Abbreviations used
- AAV
adeno associated virus
- ATP
adenosine triphosphate
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- DMSO
dimethyl sulfoxide
- ETC
electron transport chain
- ETHE1
ethylmalonic encephalopathy 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GSSSG
glutathione trisulfide
- H2S
hydrogen sulfide
- ICV
intracerebroventricular
- IN
intranasal
- IP
intraperitoneal
- LA
lipoic acid
- LASSS
lipoic acid trisulfide
- LDH
lactate dehydrogenase
- 3MST
3-mercaptopyruvate sulfurtransferase
- Na2S
sodium sulfide
- Na2S3
sodium trisulfide
- Nrf2
nuclear-factor-E2-related factor-2
- MPTP
1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium ion
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- SQOR
sulfide quinone oxidoreductase
- SUOX
sulfite oxidase
- TH
tyrosine hydroxylase
- TST
thiosulfate sulfurtransferase
Innovation
Parkinson's Disease (PD) is the second most common neurodegenerative disea1se in humans. We used a mouse model of PD to investigate the effects of upregulating sulfide:quinone oxidoreductase (SQOR) in the brain. Increasing the level of SQOR in the brain, either using sulfide preconditioning (SPC) by intermittent inhalation of H2S or by adeno-associated virus (AAV)-mediated gene transfer, inhibited neurodegeneration and improved the movement disorder in PD mice. Furthermore, administration of polysulfide, a product of oxidation of H2S mediated by SQOR, mitigated neurodegeneration in this mouse model. The results of this study suggest that increased sulfide catabolism by SQOR may ameliorate the pathogenesis of PD. We anticipate that the availability of stable polysulfide compounds will accelerate the clinical translation of these results into innovative therapies for PD.
1. Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative diseases in humans, second only to Alzheimer's disease [1]. PD is characterized by the selective loss of dopaminergic neurons in the substantia nigra and deficiency of dopamine in the striatum [2]. Although the pathogenesis of PD is incompletely understood, increased oxidative stress [3], inhibition of complex I of the mitochondrial electron transport chain (ETC) [4,5], and aggregation of α-synuclein [[6], [7], [8]] are thought to have key roles in disease progression. Despite intensive research, currently there is no cure for PD.
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that inhibits mitochondrial ETC complex I. Following systemic administration, MPTP crosses the blood brain barrier and is converted (in astrocytes) to 1-methyl-4-phenylpyridinium ion (MPP+) by monoamine oxidase-B [[9], [10], [11]]. MPP+ binds to dopamine transporters on dopaminergic neurons with high affinity, facilitating cellular uptake and resulting in inhibition of ETC complex I, impairment of ATP production and increased oxidative stress and cell death [[12], [13], [14], [15]]. The selective degeneration of dopaminergic neurons induced by MPTP partially reproduces the pathological and phenotypic features of PD in both humans [16] and rodents [17]. Administration of MPTP to mice is therefore a frequently used model of human PD.
Hydrogen sulfide (H2S), a colorless gas with a characteristic rotten-egg odor, is found in various natural and industrial products [18]. In human blood, sulfide exists as H2S (20%) and HS− (80%) [19]. In mammalian cells, sulfides or closely related reactive sulfur species such as persulfides (RSSH) and polysulfides (RSnH) are generated by enzymes that include cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) [[20], [21], [22]]. Sulfides are catabolized in mitochondria by sulfide oxidation enzymes. SQOR catalyzes the first step in H2S oxidation, converting sulfide to persulfide [23]. Sulfane sulfur, a sulfur atom with 6 valence electrons and no charge, is a component of persulfide and polysulfide. Recent studies suggest that sulfane sulfur-containing molecules, such as persulfide and polysulfide, may protect against neurodegenerative diseases such as PD [24,25]. These molecules may inhibit oxidation of tissues or may facilitate mitochondrial bioenergetics [22,26,27]. Persulfide is further oxidized to thiosulfate, sulfite, and sulfate by persulfide dioxygenases (PDO or ETHE1), thiosulfate sulfurtransferase (TST), and sulfite oxidase (SUOX). The sulfane sulfur of persulfides including GSSH and CysSSH can be transferred to disulfide molecules such as GSSG and CysSSCys to generate various polysulfides (including GSSSG and CysSSSCys) in non-enzymatic reactions [26]. H2S can also be generated from persulfide/polysulfide via enzymatic reactions or non-enzymatic reactions [28,29]. This complicated diversity of potential sulfur reactions has hindered our understanding of the protective roles of sulfide catabolism in human diseases.
Previously, we reported that chronic, intermittent H2S inhalation protects mice from neurodegeneration and the movement disorder seen in the MPTP-induced mouse model of PD [30]. We showed that the neuroprotective effects of H2S inhalation were associated with upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent antioxidant and detoxification proteins in the brain. The precise mechanisms by which H2S inhalation provided neuroprotection remained incompletely understood. In the previous study, H2S inhalation began at the same time as MPTP administration, making it difficult to separate the effects of H2S inhalation from those of MPTP. More recently, we reported that chronic intermittent H2S inhalation markedly upregulated SQOR levels in the brain of mice [31]. Upregulated SQOR enabled mice to survive in severe hypoxia and mitigated ischemic brain injury, whereas depletion of brain SQOR increased the sensitivity of mice to oxygen deprivation. Under physiological conditions, H2S oxidation by SQOR donates electrons to mitochondrial ETC complex III via coenzyme Q (CoQ), thereby potentially promoting ATP synthesis [[32], [33], [34]]. In addition, SQOR converts H2S to persulfide, a potent antioxidant [26,[35], [36], [37]]. Furthermore, SQOR deficiency was recently reported to cause a Leigh syndrome-like disease, characterized by encephalopathy and the presence of brain lesions in the basal ganglia and cortex [38]. These observations suggest that sulfide oxidation by SQOR has a critical role in cerebral energy homeostasis under normal conditions, as well as during oxidative stress. However, the role of sulfide oxidation in the pathogenesis of PD is largely unknown.
The objective of the current study was to investigate the effects of sulfide preconditioning on the pathogenesis of PD in the MPTP-induced mouse model. To elucidate the mechanisms responsible for the beneficial effects of H2S inhalation in PD, we examined the effects of chronic intermittent H2S inhalation before MPTP, so that effects of SPC could be distinguished from those of MPTP. We hypothesized that upregulation of SQOR by SPC would mitigate neurodegeneration and the movement disorder that occur in the PD mouse model. We report that SQOR upregulation, either induced by SPC or AAV-mediated gene transfer, prevented MPTP-induced degeneration of dopaminergic neurons and movement disorder in mice. We further showed that administration of polysulfide, an oxidative product of sulfide catalyzed by SQOR, attenuated MPTP-induced neurodegeneration.
2. Results
2.1. Sulfide pre-conditioning protected mice from the movement disorder induced by MPTP
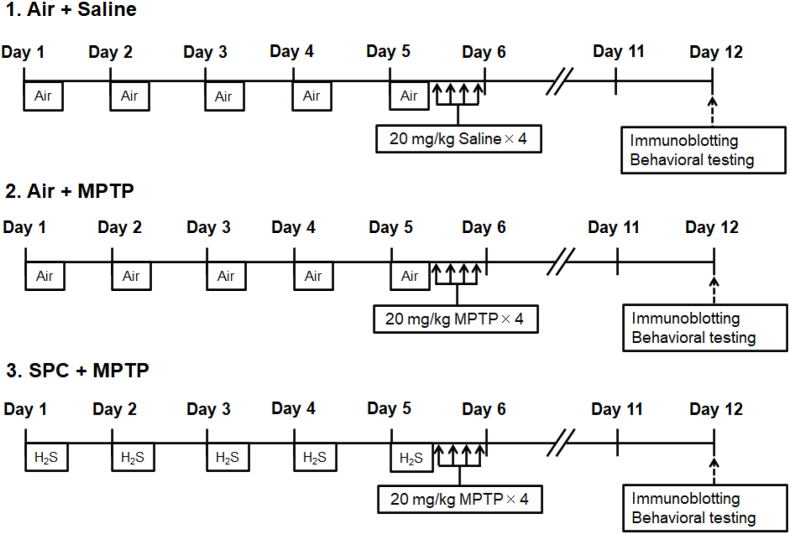
To investigate the effects of SPC on MPTP-induced neurodegeneration and movement disorder, mice were randomized into three groups; 1) mice breathing air for 5 days followed by saline administration on day 5 (Air + saline); 2) mice breathing air for 5 days followed by MPTP administration on day 5 (Air + MPTP); and 3) mice intermittently breathing H2S at 80 ppm mixed in air for 4 h daily for 5 consecutive days followed by MPTP administration on day 5 (SPC + MPTP) (Fig. 1). An investigator, who was blinded to the mouse treatment group, assessed the levels of anxiety and motor function 7 days after administration of saline or MPTP, using the open-field- and rotarod tests, respectively. The open-field test quantifies murine anxiety by measuring the amount of time that mice spend in the central area of an “open field” and the distance that mice move from the central area [39]. Increased time in the central area and decreased movement from the central area are both measures of increased anxiety. Compared to mice that were not preconditioned with H2S, mice that were treated with H2S demonstrated decreased anxiety after MPTP administration. The rotarod performance test measures the amount of time that a mouse is able to stay on a rotating rod and is a measure of coordination, grip strength and motor coordination [40]. Compared to mice that did not receive H2S, mice that were pre-treated with H2S and then administered MPTP retained the ability to balance on the rotarod (Fig. 2, A-C). These observations suggest that SPC protected mice from anxiety and the movement disorder induced by MPTP.
Fig. 1.
Experimental protocol of sulfide preconditioning (SPC).
Mice in the SPC + MPTP group breathed H2S (80 ppm) mixed in air for 4 h (8 a.m.–12 p.m.) on each day in an airtight chamber.
Fig. 2.
Effect of SPC on behavioral tests performed after administration of MPTP.
Results of central time (A) and central distance (B) in the Open-field test. Latency to fall” is the length of time that each mouse was able to stay on the Rotarod (C). Groups of mice in the study included: 1) Mice that inhaled air and were treated with saline (Air + Saline); 2) Mice that inhaled air and were treated with MPTP (Air + MPTP); 3) Mice that inhaled H2S and were treated with MPTP (SPC + MPTP). N = 7, 8, 8 mice in each group, respectively. **p < 0.01, ****p < 0.0001 vs. Air + Saline. #p < 0.05, ##p < 0.01 vs. Air + MPTP.
2.2. Sulfide pre-conditioning prevented MPTP-induced degeneration of dopaminergic neurons
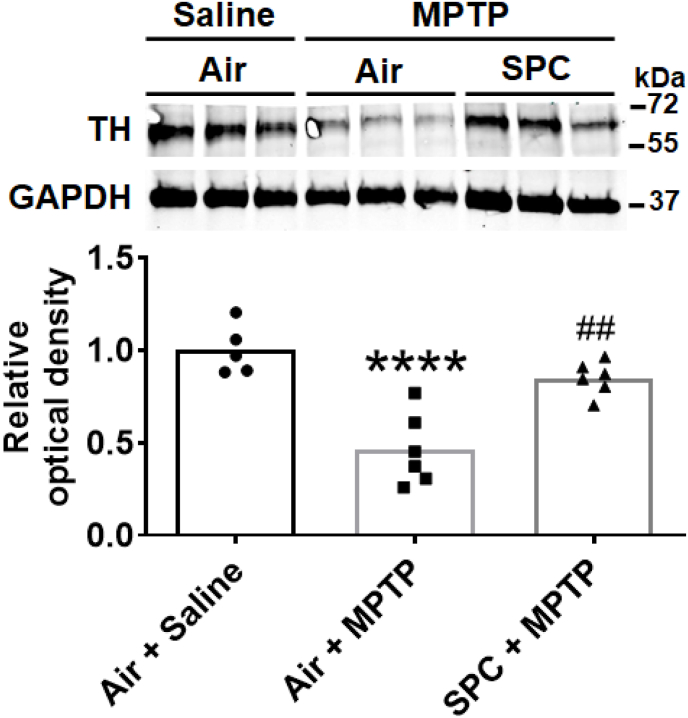
The degree of neurodegeneration induced by MPTP can be assessed by measuring the decrease in levels of tyrosine hydroxylase in the substantia nigra and striatum (the nigrostriatal region). Compared to untreated (control) mice, administration of MPTP decreased the level of tyrosine hydroxylase in the nigrostriatal region, as assessed by immunoblot. In contrast, preconditioning with H2S prevented the MPTP-induced decrease in tyrosine hydroxylase. (Fig. 3). These results show that SPC prevents MPTP-induced degeneration of dopaminergic neurons in the substantia nigra and striatum.
Fig. 3.
Tyrosine hydroxylase levels in the nigrostriatal region.
Representative immunoblots showing expression of tyrosine hydroxylase in the striatum and substantia nigra of mice 7 days after administration of MPTP or saline (n = 5, 6, 6 mice for each group, respectively). ****p < 0.0001 vs. Air + Saline. ##p < 0.01 vs. Air + MPTP.
2.3. Sulfide pre-conditioning upregulated SQOR in substantia nigra and striatum
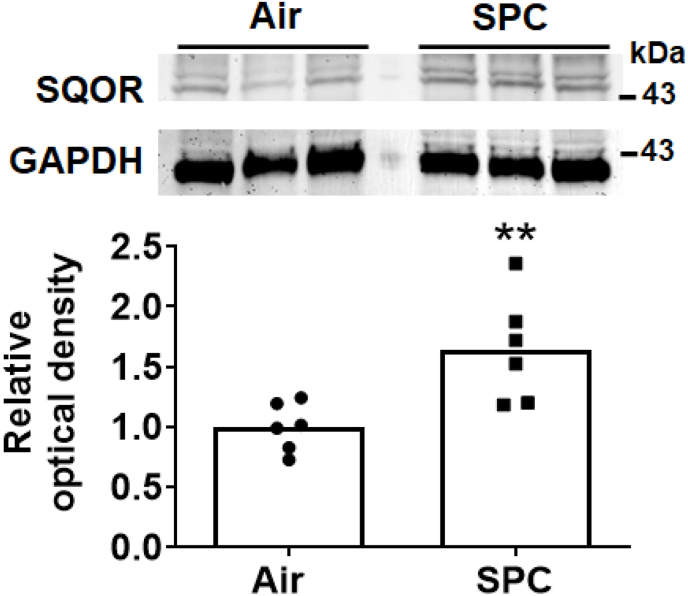
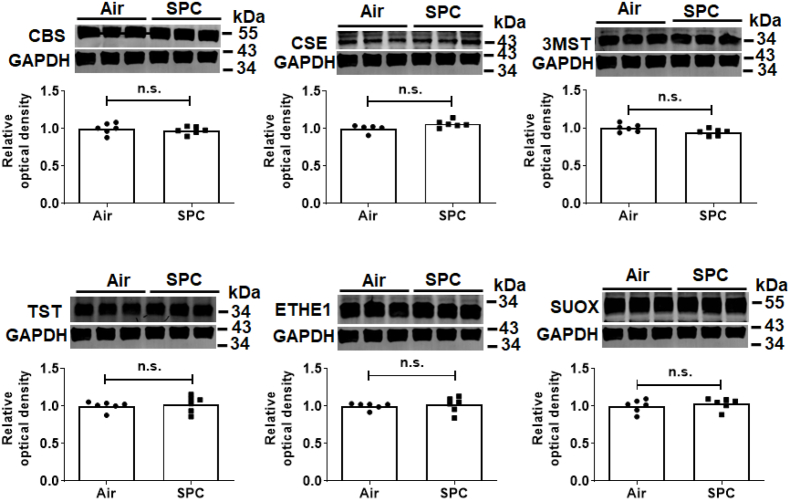
To explore the mechanisms responsible for the beneficial effects of SPC, we examined levels of SQOR in substantia nigra and striatum in mice with or without SPC. Immunoblots revealed that SPC significantly increased levels of SQOR in the nigrostriatal region (Fig. 4). In contrast, the levels of other enzymes that metabolize sulfide were not affected by SPC in this portion of the brain (Fig. 5). SQOR activity 2 h after administration of MPTP was decreased in control mice but not in mice that were pre-conditioned with H2S before administration of MPTP (Fig. 6A). SPC also prevented the decrease in levels of sulfane sulfur, a component of persulfide and polysulfide, in the nigrostriatal region 2 h after MPTP administration (Fig. 6B). These results suggest that the protective effects of SPC, in terms of preventing MPTP-induced neurodegeneration, are associated with upregulation of SQOR and increased persulfide/polysulfide levels in the nigrostriatal region.
Fig. 4.
Effect of SPC on the protein levels of SQOR in the nigrostriatal region. Representative immunoblot showing the level of SQOR in the nigrostriatal region of mice after H2S preconditioning (SPC) or control (air-breathing). N = 6 mice each. **p < 0.01 vs Air.
Fig. 5.
The effects of sulfide pre-conditioning (SPC) on the level of enzymes that metabolize sulfide in the nigrostriatal region.
Representative immunoblots and summary graphs of protein levels of enzymes in the nigrostriatal region that synthesize or metabolize sulfides in mice (n = 6 mice each).
Fig. 6.
The effects of sulfide preconditioning (SPC) on SQOR activity and sulfane sulfur levels after MPTP administration.
SQOR activity (A) and sulfane sulfur levels (B) were measured in the nigrostriatal region 2 h after MPTP administration (n = 5 mice each). *, ***p < 0.05, 0.001 vs. Air + Saline. #, ##p < 0.05, 0.01 vs. Air + MPTP.
2.4. AAV-mediated SQOR gene transfer increased SQOR levels in substantia nigra and striatum and prevented the MPTP-induced decrease in tyrosine hydroxylase
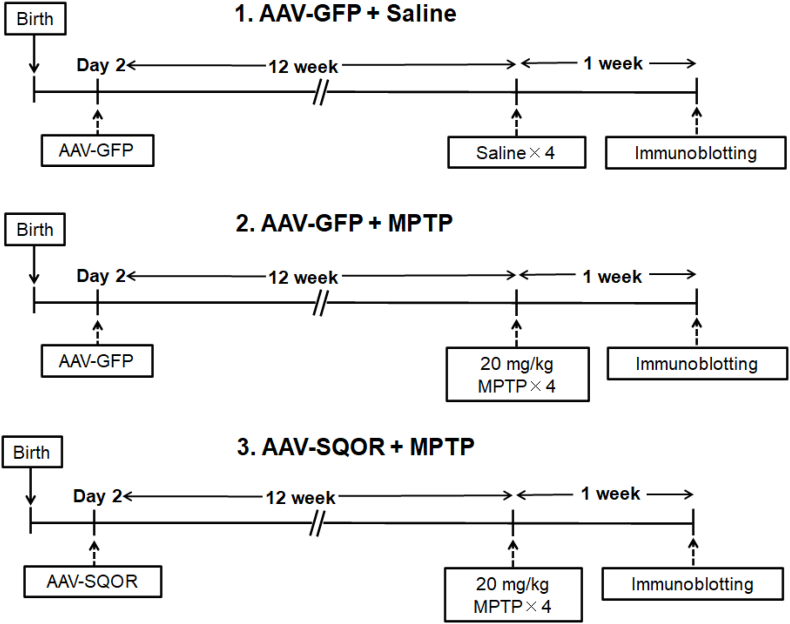
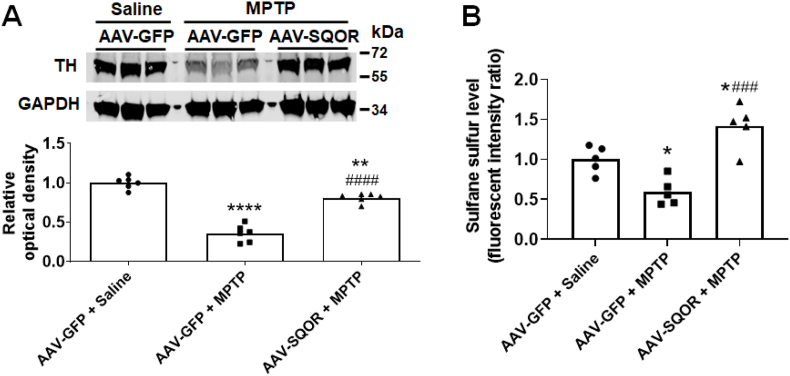
To determine whether upregulation of SQOR is sufficient to prevent neurodegeneration after MPTP administration, we examined the effects of increased levels of SQOR, induced by administration of adeno-associated virus (AAV) encoding mouse SQOR (AAV-SQOR). We used the hSYN1 promoter to express SQOR specifically in neurons of mice. AAV-SQOR was injected into the intracerebroventricular space of newborn mice and mice were used for experiments 12 weeks after injection (Fig. 7), as previously described [31]. Twelve weeks after AAV injection, AAV-SQOR-treated mice had higher levels of SQOR in substantia nigra and striatum compared to mice that received control AAV (AAV encoding green fluorescent protein, AAV-GFP) (Fig. 8). While levels of cystathionine beta synthase (CBS) were higher in mice that received AAV-SQOR compared to AAV-GFP, levels of other enzymes that metabolize sulfide were not affected by AAV-mediated SQOR gene transfer in the nigrostriatal region (Fig. 9). Compared to control, AAV-GFP-treated mice, which were treated with saline, control mice treated with MPTP had decreased levels of tyrosine hydroxylase in the nigrostriatal region. In contrast, mice that received AAV-SQOR and were treated with MPTP had normal levels of tyrosine hydroxylase (Fig. 10A) and sulfane sulfur (Fig. 10B) in the nigrostriatal region 7 days after MPTP administration. These observations support the hypothesis that the increase in persulfide/polysulfide production by increased levels of SQOR prevents MPTP-induced neurodegeneration.
Fig. 7.
Experimental protocol for AAV-mediated SQOR gene transfer.
Mice on post-natal day 2 received intracerebroventricular (ICV) injection of AAV9-hSYN1-mSQRDL-hSYN1-eGFP (AAV-SQR) or AAV9-hSYN1-eGFP (AAV-GFP) at 1010 viral particles per hemisphere. After 24 weeks, mice received four intraperitoneal injections of either MPTP (20 mg/kg) or saline. For each animal, there was a 2 h interval between each IP injection. The nigrostriatal regions were harvested 7 days after MPTP administration and the levels of tyrosine hydroxylase were determined using immunoblot.
Fig. 8.
AAV-mediated SQOR overexpression.
Representative immunoblot and quantification of SQOR levels in the striatum and substantia nigra of mice that were injected with control AAV (AAV-GFP) or AAV-SQOR. N = 6 mice each. ***p < 0.001 vs. AAV-GFP.
Fig. 9.
Level of enzymes that metabolize sulfide after AAV infection.
Representative immunoblot images and summary graphs of protein levels of enzymes in the nigrostriatal region that synthesize or metabolize sulfide in mice that received injection of control AAV or AAV-SQOR. N = 6 mice each. ***p < 0.001 vs. AAV-GFP.
Fig. 10.
Effect of SQOR overexpression on tyrosine hydroxylase and sulfane sulfur levels after MPTP administration.
Representative immunoblot and the densitometric analysis for tyrosine hydroxylase protein expression (n = 6 mice each) (A) and sulfane sufur levels (N = 5 mice each) (B) in the nigrostriatal region of mice 7 days (tyrosine hydroxylase expression) or 2 h (sulfane sulfur levels) after administration of MPTP. The level of tyrosine hydroxylase (TH) was normalized to that of GAPDH and the mean relative intensity for mice treated with AAV-GFP + saline was set to 1. *, **, ****p < 0.05, 0.01, 0.0001 vs. AAV-GFP + Saline. ###, ####p < 0.001, 0.0001 vs. AAV-GFP + MPTP.
2.5. Increased levels of SQOR inhibited cell death induced by 1-methyl-4-phenylpyridinium (MPP+) in SH-SY5Y cells
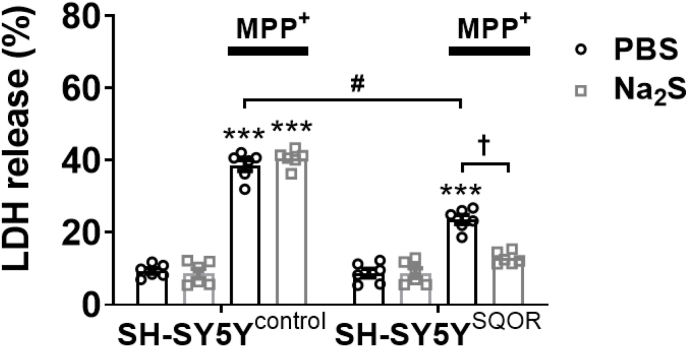
To further examine the potential protective effect of increased SQOR levels on cell viability, we expressed SQOR in SH-SY5Y neuroblastoma cells and measured the level of lactate dehydrogenase (LDH) released into tissue culture medium after addition of MPP+. Transfection of a plasmid encoding SQOR was used to induce expression of SQOR in SH-SY5Y cells, which do not normally express this protein. LDH released into the supernatant was measured 24 h after the addition of MPP+ (5 mM). Compared to control cells treated with MPP+, SQOR expression decreased the release of LDH into cell culture medium (Fig. 11). In a separate set of experiments, we examined the role of SQOR in the protective effects of H2S on the viability of SH-SY5Y cells incubated with MPP+. In SH-SY5Y cells without SQOR expression (control), Na2S (a H2S releaser) had no effect on MPP+-induced release of LDH (Fig. 11). However, in SH-SY5Y cells expressing SQOR, incubation with Na2S decreased MPP + -induced release of LDH. These results suggest that SQOR expression protects neuronal cells from MPP+-induced cytotoxicity. Because SH-SY5Y cells do not express SQOR at baseline [31], the results also indicate that SQOR is required for the cytoprotective effects of H2S against MPP+.
Fig. 11.
Effect of SQOR over expression on LDH release from SH-SY5Y cells treated with MPP+.
An LDH assay was performed to examine cell viabilities 24 h after incubation with MPP+ (5 mM) with or without Na2S (20 μM) (n = 6 each). ***p < 0.001 vs. control (no MPP+ with PBS or Na2S in the same transfection). #p < 0.05, †p < 0.05.
2.6. In the absence of SQOR, polysulfides, but not H2S, protected SH-SY5Y cells from MPP+-induced cell death
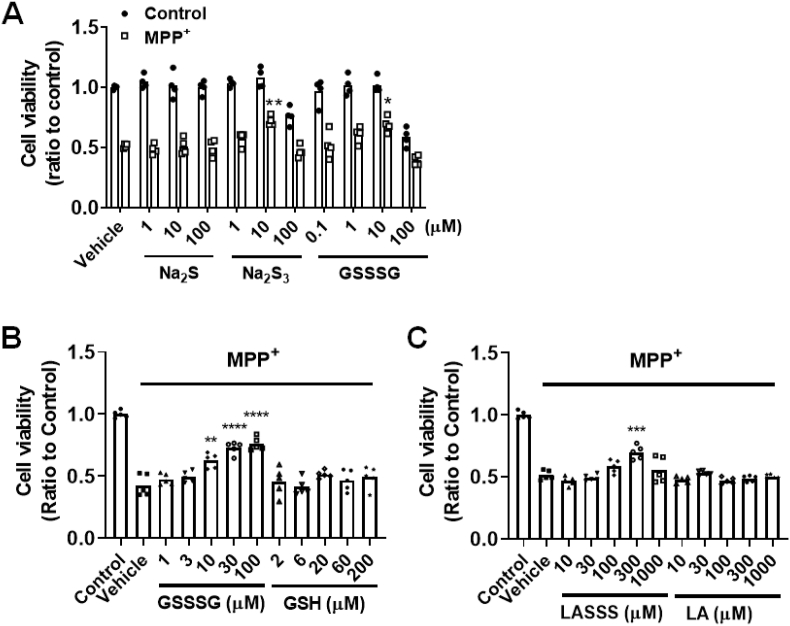
Because SQOR catalyzes the conversion of sulfide to polysulfides, and because SPC or AAV-mediated SQOR overexpression prevented the decrease in sulfane sulfur after MPTP administration, we hypothesized that the beneficial effects of upregulated SQOR after MPTP administration are mediated by increased levels of polysulfides. To consider this possibility, we examined the effects of polysulfides on the viability of SH-SY5Y cells incubated with MPP+. Cells were treated with MPP+ (5 mM) for 24 h and viability was assessed by crystal violet assay [41]. Compared to control cells, treatment with Na2S (doses between 1 and 100 μM) did not improve the viability of SH-SY5Y cells treated with MPP+ 42. In contrast, treatment with Na2S3 or GSSSG (10 μM each) increased the viability of MPP+-treated SH-SY5Y cells (Fig. 12A).
Fig. 12.
Effect of sulfide and polysulfide on cell viability of SH-SY5Y cells and primary cortical neurons incubated with MPP+
(A) SH-SY5Y cells or (B and C) murine primary cortical neurons were incubated with or without MPP+ with or without trisulfide compounds at 37 °C for 24 h. Cell viability was measured using the crystal violet assay (n = 4 or 5 each). *, **, ***p < 0.05, 0.01, 0.001 vs. control without trisulfide treatment.
To further examine the cytoprotective effects of polysulfides in a physiologically relevant context, we examined the effect of GSSSG, glutathione (GSH, a parent molecule of GSSSG without a sulfane sulfur), α-lipoic acid trisulfide (1,2,3-trithiane-4-pentanoic acid, LASSS), and α-lipoic acid (a parent molecule of LASSS without a sulfane sulfur) on murine primary cortical neurons incubated with MPP+. Cells were incubated with MPP+ (50 μM) together with each of the above compounds for 24 h. The polysulfide compounds GSSSG and LASSS, but not the parent compounds GSH and LA, protected murine primary cortical neurons from the effects of MPP+ treatment (Fig. 12B and C). These results suggest that the sulfane sulfur-releasing molecules protect neurons from MPP+-induced cytotoxicity.
2.7. Serial administration of polysulfide prevented the reduction of tyrosine hydroxylase in the substantia nigra and striatum of MPTP-treated mice
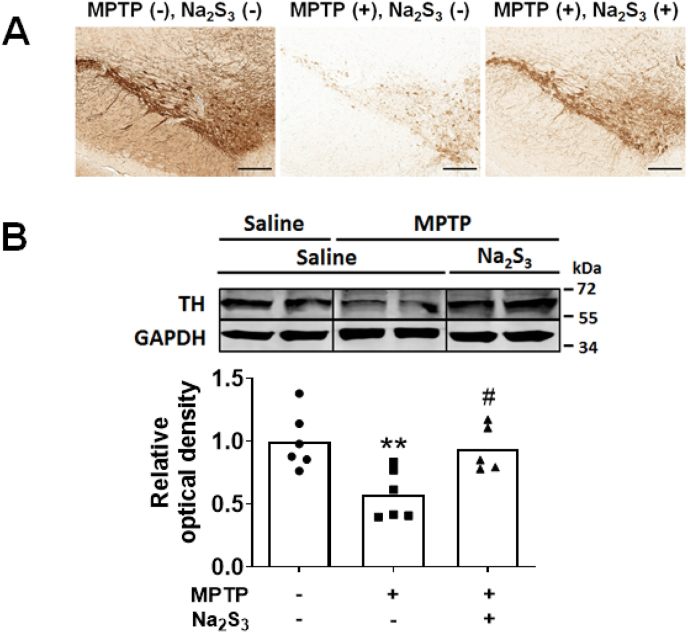
To investigate the potential therapeutic effects of polysulfides in vivo, mice were treated with or without Na2S3 after administration of MPTP or saline (control). On day 0, Na2S3 (20 mg/kg) or saline were administered IP, immediately after injection of MPTP or saline. Between days 1–6, mice received Na2S3 (20 mg/kg) or saline every 12 h. The levels of tyrosine hydroxylase in the nigrostriatal region were measured 7 days after administration of MPTP. Compared to control mice that received MPTP, administration of Na2S3 for 7 days prevented the MPTP-induced loss of tyrosine hydroxylase in substantia nigra and striatum (Fig. 13).
Fig. 13.
Effect of intraperitoneal administration of Na2S3 on MPTP-induced neurodegeneration.
The striatum and/or substantia nigra were harvested from mice 7 days after administration of MPTP or saline with or without Na2S3. Rabbit antiserum and immunohistochemistry were used to detect tyrosine hydroxylase (A). The level of tyrosine hydroxylase was quantified using immunoblot; a representative immunoblot and densitometric analysis are shown in (B). N = 6, 6, 5 mice respectively for the densitometric analysis. **p < 0.01 vs. Saline. #P < 0.05 vs. MPTP + Saline.
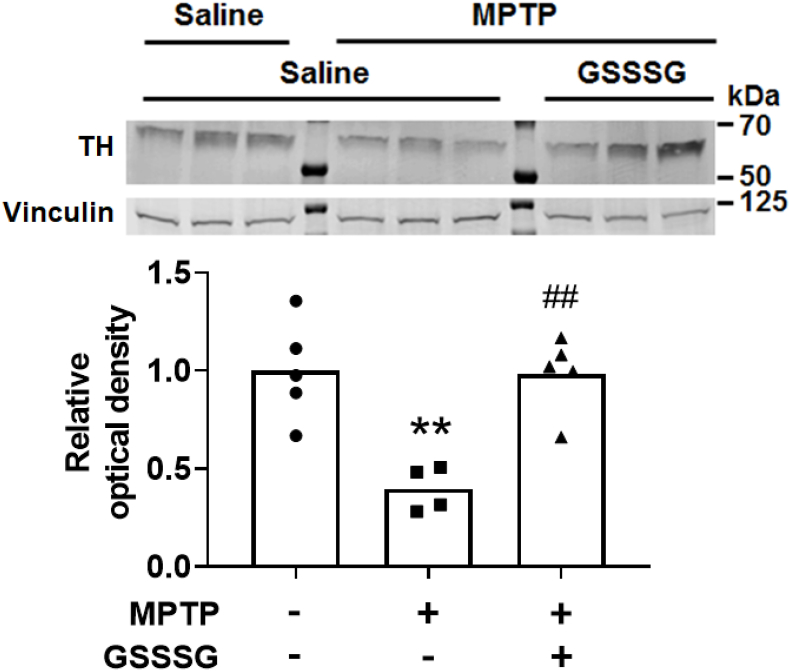
The large molecular weight of GSSSG (Mw: 644.7 DA) makes it unable to traverse the blood brain barrier after systemic administration. However, recent studies suggest that intranasal administration may permit successful passage of relatively large molecules into the central nervous system [43]. To consider the possibility that GSSSG might have a protective effect on MPTP-induced neurodegeneration when administered intranasally, GSSSG (50 mg/kg) or saline was administered IN immediately after administration of MPTP or saline (control) on day 0. Between days 1 and 6, mice received GSSSG at 50 mg/kg or saline IN every 12 h. We observed that administration of GSSSG IN prevented the MPTP-induced decrease in tyrosine hydroxylase in the nigrostriatal region (Fig. 14). Taken together, these results suggest that polysulfides have a robust therapeutic effect in a mouse model of PD.
Fig. 14.
Effect of intranasal administration of GSSSG on MPTP-induced neurodegeneration.
A representative immunoblot shows the level of tyrosine hydroxylase in the nigrostriatal region of mice 7 days after administration of MPTP or saline with or without Na2S3 treatment. Quantification of relative levels of tyrosine hydroxylase was determined using densitometry, comparing levels of tyrosine hydroxylase to vinculin (n = 5, 4, 5 mice, respectively). **p < 0.01 vs. Saline + Saline. ##p < 0.05 vs. MPTP + Saline.
3. Discussion
The current study revealed that increasing the level of polysulfides in the brain prevented neurodegenerative disease in a murine model of Parkinson's disease. Breathing H2S at 80 ppm for 4 h per day for 5 days before administration of MPTP prevented the subsequent development of anxiety and movement disorder. Preconditioning with H2S also protected mice from MPTP-induced loss of tyrosine hydroxylase-containing dopaminergic neurons in substantia nigra and striatum. The beneficial effects of SPC were associated with increased levels of SQOR and sulfane sulfur in the nigrostriatal region. AAV-mediated, neuron-specific, expression of SQOR in the central nervous system was sufficient to prevent MPTP-induced neurodegeneration in mice. In SH-SY5Y cells, expression of SQOR improved cell viability, despite the presence of MPP+. In addition, the presence of SQOR in SH-SY5Y cells was required for H2S to exert cytoprotective effects against MPP+. Administration of molecules that contain sulfane sulfur (i.e. Na2S3, GSSSG) attenuated the reduction of tyrosine hydroxylase in the nigrostriatal region in mice treated with MPTP. Taken together, these results revealed neuroprotective effects of SQOR and polysulfides in a PD model of mice.
The potential protective role of H2S in neurodegenerative diseases, including PD, has been a focus of intensive investigation. Previous, pre-clinical studies reported protective effects of exogenous H2S donor compounds and endogenous H2S in PD models [[44], [45], [46], [47]]. Anti-oxidative, anti-apoptotic, anti-inflammatory, and pro-survival effects have been suggested to explain the cytoprotective effects of H2S in these studies. In a previous study, we observed that chronic, intermittent inhalation of H2S, started immediately after MPTP administration, prevented motor dysfunction and degeneration of dopaminergic neurons in the nigrostriatal region in a mouse model of PD [30]. Although we observed modest upregulation of genes encoding antioxidant proteins, including heme oxygenase-1 and glutamate-cysteine ligase, the precise mechanism of protection remained incompletely defined.
To further characterize the beneficial effects of H2S in PD, we examined the cytoprotective effects of multiple H2S donor compounds on SH-SY5Y cells incubated with MPP+ 42. We previously found that the cytoprotective effects of H2S donor compounds correlated with their ability to increase sulfane sulfur, but not H2S per se, in cells and culture medium [42]. In fact, Na2S failed to improve survival of SH-SY5Y cells incubated with MPP + unless the cells also expressed SQOR. This observation suggests that SQOR is required for the beneficial effects of Na2S.
Although protective effects of exogenous and endogenous H2S have been studied extensively, the role of sulfide catabolism has thus far garnered little attention. We recently reported that chronic, intermittent H2S inhalation induced robust tolerance to hypoxia and ischemic brain injury in mice. The acquired tolerance to acute hypoxia/ischemia in sulfide pre-conditioned mice was associated with increased levels of SQOR and an increased capacity to catabolize sulfide in the brain [31]. Because upregulation of SQOR increases persulfide and polysulfide levels in neurons [31], we posit that the beneficial effects of SQOR upregulation in this mouse model of PD are at least in part mediated via the effects of persulfide and polysulfide compounds. Persulfide and polysulfide species have been reported to be potent antioxidants [26]. For example, Li and colleagues showed that GSSH was 50-fold more effective than H2S in terms of scavenging H2O2 at physiological pH [48]. Polysulfides, such as GSSSG can generate persulfides, such as GSSH [26,48,49]. Therefore, administration of either persulfide or polysulfide, the latter a persulfide releasing molecule, would be expected to protect against oxidative insults caused by MPTP. However, the mechanisms responsible for the protective effects of exogenous persulfide/polysulfide and SQOR upregulation may not be identical. For example, persulfide/polysulfide can scavenge reactive oxygen species (ROS) and scavenging ROS protects dopaminergic neurons against MPP+ [26,50,51]. However, it is challenging to separate the effect of scavenging ROS from the other protective effects of persulfide/polysulfide. For another example, SQOR has been shown to mediate the therapeutic effects of H2S via activation of adenosine monophosphate-activated protein kinase (AMPK) [52]. A number of preclinical studies have reported neuroprotective effects of AMPK activation in PD models [53,54]. Therefore, it is possible that SQOR upregulation mediates protective effects via AMPK activation. The role of AMPK activation in the protective effects of SQOR in PD remains to be examined in future studies.
Because oxidation of sulfide by SQOR donates electrons to mitochondrial ETC complex III via CoQ [35,55], it is also conceivable that upregulation of SQOR and the increased capacity to oxidize sulfide may support mitochondrial bioenergetic function and cell survival in the setting of the complex I inhibition observed in PD [35,55]. Our previous observation that Na2S increases intracellular ATP levels in SH-SY5Y cells expressing SQOR, but not in SH-SY5Y cells without SQOR expression, supports this hypothesis [31].
Sulfane sulfur mediates cysteine persulfidation (or S-sulfhydration), a post-translational modification that adds a sulfur atom to a cysteine residue, potentially altering the activity of the target protein. In contrast, H2S itself does not cause persulfidation [56,57]. Because cysteine persulfidation of some enzymes may prove to be beneficial in some neurodegenerative diseases [[58], [59], [60]], the relationship between cysteine persulfidation and the protective effect of SQOR and polysulfide remains to be elucidated. A model of the potential mechanism by which the SQOR/H2S/sulfane sulfur system protects against neuronal injury is shown in Fig. 15.
Fig. 15.
A potential protection mechanism of SQOR, H2S and polysulfide.
ATP: adenosine triphosphate, DAT: dopamine transporter, ETC: electron transfer chain, Keap-1: Kelch-like ECH-associated protein 1, MAO-B: monoamine oxidase B, NF-κB: nuclear factor kappa B, ROS: reactive oxygen species.
We observed protective effects of trisulfide compounds in MPTP/MPP+-induced cell injury. The small size of Na2S3 permitted us to deliver the molecule intraperitoneally, with the expectation that it would cross the blood brain barrier. Because of the large size of GSSSG, we delivered this compound intranasally and nevertheless saw protective effects. Whether GSSSG crossed the blood brain barrier after intranasal administration is uncertain. Because the sulfane sulfur of polysulfide can be transferred to thiols and persulfides to generate other polysulfides, it is possible that smaller polysulfides or persulfides (e.g. GSSH, CysSSH) are generated by GSSSG and delivered through the blood brain barrier.
There are several potential limitations of the present study. In this murine model, an exogenous chemical (MPTP) was used to acutely induce PD, which is clearly different from the chronic, slowly developing disease in humans. In addition, the behavioral tests used to study the effects of MPTP on murine anxiety and movement only crudely reflect the complex clinical symptoms of PD in humans [61].
Because MPTP and its metabolite MPP + are electrophile while persulfides are nucleophile, we cannot exclude the possibility of direct chemical reaction between MPP+ and persulfides. However, we observed that trisulfides exerted cytoprotective effects at 10 μM against 5 mM of MPP+ (Fig. 12A). This 500-fold difference in the concentration argues against the major role of direct chemical reaction in the beneficial effects of trisulfides.
An additional limitation is that although MPTP induces degeneration of dopaminergic neurons in the nigrostriatal region, this chemical does not induce accumulation of α-synuclein aggregates in Lewy bodies, which are seen in patients with PD [62,63]. Additional studies will be required to examine the potential protective effects of SQOR in PD models that involve α-synuclein aggregation.
4. Conclusion
In conclusion, in the current study, we identified SQOR as a novel therapeutic target in PD. Upregulation of SQOR induced by either SPC or AAV-mediated gene transfer protects dopaminergic neurons in the nigrostriatal regions of the brain in a mouse model of PD. We also found that administration of polysulfides, oxidative products of sulfide catabolism by SQOR, prevents neurodegeneration of dopaminergic neurons by increasing sulfane sulfur levels. These results lay the foundation for further studies examining the role of sulfide catabolism against PD and other neurodegenerative diseases.
Funding sources
This study was supported by sponsored research agreement from Kyowa Hakko Bio Co., Ltd. to Dr. Ichinose.
Authorship confirmation statement
FN, EM, and FI conceived of this study. FN, EM, YM, EK, ME, HH, KS, SB, KO and WJ performed experiments and data analysis. EM and FI supervised the study. FN, DB, FI, and EM wrote the manuscript with consultation from all authors.
Material and methods
Detailed description for the material and methods can be found in the supplement.
Statistical analysis
All data were presented as mean ± SEM and all individual values. An unpaired two-tail Student's t-test was used to compare two independent groups. Normally distributed data were analyzed with the One-way or two-way analysis of variance (ANOVA) with Tukey's multiple comparison test. Significance was considered at the level of p < 0.05. Graphpad Prism for Windows 8.4.3 and Graphpad Prism 6 for Mac OS X (Graphpad Software Inc., La Jolla, CA, USA) was used for statistical analysis.
Declaration of competing interestCOI
Dr. Ichinose receives research funding from Kyowa Hakko Bio Co., Ltd. Drs. Kanemaru, Ezaka, Ichinose, and Marutani are listed as inventors of patents filed by MGH related to GSSSG.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102562.
Contributor Information
Fumito Ichinose, Email: FICHINOSE@mgh.harvard.edu.
Eizo Marutani, Email: EMARUTANI@mgh.harvard.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Alves G., Forsaa E.B., Pedersen K.F., Dreetz Gjerstad M., Larsen J.P. Epidemiology of Parkinson's disease. J. Neurol. 2008;255(Suppl 5):18–32. doi: 10.1007/s00415-008-5004-3. [DOI] [PubMed] [Google Scholar]
- 2.Moore D.J., West A.B., Dawson V.L., Dawson T.M. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 3.Puspita L., Chung S.Y., Shim J.W. Oxidative stress and cellular pathologies in Parkinson's disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schapira A.H., Cooper J.M., Dexter D., Clark J.B., Jenner P., Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 5.Parker W.D., Jr., Parks J.K., Swerdlow R.H. Complex I deficiency in Parkinson's disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 7.Bellucci A., Zaltieri M., Navarria L., Grigoletto J., Missale C., Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson's disease. Brain Res. 2012;1476:183–202. doi: 10.1016/j.brainres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Bellucci A., Mercuri N.B., Venneri A., et al. Review: Parkinson's disease: from synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016;42:77–94. doi: 10.1111/nan.12297. [DOI] [PubMed] [Google Scholar]
- 9.Levitt P., Pintar J.E., Breakefield X.O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonsalla P.K., Wong L.Y., Winnik B., Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance. Exp. Neurol. 2010;221:329–334. doi: 10.1016/j.expneurol.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sai T., Uchida K., Nakayama H. Biochemical evaluation of the neurotoxicity of MPTP and MPP⁺ in embryonic and newborn mice. J. Toxicol. Sci. 2013;38:445–458. doi: 10.2131/jts.38.445. [DOI] [PubMed] [Google Scholar]
- 12.Javitch J.A., Snyder S.H. Uptake of MPP(+) by dopamine neurons explains selectivity of parkinsonism-inducing neurotoxin, MPTP. Eur. J. Pharmacol. 1984;106:455–456. doi: 10.1016/0014-2999(84)90740-4. [DOI] [PubMed] [Google Scholar]
- 13.Obata T. Nitric oxide and MPP+-induced hydroxyl radical generation. J. Neural. Transm. 2006;113:1131–1144. doi: 10.1007/s00702-005-0415-0. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz A., Rey P., Guerra M.J., Mendez-Alvarez E., Soto-Otero R., Labandeira-Garcia J.L. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51:112–120. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Schapira A.H. Mitochondrial dysfunction in Parkinson's disease. Cell Death Differ. 2007;14:1261–1266. doi: 10.1038/sj.cdd.4402160. [DOI] [PubMed] [Google Scholar]
- 16.Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J. Neurochem. 2016;139(Suppl 1):318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 17.Meredith G.E., Rademacher D.J. MPTP mouse models of Parkinson's disease: an update. J. Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiba M., Kajimura M., Goda N., Suematsu M. From O2 to H2S: a landscape view of gas biology. Keio J. Med. 2002;51:1–10. doi: 10.2302/kjm.51.1. [DOI] [PubMed] [Google Scholar]
- 19.Olson K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta Bioenerg. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Akaike T., Ida T., Wei F.-Y., et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landry A.P., Ballou D.P., Banerjee R.H. (2)S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J. Biol. Chem. 2017;292:11641–11649. doi: 10.1074/jbc.M117.788547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morén C., deSouza R.M., Giraldo D.M., Uff C. Antioxidant therapeutic strategies in neurodegenerative diseases. Int. J. Mol. Sci. 2022;23:9328. doi: 10.3390/ijms23169328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strope T.A., Birky C.J., Wilkins H.M. The role of bioenergetics in neurodegeneration. Int. J. Mol. Sci. 2022;23:9212. doi: 10.3390/ijms23169212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ida T., Sawa T., Ihara H., et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi S., Hisatsune A., Kurauchi Y., Seki T., Katsuki H. Polysulfide protects midbrain dopaminergic neurons from MPP+-induced degeneration via enhancement of glutathione biosynthesis. J. Pharmacol. Sci. 2018;137:47–54. doi: 10.1016/j.jphs.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Dóka É., Pader I., Bíró A., et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson K.R. H2S and polysulfide metabolism: conventional and unconventional pathways. Biochem. Pharmacol. 2018;149:77–90. doi: 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Kida K., Yamada M., Tokuda K., et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxidants Redox Signal. 2010;15:343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marutani E., Morita M., Hirai S., et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 2021;12:3108. doi: 10.1038/s41467-021-23363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong R., Searcy D.G. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;129:129–137. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 33.Goubern M., Andriamihaja M., Nübel T., Blachier F., Bouillaud F. Sulfide, the first inorganic substrate for human cells. Faseb. J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 34.Szabo C., Ransy C., Módis K., et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landry A.P., Ballou D.P., Banerjee R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem. 2021;22:949–960. doi: 10.1002/cbic.202000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson M.R., Melideo S.L., Jorns M.S. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 37.Jackson M.R., Melideo S.L., Jorns M.S. In: Methods in Enzymology. Cadenas E., Packer L., editors. Academic Press; 2015. Chapter fourteen - role of human sulfide: quinone oxidoreductase in H2S metabolism; pp. 255–270. [DOI] [PubMed] [Google Scholar]
- 38.Friederich M.W., Elias A.F., Kuster A., et al. Pathogenic variants in SQOR encoding sulfide:quinone oxidoreductase are a potentially treatable cause of Leigh disease. J. Inherit. Metab. Dis. 2020;43:1024–1036. doi: 10.1002/jimd.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrella E., Del Gallo F., Porrini V., et al. Age-dependent neuropsychiatric symptoms in the NF-κB/c-Rel knockout mouse model of Parkinson's disease. Front. Behav. Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.831664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozas G., López-Martín E., Guerra M.J., Labandeira-García J.L. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods. 1998;83:165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 41.Marutani E., Kosugi S., Tokuda K., et al. A novel hydrogen sulfide-releasing N-Methyl-d-Aspartate receptor antagonist prevents ischemic neuronal death. J. Biol. Chem. 2012;287:32124–32135. doi: 10.1074/jbc.M112.374124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marutani E., Sakaguchi M., Chen W., et al. Cytoprotective effects of hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonists mediated by intracellular sulfane sulfur. Med. Chem. Commun. 2014;5:1577–1583. doi: 10.1039/C4MD00180J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanson L.R., Frey W.H., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu M., Zhao F.F., Tang J.J., et al. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxidants Redox Signal. 2012;17:849–859. doi: 10.1089/ars.2011.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu L.F., Lu M., Tiong C.X., Dawe G.S., Hu G., Bian J.S. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 46.Tabassum R., Jeong N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019;16:1386–1396. doi: 10.7150/ijms.36516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M., Zhu J., Pan Y., et al. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson's disease. J. Neurosci. Res. 2015;93:487–494. doi: 10.1002/jnr.23504. [DOI] [PubMed] [Google Scholar]
- 48.Li H., Liu H., Chen Z., et al. Using resonance synchronous spectroscopy to characterize the reactivity and electrophilicity of biologically relevant sulfane sulfur. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wada N., Matsugo S. Revisit of the photoirradiation of α-lipoic acid—role of hydrogen sulfide produced in the reaction. BioChemistry (Rajkot, India) 2021;1:148–158. [Google Scholar]
- 50.Kim S.Y., Woo M.S., Park J.S., Kim H.S. Regulation of matrix metalloproteinase-9 gene expression in MPP+- or 6-OHDA-treated human neuroblastoma SK-N-BE(2)C cells. Neurochem. Int. 2010;56:437–442. doi: 10.1016/j.neuint.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Wu X., Ren Y., Wen Y., et al. Deacetylation of ZKSCAN3 by SIRT1 induces autophagy and protects SN4741 cells against MPP(+)-induced oxidative stress. Free Radic. Biol. Med. 2022;181:82–97. doi: 10.1016/j.freeradbiomed.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Jia J., Wang Z., Zhang M., et al. SQR mediates therapeutic effects of H(2)S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz5752. eaaz5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curry D.W., Stutz B., Andrews Z.B., Elsworth J.D. Targeting AMPK signaling as a neuroprotective strategy in Parkinson's disease. J. Parkinsons Dis. 2018;8:161–181. doi: 10.3233/JPD-171296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen Z., Zhang J., Tang P., Tu N., Wang K., Wu G. Overexpression of miR-185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson's disease. Mol. Med. Rep. 2018;17:131–137. doi: 10.3892/mmr.2017.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson K.R., Straub K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31:60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 56.Toohey J.I., Cooper A.J.L. Thiosulfoxide (sulfane) sulfur: new chemistry and new regulatory roles in biology. Molecules. 2014;19:12789–12813. doi: 10.3390/molecules190812789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toohey J.I. Sulfur signaling: is the agent sulfide or sulfane? Anal. Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 58.Hourihan J.M., Kenna J.G., Hayes J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the Keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxidants Redox Signal. 2012;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 59.Paul B.D., Sbodio J.I., Xu R., et al. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature. 2014;509:96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakinohana M., Kida K., Minamishima S., et al. Delayed paraplegia after spinal cord ischemic injury requires caspase-3 activation in mice. Stroke. 2011;42:2302–2307. doi: 10.1161/STROKEAHA.110.600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potashkin J.A., Blume S.R., Runkle N.K. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2010;2011 doi: 10.4061/2011/658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volpicelli-Daley L.A., Luk K.C., Patel T.P., et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luk K.C., Kehm V., Carroll J., et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.