, et al. Irritable bowel syndrome and visceral hypersensitivity : Risk factors and pathophysiological mechanisms. Acta Gastroenterol Belg 2016;79(1):29–38. [PubMed] [Google Scholar]
- 63.Azpiroz F, Bouin M, Camilleri M, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 2007;19(s1):62–88. [DOI] [PubMed] [Google Scholar]
- 64.Akbar A, Walters JRF, Ghosh S. Review article: Visceral hypersensitivity in irritable bowel syndrome: Molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther 2009;30(5):423–35. [DOI] [PubMed] [Google Scholar]
- 65.Eccles JA, Owens AP, Mathias CJ, et al. Neurovisceral phenotypes in the expression of psychiatric symptoms. Front Neurosci 2015;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spaziani R, Bayati A, Redmond K, et al. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol Motil 2008;20(5):576–42. [DOI] [PubMed] [Google Scholar]
- 67.Davydov DM, Naliboff B, Shahabi L, et al. Baroreflex mechanisms in irritable bowel syndrome: Part I. Traditional indices. Physiol Behav 2016;157:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Migeotte PF, Prisk GK, Paiva M. Microgravity alters respiratory sinus arrhythmia and short-term heart rate variability in humans. Am J Physiol Heart Circ Physiol 2003;284(6):H1995–2006. [DOI] [PubMed] [Google Scholar]
- 69.Polster A, Friberg P, Gunterberg V, et al. Heart rate variability characteristics of patients with irritable bowel syndrome and associations with symptoms. Neurogastroenterol Motil 2018;30(7):e13320. [DOI] [PubMed] [Google Scholar]
- 70.Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: A systematic review with meta-analysis. Saudi J Gastroenterol 2018;24(3):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choung RS, Herrick LM, Locke GR, III, et al. Irritable bowel syndrome and chronic pelvic pain: A population-based study. J Clin Gastroenterol 2010;44(10):696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hyland ME, Bacon AM, Lanario JW, et al. Symptom frequency and development of a generic functional disorder symptom scale suitable for use in studies of patients with irritable bowel syndrome, fibromyalgia syndrome or chronic fatigue syndrome. Chronic Dis Transl Med 2019;5(2):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breckan RK, Asfeldt AM, Straume B, et al. Prevalence, comorbidity, and risk factors for functional bowel symptoms: A population-based survey in northern Norway. Scand J Gastroenterol 2012;47(11):1274–82. [DOI] [PubMed] [Google Scholar]
- 74.Erdrich S, Hawrelak JA, Myers SP, et al. A systematic review of the association between fibromyalgia and functional gastrointestinal disorders. Ther Adv Gastroenterol 2020;13:1756284820977402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Hernandez A, de la Coba P, Reyes Del Paso GA. Central sensitisation pain and autonomic deficiencies in fibromyalgia. Clin Exp Rheumatol 2022;40(6):1202–9. [DOI] [PubMed] [Google Scholar]
- 76.Erdrich S, Hawrelak JA, Myers SP, et al. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet Disord 2020;21(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martins DF, Viseux FJ, Salm DC, et al. The role of the vagus nerve in fibromyalgia syndrome. Neurosci Biobehav Rev 2021;131:1136–49. [DOI] [PubMed] [Google Scholar]
- 78.Jaster JH. Gravity in the brain-how it may regulate skeletal muscle metabolism by balancing compressive ischemic changes in the weight-bearing pituitary and hypothalamus. Physiol Rep 2021;9(10):e14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009;60(1):355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 2009;16(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei L, Singh R, Ghoshal UC. Enterochromaffin cells-gut microbiota crosstalk: Underpinning the symptoms, pathogenesis, and pharmacotherapy in disorders of gut-brain interaction. J Neurogastroenterol Motil 2022;28(3):357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2(1):16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Li WG, Yu Y, et al. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci 2013;33(10):4265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125(3):926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aroniadis OC, Drossman DA, Simrén M. A perspective on brain–gut communication: The American Gastroenterology Association and American Psychosomatic Society Joint Symposium on Brain–gut interactions and the intestinal microenvironment. Psychosom Med 2017;79(8):847–56. [DOI] [PubMed] [Google Scholar]
- 86.Ishikawa C, Li H, Ogura R, et al. Effects of gravity changes on gene expression of BDNF and serotonin receptors in the mouse brain. PLoS One 2017;12(6):e0177833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damasio A, Carvalho GB. The nature of feelings: Evolutionary and neurobiological origins. Nat Rev Neurosci 2013;14(2):143–52. [DOI] [PubMed] [Google Scholar]
- 88.Chai Vasarhelyi E, Chin J. Free Solo. United Stages: National Geographic Documentary Films, 2018. [Google Scholar]
- 89.McIntyre J, Zago M, Berthoz A, et al. Does the brain model Newton's laws? Nat Neurosci 2001;4(7):693–4. [DOI] [PubMed] [Google Scholar]
- 90.Rader N, Bausano M, Richards JE. On the nature of the visual-cliff-avoidance response in human infants. Child Dev 1980;51(1):61–8. [PubMed] [Google Scholar]
- 91.Emmelkamp PMG, Krijn M, Hulsbosch AM, et al. Virtual reality treatment versus exposure in vivo: A comparative evaluation in acrophobia. Behav Res Ther 2002;40(5):509–16. [DOI] [PubMed] [Google Scholar]
- 92.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? J Pain 2009;10(11):1113–20. [DOI] [PubMed] [Google Scholar]
- 93.Mayer EA, Labus JS, Tillisch KT, et al. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015;12(10):592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cian L. Verticality and conceptual metaphors: A systematic review. J Ass Consum Res 2017;2(4):444–59. [Google Scholar]
- 95.Gottwald JM, Elsner B, Pollatos O. Good is up-spatial metaphors in action observation. Front Psychol 2015;6:1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kearney DJ, Kamp KJ, Storms M, et al. Prevalence of gastrointestinal symptoms and irritable bowel syndrome among individuals with symptomatic posttraumatic stress disorder. J Clin Gastroenterol 2022;56(7):592–6. [DOI] [PubMed] [Google Scholar]
- 97.Spiegel BMR, Khanna D, Bolus R, et al. Understanding gastrointestinal distress: A framework for clinical practice. Am J Gastroenterol 2011;106(3):380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trieschmann K, Chang L, Park S, et al. The visceral sensitivity index: A novel tool for measuring GI-symptom-specific anxiety in inflammatory bowel disease. Neurogastroenterol Motil 2022;34(9):e14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller AR, North CS, Clouse RE, et al. The association of irritable bowel syndrome and somatization disorder. Ann Clin Psychiatry 2001;13(1):25–30. [DOI] [PubMed] [Google Scholar]
- 100.Guidi J, Lucente M, Sonino N, et al. Allostatic load and its impact on health: A systematic review. Psychother Psychosom 2021;90(1):11–27. [DOI] [PubMed] [Google Scholar]
- 101.Spiegel B, Schoenfeld P, Naliboff B. Systematic review: The prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther 2007;26(2):183–93. [DOI] [PubMed] [Google Scholar]