Summary
Background
Repetitive transcranial magnetic stimulation (rTMS) can target specific neural circuits, which may allow for personalized treatment of depression. Treatment outcome is typically determined using sum scores from validated measurement scales; however, this may obscure differential improvements within distinct symptom domains. The objectives for this work were to determine: (1) whether a standard depression measure can be represented using a four symptom cluster model and (2) whether these symptom clusters had a differential response to rTMS treatment.
Methods
Data were obtained from two multi-centre randomized controlled trials of rTMS delivered to the left dorsolateral prefrontal cortex (DLPFC) for participants with treatment-resistant depression (TRD) conducted in Canada (THREE-D [Conducted between Sept 2013, and Oct 2016] and CARTBIND [Conducted between Apr 2016 and Feb 2018]). The first objective used confirmatory factor analytic techniques, and the second objective used a linear mixed effects model. Trial Registration: NCT01887782, NCT02729792.
Findings
In the total sample of 596 participants with TRD, we found a model consisting of four symptom clusters adequately fit the data. The primary analysis using the THREE-D treatment trial found that symptom clusters demonstrated a differential response to rTMS treatment (F(3,5984) = 31.92, p < 0.001). The anxiety symptom cluster was significantly less responsive to treatment than other symptom clusters (t(6001) = -8.02, p < 0.001). These findings were replicated using data from the CARTBIND trial.
Interpretation
There are distinct symptom clusters experienced by individuals with TRD that have a differential response to rTMS. Future work will determine whether differing rTMS treatment targets have distinct patterns of symptom cluster responses with the eventual goal of personalizing rTMS protocols based on an individual's clinical presentation.
Funding
Canadian Institutes of Health Research, Brain Canada.
Keywords: Depressive disorders, Repetitive transcranial magnetic stimulation, Cluster analysis, Treatment outcomes
Abbreviations: CFA, Confirmatory factor analysis; CFI, Comparative fit index; DLPFC, Dorsolateral prefrontal cortex; HDRS-17, 17-item Hamilton Depression Rating Scale; HFL, High-frequency left stimulation; iTBS, Intermittent theta-burst stimulation; MDD, Major depressive disorder; MINI, Mini International Neuropsychiatric Interview; RMSEA, Root mean square error of approximation; rTMS, Repetitive transcranial magnetic stimulation; SRMR, Standardized root mean squared residual; TRD, Treatment-resistant depression
Research in context.
Evidence before this study
We conducted a search in MEDLINE using the Pubmed interface from January 1980 to July 2022 and included all articles (English and non-English) of studies including human participants using the following search terms: (1) repetitive transcranial magnetic stimulation (rTMS), (2) statistical methods to identify latent classes or clusters of symptoms (cluster analysis, bayes theorem, principal components analysis, and factor analysis), and (3) major depressive disorder.
There is minimal information on this topic available in the current literature. To date, there has been a single published study examining symptom cluster response with rTMS amongst individuals with depression. This study used an exploratory, data-driven approach and found that rTMS treatment results in a differential impact on distinct symptom clusters.
Added value of this study
This study used two large clinical trials of rTMS to conduct a confirmatory, hypothesis-driven study to determine if rTMS delivered to the left dorsolateral prefrontal cortex (DLFPC) had a differential response on symptom clusters in patients with treatment-resistant depression (TRD). This work found that patients with TRD referred for rTMS experience four distinct symptom clusters - mood, insomnia, somatic and anxiety symptoms - and that these clusters respond differently to rTMS. Consistent with prior work, mood symptoms were significantly more responsive than anxiety symptoms. These findings were independently replicated in both clinical trials supporting the robustness of results.
Implications of all the available evidence
There appear to be distinct symptom clusters amongst individuals experiencing treatment-resistant depression and these symptom clusters have a differential response to rTMS treatment. Future work will determine if rTMS protocols can be personalized to an individual's clinical presentation.
Introduction
Major depressive disorder (MDD) is a leading source of illness burden and disability worldwide. First line treatment for depression involves pharmacotherapy, psychotherapy or a combination of the two.1 Unfortunately, many individuals do not respond to initial trials of medication or psychotherapy, and are diagnosed with treatment-resistant depression (TRD).1 Novel treatments, such as repetitive transcranial magnetic stimulation (rTMS) have demonstrated effectiveness for achieving response and remission in TRD potentially through modulating dysfunctional neural circuits.2, 3, 4
Though response and remission in depression are typically characterized using the sum score of a psychometric scale, this approach has been heavily criticized.5 Instead, depression is more accurately represented as a construct with multiple distinct symptom clusters, which is a characteristic known as multidimensionality.6 Prior work using factor analyses has identified 2 to 5 symptom dimensions on standard depression measures.6 Though the multidimensionality of depression (i.e., factor structure of standard depression measures) has been assessed in a variety of clinical samples of individuals with depression,6,7 to our knowledge it has not been assessed in a sample of adults with TRD referred for rTMS.
Given that depression has also been conceptualized as a disorder of abnormal neural circuits, it is possible that these distinct symptom domains - or clusters - may each originate from dysfunction in specific neural circuits.2 rTMS therefore has the potential, through its ability to focally stimulate cortical regions, to modulate specific dysfunctional neural circuits which may allow for targeting specific symptom clusters and achieve differential responses to treatment. Unfortunately, when sum scores of depression are used to examine treatment response, then differential responses of symptom clusters will go undetected.8 Differential patterns of response have been assessed in a variety of pharmacological trials of individuals with depression and found that particular symptoms may have greater response to treatment such as the core emotional or mood symptoms of depression,9,10 neurovegetative symptoms,11 or particular symptom groupings.12 Studies of differential symptom cluster response to brain stimulation including electroconvulsive therapy and transcranial direct current stimulation have also found that the core mood and dysphoric symptoms respond better to treatment than other symptom clusters.13,14
The knowledge of how rTMS treatment can differentially impact distinct symptom clusters in depression could eventually facilitate personalized delivery of rTMS based on an individual's presenting symptoms. However, to our knowledge, there has only been one study conducted examining differential symptom response to rTMS.15 In this study, the authors examined clustering of MRI voxel-based connectivity in subjects with depression severity measured using the Beck Depression Inventory who received rTMS with a figure-of-eight coil to the left dorsolateral prefrontal cortex (DLPFC), and found that two symptom clusters best explained their findings - a dysphoric cluster and an anxiosomatic cluster. Importantly, they also found that rTMS had a differential impact on these symptom clusters depending on the treatment target.15 In particular, treatment delivered to the left DLPFC resulted in greater improvement of dysphoric symptoms compared to anxiosomatic symptoms.15 While these results have potentially important implications, a notable caveat is that the results were based on two small samples (N = 30; N = 81) and a meta-analysis with heterogeneous rating scales.15 Furthermore, this study, as well as other symptom cluster studies10 typically use data-driven approaches to identify symptom clusters, such as exploratory factor analysis, which are suitable for hypothesis generation as opposed to hypothesis evaluation.16
To build on findings of prior work we used data from two large randomized controlled trials of rTMS delivered to the same MNI-152 coordinate in the left DLPFC and employed a hypothesis-driven approach to address two objectives.17,18 First, we sought to determine the presence of distinct symptom clusters amongst adults with TRD referred for rTMS as measured on the Hamilton Depression Rating Scale using a hypothesis evaluative framework. We hypothesized that a multiple symptom cluster model of depression would more accurately fit the observed data compared to a single symptom cluster model. Second, we sought to determine whether rTMS treatment delivered in these trials would have a differential impact on the identified symptom clusters. We hypothesized, informed by prior work,15 that the treatment target of these studies (i.e., left DLPFC) would result in significantly greater improvement in mood symptom clusters compared to anxiety symptom clusters. Confirmation of these hypotheses would therefore be an important advance in the delivery of rTMS and one step closer towards personalized treatment of depression.
Methods
Study procedures
This work was a secondary analysis combining data from two multi-centre randomized trials of rTMS delivered to the left DLPFC targeted using MRI-guided neuronavigation at the MNI-152 stereotaxic coordinate (x-38, y+44, z+26).17,18 The first study (THREE-D) was a randomized non-inferiority trial comparing two rTMS protocols applied to the left DLPFC: standard (10 Hz) high frequency left (HFL) or intermittent theta-burst (iTBS) stimulation.17 The second study (CARTBIND) was a randomized superiority trial of iTBS stimulation applied to the left DLPFC comparing once daily treatment with twice daily treatment.18
For both studies, participants were outpatients between the ages of 18–59 (up to 65 for THREE-D) with a diagnosis of unipolar MDD confirmed using the Mini International Neuropsychiatric Interview (MINI). They met the following inclusion criteria: current major depressive episode scoring ≥18 on the 17-item Hamilton Depression Rating Scale (HDRS-17)19; lack of response to at least one adequate or two inadequate antidepressant trials during the current episode; and receiving stable dosages of psychotropic medications for at least four weeks prior to screening. The exclusion criteria were: substance dependence/abuse <3 months preceding study entry; unstable medical/neurologic illness; acute suicidality; MINI diagnosis of bipolar I or II disorder, primary psychotic disorder, or psychotic symptoms in current episode; any rTMS contraindication (i.e., history of seizures; intracranial implant); lifetime history of failure to respond to an adequate course of ECT; previous rTMS treatment; receiving lorazepam >2 mg/day (or equivalent); receiving any anticonvulsant; pregnancy; or significant laboratory abnormalities. THREE-D excluded participants with >3 failed adequate trials of antidepressant medication, while CARTBIND did not. For both studies, local research ethics board approval was obtained for all three study sites and all participants provided written, informed consent.
In both cases, treatment was delivered daily for 5 days per week for either 20–30 days (THREE-D)17 or 30 days (CARTBIND)18 while individuals continued their psychotropic medications unchanged for the study duration. Randomization of participants was stratified by degree of medication resistance (>1 adequate vs 1 or fewer medication trials). While the design of both studies did not allow the rTMS technician or patient to be blinded during treatment, outcome assessors were blinded to treatment allocation.
Choice of primary measure
The primary outcome measure in both studies was the HDRS-17, which is the most commonly used clinician-rated psychometric measure of depression severity.19 Of the 17 items, nine are scored between 0 (not present) and four points (severe), while the remaining eight are scored between 0 (not present) and two points (severe) for a total score ranging from 0 to 52. Outcome assessments were completed by trained research assistants blinded to treatment allocation in both studies. In THREE-D, the HDRS-17 was collected at baseline, and every week until study completion, while CARTBIND collected this at baseline, then weekly from weeks two until six. Further details regarding study procedures for both studies are available in the original publications.17,18 A comprehensive review of the psychometric properties of HDRS-17 has previously been published.6
rTMS procedure
Before treatment, all participants underwent an anatomical MRI. rTMS treatments were guided using MRI-guided neuronavigation to optimize coil positioning. The left DLPFC was targeted using the MNI-152 stereotaxic coordinate (x-38, y+44, z+26).17,18 A MagPro X100/R30 stimulator equipped with a B70 fluid-cooled coil (MagVenture, Farum, Denmark) was used for stimulation. The resting motor threshold (RMT) was determined using visual observation. For THREE-D, HFL was delivered with the FDA-approved treatment settings (120% RMT, 10 Hz, 4 s on, 26 s off, 3000 pulses/session over 37.5 min).17 iTBS was delivered to the same site with the same intensity but used a different stimulation pattern (triplet 50 Hz bursts, repeated at 5 Hz, 2 s on, 8 s off, 600 pulses/session over 3 min).17 For CARTBIND, iTBS was delivered to same location, but one treatment arm delivered 1200 pulses consecutively with the other treatment arm delivering 600 pulses separated by 1 h (1200 pulses total).18
Statistical analyses
Confirmatory factor analysis
To assess the dimensionality - or number of symptom clusters - present in the HDRS-17, we used confirmatory factor analysis (CFA) to test our hypotheses. We first conducted a CFA using a single factor model to test the hypothesis that the HDRS-17 is a unidimensional symptom measure. We then conducted a CFA using a previously described four-factor model,20 which was a model obtained by meta-analyzing exploratory factor analyses of the HDRS published over a 30 year period. The factors present in this model were (1) mood, (2) anxiety, (3) insomnia, and (4) somatic factors, with factor loading available in the original publication.20 This model was selected because, it is the most comprehensive meta-analysis of the HDRS-17 factor structure published to date. We modified the model a priori by excluding the insight item because of (1) poor conceptual fit with the primary factor (anxiety), (2) complex factor loading onto other factors, and (3) poor item reliability.6
CFA was conducted using the lavaan package (Version 0.6.11) in R (R version 4.1.3 (2022-03-10))21 with HDRS-17 scores from THREE-D and CARTBIND pooled together to achieve sufficient sample size.22 We estimated parameters for the factors using maximum likelihood estimators with heteroskedasticity-consistent standard errors. Visual inspection of the distribution of symptom cluster sum scores demonstrated an approximately normal distribution (appendix p5). In the event of inadequate model fit, we assessed for areas of localized strain (identified by modification indices >10), and made modifications to the factor model guided by theoretical and empirical considerations.23 Where item loadings were negative on their assigned factor, these items were subtracted from (rather than added to) the symptom cluster sum score. We first assessed model fit at baseline in both studies. We next assessed for measurement invariance using data from baseline and weeks 2–4 (given these weeks had minimal non-random missing data) and sequentially assessed for configural, metric and scalar invariance.24 For the baseline model, we assessed global fit using both absolute indices (root mean square error of approximation [RMSEA] and standardized root mean squared residual [SRMR]) and relative fit indices (chi-square value, and comparative fit index [CFI]) interpreted using standard cutoffs (RMSEA≤0.06, SRMR≤0.05, CFI≥0.90).25,26 For assessment of measurement invariance, we used previously recommended cutoffs for adequate samples (N ≥ 300) indicating measurement non-invariance: ΔCFI ≥ −0.01, ΔRMSEA≥0.015, ΔSRMR≥0.03 (metric invariance), ΔSRMR≥0.015 (scalar invariance).27 Where applicable, we used robust fit measures.
Symptom cluster response
After confirming the four-factor model demonstrated adequate fit, we considered each of these dimensions to be a distinct symptom cluster and assessed whether there was a differential response within these clusters to rTMS treatment. For this analysis given its more exploratory nature, we used the THREE-D dataset for the primary analysis, and data from CARTBIND as an independent replication to assess reproducibility. To assess for differential symptom cluster response, we fit a linear mixed effects model using the lmerTest package (Version 3.1.3)28 in which the outcome (dependent variable) was the sum score of a symptom cluster. As there were four symptom clusters, each participant contributed four outcome scores, one for each cluster (i.e., mood, anxiety, insomnia, and somatic). The sum score of each symptom cluster was scaled using the proportion of maximum possible scaling method to facilitate comparisons between symptom clusters.29 This method transforms each scale to a metric ranging from 0 to 1, and avoids the problems inherent to z-standardization.30 The model incorporated time, symptom cluster type and their interaction as fixed effects. The interaction between time and symptom cluster type allows for assessing whether the effect of time (i.e., duration of rTMS treatment) differs between distinct symptom clusters. We also included treatment allocation as a fixed effect to ensure any observed estimates controlled for treatment allocation. Time was modeled as a continuous variable as number of weeks since starting rTMS treatment. Symptom cluster type was modeled as a categorical variable with four levels: anxiety, mood, insomnia, and somatic. For random effects, we compared two different models using the likelihood ratio test: (1) random effect for subject intercept, and (2) random effect for subject intercept and subject specific slope. In the primary analysis using THREE-D, we analyzed the first four weeks of treatment, as there is non-random missing data after week four due to the study design.17 In the replication analysis using CARTBIND, we analyzed the complete 6 weeks of treatment.18
The primary comparison of interest for this analysis was the interaction term between time and symptom cluster, and whether it was statistically significant. Post hoc analyses examined which, if any, symptom clusters had a significant interaction with time, which would indicate the symptom clusters with greater (or lesser) response to rTMS treatment. In these post hoc analyses, the anxiety symptom cluster was used for the reference group. We conducted additional analyses to determine if there was a study effect on symptom response by combining THREE-D with CARTBIND data and including an additional covariate in the primary analytic model denoting study. To improve interpretability of the magnitude of differential symptom cluster response, we used the emmeans package (Version 1.7.3) to calculate standardized effect sizes of each cluster after rTMS treatment relative to the anxiety symptom cluster group (i.e., reference). We also calculated the standardized effect size of each symptom cluster at treatment completion relative to baseline. Model fit was assessed by visual inspection of a density plot of residuals and degrees of freedom were estimated using Satterthwaite's method. Statistical tests were two-tailed with set to 0.05.
Role of funding
The study funders for THREE-D (Canadian Institutes of Health Research) and CARTBIND (Brain Canada) as well as the device manufacturer (MagVenture), which provided in-kind equipment support for THREE-D (two coils and two high performance coolers at each site) had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author (TSK) and senior author (DMB) had full access to the data and the corresponding author had final responsibility for the decision to submit for publication.
Results
Confirmatory factor analysis
Included in this analysis were 596 participants, 388 participants from THREE-D and 208 participants from CARTBIND. Baseline characteristics have been previously reported in the original publications.17,18 In assessing the dimensionality of the HRSD-17 using the combined THREE-D and CARTBIND datasets, we found that a one-dimensional model had an inadequate model fit ( = 428.20, CFI = 0.32, RMSEA = 0.07, SRMR = 0.07). Before a priori modifications (i.e., exclusion of insight item), the four-factor model did not meet prespecified cutoffs for adequate model fit ( = 223.90, CFI = 0.76, RMSEA = 0.04, SRMR = 0.05). After a priori modifications to the four-factor model there was still poor relative and absolute model fit ( = 216.98, CFI = 0.75, RMSEA = 0.05, SRMR = 0.05); however, this model fit was significantly improved compared to the one factor model ( (6) = 202.69, p < 0.001). Examining the areas of local strain identified significant covariance between the psychomotor agitation and retardation items, consistent with the fact that both are observer-rated items (appendix p1). Since agitation and retardation are characteristics along the same spectrum, the decision was made to allow psychomotor disturbances to load onto both mood and anxiety factors. This resulted in a significant improvement in both local strain and global model fit such that the majority of model fit indices were adequate, though CFI was marginal ( = 179.55, CFI = 0.82, RMSEA = 0.04, SRMR = 0.05) (appendix p1). This model also demonstrated a significantly improved fit compared to the a priori four-factor model ( (3) = 37.74, p < 0.001). These model modifications also resulted in negative factor loadings of psychomotor agitation onto the mood factor and psychomotor retardation onto the anxiety factor. Completely standardized factor loadings of the final model using measurements from baseline, and weeks 2–4 with factor loadings fixed at all time points are presented in Table 1 with graphical representation of our final latent variable model in the appendix (p4). In assessing measurement invariance, we found adequate model fit supporting configural invariance ( = 886.37, CFI = 0.89, RMSEA = 0.05, SRMR = 0.04) in addition to support for metric invariance (ΔCFI = −0.013, ΔRMSEA = 0.00, ΔSRMR = 0.00), though ΔCFI was marginal, as well as support for scalar invariance (ΔCFI = 0.007, ΔRMSEA = 0.00, ΔSRMR = 0.00).
Table 1.
Confirmatory factor analysis of baseline and weeks 2–4 measurements with fixed factor loadings with completely standardized factor loadings.
| Estimate | Std. Err. | Z | p | |
|---|---|---|---|---|
| Factor loadings | ||||
| Anxiety | ||||
| Psychic anxiety | 0.67 | 0.06 | 11.78 | 0.000 |
| Psychomotor agitation | 0.17 | 0.04 | 4.21 | 0.000 |
| Psychomotor retardation | −0.12 | 0.04 | −2.62 | 0.009 |
| Somatic anxiety | 0.40 | 0.03 | 11.48 | 0.000 |
| Hypochondriasis | 0.16 | 0.03 | 6.07 | 0.000 |
| Mood | ||||
| Depressed mood | 0.51 | 0.03 | 17.03 | 0.000 |
| Psychomotor agitation | −0.05 | 0.03 | −1.82 | 0.069 |
| Psychomotor retardation | 0.21 | 0.03 | 7.17 | 0.000 |
| Work and activity difficulties | 0.46 | 0.03 | 16.70 | 0.000 |
| Guilt | 0.39 | 0.02 | 15.95 | 0.000 |
| Suicidality | 0.36 | 0.02 | 15.48 | 0.000 |
| Insomnia | ||||
| Early insomnia | 0.44 | 0.03 | 12.61 | 0.000 |
| Middle insomnia | 0.62 | 0.04 | 14.48 | 0.000 |
| Late insomnia | 0.45 | 0.04 | 12.77 | 0.000 |
| Somatic | ||||
| Loss of sexual interest | 0.27 | 0.03 | 7.99 | 0.000 |
| Weight Loss | 0.14 | 0.03 | 5.36 | 0.000 |
| Somatic symptoms | 0.38 | 0.05 | 8.44 | 0.000 |
| Appetite loss | 0.31 | 0.04 | 8.30 | 0.000 |
| Residual variances | ||||
| Psychic anxiety | 0.56 | 0.08 | 6.96 | 0.000 |
| Psychomotor agitation | 0.99 | 0.07 | 14.94 | 0.000 |
| Psychomotor retardation | 0.97 | 0.06 | 17.49 | 0.000 |
| Somatic anxiety | 0.81 | 0.05 | 17.40 | 0.000 |
| Hypochondriasis | 0.97 | 0.08 | 12.71 | 0.000 |
| Depressed mood | 0.68 | 0.05 | 12.71 | 0.000 |
| Work and activity difficulties | 0.82 | 0.06 | 12.86 | 0.000 |
| Guilt | 0.85 | 0.06 | 13.78 | 0.000 |
| Suicidality | 0.87 | 0.04 | 20.45 | 0.000 |
| Early insomnia | 0.83 | 0.05 | 18.18 | 0.000 |
| Middle insomnia | 0.60 | 0.05 | 10.87 | 0.000 |
| Late insomnia | 0.78 | 0.04 | 20.30 | 0.000 |
| Loss of sexual interest | 0.94 | 0.06 | 14.80 | 0.000 |
| Weight Loss | 0.96 | 0.07 | 14.20 | 0.000 |
| Somatic symptoms | 0.87 | 0.07 | 12.47 | 0.000 |
| Appetite loss | 0.88 | 0.03 | 26.85 | 0.000 |
| Latent covariances | ||||
| Anxiety with Mood | 0.25 | 0.08 | 2.96 | 0.003 |
| Anxiety with Insomnia | 0.10 | 0.08 | 1.32 | 0.188 |
| Anxiety with Somatic | 0.13 | 0.11 | 1.22 | 0.222 |
| Mood with Insomnia | −0.05 | 0.08 | −0.60 | 0.552 |
| Mood with Somatic | 0.32 | 0.11 | 2.87 | 0.004 |
| Insomnia with Somatic | 0.06 | 0.11 | 0.55 | 0.582 |
Std. Err.: Standard error; z: z-score; CFI: comparative fit index; RMSEA: Root mean square error of approximation; SRMR: standardized root mean squared residual.
Symptom cluster response
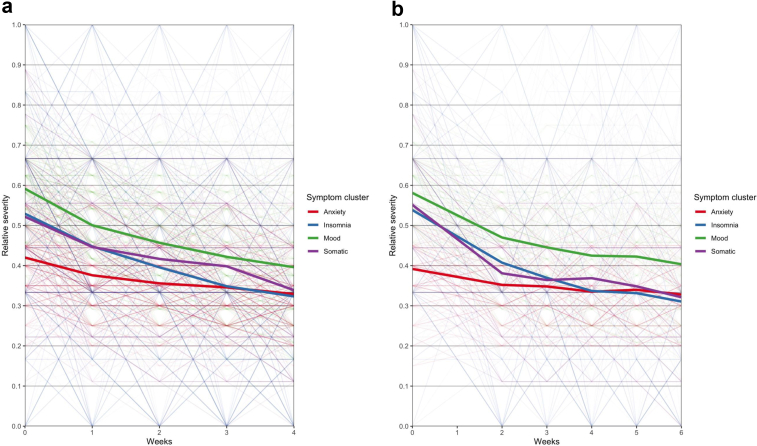
Following confirmation of the multidimensionality of the HDRS-17 we conducted analyses assessing the differential symptom response to rTMS using linear mixed effects models in the THREE-D dataset. In assessing the mixed effects models with differing random effects, we found that the model incorporating subject-specific random slopes significantly improved model fit ( (9) = 2216.98, p < 0.001). Using this model, we found a significant interaction between time and symptom cluster (F(3,5984) = 31.92, p < 0.001). In post-hoc analyses, relative to the anxiety symptom cluster, there was a statistically significant greater reduction in severity for mood (t(6001) = -8.02, p < 0.001), insomnia (t(5980) = −8.83, p < 0.001) and somatic (t(5995) = -6.12, p < 0.001) symptom clusters over time. The pattern of symptom cluster responses in THREE-D over the course of rTMS treatment are presented in Fig. 1. Standardised effect sizes for each contrast after four weeks of treatment are presented in Table 2. The effect size for each symptom cluster relative to anxiety ranged from 0.14 to 0.21, while the other contrasts were smaller in magnitude and not statistically significant. The effect size of each symptom cluster from baseline to study completion was statistically significant (also Table 2). Visual inspection of the density plot of residuals demonstrated a normal distribution suggesting adequate model fit (appendix pp6, 7).
Fig. 1.
Spaghetti plots of change in relative severity of symptom cluster with rTMS treatment in (a) THREE-D and (b) CARTBIND. Relative severity is the proportion of maximum possible value to facilitate comparisons between symptom clusters.
Table 2.
Effect sizes of contrast with rTMS treatment.
| Symptom cluster | THREE-D effect size (95% CI) | CARTBIND effect size (95% CI) |
|---|---|---|
| Baseline to treatment completion | ||
| Anxiety | 0.66 (0.52, 0.81) | 0.46 (0.26, 0.66) |
| Insomnia | 1.45 (1.31, 1.60) | 1.60 (1.40, 1.81) |
| Mood | 1.42 (1.28, 1.57) | 1.32 (1.12, 1.52) |
| Somatic | 1.32 (1.18, 1.47) | 1.72 (1.51, 1.92) |
| Weekly symptom cluster comparison | ||
| Anxiety–insomnia | 0.21 (0.16, 0.25) | 0.18 (0.14, 0.23) |
| Anxiety–mood | 0.19 (0.14, 0.23) | 0.13 (0.09, 0.18) |
| Anxiety–Somatic | 0.14 (0.10, 0.19) | 0.18 (0.14, 0.22) |
| Insomnia–Mood | −0.02 (−0.06, 0.03) | −0.05 (−0.09, −0.01) |
| Insomnia–Somatic | −0.06 (−0.11, −0.02) | −0.01 (−0.05, 0.03) |
| Mood–Somatic | −0.04 (−0.09, 0.00) | 0.04 (0.00, 0.09) |
Effect sizes of individual symptom clusters are from baseline to treatment completion, while symptom cluster comparisons are weekly effect sizes.95%CI: 95% confidence interval.
Using the CARTBIND dataset as an independent replication, we found results consistent with our primary analysis supporting the replicability of our results. We found a significant interaction between symptom cluster and time (F(3,3800) = 33.02, p < 0.001). Post hoc analyses, relative to anxiety symptom cluster, also found a statistically significant greater reduction in severity over time for all other symptom clusters - mood (t(3808) = −6.36, p < 0.001), insomnia (t(3791) = −8.72, p < 0.001), and somatic (t(3809) = −8.43, p < 0.001) - but the magnitude of effects differed slightly (see Table 2). The pattern of symptom cluster responses over the course of rTMS treatment in CARTBIND is presented in Fig. 1. We did not find an effect of study on the pattern of symptom cluster response over the course of treatment (t(648) = −0.51, p = 0.608).
Discussion
In this novel analysis of two large rTMS clinical trials we found that depression experienced by patients with TRD referred for rTMS consists of multiple distinct symptom clusters, and these clusters have differential responses to rTMS treatment. This is also the first study to demonstrate that rTMS delivered to the left DLPFC using MRI-guided neuronavigation resulted in significantly greater improvements in mood, somatic, and insomnia symptoms compared to anxiety. This finding of differential symptom cluster response was independently replicated in a separate clinical trial of rTMS targeting the same treatment location with a different protocol, which lends support to the robustness of these findings.
Our finding that distinct symptom clusters have differential responses to rTMS treatment is consistent with similar analyses conducted in pharmacotherapy trials.9, 10, 11, 12 These studies consistently find that the core “emotional” or depressed mood symptoms are more responsive to pharmacotherapy treatment compared to other items9 or symptom clusters.10 However, due to differences in analytic techniques, the symptom clusters identified in other studies measure different constructs such as “atypical” symptoms,10 or cognitive symptoms.11 As a result, direct comparisons between the present and prior work is problematic. Comparative studies of pharmacotherapy with rTMS using consistent analytic techniques will be required to determine whether our findings are unique to rTMS or are consistent across depression treatment modalities. If there are unique effects observed by treatment modality, it will then be important to consider mechanisms of observed differences and whether side effects such as pain, which is associated with anxiety in rTMS,31 mediate symptom cluster response. Similarly, it will also be important to determine whether differing rTMS treatment targets (i.e., right DLPFC or DMPFC), can achieve greater relative reductions in anxiety symptoms, as suggested by prior hypothesis generating work.15 Regardless of future study findings, our work provides clinically relevant information that can inform the current decision-making process for patients with depression. For those considering rTMS treatment with a figure-of-eight coil applied to the left DLPFC, our work indicates that patients should expect less relative improvement in anxiety symptoms compared to core mood, insomnia, and somatic symptoms.
This is a timely and clinically relevant finding because the U.S. FDA recently approved the Brainsway Deep TMS system for treating depression with comorbid anxiety.32 A possible explanation to account for the discrepancy between our findings and the approval of the Brainsway device may be related to the electric field distribution of the Brainsway coil compared to the figure-of-eight coil.33 Modeling studies indicate that the Brainsway device, even when applied to the left DLPFC generates substantial electric field in the right DLPFC as well as smaller fields bilaterally in the prefrontal and medial prefrontal cortex, while the figure-of-eight coil was largely localized to the left DLPFC.33
These findings are also congruent with the neuroimaging based findings of prior work demonstrating the differential response of symptom clusters with rTMS.15 The authors of this previous study hypothesized that rTMS treatment delivered to the left DLPFC may have preferentially greater impact on mood/dysphoric symptoms compared to anxiety symptoms, a finding which was confirmed in our study. Our results also suggest that, given the similarity between the THREE-D and CARTBIND findings, the number and timing of stimulation pulses do not have a significant effect on differential response of symptom clusters. While the effect of rTMS on depression and mood symptoms is well-established,17 the effect of rTMS on sleep disturbances and insomnia is less understood.34 To our knowledge there has been a single study examining the effect of rTMS treatment delivered to the left DLPFC on sleep disturbances related to depression, and found – consistent with our findings – that rTMS improved sleep quality independent of mood.35
Though there are strengths to our work including a large sample size, independent replication, and a hypothesis evaluation framework, there are also limitations to consider. First, while our hypotheses were guided by neuroimaging,15 in our analysis we only considered clinical measures of depression and future work will incorporate neuroimaging data available for study participants. Second, the results of this analysis are specific to the protocols used in the trials and participants had treatment delivery based on structural imaging localization. While the replication of the symptom cluster response with THREE-D and CARTBIND studies is promising, future work will be required to assess the generalizability of these results to novel rTMS protocols.36 Third, both studies included individuals with TRD and how these findings may apply to other samples (i.e., bipolar depression or depression with anxious distress) is unknown. Fourth, our primary analysis of symptom cluster response was based on a trial using two different forms of rTMS, which while they have demonstrated non-inferiority,17 could potentially differentially impact symptom clusters. However, we accounted for this analytically by including treatment allocation as a covariate in our analytic models, and also replicated these findings using an external dataset using the same form of rTMS treatment (iTBS).18 Fifth, the present work made several modifications to a pre-existing factor model,20 which while guided by theoretical and empirical considerations consistent with best practices for conducting CFA,23 means that the factor model developed in the current work requires replication in independent datasets. While we used the current datasets to replicate the symptom cluster response analysis, we were unable to do so for the CFA, which require sample large sample sizes ranging from 300-500.22 Therefore, we were required to pool data from both studies for the factor analysis such that replication analyses were not possible. Sixth, while we incorporated repeated measurements into our CFA, due to software limitations we were unable to model the multilevel nature of the observed data due to within-individual clustering and as a result the estimated standard errors should be interpreted cautiously. Last, while there were differences between the datasets in study design (e.g., non-inferiority vs superiority), duration (e.g. 4 vs 6 weeks), and selection criteria (e.g., ATHF criteria) between studies, the consistency of our symptom cluster analysis support the robustness of our findings.
In a hypothesis-driven analysis of two large rTMS clinical trials, we found that rTMS treatment delivered to the left DLPFC demonstrated a differential impact on the distinct symptom clusters of depression. In particular we found evidence for a greater effect of rTMS on reducing mood, insomnia and somatic symptoms compared to anxiety symptoms, a finding that was replicated in an external dataset. Future work will determine whether differing rTMS treatment targets have distinct patterns of symptom cluster responses with the eventual goal of personalizing rTMS protocols based on an individual's clinical presentation.
Contributors
Concept and design: Kaster, Blumberger.
Acquisition, analysis, or interpretation of data: Kaster, Downar, Vila-Rodriguez, Baribeau, Thorpe, Daskalakis, Blumberger.
Drafting of the manuscript: Kaster, Blumberger.
Critical revision of the manuscript for important intellectual content: Kaster, Downar, Vila-Rodriguez, Baribeau, Thorpe, Daskalakis, Blumberger.
Statistical analysis: Kaster, Baribeau, Thorpe.
Administrative, technical, or material support: Downar, Vila-Rodriguez, Blumberger.
Supervision: Blumberger.
Data sharing statement
Deidentified participant data along with data dictionaries is available and can be shared with researchers who provide a methodologically sound proposal that includes a protocol and a statistical analysis plan, and is not in conflict with the investigators’ publication plan. Proposals should be directed to daniel.blumberger@camh.ca. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
JD reports research grants from CIHR, the National Institute of Mental Health, Brain Canada, the Canadian Biomarker Integration Network in Depression, the Ontario Brain Institute, the Wilburforce Foundation, the Klarman Family Foundation, the Arrell Family Foundation and the Bowness Family Foundation, travel stipends from Lundbeck and ANT Neuro, in-kind equipment support for investigator-initiated trials from MagVenture, and is an advisor for BrainCheck, TMS Neuro Solutions and Restorative Brain Clinics and is co-founder of Ampa Health. FVR receives research support from CIHR, Brain Canada, Michael Smith Foundation for Health Research, Vancouver Coastal Health Research Institute and Weston Brain Institute for investigator-initiated research. Philanthropic support from Seedlings Foundation. In-kind equipment support for this investigator-initiated trial from MagVenture. He has received honoraria for participation in advisory board for Janssen. DMB receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health – US (NIH), Brain Canada Foundation and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he was the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen. He has participated in an advisory board for Welcony Inc. ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc. He is currently on the Scientific Advisory Board of Brainsway Inc. His work is supported by the Canadian Institutes of Health Research (CIHR), the National Institutes of Mental Health (NIMH), Brain Canada and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. TSK, DAB, and KET report no competing interests.
Acknowledgements
The authors thank the clinical research staff and the patient participants of both the THREE-D and CARTBIND study. THREE-D and CARTBIND received support from the Temerty Family Foundation, the Griffith Family Foundation, the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health, and Tina Buchan and the Buchan Family Fund (through the University Health Network), philanthropic donation to the NINET lab, Health Canada, Simons Foundation, the Klarman Family Foundation, the Arrell Family Foundation, and the Toronto General and Western Hospital Foundation. MagVenture provided in-kind equipment support in the form of two coils and two high performance coolers at each site; however, MagVenture had no role in the study design, data analysis, interpretation, or preparation of this manuscript and none of the investigators receive any financial compensation or have any financial interests in Magventure.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101765.
Appendix A. Supplementary data
References
- 1.Souery D., Amsterdam J., de Montigny C., et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9:83–91. doi: 10.1016/s0924-977x(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 2.Ressler K.J., Mayberg H.S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaster T.S., Downar J., Vila-Rodriguez F., et al. Trajectories of response to dorsolateral prefrontal rTMS in major depression: a THREE-D study. Am J Psychiatr. 2019;176:367–375. doi: 10.1176/appi.ajp.2018.18091096. [DOI] [PubMed] [Google Scholar]
- 4.Brunoni A.R., Chaimani A., Moffa A.H., et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatr. 2017;74:143–152. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 5.Fried E.I., Nesse R.M. Depression sum-scores don't add up: why analyzing specific depression symptoms is essential. BMC Med. 2015;13:72. doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagby R.M., Ryder A.G., Schuller D.R., Marshall M.B. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatr. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- 7.Quilty L.C., Robinson J.J., Rolland J.-P., Fruyt F.D., Rouillon F., Bagby R.M. The structure of the Montgomery-Asberg depression rating scale over the course of treatment for depression. Int J Methods Psychiatr Res. 2013;22:175–184. doi: 10.1002/mpr.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried E.I., Nesse R.M. Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR∗D study. J Affect Disord. 2015;172:96–102. doi: 10.1016/j.jad.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hieronymus F., Emilsson J.F., Nilsson S., Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatr. 2016;21:523–530. doi: 10.1038/mp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chekroud A.M., Gueorguieva R., Krumholz H.M., Trivedi M.H., Krystal J.H., McCarthy G. Reevaluating the efficacy and predictability of antidepressant treatments: a symptom clustering approach. JAMA Psychiatr. 2017;74:370–378. doi: 10.1001/jamapsychiatry.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uher R., Maier W., Hauser J., et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatr. 2009;194:252–259. doi: 10.1192/bjp.bp.108.057554. [DOI] [PubMed] [Google Scholar]
- 12.Bondar J., Caye A., Chekroud A.M., Kieling C. Symptom clusters in adolescent depression and differential response to treatment: a secondary analysis of the Treatment for Adolescents with Depression Study randomised trial. Lancet Psychiatr. 2020;7:337–343. doi: 10.1016/S2215-0366(20)30060-2. [DOI] [PubMed] [Google Scholar]
- 13.Goerigk S.A., Padberg F., Chekroud A., Kambeitz J., Bühner M., Brunoni A.R. Parsing the antidepressant effects of non-invasive brain stimulation and pharmacotherapy: a symptom clustering approach on ELECT-TDCS. Brain Stimul. 2021;14:906–912. doi: 10.1016/j.brs.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Wade B.S.C., Hellemann G., Espinoza R.T., et al. Depressive symptom dimensions in treatment-resistant major depression and their modulation with electroconvulsive therapy. J ECT. 2020;36:123–129. doi: 10.1097/YCT.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqi S.H., Taylor S.F., Cooke D., Pascual-Leone A., George M.S., Fox M.D. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatr. 2020;177:435–446. doi: 10.1176/appi.ajp.2019.19090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haig B.D. Exploratory factor Analysis, theory generation, and scientific method. Multivariate Behav Res. 2005;40:303–329. doi: 10.1207/s15327906mbr4003_2. [DOI] [PubMed] [Google Scholar]
- 17.Blumberger D.M., Vila-Rodriguez F., Thorpe K.E., et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 18.Blumberger D.M., Vila-Rodriguez F., Wang W., et al. A randomized sham controlled comparison of once vs twice-daily intermittent theta burst stimulation in depression: a Canadian rTMS treatment and biomarker network in depression (CARTBIND) study. Brain Stimul. 2021;14:1447–1455. doi: 10.1016/j.brs.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafer A.B. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 21.Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Software. 2012;48:1–36. [Google Scholar]
- 22.Wolf E.J., Harrington K.M., Clark S.L., Miller M.W. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ Psychol Meas. 2013;76:913–934. doi: 10.1177/0013164413495237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher M.W., Brown T.A. Handbook of quantitative methods for educational research. Brill; 2013. Introduction to confirmatory factor analysis and structural equation modeling; pp. 287–314. [Google Scholar]
- 24.Putnick D.L., Bornstein M.H. Measurement invariance conventions and reporting: the state of the art and future directions for psychological research. Develop Review. 2016;41:71–90. doi: 10.1016/j.dr.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei P.-W., Wu Q. Introduction to structural equation modeling: issues and practical considerations. Educ Meas. 2007;26:33–43. [Google Scholar]
- 26.Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 27.Chen F.F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct Equ Model: A Multidiscip J. 2007;14:464–504. [Google Scholar]
- 28.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. J Stat Software. 2017;82:1–26. [Google Scholar]
- 29.Little T.D. Guilford press; 2013. Longitudinal structural equation modeling. [Google Scholar]
- 30.Moeller J. A word on standardization in longitudinal studies: don't. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.01389. https://www.frontiersin.org/article/10.3389/fpsyg.2015.01389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humaira A., Gao S., Gregory E., et al. A patient-oriented analysis of pain side effect: a step to improve the patient's experience during rTMS? Brain Stimul. 2021;14:1147–1153. doi: 10.1016/j.brs.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S.L., Bikson M., Badran B.W., George M.S. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2022;15:73–75. doi: 10.1016/j.brs.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parazzini M., Fiocchi S., Chiaramello E., Roth Y., Zangen A., Ravazzani P. Electric field estimation of deep transcranial magnetic stimulation clinically used for the treatment of neuropsychiatric disorders in anatomical head models. Med Eng Phys. 2017;43:30–38. doi: 10.1016/j.medengphy.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Oroz R., Kung S., Croarkin P.E., Cheung J. Transcranial magnetic stimulation therapeutic applications on sleep and insomnia: a review. Sleep Sci Pract. 2021;5:3. [Google Scholar]
- 35.Collins A.R., Cheung J., Croarkin P.E., Kolla B.P., Kung S. Effects of transcranial magnetic stimulation on sleep quality and mood in patients with major depressive disorder. J Clin Sleep Med: JCSM. 2022;18:1297–1305. doi: 10.5664/jcsm.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumberger D.M., Daskalakis Z.J., Vila-Rodriguez F., et al. Accelerated intermittent theta burst as a substitute for patients needing electroconvulsive therapy during the COVID-19 pandemic: study protocol for an open-label clinical trial. medRxiv. 2020 doi: 10.1101/2020.12.15.20248260. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.