Abstract
Background:
Alcohol use is a major global healthcare burden which contributes to numerous adverse health outcomes, including liver disease. Many factors influence individual susceptibility to alcohol-associated diseases, including nutritional factors. The objective of the current study was to examine inter-relations between alcohol, dietary micro- and macronutrient consumption, and liver health by analyzing data from the 2017-2018 National Health and Nutrition Examination Survey (NHANES).
Methods:
Based on self-reported alcohol consumption, NHANES respondents were assigned into four categories: never drinkers (lifetime abstainers), non-drinkers (past-year abstainers), moderate drinkers (1/2 drinks per day for females/males, respectively), and heavy drinkers (>1/>2 drinks per day for females/males, respectively, and/or frequent binge drinking). Survey-weighted regression analyses (adjusted for gender, age, race, education, and body mass index) were performed to examine associations between alcohol intake, dietary, and liver health characteristics.
Results:
Individuals categorized as heavy drinkers were significantly younger, most often well-educated males with low incidences of diabetes and other comorbidities. They consumed the most overall calories and various micronutrients, indicating a diet which was not necessarily nutrient-poor. Neither moderate nor heavy drinkers had liver steatosis or fibrosis as measured by liver elastography, although heavy drinkers had modestly elevated plasma biomarkers of liver injury, including ALT, AST, and GGT, compared to the other groups.
Conclusions:
Our findings suggest that the category of heavy drinkers in the 2017-2018 NHANES consisted of generally healthy individuals with high energy intake and no evidence of liver steatosis or fibrosis. However, slightly increased plasma liver markers may indicate a risk of future progression to more advanced stages of liver disease over time in some individuals. Several limitations should be considered when interpreting these data, including the potential misclassification of drinking categories and the lack of standardized cutoff scores for fatty liver as assessed by elastography, among several others.
Keywords: National Health and Nutrition Examination Survey, alcohol consumption, dietary macro- and micronutrients, liver health and disease
Introduction
Excessive alcohol consumption has significant worldwide public health and economic implications. According to the 2018 World Health Organization report on Alcohol and Health, alcohol use contributes to three million deaths each year globally, and plays a significant role in the development of poor health outcomes for millions of individuals (Organization, 2018). Overall, 5.1% of global disease burden and injury is attributable to alcohol (Organization, 2018), and the healthcare burden of alcohol consumption in the United States in 2010 alone was estimated at $28.4 billion (Sacks et al., 2015). Harmful alcohol consumption can lead to multiple pathological conditions, including alcohol-associated liver disease (ALD), which is currently recognized as a major cause of alcohol-related morbidity and mortality in the United States and worldwide (Seitz et al., 2018, Rehm and Shield, 2019). ALD is characterized by a wide spectrum of liver pathology, ranging from reversible liver steatosis to steatohepatitis, fibrosis, and cirrhosis, with a subsequent risk of hepatocellular carcinoma (Louvet and Mathurin, 2015, Seitz et al., 2018). Although alcohol intake is the most important risk factor for developing ALD (Savolainen et al., 1993), there are several other modifiable and non-modifiable factors contributing to disease risk including sex (Eagon, 2010), genetics (Anstee et al., 2015, Lazo et al., 2021, Buch et al., 2015), epigenetics (Page et al., 2015, Habash et al., 2022), various comorbidities such as obesity (Alatalo et al., 2008, Patra et al., 2021), and multiple environmental factors, such as nutrition (Kirpich et al., 2016, Mendenhall et al., 1995, Mendenhall et al., 1984, Wu and Meng, 2020, Nanji and French, 1986, Dasarathy, 2016, Zirnheld et al., 2019, Lieber, 2000).
Accumulating evidence suggests that alcohol-related factors such as daily intake amount, duration of alcohol consumption, and drinking pattern play a role in ALD development and contribute to disease progression and severity (Aberg et al., 2017, Delacote et al., 2020, Askgaard et al., 2015, Zakhari and Li, 2007, Simpson et al., 2019). Most individuals chronically consuming large amounts of alcohol over the course of the day for >2 weeks develop reversible hepatic steatosis as shown in early studies by Lieber and coworkers in healthy volunteers (Rubin and Lieber, 1968), however, only 10-20% will further progress to advanced ALD (Seitz et al., 2018). Analysis of chronic liver disease prevalence in the general population revealed that daily intake of 30 g ethanol may increase the risk of developing cirrhosis or non-cirrhotic liver damage in men and women (Bellentani et al., 1997). In the United States, one standard drink contains approximately 14 g of ethanol. As per the National Institute on Alcohol Abuse and Alcoholism (NIAAA), more than 4 drinks on any day or more than 14 drinks per week for men, and more than 3 drinks on any day or more than 7 drinks per week for women is considered heavy drinking (NIAAA, 2022). A binge drinking pattern, which is defined by the NIAAA as consumption of ≥ 5 drinks for men and ≥ 4 for women in a 2-hour period, can also increase the risk of developing ALD (Aberg et al., 2017).
Imbalance in dietary macro- and micronutrients has been also recognized as a significant risk factor for the development and progression of ALD. Research in preclinical animal models showed that an ethanol-containing diet rich in n6-polyunsaturated fatty acids (PUFAs) resulted in more severe manifestations of liver injury as compared to an ethanol-containing diet rich in saturated fats (Kirpich et al., 2012). It has been also shown that n3-PUFA supplementation (Huang et al., 2013, Wada et al., 2008) or endogenous reduction in the n6/n3 PUFA ratio (Warner et al., 2019, Warner et al., 2021) were beneficial in experimental ALD. In humans, significant correlations have been observed between rates of alcohol-associated cirrhosis mortality and consumption of dietary animal fat and alcohol (Nanji and French, 1986). Further, inadequate dietary protein intake was associated with poor outcome in patients with severe alcohol-associated hepatitis (Mendenhall et al., 1995, Mendenhall et al., 1984, Moreno et al., 2016). In addition, dietary and metabolic imbalances in micronutrients including zinc, iron, copper, magnesium, selenium, vitamin D, and vitamin E are common in ALD patients, and may contribute to ALD pathogenesis (Wu and Meng, 2020).
The objective of the current study was to analyze the latest 2017-2018 National Health and Nutrition Examination Survey (NHANES) with the goal of evaluating alcohol use and dietary intake of macro- and micronutrients in a representative cohort of the US population, and to examine the association between alcohol consumption, dietary factors, and liver health using liver elastography endpoints new to the 2017-2018 NHANES.
Materials and Methods
Study population and exclusion criteria
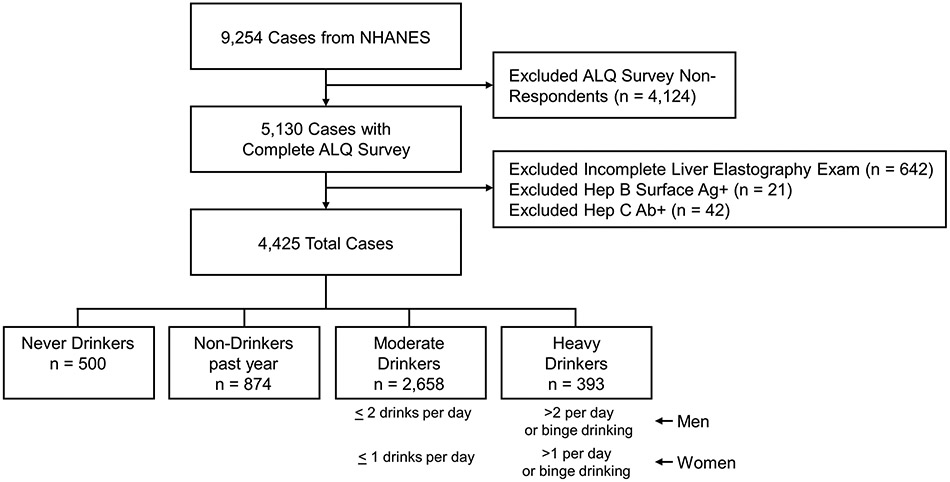
The current study used data from the 2017-2018 NHANES, the latest iteration of the National Health and Nutrition Examination Survey, administered by the Centers for Disease Control and Prevention to noninstitutionalized civilian adult residents of the United States. 9,254 individuals participated in the 2017-2018 NHANES. During their visits to the Mobile Examination Center, participants 18 years of age and older were administered an Alcohol Use Questionnaire (ALQ), a computer-assisted personal interview questionnaire focusing on lifetime and current alcohol use over the last 12 months. The ALQ was administered by a trained Examination Center interviewer. There were 4,124 ALQ non-responders, which were excluded from our study population. Further, of the 5,130 ALQ survey respondents, individuals without a complete liver elastography exam (n = 642) and subjects with positive hepatitis B (n = 21) or hepatitis C (n = 42) antibody serology were excluded for a final sample of 4,425 individuals (Fig. 1).
Figure 1.
Establishment of respondent sample population. Of the 9,254 total NHANES survey participants, we excluded individuals who did not participate in the Alcohol Use Questionnaire, individuals with a partial elastography exam, and individuals with a positive hepatitis B surface antigen or hepatitis C antibody status. Following these exclusion criteria, 4,425 total survey respondents remained, comprising our final sample population. Abbreviations: Ab, antibody; Ag, antigen; ALQ, Alcohol Use Questionnaire; Hep B, hepatitis B; Hep C, hepatitis C; NHANES, National Health and Nutrition Examination Survey.
Categorization of participants into drinking groups
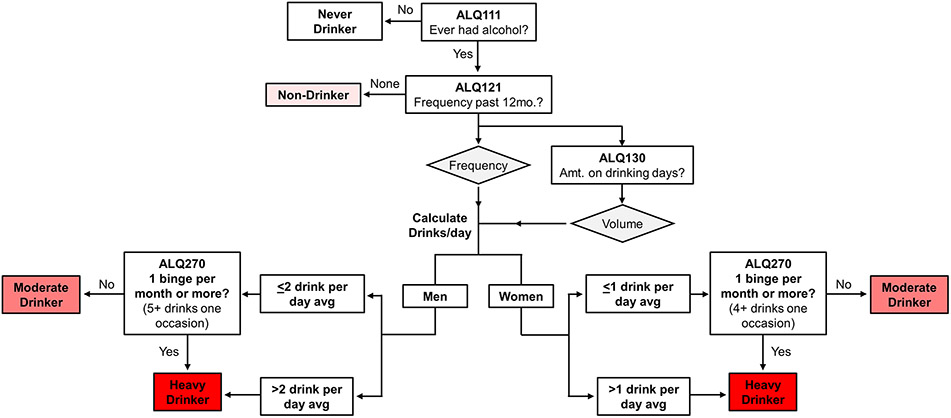
Based on questionnaire responses, ALQ respondents were assigned to four drinking groups: never drinkers, non-drinkers, moderate drinkers, and heavy drinkers (Fig. 1 and Fig. 2). “Never drinkers” were defined as individuals who responded “no” when asked if they had consumed at least one alcoholic drink during their lifetime, excluding small sips. “Non-drinkers” were defined as individuals who reported some degree of alcohol consumption in their lifetime but responded that they had “never in the last year” consumed alcohol when asked how often alcoholic drinks were consumed during the past 12 months. “Never drinkers” and “Non-drinkers” were considered separately based on previous recommendations (Butler et al., 2018).
Figure 2.
Schematic of strategy to categorize included respondents into drinking categories based on the NHANES ALQ. NHANES respondents who completed the ALQ were categorized into drinking groups based on questionnaire responses. Never drinkers were individuals who denied consumption of alcohol in their lifetime, excluding small sips (ALQ111). Non-drinkers were those who reported consumption of alcohol in their lifetime but denied alcohol use in the past year (ALQ121). Of the respondents who reported alcohol use within the past year, women and men with a daily average of ≤ 1 or ≤ 2 drinks, respectively, were considered moderate drinkers as calculated based on responses to ALQ121 and ALQ130. Heavy drinkers were any individuals with one or more self-reported binge drinking episodes per month (ALQ270) and individuals exceeding the daily average consumption limits for moderate drinking. Abbreviations: ALQ, Alcohol Use Questionnaire; avg, average; d, day; NHANES, National Health and Nutrition Examination Survey.
In order to classify moderate and heavy drinkers, we took into account guidelines from both the 2020-2025 Dietary Guidelines for Americans (DGA) (DGA, 2020) and the NIAAA (NIAAA, 2022). Based on the 2020-2025 DGA guidelines, moderate drinkers were defined as men who reported an average consumption of 2 drinks or less and women who reported an average of 1 drink or less per day. Heavy drinkers were classified by taking into account the NIAAA definition of heavy drinking as more than 4 drinks on any day or more than 14 drinks/week for men, and more than 3 drinks on any day or more than 7 drinks/week for women (NIAAA, 2022). However, the NHANES ALQ provides only a limited amount of information on the actual pattern of drinking among respondents, which makes evaluation of whether individuals drank >4/3 drinks on a single day but less than 14/7 per week for males/females difficult. Specifically, the questionnaire asks only the frequency of alcohol consumption (survey question ALQ121) and the number of standard drinks consumed on those drinking days (survey question ALQ130). To best match this information to DGA and NIAAA guidelines, then, we derived drinks per day from these two variables by multiplying them and adjusting for time. For example, if an individual reported drinking 3-4 times per week and consuming two drinks on those occasions, drinks per day was calculated as [(2 drinks per occasion x 3.5 occasions per week) / (7 days per week)] = 1 drink per day average (a moderate drinker). Heavy drinkers were men who drank on average > 2 drinks/day, and women who drank on average > 1 drink/day (equal to the 14/7 drinks per week for men/women in accordance with NIAAA guidance). Therefore, we assigned our study population into drinking groups based on calculated alcohol consumption as average number of standard drinks consumed per day (not the number of drinks consumed on any single day). In addition, individuals who reported binge drinking once or more per month on a separate survey question (survey question ALQ270, which asks about drinking ≥ 5 drinks for men and ≥ 4 for women in a 2-hour period, as per the NIAAA definition of binge drinking) were considered as Heavy drinkers.
Analysis of dietary intake
All NHANES participants were eligible for two 24-hour dietary recall interviews. The first dietary interview was administered in person in a Mobile Examination Center. A set of measuring guides, including measuring cups and spoons, was available in the interview room for reference for participants to use when reporting the amount of food items consumed. The second dietary interview was obtained by telephone three to ten days after the initial interview and was generally scheduled on a different day of the week than the initial in-person interview. Participants were given measuring cups, spoons, a ruler, and a food model booklet upon completion of the in-person interview to take home and use for reference when reporting amount of food items consumed during the subsequent telephone interview. In the current study, dietary recalls obtained during the initial in-person interview (specifically Day 1 Dietary Recalls) were used for analysis of nutritional intake, since self-reporting is likely more reliable when the interview is administered in-person.
Parameters used to characterize liver health
NHANES “Laboratory Data” variables such as alanine aminotransferase (ALT, LBXSATSI), aspartate aminotransferase (AST, LBXSASSI), gamma glutamyl transferase (GGT, LBXSGTSI), and “Examination Data”, specifically liver transient elastography variables, such as Controlled Attenuation Parameter (CAP, LUXCAPM) and Liver Stiffness Measurement (LSM, LUXSMED), were utilized to characterize liver health. Since the 2017-2018 NHANES survey was the first to include liver transient elastography, only data from these years were included. To define different stages of steatosis, CAP scores were categorized into four categories: S0 < 300 dB/m (no steatosis), S1 300-331 dB/m (5-33% steatosis), S2 332-337 dB/m (34-66% steatosis), and S3 > 337 dB/m (≥67% steatosis) (Eddowes et al., 2019). Liver stiffness values were categorized as: F0-1 < 8.2 kPa (no fibrosis), and F2 8.2-9.6 kPa, F3 9.7-13.5 kPa, or F4 > 13.6 kPa (varying degrees of fibrosis) (Eddowes et al., 2019). Equipment and techniques used for elastography are described in detail in the NHANES Liver Ultrasound Transient Elastography Procedures Manual (accessible via https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2017).
Statistical analysis
Data were reported as survey-weighted mean ± standard error. Means were survey-weighted using the WTMEC2YR variable based on established methods (Heeringa et al., 2017), as recommended by the NHANES Analytical Guidelines (Fakhouri et al., 2020). Survey-weighted multiple linear regression models were used to test associations between nutritional factors, alcohol consumption and liver elastography parameters, adjusted by demographic variables including gender, age, race, education, and body mass index (BMI). For continuous variables, statistical significance was determined by a survey-weighted Student’s t-test for comparisons between two groups or a survey-weighted one-way analysis of variance (ANOVA) with post-hoc tests for comparisons between more than two groups. For categorical variables, statistical significance was determined by a survey-weighted Chi-square test. A p value < 0.05 (2-tailed) was considered statistically significant. All p values were adjusted for demographic variables, specifically, gender, age, ethnicity, education, and BMI. Statistical analyses were conducted using R version 3.5.3 and GraphPad Prism software version 9.0.1 (San Diego, CA).
Results
Characterization of the study population
Our study population of 4,425 individuals was divided into four categories based on drinking behavior. There were 11.3% never drinkers (lifetime abstainers), 19.8% non-drinkers (past-year abstainers), 60% moderate drinkers (1/2 or less drinks per day for females/males, respectively), and 8.9% “heavy drinkers” (>1 or >2 drinks per day for females and males, respectively, or at least monthly binge drinking). Demographic characteristics of our study participants, including gender, age, ethnicity, education, physical activity, and diabetes status are shown in Table 1. The proportion of males generally tended to increase as drinking category advanced (i.e., from never drinkers to non-drinkers to moderate, to heavy), while the proportion of females generally tended to decrease. With respect to age, apart from never drinkers, age generally decreased with increasing alcohol use, with heavy drinkers being the youngest at ~41/42 years old for males/females. Notably, the average age of male never-drinkers was 22 years lower than the average age of female never-drinkers, suggesting that men may start drinking at an earlier age than women. With respect to physical activity (work-related), the proportion of individuals performing adequate physical work increased with increasing alcohol use from ~37-41% in never- and non-drinkers to ~45% and ~54% in moderate and heavy drinkers, respectively. The proportion of individuals performing adequate physical recreation (leisure-related), however, was overall lower and increased from never/non-drinkers (~36 and ~24%, respectively) to moderate (43%), then declined in heavy drinkers (~38%). There were also some differences in the prevalence of several comorbidities such as diabetes, cancer, and cardiovascular disease between drinking categories. Never drinkers had a lower proportion of individuals with diabetes as compared to non-drinkers by roughly 4% (21.4% to 16.9%), and interestingly, this proportion decreased further in moderate and heavy drinkers (to ~11% and 7%, respectively). The proportion of individuals with pre-diabetes, and the incidence of any type of cancer and cardiovascular disease (specifically coronary artery disease, congestive heart failure, angina, or heart attack) followed a similar trend. Initial analysis of body measures and nutrition revealed that heavy drinkers were the tallest group on average, and despite consuming the greatest number of calories had no differences in BMI.
Table 1.
Demographics of sample population. Gender, age, ethnicity, education, adequacy of physical work and physical recreation, and diabetes distributions for total sample population and individual drinking groups. All variables except age, cancer incidence, cardiovascular disease incidence, height, body mass index, and energy intake are reported as weighted mean percentage (SEM) of total subpopulation. Remaining variables are reported as weighted mean value (SEM) in the indicated units. Cardiovascular disease incidence represents the combined incidences of any of the following: congestive heart failure, coronary heart disease, angina, or heart attack. P values reported for diabetes, comorbidities, body measures, and nutrition endpoints are calculated from multivariate analysis where data were adjusted for gender, age, race, education, and BMI. Abbreviations: N, number; SEM, standard error of the mean.
| Total number |
Never Drinkers |
Non- Drinkers |
Moderate Drinkers |
Heavy Drinkers |
P Value | ||
|---|---|---|---|---|---|---|---|
| 4425 | 500 | 874 | 2658 | 393 | |||
| Gender | Male | 49.9 (1.0) | 39.0 (2.9) | 50.8 (2.0) | 49.1 (1.8) | 62.2 (2.9) | <0.01 |
| Female | 50.1 (1.0) | 61.0 (2.9) | 49.2 (2.0) | 50.9 (1.8) | 37.8 (2.9) | ||
| Age (years) | Male | 46.0 ± 0.7 | 38.1 ± 2.4 | 56.7 ± 1.1 | 45.1 ± 0.9 | 40.8 ± 1.3 | <0.01 |
| Female | 47.8 ± 0.8 | 50.9 ± 2.0 | 57.3 ± 1.2 | 45.9 ± 0.9 | 41.5 ± 1.8 | <0.01 | |
| Ethnicity | Non-Hispanic White | 62.9 (2.5) | 49.3 (5.3) | 63.5 (3.0) | 64.3 (2.5) | 63.3 (3.3) | <0.01 |
| Non-Hispanic Black | 11.0 (1.6) | 13.5 (2.7) | 11.6 (1.6) | 10.5 (1.6) | 11.1 (2.1) | ||
| Non-Hispanic Asian | 5.3 (0.9) | 17.9 (2.4) | 4.5 (0.9) | 4.5 (0.8) | 1.9 (0.7) | ||
| Other Hispanic | 6.8 (0.8) | 6.5 (1.3) | 6.9 (1.3) | 7.2 (0.9) | 4.6 (1.4) | ||
| Mexican American | 9.3 (1.6) | 9.1 (2.6) | 7.7 (1.5) | 9.1 (1.6) | 12.7 (2.4) | ||
| Other race-including Multi-Racial | 4.8 (0.6) | 3.7 (1.8) | 5.7 (1.0) | 4.4 (0.6) | 6.4 (1.6) | ||
| Education | Less than 9th grade | 3.4 (0.6) | 8.9 (1.4) | 6.7 (1.3) | 2.1 (0.4) | 2.8 (0.7) | <0.01 |
| 9-12 grade | 8.7 (0.5) | 13.5 (1.9) | 11.9 (1.2) | 7.0 (0.6) | 10.9 (1.6) | ||
| High school graduate | 27.2 (1.6) | 27.1 (3.3) | 30.9 (1.9) | 26.6 (1.9) | 25.3 (3.9) | ||
| College or AA | 29.6 (1.4) | 21.4 (3.5) | 27.4 (2.4) | 30.5 (1.7) | 33.7 (2.9) | ||
| College graduate or above | 31.1 (2.7) | 29.2 (5.0) | 23.0 (3.0) | 33.8 (3.1) | 27.3 (3.5) | ||
| Physical Work | Adequate | 44.7 (1.5) | 36.7 (3.5) | 40.6 (2.2) | 45.2 (1.6) | 54.4 (4.2) | 0.01 |
| Inadequate | 55.3 (1.5) | 63.3 (3.5) | 59.4 (2.2) | 54.8 (1.6) | 45.6 (4.2) | ||
| Physical Recreation | Adequate | 38.9 (1.8) | 35.8 (3.4) | 23.7 (2.9) | 43.0 (1.9) | 38.1 (4.0) | <0.01 |
| Inadequate | 61.1 (1.8) | 64.2 (3.4) | 76.3 (2.9) | 57.0 (1.9) | 61.9 (4.0) | ||
| Diabetes | Prediabetes | 21.1 (0.9) | 24.7 (4.5) | 29.5 (3.0) | 19.2 (1.0) | 17.0 (2.2) | <0.01 |
| Normal | 66.1 (1.0) | 58.4 (4.1) | 49.1 (2.8) | 69.5 (1.1) | 76.1 (2.5) | ||
| Diabetes | 12.9 (0.7) | 16.9 (3.0) | 21.4 (2.3) | 11.3 (0.9) | 6.9 (1.7) | ||
| Comorbidities | Cancer Incidence (%) | 10.7 (1.4) | 10.6 (1.8) | 16 (1.8) | 10.3 (1.2) | 2.2 (0.9) | <0.01 |
| Cardiovascular Disease Incidence (%) | 6.4 (0.9) | 4.3 (1.4) | 12.2 (1.4) | 5.2 (0.6) | 3.9 (1.5) | <0.01 | |
| Body Measures | Height (cm) | 168.0 (0.4) | 164.7 (0.7) | 167.0 (0.4) | 168.4 (0.3) | 171.7 (0.7) | <0.01 |
| Body Mass Index (kg/m2) | 29.4 (0.3) | 28.3 (0.5) | 29.6 (0.5) | 29.6 (0.3) | 29.2 (0.4) | 0.10 | |
| Nutrition | Energy Intake (kcal) | 2177.6 (44.5) | 1899.4 (74.6) | 2060.3 (59.2) | 2203.1 (27.8) | 2619.7 (86.4) | <0.01 |
Characterization of alcohol consumption
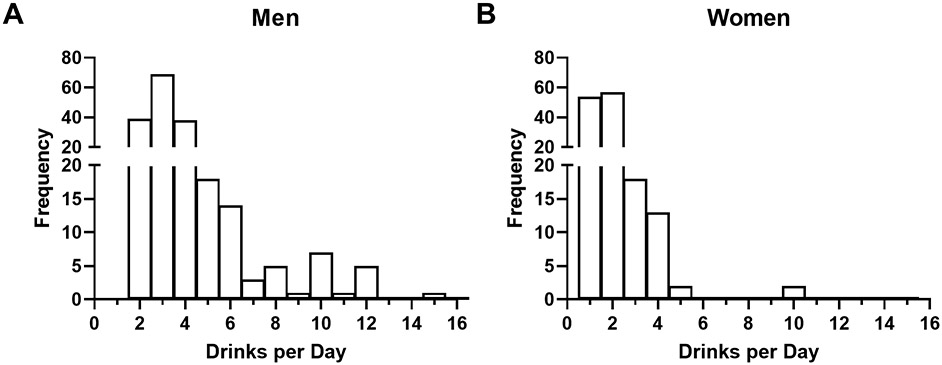
Among moderate drinkers, both males and females consumed a mean of less than one standard drink per day, with men consuming on average more than twice as much as women (Table 2). Heavy drinkers, by comparison, consumed an average of roughly 3 or 2 standard drinks per day for males and females, respectively, indicating that even in this drinking category, the average alcohol consumption only just exceeded the lower cutoff of heavy drinking as per our definition. Further, among male and female heavy drinkers, the distribution of average drinks per day, while skewed right for both genders, had a higher number of male individuals consuming 3+ drinks per day than females (Fig. 3 A-B, respectively), further confirming higher alcohol consumption in males.
Table 2.
Alcohol consumption in moderate and heavy drinkers reported as average standard drinks consumed per day. Data reported as both mean value ± SEM and median.
| Drinks per Day | Moderate Drinkers | Heavy Drinkers | |
|---|---|---|---|
| Men | Mean | 0.362 ± 0.013 | 3.164 ± 0.165 |
| Median | 1 | 3 | |
| Women | Mean | 0.159 ± 0.007 | 2.040 ± 0.301 |
| Median | 1 | 2 | |
Figure 3.
Distribution of volume of alcohol consumption in heavy drinkers. (A and B) Frequency distribution of average daily standard drink consumption for men and women, respectively.
Analysis of nutrient intake and anthropometric measurements
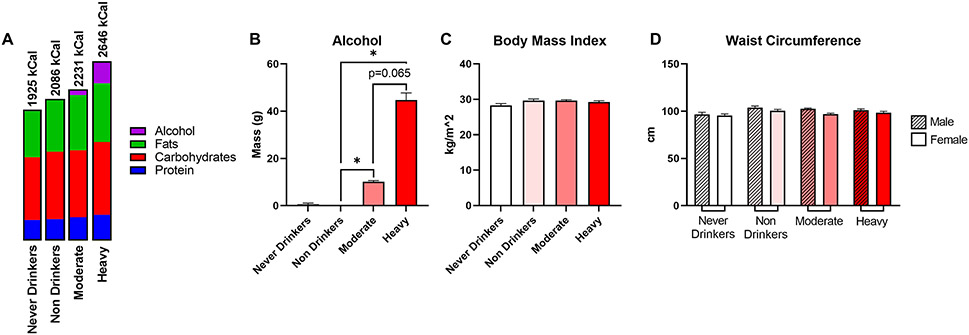
Analysis of nutrient intake revealed that heavy drinkers consumed more total calories than any other drinking category (Fig. 4A). While this difference was expected due to significantly increased alcohol intake in this group (Fig. 4B), heavy drinkers also consumed a slightly higher amount of all three macronutrients (protein, carbohydrates, and total fat) compared to other drinking groups (Table 3). Notably, despite a slight increase in calorie consumption and macronutrient content between heavy drinkers and other groups, there were no significant differences in anthropometric measurements including BMI (Fig. 4C) and waist circumference in males and females (Fig. 4D).
Figure 4.
Characterization of macronutrient intake and anthropometric variables between respondent groups. (A) Overview of daily energy intake with percentage of kcal from carbohydrates, fat, protein, and alcohol. (B) Average daily alcohol consumption as reported in the 24-hour dietary recall data, not data obtained from the alcohol use questionnaire. (C) Body mass index. (D) Waist circumference by gender. Data are presented as weighted mean value ± SEM. All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: cm, centimeters; g, grams; kcal, kilocalories; kg, kilogram, m, meter.
Table 3.
Macronutrient and fatty acid intake values between drinking categories. Data are reported as weighted mean value (SEM). All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: g, gram; kcal, kilocalories; SEM, standard error of the mean.
| Never Drinkers |
Non- Drinkers |
Moderate Drinkers |
Heavy Drinkers |
Overall P Value |
Never vs. Non P Value |
Never vs. Moderate P Value |
Never vs. Heavy P Value |
Non vs. Moderate P Value |
Non vs. Heavy P Value |
Moderate vs. Heavy P Value |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | 1899.4 (74.6) | 2060.3 (59.2) | 2203.1 (27.8) | 2619.7 (86.4) | <0.01 | 0.08 | 0.01 | 0.12 | 0.40 | 0.15 | 0.30 |
| Protein (g) | 74.1 (3.2) | 77.1 (3.2) | 84.6 (1.3) | 93.5 (3.8) | 0.01 | 0.18 | 0.01 | 0.11 | 0.37 | 0.26 | 0.39 |
| Carbohydrate (g) | 234.1 (8.3) | 251.4 (7.0) | 249.6 (3.8) | 270.9 (11.5) | 0.26 | 0.56 | 0.01 | 0.42 | 0.72 | 0.71 | 0.68 |
| Total fat (g) | 76.4 (4.4) | 85.7 (2.7) | 91.4 (1.3) | 97.2 (3.4) | 0.03 | 0.31 | 0.01 | 0.21 | 0.51 | 0.47 | 0.37 |
| Total saturated fatty acids (g) | 25.4 (1.7) | 28.1 (0.9) | 30.1 (0.4) | 31.6 (1.3) | 0.15 | 0.51 | 0.01 | 0.08 | 0.49 | 0.63 | 0.47 |
| Total monounsaturated fatty acids (g) | 25.6 (1.6) | 29.4 (1.0) | 31.3 (0.6) | 33.2 (1.2) | 0.02 | 0.26 | 0.01 | 0.22 | 0.49 | 0.47 | 0.32 |
| Total polyunsaturated fatty acids (g) | 17.8 (1.0) | 19.8 (0.8) | 21.2 (0.5) | 22.9 (1.0) | 0.030 | 0.31 | 0.02 | 0.41 | 0.60 | 0.38 | 0.38 |
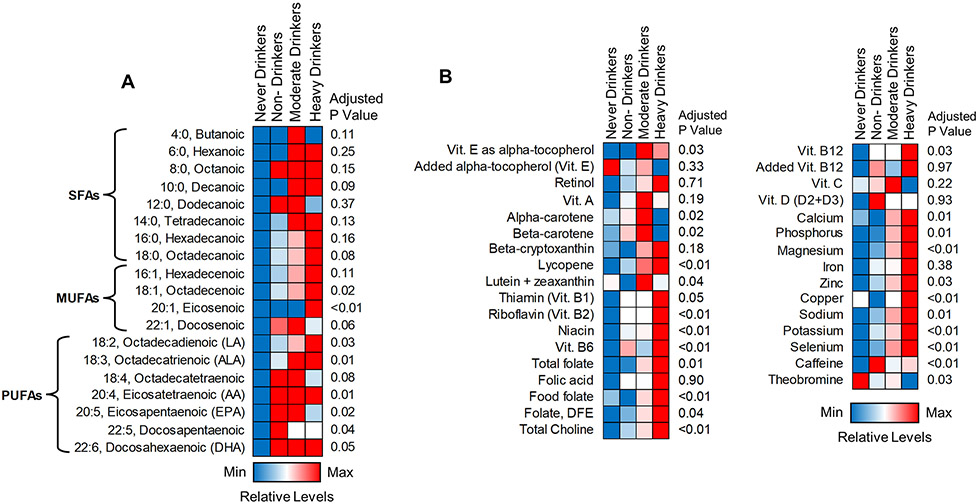
Further analysis of individual types of dietary fatty acids (FAs, including saturated, mono- and poly-unsaturated FAs [SFAs, MUFAs and PUFAs, respectively]) revealed a similar trend with total fat consumption, where never drinkers consumed the lowest amount and heavy drinkers consumed the highest amount of each type of FAs (Table 3, Fig. 5A). However, there were several individual FAs, including dodecanoic (12:0), docosenoic (22:1), octadecatetraenoic (18:4), eicosapentaenoic (20:5), and docosapentaenoic acids (22:5), whose consumption was decreased (although not statistically significant) in heavy drinkers relative to moderate or non-drinkers. Of note, there was an overall non-significant upward trend in consumption of total n3-PUFAs (apart from eicosapentaenoic [20:5]) as well as total n6-PUFAs, with no significant differences in the n6/n3 PUFA ratio between drinking categories (Table 4). Lastly, consistent with macronutrient intake, consumption/supplementation of most micronutrients trended upwards across drinking groups with the highest levels in heavy drinkers (Fig. 5B, Table 5). However, some notable exceptions were observed. Specifically, the intake of several micronutrients with antioxidant properties, such as added alpha tocopherol (Vitamin E), vitamin C, and several carotenoids (e.g., alpha- and beta-carotene) were the lowest (although not statistically significant) in heavy drinkers as compared to other groups.
Figure 5.
Visualization of fatty acid and micronutrient intake between respondent groups. (A) Heatmap showing daily intake trends in individual dietary fatty acids. (B) Heatmap showing daily intake of individual micronutrients. Colors represent relative consumption levels, with red being the maximum level and red being the minimum level for each individual variable. All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: ANOVA, analysis of variance; DFE, dietary food equivalents; MFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; SFAs, saturated fatty acids; vit., vitamin.
Table 4.
Individual fatty acid intake values and n3/n6-PUFA ratio between drinking categories. The n3/n6 PUFA ratio was calculated as (ALA + EPA + DHA) / (LA + AA). Data are weighted mean (SEM). All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; g, grams; LA, linoleic acid; n3, omega 3; n6, omega 6; PUFA, polyunsaturated fatty acid.
| Never Drinkers |
Non- Drinkers |
Moderate Drinkers |
Heavy Drinkers |
Overall P Value |
Never vs. Non P Value |
Never vs. Moderate P Value |
Never vs. Heavy P Value |
|
|---|---|---|---|---|---|---|---|---|
| 4:0, Butanoic (g) | 0.5 (0.043) | 0.5 (0.022) | 0.6 (0.02) | 0.5 (0.035) | 0.11 | 0.57 | 0.02 | 0.04 |
| 6:0, Hexanoic (g) | 0.3 (0.027) | 0.3 (0.014) | 0.4 (0.012) | 0.4 (0.026) | 0.25 | 0.54 | 0.02 | 0.04 |
| 8:0, Octanoic (g) | 0.2 (0.016) | 0.3 (0.014) | 0.3 (0.008) | 0.3 (0.019) | 0.15 | 0.72 | 0.06 | 0.38 |
| 10:0, Decanoic (g) | 0.5 (0.038) | 0.5 (0.02) | 0.6 (0.017) | 0.6 (0.039) | 0.08 | 0.36 | 0.02 | 0.04 |
| 12:0, Dodecanoic (g) | 0.8 (0.1) | 1.1 (0.1) | 1.1 (0.033) | 0.9 (0.1) | 0.37 | 0.82 | 0.22 | 0.80 |
| 14:0, Tetradecanoic (g) | 2.1 (0.2) | 2.3 (0.1) | 2.6 (0.1) | 2.6 (0.2) | 0.13 | 0.50 | 0.01 | 0.04 |
| 16:0, Hexadecanoic (g) | 14.1 (1) | 15.3 (0.5) | 16.2 (0.2) | 17.4 (0.7) | 0.16 | 0.55 | 0.01 | 0.12 |
| 18:0, Octadecanoic (g) | 6.0 (0.4) | 6.8 (0.2) | 7.2 (0.1) | 7.8 (0.3) | 0.08 | 0.47 | 0.01 | 0.07 |
| 16:1, Hexadecenoic (g) | 1.1 (0.1) | 1.2 (0.046) | 1.3 (0.038) | 1.4 (0.1) | 0.11 | 0.51 | 0.02 | 0.79 |
| 18:1, Octadecenoic (g) | 24.1 (1.5) | 27.7 (1.0) | 29.4 (0.5) | 31.2 (1.1) | 0.02 | 0.25 | 0.01 | 0.21 |
| 20:1, Eicosenoic (g) | 0.3 (0.015) | 0.3 (0.023) | 0.3 (0.009) | 0.4 (0.014) | 0.01 | 0.19 | 0.02 | 0.64 |
| 22:1, Docosenoic (g) | 0.023 (0.004) | 0.037 (0.004) | 0.038 (0.005) | 0.034 (0.005) | 0.06 | 0.41 | 0.11 | 0.74 |
| 18:2 (n6), Octadecadienoic (g) | 15.8 (0.9) | 17.5 (0.7) | 18.8 (0.4) | 20.5 (0.9) | 0.03 | 0.32 | 0.02 | 0.40 |
| 18:3 (n3), Octadecatrienoic (g) | 1.6 (0.1) | 1.9 (0.1) | 2.0 (0.1) | 2.0 (0.1) | 0.01 | 0.38 | 0.02 | 0.56 |
| 18:4 (n3), Octadecatetraenoic (g) | 0.007 (0.001) | 0.01 (0.002) | 0.01 (0.001) | 0.009 (0.002) | 0.08 | 0.90 | 0.11 | 0.37 |
| 20:4 (n6), Eicosatetraenoic (g) | 0.1 (0.008) | 0.2 (0.011) | 0.2 (0.004) | 0.2 (0.012) | 0.01 | 0.27 | 0.02 | 0.64 |
| 20:5 (n3), Eicosapentaenoic (g) | 0.023 (0.004) | 0.032 (0.005) | 0.032 (0.003) | 0.028 (0.008) | 0.02 | 0.48 | 0.22 | 0.14 |
| 22:5 (n3), Docosapentaenoic (g) | 0.023 (0.002) | 0.032 (0.005) | 0.028 (0.002) | 0.028 (0.002) | 0.04 | 0.48 | 0.02 | 0.77 |
| 22:6 (n3), Docosahexaenoic (g) | 0.046 (0.007) | 0.1 (0.011) | 0.1 (0.004) | 0.1 (0.013) | 0.05 | 0.50 | 0.17 | 0.23 |
| n6/n3 PUFA Ratio | 9.527 (0.787) | 8.711 (0.552) | 8.912 (0.459) | 9.727 (0.627) | 0.03 | 0.41 | 0.39 | 0.41 |
Table 5.
Individual micronutrient intake values between drinking categories. Data are reported as weighted mean (SEM). All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: mg, milligram; μg, microgram; SEM, standard error of the mean.
| Never Drinkers |
Non-Drinkers | Moderate Drinkers |
Heavy Drinkers |
Overall P Value |
Never vs. Non P Value |
Never vs. Moderate P Value |
Never vs. Heavy P Value |
|
|---|---|---|---|---|---|---|---|---|
| Vitamin E as alpha-tocopherol (mg) | 8.7 (0.6) | 8.7 (0.3) | 9.7 (0.3) | 9.3 (0.5) | 0.03 | 0.93 | 0.03 | 0.25 |
| Added alpha-tocopherol (Vitamin E) (mg) | 1.1 (0.3) | 0.9 (0.1) | 1.0 (0.1) | 0.7 (0.3) | 0.33 | 0.17 | 0.41 | 0.14 |
| Retinol (μg) | 398.4 (22.2) | 409.4 (17.4) | 420 (10.1) | 451.0 (40.1) | 0.71 | 0.98 | 0.02 | 0.07 |
| Vitamin A (μg) | 567.2 (31.7) | 604.4 (30.3) | 667.0 (25.9) | 609.3 (43.5) | 0.19 | 0.51 | 0.06 | 0.19 |
| Alpha-carotene (μg) | 306.6 (48.1) | 323.7 (52.8) | 419.1 (51.3) | 284.2 (37.7) | 0.02 | 0.56 | 0.82 | 0.40 |
| Beta-carotene (μg) | 1831.8 (207.6) | 2138.1 (187.3) | 2711.7 (219.8) | 1713.5 (158.6) | 0.02 | 0.33 | 0.24 | 0.58 |
| Beta-cryptoxanthin (μg) | 80.2 (12.3) | 78.7 (6.9) | 84.7 (6.3) | 89.1 (17.7) | 0.18 | 0.55 | 0.14 | 0.48 |
| Lycopene (μg) | 3389.9 (355.1) | 4361.9 (484.3) | 5270.7 (350.7) | 5617.4 (516.4) | <0.01 | 0.11 | 0.08 | 0.38 |
| Lutein + zeaxanthin (μg) | 1443.7 (257.6) | 1271.5 (95.8) | 1751.9 (172.4) | 1423.6 (130.2) | 0.04 | 0.35 | 0.27 | 0.50 |
| Thiamin (Vitamin B1) (μg) | 1.5 (0.1) | 1.6 (0.1) | 1.6 (0.032) | 1.8 (0.1) | 0.05 | 0.54 | 0.49 | 0.34 |
| Riboflavin (Vitamin B2) (μg) | 1.8 (0.1) | 2.1 (0.1) | 2.1 (0.041) | 2.5 (0.1) | <0.01 | 0.26 | 0.25 | 0.15 |
| Niacin (mg) | 21.8 (0.7) | 25.8 (1.9) | 26.4 (0.4) | 33 (1.4) | <0.01 | 0.34 | 0.17 | 0.12 |
| Vitamin B6 (mg) | 1.8 (0.1) | 2.4 (0.4) | 2.1 (0) | 2.7 (0.2) | <0.01 | 0.39 | 0.33 | 0.17 |
| Total Folate (μg) | 358.5 (14.9) | 360.5 (7.6) | 383.4 (8.8) | 442.5 (18.1) | 0.01 | 0.75 | 0.68 | 0.28 |
| Folic Acid (μg) | 159.7 (11.4) | 171.3 (7.7) | 171.1 (4.1) | 190.8 (16.4) | 0.90 | 0.64 | 0.80 | 0.64 |
| Food Folate (μg) | 198.9 (10.1) | 189.4 (6.3) | 213.3 (7.1) | 251.8 (8.7) | <0.01 | 0.39 | 0.66 | 0.19 |
| Folate as Dietary Folate Equivalents (μg) | 469.9 (21.5) | 480 (11.9) | 502.8 (10.8) | 575.6 (28.7) | 0.04 | 0.97 | 0.71 | 0.35 |
| Choline (mg) | 284.3 (12.7) | 315 (15.1) | 341.5 (5.2) | 422.7 (21.7) | <0.01 | 0.72 | 0.25 | 0.13 |
| Vitamin B12 (μg) | 3.9 (0.2) | 4.9 (0.4) | 4.9 (0.1) | 5.9 (0.5) | 0.03 | 0.41 | 0.29 | 0.24 |
| Added Vitamin B12 (μg) | 0.7 (0.1) | 1.2 (0.3) | 0.9 (0.1) | 1.4 (0.3) | 0.97 | 0.35 | 0.69 | 0.37 |
| Vitamin C (mg) | 70.1 (4.7) | 71.8 (2.9) | 75.7 (3.6) | 64.8 (4.7) | 0.22 | 0.81 | 0.04 | 0.05 |
| Vitamin D (D2 + D3) (μg) | 4.1 (0.3) | 4.5 (0.5) | 4.2 (0.2) | 4.2 (0.4) | 0.93 | 0.90 | 0.98 | 0.98 |
| Calcium (mg) | 919.3 (44.6) | 880.8 (27.1) | 992.4 (19.4) | 1071.2 (45.1) | 0.01 | 0.23 | 0.01 | 0.05 |
| Phosphorous (mg) | 1283.3 (52.9) | 1296.4 (45.3) | 1427.3 (23.4) | 1594 (51.9) | 0.01 | 0.56 | 0.58 | 0.28 |
| Magnesium (mg) | 273.7 (10.5) | 282.6 (7.3) | 308.1 (7.0) | 342.1 (10.6) | <0.01 | 0.07 | 0.01 | 0.04 |
| Iron (mg) | 13.6 (0.7) | 14.1 (0.4) | 14.2 (0.2) | 15.8 (0.7) | 0.38 | 0.65 | 0.01 | 0.11 |
| Zinc (mg) | 9.9 (0.4) | 10.7 (0.5) | 11.4 (0.2) | 12.6 (0.7) | 0.03 | 0.30 | 0.01 | 0.04 |
| Copper (mg) | 1.2 (0.1) | 1.1 (0.029) | 1.2 (0.028) | 1.3 (0.049) | 0.00 | 0.35 | 0.74 | 0.71 |
| Sodium (mg) | 3067 (132.4) | 3302.8 (105.4) | 3669.1 (66.2) | 4027.7 (133.6) | 0.01 | 0.59 | 0.25 | 0.22 |
| Potassium (mg) | 2323.1 (79.6) | 2538.1 (52.7) | 2633.3 (53.3) | 2838.8 (83.7) | <0.01 | 0.60 | 0.29 | 0.18 |
| Selenium (μg) | 103.3 (3.2) | 107.7 (4.8) | 119.6 (1.6) | 128.7 (5.2) | <0.01 | 0.15 | 0.01 | 0.18 |
| Caffeine (mg) | 85.4 (6.4) | 213.7 (14.5) | 170.2 (6.9) | 182.5 (12.2) | <0.01 | 0.09 | 0.08 | 0.10 |
| Theobromine (mg) | 57.1 (11.6) | 38.6 (3.5) | 41.3 (1.9) | 29.6 (5.1) | 0.03 | 0.31 | 0.34 | 0.23 |
Evaluation of parameters characterizing liver health
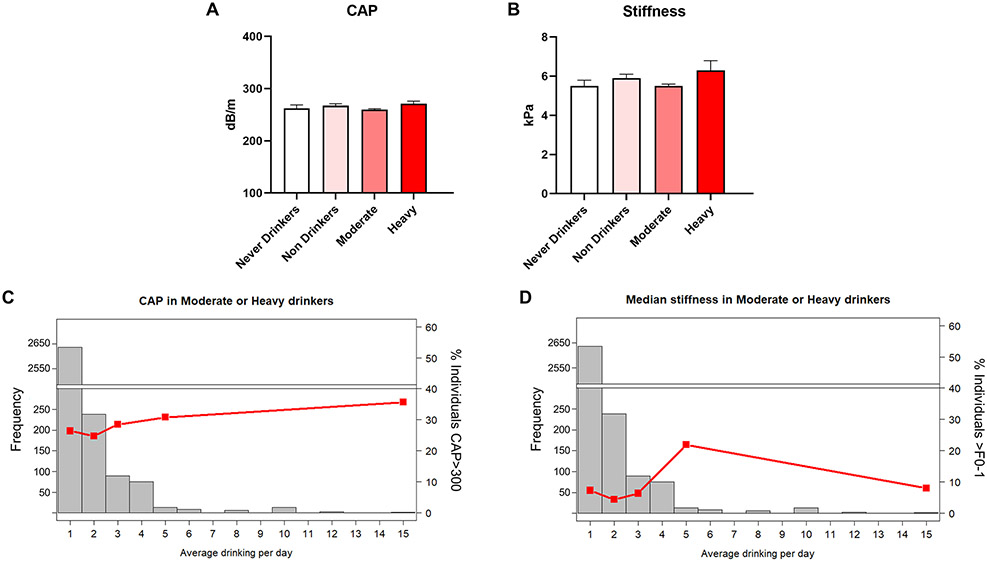
Given the critical role of alcohol consumption and nutrition in individual susceptibility to liver disease, we next analyzed measures of liver pathology reported in the NHANES database. Specifically, we accessed liver elastography data (a new addition to the NHANES survey in 2017-2018) to identify differences in liver fat content and liver fibrosis, as reported by CAP score and liver stiffness variables, respectively. The mean CAP and stiffness values were within S0 and F0-1 categories, respectively, across all drinking groups, despite some modest differences between never and heavy drinkers (Fig. 6A-B), indicating that heavy drinking status, as per our definition, was still not sufficient to be associated with a clinically relevant increase in liver elastography measures on average. Further analysis demonstrated limited effects of an increase in average drinks per day on either CAP score or liver stiffness (Fig. 6C-D). When stratifying individuals into two or four groups based on CAP score (S0 and >S0, or S0, S1, S2, and S3), we still observed only weak trends in the proportion of individuals in each CAP score category between drinking groups (Table 6). Similarly, when stratifying individuals into four categories based on liver stiffness (F0-1, F2, F3, and F4), there were no consistent trends that would indicate a meaningful increase in liver fibrosis with increased alcohol use (Table 6).
Figure 6.
Characterization of liver steatosis and fibrosis. (A) Liver CAP score. (B) Liver stiffness. (C-D) Liver steatosis and fibrosis in heavy drinkers with increasing average daily alcohol consumption in standard drinks, respectively. Histogram bars represent frequency of individuals in each bin (left Y axis), lines represent percent of individuals exceeding a CAP of 300 dB/m or a stiffness measurement of 8.6 kPa (right Y axes), respectively. Data are presented as weighted mean value ± SEM. All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: CAP, controlled attenuation parameter; dB, decibel; GGT, gamma-glutamyl transferase; kPa, kilopascal; m, meter.
Table 6.
Distribution of CAP and stiffness categories across drinking categories. Proportions of individuals in each CAP category (broken down into two or four levels) or in each fibrosis category (broken down into four levels) are shown. Data are reported as weighted mean percentage (SEM) of subpopulation. All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: CAP, controlled attenuation parameter; N, number; SEM, standard error of the mean.
| Overall | Never Drinkers | Non-Drinkers | Moderate Drinkers |
Heavy Drinkers | P Value | ||
|---|---|---|---|---|---|---|---|
| CAP (2 Levels) | S0 | 72.1 (1.1) | 67.3 (4.5) | 68.5 (2.3) | 73.8 (1.3) | 70.8 (3.5) | 0.17 |
| >S0 | 27.9 (1.1) | 32.7 (4.5) | 31.5 (2.3) | 26.2 (1.3) | 29.2 (3.5) | ||
| CAP (4 Levels) | S0 | 72.1 (1.1) | 67.3 (4.5) | 68.5 (2.3) | 73.8 (1.3) | 70.8 (3.5) | 0.15 |
| S1 | 12.8 (0.6) | 16.6 (2.5) | 14.2 (1.3) | 12.4 (0.9) | 10.1 (1.7) | ||
| S2 | 1.7 (0.3) | 1.6 (0.6) | 1.8 (0.7) | 1.7 (0.3) | 1.5 (0.5) | ||
| S3 | 13.4 (1.0) | 14.5 (3.6) | 15.6 (1.8) | 12.1 (1.0) | 17.6 (2.8) | ||
| Stiffness | F0-1 | 92.2 (0.8) | 92.6 (2.2) | 90.3 (1.3) | 92.8 (0.8) | 91.2 (2.7) | 0.43 |
| F2 | 2.6 (0.4) | 2.3 (0.6) | 3.6 (0.8) | 2.6 (0.5) | 1.5 (0.5) | ||
| F3 | 2.8 (0.3) | 1.8 (0.8) | 3.4 (0.8) | 2.7 (0.4) | 3.0 (1.5) | ||
| F4 | 2.4 (0.3) | 3.2 (2.0) | 2.7 (0.9) | 1.9 (0.3) | 4.3 (1.8) |
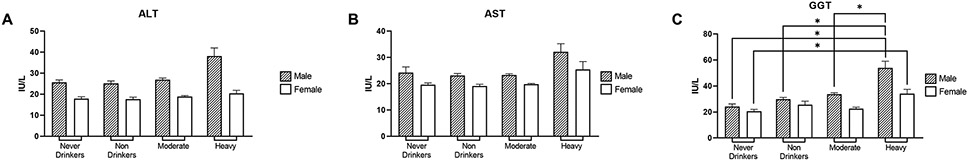
We next examined plasma biochemical markers of liver injury such as ALT, AST, and GGT to further characterize liver health in our population. In contrast to liver steatosis and fibrosis, all three liver biomarkers were elevated (particularly GGT being statistically significant) in heavy drinkers compared to moderate drinkers and were always higher in males than in females (Fig. 7A-C).
Figure 7.
Characterization of liver injury markers. (A-C) Plasma levels of liver injury biomarkers ALT, AST, and GGT, respectively, by gender. Data are presented as weighted mean value ± SEM. All data and associated p values were adjusted for gender, age, race, education, and BMI using multivariate analysis. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; IU, international unit; L, liter.
Discussion
The current study analyzed alcohol consumption and dietary intake of macro- and micronutrients and their associations with parameters that characterize liver health using data from the 2017-2018 NHANES. Based on self-reported drinking behavior, our study cohort was divided into four categories, including “moderate drinkers”, “heavy drinkers”, and two nondrinking comparison groups, “never drinkers” and “non-drinkers”, which were considered separately based on previous recommendations as inclusion of these two groups in one category may confound results (Butler et al., 2018). The moderate drinking category included almost 60% of our study population, whereas ~9% belonged to heavy drinking group. Among moderate drinkers, both males and females consumed a mean of less than one standard drink per day, with men consuming on average more than twice as much as women. Heavy drinkers, by comparison, consumed an average of roughly 3 or 2 standard drinks per day, which could reach 21 or 14 standard drinks per week for men/women, respectively, in agreement with the NIAAA heavy drinking definition of more than 14/7 standard drinks per week for men/women, respectively. Of note, there is evidence that the gap in alcohol consumption between males and females in the US is decreasing due to several reasons, including increased drinking in females with no change in males’ behavior (White, 2020).
Alcohol consumption and nutrition are both well-known modifying factors of alcohol-associated multi-organ pathology (Lieber, 2000). Our analysis revealed that individuals categorized as heavy drinkers consumed the highest amounts of each macronutrient (carbohydrates, protein, and fat), and consequently, had the highest total calorie intake (~2600 kcal on average), even when disregarding extra calories from alcohol. However, this increased energy intake is made less remarkable when considering the greater energy intake requirements of the heavy drinking group based on their demographic characteristics. Given that individuals categorized as heavy drinkers were most males of roughly 40 years old, the FDA-recommended energy intake would be estimated at ~2400 kcal or ~2600 kcal daily for ‘sedentary’ and ‘moderately active’ individuals, respectively (DGA, 2020). Of note, no significant differences in anthropometric measurements including BMI and waist circumference were observed between drinking category groups in the current analysis, similar to analyses of 2003-2012 NHANES datasets (Butler et al., 2018). In contrast, studies on very heavy drinking patients with alcohol-associated hepatitis (consuming ~15 drinks per day) show that “empty calories” are a major concern (Barve et al., 2017). In addition, analysis of same-day associations between alcohol and diet in NHANES 2003-2008 revealed that even moderate drinkers had poorer diets on drinking days (Breslow et al., 2013), suggesting the need for dietary education in all subjects consuming alcohol. The findings in the current study, though, are in line with recent reports establishing a novel disease state known as “both alcohol-associated and non-alcohol-associated steatohepatitis”, or “BASH”, wherein individuals are consuming high calories and drinking alcohol, resulting in a liver disease state of mixed etiologies (Sanchez-Jimenez et al., 2018). Our analysis further demonstrated that heavy drinkers consumed larger amounts of multiple PUFAs with an n6/n3 PUFA ratio of ~9:1 across all studied groups. A previous analysis from our group of 2015-2016 NHANES data found an n6/n3 PUFA ratio of roughly 9:1 for US citizens 20 years and older (Zirnheld et al., 2019), suggesting that intake of n6 PUFAs in the American diet relative to n3 PUFAs remains highly imbalanced in favor of n6 PUFAs. This skewed PUFA intake pattern may facilitate inflammatory processes in the liver, predisposing individuals to or advancing the progression of diseases such as non-alcohol-associated fatty liver disease (NAFLD) and ALD (or BASH, for that matter) (Valenzuela and Videla, 2011, Zirnheld et al., 2019). In the current study, we further found that heavy drinkers also had the highest intake of most micronutrients, including zinc, which has been suggested to exert beneficial effects in liver diseases (Mohammad et al., 2012). Notable exceptions to this trend, however, included certain antioxidant micronutrients such as vitamin E and several carotenoids. Numerous pre-clinical and clinical studies support the function of these micronutrients as antioxidants which play a role in ameliorating the oxidative stress that contributes to the initiation and progression of liver injury (Li et al., 2015, Sanyal et al., 2010, Christensen et al., 2019, Senoo et al., 2010). However, these findings should be considered in an exploratory context, given the increased likelihood of identifying statistically significant results due to multiple testing across numerous micronutrients. Overall, our results complement previous reports of increased caloric and nutrient intake in heavy drinkers as demonstrated in older NHANES iterations as far back as 1999 (Breslow et al., 2010).
Analysis of the relation between alcohol consumption, dietary factors, and liver health revealed that although alcohol intake and total energy were the highest in heavy drinkers, there were still no clinically relevant differences in steatosis and liver fibrosis markers (assessed by elastography, a new measurement for the 2017-2018 NHANES) as compared to other drinking groups. This relative lack of overt liver disease in these heavy drinkers could be due, in part, to the overall lack of macro- or micronutrient deficiencies (e.g., protein or zinc deficiency) reported in some studies of patients with more advanced ALD (Mendenhall et al., 1995, Mendenhall et al., 1984, Moreno et al., 2016, McClain et al., 1979, Mohammad et al., 2012). The demographic characteristics and health status of individuals identified as heavy drinkers need also be considered, since compared to individuals in other groups, these respondents were significantly younger and were most often well-educated, healthy males with low incidences of diabetes and other comorbidities. Therefore, these overrepresented individuals simply may not have yet suffered the serious consequences of their heavy alcohol consumption. However, it is important to note that biochemical markers of liver injury, including AST, ALT, and GGT were modestly higher in heavy drinkers compared to other groups, indicating that while heavy drinkers on average may not progress to a state of liver disease advanced enough to be associated with elevated CAP or liver stiffness, the development of liver injury may already be initiated and could potentially further progress over time in some individuals. This idea is supported by previous evidence that daily alcohol consumption exceeding 40 to 80 g/day for males and 20 to 40 g/day for females (equivalent to ~2.8 to 5.6 and ~1.4 to 2.8 drinks/day for males and females, respectively, as in the United States one standard drink contains approximately 14 g of ethanol) for 10 years or more will likely lead to ALD (Becker et al., 1996). Unfortunately, the NHANES ALQ only allows identification of a respondent’s drinking pattern over the last 12 months and could not discriminate individuals drinking for longer periods of time. In addition, multiple other factors may impact the onset of ALD including genetics, epigenetics, comorbidities, and alterations in intestinal barrier function and gut microbiota. For example, it has been shown that among long-term alcohol consumers with similar levels of alcohol consumption (~15 drinks/day), the subset of individuals who developed liver injury had greater endotoxemia/intestinal permeability – two factors which are considered to be critical in ALD pathogenesis (Kirpich et al., 2017).
There are several other recently published reports which analyzed associations between alcohol consumption and liver health using 2017-2018 NHANES data. One study reported results comparable to ours, showing that heavy alcohol consumption as defined similarly to our study (> 2 drinks per day for men or > 1 drink per day for women) was not associated with fatty liver as measured by CAP (Unalp-Arida and Ruhl, 2022). However, when taking into account key aspects of drinking behavior (the frequency of drinking [days per week], the quality of drinking [drinks per drinking day], and binge drinking), positive associations between alcohol consumption and CAP were revealed in multivariable models (Niezen et al., 2021). Specifically, drinking 5+ drinks or 1-2 drinks 5-7 times per week in the past 12 months was significantly associated with liver fat (CAP score) (Niezen et al., 2021). Of note, there is some controversy regarding the effects of different amounts of alcohol on NAFLD. Several studies have suggested some potential benefits of low-to-moderate alcohol intake in NAFLD; however, there is also evidence that even moderate amounts of ethanol may increase the risk of liver disease progression and hepatocellular carcinoma (elegantly reviewed by (Idalsoaga et al., 2020)).
The current study had several limitations to consider when interpreting the results. First, there is no official consensus on how to categorize individuals based on alcohol consumption patterns, and alcohol consumption criteria recommended by different federal agencies (e.g., DGA and NIAAA) can be difficult to apply universally in objective studies. In addition, there are some discrepancies in how drinking levels are described between agencies. For example, a female who has consumed 2 drinks in a single drinking occasion has exceeded DGA criteria for moderate drinking, but has not yet met the NIAAA criteria for heavy drinking as long as she does not exceed 7 drinks during that week. This issue is exacerbated by the fact that drinking is self-reported in the NHANES ALQ, and self-report surveys are thought to generally underestimate an individual’s true volume of alcohol consumption (Stockwell et al., 2016) potentially due to social desirability bias wherein heavy drinkers perceive their alcohol consumption habits in a negative light and choose to conceal them from surveyors (Davis et al., 2010). Clearly, it is necessary to standardize the terminology and more clearly categorize drinking patterns before the role of alcohol use and alcohol intake pattern can be properly assessed and results between studies can be correctly compared. In addition, due to the nature of the NHANES ALQ survey questions, heavy drinkers who have ceased alcohol consumption over 12 months ago due to chronic health issues, i.e., the ‘sick-quitter’ effect (Sarich et al., 2019, Park et al., 2017), may have been misclassified as non-drinkers. Misclassification of drinking categories may lead to incorrect conclusions regarding the effects of alcohol consumption on different aspects of health and wellness (Fekjaer, 2013). Next, our study included individuals 18 year of age or older, since these were the only individuals eligible to complete the alcohol use questionnaire of the NHANES. Numerous studies have shown a high prevalence of underage drinking in individuals under 18 who may, in fact, be more susceptible to the toxic effects of ethanol (Silveri, 2012). Analyzing the correlations between diet, alcohol use, and liver health in an underage population would be useful to elucidate the differential effects of alcohol use in different age groups. Additionally, there is no consensus on cutoff score for fatty liver as assessed by CAP. We used a value of 300, which is conservative but validated by liver biopsy (Eddowes et al., 2019), whereas other groups analyzing the 2017-2018 NHANES dataset used values in the 260-280 range (Vilar-Gomez et al., 2021, Kim et al., 2021, Kim et al., 2022), and as low as 248 (Zhang et al., 2021). Lastly, we evaluated potential alcohol-nutrient interactions based only on dietary history and not on blood nutrient levels, which are not available in the 2017-2018 NHANES.
In summary, analysis of alcohol use, consumption of micro- and macronutrients, and liver health in 2017-2018 NHANES revealed that the categories of moderate and heavy drinkers consisted of generally healthy individuals with no meaningful liver steatosis or fibrosis. Slightly increased plasma liver injury markers such as ALT, AST, and GGT in the heavy drinking group suggest some concerns about optimal liver health in these individuals. Several limitations in the analysis should be considered including the potential misclassification of drinking categories and the lack of standardized cutoff scores for fatty liver as assessed by elastography, among several others. Future studies should continue to explore the inter-relations between alcohol use, diet, and liver health not only in NHANES data, but also in other sources including subjects consuming higher amounts of alcohol.
Acknowledgments
The authors acknowledge Marion McClain for manuscript editing support.
Funding:
This study was supported by the National Institutes of Health through multiple research grants, including R01AA024102 (I.A.K.), R01AA028905 (I.A.K.); U01AA026934, 1U01AA026926-01, 1U01AA026980-01; F31AA028423 (J.B.W); and the U.S. Department of Veterans Affairs Grant 1I01BX002996 (C.J.M.). This work was also supported by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant P20GM113226 (to CJM); and by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Grant P50AA024337 (to CJM). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest Statement
The authors declare no conflicts of interest.
References
- Aberg F, Helenius-Hietala J, Puukka P, Jula A (2017) Binge drinking and the risk of liver events: A population-based cohort study. Liver Int 37:1373–1381. [DOI] [PubMed] [Google Scholar]
- Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemela OJ (2008) Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr 88:1097–1103. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Daly AK, Day CP (2015) Genetics of Alcoholic Liver Disease. Semin Liver Dis 35:361–374. [DOI] [PubMed] [Google Scholar]
- Askgaard G, Gronbaek M, Kjaer MS, Tjonneland A, Tolstrup JS (2015) Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol 62:1061–1067. [DOI] [PubMed] [Google Scholar]
- Barve S, Chen SY, Kirpich I, Watson WH, McClain C (2017) Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res 38:289–302. [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P, Jensen G (1996) Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 23:1025–1029. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G (1997) Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Chen CM, Graubard BI, Jacobovits T, Kant AK (2013) Diets of drinkers on drinking and nondrinking days: NHANES 2003-2008. Am J Clin Nutr 97:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Guenther PM, Juan W, Graubard BI (2010) Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population, 1999-2006. J Am Diet Assoc 110:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Bruckner S, Zeissig S, Stephan AM, Wodarz N, Deviere J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Volzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nothen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J (2015) A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 47:1443–1448. [DOI] [PubMed] [Google Scholar]
- Butler L, Popkin BM, Poti JM (2018) Associations of Alcoholic Beverage Consumption with Dietary Intake, Waist Circumference, and Body Mass Index in US Adults: National Health and Nutrition Examination Survey 2003-2012. J Acad Nutr Diet 118:409–420 e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Lawler T, Mares J (2019) Dietary Carotenoids and Non-Alcoholic Fatty Liver Disease among US Adults, NHANES 2003(-)2014. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasarathy S (2016) Nutrition and Alcoholic Liver Disease: Effects of Alcoholism on Nutrition, Effects of Nutrition on Alcoholic Liver Disease, and Nutritional Therapies for Alcoholic Liver Disease. Clin Liver Dis 20:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CG, Thake J, Vilhena N (2010) Social desirability biases in self-reported alcohol consumption and harms. Addict Behav 35:302–311. [DOI] [PubMed] [Google Scholar]
- Delacote C, Bauvin P, Louvet A, Dautrecque F, Ntandja Wandji LC, Lassailly G, Voican C, Perlemuter G, Naveau S, Mathurin P, Deuffic-Burban S (2020) A Model to Identify Heavy Drinkers at High Risk for Liver Disease Progression. Clin Gastroenterol Hepatol 18:2315–2323 e2316. [DOI] [PubMed] [Google Scholar]
- DGA (2020) Dietary Guidelines for Americans, 2020-2025, in Series Dietary Guidelines for Americans, 2020-2025, U.S. Department of Agriculture and U.S. Department of Health and Human Services. [Google Scholar]
- Eagon PK (2010) Alcoholic liver injury: influence of gender and hormones. World J Gastroenterol 16:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN (2019) Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 156:1717–1730. [DOI] [PubMed] [Google Scholar]
- Fakhouri T, Martin C, Chen T, Akinbami L, Ogden C, Paulose-Ram R (2020) An investigation of nonresponse bias and survey location variability in the 2017–2018 National Health and Nutrition Examination Survey. National Center for Health Statistics Vital Health Stat 2 185. [PubMed] [Google Scholar]
- Fekjaer HO (2013) Alcohol-a universal preventive agent? A critical analysis. Addiction 108:2051–2057. [DOI] [PubMed] [Google Scholar]
- Habash NW, Sehrawat TS, Shah VH, Cao S (2022) Epigenetics of alcohol-related liver diseases. JHEP Rep 4:100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa S, West BT, Berglund PA (2017) Applied survey data analysis. Second edition. ed., CRC Press, Taylor & Francis Group, Boca Raton, FL. [Google Scholar]
- Huang LL, Wan JB, Wang B, He CW, Ma H, Li TW, Kang JX (2013) Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot Essent Fatty Acids 88:347–353. [DOI] [PubMed] [Google Scholar]
- Idalsoaga F, Kulkarni AV, Mousa OY, Arrese M, Arab JP (2020) Non-alcoholic Fatty Liver Disease and Alcohol-Related Liver Disease: Two Intertwined Entities. Front Med (Lausanne) 7:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cholankeril G, Loomba R, Ahmed A (2021) Prevalence of Fatty Liver Disease and Fibrosis Detected by Transient Elastography in Adults in the United States, 2017-2018. Clin Gastroenterol Hepatol 19:1499–1501 e1492. [DOI] [PubMed] [Google Scholar]
- Kim D, Cholankeril G, Loomba R, Ahmed A (2022) Prevalence of Nonalcoholic Fatty Liver Disease and Hepatic Fibrosis Among US Adults with Prediabetes and Diabetes, NHANES 2017-2018. J Gen Intern Med 37:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ (2012) The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res 36:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, McClain CJ, Vatsalya V, Schwandt M, Phillips M, Falkner KC, Zhang L, Harwell C, George DT, Umhau JC (2017) Liver Injury and Endotoxemia in Male and Female Alcohol-Dependent Individuals Admitted to an Alcohol Treatment Program. Alcohol Clin Exp Res 41:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Miller ME, Cave MC, Joshi-Barve S, McClain CJ (2016) Alcoholic Liver Disease: Update on the Role of Dietary Fat. Biomolecules 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo M, Bilal U, Mitchell MC, Potter J, Hernaez R, Clark JM (2021) Interaction Between Alcohol Consumption and PNPLA3 Variant in the Prevalence of Hepatic Steatosis in the US Population. Clin Gastroenterol Hepatol 19:2606–2614 e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci 16:26087–26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS (2000) ALCOHOL: its metabolism and interaction with nutrients. Annu Rev Nutr 20:395–430. [DOI] [PubMed] [Google Scholar]
- Louvet A, Mathurin P (2015) Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 12:231–242. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Van Thiel DH, Parker S, Badzin LK, Gilbert H (1979) Alterations in zinc, vitamin A, and retinol-binding protein in chronic alcoholics: a possible mechanism for night blindness and hypogonadism. Alcohol Clin Exp Res 3:135–141. [DOI] [PubMed] [Google Scholar]
- Mendenhall C, Roselle GA, Gartside P, Moritz T (1995) Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res 19:635–641. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA (1984) Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med 76:211–222. [DOI] [PubMed] [Google Scholar]
- Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ (2012) Zinc and liver disease. Nutr Clin Pract 27:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, Hittelet A, Piquet MA, Laleman W, Orlent H, Lasser L, Serste T, Starkel P, De Koninck X, Negrin Dastis S, Delwaide J, Colle I, de Galocsy C, Francque S, Langlet P, Putzeys V, Reynaert H, Degre D, Trepo E (2016) Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology 150:903–910 e908. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW (1986) Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res 10:271–273. [DOI] [PubMed] [Google Scholar]
- NIAAA. Drinking Levels Defined. Available at: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Niezen S, Trivedi HD, Mukamal KJ, Jiang ZG (2021) Associations between alcohol consumption and hepatic steatosis in the USA. Liver Int 41:2020–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. Alcohol. Available at: https://www.who.int/news-room/fact-sheets/detail/alcohol. Accessed November 5, 2021.
- Page A, Paoli PP, Hill SJ, Howarth R, Wu R, Kweon SM, French J, White S, Tsukamoto H, Mann DA, Mann J (2015) Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol 62:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Ryu Y, Cho SI (2017) The Association Between Health Changes and Cessation of Alcohol Consumption. Alcohol Alcohol 52:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Buckley C, Kerr WC, Brennan A, Purshouse RC, Rehm J (2021) Impact of body mass and alcohol consumption on all-cause and liver mortality in 240 000 adults in the United States. Drug Alcohol Rev 40:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD (2019) Global Burden of Alcohol Use Disorders and Alcohol Liver Disease. Biomedicines 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E, Lieber CS (1968) Alcohol-induced hepatic injury in nonalcoholic volunteers. N Engl J Med 278:869–876. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49:e73–e79. [DOI] [PubMed] [Google Scholar]
- Sanchez-Jimenez BA, Brizuela-Alcantara D, Ramos-Ostos MH, Alva-Lopez LF, Uribe-Esquivel M, Chavez-Tapia NC (2018) Both alcoholic and non-alcoholic steatohepatitis association with cardiovascular risk and liver fibrosis. Alcohol 69:63–67. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich P, Canfell K, Banks E, Paige E, Egger S, Joshy G, Korda R, Weber M (2019) A Prospective Study of Health Conditions Related to Alcohol Consumption Cessation Among 97,852 Drinkers Aged 45 and Over in Australia. Alcohol Clin Exp Res 43:710–721. [DOI] [PubMed] [Google Scholar]
- Savolainen VT, Liesto K, Mannikko A, Penttila A, Karhunen PJ (1993) Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res 17:1112–1117. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H (2018) Alcoholic liver disease. Nat Rev Dis Primers 4:16. [DOI] [PubMed] [Google Scholar]
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y (2010) Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int 34:1247–1272. [DOI] [PubMed] [Google Scholar]
- Silveri MM (2012) Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry 20:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral V, Floud S, Million Women Study C (2019) Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health 4:e41–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell T, Zhao J, Greenfield T, Li J, Livingston M, Meng Y (2016) Estimating under- and over-reporting of drinking in national surveys of alcohol consumption: identification of consistent biases across four English-speaking countries. Addiction 111:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unalp-Arida A, Ruhl CE (2022) Transient Elastography-Assessed Hepatic Steatosis and Fibrosis Are Associated With Body Composition in the United States. Clin Gastroenterol Hepatol 20:e808–e830. [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Videla LA (2011) The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct 2:644–648. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E, Vuppalanchi R, Mladenovic A, Samala N, Gawrieh S, Newsome PN, Chalasani N (2021) Prevalence of High-risk Nonalcoholic Steatohepatitis (NASH) in the United States: Results From NHANES 2017-2018. Clin Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O (2008) Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J Hepatol 49:441–450. [DOI] [PubMed] [Google Scholar]
- Warner DR, Warner JB, Hardesty JE, Song YL, King TN, Kang JX, Chen CY, Xie S, Yuan F, Prodhan MAI, Ma X, Zhang X, Rouchka EC, Maddipati KR, Whitlock J, Li EC, Wang GP, McClain CJ, Kirpich IA (2019) Decreased omega-6:omega-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury. J Lipid Res 60:2034–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J, Hardesty J, Song Y, Sun R, Deng Z, Xu R, Yin X, Zhang X, McClain C, Warner D, Kirpich I (2021) Fat-1 Transgenic Mice With Augmented n3-Polyunsaturated Fatty Acids Are Protected From Liver Injury Caused by Acute-On-Chronic Ethanol Administration. Front Pharmacol 12:711590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM (2020) Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res 40:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Meng QH (2020) Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J Gastroenterol 26:4567–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S, Li TK (2007) Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology 46:2032–2039. [DOI] [PubMed] [Google Scholar]
- Zhang X, Heredia NI, Balakrishnan M, Thrift AP (2021) Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017-2018. PLoS One 16:e0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnheld KH, Warner DR, Warner JB, Hardesty JE, McClain CJ, Kirpich IA (2019) Dietary fatty acids and bioactive fatty acid metabolites in alcoholic liver disease. Liver Research 3:206–217. [Google Scholar]