Abstract
Rationale & Objective
Dysnatremias have been associated with an increased risk of mortality in the chronic kidney disease (CKD) population. Our objective is to identify the prevalence of and risk factors associated with dysnatremias in a CKD population and assess the association of dysnatremias with kidney failure and mortality among patients with CKD enrolled in the Chronic Renal Insufficiency Cohort Study.
Study Design
Analysis of prospective cohort study.
Setting & Participants
Adult patients aged 21-74 years with CKD from the Chronic Renal Insufficiency Cohort study.
Predictors
Baseline and time-dependent hyponatremia and hypernatremia.
Outcomes
All-cause mortality and kidney failure.
Analytical Approach
Baseline characteristics were compared using χ2 tests for categorical variables, analysis of variance for age, and Kruskal-Wallis tests for laboratory variables. Cox proportional hazards models and competing risk models were used to evaluate the association between baseline sodium level and overall mortality.
Results
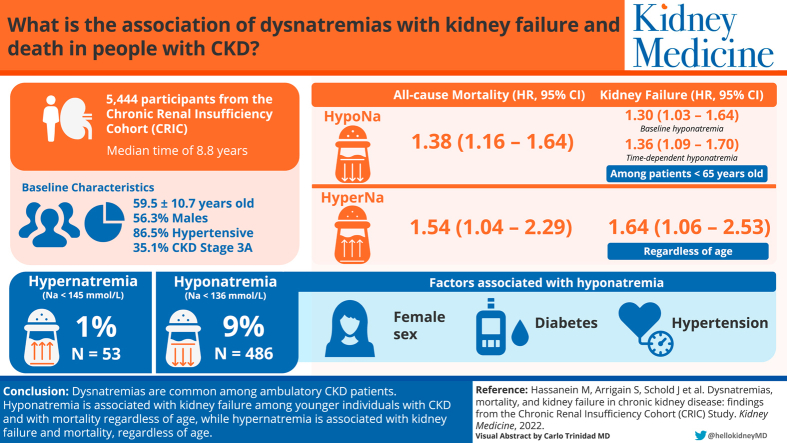
Of a total of 5,444 patients with CKD, 486 (9%) had hyponatremia and 53 (1%) had hypernatremia. Altogether, 1,508 patients died and 1,206 reached kidney failure. In adjusted Cox models, time-dependent dysnatremias were strongly associated with mortality for both hyponatremia (HR, 1.38; 95% CI, 1.16-1.64) and hypernatremia (HR, 1.54; 95% CI, 1.04-2.29). Factors associated with hyponatremia included female sex, diabetes, and hypertension. Regardless of age, time-dependent hypernatremia was associated with an increased risk of kidney failure (HR, 1.64; 95% CI, 1.06-2.53). Baseline and time-dependent hyponatremia were associated with an increased risk of kidney failure in patients younger than 65 (baseline hyponatremia HR, 1.30; 95% CI, 1.03-1.64 and time-dependent hyponatremia HR, 1.36; 95% CI, 1.09-1.70) but not among patients aged >65 years.
Limitations
Inability to establish causality and lack of generalizability to hospitalized patients.
Conclusions
Dysnatremias are prevalent among ambulatory CKD patients and are associated with mortality and kidney failure. Time-dependent dysnatremias were significantly associated with mortality in patients with CKD. Time-dependent hypernatremia was associated with progression to kidney failure. Baseline and time-dependent hyponatremia were associated with an increased risk of progression to kidney failure in those younger than 65 years.
Index Words: Chronic kidney disease, Chronic Renal Insufficiency Cohort, CRIC, death, dysnatremias, hypernatremia, hyponatremia, kidney failure, mortality
Visual Abstract
Hyponatremia is the most common electrolyte disorder worldwide, with a prevalence ranging from 14.5% to 58% in hospitalized patients without kidney disease.1, 2, 3, 4 Hospitalized patients with hyponatremia have a 50% higher risk of death compared with patients with eunatremia.5, 6, 7, 8 Hyponatremia may contribute to increased mortality secondary to organ dysfunction.9 The presence of hyponatremia has been associated with more deleterious outcomes in congestive heart failure (CHF) and cirrhosis.10 Hypernatremia is less common with a reported prevalence of 2% in hospitalized patients.11 Hypernatremia is an independent risk factor for mortality, particularly in the intensive care unit.12
The kidneys play a central role in the regulation of water homeostasis.13 Dysregulation in electrolyte-free water balance predisposes individuals with kidney disease to dysnatremias.14 In advanced chronic kidney disease (CKD), the kidneys lose their ability to concentrate and/or dilute urine, which may predispose individuals to hypernatremia and hyponatremia, respectively.15 Dysnatremias in patients with CKD have been associated with increased all-cause mortality, cardiovascular, malignancy, and non-cardiovascular/non-malignancy-related deaths.16,17 Individuals with CKD at risk for hyponatremia include those with younger age, diabetes, CHF, and earlier stages of CKD.18 There is a void of literature on the long-term effects of sodium in an extensively studied CKD population, and therefore, we sought to evaluate the effect of dysnatremias on mortality and kidney failure from patients enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study.19
Methods
Study Population
CRIC is a multicenter, prospective study in the United States that was designed to examine the determinants of CKD progression, cardiovascular disease, and mortality but has evolved into a national database for the investigation of a wide range of CKD-related topics. Details of the CRIC study have been published previously.20,21
Briefly, the CRIC study started in 2003 and has enrolled a total of 5,499 participants from 13 recruitment sites, with ages 21-74 and an estimated glomerular filtration rate (eGFR) of 20-70 mL/min/1.73 m2 (Item S1). Participants are still actively being followed every 6 months. General exclusion criteria for CRIC participant enrollment included New York Heart Association class III-IV CHF, cirrhosis, chemotherapy for systemic cancer, immunosuppression for primary kidney disorders within 6 months, prior kidney transplant, and polycystic kidney disease. Informed consent was obtained during initial screening visits.
We included all CRIC study participants with baseline levels of both serum sodium and glucose. We used the 2021 race-agnostic Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for eGFR.22 CKD was classified into: CKD stage 1 and 2 (eGFR ≥60 mL/min/1.73 m2), CKD stage 3a (eGFR 45-59 mL/min/1.73 m2), CKD stage 3b (eGFR 30-44 mL/min/1.73 m2), CKD stage 4 (eGFR 15-29 mL/min/1.73 m2), and CKD stage 5 (eGFR <15 mL/min/1.73 m2).
Serum Sodium and Glucose
Measurements of serum sodium (mmol/L) were processed at the CRIC Central Laboratory using an ion-selective electrode for all samples collected through October 2018. Samples collected after October 2018 were processed using ion-selective electrodes at participating CRIC sites. Sodium values were adjusted for glucose levels > 200 mg/dL as follows: adjusted sodium = sodium + (1.6 × (glucose-100)/100)). Adjusted sodium levels were grouped into hyponatremia (<136 mmol/L), eunatremia (136 to <146 mmol/L), and hypernatremia (≥146 mmol/L). These values were chosen based on cutoff values reported by Huang et al17 who conducted a similar study in a tertiary referral center. Sodium and glucose values were measured at baseline and yearly during follow-up visits.
Outcomes
Primary measured outcomes included all-cause mortality and kidney failure. Kidney failure was defined as the initiation of dialysis or pre-emptive transplant. All CRIC participants complete annual in-person follow-up visits that include collection of medical history, hospital events, medication review, physical measures, and laboratory testing. Time of death is ascertained through reports from family, medical records, obituaries, and queries of the National Death Index.21 Kidney failure status and timing are confirmed using the US Renal Data System. The CRIC Adjudication Committee reviews all de-identified medical records to determine kidney clinical endpoints. The data used for our analysis was ascertained from 2003 through 2020.
Statistical Analysis
We compared baseline characteristics among patients with hyponatremia, eunatremia, and hypernatremia using χ2 tests for categorical variables, analysis of variance for age, and Kruskal-Wallis tests for laboratory variables. Generalized estimating equations were used to evaluate factors associated with hyponatremia compared to eunatremia and hypernatremia using repeated measures of sodium over time. In addition to sodium and glucose measurements, other data was updated during follow-up including laboratory measures, body mass index, medications, and comorbid conditions. Covariates were chosen a priori based on factors previously shown or thought to be related to serum sodium levels and mortality. We adjusted the model for age, sex, race, body mass index (<25, 25-30, ≥30 kg/m2), history of smoking, diabetes, hypertension, stroke, cancer, CHF, cardiovascular disease, peripheral vascular disease, CKD stage, serum bicarbonate, serum albumin, serum potassium level, log blood urea nitrogen, selective serotonin reuptake inhibitors, angiotensin-converting enzyme inhibitors, aldosterone receptor blockers, β-blockers, loop diuretics, and thiazides. For the time-dependent data of factors associated with hyponatremia, body mass index was missing on 9% of follow-ups, whereas less than 1% of data was missing for hypertension, cancer, serum potassium, blood urea nitrogen, serum albumin, serum bicarbonate, eGFR, angiotensin-converting enzyme inhibitors, aldosterone receptor blockers, β-blockers, selective serotonin reuptake inhibitors, loop, and thiazide diuretics. We used the carry-forward method when covariates were missing in follow-up data. For patients that reached kidney failure, we included their data before reaching kidney failure and excluded their data after reaching kidney failure. To evaluate whether factors associated with hyponatremia remained consistent even when including data for patients already on dialysis, we did a sensitivity analysis in which we included all measurements per patient and assigned an eGFR of 10 mL/min/1.73 m2 to patients after they went on dialysis.
We estimated and graphed time to mortality and kidney failure using competing risks accounting for potential informative censoring.23 We used Cox proportional hazards models to evaluate the association between baseline sodium level and overall mortality. We also used Fine and Gray’s extension of Cox regression24 to evaluate the association between baseline serum sodium levels and kidney failure with death as a competing risk. We adjusted the models for the following variables: age, sex, race, smoking status, eGFR, body mass index, diabetes, hypertension, coronary artery disease, CHF, stroke, all cancer, peripheral vascular disease, hypercholesterolemia, serum albumin, serum bicarbonate, serum potassium, blood urea nitrogen, and the use of each of the following: angiotensin-converting enzyme inhibitors, aldosterone receptor blockers, beta-blockers, selective serotonin reuptake inhibitors, loop diuretics, and thiazides. We used natural cubic splines with 3 equally spaced knots (at the 25th, 50th, and 75th percentiles) to relax linearity assumptions for continuous covariates when appropriate. We tested 2-way interactions between hyponatremia and eunatremia with each of the following variables: eGFR, age, and CHF. Interactions with age were subsequently evaluated as age <65 versus ≥65 years for ease of interpretation. We did not test interactions with hypernatremia because of the small sample size. In addition, we also fitted models including baseline continuous sodium with splines at the 10th, 50th, and 90th percentiles while adjusting for the above-mentioned covariates. When significant associations were found, we plotted sodium versus the hazard ratio of the outcome (using the median sodium value in our data as reference value). Urine albumin to creatinine ratio was a potentially important covariate that was missing in 31% of patients, so we evaluated whether results were consistent when adjusting for baseline urine albumin to creatinine ratio in sensitivity analyses.
To evaluate the relationship between serum sodium levels and outcomes, we fitted a Cox proportional hazards model of all-cause mortality with time-dependent repeated measures of sodium. For each yearly serum sodium measurement, the corresponding serum glucose measurement was used for adjustment. For completed visits over the entire study, approximately 6% of sodium and glucose measurements were missing. We used carry-forward values to fill in data when serum sodium and glucose values were not obtained until the next yearly follow-up. It seemed reasonable to assume constant sodium level for that patient until the next completed visit. We also fitted a similar adjusted Cox model of time-dependent sodium level and kidney failure censored at death.
Less than 1% of data was missing for some baseline variables (Table 1). We used multiple imputations (SAS proc MI) with the Markov Chain Monte Carlo method and a single chain to impute 5 datasets with complete data. All the covariates from the multivariable model were included in the imputation. All models were performed on each of the 5 imputed datasets, and parameter estimates were combined using SAS MI analyze.
Table 1.
Patient Characteristics at Baseline by Sodium Category
| Factor | N missing | Overall (N = 5,444) | Hyponatremia <136 mmol/L (N = 486) |
Eunatremia 136-145 mmol/L (N = 4,905) |
Hypernatremia ≥145 mmol/L (N = 53) |
P |
|---|---|---|---|---|---|---|
| Age (y) | 0 | 59.5 ± 10.7 | 58.1 ± 11.7 | 59.7 ± 10.6 | 60.9 ± 7.6 | 0.004a |
| Age (y) | 0 | 0.34b | ||||
| <65 | 3,510 (64.5%) | 327 (67.3%) | 3,147 (64.2%) | 36 (67.9%) | ||
| ≥65 | 1,934 (35.5%) | 159 (32.7%) | 1,758 (35.8%) | 17 (32.1%) | ||
| Sex | 0 | 0.001b | ||||
| Male | 3,066 (56.3%) | 249 (51.2%) | 2,797 (57.0%) | 20 (37.7%) | ||
| Female | 2,378 (43.7%) | 237 (48.8%) | 2,108 (43.0%) | 33 (62.3%) | ||
| Race | 0 | <0.001b | ||||
| White | 2,502 (46.0%) | 236 (48.6%) | 2,246 (45.8%) | 20 (37.7%) | ||
| Black | 2,411 (44.3%) | 163 (33.5%) | 2,217 (45.2%) | 31 (58.5%) | ||
| Other | 531 (9.8%) | 87 (17.9%) | 442 (9.0%) | 2 (3.8%) | ||
| BMI (kg/m2) | 29 | <0.001b | ||||
| <25 | 785 (14.5%) | 112 (23.1%) | 663 (13.6%) | 10 (19.2%) | ||
| 25-30 | 1,562 (28.8%) | 151 (31.1%) | 1,400 (28.7%) | 11 (21.2%) | ||
| >30 | 3,068 (56.7%) | 222 (45.8%) | 2,815 (57.7%) | 31 (59.6%) | ||
| Comorbid conditions | ||||||
| Current smoker | 0 | 683 (12.5%) | 67 (13.8%) | 606 (12.4%) | 10 (18.9%) | 0.25b |
| Diabetes mellitus | 0 | 2,794 (51.3%) | 280 (57.6%) | 2,490 (50.8%) | 24 (45.3%) | 0.01b |
| Hypertension | 2 | 4,706 (86.5%) | 416 (85.6%) | 4,243 (86.5%) | 47 (88.7%) | 0.76b |
| CV disease | 0 | 1,833 (33.7%) | 160 (32.9%) | 1,646 (33.6%) | 27 (50.9%) | 0.03b |
| Stroke | 0 | 567 (10.4%) | 45 (9.3%) | 513 (10.5%) | 9 (17.0%) | 0.21b |
| CHF | 0 | 527 (9.7%) | 38 (7.8%) | 482 (9.8%) | 7 (13.2%) | 0.25b |
| Diagnosed or treated for any cancer 5 years before enrollment | 0 | 452 (8.3%) | 40 (8.2%) | 409 (8.3%) | 3 (5.7%) | 0.78b |
| Hyperlipidemia | 0 | 4,342 (79.8%) | 384 (79.0%) | 3,912 (79.8%) | 46 (86.8%) | 0.41b |
| Peripheral vascular disease | 0 | 363 (6.7%) | 37 (7.6%) | 323 (6.6%) | 3 (5.7%) | 0.66b |
| Laboratory tests | ||||||
| 2021 CKD-EPI Stage: eGFR (mL/min/1.73 m2) | 0 | 0.004b | ||||
| Stage 1 and 2: ≥60 | 1,253 (23.0%) | 119 (24.5%) | 1,131 (23.1%) | 3 (5.7%) | ||
| Stage 3a: 45-59 | 1,840 (33.8%) | 161 (33.1%) | 1,664 (33.9%) | 15 (28.3%) | ||
| Stage 3b: 30-44 | 1,616 (29.7%) | 149 (30.7%) | 1,447 (29.5%) | 20 (37.7%) | ||
| Stage 4 and 5: <30 | 735 (13.5%) | 57 (11.7%) | 663 (13.5%) | 15 (28.3%) | ||
| Hemoglobin (g/dL) | 28 | 12.6 [11.5,13.9] | 12.4[11.2,13.5] | 12.6[11.5,13.9] | 12.1[11.2,13.6] | 0.001c |
| CO2 (mmol/L) | 0 | 25 [23,26] | 23.5 [22,26] | 25 [23,26] | 25 [22,26] | <0.001c |
| Serum albumin (g/dL) | 32 | 4 [3.7,4.2] | 3.9 [3.6,4.2] | 4 [3.7,4.2] | 4.2 [4.0,4.5] | <0.001c |
| Total bilirubin (mg/dL) | 34 | 0.3 [0.2,0.5] | 0.3 [0.2,0.5] | 0.3 [0.2,0.5] | 0.2 [0.2,0.3] | <0.001c |
| Aspartate aminotransferase (U/L) | 39 | 22 [18,28] | 24 [19,30.5] | 22 [18,28] | 24 [21,32] | <0.001c |
| Alanine aminotransferase (U/L) | 38 | 27 [20,35] | 29 [20,37] | 26 [20,35] | 31 [24,40] | <0.001c |
| Alkaline phosphatase (U/L) | 36 | 81 [66,100] | 83 [65,103.5] | 80 [66,100] | 89 [79,104] | 0.002c |
| UAlb/UCreat (μg/mg) | 1,687 | 52.3 [8.7,461.4] | 51.5 [7.6,890.4] | 52.8 [8.9,452] | 26.1 [10.7,198.4] | 0.16c |
| Potassium (mmol/L) | 44 | 4.3 [4,4.6] | 4.3 [4,4.7] | 4.3 [4,4.6] | 4.5 [4.2,4.7] | <0.001c |
| Blood urea nitrogen (mg/dL) | 2 | 24 [19,33] | 25 [19,34] | 24 [19,33] | 30 [20,42] | 0.11c |
| Medications | ||||||
| Antidepressants | 39 | 1,052 (19.5%) | 103 (21.3%) | 939 (19.3%) | 10 (18.9%) | 0.55b |
| SSRIs | 39 | 593 (11%) | 55 (11.4%) | 533 (10.9%) | 5 (9.4%) | 0.90b |
| β-Blockers | 39 | 2,682 (49.6%) | 238 (49.3%) | 2,407 (49.4%) | 37 (69.8%) | 0.01b |
| ACEi or ARBs | 39 | 3,720 (68.8%) | 316 (65.4%) | 3,366 (69.1%) | 38 (71.7%) | 0.22b |
| Statins | 39 | 3,135 (58%) | 284 (58.8%) | 2,817(57.9%) | 34(64.2%) | 0.61b |
| Loops | 39 | 1,800 (33.3%) | 169 (35%) | 1,608(33%) | 23(43.4%) | 0.20b |
| Thiazides | 39 | 1,481(27.4%) | 153(31.7%) | 1,312(26.9%) | 16(30.2%) | 0.08b |
| Potassium sparing diuretics | 39 | 548 (10.1%) | 63 (13%) | 477 (9.8%) | 8 (15.1%) | 0.04b |
| All diuretics | 39 | 3,021 (55.9%) | 283 (58.6%) | 2,701 (55.5%) | 37 (69.8%) | 0.05b |
Note: Statistics presented as mean ± SD, median [P25, P75], or n (%).
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, aldosterone receptor blocker; BMI, body mass index; CV, cardiovascular; CHF, congestive heart failure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; SSRI, selective serotonin reuptake inhibitor; UAlb, urinary albumin; UCreat, urinary creatinine, CO2: Bicarbonate.
Analysis of variance
Pearson’s χ2 test
Kruskal-Wallis test
All analyses were conducted using Linux SAS version 9.4 (SAS Institute), and figures were created using R 3.5.1 (The R Foundation for Statistical Computing).
Results
Out of 5,499 patients that participated in the CRIC study, 5,444 (99%) had baseline serum sodium and glucose measurements and were included in this study. Of the 5,444 patients, n = 486 (9%) had hyponatremia and n = 53 (1%) had hypernatremia, which was within the previously reported prevalence in CKD patients.16, 17, 18,25, 26, 27 Baseline characteristics showed a mean age of 59.5 ± 10.7 years, male (56.3%), African American (44.3%), hypertension (86.5%), diabetes (51.3%), with CKD stage 3a (35.1%) predominance. Many patient characteristics were different across sodium levels (Table 1). Patients with hyponatremia were slightly younger than those with eunatremia and hypernatremia (58.1 vs 59.7 vs 50.9, P = 0.004). The percent of African American patients across sodium categories was 33.5%, 45.2%, and 58.5%, respectively (P < 0.001). The percentage of patients having CHF, taking loops, thiazides, or selective serotonin reuptake inhibitors at each of the sodium categories was not significantly different.
Factors Associated With Hyponatremia Over Time
Ninety percent of patients (n = 4,910), had at least 1 annual in-person follow-up visit with ambulatory laboratory data. The median number of serum sodium and glucose measurements was 5 per patient and ranged from 1-15 years. Among these, 7% of the measurements indicated hyponatremia, whereas 1% indicated hypernatremia, with the remaining 92% being eunatremic. In the adjusted model, we found the following variables were associated with higher odds of hyponatremia: females compared to males, diabetes, hypertension, CHF, and thiazide diuretic use (Table 2). There was a protective effect toward eunatremia in African American compared to White patients, CKD stage 3b compared to CKD stages 1 and 2, lower eGFR < 30 mL/min/1.73 m2, higher albumin, and higher bicarbonate (CO2). Many of these associations remained consistent in our sensitivity analysis.
Table 2.
Multivariable Model of Factors Associated With Hyponatremia Including Repeated Measures per Patient
| Primary Analysis Excluding Measurements Taken After Kidney Failure OR (95% CI)a N = 5,444 patients and 33,138 Records |
P | Sensitivity Analysis Including Measurements Taken After Kidney Failure OR (95% CI)a N = 5,444 Patients and 36,897 Records |
P | |
|---|---|---|---|---|
| Age per 10 y | 0.97 (0.92-1.01) | 0.12 | 0.95 (0.92-0.99) | 0.02 |
| Female vs male | 1.26 (1.14-1.38) | <0.001 | 1.26 (1.15-1.37) | <0.001 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 0.66 (0.59-0.74) | <0.001 | 0.70 (0.63-0.77) | <0.001 |
| Other | 1.24 (1.08-1.42) | 0.01 | 1.24 (1.09-1.41) | 0.001 |
| BMI (kg/m2) | ||||
| <25 | Ref | Ref | ||
| 25-<30 | 0.75 (0.66-0.85) | <0.001 | 0.77 (0.68-0.87) | <0.001 |
| >30 | 0.52 (0.46-0.59) | <0.001 | 0.54 (0.48-0.61) | <0.001 |
| Comorbid conditions | ||||
| Current smoker | 0.98 (0.84-1.13) | 0.76 | 0.96 (0.84-1.11) | 0.59 |
| Diabetes mellitus | 1.29 (1.17-1.43) | <0.001 | 1.30 (1.18-1.42) | <0.001 |
| Hypertension | 1.20 (1.002-1.45) | 0.05 | 1.21 (1.01-1.44) | 0.04 |
| CV disease | 0.95 (0.84-1.08) | 0.44 | 0.98 (0.87-1.10) | 0.74 |
| Stroke | 0.99 (0.84-1.16) | 0.88 | 0.93 (0.80-1.07) | 0.31 |
| CHF | 1.26 (1.07-1.49) | 0.01 | 1.46 (1.27-1.67) | <0.001 |
| Cancer | 0.94 (0.82-1.07) | 0.33 | 0.97 (0.86-1.09) | 0.56 |
| Hyperlipidemia | 0.93 (0.80-1.07) | 0.32 | 0.97 (0.84-1.11) | 0.64 |
| Peripheral vascular disease | 0.99 (0.82-1.19) | 0.94 | 1.06 (0.90-1.24) | 0.47 |
| Laboratory tests | ||||
| eGFR (mL/min/1.73 m2) | ||||
| >60 | Ref | Ref | ||
| 45-59 | 0.87 (0.77-0.99) | 0.04 | 0.89 (0.78-1.01) | 0.08 |
| 30-44 | 0.77 (0.67-0.90) | <0.001 | 0.81 (0.71-0.94) | 0.004 |
| <30 | 0.64 (0.52-0.79) | <0.001 | 0.85 (0.72-1.00) | 0.05 |
| CO2 (per 1 mmol/L) | 0.90 (0.89-0.92) | <0.001 | 0.92 (0.90-0.93) | <0.001 |
| Serum albumin (per 1 g/dL) | 0.54 (0.48-0.60) | <0.001 | 0.52 (0.47-0.57) | <0.001 |
| Potassium | ||||
| Hypokalemia (<3.6 mmol/L) | 1.11 (0.92-1.34) | 0.29 | 1.20 (1.02-1.42) | 0.03 |
| Normal (3.6-5.2 mmol/L) | Ref | Ref | ||
| Hyperkalemia (>5.2 mmol/L) | 1.17 (0.95-1.43) | 0.14 | 1.19 (0.99-1.43) | 0.06 |
| Log BUN (per 1 unit) | 1.00 (0.86-1.16) | 0.98 | 0.97 (0.85-1.09) | 0.59 |
| Medications | ||||
| SSRIs | 1.12 (0.98-1.28) | 0.10 | 1.16 (1.02-1.31) | 0.02 |
| β-Blockers | 1.04 (0.94-1.14) | 0.48 | 1.03 (0.94-1.13) | 0.52 |
| ACEi/ARB | 1.08 (0.97-1.20) | 0.15 | 1.05 (0.96-1.15) | 0.30 |
| Loops | 1.09 (0.98-1.22) | 0.11 | 1.00 (0.90-1.10) | 0.96 |
| Thiazides | 1.61 (1.45-1.79) | <0.001 | 1.54 (1.39-1.70) | <0.001 |
Note: For sensitivity analysis, patients were assigned eGFR = 10 after reaching kidney failure (for those that were not on dialysis)
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, aldosterone receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; CV, cardiovascular; CHF, congestive heart failure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; OR, odds ratio; SSRI, selective serotonin reuptake inhibitor.
Parameter estimates obtained with multiple imputations analyzed from 5 imputed datasets.
Baseline and Time-Dependent Dysnatremias Associated With Mortality
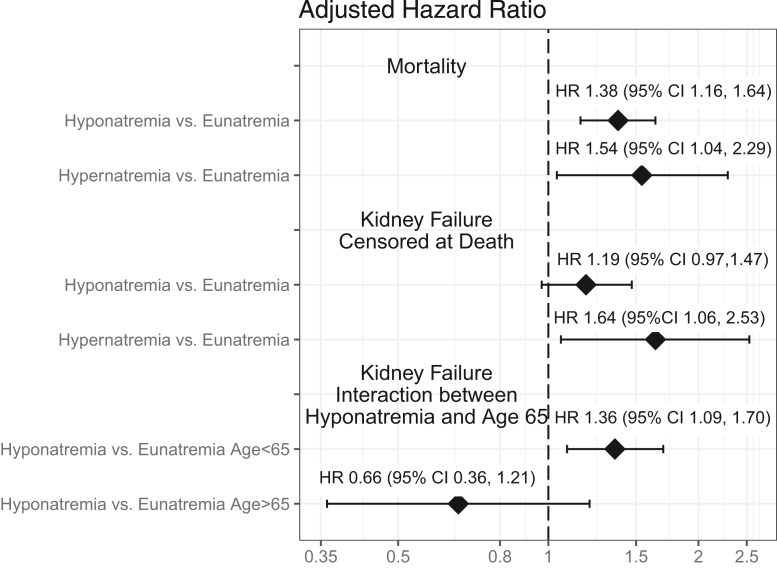
During a median follow-up of 8.8 years, 1,508 (28%) patients died. Table 3 shows results from the adjusted Cox proportional modeling for all-cause mortality with baseline sodium. After adjustment, sodium was not associated with all-cause death among the entire cohort. However, there was a significant interaction between baseline hyponatremia and continuous age (P = 0.01). When we evaluated the interaction grouping age as <65 versus >65 years for interpretability, it was no longer significant (P = 0.06), but it showed the direction of a non-significant increased risk of mortality in participants aged <65 years (hazard ratio [HR], 1.19; 95% confidence interval [CI], 0.96-1.48). Baseline hypernatremia was not associated with mortality (HR, 1.17; 95% CI, 0.77-1.77). However, time-dependent hypernatremia did increase the risk of death. Due to the small sample size, we did not evaluate whether there was any interaction with age.
Table 3.
Adjusted Models of Baseline Sodium Level and Time-Dependent Sodium Versus Outcomes (N = 5,444)
| Baseline Hyponatremia Versus Eunatremiaa |
Baseline Hypernatremia Versus Eunatremiaa |
|
|---|---|---|
| All-Cause Death HR (95% CI) | All-Cause Death HR (95% CI) | |
| Overall | 1.09 (0.91-1.30) | 1.17 (0.77-1.77) |
| Interaction with age 65 y (P = 0.06) | ||
| Age <65 | 1.19 (0.96-1.48) | -- |
| Age >65 | 0.83 (0.62-1.13) | -- |
| Kidney Failure With Death as a Competing Risk SHR (95% CI) |
Kidney Failure With Death as a Competing Risk SHR (95% CI) |
|
|---|---|---|
| Overall | 1.09 (0.88-1.34) | 0.61 (0.26-1.41) |
| Interaction with age 65 (P = 0.004) | ||
| Age <65 | 1.30 (1.03, 1.64) | -- |
| Age >65 | 0.54 (0.30, 0.97) | -- |
| Time-Dependent Hyponatremia Versus Eunatremiaa |
Time-Dependent Hypernatremia Versus Eunatremiaa |
|
|---|---|---|
| All-Cause Death HR (95% CI) |
All-Cause Death HR (95% CI) |
|
| Overall (P < 0.001) | 1.38 (1.16-1.64) | 1.54 (1.04-2.29) |
| Interaction with age 65 y (P = 0.09) | ||
| Age <65 | 1.54 (1.24-1.90) | -- |
| Age >65 | 1.12 (0.84-1.50) | -- |
| Kidney Failure Censored at Death HR (95% CI) |
Kidney Failure Censored at Death HR (95% CI) |
|
|---|---|---|
| Overall (P = 0.03) | 1.19 (0.97-1.47) | 1.64 (1.06-2.53) |
| Interaction with age 65 y (P = 0.03) | ||
| Age <65 | 1.36 (1.09-1.70) | -- |
| Age >65 | 0.66 (0.36-1.21) | -- |
Note: Parameter estimates obtained with MI analyzed from 5 imputed datasets.
Abbreviations: CI, confidence interval; HR, hazard ratio; SHR, subhazard ratio.
Adjusted for: age (only models with age 65 y interaction are adjusted for age >65 vs <65), sex, race, smoking status, estimated glomerular filtration rate, body mass index, diabetes, hypertension, coronary artery disease, congestive heart failure, stroke, all cancer, peripheral vascular disease, angiotensin-converting enzyme inhibitor, aldosterone receptor blocker, β-blocker, selective serotonin reuptake inhibitor, loop, and thiazide diuretic, hypercholesterolemia, serum albumin, serum bicarbonate, serum potassium, and log blood urea nitrogen. Note that interaction with age was only tested for hyponatremia vs eunatremia. Hypernatremia was excluded from this test because of small sample size (N).
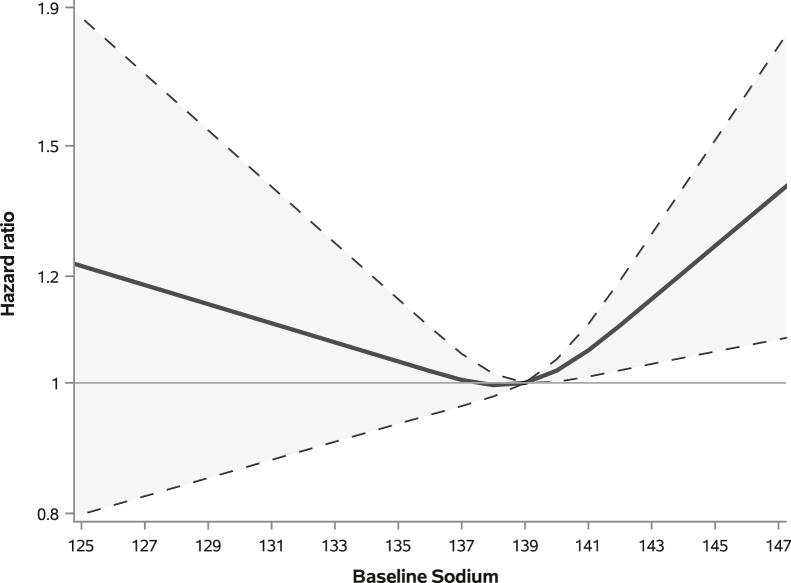
When we evaluated continuous sodium value at baseline versus mortality, we found a significant relation with sodium (overall sodium P = 0.04) with the non-linear term being significant (P = 0.03). When compared to the median sodium value of 139, we found that there was some significantly increased risk of mortality at higher levels of sodium, probably starting at around sodium values of 141 (Fig 1). In this same analysis, we saw that lower sodium was associated with higher but non-significant HRs when compared to the median sodium value.
Figure 1.
Association between continuous sodium at baseline with mortality.
In sensitivity analysis adjusting for urine albumin to creatinine ratio (log value), we found that the association between baseline sodium level and mortality was similar to the main analysis. The HR for hyponatremia compared to eunatremia was 1.11 (95% CI, 0.91-1.34), and for hypernatremia 1.16 (95% CI, 0.75-1.77).
Time-dependent hyponatremia was associated with an increased risk of all-cause death (HR, 1.37; 95% CI, 1.15-1.63; P < 0.001, Fig 2). The interaction with age ≤ 65 years was not statistically significant (P = 0.09).
Figure 2.
Forest plot showing association of time-dependent sodium levels with mortality and kidney failure. CI, confidence interval; HR, hazard ratio.
Baseline and Time-Dependent Hyponatremia Associated With Progression to Kidney Failure
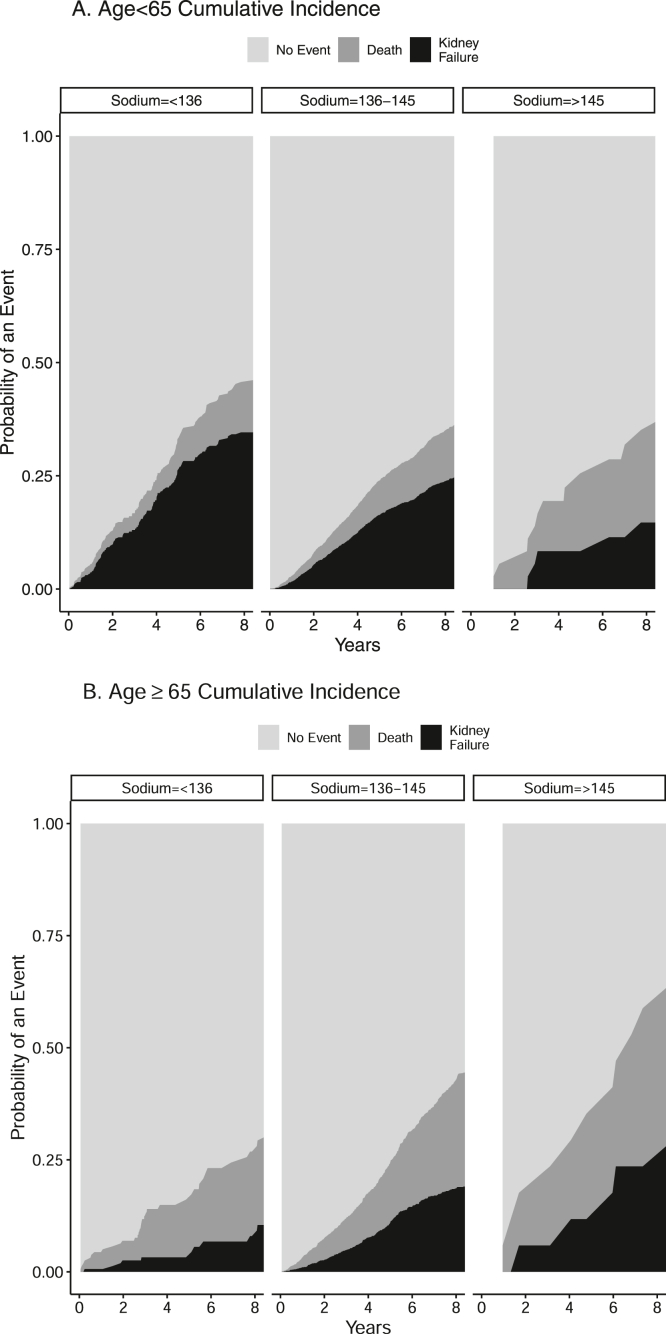
After a median follow-up of 5.1 years, 1,206 patients reached kidney failure and 951 patients died before reaching the endpoint. The cumulative incidence of kidney failure with death as a competing risk was different across sodium levels at 5 years, 19%, (95% CI, 16-23), 14% (95% CI, 13-16), and 9% (95% CI, 3-19) for hyponatremia, eunatremia, and hypernatremia, respectively, P = 0.04). Figure 3 shows the cumulative incidence of kidney failure and death by baseline sodium category among those with ages <65 and ≥65 years (Fig 3A,B).
Figure 3.
Cumulative incidence of kidney failure and death by baseline sodium category among those with age <65 y (A) and ≥65 y (B).
In an adjusted competing risks regression model of kidney failure with death as a competing risk, baseline sodium was not significantly associated with kidney failure (HR, 1.09; 95% CI, 0.88-1.34) (Table 3). We found that baseline hyponatremia was associated with an increased risk of kidney failure among patients aged <65 years, but a decreased risk in patients aged ≥ 65 years (interaction P = 0.004). A similar effect can be observed in the unadjusted cumulative incidence graphs (Fig 3A, B). No other significant interactions were found in the analysis of kidney failure with death as a competing risk of baseline hyponatremia.
For kidney failure, we found no significant association when modeling continuous baseline sodium with splines (overall P = 0.45, and non-linear term P = 0.22) or when modeling sodium linearly (P = 0.68).
In sensitivity analysis adjusting for urine albumin to creatinine ratio (log value), we found that the association between baseline sodium level and kidney failure with death as a competing risk was similar to the main analysis. The subhazard ratio for hyponatremia was 1.19 (95% CI, 0.94-1.50) and for hypernatremia, 0.63 (95% CI, 0.28-1.41).
The adjusted model of time-dependent sodium level with risk of kidney failure progression death censored showed an interaction between hyponatremia and age <65 versus >65 years (P = 0.03, Fig 2). Hyponatremia was associated with a higher risk of kidney failure in subjects aged <65 years (HR, 1.36; 95%CI, 1.09-1.70). Time-dependent hypernatremia was significantly associated with an increased risk of kidney failure (HR, 1.64; 95%CI, 1.06-2.53).
Discussion
In our analysis of the CRIC data, we found that time-dependent dysnatremias were strongly associated with mortality. Factors associated with hyponatremia included female sex, diabetes, and hypertension. Regardless of age, time-dependent hypernatremia was associated with an increased risk of kidney failure. Baseline and time-dependent hyponatremia were associated with an increased risk of kidney failure in patients younger than 65 but not among patients aged >65 years.
The purpose of the study is to identify the prevalence of dysnatremias in an ambulatory CKD population and its risk factors and associations with kidney failure and mortality so that clinicians may better risk-stratify patients. Previously aforementioned studies may have asserted associations; however, we believe that using the CRIC database gives more credence to the results because the endpoints are adjudicated and the laboratory data is procured in the ambulatory setting without medical indication.
The prevalence of hyponatremia and hypernatremia was 8.9% and 0.9%, respectively. In patients with CKD, all-cause death was associated with time-dependent hyponatremia, and in younger patients aged <65 years. Age is an effect modifier for adverse risk, which could be due to younger, sicker patients in our cohort. Our results are consistent with a meta-analysis and systematic review that showed that baseline hyponatremia and time-averaged hyponatremia were associated with a higher risk of all-cause mortality.16 Our studied population excluded patients on peritoneal and hemodialysis. Others have confirmed the associations between hyponatremia and mortality, but the African American population was underrepresented.25,28
Our study demonstrates that African American race is associated with a lower risk of hyponatremia, which could be related to differences in diet and fluid intake. African Americans have lower levels of plasma renin activity28 and aldosterone,29 leading to an increase in the risk of salt sensitivity.30 Historically, there has been a significant focus on the renin-angiotensin-aldosterone system on the epithelial sodium channel on the distal convoluted tubule. Chun et al31 demonstrated ethnic differences in the activity of the NaK2Cl co-transporter in the thick ascending loop, a key contributor to the creation and maintenance of the countercurrent mechanism, which is critical to producing a final concentration of urine, a promoter for dysnatremias. Administration of furosemide increased the activity of the NaK2Cl co-transporter activity in African American individuals when compared to White individuals as demonstrated by a slower restoration of Uosm (P < 0.001), which could contribute to more free water clearance through upregulation of the countercurrent multiplier and the decreased likelihood of hyponatremia.31 Additionally, time-varying hypernatremia was associated with all-cause mortality, but because of the small sample size, interactions were not tested.
The presence of hyponatremia associated with CHF, cirrhosis, myocardial infarction, pulmonary embolism, and cancer all increased the risk of death.17,32,33 Dysnatremias in patients with CKD have been associated with increased mortality in a U-shaped pattern.18 Because these laboratory values have been obtained in an ambulatory setting, we suspect that our cohort has chronic hyponatremia (≥ 48 hours) which has been associated with osteoporosis, falls, fractures, and a prolonged reaction time.34, 35, 36 Severe hypernatremia and rapid correction of chronic hypernatremia can lead to neurological manifestations including seizures and coma due to the shifting of water from the brain cells and cerebral edema, respectively.37
We identified risk factors for hyponatremia including female sex, CHF, diabetes, hypertension, and diuretic use, which has been confirmed in other studies.17,18,32,38, 39, 40 In patients with hyponatremia, evidence regarding the role of sex on mortality is contradicting because of differences in comorbidity burden.3,41, 42, 43 Contrary to androgens, estrogens are thought to inhibit the sodium-potassium ATPase and thus impair brain adaptation to hyponatremia, predisposing to cerebral edema.42 In diuretic users, Lim and colleagues26 reported that hyponatremia was associated with an increased risk for kidney replacement therapy but not mortality compared with diuretic non-users. This could be attributed to diuretic-induced fluid imbalances, including volume depletion, which could contribute to worse outcomes.26,44
Interestingly, multivariable modeling consistently demonstrated that patients with CKD stages 3b and 4 had a lower risk of hyponatremia compared with patients with eGFR ≥ 60 mL/min/1.73 m2. This has been corroborated with other studies, and it has been suggested that patients with preserved eGFR may have a reduced ability to excrete free water from vasopressin release, rather than a diluting capacity defect, which is seen more in advanced CKD as evidence of isosthenuria (urine osmolality ∼ 300 mOsm/L) regardless of water consumption. Our study also shows that elevated CO2 levels, elevated serum albumin, and African American race reduce the risk of hyponatremia, but the physiological determinants are unknown and warrant further investigation.
Our study adds to the literature because it affirms some findings from previously published work but also explores the associations of dysnatremias with progression to kidney failure.45 Baseline and time-dependent hyponatremia, when kidney failure is censored at death, was not associated with kidney failure overall. However, in patients aged <65 years, baseline hyponatremia (HR, 1.27; 95% CI,1.01-1.61) and time-dependent hyponatremia (HR, 1.38; 95% CI, 1.10-1.72) were associated with progression to kidney failure. Time-dependent hypernatremia was also associated with progression to kidney failure. Our larger and more inclusive study population was corroborated by the findings of Han et al,45 the only other study that found an association between hyponatremia and kidney failure. One plausible explanation is that arginine vasopressin and plasma copeptin, derived from the C-terminal portion of arginine vasopressin, likely contribute to CKD progression by their V1a- and V2-mediated effects of renal hemodynamics, leading to increased intraglomerular capillary pressure and mesangial proliferation and progression of CKD.46, 47, 48 Suppression of arginine vasopressin in the 5/6 nephrectomized rat by water ingestion was shown to reduce renal hypertrophy, glomerulosclerosis, and tubulointerstitial fibrosis.49 This theory, however, warrants further investigation.
Our study has several strengths, which include the analysis of the CRIC study dataset. The CRIC study is a large, diverse population of patients with CKD who have been followed for many years with defined cardiac and kidney outcomes that have been adjudicated by the CRIC Adjudication Committee. This study sample is an accurate representation of a US kidney failure population. The purposeful oversampling of African American individuals and people with diabetes in the cohort offers a good representation of a general CKD population. Using CRIC lab data permitted us to exclude bloodwork that was obtained for cause indications used in other studies and may have altered results. Our long length of follow-up and serial lab measurements allowed us to confidently explore the risks of mortality and kidney failure over time. Limitations to our study include the inability to establish causality, the lack of data that would better characterize hyponatremia, including serum and urine osmolality, and the inability to generalize the results to hospitalized patients. Despite our robust associations of dysnatremias with mortality and progression to kidney failure, we suspect that there may be unmeasured confounders and competing risk factors that have contributed to some of our results. The CRIC excluded individuals with cirrhosis, New York Heart Association class III/IV CHF, and nephrotic syndromes so results cannot be generalizable to these populations. Additionally, this does not shed light on the mitigation of these outcomes if sodium was corrected.
In summary, our results suggest an increased risk of mortality and progression to kidney failure in patients with baseline hyponatremia who are younger than 65 years. Time-dependent hyponatremia and hypernatremia were both associated with increased mortality, whereas time-dependent hyponatremia was associated with progression to kidney failure in patients younger than 65 years. Treating physicians should recognize the potential risks of dysnatremias in patients with CKD in the hopes of potentially improving outcomes. More clarity is required to better understand the association between dysnatremias, mortality, and kidney failure, and if correction can improve outcomes.
Article Information
CRIC Study Investigators
A list of the CRIC study investigators is available at www.cristudy.org.
Authors’ Full Names and Academic Degrees
Mohamed Hassanein, Susana Arrigain, Jesse D. Schold, Georges N. Nakhoul, Sankar D. Navaneethan, Ali Mehdi, Arjun Sekar, Jad Tabbara, Jonathan J Taliercio on behalf of the CRIC Investigators
Authors’ Contributions
Research Idea: MH and JTT; Data acquisition/interpretation and statistical analysis: SA and JDS. Critical appraisal and data interpretation: MH, GNN, SDN, JT, AS, AM, and JJT. Supervision and mentorship: GNN, SDN, AM, JJT. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding for the CRIC study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902, and U24DK060990). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health/National Center for Advancing Translational Sciences UL1TR000003, Johns Hopkins University UL1TR000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NIH/NCATS and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, the University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1RR024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received March 20, 2022 as a submission to the expedited consideration track with 4 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form August 31, 2022.
Footnotes
Complete author and article information provided before references.
Item S1: Detailed Methods.
Supplementary Material
. Item S1.
References
- 1.Burst V. Etiology and epidemiology of hyponatremia. Front Horm Res. 2019;52:24–35. doi: 10.1159/000493234. [DOI] [PubMed] [Google Scholar]
- 2.Waikar S.S., Mount D.B., Curhan G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald R., Jaber B.L., Price L.L., Upadhyay A., Madias N.E. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170(3):294–302. doi: 10.1001/archinternmed.2009.513. [DOI] [PubMed] [Google Scholar]
- 4.Hao J., Li Y., Zhang X., et al. The prevalence and mortality of hyponatremia is seriously underestimated in Chinese general medical patients: an observational retrospective study. BMC Nephrol. 2017;18(1):328. doi: 10.1186/s12882-017-0744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadhyay A., Jaber B.L., Madias N.E. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7) doi: 10.1016/j.amjmed.2006.05.005. 10.1016/j.amjmed.2006.05.005 (suppl 1):S30-S35. [DOI] [PubMed] [Google Scholar]
- 6.Clayton J.A., Le Jeune I.R., Hall I.P. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. QJM. 2006;99(8):505–511. doi: 10.1093/qjmed/hcl071. [DOI] [PubMed] [Google Scholar]
- 7.Gill G., Huda B., Boyd A., et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol (Oxf) 2006;65(2):246–249. doi: 10.1111/j.1365-2265.2006.02583.x. [DOI] [PubMed] [Google Scholar]
- 8.Zilberberg M.D., Exuzides A., Spalding J., et al. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin. 2008;24(6):1601–1608. doi: 10.1185/03007990802081675. [DOI] [PubMed] [Google Scholar]
- 9.Hoorn E.J., Zietse R. Hyponatremia and mortality: how innocent is the bystander? Clin J Am Soc Nephrol. 2011;6(5):951–953. doi: 10.2215/CJN.01210211. [DOI] [PubMed] [Google Scholar]
- 10.Corona G., Giuliani C., Parenti G., et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLOS ONE. 2013;8(12) doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arampatzis S., Frauchiger B., Fiedler G.M., et al. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 2012;125(11):1125.e1–1125.e7. doi: 10.1016/j.amjmed.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Lindner G., Funk G.C., Schwarz C., et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50(6):952–957. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Berl T. In: Atlas of Diseases of the Kidney. Schrier R., editor. Blackwell Science; 1999. Diseases of water metabolism; pp. 1.1–1.19. [Google Scholar]
- 14.Seay N.W., Lehrich R.W., Greenberg A. Diagnosis and management of disorders of body tonicity-hyponatremia and hypernatremia: core curriculum 2020. Am J Kidney Dis. 2020;75(2):272–286. doi: 10.1053/j.ajkd.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Combs S., Berl T. Dysnatremias in patients with kidney disease. Am J Kidney Dis. 2014;63(2):294–303. doi: 10.1053/j.ajkd.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L., Hou Y., Xiao Q., Du Y. Association of serum sodium and risk of all-cause mortality in patients with chronic kidney disease: a meta-analysis and sysematic review. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-16242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H., Jolly S.E., Airy M., et al. Associations of dysnatremias with mortality in chronic kidney disease. Nephrol Dial Transplant. 2017;32(7):1204–1210. doi: 10.1093/ndt/gfw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovesdy C.P., Lott E.H., Lu J.L., et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125(5):677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash J.P., Go A.S., Appel L.J., et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denker M., Boyle S., Anderson A.H., et al. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10(11):2073–2083. doi: 10.2215/CJN.04260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman H.I., Appel L.J., Chertow G.M., et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7) doi: 10.1097/01.asn.0000070149.78399.ce. 10.1097/01.ASN.0000070149.78399.CE (suppl 2):S148-S153. [DOI] [PubMed] [Google Scholar]
- 22.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 24.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.2307/2670170. [DOI] [Google Scholar]
- 25.Chiu D.Y.Y., Kalra P.A., Sinha S., Green D. Association of serum sodium levels with all-cause and cardiovascular mortality in chronic kidney disease: results from a prospective observational study. Nephrology (Carlton) 2016;21(6):476–482. doi: 10.1111/nep.12634. [DOI] [PubMed] [Google Scholar]
- 26.Lim L.M., Tsai N.C., Lin M.Y., et al. Hyponatremia is associated with fluid imbalance and adverse renal outcome in chronic kidney disease patients treated with diuretics. Sci Rep. 2016;6(1) doi: 10.1038/srep36817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shavit L., Merin O., Grenader T., et al. Hyponatremia predicts poor outcomes in patients with chronic kidney disease undergoing heart operation. Ann Thorac Surg. 2018;106(3):696–701. doi: 10.1016/j.athoracsur.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Helmer O.M. The renin-angiotensin system and its relation to hypertension. Prog Cardiovasc Dis. 1965;8(2):117–128. doi: 10.1016/s0033-0620(65)80003-2. [DOI] [PubMed] [Google Scholar]
- 29.Pratt J.H., Jones J.J., Miller J.Z., Wagner M.A., Fineberg N.S. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med. 1989;321(17):1152–1157. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger M.H., Miller J.Z., Luft F.C., Grim C.E., Fineberg N.S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 Pt 2):II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 31.Chun T.Y., Bankir L., Eckert G.J., et al. Ethnic differences in renal responses to furosemide. Hypertension. 2008;52(2):241–248. doi: 10.1161/HYPERTENSIONAHA.108.109801. [DOI] [PubMed] [Google Scholar]
- 32.Hoorn E.J., Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62(1):139–149. doi: 10.1053/j.ajkd.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Abu Zeinah G.F., Al-Kindi S.G., Hassan A.A., Allam A. Hyponatraemia in cancer: association with type of cancer and mortality. Eur J Cancer Care (Engl) 2015;24(2):224–231. doi: 10.1111/ecc.12187. [DOI] [PubMed] [Google Scholar]
- 34.Verbalis J.G., Barsony J., Sugimura Y., et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–563. doi: 10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renneboog B., Musch W., Vandemergel X., Manto M.U., Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1–71.e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Renneboog B., Sattar L., Decaux G. Attention and postural balance are much more affected in older than in younger adults with mild or moderate chronic hyponatremia. Eur J Intern Med. 2017;41:e25–e26. doi: 10.1016/j.ejim.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Sonani B., Naganathan S., Al-Dhahir M.A. StatPearls. StatPearls Publishing; 2022. Hypernatremia. [PubMed] [Google Scholar]
- 38.Rudkovskaia A.A., Tonelli A.R., Rao Y., et al. Is hyponatremia associated with mortality in pulmonary arterial hypertension? Pulm Circ. 2018;8(2) doi: 10.1177/2045894018776888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahin O.Z., Asci G., Kircelli F., et al. The impact of low serum sodium level on mortality depends on glycemic control. Eur J Clin Invest. 2012;42(5):534–540. doi: 10.1111/j.1365-2362.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- 40.Qiu Y., Ye H., Fan L., et al. Serum sodium modifies the association of systolic blood pressure with mortality in peritoneal dialysis patients. Kidney Blood Press Res. 2020;45(6):916–925. doi: 10.1159/000510478. [DOI] [PubMed] [Google Scholar]
- 41.Mohan S., Gu S., Parikh A., Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med. 2013;126(12):1127–1137.e1. doi: 10.1016/j.amjmed.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayus J.C., Achinger S.G., Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Ren Physiol. 2008;295(3):F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 43.Barsony J., Manigrasso M.B., Xu Q., Tam H., Verbalis J.G. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age (Dordr) 2013;35(2):271–288. doi: 10.1007/s11357-011-9347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan Y.H., Sarriff A., Adnan A.S., Khan A.H., Mallhi T.H. Chronic kidney disease, fluid overload and diuretics: a complicated triangle. PLOS ONE. 2016;11(7) doi: 10.1371/journal.pone.0159335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han S.W., Tilea A., Gillespie B.W., et al. Serum sodium levels and patient outcomes in an ambulatory clinic-based chronic kidney disease cohort. Am J Nephrol. 2015;41(3):200–209. doi: 10.1159/000381193. [DOI] [PubMed] [Google Scholar]
- 46.Choi H.Y., Park H.C., Ha S.K. High water intake and progression of chronic kidney diseases. Electrolyte Blood Press. 2015;13(2):46–51. doi: 10.5049/EBP.2015.13.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golembiewska E., Machowska A., Stenvinkel P., Lindholm B. Prognostic value of copeptin in chronic kidney disease: from general population to end-stage renal disease. Curr Protein Pept Sci. 2017;18(12):1232–1243. doi: 10.2174/1389203718666170717095301. [DOI] [PubMed] [Google Scholar]
- 48.Torres V.E. Vasopressin in chronic kidney disease: an elephant in the room? Kidney Int. 2009;76(9):925–928. doi: 10.1038/ki.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouby N., Bachmann S., Bichet D., Bankir L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol. 1990;258(4 Pt 2):F973–F979. doi: 10.1152/ajprenal.1990.258.4.F973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Item S1.