OBJECTIVES:
To outline the postoperative management of a long segment tracheal transplant in the ICU setting.
DESIGN:
The recipient required reconstruction of a long segment tracheal defect from a previous prolonged intubation. A male donor was chosen for a female recipient to enable analysis of the reepithelialization kinetics using fluorescence in situ hybridization to analyze the source of the new ciliated epithelium.
SETTING:
Transplant ICU at the Mount Sinai Hospital, New York, NY.
PATIENTS:
The female recipient was previously intubated for an asthma exacerbation and subsequently developed long segment tracheal stenosis and failed conventional management including dilatation, stenting, and six major surgical procedures rendering her chronically tracheostomy-dependent. The male donor suffered a massive subarachnoid hemorrhage and was subsequently pronounced brain dead. Organ procurement occurred after obtaining appropriate consent from the patient’s family.
INTERVENTIONS:
The patient received a deceased donor tracheal allograft that included the thyroid gland, parathyroid glands, and the muscularis of the cervical and thoracic esophagus. Triple therapy immunosuppression (tacrolimus, mycophenolate mofetil, and a corticosteroid taper) was maintained.
MEASUREMENTS AND MAIN RESULTS:
The patient was initially managed postoperatively with deep sedation on ventilator via armored/reinforced endotracheal tube placed through a small tracheostomy located along the superior tracheal anastomosis. Serial bronchoscopies were performed for graft assessment, pulmonary toilet, and biopsies, which initially showed acute inflammatory changes but no features of acute allograft rejection. A euthyroid state was maintained but hypercalcemia developed.
CONCLUSIONS:
The ICU management of this first long segment orthotopic tracheal transplant required a multidisciplinary approach involving critical care, otolaryngology, transplant surgery, interventional pulmonary, endocrinology, 1:1 nursing throughout the recipient’s transplant ICU stay, and respiratory therapy that resulted in the successful establishment of a viable tracheal airway and heralded the end of chronic tracheostomy dependence.
Keywords: critical care, human, respiratory insufficiency, trachea, transplantation
KEY POINTS
Question: Critical care issues and postoperative management after the first successful orthotopic tracheal transplant using donor trachea with thyroid gland and associated vasculature.
Findings: The ICU management included low airway pressures using pressure support ventilation, aggressive pulmonary toilet, hypercalcemia treatment due to additional parathyroid glands, maintaining immunosuppression, and serial bronchoscopies for surveillance and monitoring for acute rejection. The successful establishment of a viable tracheal airway heralded the end of chronic tracheostomy dependence.
Meaning: The management of patients after orthotopic tracheal transplant will be of increasing importance for intensivists in the near future, as this procedure becomes more prevalent.
A first of its kind orthotopic tracheal transplant was performed at Mount Sinai Hospital in a patient with long segment tracheal stenosis under an institutional review board-approved research protocol. The following article begins with a background about tracheal transplantation followed by a brief overview of the orthotopic tracheal transplantation procedure with a detailed look at the critical care issues and postoperative monitoring in the transplant ICU (TICU).
Historically, tracheal allotransplantation has been a daunting endeavor, particularly due to challenges in reestablishing blood supply to the transplanted tracheal graft (1, 2). It has been scientific dogma for more than 70 years that the trachea harbors no organ-specific vascular supply to allow for allograft revascularization. As a result, a series of staged procedures have been proposed to initially reconstitute tracheal blood supply followed by a second stage for tracheal implantation. In 1979, a donor trachea was implanted into the sternocleidomastoid muscle of the recipient as the initial stage and the trachea with the associated sternocleidomastoid muscle was then transferred to its orthotopic position 3 weeks later (3). In 1993, a one-stage allotransplantation of the trachea requiring omentopexy of the graft was performed with features of graft stenosis developing after 4 months due to progressive fibrosing mediastinitis (4).
The segmental arrangement of blood flow in the trachea limits the margins for circumferential dissection of the trachea beyond 1–2 cm on either side, rendering it vulnerable to ischemia with more extensive dissections (5). The luminal surface of the trachea is lined by pseudostratified columnar epithelium comprised of highly specialized ciliated cells that help to coordinate mucociliary clearance to protect the respiratory tract from inhaled particulates (6, 7). Prosthetic tracheal transplants that are devoid of this protective mucociliary mechanism have not served as a viable means of repairing long segment tracheal defects (8) due to stasis of secretions leading to airway obstruction (9).
Traditionally, airway defects that are between 1 and 5 cm can be bridged with end-to-end anastomoses (1). However, defects that are greater than 5 cm large defects are not amenable to conventional reconstructive techniques and would require more permanent airway alternatives, including mediastinal tracheostomies.
The use of decellularized and polymeric constructs in patients with tracheal defects was introduced in 2008 (10). However, multiple such failed allograft procedures in human subjects led to this practice becoming shrouded in ethical controversy when it was determined that there was insufficient preclinical data substantiating the safety of these techniques (10, 11).
ORTHOTOPIC TRACHEAL TRANSPLANT
Written consent was obtained from the patient for this case presentation. A 56-year-old female with past medical history (PMH) of hypertension, asthma, insulin-dependent diabetes, coronary artery disease, hyperlipidemia, and sarcoidosis with extensive smoking history suffered an asthma exacerbation in 2014 that required prolonged intubation. She subsequently developed long segment tracheal stenosis as well as a complete cricoid stenosis. The patient underwent a series of tracheal resections followed by multiple unsuccessful stent and dilatation procedures rendering her permanently tracheostomy-dependent.
The donor was a 37-year-old male with PMH of end-stage renal disease with prior renal transplant who suffered an acute subarachnoid hemorrhage and had been declared brain dead. The patient was then transferred to Mount Sinai Hospital for organ procurement.
A NOVEL SURGICAL APPROACH
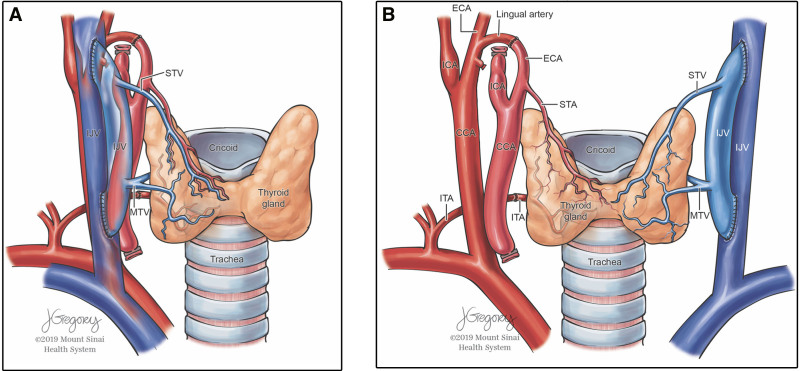
Donor procurement involved removal of the donor cricoid cartilage and 12 cm of donor trachea along with the associated vasculature, intact tracheoesophageal complex, thyroid gland, and infra-hyoid muscles (Fig. 1A).
Figure 1.
Tracheal graft blood supply. A, Blood supply to the trachea and thyroid gland B, Microvascular anastomoses between donor and recipient blood vessels. (Used with permission from ©Mount Sinai Health System). CCA = common carotid artery, ECA = external carotid artery, ICA = internal carotid artery, IJV = internal jugular vein, ITA = inferior thyroid artery, MTV = middle thyroid vein, STA = superior thyroid artery, STV = superior thyroid vein.
The recipient was prepared with a near-complete cricoidectomy, preserving the recurrent laryngeal nerves. Approximately 9.5 cm of diseased trachea was separated from the esophagus and removed.
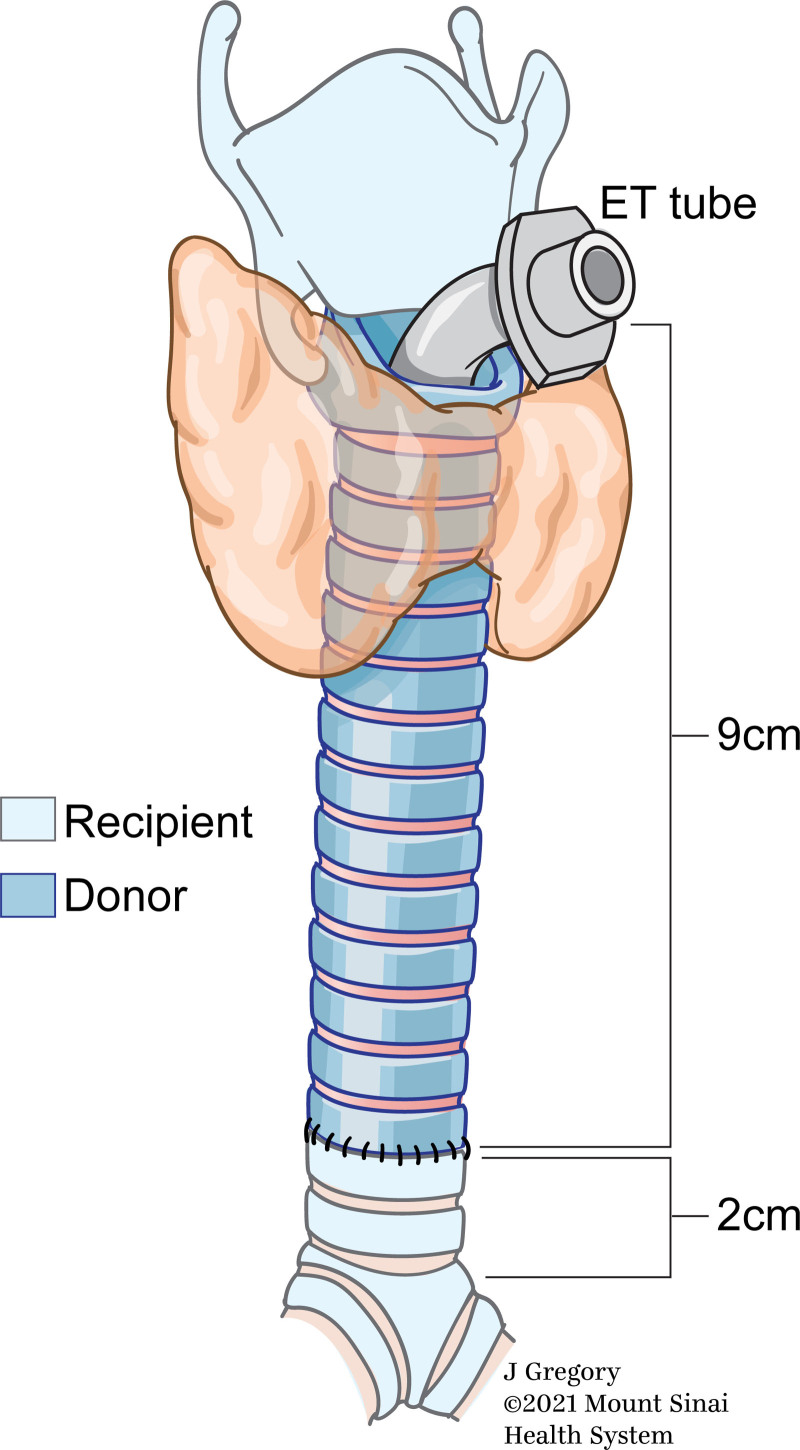
The allograft was placed inside the recipient bed and microvascular anastomoses were performed (Fig. 1B). Since the trachea derives its blood supply from the superior thyroid artery and vein as well as the inferior thyroid vessels, in order to prevent interruption of blood supply to the donor trachea, the thyroid gland was transplanted with the trachea. The parathyroid glands could be removed; however, this may also compromise the vascular supply to the donor organ. Therefore, the parathyroid and thyroid glands were transplanted with the trachea.” Ventilation was achieved via a 6.5F cuffed armored/reinforced endotracheal tube passing through the allograft via the superior tracheal anastomosis. The cuff of the endotracheal tube was inflated below the inferior tracheal anastomosis to prevent pressure necrosis of the allograft (Fig. 2).
Figure 2.
Positioning of the endotracheal (ET) tube through the superior tracheal anastomosis to maintain mechanical ventilation as well as to facilitate serial bronchoscopic examinations in the immediate postoperative period. (Used with permission from ©Mount Sinai Health System).
Postoperative Course
The patient was monitored in the TICU. She was maintained on pressure control ventilation during the surgical procedure and transitioned to pressure support ventilation (PSV) postoperatively. As the patient required continued mechanical ventilation, spontaneous breathing via PSV mode with an inspiratory pressure of 10 cm H2O above a baseline positive end-expiratory pressure of 5 cm H2O was selected to avoid high airway pressures that might disrupt the integrity of the tracheal allograft or injure the mucosa. The armored/reinforced tube cuff pressures were maintained less than 20 mm Hg. Serial arterial blood gas measurements were obtained every hour in the immediate postoperative period to monitor for hypoxia or hypercapnia while sedated.
The patient was lightly sedated with a Richmond Agitation-Sedation Scale between –3 and –2 in order to allow for patient comfort and maintain the neck in neutral position.
The patient’s neck was maintained in neutral position to prevent flexion, extension, or left or right head movement. The head end of the bed was kept elevated at 30 degrees to prevent oral or gastric secretion aspiration. The patient’s chin was left mobile and did not require any retention sutures.
In the absence of mature ciliated columnar epithelium, the armored/reinforced endotracheal tube was irrigated with saline bullets and suctioned every hour in the immediate postoperative period to allow for upper airway secretion clearance and prevention of mucous plugging. On postoperative day (POD) number 31, patient developed mucous plugging requiring emergent bedside bronchoscopy by the ear, nose, and throat team for removal.
On POD number 6, the patient underwent surveillance bronchoscopy via the armored/reinforced endotracheal tube. The tracheal mucosa appeared grossly normal, and biopsies were deferred during this initial inspection. The endotracheal tube was removed and replaced with a soft laryngectomy tube to cannulate the stoma. Oxygen was delivered by face tent just below the stoma and sedation was discontinued at this point. All subsequent bronchoscopies were performed via the stomal T-tube. A heat and moisture exchanger cap was placed over the open tracheal stoma on POD number 16 to allow for phonation, filtering of air particles and providing humidification to respiratory mucosa.
Physical and occupational therapy (PT/OT) commenced after extubation on POD number 6 as the patient remained intubated and sedated to maintain the neck in neutral position and to avoid dislodging the armored/reinforced ETT. PT/OT consisted of range of motion exercises and neuromuscular reeducation.
On POD number 19, the patient developed swelling of the right ankle. Lower extremity venous ultrasound revealed a nonocclusive right popliteal vein thrombus. Of note, the patient was receiving prophylactic deep vein thrombosis (DVT) prophylaxis with subcutaneous heparin since POD number 1 as per usual protocol. Therapeutic dose enoxaparin was subsequently started for treatment of right lower extremity DVT and continued after hospital discharge.
Postoperative course was also complicated by the development of acute kidney injury (AKI) with peak creatinine of 2.3 on POD number 27. AKI was attributed to a combination of supratherapeutic tacrolimus level, concurrent use of trimethoprim-sulfamethoxazole and dehydration. Renal function improved after IV fluid resuscitation and atovaquone was substituted for trimethoprim-sulfamethoxazole.
IMMUNOSUPPRESSION/ANTIMICROBIAL PROPHYLAXIS
Immunosuppression was maintained throughout the peri- and post-transplant period. Induction immunosuppression consisted of three doses of thymoglobulin, with the first dose administered intraoperatively. A conservative immunosuppression regimen was adopted consisting of tacrolimus (FK506), mycophenolate mofetil, and a methylprednisolone taper. Plasma FK506 levels of 10–12 ng/mL was considered therapeutic. The patient continues to be on triple immunosuppression 20 months postoperatively and will continue the same regimen for the near future. Sulfamethoxazole-trimethoprim, valganciclovir, and fluconazole were used as antimicrobial prophylaxis.
MONITORING FOR ACUTE REJECTION USING CYTOPATHOLOGY
Animal studies of tracheal allotransplant performed in rabbits in 1993 discovered that tracheal rejection was lymphocyte-mediated, specifically targeting allograft endothelium (12). In our patient, bedside bronchoscopies were performed for direct airway visualization and endobronchial biopsies were obtained on POD numbers 0, 9, 16, 30, 42, 72, and 86. The appearance of ciliated squamous epithelium in the transplanted trachea was an encouraging finding indicating an improved likelihood of effective mucus clearance and maintenance of airway hygiene.
A male donor was designed for this female recipient to enable analysis of the reepithelialization kinetics (9) and fluorescence in situ hybridization (FISH) was used to analyze the source of the new ciliated epithelium. Interphase FISH analysis on POD number 51 were consistent with 5.6% host (XX) cells and 94.5% donor (XY) cells. This was compared with FISH analysis on POD number 30, which was consistent with 0.4% host (XX) cells and 99.6% donor (XY) cells. This data suggested that the donor allograft was being reepithelialized with recipient-derived epithelium.
Nutrition
Postoperatively, the patient was experiencing persistent nausea despite anti-emetics and therefore remained nothing by mouth to prevent vomiting and aspiration. On POD number 7, patient was started on peripheral parenteral nutrition (PPN). She underwent a functional endoscopic swallowing evaluation and a modified barium swallow that showed no esophagopharyngeal reflux or aspiration. Patient was started on a soft, carbohydrate-restricted diet on POD number 13, at which time her PPN was discontinued.
Endocrinopathies
In the immediate postoperative period, hyperglycemia due to diabetes and immunosuppression required an insulin drip to maintain blood glucose 120–180 mg/dL. The patient’s native thyroid and parathyroid glands were not removed during surgery and the patient developed persistent hypercalcemia postoperative that was refractory to calciuresis. She received IV pamidronate with improvement of serum calcium. The cause of primary hyperparathyroidism and resultant hypercalcemia was attributed to “8-gland disease,” suggesting the role of increased parathyroid hormone production by two sets of parathyroid glands.
POST-HOSPITAL DISCHARGE FOLLOW-UP
The patient was followed in outpatient otolaryngology clinic after hospital discharge. She phonated well without persistent productive cough or respiratory infection.
CANDIDATES FOR FUTURE TRACHEAL TRANSPLANTS
This case of orthotopic tracheal transplant is the first of its kind as donor trachea was transplanted en masse with its complex network of blood vessels into the recipient. The preliminary cytopathology findings were consistent with successful integration of the donor trachea into the recipient without features of acute graft rejection. Patients with long segment tracheal defects who are otherwise not amenable to conventional reconstruction may now be potential candidates for tracheal transplant. In the current state of global pandemic due to COVID-19, the number of cases of hypoxic respiratory failure requiring tracheostomy have increased exponentially. Management of patients who have undergone orthotopic tracheal transplant will be of increasing importance for intensivists in the near future as this procedure becomes more prevalent.
ACKNOWLEDGMENTS
We thank Jeffrey Mechanick, MD, for his care and insights into the endocrine management of the patient.
Footnotes
Dr. Chopra was involved in conceptualization, investigation, visualization, writing—original and final draft, and writing—reviewing and editing. Dr. Oropello was involved in conceptualization, investigation, visualization, supervision, and writing—reviewing and editing. Dr. Wang was involved in conceptualization, visualization, and writing—reviewing and editing. Dr. Mo was involved in writing—reviewing and editing. Dr. Kohli-Seth was involved in conceptualization and planning. Dr. Genden is a chief ear, nose, and throat surgeon who performed tracheal transplant and he was involved in writing—reviewing and editing.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Delaere P, Vranckx J, Verleden G, et al. ; Leuven Tracheal Transplant Group: Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010; 362:138–145 [DOI] [PubMed] [Google Scholar]
- 2.Delaere P, Lerut T, Van Raemdonck D: Tracheal transplantation: State of the art and key role of blood supply in its success. Thorac Surg Clin 2018; 28:337–345 [DOI] [PubMed] [Google Scholar]
- 3.Rose KG, Sesterhenn K, Wustrow F: Tracheal allotransplantation in man. Lancet 1979; 1:433. [DOI] [PubMed] [Google Scholar]
- 4.Levashov YN, Yablonsky PK, Cherny SM, et al. : One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 1993; 7:383–386 [DOI] [PubMed] [Google Scholar]
- 5.Furlow PW, Mathisen DJ: Surgical anatomy of the trachea. Ann Cardiothorac Surg 2018; 7:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand-Saberi BEM, Schäfer T: Trachea: Anatomy and physiology. Thorac Surg Clin 2014; 24:1–5 [DOI] [PubMed] [Google Scholar]
- 7.Ostrowski LE, Bennett WD: Cilia and mucociliary clearance. In: Encyclopedia of Respiratory Medicine. Laurent GJ, Shapiro SD. (Eds). Cambridge, MA, Academic Press Elsevier, 2006, pp 466–470 [Google Scholar]
- 8.Vranckx JJ, Delaere P: The current status and outlook of trachea transplantation. Curr Opin Organ Transplant 2020; 25:601–608 [DOI] [PubMed] [Google Scholar]
- 9.Genden EM, Miles BA, Harkin T, et al. : Single-stage long-segment tracheal transplantation. Am J Transplant 2021; 21:3421–3427 [DOI] [PubMed] [Google Scholar]
- 10.Greaney AM, Niklason LE: The history of engineered tracheal replacements: Interpreting the past and guiding the future. Tissue Eng Part B Rev 2020; 27:341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Lancet: The final verdict on Paolo Macchiarini: Guilty of misconduct. Lancet 2018; 392:2. [DOI] [PubMed] [Google Scholar]
- 12.Delaere PR, Liu Z, Sciot R, et al. : The role of immunosuppression in the long-term survival of tracheal allografts. Arch Otolaryngol Head Neck Surg 1996; 122:1201–1208 [DOI] [PubMed] [Google Scholar]