Abstract
Introduction:
Bile duct involvement is a key finding of primary biliary cholangitis (PBC). The aim of this study was to evaluate baseline ductopenia and disease progression.
Methods:
Retrospective longitudinal histological follow-up of treatment-naïve patients with PBC.
Results:
83 patients were included, with ductopenia correlated to fibrosis stage at baseline. The cumulative incidence of severe ductopenia remained stable after 5 years while fibrosis continually increased over time. Baseline APRI and elevated alkaline phosphatase >2 times the normal with abnormal bilirubin were associated with ductopenia progression.
Conclusion:
Bile duct injury does not appear to follow the same course as fibrosis in PBC.
Keywords: Ductopenia, fibrosis, APRI, histological progression
Introduction
Primary biliary cholangitis (PBC) is a slowly progressive chronic cholestatic liver disease which can ultimately result in cirrhosis and end-stage liver disease.(1) Histological findings of disease progression include bile duct paucity (“ductopenia”) and fibrosis.(2) However, diagnosis of PBC can be made in the presence of chronic cholestasis and anti-mitochondrial antibody (AMA).(3, 4) While non-invasive markers have increasingly replaced liver biopsy in clinical management of liver disease,(5-7) histological findings, predominantly fibrosis, are still considered objective, predictive factors.(8) In PBC, ductopenia has also been linked to disease progression and is a predictive factor for response to treatment.(9, 10) The aim of this study was to evaluate baseline ductopenia and to determine factors associated with PBC histological progression in a historical cohort.
Methods
This was a retrospective study of treatment-naïve patients with PBC followed at the National Institutes of Health (NIH) between 1979 and 2020. Diagnosis of PBC was established by liver biopsy in all cases, and patients with evidence of other chronic liver disease on biopsy were excluded. The AST-to-Platelet Ratio Index (APRI) cut-off value of 0.54 was retained as elevated APRI.(7) Blinded evaluation of biopsies was performed by a single hepatopathologist. Fibrosis was evaluated using the Ishak scoring system. Presence of bile ducts in portal areas was expressed as a ratio ranging from 0% (no ducts identified) to 100% (a bile duct in each portal area). Severity of ductopenia was scored as 0 when ≥75% of portal areas had bile ducts, 1+ for 50-75%, 2+ for 25-50% or 3+ for <25%.(11) Patients with follow-up liver biopsies were included in the longitudinal histological follow-up cohort. Information regarding data collection and statistical analysis can be found in the supplementary section (see Supplemental Digital Content 1).
Results:
83 treatment-naïve patients with biopsy proven PBC were considered for analysis. The average age at biopsy was 50.7 years (range 32 to 68 years); 89% were women and 88% were white. AMA was present in 71 patients (86%), and 12 of the 15 AMA-negative subjects were positive for antinuclear antibody (ANA). 63% of patients had minimal fibrosis (Ishak 0-2), 31% moderate fibrosis (Ishak 3-4) and 6% cirrhosis (Ishak 5-6). Bile duct loss was scored as 0 in 26%, 1+ in 27%, 2+ in 25% and 3+ in 22%. Average biopsy size was 19.5 mm (standard deviation (SD) 9.9) with 15.9 portal tracts (SD 10.1) per sample on average. Baseline characteristics stratified by degree of ductopenia are presented in Table 1 (See Table, Supplemental Digital Content 2, for baseline characteristics by fibrosis stage). Strikingly, while liver tests were statistically different between the four groups, platelet counts did not differ significantly and no correlation was found with magnitude of ductopenia (p=0.7) Overall, a strong correlation between fibrosis and ductopenia was observed (See Figure, Supplemental Digital Content 3). However a subgroup of 6 patients with severe ductopenia (<25%) and minimal fibrosis (Ishak fibrosis stage <3) was identified, of which 3 progressed to cirrhosis within 5 years, and 3 underwent liver transplantation within 10 years.
Table 1.
Baseline characteristics of entire cohort based on ductopenia score on index biopsy
| Score=0 n=21 |
Score=1 n=22 |
Score=2 n=20 |
Score=3 n=18 |
P | |
|---|---|---|---|---|---|
| Age (years) | 51 (35-67) | 52 (34-68) | 51 (32-67) | 49 (35-66) | 0.83 |
| White blood cell count (x 109/L) | 7.9 (3.2-13.8) | 7.2 (2.4-10.0) | 6.0 (3.1-10.9) | 6.6 (2.5-10.2) | 0.07 |
| Absolute neutrophil count (x 109/L) | 4.3 (1.3-8.3) | 4.2 (1.2-5.7) | 3.3 (1.6-6.4) | 3.7 (1.5-6.4) | 0.10 |
| Absolute lymphocyte count (x 109/L) | 2.5 (1.2-6.1) | 2.1 (0.7-3.9) | 2.0 (0.8-3.5) | 2.2 (0.7-4.0) | 0.43* |
| Hemoglobin (g/dL) | 13.8 (11.8-16.0) | 13.6 (11.9-16.1) | 13.1 (10.5-15.0) | 12.8 (10.9-14.5) | 0.05 |
| Platelet count (x 109/L) | 276 (85-403) | 282 (126-439) | 236 (94-357) | 274 (108-432) | 0.35 |
| Prothrombin time (sec.) | 11.9 (10.8-14.4) | 11.7 (10.6-13.7) | 11.5 (10.0-12.8) | 11.6 (10.4-12.8) | 0.57 |
| Total bilirubin (mg/dL) | 0.8 (0.3-2.6) | 0.8 (0.3-2.9) | 1.1 (0.5-5.0) | 2.0 (0.4-7.1) | 0.01 * |
| Direct bilirubin (mg/dL) | 0.3 (0-1.9) | 0.3 (0-2.9) | 0.5 (0-3.1) | 1.3 (0-5) | 0.002 * |
| ALT (U/L) | 99 (23-298) | 77 (26-137) | 100 (28-192) | 152 (77-314) | 0.002 # |
| AST (U/L) | 72 (20-259 | 60 (15-112) | 79 (26-149) | 121 (53-246) | 0.001 # |
| ALP (U/L) | 284 (78-1010 | 399 (68-1350) | 544 (113-1290) | 995 (256-2620) | 0.001 # |
| GGT (U/L) | 416 (26-1110) | 473 (36-1140) | 543 (49-1488) | 1083 (425-2095) | 0.001 |
| Albumin (mg/dL) | 4.1 (3.6-4.5) | 4.0 (2.9-4.8) | 3.9 (3.3-4.6) | 3.9 (3.3-4.7) | 0.63 |
| Creatinine (mg/dL) | 0.9 (0.7-1.2) | 1.0 (0.7-1.3) | 0.9 (0.6-1.3) | 0.9 (0.7-1.1) | 0.49 |
| IgM (mg/dL) | 460 (144-1200) | 480 (140-928) | 535 (144-1520) | 518 (95-1410) | 0.92* |
| APRI | 0.73 (0.15-2.15) | 0.62 (0.14-1.51) | 0.98 (0.22-2.60) | 1.25 (0.42-2.73) | 0.008 * |
| Mayo risk score | 3.97 (2.49-5.31) | 4.09 (2.71-6.35) | 4.21 (2.70-6.36) | 4.55 (3.38-6.11) | 0.23 |
| Ishak fibrosis | 1.57 (0-4) | 2.05 (0-4) | 2.30 (0-6) | 3.17 (1-6) | 0.009 * |
| Mean Ductular reaction | 0.48 (0-0.88) | 0.74 (0.43-1.33) | 0.64 (0.15-1.1) | 0.79 (0.38-1.33) | 0.28 |
| HAI Periportal inflammation | 2.7 (1-5) | 3.3 (1-6) | 3 (1-6) | 2.8 (1-6) | 0.55* |
| HAI Portal inflammation | 1.7 (1-4) | 1.5 (0-3) | 2.1 (0-3) | 1.7 (1-3) | 0.46* |
| Copper | 0.82 (0-3) | 0.60 (0-2) | 1.62 (1-3) | 1.46 (0-3) | 0.01 * |
| CK7 staining periportal | 1.20 (0-3) | 1.67 (1-3) | 1.67 (1-3) | 2.00 (1-3) | 0.16* |
| CK7 staining zone 3 | 0.80 (0-2) | 0.83 (0-1) | 1.22 (0-2) | 1.22 (0-2) | 0.36* |
All values are presented as mean and range and p values are obtained by ANOVA unless specified otherwise
Kruskal-Wallis test
log transform
Paired liver biopsies were available in 59 patients (71%), performed on average 5.4 years apart (Figure 1). Prior to the last biopsy, 87% of patients had received treatment, including 31% receiving UDCA.
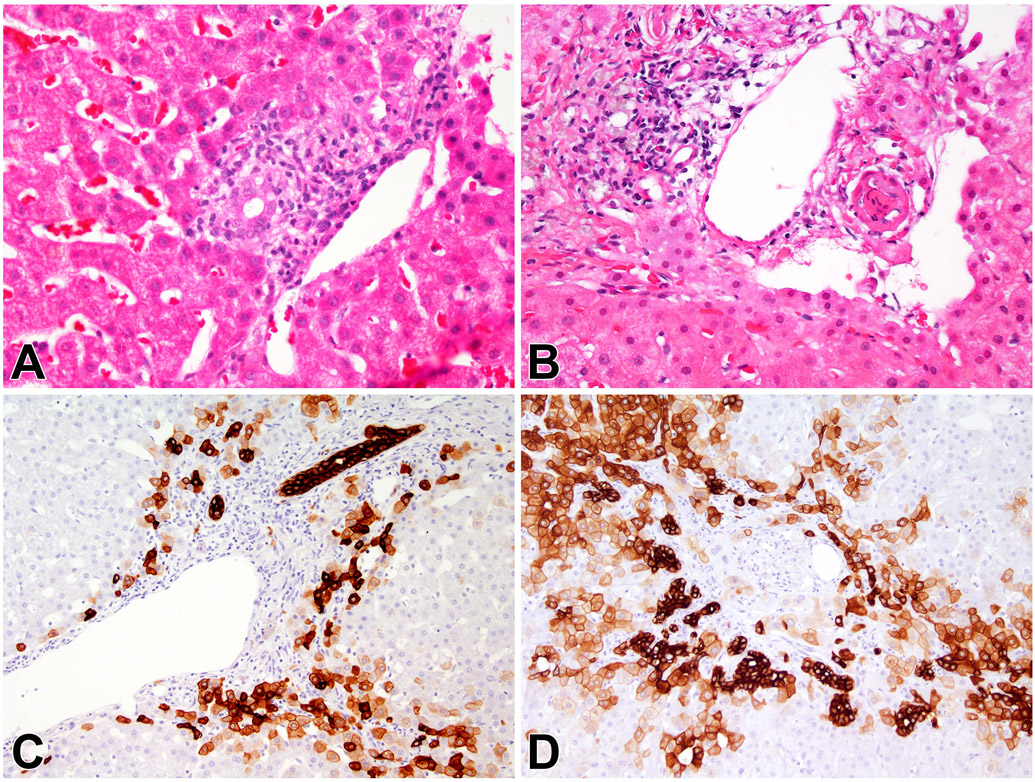
Figure 1.
Progression of Ductopenia. A, C. The initial biopsy showed inflamed portal areas with duct injury. (Panel A, H&E, 400x), while keratin 7 staining shows positive staining in periportal hepatocytes (Panel C, anti-keratin 7, 200x). B, D. A second biopsy obtained four years later shows ductopenic portal areas. (Panel B, H&E, 400x) and the keratin 7 stain shows ductular reaction and more extensive hepatocyte staining (Panel D, anti-keratin 7, 200x).
Ductopenia did not differ significantly in paired samples (53% to 48%, p=0.43). Average Ishak fibrosis score was 2.9 (SD 1.6), with significant progression of fibrosis in paired samples (p=0.006) and more advanced fibrosis stage overall (See Figure, Supplemental Digital Content 4).
Cumulative incidence of progression of fibrosis by two stages and bile duct loss to severe ductopenia in the overall cohort was 5% in each group at 1 year and increased to 20% and 22% at 5 years for fibrosis and ductopenia respectively. However, the cumulative incidence of increase in fibrosis reached 37% at 10 years and 55% at 15 years while ductopenia progression remained stable (See Figure, Supplemental Digital Content 5).
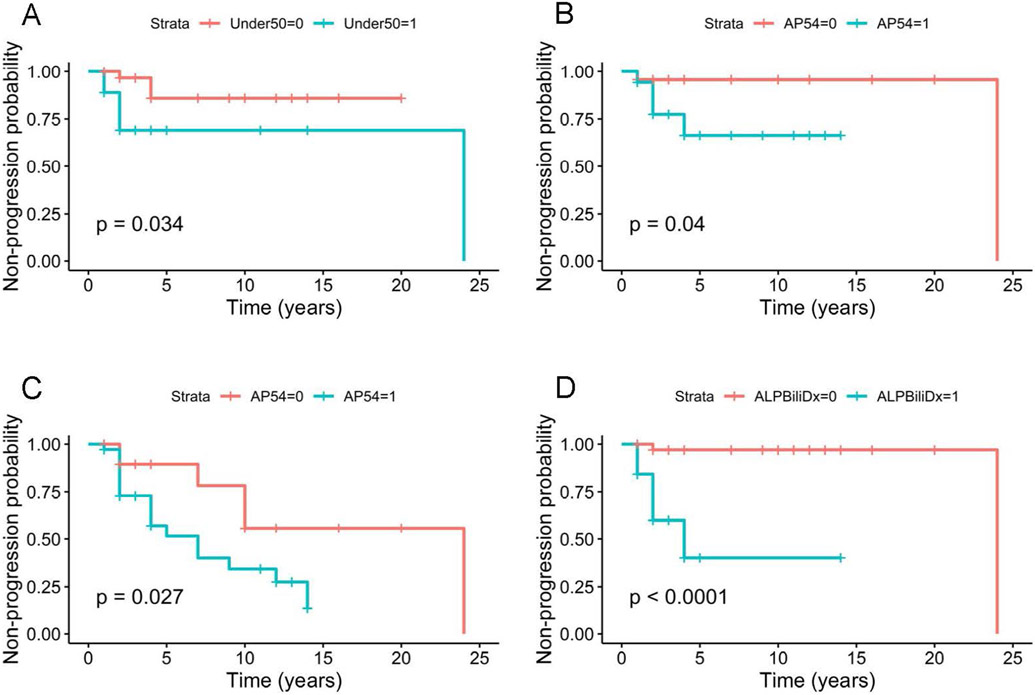
Patients with baseline ductopenia score 2+ progressed significantly to severe ductopenia as opposed to patients with a score of 0 or 1+ (Figure 2A). In addition, elevated baseline APRI, as well as baseline ALP > 2 times the normal with an elevated bilirubin were associated with a significant progression of ductopenia on follow-up biopsy (Figures 2B-D).
Figure 2.
Kaplan Meier analysis of progression of ductopenia. (A) Progression to severe ductopenia (<25%) stratified by initial ductopenia score 0 or 1 (>50% bile ducts) in red and initial ductopenia score 2+ (25 to 50%) in blue, (B) progression to severe ductopenia stratified by baseline elevated baseline APRI in blue, (C) overall decrease in bile duct count from first biopsy on final biopsy stratified by baseline elevated APRI in blue and (D) progression to severe ductopenia with baseline ALP > 2 times the upper limit of normal with an elevated bilirubin in blue. P value log rank score.
Discussion
This study demonstrates the correlation between the severity of ductopenia and common markers of disease severity at diagnosis. In addition, while fibrosis and ductopenia remain strongly associated, some patients present with ductopenia out of proportion to their fibrosis stage.
Disease outcomes in PBC are also influenced by factors other than fibrosis, most notably a pre-sinusoidal component of portal hypertension and ductopenia.(12-14) Cases with ductopenia out of proportion to fibrosis have been identified, deemed a “premature ductopenic variant”, with a severe clinical course.(9) These clinically relevant findings can only be confirmed through liver biopsy which provides an overall histological assessment of PBC, as highlighted in expert opinion.(15)
The prognostic significance of histological features should be correlated to surrogate serologic markers. Baseline APRI has been shown as a marker of adverse clinical events in PBC.(7) In our cohort, baseline APRI as well as elevated ALP and bilirubin correlated with ductopenia on initial biopsy as well as progression of duct injury. A striking finding, however, was the lack of association of severity of ductopenia and platelet counts, which might be accounted for by the mild severity of disease at initial biopsy.
This single center retrospective study is limited by a relatively small number of subjects. Furthermore, clinical events occurred infrequently as most patients had early stage disease and were enrolled before the availability of UDCA. Nonetheless, this cohort benefited from long follow-up as well as longitudinal histological data with careful assessment of features.
In PBC, fibrosis and ductopenia, while remaining associated, may run different courses in select individuals. A subgroup of patients present with ductopenia out of proportion to fibrosis stages. Overall, the progression of bile duct loss appears to be slower than fibrosis progression. In addition, commonly used serological prognostic markers are associated with progression of ductopenia over time.
Supplementary Material
Financial support:
This work was supported by the NIDDK Intramural Research Program.
Footnotes
Potential competing interests: The authors conducted this work as employees of the National Institutes of Health and have no conflicts of interest to disclose.
References
- 1.Lleo A, Leung PSC, Hirschfield GM, et al. The Pathogenesis of Primary Biliary Cholangitis: A Comprehensive Review. Semin Liver Dis 2020;40:34–48. [DOI] [PubMed] [Google Scholar]
- 2.Poupon R, Chazouilleres O, Balkau B, et al. Clinical and biochemical expression of the histopathological lesions of primary biliary cirrhosis. UDCA-PBC Group. J Hepatol 1999;30:408–12. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–172. [DOI] [PubMed] [Google Scholar]
- 4.Lindor KD, Bowlus CL, Boyer J, et al. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69:394–419. [DOI] [PubMed] [Google Scholar]
- 5.Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015;149:1804–1812 e4. [DOI] [PubMed] [Google Scholar]
- 6.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology 2014;147:1338–49 e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi PJ, Bruns T, Cheung A, et al. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J Hepatol 2014;60:1249–58. [DOI] [PubMed] [Google Scholar]
- 8.Murillo Perez CF, Hirschfield GM, Corpechot C, et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther 2019;50:1127–1136. [DOI] [PubMed] [Google Scholar]
- 9.Vleggaar FP, van Buuren HR, Zondervan PE, et al. Jaundice in non-cirrhotic primary biliary cirrhosis: the premature ductopenic variant. Gut 2001;49:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010;105:2186–94. [DOI] [PubMed] [Google Scholar]
- 11.Bonkovsky HL, Kleiner DE, Gu J, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017;65:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colina F, Pinedo F, Solis JA, et al. Nodular regenerative hyperplasia of the liver in early histological stages of primary biliary cirrhosis. Gastroenterology 1992;102:1319–24. [PubMed] [Google Scholar]
- 13.Navasa M, Pares A, Bruguera M, et al. Portal hypertension in primary biliary cirrhosis. Relationship with histological features. J Hepatol 1987;5:292–8. [DOI] [PubMed] [Google Scholar]
- 14.Huet PM, Vincent C, Deslaurier J, et al. Portal hypertension and primary biliary cirrhosis: effect of long-term ursodeoxycholic acid treatment. Gastroenterology 2008;135:1552–60. [DOI] [PubMed] [Google Scholar]
- 15.Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D, et al. The challenges of primary biliary cholangitis: What is new and what needs to be done. J Autoimmun 2019;105:102328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.