Abstract
As one of the typical emerging contaminants, microplastics exist widely in the environment because of their small size and recalcitrance, which has caused various ecological problems. This paper summarizes current adsorption and removal technologies of microplastics in typical aquatic environments, including natural freshwater, marine, drinking water treatment plants (DWTPs), and wastewater treatment plants (WWTPs), and includes abiotic and biotic degradation technologies as one of the removal technologies. Recently, numerous studies have shown that enrichment technologies have been widely used to remove microplastics in natural freshwater environments, DWTPs, and WWTPs. Efficient removal of microplastics via WWTPs is critical to reduce the release to the natural environment as a key connection point to prevent the transfer of microplastics from society to natural water systems. Photocatalytic technology has outstanding pre-degradation effects on microplastics, and the isolated microbial strains or enriched communities can degrade up to 50% or more of pre-processed microplastics. Thus, more research focusing on microplastic degradation could be carried out by combining physical and chemical pretreatment with subsequent microbial biodegradation. In addition, the current recovery technologies of microplastics are introduced in this review. This is incredibly challenging because of the small size and dispersibility of microplastics, and the related technologies still need further development. This paper will provide theoretical support and advice for preventing and controlling the ecological risks mediated by microplastics in the aquatic environment and share recommendations for future research on the removal and recovery of microplastics in various aquatic environments, including natural aquatic environments, DWTPs, and WWTPs.
Keywords: Aquatic environment, Enrichment and removal, Microplastics, Wastewater treatment plants
Graphical abstract
Highlights
-
•
Microplastics enrichment and removal are pivotal to controlling contamination.
-
•
Coagulation-flocculation-sedimentation works in microplastics removal.
-
•
Membrane bioreactor achieves higher microplastics removal efficiency.
-
•
Photocatalytic degradation of microplastics exhibits significantly high efficiency.
-
•
Abiotic pretreatment of microplastics is recommended before biodegradation.
1. Introduction
The mass production of plastics began around 1950 because of its superior properties [1], and society has been using plastics for about a hundred years. Over these decades, the world's plastic production increased from two million tons in 1950 to 370 million tons in 2019 [2] and will continue to increase in the future. Studies predict that the output of plastics will double again in 20 years and almost quadruple by 2050 [3]. With the mass production and widespread use of plastics, it is estimated that more than 400 million tons of plastic waste will be produced each year after 2020 [4]. Approximately 76% of the total plastic production is treated as waste of which 12% is burned, 79% is buried or released into the environment, and only 9% is recycled [5]. The incineration of plastics releases carbon monoxide, dioxins and dioxin-related compounds, and nitrogen oxides into the atmosphere [6], resulting in air pollution and the inability to completely remove plastic waste [7].
Plastics with different sources, shapes, and types decomposed into microplastics (MPs) with diameters less than 5 mm through photodegradation, thermal oxidation, thermal degradation, and possible biodegradation [8,9]. MPs have the characteristics of small size, large specific surface area, and remarkable chemical stability [10]. When MPs enter the food chain, the pollutants and toxic substances attached to the surface of MPs which will be ingested by animals. These MPs are toxic and ultimately threaten animal and human health [[11], [12], [13], [14], [15]].
Studies have focused on the sources, abundance, degradation, and interaction of MPs with their surface organisms in aquatic environments [[16], [17], [18], [19]]. MPs particles and plastic fibers produced in the process of human life enter the wastewater treatment plants (WWTPs) through domestic wastewater, but they are difficult to be effectively captured and removed completely by conventional WWTPs processes because of their small sizes [20]. MPs enter rivers or accumulate into river sediments with the effluent of the WWTPs, and finally enter the marine environment which will exist in the environment for decades [[21], [22], [23], [24]]. MPs can be transported far away by ocean currents, so MPs are widely distributed in the ocean, sediments, and even in the deep sea [25,26].
MPs pollution has gradually spread from aquatic environments to the rest of the environment and has caused adverse effects. Therefore, it is important to develop removal, recycling, and degradation technologies for MPs and prevent the potential ecological risks.
This paper takes natural aquatic environments, drinking water treatment plants (DWTPs), and WWTPs as typical aquatic scenarios, and comprehensively summarizes and discusses the research progress of MPs enrichment, removal and degradation technologies in various aquatic environments. In this review, we compared and analyzed the existing removal and degradation technologies of MPs to offer valuable scientific strategies for the development of new MPs removal, degradation, and recycling technologies to achieve efficient MPs pollution remediation.
2. Enrichment and removal technologies of microplastics in aquatic environments
2.1. Natural aquatic environments
2.1.1. Adsorption
Different removal technologies based on adsorption mechanisms have been proven to be effective approaches to remove MPs and nanoplastics (NPs) in aquatic environments. The chemically synthesized sponge materials [[27], [28], [29], [30]], graphene materials [31], and biochar materials [32] can be used to remove MPs and NPs in natural waters. Sponge materials can effectively remove various pollutants in water. Currently, some studies have used natural organic compounds as raw materials to produce sponge materials to adsorb MPs in the aquatic environment. A natural and biodegradable green sponge material with high mechanical properties was prepared by chemically crosslinking plant proteins. The abundant active side chains on the amino acid residues of the sponge allows for the protein sponge to have excellent adsorption capacity for MPs (the removal efficiency is as high as 81.2%) as well as reusability and biodegradability properties [29]. Sponge materials with chitin and graphene oxide (ChGO), chitin-based sponges combined with O–C3N4, chitosan were prepared, and all of them had acceptable performance and reusability. Each material had more than 70% removal efficiency for pure polystyrene (PS), carboxylate-modified polystyrene (PS–COOH) and amine-modified polystyrene (PS–NH2) MPs [27,30]. Yuan et al. used the chemical action of three-dimensional reduced graphene oxide (3D RGO) in water to complete the adsorption of PS−MPs. The analysis showed that under the optimal conditions (pH 6, ion concentration 600 mg L−1, adsorption time 120 min, and temperature 26 °C), the 3D RGO reached the maximal adsorption (617.28 mg g−1) on PS−MPs [31]. MPs would re-enter the environment by desorption from the adsorbents when rinsed, Mg/Zn modified magnetic biochar (Mg/Zn−MBCs) could degrade MPs by thermal treatment in situ, thus achieving Mg/Zn−MBC recycling, which can avoid the risk of MPs desorption [32].
Some metals combined with other chemical substances or metal compounds have also been reported for adsorption and removal of MPs. Micro/nanomotors (MNMs) are nano or micro-scale substances that move in a way similar to biological motor proteins, which can transport other objects and/or catalyze [33,34]. There is a broad application potential in the field of environment and some articles have reviewed the use of MNMs to remove MPs in the environment [35]. Self-propelled micromotors have certain academic value and application potential in microenvironmental remediation, such as the ability to overcome the diffusion limitation in the catalytic process and the ability to closely interact with the environment. For example, photocatalytic TiO2-based micromotors (Au@mag@TiO2, mag = Ni, Fe) could effectively move in peroxides and water under UV light irradiation and be used to remove MPs in aquatic environments [36]. The Fe2O3–MnO2 micromotor exhibits efficient removal of both aquatic organics and suspended MPs via the synergetic effect of catalytic degradation, surface adsorption, and adsorptive bubbles separation mechanisms [37]. A series of zirconium metal–organic framework-based foam materials have been successfully fabricated and applied in simulated MPs removal in water or seawater conditions [38]. Interactions between NPs and Zn–Al layered double hydroxide (LDH) confirmed that Zn–Al LDH can be considered as a potential adsorbent for NPs removal in freshwater systems [39].
Because of the surface charge between substances, some common organic substances in the environment can also adsorb MPs in ultrapure water, such as granular activated carbon (GAC) [40]. Except activated carbon, untreated coffee grounds can also effectively remove NPs from aquatic solutions [41]. In addition, there are some studies on magnetic adsorption of MPs in aquatic environments. For example, hydrophobic Fe nanoparticles modified with hexadecyltrimethoxysilane (HDTMS) could remove MPs due to hydrophobic interactions [42]. Based on the characteristics that magnetic carbon nanotubes (M−CNTs) could be adsorbed on MPs, such as polyethylene (PE), polyethylene terephthalate (PET), and polyamide (PA), in aquatic environments, all the MPs/M−CNTs composites could be readily separated from aquatic solutions by magnetic force [43].
The adsorption mechanisms of MPs by chemically synthesized sponge materials with larger sizes are based on hydrogen bonds, electrostatic between MPs and sponge materials are π−π interactions [27,30]. MPs in aquatic environments generally have a negative charge and can easily interact electrostatically with positively charged substances and be adsorbed on the surface. In comparison, the adsorption mechanisms of MPs via micromotors with smaller sizes are generally based on the phoretic interactions and shoveling, noncontact shoveling, and adsorptive bubble separation. Previous studies have reported that the synergetic adsorption mechanisms of surface and bubble separations also played a critical role in MPs adsorption [[35], [36], [37],44,45].
Many factors can also affect the MPs removal efficiency including pH, temperature, adsorbents types, dissolved organic matter (DOM), and ions, but pH and temperature are the two most important factors. pH affects the adsorption efficiency mainly by influencing the charge on the surface of MPs and the adsorbent. Temperature can affect the adsorbate diffusion rate and equilibrium capacity, and higher temperatures achieve more MPs adsorption [29]. Different adsorbents types have different electrostatic and hydrogen bonding interactions with MPs, thereby altering the adsorption capacity. DOM changes the interaction of absorbents with MPs [30]. On top of this, ions can influence the electrostatic attraction between MPs and the adsorbent, which in turn alters the MPs adsorption by adsorbent [31,46]. In other words, the adsorption removal of MPs has the advantages of high adsorption capacity, high removal efficiency, low energy consumption and reusability. However, the adsorbents need to be eluted from the adsorbed MPs after use, which allows for the potential risk that the MPs would re-enter the environment. The advantages or disadvantages of other emerging technologies are yet to be clarified as they are still in the early age of research.
2.1.2. Filtering
Filtration could also be applied to MPs removal in aquatic environments. The biofilter prepared by Kuoppamäki et al. not only removes nutrients and heavy metals in rainwater, but also remove MPs [47]. Cost-effective guinea cornhusk ash (GCHA) was fabricated, which supports thin film composite (TFC) membranes via interfacial polymerization, and can be used to remove MPs from aqueous solutions and has good stability in seawater pretreatment [48].
2.1.3. Other enrichment and removal technologies
In addition to adsorption and filtration, some other technologies also show excellent application prospects in the removal of MPs in the aquatic environment. Electrocoagulation (EC) removes MPs through a series of physical-chemical reactions, which usually follows three consecutive steps. Firstly, the metal anode produces metal cations and forms micro coagulants in the presence of an electric field. Then, the charged micro coagulants trap and engulf suspended particles, such MPs forming colliding flocs. Thirdly, the microflocs size continues to build with more collisions and finally achieve MPs removal via physical and/or chemical reactions [49,50].
Lysozyme amyloid fibrils serves as a novel natural bio-flocculant for removing dispersed MPs from water [51]. MPs can also be enriched and removed from water by using adhesives. Chazovachii et al. indicated that shaking zirconium silicate beads coated with poly(2-ethylhexyl acrylate) in aqueous suspensions containing PS−MPs (10 μm) could remove up to 99% of the MPs within 5 min[52]. Solar energy was used to drive the removal of MPs in aquatic environments, in which the sunlight was focused by high-density glass spheres to induce convection and form microbubbles at the interface. MPs were then driven to the bubbles through convection. The temperature in the bubbles was much higher than in the solution, so that the aggregated MPs were fused to form a large block. This method could remove MPs in water without biological or chemical energy which can cause secondary pollution [53].
In the natural aquatic environment, MPs removal technologies based on the principle of adsorption has been widely studied and showed obvious advantages. Enriched MPs can be combined with recycling technology to achieve harmless treatment of MPs. Many materials that can adsorb and enrich MPs in aquatic environments are reusable, and they still have high adsorption efficiency after repeated use. Adsorption materials are easy to separate from environmental substances without introducing new pollutants. Compared with other methods, the energy consumption of MPs removal by adsorption enrichment is significantly lower. Among various MPs removal methods, the sponge material prepared with chitin not only had higher MPs removal efficiency, but can also be reused. Moreover, chitin does not cause environmental pollution and is an environmentally friendly and readily available material. The sponge does not consume energy and causes no pollution. As a green environmentally friendly and convenient MPs adsorption method, it has broad and promising application prospects. In the future, more attention should be paid to the adsorption and enrichment of MPs by using materials easily available in the environment or materials such as domestic waste. MPs removal devices driven by low energy consumption or solar energy, will also help to achieve material recycling under carbon constraints. The removal technologies of MPs in natural freshwater environments in the last five years were summarized in Table 1, Table 2.
Table 1.
Adsorption removal technologies of MPs in natural water.
| Removal mechanism | Removal technologies | Material | Types of MPs | Maximum adsorption capacity | Removal efficiency | Reference |
|---|---|---|---|---|---|---|
| Adsorption | Oat protein sponges | Oat protein isolate | 1 μm PS (1 mg per L suspension) | 5.7 mg L−1 (25 °C, MPs = 15 mg L−1) | The highest rate was 81.2% | [29] |
| Sponge | ChGO | 1 μm PS (1 mg L−1) | 8.461 mg L−1 (45 °C) | The highest rate was 92.2% | [27] | |
| 1 μm PS–COOH (1 mg L−1) | N/A | The highest rate was 74.9% | ||||
| 1 μm PS–NH2 (1 mg L−1) | N/A | The highest rate was 90.2% | ||||
| Chitin-based sponge | ChCN, ChGO, and ChGO−CT | 1 μm PS (1 mg L−1) | 9.67 mg L−1 (ChGO, 45 °C) | 89.6–92.1% | [30] | |
| 1 μm PS–COOH (1 mg L−1) | 8.86 mg L−1 (ChGO, 45 °C) | 80.4–81.3% | ||||
| 1 μm PS–NH2 (1 mg L−1) | 12.9 mg L−1 (ChCN, 45 °C) | 83.2–87.1% | ||||
| Biochar and modified biochar | MBCs | 1 μm, 100 mg mL−1 PS | 100.6 mg g−1 | 94.81% | [32] | |
| Mg−MBCs | 98.52 mg g−1 | 98.75% | ||||
| Zn−MBCs | 99.21 mg g−1 | 99.46% | ||||
| Three-dimensional graphene | 3D RGO | 5 μm monodisperse PS microspheres (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 g L−1) | 617.28 mg g−1 (pH = 6, 26 °C, C0 = 600 mg L−1, t = 120 min) | Tap water (56.08%, 448.60 mg g−1); micropolluted water (53.85%, 430.78 mg g−1); distilled water (66.63%, 533.06 mg g−1) | [31] | |
| Photocatalytic TiO2-based Micromotor | Au@mag@TiO2, mag = Ni, Fe | Separated and extracted from personal care products and Baltic Sea and Warnow river | N/A | 71% in 0.2% H2O2 solution | [36] | |
| 67% in Warnow river | ||||||
| Bubble-propelled iron oxides−MnO2 core-shell MNMs | Fe2O3–MnO2 MNMs |
separated and extracted from the facial cleanser | N/A | Separated more than 10% of the suspended MPs from the polluted water in 2 h | [37] | |
| Zirconium metal–organic framework-based foam | UiO-66-OH@MF-3 | PVDF (∼260 nm), PMMA (∼325 nm), PS (∼183 nm) |
N/A | The highest rate was 95.5 ± 1.2% | [38] | |
| Zn–Al LDH | Zn–Al LDH | 55 nm PS (250 mg L−1) | 164.49 mg g−1 (deionized water); 162.62 mg g−1 (synthetic freshwater); 53.27 mg g−1 (synthetic hard water) | 100% (pH 4); 37% (pH 9) | [39] | |
| GAC | Granular coconut shell-based Activated Carbon | PS latex NPs (90 ± 7 nm, 3 g L−1) | 2.20 ± 0.06 mg g−1 in ultrapure water | 98% | [40] | |
| 6.33 ± 0.20 mg g−1 in natural surface water from Lake Geneva | 90% | |||||
| Coffee grounds | Coffee grounds biowaste | Fluorescent-orange amine-modified PS beads (fluo-NP, 100 nm, 25,000 mg L−1) | 4 mg g−1 (t = 40 min) | The maximum adsorption efficiency 74% | [41] | |
| Magnetic adsorption | Hydrophobic Fe nanoparticles | Modified Fe nanoparticles binding MPs | MPs in three size ranges, large (1–8 mm), medium (200 μm–1 mm), and small (<20 μm) | N/A | For the large size MPs: 74–105%, for the medium size MPs: 59–100% for RO water and 49–90% for sediment, for the small MPs: ∼90% | [42] |
| M−CNTs | M−CNTs | PE, PET, and PA (diameter 48 μm, 5 g L−1) | 1650 mg M−CNTs per g PE; 1400 mg M−CNTs per g PET; 1100 mg M−CNTs per g PA | 100% | [43] |
Table 2.
Enrichment and removal technologies of MPs in natural water.
| Removal mechanism | Removal technologies | Material | Types of MPs | Removal efficiency | Reference |
|---|---|---|---|---|---|
| Filtration | Biofilter structures | (a) Crushed light-expanded clay aggregates without biochar or amended with biochar, (b) Filtralite P clay aggregates, (c) Crushed concrete, or (d) Filter sand | Fluorescent PE MPs beads up to 10 μm in diameter, coated with luminescence dye (0.02 g mL−1) | 100% | [47] |
| Silica-based ceramic hollow fiber (HF) microporous membrane | guinea cornhusk ash (GCHA) | PVC, polyvinylpyrrolidone (PVP), polyacrylonitrile (PAN), polymethylmethacrylate (PMMA) (50 mg L−1) | 88.8–97.2% | [48] | |
| Electrocoagulation | Electrocoagulation | Reactor, electrodes | PE (Fluorescent green, spherical microbeads of 300–355 μm 0.997 g cm−3, 0.1 g L−1) | The highest removal efficiency being 90–100% | [54] |
| Flocculation | Natural bio-flocculant | Lysozyme amyloid fibrils | Carboxylated PS particles (500 nm, 50 mg mL−1) | Turbidity and TOC decreased by 98.2 and 93.4%, respectively | [51] |
| Noncovalent interactions | Pressure-sensitive adhesive | Zirconium silicate beads coated with poly (2-ethylhexyl acrylate) | PS (10 μm, 2 mg mL−1) | 99% | [52] |
| Collect and fuse plastic particles into large bulks in the microbubble | Solar energy | Spherical K5 glass balls | Monodisperse PS colloids (60 nm, 90 nm, 200 nm, 500 nm, 1 μm, and 3 μm PS) | Maximum collection efficiency over 70% | [53] |
2.2. Marine environment
Feasible and effective methods to enrich and remove MPs in the marine environment are still lacking. Marine organisms have great potential for the removal of marine MPs. From the perspective of marine MPs removal, we believe that the adsorption and ingestion behavior of marine organisms on MPs is an important way to partially remove marine MPs.
The feeding and adsorption ability of marine organisms to MPs has been investigated and confirmed recently. For example, corals in the ocean remove MPs from the seawater through active (ingestion) and passive (adhesion to the surface) mechanisms [55,56]. Red Sea giant clam (Tridacna maxima) can ingest MPs on its own and its shell has the ability to adsorb MPs [57]. MPs were detected in the gut of gooseneck barnacles (Lepas spp.) in the North Pacific Subtropical Gyre [58]. Although the ingestion and adsorption of MPs by marine organisms temporarily enriches MPs from seawater, MPs enriched within the food chain could eventually move up to the human body as the marine organisms that ingested or adsorbed MPs were preyed on by higher trophic organisms or released to the marine environment upon degradation by a decomposer once they died. Therefore, the ingestion and adsorption of MPs by marine organisms can only migrate marine MPs rather than effectively removal, which increases the potential risk of MPs passing and accumulating along the food chain. Therefore, the removal of MPs in the marine environment is important while the relevant technologies are still lacking.
Compared with natural aquatic environments, MPs have different fates in the marine environment. MPs in the ocean are easily affected by ocean currents, as well as various small fouling organisms adhering to the surface of MPs to facilitate their settlement. Rius-Ayra et al. presented a superhydrophobic surface obtained by combining anode oxidation and the liquid-phase deposition of lauric acid which could remove MPs from simulated marine water [59]. An MP concentrator (MPC) was designed and optimized, which could balance inertial lift forces and Dean drag forces in a fully enclosed system to concentrate MPs ≥19 μm. The MPC achieved more than 90% recovery of MPs from seawater samples and polypropylene (PP) food container extracts, and also obtained MPs counts (per gram wet sediment) for environmental ocean floor samples within the range (Polyurethane, Cis-polyisoprene, PS, PE, Nylon, Polyester, Ammonium polyacrylate, Alkyd resin, Melamine-formaldehyde resin, Anionic polymer (Hydraid 771), Adhesive (Tuff-Bond®)) previously achieved by filtration [60].
Apart from large marine organisms, marine microbes such as algae can also enrich MPs and NPs. The extracellular polymeric substances (EPS) secreted by algae form aggregates with MPs particles, making MPs easy to be deposited and separated. Current studies have found that EPS of two marine microalgae (Tetraselmis sp. and Gloeocapsa sp.) have excellent effects on the aggregation of MPs. EPS isolated from other algae in fresh water, such as Cyanothece sp., Microcystis panniformis, and Scenedesmus sp., also exerted similar effects [61,62]. A bacterial biofilm with a “capture-release mechanism” was designed, whose EPS could firstly cause bioaggregation of MPs, followed by an inducible biofilm dispersal mechanism that releases trapped MPs for downstream resource recovery [63].
Other organisms in marine environments can also share new insights for MPs removal. Based on the basic characteristics of the adhesive chemistry practiced by marine mussels, adhesive polydopamine (PDA)@Fe3O4 magnetic microrobots (MagRobots) were prepared by coating Fe3O4 nanoparticles with a polymeric layer of dopamine via one-step self-polymerization. Such adhesive MagRobots are promising to remove MPs from aquatic environments at a large scale [64]. Jellyfish is a common marine organism that can produce a large amount of mucus, which could be as a new type of biological flocculation material, and the jellyfish mucus has been shown to be able to chelate PS−MPs in the aquatic environment [65]. Table 3 summarized the ocean MPs removal techniques over the past three years.
Table 3.
Removal technology of MPs in marine.
| Removal technologies | Material | Removal mechanism | Types of MPs | Removal efficiency | Reference |
|---|---|---|---|---|---|
| Non-fluorinated superhydrophobic aluminium surface | Combining anodisation and the liquid-phase deposition of lauric acid | Superhydrophobic/superoleophilic wetting properties | 53 ± 7 MP mL−1 of PP−MPs (size = 262 ± 4 μm) (the solvent is 3.5 wt% NaCl aqueous solution) | >99% | [59] |
| MPC | Patterned PDMS with inlet and outlet holes bonded to a slide via oxygen plasma | Concentrate particles of specific sizes with the balance of inertial lift force and Dean drag force in a fully enclosed system | Blank NaCl solutions containing 20 μm green PS beads, 5 μm red PS beads, and 1 μm green PS beads (3 × 10−5 g mL−1) | ≥90% | [60] |
| Tetraselmis sp., Gloeocapsa sp., Microcystis panniformis, Scenedesmus sp. | EPS | EPS and MPs form hetero-aggregates | PMMA, PS (<106 μm; 106–250 μm), density (high and low) (12.5 and 125 mg L−1) | N/A | [61] |
| Cyanothece sp. | EPS | EPS to aggregate NPs and MPs | 0.1 μm PS−NPs and 10 μm PS−MPs (solution in deionized water containing 0.1% Tween 20, 1 and 10 mg L−1) | N/A | [62] |
| A bacterial biofilm with a “capture-release mechanism” | EPS | EPS can cause bioaggregation of MPs | MPs (106–300 μm) in seawater | N/A | [63] |
| PDA@Fe3O4 (MagRobots) | Coating Fe3O4 nanoparticles with a polymeric layer of dopamine via one-step self-polymerization. | Mimicking basic characteristics of the adhesive chemistry practiced by marine mussels | MPs solution (2 mg mL−1) | N/A | [64] |
| Jellyfish mucus | C. tuberculata, A. aurita, and R. pulmo jellyfish and M. leidyi | Jellyfish mucus can efficiently sequester PS−MPs particles from the suspension | PS microspheres, dyed with Fluorescent Green with the average particle size of 48 μm, particle density of 1.05 g cm−3, refractive index 1.59 | N/A | [65] |
Most of MPs accumulated in the aquatic environment eventually gathered in the marine environment. Compared with the freshwater environment, the marine environment is more complex and larger, and many MPs removal methods are difficult to apply to the marine environment broadly. Moreover, MPs can easily settle in the ocean caused by the attachment of marine microbes and fouling organisms. Nevertheless, all kinds of valuable marine organisms can provide MPs removal methods potential and suggestions. The removal technologies of MPs in the ocean are still being challenged to be utilized in the field. Further in-depth investigations and technical improvements are necessary, especially for the heavily polluted coastlines and estuaries.
2.3. Drinking water treatment plants (DWTPs)
The fate and environmental behavior of MPs in DWTPs have received increasing attention to ensure the safety of drinking water. Recent studies indicated that removal efficiency of MPs in DWTPs depends on raw water quality and treatment process in each catchment [[66], [67], [68], [69], [70]]. The abundance of MPs in raw water and treated water of two DWTPs with different treatment processes varied significantly. The number of MPs ranges from less than 20 to more than 1200 pieces per liter with sizes mainly less than 10 μm[71].
2.3.1. Coagulation - flocculation - sedimentation (CFS) technology
Coagulation-flocculation-sedimentation (CFS) are used by many DWTPs to remove particulates and colloidal substances, and this process is also used to remove MPs and NPs. Under the action of coagulants, the colloids and fine suspended matter in the water coalesce into flocs and precipitate. During the removal by coagulation/flocculation processes, particles are separated from the water phase, largely by the use of metallic salts (iron and aluminium) and polymeric flocculant aids like polyacrylamide (PAM) [72]. The previous work indicated that PS−MPs less than 90 μm were more easily removed by alum CFS treatment [73]. Alum flocculation was proposed to be the main mechanism for removing MPs from drinking water, and it can remove MPs from solutions with surfactants [74]. In addition, approximately 80% of MPs were removed when using ferric and aluminium sulphate as coagulants. Polyvinyl chloride (PVC) MPs less than 50 μm were coagulated by ferric and aluminium sulphate, and MPs no less than 15 μm were completely removed under optimized coagulation conditions (ferric sulphate at 20 mg L−1 and pH 7 or aluminium sulphate at 40 mg L−1 and pH 7) [75]. When Fe-based salts worked as coagulants to remove PE−MPs, the removal efficiency was greatly improved by the addition of PAM [76]. Coagulation and sedimentation could also be combined with other technologies to remove MPs in drinking water. For example, MPs with larger size were better removed by coagulation and filtration, and the coagulation with AlCl3 is better than that of FeCl3. When the dosage of coagulant AlCl3 was 10 mg L−1 and the settling time was 1 h, the maximum MPs removal rate (about 90%) was achieved under slightly acidic conditions (pH 6). By filtration, the MPs more than 20 μm could be completely retained in sand, while the MPs <20 μm were likely passed through the sand [77].
The removal efficiency of MPs by traditional drinking water treatment processes varies in different DWTPs. For example, the removal efficiencies of MPs by CFS and filtration were only 54% and 76%, respectively, in two DWTPs located in Indonesia [78]. This difference could be explained based on the fact that different types and sizes of MPs have different affinity to flocculants. The aged MPs also increased the affinity to coagulants and flocculants because of the changed chemical properties and increased roughness [79]. Although the CFS process has been widely applied in the removal of MPs in DWTPs, the road ahead is still long and difficult to use CFS process to completely remove all sizes of MPs since there is a threshold for MPs size, that is, when the MPs size is about 10–20 μm, the removal efficiency of CFS process for MPs is lower [80].
2.3.2. Other removal technologies
In addition to widely used CFS technology, other processes also showed high MPs removal efficiency in DWTPs. Ultrafiltration/reverse osmosis (advanced treatment) exhibited more effective MPs removal than that of ozonation/carbon filtration stage (upgraded conventional treatment) [70]. Moreover, DWTPs with pulse cleaners achieved MPs removal efficiency of 85% in raw water [81]. Although different treatment processes of DWTPs can remove most MPs in raw water, MPs can still be detected in the pipelines of DWTPs and distribution system during transportation. Nylon and PVC were predominant in the waterways and pipe scale samples in all identified MPs [82].
MPs in drinking water are undoubtedly closely related to human health, therefore it is particularly important to ensure its efficient removal in raw water by DWTPs. Currently, increasing attention has been paid to the removal of MPs. The traditional CFS technology can achieve high removal efficiency of MPs. The substrates of coagulants, abundance and properties of MPs in raw water, and the CFS treatment process of different DWTPs would affect the removal efficiency of MPs. In comparison with other processes such as ultrafiltration and reverse osmosis for MPs removal in DWTPs, the CFS process is more widely used. The MPs removal technology and treatment efficiency in DWTPs are outlined in Table 4. Different treatment processes can be combined to ensure the efficient removal of MPs when necessary. Moreover, whether the treated drinking water would be polluted by MPs during the transportation process or not, the avoidance of MPs pollution in the pipeline are still a problem that needs to be further explored.
Table 4.
MPs removal in drinking water treatment plants.
| Removal technology | Water source | Types of MPs | Removal efficiency | Reference |
|---|---|---|---|---|
| Alum-based CFS | Grand River water (Ontario, Canada) and Lake Erie water (provided by the Lake Huron and Elgin Area Water Systems) | Fluoresbrite yellow-green (YG) carboxylated PS microspheres (3, 6, 25, 45, and 90 μm) | Approximately 60–100% | [73] |
| Alum coagulation | Tap water, ultrapure water | a) PE; b) rayon; c) polyester; d) Fluorescent red microspheres (1–5 μm diameter) | N/A | [74] |
| Ferric and aluminium Sulphate coagulation | Model MP-containing water (10 mg L−1 of PVC−MPs; alkalinity of 1 mmol L−1) | Pristine PVC−MPs (chlorine content of 57%, <50 μm) | Ferric sulphate: approximately 80% | [75] |
| aluminium sulphate: not exceeded approximately 80% | ||||
| Fe-based coagulants (PAM) coagulation | PE solution | PE (<0.5 mm, 0.5 < d < 1 mm, 1 < d < 2 mm, 2 < d < 5 mm) | PE (d < 0.5 mm) without PAM: 13.27 ± 2.19% | [76] |
| 89.23 ± 3.22%, 87.66 ± 1.89%, 85.21 ± 2.12%, 89.32 ± 3.96%, and 90.91 ± 1.01% with 3, 6, 9, 12, 15 mg L−1 anionic PAM, respectively | ||||
| Coagulation/sedimentation | Han River in Yangpyeong, Korea | PS microbeads with four different sizes (10, 20, 45, and 90 μm) and amidine PS microbeads (1 μm diameter) | 20, 45, and 90 μm MPs: 77.4–95.3%; 10 μm MPs: 33.0–41.1% | [77] |
| Sand filtration | 45 and 90 μm MPs: 100%; 10 μm MPs: 83.4%; 20 μm MPs: 98.8%; |
|||
| UV-based oxidation | N/A | |||
| Aeration, pre-sedimentation, coagulation, flocculation-sedimentation, filtration, disinfection | Surabaya River | MPs are separated from the raw water | The total MPs removal efficiencies in Sub-DWTPs I and II were 54 and 76%, respectively. | [78] |
| Coagulants and flocculants | Prairies River (Laval, Canada) | PE microspheres (10–20, 125–150 μm); PS microspheres (130–150 μm); PEST fibers (width: 12–16 μm, length: 105–1325 μm) | PE microspheres: 82%; PEST fibers: 99%; PS microspheres: 84% |
[79] |
| CFS | Lake Huron and the Great Lakes tributary | PE: 10–20 μm, 45–53 μm, and 106–125 μm; PS: 180 nm and 1.2 μm | 1 μm: < 0.1% | [80] |
| 10–20 μm: 1.8 ± 1.2% | ||||
| 45–53 μm: 0.3 ± 0.3% | ||||
| 106–125 μm: 1.4 ± 1.2% | ||||
| Granular filtration | 180 nm: 98.9 ± 0.7% | |||
| 1 μm: 94.9 ± 0.4% | ||||
| 10–20 μm: 86.9 ± 4.9% | ||||
| 45–54 μm: 97.0 ± 3.0% | ||||
| 106–125 μm: 99.9 ± 0.1% | ||||
| Sand filtration | Llobregat river (NE Spain) | MPs are separated from the raw water | 78 ± 9% | [70] |
| GAC filtration | 18 ± 46% | |||
| Reverse osmosis | 54 ± 27% | |||
| Finished water | Overall removal efficiency of 93 ± 5% | |||
| Pre-disinfection | Ganga river | MPs are separated from the raw water | 2.0% | [81] |
| Flocculation | 2.4% | |||
| Pulse clarification | 63% | |||
| Sand filtration | 85% | |||
| Finished water | 84.6% |
2.4. Wastewater treatment plants (WWTPs)
WWTPs is the main way for MPs to be transferred from wastewater to the natural aquatic environment [[83], [84], [85], [86]]. The sludge from WWTPs and the sludge-based fertilizer used in agriculture would also introduce a large number of MPs into the soil [[87], [88], [89], [90]]. Sludge absorbed MPs is an important source of MPs to the environment [91]. Therefore, it is essential to enrich and remove MPs from sewage in WWTPs.
The occurrence and removal technologies of MPs in WWTPs have been reviewed from different treatment processes including physical, chemical and biological treatment technologies [[92], [93], [94]]. Currently, the environmental pollution and ecological risk caused by MPs emissions in WWTPs have been highlighted, and research on removal technologies have emerged rapidly. This paper goes beyond previous reviews and mainly introduces the newly published removal technologies of MPs in WWTPs in terms of wastewater and sludge. A membrane bioreactor (MBR) is one of the most widely used secondary treatment technologies in WWTPs. Many studies have confirmed that MBR has a higher MPs removal efficiency relative to other technologies. In this review, we focused on the differences in MPs removal efficiency between MBR and other biological treatment technologies.
2.4.1. Removal of MPs in wastewater
The abundance and characteristics of MPs in different WWTPs are usually different, which is generally related to the regional and surrounding industrial properties [95]. The shape of MPs as well as the treatment processes of WWTPs affect the removal efficiency. In general, primary treatment and secondary treatment are the main processes to remove MPs. Moreover, the abundance and characteristics of MPs in the same WWTPs varies throughout the day. The abundance and characteristics of MPs might also be affected by different WWTPs [95], treatment stages [96] and influent periods [97]. The efficiency of MPs removal in WWTPs is mainly influenced by the applied treatment process, especially biological treatment (e.g., MBR) [92].
Different membrane technologies, such as MBR, usually has a higher removal efficiency [[98], [99], [100]], and widely used in WWTPs to remove MPs. MBR could achieve ∼99% of MPs removal efficiency [101], which was significantly higher than an oxidation ditch (OD) and conventional activated sludge (CAS) treatment. A higher MPs removal efficiency using MBR combined with rapid sand filtration (RSF) as a tertiary treatment technology [100] was accomplished (99.5% of influent MPs were removed in MBR system vs. 97% in OD system) [99]. Compared with CAS, the MBR process had a higher retention rate for MPs than the secondary CAS process [98]. Talvitie et al. investigated the removal of MPs from effluent in four different municipal WWTPs utilizing different advanced final-stage treatment technologies. The study included a MBR treating primary effluent and different tertiary treatment technologies treating secondary effluent (e.g., discfilter, RSF, and dissolved air flotation). The MBR removed 99.9% of MPs during treatment [102]. However, MPs can also change the performance of the MBR when removing MPs efficiently. PP−MPs in the range of 0.14–0.30 g L−1 could inhibit the microbial growth in a MBR, but PP−MPs accumulation in the range of 2.34–5.00 g L−1 improved the diversity and enrichment of the microbial community [103]. The MBR technology showed excellent MPs removal efficiency in wastewater treatment. However, membrane contamination is an inevitable challenge, which needs to be further investigated and solved.
Sedimentation technology was also utilized to remove MPs in wastewater. A study on the MPs removal by flotation vs. sedimentation processes showed that steady flow conditions of settling tanks favored MPs removal and sedimentation outperformed flotation in capturing MPs of all shapes and sizes. Despite this removal efficiency, small size fibers (<1 mm) were the most challenging MPs emissions from WWTPs [104]. The efficiency of different treatment processes for MPs in WWTPs were reviewed in this paper and summarized in Table 5.
Table 5.
MPs removal in wastewater treatment plants.
| Removal technology | MPs concentration in influent | MPs concentration in effluent | Removal efficiency | Reference |
|---|---|---|---|---|
| Primary treatment | 288.5 ± 32.8 n L−1 | 108.4 ± 15.2 n L−1 | 62.4% | [96] |
| Secondary treatment | 108.4 ± 15.2 n L−1 | 30.1 ± 8.2 n L−1 | 72.1% | |
| Tertiary treatment | 30.1 ± 8.2 n L−1 | 22.9 ± 7.2 n L−1 | 24.2% | |
| MBR | 4.40 ± 1.01 MP L−1 | 0.92 ± 0.21 MP L−1 | 79.01% | [100] |
| RSF | 1.08 ± 0.28 MP L−1 | 75.49% | ||
| MBR | 5.6 ± 0.09 mg L−1 | 0.028 ± 0.01 mg L−1 | 99.5% | [99] |
| OD | 0.168 ± 0.02 mg L−1 | 97% | ||
| CAS | 57.6 ± 12.4 MP L−1 | 1.0 ± 0.4 MP L−1 | 98.3% | [98] |
| MBR | 0.4 ± 0.1 MP L−1 | 99.4% | ||
| DF1 | 0.5 ± 0.2 MP L−1 | 0.3 ± 0.1 MP L−1 | 40.0% | [102] |
| DF2 | 2.0 ± 1.3 MP L−1 | 0.03 ± 0.01 MP L−1 | 98.5% | |
| RSF | 0.7 ± 0.1 MP L−1 | 0.02 ± 0.007 MP L−1 | 97.1% | |
| DAF | 2.0 ± 0.07 MP L−1 | 0.1 ± 0.04 MP L−1 | 95.0% | |
| MBR | 6.9 ± 1.0 MP L−1 | 0.005 ± 0.004 MP L−1 | 99.9% |
Research on MPs removal in WWTPs is still in its infancy, and there are no targeted MPs removal processes applied in real WWTPs. Although the current processes in WWTPs could removal most of the MPs via being adsorbed to sludge, the emissions of MPs would still be considerable an impact because of the high daily treatment volume. Most WWTPs are still on the way to develop new removal processes for MPs, but currently use traditional wastewater treatment processes to remove MPs. The traditional wastewater treatment methods usually have high removal efficiency for large size MPs, but there are still many smaller size MPs in the effluent. In order to improve the removal efficiency, the existing treatment processes could be advanced, such as adding a targeted filtration section process for small-size MPs before discharge, which can effectively reduce smaller MPs entering the natural water environment.
Among all the ways to prevent MPs from human exposure to the natural environment system, the wastewater treatment process is undoubtedly the most important point to reduce emissions. As the critical connection node, WWTPs are of great importance to control and avoid MPs emissions. It is of high research value to improve the removal efficiency of MPs by advancing traditional treatment processes, and thus reduce emissions. Most MPs intercepted by WWTPs are retained in activated sludge and discharged into subsequent sludge treatment process, which can be partially released into the environment and causes further pollution. Therefore, more attention should be paid to MPs in sludge and sludge treatment processes, and new targeted technologies for MPs removal, degradation or recycling should be further explored.
2.4.2. Removal of MPs in sludge
After different treatment processes in WWTPs, most MPs are adsorbed in the sludge. Approximately 160 million MPs were released into the aquatic environment and 3.4 billion MPs were accumulated into 30 tons of sludge daily by one WWTP in Italy [105]. Of all the MPs entering the WWTPs, 2% were discharged through wastewater effluent, only 4% were detected in the sludge of the biological treatment section, and about 94% of the other MPs might exist in the excess sludge, but not detected by the currently available analytical methods. Therefore, nearly 96% of the MPs are released into the environment and whereabouts are unknown [106]. Effective recycling or removal of MPs in sludge would facilitate further reducing MPs discharged into water and soil environments [107]. MPs in sludge can be removed by in situ degradation or by subsequent sludge treatment technologies.
Currently, the reported MPs removal methods in sludge are mostly based on the degradation of MPs by bacteria in activated sludge, but cannot be used as the mainstream technology because of the lower efficiency. For example, the bacteria strain isolated from activated sludge degraded 17% of PET−MPs of 2.63 g L−1, which was incubated at 30 °C under a pH 7–7.5 with a reactor residence time of 168 days [108]. Hyperthermophilic composting (hTC) technology was used for in situ degradation of MPs in sludge, hTC significantly enhanced biodegradation of sludge-based MPs and after 45 days of hTC treatment, 43.7% of the MPs were removed from the sewage sludge, which was the highest ever reported for MPs biodegradation [109].
The physical and chemical properties of sludge make it difficult to separate and remove MPs once trapped. Therefore, the removal methods of MPs in sludge needs to be further development. Since the removal of MPs in sludge is considerably challenging, there are a few relevant published studies, thus the development of MPs removal technologies in sludge is quite demanding but emerging. We suggest that the starting point could be investigating the effect of different sludge treatment technologies on MPs removal. Obviously, it is way better to prevent MPs pollution via exploring methods to separate most of the MPs before being trapped in the sludge.
2.5. Other aquatic related environments
According to previous published works, the MPs in the soil environment is 4–23 times than that of in marine systems [85,94,110]. Compared with the aquatic environment, the soil environment is prone to absorb MPs because of its complex components, including but not limited to, organic matter, sand, clay and silt. These factors make it difficult to enrich and remove MPs. Many studies demonstrated that plants could absorb MPs in soil through rhizomes. Growing evidence indicated that many terrestrial plants could potentially take up MPs/NPs via roots and translocate them to aboveground portions via the vascular system, primarily driven by the transpiration stream [111,112]. Subsequently, MPs adsorbed by plant roots are also easily absorbed by animals and enter the aquatic food network [113]. Similar to the feeding and adsorption of MPs by marine organisms, the adsorption of MPs by plant roots is not only difficult to completely remove or degrade, but also can be used as a carrier to make MPs enter the food web, and ultimately cause harm to human health.
Recent research about removing the MPs were mainly focused on various aquatic environments, drinking water treatment, and wastewater treatment scenarios. Some studies have found that there are also different shapes of MPs in marine sediments [114], but there were few studies on MPs removal from river and marine sediments. For MPs in marine sediments, they can be efficiently separated and removed by adsorption. This method uses the lipophilic characteristics of MPs and has the advantage of low cost [115]. The degree of MPs pollution in other natural aquatic ecosystems has also been widely concerned, some natural aquatic ecosystems also have the ability to remove MPs. Natural wetland systems were capable of removing 50% of surface water MPs [116]. As a nature-based wastewater treatment system, vertical-flow constructed wetlands (VFCWs) had the function of removing MPs with porous media, where MPs were distributed throughout the full height of gravel-filled VFCWs, and earthworms could transport MPs to the bottom of VFCWs and ingest them [117].
2.6. Comparison of microplastic removal technologies in different aquatic environments
MPs in different aquatic environments have different occurrence patterns, therefore different technologies applied to different scenarios. Many enrichment and removal technologies based on adsorption were applied in the natural water environment. Usually, materials available in the aquatic environment were used as raw materials for MPs adsorption to reduce the cost. The natural water and the marine system are both an open and broad area, where adsorption is more applicable than filtration. While in comparison with natural water and marine environments, DWTPs and WWTPs are both closed and controlled environments. MPs removal is easier than that of an open area, although more attention should be paid to thoroughly remove MPs in DWTPs and WWTPs. The traditional treatment methods were used in DWTPs and WWTPs, in which the CFS technology was the most widely used process in DWTPs, and the removal effect of different coagulants on MPs varied. According to previous studies, a MBR had better performance on MPs removal in WWTPs. Additionally, current research on MPs are focusing on sources, analytical methods, ecological risks of MPs in soil environment [110,118,119] and identification techniques and transportable ecological risks in atmosphere [[120], [121], [122]]. In general, it is difficult to recycle or extract MPs from the soil environment and atmosphere artificially because of environmental differences and complexities.
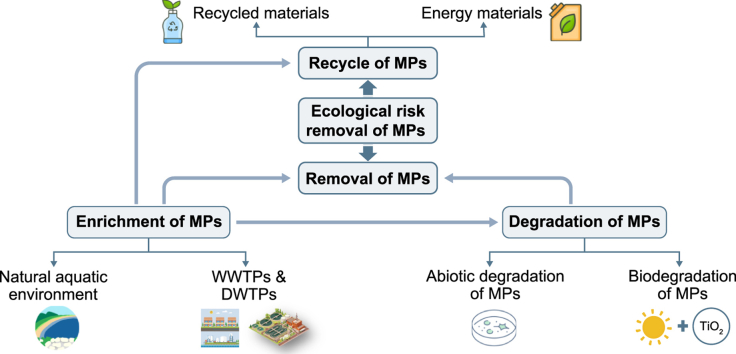
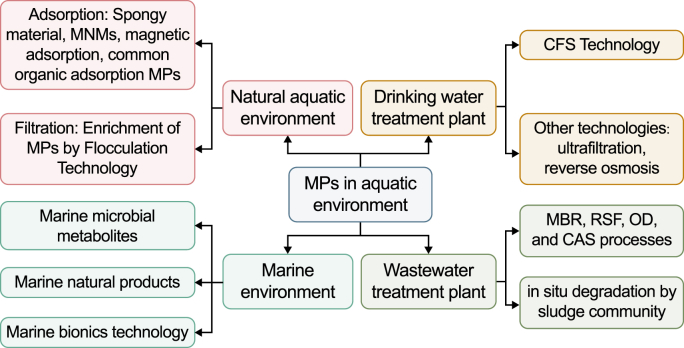
In summary, research on the removal of MPs in the aquatic environment is still in the early stages, and most of the results have not yet been really put into practice on a relatively large scale. In the future, various treatment technologies need to be developed, improved and applied to the environment. In particular, DWTPs and WWTPs are inclined to improve the preexisting traditional treatment processes, such as adding some treatment to enhance MPs removal. For WWTPs, the removal of MPs in sludge is of great importance. Fig. 1 summarized the removal techniques of MPs in different aquatic environments.
Fig. 1.
Removal technologies of microplastics in different aquatic environments.
3. MPs degradation
The degradation of MPs has been previously reviewed [4,123,124], mostly focusing on biodegradation [[125], [126], [127], [128]]. The degradation of MPs in this paper mainly focuses on the newly published work not summarized by other reviews yet, including abiotic and biotic degradation. Abiotic degradation of MPs refers to the degradation because of various abiotic factors such as ultraviolet radiation, temperature, air, water and mechanical force. Different abiotic degradation pathways include mechanical, thermal and chemical degradation [124,129]. Biodegradation is the process of depolymerization of MPs through microbial digestion until they are mineralized to carbon dioxide [124,130]. The summary of abiotic and biotic degradation technologies of MPs was reviewed and shown in Table 6, Table 7.
Table 6.
Abiotic degradation of MPs.
| Degradation technology | Degradation mechanism | Types of MPs | Degradation efficiency | Reference |
|---|---|---|---|---|
| Hydroxy-rich ultrathin BiOCl (BiOCl−X) degrades MPs | Photocatalytic degradation | 200–250 μm HDPE microspheres (PE−S), 2.38 mm Nylon-66 MPs, 3 mm POM microspheres, 2.6 mm white PP microspheres, 3 mm red PP microspheres, 5 mm black PP microspheres, 4 mm recycled HDPE |
PE-S mass loss 5.38% (BiOCl−1); PE−S mass loss 0.22% (BiOCl) | [139] |
| ZnO–Pt nanocomposite photocatalysts degrades MPs | Photocatalytic degradation | LDPE film | N/A | [140] |
| visible light photocatalysis of NPs using anodized CuxO | Visible-light photocatalytic degradation | 9 mg mL−1 PS−NPs solutions | The concentration of PS−NPs was reduced by 23% after 50 h | [141] |
| TiO2 nanoparticle film made with Triton X−100 | Photocatalytic degradation | 400 nm PS | Mineralization 98.40% of 400 nm PS in 12 h | [142] |
| Photocatalysis with TiO2–P25/β−SiC foams under UV-A radiation | Photocatalytic degradation | Three monodisperse suspensions of nanobeads:105 nm PMMA nanobeads; 140 nm PS nanobeads; 508 nm PS nanobeads | N/A | [143] |
| Poly(styrene-block-acrylic acid) containing TiO2 gel (PS−b−PAA/TiO2) polymer could provoke photocatalytic activity to PS particles in water | Photocatalytic degradation | PS containing a N–H type hindered amine light stabilizer (PS/LA-77) in water | The molecular weight decreases were from 10% to 11% | [144] |
| Green photocatalysis using a protein-based porous N–TiO2 semiconductor | Photocatalytic degradation | Extracted from a commercially available exfoliating scrub with diameters ranging 700–1000 μm | A total mass loss of 1.85% during the first 16 h of irradiation | [145] |
| Mesoporous N–TiO2 coating | Photocatalytic degradation | Primary HDPE and LDPE MPs of two sizes were obtained from two commercial facial scrubs of different brand | Mass Loss (%): HDPE_A: 0.22 ± 0.02; HDPE_B: 4.65 ± 0.35; (5 ± 0.01) mm × (5 ± 0.01) mm LDPE: 0.97 ± 0.32; (3 ± 0.01) mm × (3 ± 0.01) mm: 1.38 ± 0.13 | [137] |
| Electro-Fenton like (EF-like) technology based on TiO2/graphite (TiO2/C) cathode | Cathodic reduction dechlorination and hydroxyl radical (Oradical dotH) oxidation simultaneously | 100–200 μm PVC−MPs | Degrade PVC−MPs with 56 wt % removal after potentiostatic electrolysis at −0.7 V vs. Ag/AgCl at 100 °C for 6 h | [148] |
| Hydrothermal coupled Fenton system | Thermal fenton reaction | 1 g L−1 certain types of MPs (UHMWPE, LDPE, HDPE, PS, PVC, PP, or PET) dispersed in 150 mL of ultrapure water | 95.9% weight loss of MPs in 4–16 h | [149] |
| Functionalized carbon nanosprings (Mn@NCNTs/PMS) degrade MPs | The magnetic nanohybrids were applied for peroxymonosulfate activation to generate highly oxidizing radicals to decompose MPs under hydrothermal conditions | Extracted from facial cleanser paste | The Mn@NCNTs/PMS system can realize 50 wt % of MPs removal by assisting with hydrolysis | [150] |
| ZnO nanorod photocatalysts | Photocatalytic degradation | Fragmented LDPE MPs residues | N/A | [151] |
Table 7.
Biodegradation of MPs.
| MPs-degrading bacteria | Source | Degradation mechanism | Types of MPs | Degradation efficiency | Reference |
|---|---|---|---|---|---|
| Rhodococcus sp. and Bacillus sp. | Mangrove sediment | The two bacterial isolates possibly possessed the enzymatic components needed to degrade PP | Isotactic PP−MPs granules (white, spherical) with a density of 0.9 g ml−1 at 25 °C, molecular weight of 250,000 Mw, average Mn of 67,000 | The weight loss of PP after 40 days: Rhodococcus sp. 4.0% and Bacillus sp. 6.4% | [156] |
| B. cereus and B. gottheilii | Mangrove sediment | The bacterial isolates possess functional groups that can attach to the microplastic surfaces | PE powder (white/75 μm, 0.94 g mL−1), PP granules (white/spherical, 0.9 g mL−1), PS granules (white/spherical, 1.59 g mL−1), PET granules (granular/milky white, 1.68 g mL−1) | After 40 days, the percentage weight loss of PE, PET, and PS by B. cereus was 1.6%, 6.6%, and 7.4%, respectively; the percentage weight loss of PE, PET, PP, and PS by B. gottheilii was 6.6%, 3.0%, 3.6%, and 5.8%, respectively | [157] |
| Zalerion maritimum | Maritime coastal waters | Zalerion maritimum used PE-MPs as a nutrient source | PE−MPs (250–1000 μm) | The weight loss of PE−MPs in 14 days was 56.7 ± 2.9% | [158] |
| Pure bacterial strains, Bacillus licheniformis and Lysinibacillus massiliensis, and a mixed bacterial Culture of Delftia acidovorans and Bacillus sp. | Activated sludge and sediment | There was a release of additives from the surface of LDPE−MPs and PS−MPs and disruption of its structure |
LDPE, PS−MPs (300–500 μm) | N/A | [159] |
| Aspergillus flavus named PEDX3 | The guts of wax moth Galleria mellonella | Two LMCOs that isolated from Aspergillus flavus were considered as the potential PE-degrading enzymes after preliminary screen | LDPE with density of 0.921 g cm−3, HDPE with density of 0.955 g cm−3 (<200 μm) | The mass loss percentage (Δm/m0) was 3.9025 ± 1.18% after 28 days | [161] |
| Greater wax moth (Galleria mellonella) larvae | N/A | Through the styrene oxide–phenylacetaldehyde, and 4-methylphenol–4-hydroxybenzaldehyde–4-hydroxybenzoate metabolic pathways | PS microbead suspensions with and without red fluorescence labeling (25 μm, provided as mono-spheres suspended in distilled water at a concentration of 2.5% w/v) | 27%, 56%, 66%, and 80%, respectively after 3, 6, 12, and 18 h | [162] |
| Activated sludge and the compost cell suspension | Activated sludge | Hyperthermo-philic composting (hTC) technology | Extracted from activated sludge (<0.5 mm) | 43.7% of the MPs was removed from the sewage sludge after 45 d; the hTC in-oculum degraded 7.3% of the PS−MPs at 70 °C in 56 days in lab-scale | [109] |
| Bacterial communities in activated sludge | Activated sludge | Two bacterial strains within the consortium were isolated and identified as B. cereus SEHD031MH and A. mediolanus PNP3 demonstrated a great potential to degrade PET | Polycaprolactone diol (PCL) (Mn 2000) and PET−MPs (>40% crystallinity and inherent viscosity 0.80 dL g−1) (300–425 μm) | The consortium degraded 17% of PET and 34% of PCL (at 30 °C, pH 7–7.5, reactor residence time 168 days, and PET concentration of 2.63 g L−1) | [108] |
| Mixed microbial consortium | Landfill site | A mixed bacterial culture mainly consisting of Bacillus sp. and Paenibacillus sp. isolated from a landfill site could help accelerate PE−MPs degradation | PE−MPs granules (white and amorphous) with a density of 0.94 g mL−1 at 25 °C | The weight loss of PE microplastic was 14.7% after 60 d | [160] |
| Bacterial community on microplastics | Urban river sediments | The plastic-degrading bacteria were the crucial factor for the degradation of MPs and the deeper sediment conditions may promote the biodegradation of MPs | Extracted from urban river sediments (<1, 1–2, 2–3, 3–4, 4–5, and >5 mm) | N/A | [164] |
| Periphytic biofilm in various backgrounds of carbon sources (glucose, peptone, and glucose and peptone) | Xuan Wu Lake, Nanjing, to obtain a natural microbial entity of complex structure | Adding and/or changing a C-source changes the density and diversity of periphytic biofilms and influences the biodegradation of MPs by periphytic biofilms | PP, PE, PET−MPs (dimensions <1000 μm) | 9.52–18.02%, 5.95–14.02%, and 13.24–19.72% for PP, PE, and PET respectively, after 60 d | [165] |
| Human colonic microbiota | Human colonic | Gastrointestinal digestion and colonic fermentation | PET−MPs (160 ± 110 μm) | N/A | [167] |
3.1. Abiotic degradation
The spontaneous abiotic degradation of MPs in the natural environment includes mechanical degradation of MPs by various mechanical forces, which breaks large MPs into smaller MPs. In this process, the relative molecular weight of the polymer will not decrease, and the decrease in the relative molecular weight usually occurs in the chemical degradation process [123,131].
The commonly used plastic degradation methods are not suitable for MPs degradation due to its small size. Among various abiotic degradable methods for MPs degradation, photocatalytic degradation has been extensively investigated. Visible light photocatalysis was considered as an environmental protection process. It uses light irradiation to stimulate photocatalyst and generates a pair of electrons and holes in the redox reaction [[132], [133], [134]], in which organic pollutants could be degraded into water, CO2 and inorganic acids [135]. When the semiconductor, such as TiO2, is bombarded with photons with E ≥ Eg (band gap), electrons (e−) on the valance band are transferred into the conduction band, leaving behind positive holes (h+). Holes react with water or hydroxyl groups adsorbed on the surface of the semiconductor, generating hydroxyl radicals (•OH). Electrons react with adsorbed oxygen to form superoxide anion radicals (O2•−) [136]. Those ROS are powerful oxidizing agents that are capable of mineralizing organic pollutants adsorbed in the surface of the semiconductor into H2O and CO2 [137]. Two metal-based catalysts, ZnO and BiOCl, were currently used in the photocatalytic degradation of MPs [138]. The novel hydroxy-rich ultrathin BiOCl (BiOCl−X) surface hydroxyl could effectively enhance the generation of hydroxyl radicals, thereby improving the photocatalytic degradation efficiency of MPs [139]. Fragmented MPs, particularly low-density polyethylene (LDPE) film, in water could be degraded through visible light-induced plasmonic photocatalysts comprising of platinum nanoparticles deposited on zinc oxide (ZnO) nanorods (ZnO–Pt) [140]. Different p-type copper oxide film semiconductors were synthesized in anode, with photocatalytic activity under visible-light for the degradation of PS−NPs [141].
Ti or TiO2 were also widely used in the preparation of MPs photodegradation catalysts. TiO2 nanoparticle films made with Triton X−100 showed complete mineralization (98.40%) of 400 nm PS in 12 h [142]. The degradation of poly(methyl methacrylate) (PMMA) and PS nanoparticles by photocatalysis with TiO2–P25/β−SiC foams under UV-A radiation were tested and up to 50% of total organic carbon (TOC) conversion after 7 h were achieved [143]. A poly(styrene-block-acrylic acid) containing TiO2 gel (PS−b−PAA/TiO2) polymer photocatalyst had the same density as PS and could provoke photocatalytic activity to PS particles in water [144]. Two semiconductors based on N–TiO2 were synthesized by extrapallial fluid of saltwater mussels and a conventional less sustainable sol-gel route, respectively, and the photodegradation of HDPE−MPs extracted from commercial facial grinding paste was successfully realized [145]. The degradation of HDPE and LDPE−MPs by mesoporous N–TiO2 coating under visible light irradiation could be affected by the shape and size of the MPs. The degradation rate of the MPs with a smaller size was higher than that of MPs with thin film shape, which could be related to the light intensity and oxygen-containing reaction medium [137]. Furthermore, it has also been confirmed that environmental factors such as UV, temperature, pH and humidity can also affect the degradation of MPs. Low temperatures (0 °C) cause fragmentation of MPs, thereby increasing their surface area and interaction with N–TiO2. Low pH introduces H+ into the system, contributing to accelerated interaction between TiO2 nanoparticles and MPs [146,147].
In addition, an electro-Fenton like (EF-like) technology based on a TiO2/graphite (TiO2/C) cathode was used to degrade typical PVC−MPs in water, and it exhibited a remarkable performance on PVC degradation via cathodic reduction dichlorination and hydroxyl radical oxidation simultaneously [148]. A hydrothermal coupled Fenton system was developed for the decomposition of ultrahigh-molecular-weight polyethylene, achieving a 95.9% weight loss in 16 h and 75.6% mineralization efficiency in 12 h. The high effectiveness was attributed to the synergy of hydrothermal hydrolysis, proton-rich environment, and massive production of hydroxyl radicals [149].
Helical carbon nanotubes were engineered with high-level nitrogen dopants and encapsulated metal nanoparticles and applied for peroxymonosulfate activation to generate highly oxidizing radicals to decompose MPs under hydrothermal conditions [150]. In addition, heterogeneous ZnO photocatalyst excited by visible light could also degrade MPs fragments and LDPE films in water [151].
3.2. Biotic degradation
MPs are cleaved into smaller molecules, such as monomers, dimers, or oligomers, by the action of extracellular enzymes produced by microbes, and these smaller molecules pass through the cell membrane to be used by bacteria as a source of energy or carbon [152]. Therefore, the environmental conditions, such as temperature and pH, can impact the biodegradation of MPs by influencing the growth and metabolic processes of microbes. Furthermore, aged MPs are more easily degraded by microbes [146,[153], [154], [155]]. This paper has briefly introduced the research progress of biodegradation of MPs by microbial strains or community, and reviews the biodegradation of MPs by strains or community isolated from sediment environment, marine environment and biological intestinal tract during the last three years. We mainly introduced the degradation effects of MPs by microbes in various environments from the perspective of strains or community in different environments.
Many studies have documented isolated bacteria or fungi capable of degrading MPs isolated from the environment. For example, Rhodococcus sp. and Bacillus sp. isolated from mangrove sediments had the ability to degrade PP−MPs [156]; Bacillus cereus and Bacillus gottheilii isolated from a mangrove sediment system in Malaysia Peninsula could grow and degrade MPs on synthetic medium containing PE, PET and PS−MPs as the sole carbon sources. Both the these two strains showed the potential to repair MPs pollution [157]. Zalerion maritimum, a fungus isolated from the marine environment by Paço et al., was cultured in PE−MPs medium and showed PE biodegradation potential [158]. LDPE−MPs and PS−MPs were reported to be degraded by Bacillus licheniformis, Lysinibacillus massiliensis, and mixed cultures of Delftia acidovorans and Bacillus sp. [159]. Hyperthermophilic bacteria in hTC accelerated PS−MPs biodegradation through excellent bio-oxidation performance of Thermus, Bacillus, and Geobacillus, which were the dominant bacteria responsible for the highly efficient biodegradation [109]. Bacillus cereus SEHD031MH and Agromyces mediolanus PNP3 were isolated from the activated sludge and both of them thrived with PET as the sole carbon source [108]. A mixed bacterial culture mainly consisting of Bacillus sp. and Paenibacillus sp. isolated from a landfill site could also contribute accelerating PE−MPs degradation [160].
Biodegradation of plastic polymers by insects, such as wax moth (Galleria mellonella), might be a solution to reduce plastic pollution. A PE-degrading fungus Aspergillus flavus named PEDX3, which was isolated from the gut contents of G. mellonella, has two laccase-like multicopper oxidases (LMCOs) genes, AFLA_006190 and AFLA_053930 with up-regulated expression during the degradation process, which might contribute to PE-degradation [161]. Two potential metabolic pathways of PS in the intestine of G. mellonella larvae was discovered, including the styrene oxide–phenylacetaldehyde and 4-methylphenol–4-hydroxybenzaldehyde–4-hydroxybenzoate pathways [162]. Furthermore, a new enzyme FAST-PETase was discovered by using artificial intelligence, showed great potential to promote PET plastics degradation. It has been proved that untreated postconsumer-PET from 51 different thermoformed products could all be almost completely degraded by FAST-PETase in one week [163].
The biofilm attached to the surface of MPs also plays a significant role in MPs degradation. Take MPs in sediments for example, according to the homologous theory, bacterial genera containing plastic-degrading bacteria isolated from other literatures were defined as potential plastic-degrading bacteria genera and bacterial community on MPs has less stability than that of in sediments. Compared with sediments, more potential plastic degrading bacteria were colonized in deeper layers [164]. The addition of other carbon sources could affect the bacterial community structure of the biofilm during the degradation of MPs by biofilm attached to MPs, which might lead to the change of biodegradation ability [165]. In the marine environment, marine microbes have adapted to plastic as a surface for colonization, when comparing the taxonomic patterns of plastic-associated marine bacteria, recurring groups and families included Erythrobacteraceae, and Rhodobacteraceae (Alphaproteobacteria), Flavobacteriaceae (Bacteriodetes), and the phylum of cyanobacteria (such as the Phormidium genus). A new viewpoint proposed that some indicator bacteria could attach to the surface of marine MPs and played a role in degradation [166]. A recent study on the effect of MPs on human enteric microbial community showed that PET−MPs could change its structure, and PET−MPs also showed aging evidence, potentially because of the gastrointestinal digestion and enteric fermentation [167].
Biodegradation is a highly desirable and environmentally friendly way to remove MPs. Firstly, it allows for complete mineralization. In addition, some microbes can convert MPs into other valuable byproducts. However, MPs biodegradation also have obvious limitations in degradation efficiency in natural conditions. Nevertheless, the MPs biodegradation is promising, and how to improve the MPs biodegradation efficiency is an issue of great importance that deserves more attention. Depolymerization by physical-chemical degradation techniques followed by possible complete mineralization of MPs through biodegradation are highly recommended to obtain more efficient MPs removal.
3.3. Existing problems and possible breakthroughs
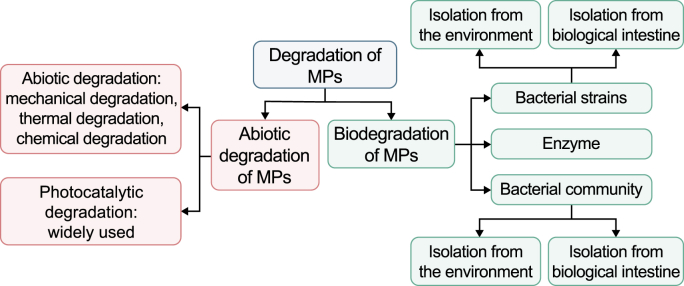
Due to the difficulty in degradation and the large amount of plastic used, the MPs produced during aging process easily accumulate in the environment over time. Among various MPs removal technologies, MPs mineralization are the technologies that can completely eliminate MPs. We suggest that abiotic degradation technology, such as physical and chemical degradation processes, could be combined with biodegradation technologies to achieve a higher efficiency or complete degradation. The pretreatment of MPs by abiotic degradable technology reduces the particle size and molecular weight of MPs, and increases the surface roughness of MPs. In the subsequent biodegradation stage, plastic degrading bacteria are more likely to attach to the surface of MPs to increase its degradation efficiency. Finally, MPs are completely mineralized to CO2 by plastic degrading bacteria using MPs as a carbon source through metabolism. In comparison with abiotic degradable technology, the biodegradation of MPs is greener, and requires less additional energy, which is in line with the current promotion of sustainable development under low carbon constraints. However, compared with abiotic degradable technologies, such as photocatalytic degradation, the degradation efficiency of MPs by plastic degrading bacteria is much lower. Moreover, most of the abiotic degradable technologies and biodegradation of MPs are still in the laboratory stage. Further research and technical improvements are still needed for the field applications. In the future, further separation of plastic degrading bacteria/communities in the environment should be used to explore its degradation mechanisms, to develop efficient degradation enzymes, to improve the biodegradation efficiency, to advance MPs degradation technologies, and to increase the possibility of practical environmental applications. Meanwhile, biodegradable plastic production would also be highlighted and investigated in. We can pay more attention to the efficient degradation and mineralization of MPs, and linking the enrichment and degradation of MPs to develop a technical process to remove the ecological risks of MPs. The abiotic degradable and biodegradable methods of MPs are summarized in Fig. 2.
Fig. 2.
Abiotic degradation and biodegradation methods of microplastics.
4. Recycling of MPs
Many studies have focused on the resource utilization and recycling of plastic wastes [[168], [169], [170]], but the research on MPs recycling methods is still relatively scarce. According to statistics, 19–23 million tons (11%) of plastic waste generated globally in 2016 entered the aquatic ecosystem. By 2030, the global annual emissions of plastics may reach 53 million tons per year, of which more than 10 million tons of plastics would enter the global ocean every year. About 13.5% of the global marine plastics exist in the form of MPs [1,[171], [172], [173], [174]]. Compared with plastic waste, MPs in aquatic environment have smaller size and potentially higher ecological toxicity and risks, and should be removed or recycled. MPs can be recycled as materials, mixed with other materials and reused, or degraded to produce new energy sources.
Although there have been some studies on the recycling of plastic waste, the recycling of MPs is still in the initial stage and needs further research. A brand-new sustainable material, an eco-friendly foam made of waste MPs was incorporated into a bio-matrix and this novel open-cell material can be used as acoustic and thermal insulation for industrial, civil and maritime applications [175]. In another study, small plastic fragments on the northeast Brazilian coast were collected and recycled to prepare the recycled materials [176]. A Ni–Pd/TNPs nanocatalyst was prepared, which could be used for catalytic cracking MPs-phenol and steam reforming reaction to generate valuable liquid products and hydrogen fuel [177]. A new study reported an electrocatalytic upcycling strategy for PET waste to produce valuable H2, terephthalic acid (PTA), and potassium diformate (KDF) [178].
Most of the research on MPs recycling was mainly focused on the recycling technology and degradation method of MPs in the environment. However, there were few studies on the recycling and reuse of MPs after recovery. Although MPs recycling is a means of eliminating MPs ecological risks, it is also expected to takes into account the economic benefits between recycling costs and utilization of MPs.
5. Conclusions and future perspectives
Plastic products have undoubtedly brought great convenience to human society since invented, and the environmental pollution and ecological risks behind them have become increasingly noticeable. MPs, which are broken from plastic waste in the environment, have spread all over the world. Because of their difficult degradation, recycling challenges, large specific surface area, and the ability of adsorbing other pollutants, they can easily enter the food chain and other environments, which cause more ecological risks than plastics. One of the best ways to remove the ecological risks caused by MPs is to enrich them from the environment and recycle them, or the complete degradation and mineralization.
Many research have studied on the enrichment and removal of MPs in aquatic environment, such as the preparation of materials with adsorption by using various natural products of animals and plants to achieve efficient adsorption of MPs in water. DWTPs and WWTPs are important ways for MPs to be transported to humans. They both play an important role in controlling MPs pollution. The main removal method of MPs by DWTPs is CFS technology, which can remove most MPs in raw water. The traditional treatment technology of WWTPs can also remove most of the MPs, but the number of MPs entering the aquatic environment through WWTPs is still very considerable due to the large amount of effluent from WWTPs. In general, the MBR technology has the highest removal efficiency of MPs relative to different secondary treatment technologies used in wastewater treatment processes. Therefore, it is necessary to develop more innovative treatment technologies for DWTPs and WWTPs in order to improve the removal efficiency of MPs.
Photocatalytic degradation in abiotic degradation of MPs is a widely studied degradation technology, which has relatively high catalytic efficiency. Photocatalytic degradation and mineralization of MPs have the advantages of low energy consumption. The biodegradation of MPs was mainly focused on the degradation of MPs by plastic degrading bacteria or microbial community isolated from the environment. However, most of the biodegradation of MPs is still in the laboratory research stage. In the future, abiotic degradation technology as a pretreatment and biodegradation technology as subsequent mineralization can be combined to achieve complete degradation in the field.
The previous work on MPs has been involved in many aspects globally. The removal technologies of MPs in the environment have become a research hotspot, but still needs further research in order to achieve real application in the field. With the emergence and maturity of new technologies, MPs may become a new type of waste energy and attribute to sustainable development. With regard to MPs removal, more research and technologies are required in soil, atmosphere, and various sediments. DWTPs and WWTPs are of paramount importance in MPs removal as the key point to connect urban and social water cycling. Similarly, MPs removal in sludge should be highlighted to prevent it from entering into the soil environment. In addition, more research could be conducted on how to recycle and reuse MPs in the environment under the low carbon constraints. Plastic production processes should also be improved to make plastics and the byproducts more environmentally friendly. It is also important to increase the awareness of the benefits of living a plastic-free life. Depolymerization by physical-chemical degradation techniques followed by possible complete mineralization of MPs through biodegradation are highly recommended to obtain more efficient MPs removal.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 52070060 and No.52230004), Shenzhen Overseas High-level Talents Research Startup Program (No. 20200518750C), Shenzhen Science and Technology Program (Grant No. KQTD20190929172630447), and State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. 2021TS29), and Open Project of Key Laboratory of Environmental Biotechnology, CAS (Grant No KF2021006).
Contributor Information
Shu-Hong Gao, Email: gaoshuhong@hit.edu.cn.
Ai-Jie Wang, Email: waj0578@hit.edu.cn.
References
- 1.Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3(7) doi: 10.1126/SCIADV.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade H., Glüge J., Herzke D., Ashta N.M., Nayagar S.M., Scheringer M. Oceanic long-range transport of organic additives present in plastic products: an overview. Environ. Sci. Eur. 2021;33(1) doi: 10.1186/S12302-021-00522-X. [DOI] [Google Scholar]
- 3.Barra R., Leonard S.A. 54th Global Environment Facility Council Meeting, Da Nang, Viet Nam. June 24-26. 2018. Plastics and the circular economy. [Google Scholar]
- 4.Ali S.S., Elsamahy T., Al-Tohamy R., et al. Plastic wastes biodegradation: mechanisms, challenges and future prospects. Sci. Total Environ. 2021:780. doi: 10.1016/J.SCITOTENV.2021.146590. [DOI] [PubMed] [Google Scholar]
- 5.Association for the Advancement of Science A . 2017. The Future of Plastics Recycling. Published online. [DOI] [Google Scholar]
- 6.Ru J., Huo Y., Yang Y. Microbial degradation and valorization of plastic wastes. Front. Microbiol. 2020:442. doi: 10.3389/FMICB.2020.00442. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z., Lü F., Zhang H., et al. Is incineration the terminator of plastics and microplastics? J. Hazard Mater. 2021;401 doi: 10.1016/J.JHAZMAT.2020.123429. [DOI] [PubMed] [Google Scholar]
- 8.Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62(8):1596–1605. doi: 10.1016/J.MARPOLBUL.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Briassoulis D., Pikasi A., Briassoulis C., Mistriotis A. Disintegration behaviour of bio-based plastics in coastal zone marine environments: a field experiment under natural conditions. Sci. Total Environ. 2019;688:208–223. doi: 10.1016/j.scitotenv.2019.06.129. [DOI] [PubMed] [Google Scholar]
- 10.Matjašič T., Simčič T., Medvešček N., Bajt O., Dreo T., Mori N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021;752 doi: 10.1016/J.SCITOTENV.2020.141959. [DOI] [PubMed] [Google Scholar]
- 11.Shah A.A., Hasan F., Hameed A., Ahmed S. Biological degradation of plastics: a comprehensive review. Biotechnol. Adv. 2008;26(3):246–265. doi: 10.1016/J.BIOTECHADV.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Baztan J., Carrasco A., Chouinard O., et al. Protected areas in the Atlantic facing the hazards of micro-plastic pollution: first diagnosis of three islands in the Canary Current. Mar. Pollut. Bull. 2014;80(1–2):302–311. doi: 10.1016/J.MARPOLBUL.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor I.A., Golsteijn L., Hendriks A.J. Review of the partitioning of chemicals into different plastics: consequences for the risk assessment of marine plastic debris. Mar. Pollut. Bull. 2016;113(1–2):17–24. doi: 10.1016/J.MARPOLBUL.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Hermabessiere L., Dehaut A., Paul-Pont I., et al. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–793. doi: 10.1016/J.CHEMOSPHERE.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 15.Raddadi N., Fava F. Biodegradation of oil-based plastics in the environment: existing knowledge and needs of research and innovation. Sci. Total Environ. 2019;679:148–158. doi: 10.1016/J.SCITOTENV.2019.04.419. [DOI] [PubMed] [Google Scholar]
- 16.Rogers K.L., Carreres-Calabuig J.A., Gorokhova E., Posth N.R. Micro-by-micro interactions: how microorganisms influence the fate of marine microplastics. Limnol Oceanogr Lett. 2020;5(1):18–36. doi: 10.1002/LOL2.10136. [DOI] [Google Scholar]
- 17.Sánchez C. Fungal potential for the degradation of petroleum-based polymers: an overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020;40 doi: 10.1016/J.BIOTECHADV.2019.107501. [DOI] [PubMed] [Google Scholar]
- 18.Priya A., Dutta K., Daverey A. A comprehensive biotechnological and molecular insight into plastic degradation by microbial community. J. Chem. Technol. Biotechnol. 2022;97(2):381–390. doi: 10.1002/JCTB.6675. [DOI] [Google Scholar]
- 19.Qin Z.H., Mou J.H., Chao C.Y.H., et al. Biotechnology of plastic waste degradation, recycling, and valorization: current advances and future perspectives. ChemSusChem. 2021;14(19):3981. doi: 10.1002/CSSC.202101825. 3981. [DOI] [PubMed] [Google Scholar]
- 20.Browne M.A., Crump P., Niven S.J., et al. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 2011;45(21):9175–9179. doi: 10.1021/ES201811S/ASSET/IMAGES/MEDIUM/ES-2011-01811S_0003.GIF. [DOI] [PubMed] [Google Scholar]
- 21.Cole M., Lindeque P., Halsband C., Galloway T.S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011;62(12):2588–2597. doi: 10.1016/J.MARPOLBUL.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Castañeda R.A., Avlijas S., Simard M.A., Ricciardi A. Microplastic pollution in st. Lawrence river sediments. Can J Fish Aquat Sci. 2014 doi: 10.1139/cjfas-2014-0281. Published online. [DOI] [Google Scholar]
- 23.McCormick A., Hoellein T.J., Mason S.A., Schluep J., Kelly J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014;48(20):11863–11871. doi: 10.1021/ES503610R. [DOI] [PubMed] [Google Scholar]
- 24.Mccormick A.R., Hoellein T.J., London M.G., et al. Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere. 2016;7(11) doi: 10.1002/ecs2.1556. [DOI] [Google Scholar]
- 25.Galgani F., Hanke G., Maes T. Global distribution, composition and abundance of marine litter. Mar Anthropog Litter. 2015:29–56. doi: 10.1007/978-3-319-16510-3_2. Published online January 1. [DOI] [Google Scholar]
- 26.Carroll Chris, Sousa João T.F. International Union for Conservation of Nature; 2015. Plastic Debris in the Ocean: the Characterization of Marine Plastics and Their Environmental Impacts, Situation Analysis Report. [DOI] [Google Scholar]
- 27.Sun C., Wang Z., Chen L., Li F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020;393 doi: 10.1016/J.CEJ.2020.124796. [DOI] [Google Scholar]
- 28.Risch P., Adlhart C. A chitosan nanofiber sponge for oyster-inspired filtration of microplastics. ACS Appl Polym Mater. 2021;2021:4685–4694. doi: 10.1021/ACSAPM.1C00799/SUPPL_FILE/AP1C00799_SI_001.PDF. [DOI] [Google Scholar]
- 29.Wang Z., Sun C., Li F., Chen L. Fatigue resistance, re-useable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chem. Eng. J. 2021;415 doi: 10.1016/J.CEJ.2021.129006. [DOI] [Google Scholar]
- 30.Sun C., Wang Z., Zheng H., Chen L., Li F. Biodegradable and re-useable sponge materials made from chitin for efficient removal of microplastics. J. Hazard Mater. 2021;420 doi: 10.1016/J.JHAZMAT.2021.126599. [DOI] [PubMed] [Google Scholar]
- 31.Yuan F., Yue L., Zhao H., Wu H. Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide. Water Sci. Technol. 2020;81(10):2163–2175. doi: 10.2166/WST.2020.269. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Sun C., Huang Q.X., Chi Y., Yan J.H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard Mater. 2021;419 doi: 10.1016/J.JHAZMAT.2021.126486. [DOI] [PubMed] [Google Scholar]
- 33.Vale R.D., Milligan R.A. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288(5463):88. doi: 10.1126/science.288.5463.88. (80-) [DOI] [PubMed] [Google Scholar]
- 34.Safdar M., Khan S.U., Jänis J. Progress toward catalytic micro- and nanomotors for biomedical and environmental applications. Adv. Mater. 2018;30(24):1–27. doi: 10.1002/adma.201703660. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Villa K. Self-propelled micro/nanomotors for removal of insoluble water contaminants: microplastics and oil spills. Environ Sci Nano. 2021;8(12):3440–3451. doi: 10.1039/D1EN00663K. [DOI] [Google Scholar]
- 36.Wang L., Kaeppler A., Fischer D., Simmchen J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl. Mater. Interfaces. 2019;11(36):32937–32944. doi: 10.1021/ACSAMI.9B06128. [DOI] [PubMed] [Google Scholar]
- 37.Ye H., Wang Y., Liu X., et al. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021;588:510–521. doi: 10.1016/J.JCIS.2020.12.097. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y.-J., Chen Y., Miao C., et al. Metal–organic framework-based foams for efficient microplastics removal. J. Mater. Chem. 2020;8(29):14644–14652. doi: 10.1039/D0TA04891G. [DOI] [Google Scholar]
- 39.Tiwari E., Singh N., Khandelwal N., Monikh F.A., Darbha G.K. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J. Hazard Mater. 2020;397 doi: 10.1016/J.JHAZMAT.2020.122769. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez Arenas L., Ramseier Gentile S., Zimmermann S., Stoll S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021;791 doi: 10.1016/J.SCITOTENV.2021.148175. [DOI] [PubMed] [Google Scholar]
- 41.Yen P.L., Hsu C.H., Huang M.L., Liao V.H.C. Removal of nano-sized polystyrene plastic from aqueous solutions using untreated coffee grounds. Chemosphere. 2022;286 doi: 10.1016/J.CHEMOSPHERE.2021.131863. [DOI] [PubMed] [Google Scholar]
- 42.Grbic J., Nguyen B., Guo E., You J.B., Sinton D., Rochman C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019;6(2):68–72. doi: 10.1021/ACS.ESTLETT.8B00671. [DOI] [Google Scholar]
- 43.Tang Y., Zhang S., Su Y., Wu D., Zhao Y., Xie B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021;406 doi: 10.1016/J.CEJ.2020.126804. [DOI] [Google Scholar]
- 44.Sun M., Chen W., Fan X., Tian C., Sun L., Xie H. Cooperative recyclable magnetic microsubmarines for oil and microplastics removal from water. Appl. Mater. Today. 2020;20 doi: 10.1016/J.APMT.2020.100682. [DOI] [Google Scholar]
- 45.Villa K., Děkanovský L., Plutnar J., Kosina J., Pumera M. Swarming of perovskite-like Bi2WO6 microrobots destroy textile fibers under visible light. Adv. Funct. Mater. 2020;30(51) doi: 10.1002/ADFM.202007073. [DOI] [Google Scholar]
- 46.Wu J., Jiang R., Lin W., Ouyang G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2019;245:836–843. doi: 10.1016/J.ENVPOL.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 47.Kuoppamäki K., Pflugmacher Lima S., Scopetani C., Setälä H. The ability of selected filter materials in removing nutrients, metals, and microplastics from stormwater in biofilter structures. J. Environ. Qual. 2021;50(2):465–475. doi: 10.1002/JEQ2.20201. [DOI] [PubMed] [Google Scholar]
- 48.Yogarathinam L.T., Usman J., Othman M.H.D., et al. Low-cost silica based ceramic supported thin film composite hollow fiber membrane from Guinea corn husk ash for efficient removal of microplastic from aqueous solution. J. Hazard Mater. 2022;424 doi: 10.1016/J.JHAZMAT.2021.127298. [DOI] [PubMed] [Google Scholar]
- 49.Shen M., Zhang Y., Almatrafi E., et al. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022;428 doi: 10.1016/J.CEJ.2021.131161. [DOI] [Google Scholar]
- 50.Chen Z., Wei W., Liu X., Ni B.-J. Emerging electrochemical techniques for identifying and removing micro/nanoplastics in urban waters. Water Res. 2022;221 doi: 10.1016/J.WATRES.2022.118846. [DOI] [PubMed] [Google Scholar]
- 51.Peydayesh M., Suta T., Usuelli M., et al. Sustainable removal of microplastics and natural organic matter from water by coagulation-flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021;55(13):8848–8858. doi: 10.1021/ACS.EST.1C01918/SUPPL_FILE/ES1C01918_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 52.Chazovachii P.T., Rieland J.M., Sheffey V.V., et al. Using adhesives to capture microplastics from water. ACS ES&T Eng. 2021;1(12):1698–1704. doi: 10.1021/ACSESTENGG.1C00272. [DOI] [Google Scholar]
- 53.Wang P., Huang Z., Chen S., et al. Sustainable removal of nano/microplastics in water by solar energy. Chem. Eng. J. 2022;428 doi: 10.1016/J.CEJ.2021.131196. [DOI] [Google Scholar]
- 54.Perren W., Wojtasik A., Cai Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega. 2018;3(3):3357–3364. doi: 10.1021/ACSOMEGA.7B02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin C., Corona E., Mahadik G.A., Duarte C.M. Adhesion to coral surface as a potential sink for marine microplastics. Environ. Pollut. 2019;255 doi: 10.1016/J.ENVPOL.2019.113281. [DOI] [PubMed] [Google Scholar]
- 56.Corona E., Martin C., Marasco R., Duarte C.M. Passive and active removal of marine microplastics by a mushroom coral (danafungia scruposa) Front. Mar. Sci. 2020:128. doi: 10.3389/FMARS.2020.00128. 0. [DOI] [Google Scholar]
- 57.Arossa S., Martin C., Rossbach S., Duarte C.M. Microplastic removal by Red Sea giant clam (Tridacna maxima) Environ. Pollut. 2019;252:1257–1266. doi: 10.1016/J.ENVPOL.2019.05.149. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein M.C., Goodwin D.S. Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the north Pacific subtropical gyre. PeerJ. 2013;2013(1):1–17. doi: 10.7717/peerj.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rius-Ayra O., Llorca-Isern N. A robust and anticorrosion non-fluorinated superhydrophobic aluminium surface for microplastic removal. Sci. Total Environ. 2021;760 doi: 10.1016/J.SCITOTENV.2020.144090. [DOI] [PubMed] [Google Scholar]
- 60.Chen C.K., Zhang J., Bhingarde A., et al. A portable purification system for the rapid removal of microplastics from environmental samples. Chem. Eng. J. 2022;428 doi: 10.1016/J.CEJ.2021.132614. [DOI] [Google Scholar]
- 61.Cunha C., Faria M., Nogueira N., Ferreira A., Cordeiro N. Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution. Environ. Pollut. 2019;249:372–380. doi: 10.1016/J.ENVPOL.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 62.Cunha C., Silva L., Paulo J., Faria M., Nogueira N., Cordeiro N. Microalgal-based biopolymer for nano- and microplastic removal: a possible biosolution for wastewater treatment. Environ. Pollut. 2020;263 doi: 10.1016/J.ENVPOL.2020.114385. [DOI] [PubMed] [Google Scholar]
- 63.Liu S.Y., Leung M.M.L., Fang J.K.H., Chua S.L. Engineering a microbial ‘trap and release’ mechanism for microplastics removal. Chem. Eng. J. 2021;404 doi: 10.1016/J.CEJ.2020.127079. [DOI] [Google Scholar]
- 64.Zhou H., Mayorga-Martinez C.C., Pumera M. Microplastic removal and degradation by mussel-inspired adhesive magnetic/enzymatic microrobots. Small Methods. 2021;5(9) doi: 10.1002/SMTD.202100230. [DOI] [PubMed] [Google Scholar]
- 65.Lengar Ž., Klun K., Dogsa I., Rotter A., Stopar D. Sequestration of polystyrene microplastics by jellyfish mucus. Front. Mar. Sci. 2021;8:854. doi: 10.3389/FMARS.2021.690749/BIBTEX. [DOI] [Google Scholar]
- 66.Pivokonsky M., Cermakova L., Novotna K., Peer P., Cajthaml T., Janda V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018;643:1644–1651. doi: 10.1016/J.SCITOTENV.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 67.Mintenig S.M., Löder M.G.J., Primpke S., Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019;648:631–635. doi: 10.1016/J.SCITOTENV.2018.08.178. [DOI] [PubMed] [Google Scholar]
- 68.Novotna K., Cermakova L., Pivokonska L., Cajthaml T., Pivokonsky M. Microplastics in drinking water treatment – current knowledge and research needs. Sci. Total Environ. 2019;667:730–740. doi: 10.1016/J.SCITOTENV.2019.02.431. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z., Lin T., Chen W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP) Sci. Total Environ. 2020;700 doi: 10.1016/J.SCITOTENV.2019.134520. [DOI] [PubMed] [Google Scholar]
- 70.Dalmau-Soler J., Ballesteros-Cano R., Boleda M.R., Paraira M., Ferrer N., Lacorte S. Microplastics from headwaters to tap water: occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain) Environ. Sci. Pollut. Res. 2021;28(42):59462–59472. doi: 10.1007/S11356-021-13220-1/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 71.Pivokonský M., Pivokonská L., Novotná K., Čermáková L., Klimtová M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020;741 doi: 10.1016/J.SCITOTENV.2020.140236. [DOI] [PubMed] [Google Scholar]
- 72.Siegel H., Fischer F., Lenz R., Fischer D., Jekel M., Labrenz M. Identification and quantification of microplastic particles in drinking water treatment sludge as an integrative approach to determine microplastic abundance in a freshwater river. Environ. Pollut. 2021;286 doi: 10.1016/J.ENVPOL.2021.117524. [DOI] [PubMed] [Google Scholar]
- 73.Xue J., Peldszus S., Van Dyke M.I., Huck P.M. Removal of polystyrene microplastic spheres by alum-based coagulation-flocculation-sedimentation (CFS) treatment of surface waters. Chem. Eng. J. 2021;422 doi: 10.1016/J.CEJ.2021.130023. [DOI] [Google Scholar]
- 74.Skaf D.W., Punzi V.L., Rolle J.T., Kleinberg K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020;386 doi: 10.1016/J.CEJ.2019.123807. [DOI] [Google Scholar]
- 75.Prokopova M., Novotna K., Pivokonska L., Cermakova L., Cajthaml T., Pivokonsky M. Coagulation of polyvinyl chloride microplastics by ferric and aluminium sulphate: optimisation of reaction conditions and removal mechanisms. J. Environ. Chem. Eng. 2021;9(6) doi: 10.1016/J.JECE.2021.106465. [DOI] [Google Scholar]
- 76.Ma B., Xue W., Ding Y., Hu C., Liu H., Qu J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019;78:267–275. doi: 10.1016/J.JES.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Na S.H., Kim M.J., Kim J.T., et al. Microplastic removal in conventional drinking water treatment processes: performance, mechanism, and potential risk. Water Res. 2021;202 doi: 10.1016/J.WATRES.2021.117417. [DOI] [PubMed] [Google Scholar]
- 78.Radityaningrum A.D., Trihadiningrum Y., Mar’atusholihah, Soedjono E.S., Herumurti W. Microplastic contamination in water supply and the removal efficiencies of the treatment plants: a case of Surabaya City, Indonesia. J. Water Proc. Eng. 2021;43 doi: 10.1016/J.JWPE.2021.102195. [DOI] [Google Scholar]
- 79.Lapointe M., Farner J.M., Hernandez L.M., Tufenkji N. Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environ. Sci. Technol. 2020;54(14):8719–8727. doi: 10.1021/ACS.EST.0C00712/SUPPL_FILE/ES0C00712_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Diehl A., Lewandowski A., Gopalakrishnan K., Baker T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020;720 doi: 10.1016/J.SCITOTENV.2020.137383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarkar D.J., Das Sarkar S., Das B.K., et al. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J. Hazard Mater. 2021;413 doi: 10.1016/J.JHAZMAT.2021.125347. [DOI] [PubMed] [Google Scholar]
- 82.Chu X., Zheng B., Li Z., et al. Occurrence and distribution of microplastics in water supply systems: in water and pipe scales. Sci. Total Environ. 2022;803 doi: 10.1016/J.SCITOTENV.2021.150004. [DOI] [PubMed] [Google Scholar]
- 83.Mason S.A., Garneau D., Sutton R., et al. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016;218:1045–1054. doi: 10.1016/J.ENVPOL.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 84.Murphy F., Ewins C., Carbonnier F., Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;50(11):5800–5808. doi: 10.1021/ACS.EST.5B05416/SUPPL_FILE/ES5B05416_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 85.Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/J.SCITOTENV.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 86.Leslie H.A., Brandsma S.H., van Velzen M.J.M., Vethaak A.D. Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017;101:133–142. doi: 10.1016/J.ENVINT.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 87.Li X., Chen L., Mei Q., et al. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018;142:75–85. doi: 10.1016/J.WATRES.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 88.Corradini F., Meza P., Eguiluz R., Casado F., Huerta-Lwanga E., Geissen V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019;671:411–420. doi: 10.1016/J.SCITOTENV.2019.03.368. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L., Xie Y., Liu J., Zhong S., Qian Y., Gao P. An overlooked entry pathway of microplastics into agricultural soils from application of sludge-based fertilizers. Environ. Sci. Technol. 2020;54(7):4248–4255. doi: 10.1021/ACS.EST.9B07905/SUPPL_FILE/ES9B07905_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q., Adams C.A., Wang F., Sun Y., Zhang S. Interactions between microplastics and soil fauna: a critical review. Crit Rev Environ Sci Technol. Published online. 2021 doi: 10.1080/10643389.2021.1915035. [DOI] [Google Scholar]
- 91.Alavian Petroody S.S., Hashemi S.H., van Gestel C.A.M. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere. 2021;278 doi: 10.1016/J.CHEMOSPHERE.2021.130471. [DOI] [PubMed] [Google Scholar]
- 92.Sun J., Dai X., Wang Q., van Loosdrecht M.C.M., Ni B.J. Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res. 2019;152:21–37. doi: 10.1016/J.WATRES.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z., Gao S.H., Luo G., et al. The contamination of microplastics in China's aquatic environment: occurrence, detection and implications for ecological risk. Environ. Pollut. 2022;296 doi: 10.1016/J.ENVPOL.2021.118737. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed R., Hamid A.K., Krebsbach S.A., He J., Wang D. Critical review of microplastics removal from the environment. Chemosphere. 2022;293(November 2021) doi: 10.1016/j.chemosphere.2022.133557. [DOI] [PubMed] [Google Scholar]
- 95.Zou Y., Ye C., Pan Y. Abundance and characteristics of microplastics in municipal wastewater treatment plant effluent: a case study of Guangzhou, China. Environ. Sci. Pollut. Res. 2020;28(9):11572–11585. doi: 10.1007/S11356-020-11431-6. 2020 289. [DOI] [PubMed] [Google Scholar]
- 96.Yang Z., Li S., Ma S., et al. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021;771 doi: 10.1016/J.SCITOTENV.2021.145377. [DOI] [PubMed] [Google Scholar]
- 97.Cao Y., Wang Q., Ruan Y., et al. Intra-day microplastic variations in wastewater: a case study of a sewage treatment plant in Hong Kong. Mar. Pollut. Bull. 2020;160 doi: 10.1016/J.MARPOLBUL.2020.111535. [DOI] [PubMed] [Google Scholar]
- 98.Lares M., Ncibi M.C., Sillanpää M., Sillanpää M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–246. doi: 10.1016/J.WATRES.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 99.Lv X., Dong Q., Zuo Z., Liu Y., Huang X., Wu W.M. Microplastics in a municipal wastewater treatment plant: fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019;225:579–586. doi: 10.1016/J.JCLEPRO.2019.03.321. [DOI] [Google Scholar]
- 100.Bayo J., López-Castellanos J., Olmos S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020;156 doi: 10.1016/J.MARPOLBUL.2020.111211. [DOI] [PubMed] [Google Scholar]
- 101.Kundu A., Shetti N.P., Basu S., Raghava Reddy K., Nadagouda M.N., Aminabhavi T.M. Identification and removal of micro- and nano-plastics: efficient and cost-effective methods. Chem. Eng. J. 2021;421 doi: 10.1016/J.CEJ.2021.129816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talvitie J., Mikola A., Koistinen A., Setälä O. Solutions to microplastic pollution – removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017;123:401–407. doi: 10.1016/J.WATRES.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q.Y., Li Y.L., Liu Y.Y., et al. Effects of microplastics accumulation on performance of membrane bioreactor for wastewater treatment. Chemosphere. 2022;287 doi: 10.1016/J.CHEMOSPHERE.2021.131968. [DOI] [PubMed] [Google Scholar]
- 104.Bilgin M., Yurtsever M., Karadagli F. Microplastic removal by aerated grit chambers versus settling tanks of a municipal wastewater treatment plant. J. Water Proc. Eng. 2020;38 doi: 10.1016/J.JWPE.2020.101604. [DOI] [Google Scholar]
- 105.Magni S., Binelli A., Pittura L., et al. The fate of microplastics in an Italian wastewater treatment plant. Sci. Total Environ. 2019;652:602–610. doi: 10.1016/J.SCITOTENV.2018.10.269. [DOI] [PubMed] [Google Scholar]
- 106.Koutnik V.S., Alkidim S., Leonard J., et al. Unaccounted microplastics in wastewater sludge: where do they go? ACS ES&T Water. 2021;1(5):1086–1097. doi: 10.1021/ACSESTWATER.0C00267. [DOI] [Google Scholar]
- 107.Carr S.A., Liu J., Tesoro A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016;91:174–182. doi: 10.1016/J.WATRES.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Torena P., Alvarez-Cuenca M., Reza M. Biodegradation of polyethylene terephthalate microplastics by bacterial communities from activated sludge. Can. J. Chem. Eng. 2021;99(S1):S69–S82. doi: 10.1002/CJCE.24015. [DOI] [Google Scholar]
- 109.Chen Z., Zhao W., Xing R., et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard Mater. 2020;384 doi: 10.1016/J.JHAZMAT.2019.121271. [DOI] [PubMed] [Google Scholar]
- 110.Xu B., Liu F., Cryder Z., et al. Microplastics in the soil environment: occurrence, risks, interactions and fate – a review. Crit. Rev. Environ. Sci. Technol. 2019;50(21):2175–2222. doi: 10.1080/10643389.2019.1694822. [DOI] [Google Scholar]
- 111.Wang W., Yuan W., Xu E.G., Li L., Zhang H., Yang Y. Uptake, translocation, and biological impacts of micro(nano)plastics in terrestrial plants: progress and prospects. Environ. Res. 2022;203 doi: 10.1016/J.ENVRES.2021.111867. [DOI] [PubMed] [Google Scholar]
- 112.Li L., Luo Y., Li R., et al. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020;3(11):929–937. doi: 10.1038/s41893-020-0567-9. 2020 311. [DOI] [Google Scholar]
- 113.Kalčíková G. Aquatic vascular plants - a forgotten piece of nature in microplastic research. Environ. Pollut. 2020;262 doi: 10.1016/J.ENVPOL.2020.114354. [DOI] [PubMed] [Google Scholar]
- 114.Culligan N., Liu K biu. Ribble K., Ryu J., Dietz M. Sedimentary records of microplastic pollution from coastal Louisiana and their environmental implications. J. Coast Conserv. 2022;26(1):1–14. doi: 10.1007/S11852-021-00847-Y/FIGURES/9. [DOI] [Google Scholar]
- 115.Reineccius J., Bresien J., Waniek J.J. Separation of microplastics from mass-limited samples by an effective adsorption technique. Sci. Total Environ. 2021;788 doi: 10.1016/J.SCITOTENV.2021.147881. [DOI] [PubMed] [Google Scholar]
- 116.Sarkar D.J., Das Sarkar S., Das B.K., et al. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021;192 doi: 10.1016/J.WATRES.2021.116853. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q., Hernández-Crespo C., Du B., Van Hulle S.W.H., Rousseau D.P.L. Fate and removal of microplastics in unplanted lab-scale vertical flow constructed wetlands. Sci. Total Environ. 2021;778 doi: 10.1016/J.SCITOTENV.2021.146152. [DOI] [PubMed] [Google Scholar]
- 118.Guo J.J., Huang X.P., Xiang L., et al. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020;137 doi: 10.1016/J.ENVINT.2019.105263. [DOI] [PubMed] [Google Scholar]
- 119.Li J., Song Y., Cai Y. Focus topics on microplastics in soil: analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020;257 doi: 10.1016/J.ENVPOL.2019.113570. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y., Kang S., Allen S., Allen D., Gao T., Sillanpää M. Atmospheric microplastics: a review on current status and perspectives. Earth Sci. Rev. 2020;203 doi: 10.1016/J.EARSCIREV.2020.103118. [DOI] [Google Scholar]
- 121.Mbachu O., Jenkins G., Pratt C., Kaparaju P. A new contaminant superhighway? A review of sources, measurement techniques and fate of atmospheric microplastics. Water Air Soil Pollut. 2020;231(2):1–27. doi: 10.1007/S11270-020-4459-4/FIGURES/5. [DOI] [Google Scholar]
- 122.Chen G., Feng Q., Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020;703 doi: 10.1016/J.SCITOTENV.2019.135504. [DOI] [PubMed] [Google Scholar]
- 123.Ali S.S., Elsamahy T., Koutra E., et al. Degradation of conventional plastic wastes in the environment: a review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021;771 doi: 10.1016/j.scitotenv.2020.144719. [DOI] [PubMed] [Google Scholar]
- 124.Arpia A.A., Chen W.H., Ubando A.T., Naqvi S.R., Culaba A.B. Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environmental effects: a state-of-the-art review. J. Hazard Mater. 2021;418 doi: 10.1016/J.JHAZMAT.2021.126381. [DOI] [PubMed] [Google Scholar]
- 125.Miloloža M., Cvetnić M., Kučić Grgić D., et al. Biotreatment strategies for the removal of microplastics from freshwater systems. A review. Environ. Chem. Lett. 2022;20(2):1377–1402. doi: 10.1007/S10311-021-01370-0. 2022 202. [DOI] [Google Scholar]
- 126.Oberbeckmann S., Labrenz M. Marine microbial assemblages on microplastics: diversity, adaptation, and role in degradation. Ann. Rev. Mar. Sci. 2020;12:209–232. doi: 10.1146/ANNUREV-MARINE-010419-010633. [DOI] [PubMed] [Google Scholar]
- 127.Othman A.R., Hasan H.A., Muhamad M.H., ’Izzati Ismail N., Abdullah S.R.S. Microbial degradation of microplastics by enzymatic processes: a review. Environ. Chem. Lett. 2021;19(4):3057–3073. doi: 10.1007/S10311-021-01197-9. 2021 194. [DOI] [Google Scholar]
- 128.Sun Q., Li J., Wang C., et al. Research progress on distribution, sources, identification, toxicity, and biodegradation of microplastics in the ocean, freshwater, and soil environment. Front. Environ. Sci. Eng. 2021;16(1):1–14. doi: 10.1007/S11783-021-1429-Z. 2021 161. [DOI] [Google Scholar]
- 129.Klein S., Dimzon I.K., Eubeler J., Knepper T.P. Analysis, occurrence, and degradation of microplastics in the aqueous environment. Handb. Environ. Chem. 2018;58:51–67. doi: 10.1007/978-3-319-61615-5_3. [DOI] [Google Scholar]
- 130.Pathak V.M. Navneet. Review on the current status of polymer degradation: a microbial approach. Bioresour Bioprocess. 2017;4(1):1–31. doi: 10.1186/S40643-017-0145-9. 2017 41. [DOI] [Google Scholar]
- 131.Lambert S., Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016;145:265–268. doi: 10.1016/J.CHEMOSPHERE.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li G., Hou Z., Zhang R., Chen X., Lu Z. Nanometer titanium dioxide mediated high efficiency photodegradation of fluazifop-p-butyl. Int. J. Environ. Res. Publ. Health. 2019;16(19):3600. doi: 10.3390/IJERPH16193600. Vol 16, Page 3600. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ateia M., Alalm M.G., Awfa D., Johnson M.S., Yoshimura C. Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: a critical review. Sci. Total Environ. 2020;698 doi: 10.1016/J.SCITOTENV.2019.134197. [DOI] [PubMed] [Google Scholar]
- 134.Ge J., Zhang Z., Ouyang Z., et al. Photocatalytic degradation of (micro)plastics using TiO2-based and other catalysts: properties, influencing factor, and mechanism. Environ. Res. 2022;209 doi: 10.1016/J.ENVRES.2022.112729. [DOI] [PubMed] [Google Scholar]
- 135.Ariza-Tarazona M.C., Villarreal-Chiu J.F., Hernández-López J.M., et al. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: effect of pH and temperature in the photocatalytic degradation process. J. Hazard Mater. 2020;395 doi: 10.1016/J.JHAZMAT.2020.122632. [DOI] [PubMed] [Google Scholar]
- 136.Aziz K.H.H., Omer K.M., Mahyar A., Miessner H., Mueller S., Moeller D. Application of photocatalytic falling film reactor to elucidate the degradation pathways of pharmaceutical diclofenac and ibuprofen in aqueous solutions. Coatings. 2019;9(8):465. doi: 10.3390/COATINGS9080465. 2019, Vol 9, Page 465. [DOI] [Google Scholar]
- 137.Llorente-García B.E., Hernández-López J.M., Zaldívar-Cadena A.A., Siligardi C., Cedillo-González E.I. First insights into photocatalytic degradation of HDPE and LDPE microplastics by a mesoporous N-TiO2 coating: effect of size and shape of microplastics. Coatings. 2020;10(7) doi: 10.3390/coatings10070658. [DOI] [Google Scholar]
- 138.Xu Q., Huang Q.S., Luo T.Y., Wu R.L., Wei W., Ni B.J. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chem. Eng. J. 2021;416 doi: 10.1016/J.CEJ.2021.129123. [DOI] [Google Scholar]
- 139.Jiang R., Lu G., Yan Z., Liu J., Wu D., Wang Y. Microplastic degradation by hydroxy-rich bismuth oxychloride. J. Hazard Mater. 2021;405 doi: 10.1016/J.JHAZMAT.2020.124247. [DOI] [PubMed] [Google Scholar]
- 140.Tofa T.S., Ye F., Kunjali K.L., Dutta J. Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalyst. 2019;9(10):819. doi: 10.3390/CATAL9100819. Vol 9, Page 819. 2019. [DOI] [Google Scholar]
- 141.Acuña-Bedoya J.D., Luévano-Hipólito E., Cedillo-González E.I., Domínguez-Jaimes L.P., Hurtado A.M., Hernández-López J.M. Boosting visible-light photocatalytic degradation of polystyrene nanoplastics with immobilized CuxO obtained by anodization. J. Environ. Chem. Eng. 2021;9(5) doi: 10.1016/J.JECE.2021.106208. [DOI] [Google Scholar]
- 142.Nabi I., Bacha A.U.R., Li K., et al. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience. 2020;23(7) doi: 10.1016/J.ISCI.2020.101326/ATTACHMENT/6D5ED3F7-EA62-446A-A584-7FC4ADB5A38D/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allé P.H., Garcia-Muñoz P., Adouby K., Keller N., Robert D. Efficient photocatalytic mineralization of polymethylmethacrylate and polystyrene nanoplastics by TiO2/β-SiC alveolar foams. Environ. Chem. Lett. 2021;19(2):1803–1808. doi: 10.1007/S10311-020-01099-2/FIGURES/4. [DOI] [Google Scholar]
- 144.Nakatani H., Kyan T., Urakawa Y. Novel recycling system of polystyrene water debris with polymer photocatalyst and thermal treatment. J. Polym. Environ. 2021;29(5):1467–1476. doi: 10.1007/S10924-020-01976-5/FIGURES/10. [DOI] [Google Scholar]
- 145.Ariza-Tarazona M.C., Villarreal-Chiu J.F., Barbieri V., Siligardi C., Cedillo-González E.I. New strategy for microplastic degradation: green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019;45(7):9618–9624. doi: 10.1016/J.CERAMINT.2018.10.208. [DOI] [Google Scholar]
- 146.Lv M., Jiang B., Xing Y., Ya H., Zhang T., Wang X. Recent advances in the breakdown of microplastics: strategies and future prospectives. Environ. Sci. Pollut. Res. 2022;1:1–17. doi: 10.1007/S11356-022-22004-0. 2022. [DOI] [PubMed] [Google Scholar]
- 147.Tsiota P., Karkanorachaki K., Syranidou E., Franchini M., Kalogerakis N. Springer Water; 2018. Microbial Degradation of HDPE Secondary Microplastics: Preliminary Results; pp. 181–188. [DOI] [Google Scholar]
- 148.Miao F., Liu Y., Gao M., et al. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard Mater. 2020;399 doi: 10.1016/J.JHAZMAT.2020.123023. [DOI] [PubMed] [Google Scholar]
- 149.Hu K., Zhou P., Yang Y., et al. Degradation of microplastics by a thermal Fenton reaction. ACS ES&T Eng. 2021;2(1):110–120. doi: 10.1021/ACSESTENGG.1C00323. [DOI] [Google Scholar]
- 150.Kang J., Zhou L., Duan X., Sun H., Ao Z., Wang S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter. 2019;1(3):745–758. doi: 10.1016/J.MATT.2019.06.004. [DOI] [Google Scholar]
- 151.Tofa T.S., Kunjali K.L., Paul S., Dutta J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019;17(3):1341–1346. doi: 10.1007/S10311-019-00859-Z/TABLES/1. [DOI] [Google Scholar]
- 152.Gu J.G., Gu J.D. Methods currently used in testing microbiological degradation and deterioration of a wide range of polymeric materials with various degree of degradability: a review. J. Polym. Environ. 2005;13(1):65–74. doi: 10.1007/S10924-004-1230-7. 2005 131. [DOI] [Google Scholar]
- 153.Awasthi A.K., Tan Q., Li J. Biotechnological potential for microplastic waste. Trends Biotechnol. 2020;38(11):1196–1199. doi: 10.1016/j.tibtech.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 154.Harrison J.P., Boardman C., O'Callaghan K., Delort A.M., Song J. Biodegradability standards for carrier bags and plastic films in aquatic environments: a critical review. R. Soc. Open Sci. 2018;5(5) doi: 10.1098/RSOS.171792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yin W., Zhang B., Shi J., Liu Z. Microbial adaptation to co-occurring vanadium and microplastics in marine and riverine environments. J. Hazard Mater. 2022;424 doi: 10.1016/J.JHAZMAT.2021.127646. [DOI] [PubMed] [Google Scholar]
- 156.Auta H.S., Emenike C.U., Jayanthi B., Fauziah S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018;127:15–21. doi: 10.1016/J.MARPOLBUL.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 157.Auta H.S., Emenike C.U., Fauziah S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017;231:1552–1559. doi: 10.1016/J.ENVPOL.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 158.Paço A., Duarte K., da Costa J.P., et al. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017;586:10–15. doi: 10.1016/J.SCITOTENV.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 159.Kucic Grgic D., Miloloza M., Lovrincic E., et al. Bioremediation of mp-polluted waters using bacteria bacillus licheniformis, lysinibacillus massiliensis, and mixed culture of bacillus sp. and delftia acidovorans. Chem. Biochem. Eng. Q. 2021;35(2):205–224. doi: 10.15255/CABEQ.2021.1915. [DOI] [Google Scholar]
- 160.Park S.Y., Kim C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019;222:527–533. doi: 10.1016/J.CHEMOSPHERE.2019.01.159. [DOI] [PubMed] [Google Scholar]
- 161.Zhang J., Gao D., Li Q., et al. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020;704 doi: 10.1016/J.SCITOTENV.2019.135931. [DOI] [PubMed] [Google Scholar]
- 162.Wang S., Shi W., Huang Z., et al. Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J. Hazard Mater. 2022;423 doi: 10.1016/J.JHAZMAT.2021.127213. [DOI] [PubMed] [Google Scholar]
- 163.Lu H., Diaz D.J., Czarnecki N.J., et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. 2022;604(7907):662–667. doi: 10.1038/s41586-022-04599-z. [DOI] [PubMed] [Google Scholar]
- 164.Niu L., Li Y., Li Y., et al. New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments. Water Res. 2021;188 doi: 10.1016/J.WATRES.2020.116449. [DOI] [PubMed] [Google Scholar]
- 165.Shabbir S., Faheem M., Ali N., et al. Periphytic biofilm: an innovative approach for biodegradation of microplastics. Sci. Total Environ. 2020;717 doi: 10.1016/J.SCITOTENV.2020.137064. [DOI] [PubMed] [Google Scholar]
- 166.Roager L., Sonnenschein E.C. Bacterial candidates for colonization and degradation of marine plastic debris. Environ. Sci. Technol. 2019;53(20):11636–11643. doi: 10.1021/ACS.EST.9B02212. [DOI] [PubMed] [Google Scholar]
- 167.Tamargo A., Molinero N., Reinosa J.J., et al. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022;12(1):1–15. doi: 10.1038/s41598-021-04489-w. 2022 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thiounn T., Smith R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020;58(10):1347–1364. doi: 10.1002/POL.20190261. [DOI] [Google Scholar]
- 169.Vollmer I., Jenks M.J.F., Roelands M.C.P., et al. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 2020;59(36):15402–15423. doi: 10.1002/ANIE.201915651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schyns ZO. G. Shaver M.P. Mechanical recycling of packaging plastics: a review. Macromol. Rapid Commun. 2021;42(3) doi: 10.1002/MARC.202000415. G Schyns ZO, Shaver MP, [DOI] [PubMed] [Google Scholar]
- 171.Jambeck J.R., Geyer R., Wilcox C., et al. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi: 10.1126/SCIENCE.1260352. 80- [DOI] [PubMed] [Google Scholar]
- 172.Lebreton L.C.M., Van Der Zwet J., Damsteeg J.W., Slat B., Andrady A., Reisser J. River plastic emissions to the world's oceans. Nat. Commun. 2017;8(1):1–10. doi: 10.1038/ncomms15611. 2017 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kane I.A., Clare M.A., Miramontes E., et al. Seafloor microplastic hotspots controlle by deep-sea circulation. Science. 2020;368(6495):1140–1145. doi: 10.1126/SCIENCE.ABA5899/SUPPL_FILE/ABA5899_KANE_SM.PDF. 80- [DOI] [PubMed] [Google Scholar]
- 174.Borrelle S.B., Ringma J., Lavender Law K., et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science. 2020;369(6509):1515–1518. doi: 10.1126/SCIENCE.ABA3656/SUPPL_FILE/ABA3656-BORRELLE-SM-DATA-S4.CSV. 80- [DOI] [PubMed] [Google Scholar]
- 175.Caniato M., Cozzarini L., Schmid C., Gasparella A. Acoustic and thermal characterization of a novel sustainable material incorporating recycled microplastic waste. Sustain Mater Technol. 2021;28 doi: 10.1016/J.SUSMAT.2021.E00274. [DOI] [Google Scholar]
- 176.Palombini F.L., Demori R., Cidade M.K., Kindlein W., de Jacques J.J. Occurrence and recovery of small-sized plastic debris from a Brazilian beach: characterization, recycling, and mechanical analysis. Environ. Sci. Pollut. Res. 2018;25(26):26218–26227. doi: 10.1007/S11356-018-2678-7/FIGURES/3. [DOI] [PubMed] [Google Scholar]
- 177.Nabgan W., Nabgan B., Abdullah T.A.T., et al. Highly active biphasic anatase-rutile Ni-Pd/TNPs nanocatalyst for the reforming and cracking reactions of microplastic waste dissolved in phenol. ACS Omega. 2022;7(4):3324–3340. doi: 10.1021/ACSOMEGA.1C05488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou H., Ren Y., Li Z., et al. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-25048-x. 2021 121. [DOI] [PMC free article] [PubMed] [Google Scholar]