Background.
The success of orthotopic liver transplantation as a life-saving treatment has led to new indications and a greater competition for organ grafts. Pediatric patients with acute liver-related crises can benefit from orthotopic liver transplantation, but organ availability in the limited time can be a major obstacle. Crossing ABO blood group barriers could increase the organs available to such patients
Methods.
From November 2010 to June 2015, 176 children aged 0.2−to18 y were transplanted in the King Faisal Specialist Hospital and Research Center. Out of those, 19 children were transplanted across blood group barriers (ABO incompatible). The underlying diseases were biliary atresia (n = 6); progressive familial intrahepatic cholestasis type 2 (n = 4); Crigler-Najjar syndrome (n = 3); hepatoblastoma (n = 2); and urea cycle disorder, Caroli disease, cryptogenic cirrhosis, and neonatal sclerosing cholangitis (n = 1 each). Immunosuppression consisted of basiliximab, mycophenolate, tacrolimus, and steroids. Pretransplant prophylactic plasmapheresis, high-dose immunoglobulins, and rituximab were not administered.
Results.
The grafts were from living donors (n = 17) and deceased donors (n = 2). Living donor morbidity was nil. The recipient median age was 21 mo (5−70 mo). After a median follow-up of 44 mo, 2 recipients (10%) died because of sepsis, 1 because of uncontrolled acute myeloid leukemia. The overall rejection rate was 7%, and no grafts were lost because of antibody-mediated rejection (AMR). HLA matching was 3.8 of 6 (A, B, DR), and there were 2 patients presented with acute cellular rejection, 1 patient with AMR, and 1 patient with biliary strictures.
Conclusions.
ABO incompatible liver transplantation is a feasible and life-saving option even with antibody and B-cell depletion-free protocol without increasing the risks for AMR. We speculate that this excellent result is most likely because of presence of relatively low titer ABO isoagglutinins and the high HLA match compatibility caused by habit of longstanding interfamilial marriages as typical of Saudi Arabia.
Liver transplantation against blood group type (ABO incompatible [ABOi]) is not frequently done because of increased risks of acute rejection (cellular and humoral rejection), vascular thrombosis, and intrahepatic bile duct complications, eventually leading to graft and patient loss. In the pediatric population, particularly in small children, ABOi transplantation has been successively performed with good results managed by more or less invasive immunosuppressive treatment protocols.1-13 Studies in both children and adults undergoing ABOi liver transplantation have described aggressive pretreatments such as splenectomy, portal vein injection of prostaglandin E1, gabexate mesylate, reduction of isoagglutinin titers by plasmapheresis, and intravenous (IV) treatment with rituximab.1-3,8,9,13 These measures risk early and late complications such as vascular thrombosis, severe infections, and irreversible B-cell depletion.13-17 An experience published by single-center series in Atlanta described good results gained by an ABOi protocol without pretransplant antibody treatment or B-cell depletion with plasmapheresis, immunoadsorption, or rituximab.1 It is well known that children <2 y of age may undergo an ABOi liver transplantation with no special desensitization protocol with good outcomes, most likely because of the immaturity of their immune system unable to produce isohemagglutinins. Maternal isoagglutinins may exert a suppressive influence on the development of specific isoagglutinins in the first year of life.4 In Saudi Arabia, there is a lesser tradition of deceased organ donation than other countries. More than 90% of liver transplantations are therefore living-related liver transplantations using either ABO identical or compatible organs. Nevertheless, at least in emergency situations, the need for ABOi transplantation is evident. Encouraged by these experiences, we started a prospective program of ABOi in patients with severe end-stage liver disease without ABO identical or compatible donor organ available. After 6 successful ABOi transplantations in end-stage liver disease, we extended indications to patients with noncirrhotic inherited liver disorders such as Crigler-Najjar syndrome, urea cycle disorders, and nonresectable hepatoblastoma because of high risk of metabolic crisis and tumor spread. Perioperative outcomes and long-term follow-up are herein reported.
MATERIALS AND METHODS
Patients and Data
A retrospective, observational, single-center study was conducted after acquiring the necessary approval of the Research Advisory Council (N°2131119) at the King Faisal Specialist Hospital and Research Center. Demographic, intraoperative, and outcome data from the Electronic Health Record on pediatric liver transplant recipients were extracted and analyzed to evaluate the outcomes.
From November 2010 to June 2015, 19 children out of a total of 176 pediatric liver transplant patients were enrolled in a prospective ABOi liver transplantation protocol after obtaining a parental informed consent. Age, diagnosis, operation technique, and status are shown in Table 1. The median age was 21 mo (range, 5−70 mo), and the median weight was 9.75 ± 3.5 kg (range, 4.3−15.6 kg). The blood group constellation donor/recipient was n = 6 A/0; n = 5 A/B; n = 4 B/0; n = 2 AB/B; n = 1 B/A; and n = 1 AB/A, respectively.
TABLE 1.
Donor and recipient characteristics
| No | Date of Tx | Age (mo) | ABO R | Donor | Degree of parental Kinship | ABO D | Diagnosis | Technique | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | July 12 | 34 | 0 +ve | Mother | Unrelated | A +ve | BA | OS | A |
| 2 | November 12 | 11 | B +ve | Nephew | 2nd degree | A +ve | BA | OS | A |
| 3 | December 12 | 70 | B +ve | Mother | 2nd degree | AB +ve | NSC | OS | A |
| 4 | December 12 | 39 | B +ve | Mother | 1st degree | A +ve | PFIC2 | OS | A |
| 5 | February 13 | 6 | B +ve | Cousin | 1st degree | A +ve | BA | OS | A |
| 6 | April 13 | 12 | A +ve | Uncle | Unrelated | B +ve | BA | OS | A |
| 7 | May 13 | 7 | 0 +ve | Uncle | 1st degree | B +ve | CNS | OS | D |
| 8 | September 13 | 8 | B +ve | Father | 1st degree | A +ve | CNS | OS | A |
| 9 | October 13 | 7 | 0 +ve | BDD | Unrelated | A +ve | BA | Split | D |
| 10 | November 13 | 21 | 0 +ve | Mother | 1st degree | A +ve | CNS | OS | A |
| 11 | July 14 | 6 | A −ve | Mother | 2nd degree | AB +ve | PFIC2 | OS | A |
| 12 | October 14 | 17 | 0 +ve | Mother | 1st degree | A +ve | Caroli disease | LS | A |
| 13 | October 14 | 33 | 0 +ve | BDD | Unrelated | A +ve | HB | Split | D |
| 14 | November 14 | 42 | 0 +ve | Mother | 1st degree | B +ve | PFIC2 | LS | A |
| 15 | November 14 | 40 | 0 +ve | Father | 1st degree | B +ve | UCD | LS | A |
| 16 | November 14 | 22 | 0 +ve | Brother | Unrelated | B +ve | BA | LS | A |
| 17 | January 15 | 21 | 0 +ve | Uncle | Unrelated | A +ve | HB | LS | A |
| 18 | May 2015 | 7 | B +ve | Aunt | 2nd degree | AB +ve | PFIC2 | LS | A |
| 19 | May 2015 | 50 | B +ve | Father | 2nd degree | A +ve | CLC | OS | A |
A, alive; BA, extrahepatic biliary atresia; BDD, brain death donor; CLC, cryptogenic liver cirrhosis; CNS, Crigler-Najjar syndrome; D, dead; HB, hepatoblastoma; LS, laparoscopic donor surgery; NSC, neonatal sclerosing cholangitis; OS, donor operation with open surgery; PFIC2, progressive familial intrahepatic cholestasis type 2; Split, split liver with a left lateral segment; Tx, transplantation; UCD, urea cycle defect.
The immunosuppression protocol consisted in methylprednisolone 10 mg/kg IV administered during the anhepatic phase and then 2 mg/kg (maximum 40 mg) IV from postoperative day (POD) 1 tapered down to 1 mg/d in weekly steps within 6 mo after transplantation. Basiliximab (10 mg IV for children <30 kg) was administered at days 1 and 4. Mycophenolate mofetil was started at 40 mg/m2/dose BID orally or via nasogastric tube on POD 1 and increased to 600 mg/m2/dose.18 The target range trough level was 1.6to3.5 mg/L. Oral tacrolimus (0.05 mg/kg/dose) was started on POD 7, aiming a trough level of 10to12 ng/mL in the first 3 mo. Later on, the trough level was reduced to 6to8 ng/mL. After 12 mo, the target trough level was 4to6 ng/mL.19 Steroids were definitely withdrawn at 12 mo following transplantation. During the perioperative phases, if transfusions were required, red cells from blood group O and fresh frozen plasma products from AB donors were given, respectively. Liver function tests, haptoglobin, and lactic dehydrogenase were performed on a daily basis during intensive care unit stay and 3 times a week in the normal ward. Isoagglutinins were measured on days 0, 1, 3, 5, 7, 10, 14, and 28.
Histological and Immunological Monitoring
Liver biopsies were not systematically done during the early posttransplant period because of the risk of bleeding while the liver was regenerating. However, we considered it in the case of deterioration of transaminases. Acute cellular rejection was defined according to the Banff criteria.20
In the case of rising isoagglutinins, hemolysis, and impaired liver function, a methylprednisolone bolus therapy 10 mg/kg up to a total of 45 mg/kg was administered. In the case of unchanged or even rising tests, high-dose IgG 0.8 mg/kg/d for 7 d was given21), and the tacrolimus trough level was adjusted to 12to14 ng/mL. Plasmapheresis (1.5 × plasma volume) was considered if there was a further rise in isoagglutinins followed by immunoadsorption and rituximab (375 mg/m2 IV).14-17 Liver biopsies were taken for C4d staining as evidence of antibody-mediated rejection (AMR) when the rejection was suspected.22,23 Anti-HLA class I and II donor-specific antibodies (DSAs) were measured in case of C4d positive staining.24
RESULTS
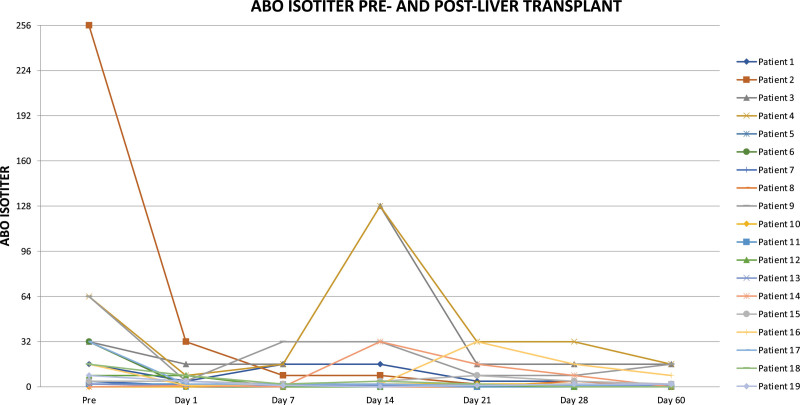
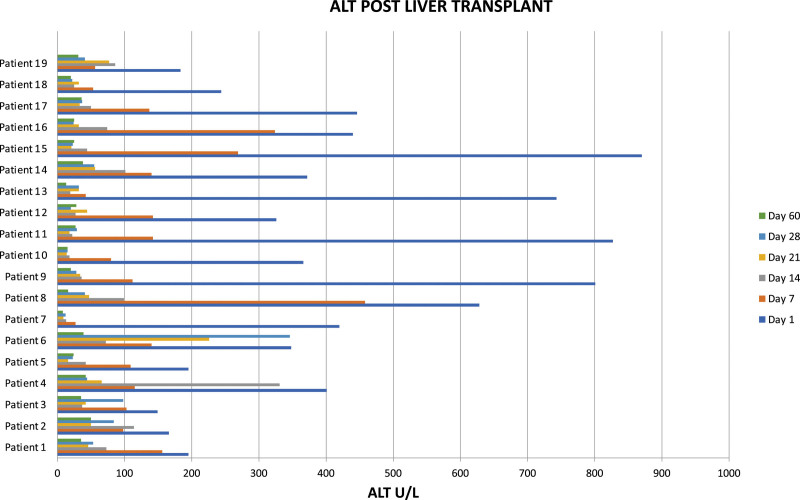
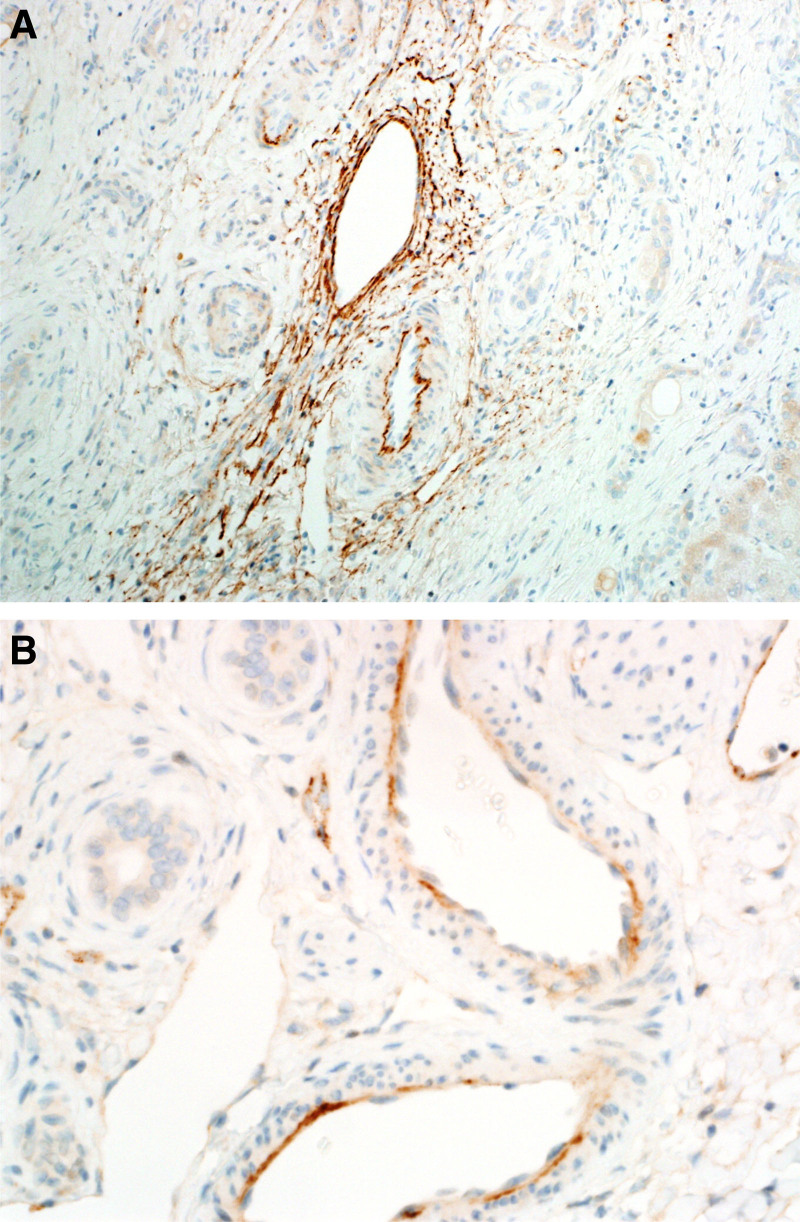
At the time of the study, 16 out of 19 patients were alive after ABOi liver transplantation. There were no surgical complications and neither primary nonfunction nor major early dysfunction of the grafts. The patients losses were not related to ABOi transplantation: 1 patient died of overwhelming septicemia in a remote area without adequate medical care facilities. A second patient died from aspiration pneumonia, and the third patient died from uncontrolled acute myeloid leukemia. In comparison with aged-matched controls, there was no difference in survival. During the same period, the remaining 157 patients who underwent ABO compatible or identical transplants in our center and who were considered the control group showed a crude survival rate at 54 mo of 90.5%, with a rate of acute cellular rejection and AMR of 7% and 0%, respectively. The results of isoagglutinin monitoring are shown in Figure 1. Three recipients with isoagglutinin titers >1:32 before transplantation were too unstable to undergo preoperative plasmapheresis but recovered without any additional medication after transplantation. Three patients had a rise in isoagglutinins on days 14 and 21, respectively, and were shown to have acute cellular rejection proven by liver biopsy. The level of HLA matching was 3.8 out of 6 (A, B, DR). Out of the 19 patients, 10 had available serum that was tested for HLA class I and class II anti-HLA antibodies. Three patients (21.1%) were sensitized for HLA class I only, 2 patients (14.3%) were sensitized for HLA class II only, and 4 patients (28.6%) were sensitized for both HLA classes I and II. One (7.1%) of the tested patient was not sensitized for HLA classes I and II. Of the sensitized patients, there were 2 patients with DSA specific for HLA classes I and II with the highest cumulative mean fluorescence intensity of 3025 and 14 673, respectively. None of these patients with DSA were associated with AMR episodes. Results of alanine aminotransferase (ALT) monitoring after transplantation in all 19 patients are shown in Figure 2. There were 4 patients with ALT >700 U/L (pt. 9, 11, 13, and 15). Patients 9 and 13 received a split organ from 1 deceased donor with prolonged cold ischemic time, patient 11 was transplanted with a left lateral segment of her mother by open surgery, and patient 15 was transplanted with an organ procured by laparoscopic surgery. All these patients normalized their ALT values within 14 d after transplantation. Patient 9 suffered from AMR 4 mo after transplantation with elevated alloantibodies, C4d positivity, and a histological feature of rejection (Figure 3). She was HLA sensitized but did not show DSAs. Steroid bolus therapy, high-dose IV IgG, plasmapheresis, and 1 dose of rituximab were administered. In the follow-up of 6 mo, she continued to need FK506 (TACROLIMUS) levels between 8 and 10 ng/mL to maintain normal transaminase levels. There was 1 patient with late manifestation of a biliary stricture requiring dilatation without need for further intervention in the long-term run.25
FIGURE 1.
Evolution of isoagglutinin titers during the time.
FIGURE 2.
Evolution of alanine aminotransferase (ALT) monitoring shortly after liver transplantation.
FIGURE 3.
Liver biopsy for diagnosis of humoral rejection: C4d positivity (A) and histological feature of rejection (B).
Donors’ Outcome
No donor death was recorded in living donor procedures. Eight out of 19 procedures (42%) were done laparoscopically.26 The overall morbidity was nil.
DISCUSSION
To the best of our knowledge, this is the largest single-center report of pediatric ABO-I liver transplantation with antibody and B-cell depletion-free immunosuppressive protocol. In contrast to reports of successful ABOi liver transplantation, we did not perform plasmapheresis or endo-beta galactosidase to remove isoagglutinins.1,24,25,27-29 Rituximab therapy was only necessary in 1 patient. The ABOi liver transplantation in pediatric recipients has been shown to have superior survival rate and lower incidence of acute rejection episodes posttransplantation compared with adult ABOi liver transplantation. The favorable results seen in pediatric ABOi liver transplant might be because of the differences in the immune system between pediatrics and adults. In pediatrics, it has been reported that the level of ABO isohemagglutinins level is lower than in adults and the complement system less active, and the innate and adaptive immune system are less mature.4,30,31
However, our oldest patient aged 70 mo did as well as the younger ones.32-34 The Atlanta group showed that 1-y actuarial patient survival for ABO-matched grafts versus ABO-I grafts was 93.0% and 100%, respectively, with a graft survival of 83.4% and 92.3%, respectively. In this study, significant differences in rejection or in vascular or biliary complications were not observed in relation to ABO compatibility. However, acute cellular rejection episodes were 37.5% versus 44.8%, respectively, in ABO-I and ABO-compatible groups.1 According to our results, the rejection rate was extremely low. The highest pretransplant isoagglutinin titers were seen in children with previous blood transfusions after variceal bleeding episodes, but they were not predictive of immunological graft dysfunction. DSAs were also not predictive of rejection in contrast to reports in the literature because the only patient with C4d positive rejection was DSA negative.27 The highest ALT and lactic dehydrogenase serum activities were seen in patients after split liver transplantation or after laparoscopic donor operation. In split and laparoscopically procured organs, the prolonged long warm ischemic time or mechanical injury may have caused a perfusion injury; however, no patient suffered vascular complications, nonanastomotic cholangiopathy, or other preservation-related complications.
Another possible explanation of such good outcomes with a relatively low immunosuppressive regimen and 7% of acute cellular rejection episodes may be based on the fact that there was a high concordance in the HLA system of our donor and recipient pairs. Six patients displayed the highest match level; this is most likely because of the long-term effect of consanguinity in the Saudi families, as we reported in Table 1. The consanguinity rate in pregnant Saudi women was found to be 54.4% in a study of 4500 investigated women.35
Although responsible for the high prevalence of many genetic and metabolic disorders indicating familial liver diseases, it may be beneficial with regard to live-related pediatric liver transplantation, which is performed in 92% of Saudi pediatric cases.19,36 HLA matching in liver transplantation is complex and unclear but not a strong predictor of tolerance or rejection among UK patients. The effect of HLA matching in this context may be the downregulation of the donor`s ABO glycosyltransferase activity, explaining the early immune accommodation to ABOi, the relative low frequency of AMR, and the dramatic decrease in preexisting ABO-isotiters as shown in our patients.37
These findings need further confirmation by investigating the HLA matching in both ABO compatible and ABOi in Saudi Arabia and comparing these results with findings in genetically more polymorphic populations.35 Finally, another reason for the favorable results may be the relatively low preoperative isoagglutinin titer, which was controlled with standard immunosuppression.
Given these results, the role of immunosuppression in pediatric liver transplantation has to be reconsidered.32,33 The cumulative load of immunosuppression during and after the transplantation in ABO identical or compatible and in ABOi situation is almost the same. The difference is the early start of tacrolimus in the standard group, whereas it was started only on day 7 in the ABOi group. Further on, mycophenolate is not started in our ABO identical and compatible patients. Because the effect of mycophenolate isonly manifests after 1 wk, a lower immunosuppression seems to have no negative impact on the outcome, even in the high- risk state of ABOi transplantation.18,28
Our study has some limitations. First, it is a prospective but single-center cohort study of a relatively small patient population. Second, it involves a specific subgroup of patients in a well-defined area with a high level of consanguinity, which may enhance the “accommodation” phenomenon in the case of ABOi liver transplants, even in the case of nonrelated living donors. However, upon our clinical results, we conclude that, in the immunological environment of Saudi Arabia, ABOi liver transplantation from living and deceased donors in children up to the age of 6 y is a safe and a life-saving option and thus an important additional source of transplant organs. Further investigations are required to address the limitations of the study.
Footnotes
R.I.T. and D.C.B. shared the senior authorship.
The authors declare no funding or conflicts of interest.
M.S., M.B., and R.I.T. participated in study conception and design. K.K., M.A., and H. Al-Khabbaz participated in acquisition of data. A.B., H. Alhussaini, H. Almanea, and H. Alhumaidan participated in analysis and interpretation of data. M.S., K.K., and R.I.T. participated in drafting of the article. R.I., D.C.B., R.I.T., A.B., and M.B. participated in critical revision. R.I.T. and D.B. participated in final supervision.
REFERENCES
- 1.Heffron T, Welch D, Pillen T, et al. Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis. Liver Transpl. 2006;12:972–978. [DOI] [PubMed] [Google Scholar]
- 2.Egawa H, Ohdan H, Haga H, et al. Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg. 2008;15:131–138. [DOI] [PubMed] [Google Scholar]
- 3.Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41:317–322. [DOI] [PubMed] [Google Scholar]
- 4.Fong SW, Qaqundah BY, Taylor WF. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex, and maternal isoagglutinins. Transfusion. 1974;14:551–559. [DOI] [PubMed] [Google Scholar]
- 5.Stewart ZA, Locke JE, Montgomery RA, et al. ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver Transpl. 2009;15:883–893. [DOI] [PubMed] [Google Scholar]
- 6.Gelas T, McKiernan PJ, Kelly DA, et al. ABO-incompatible pediatric liver transplantation in very small recipients: Birmingham’s experience. Pediatr Transplant. 2011;15:706–711. [DOI] [PubMed] [Google Scholar]
- 7.Markiewicz-Kijewska M, Kaliciński P, Teisseyre J, et al. Liver transplantation with ABO incompatible graft under immunoadsorption protocol–case report. Ann Transplant. 2010;15:68–71. [PubMed] [Google Scholar]
- 8.Shimazu M, Kitajima M. Living donor liver transplantation with special reference to ABO-incompatible grafts and small-for-size grafts. World J Surg. 2004;28:2–7. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Lee JG, Lee JJ, et al. Results of ABO-incompatible liver transplantation using a simplified protocol at a single institution. Transplant Proc. 2015;47:723–726. [DOI] [PubMed] [Google Scholar]
- 10.Okada N, Sanada Y, Hirata Y, et al. The impact of rituximab in ABO-incompatible pediatric living donor liver transplantation: the experience of a single center. Pediatr Transplant. 2015;19:279–286. [DOI] [PubMed] [Google Scholar]
- 11.Vodo M, Yasuda Y, Mizuta K. The impact of rituximab in ABO-incompatible pediatric living donor liver transplantation: the experience of a single center. Pediatr Transplant. 2015;19:279–286. [DOI] [PubMed] [Google Scholar]
- 12.Schukfeh N, Lenz V, Metzelder ML, et al. First case studies of successful ABO-incompatible living-related liver transplantation in infants in Germany. Eur J Pediatr Surg. 2015;25:77–81. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari AK, Pandey P, Aggarwal G, et al. Cascade plasmapheresis (CP) as a preconditioning regime in ABO-incompatible live related donor liver transplants (ABOi-LDLT). Transplant Res. 2014;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plate A, Havla J, Kuempfel T. Late-onset neutropenia during long-term rituximab therapy in neuromyelitis optica. Mult Scler Relat Sisord. 2014;3:2690272. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan B, Kopyltsova Y, Khokhar A, et al. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract. 2014;2:594–600. [DOI] [PubMed] [Google Scholar]
- 16.Clatworthy MR. B-cell regulation and its application to transplantation. Transpl Int. 2014;27:117–128. [DOI] [PubMed] [Google Scholar]
- 17.Roll P, Mahmood Z, Muhammad K, et al. Long-term repopulation of peripheral B-cell subsets after single and repeated rituximab infusions in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2015;33:347–353. [PubMed] [Google Scholar]
- 18.Quirós-Tejeira RE, Chang IF, Scott JD, et al. Mycophenolate mofetil in the management of alloimmune hemolytic anemia in ABO-compatible but non-identical pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2005;41:125–128. [DOI] [PubMed] [Google Scholar]
- 19.Fayyad A, Shagrani M, AlGoufi T, et al. Progress and outcomes of the first high-volume pediatric liver transplantation program in Saudi Arabia. Clinical Transpl. 2013, 77–83. [PubMed] [Google Scholar]
- 20.Demetris AJ, Bellamy C, Hübscher SG, et al. 2016 comprehensive update of the Banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16:2816–2835. [DOI] [PubMed] [Google Scholar]
- 21.Urbani L, Mazzoni A, Simone PD, et al. Treatment of antibody-mediated rejection with high-dose immunoglobulins in ABO-incompatible liver transplant recipients. Transplant Internat. 2007;20:467. [DOI] [PubMed] [Google Scholar]
- 22.Salah A, Fujimoto M, Yoshizawa A, et al. Application of complement component 4d immunohistochemistry to ABO-compatible and ABO-incompatible liver transplantation. Liver Transpl. 2014;20:200–209. [DOI] [PubMed] [Google Scholar]
- 23.Bellamy COC. Complement C4d immunohistochemistry in the assessment of liver allograft biopsy samples applications and pitfalls. Liver Transplant. 2011;17:747–750. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T, Liu D, Ogawa H, et al. Removal of blood group A/B antigen in organs by ex vivo and in vivo administration of endo-beta-galactosidase (ABase) for ABO-incompatible transplantation. Transpl Immunol. 2009;20:132–138. [DOI] [PubMed] [Google Scholar]
- 25.Song GW, Lee SG, Hwang S, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61:575–582. [DOI] [PubMed] [Google Scholar]
- 26.Broering DC, Elsheikh Y, Shagrani M, et al. Pure laparoscopic living donor left lateral sectionectomy in pediatric transplantation: a propensity score analysis on 220 consecutive patients. Liver Transpl. 2018;24:1019–1030. [DOI] [PubMed] [Google Scholar]
- 27.Kawagishi N, Takeda I, Miyagi S, et al. Long-term outcome of ABO-incompatible living-donor liver transplantation: a single-center experience. J Hepatobiliary Pancreat Surg. 2009;16:468–472. [DOI] [PubMed] [Google Scholar]
- 28.Honda M, Sugawara Y, Kadohisa M, et al. Long-term outcomes of ABO-incompatible pediatric living donor liver transplantation. Transplantation. 2018;102:1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferriani VP, Barbosa JE, de Carvalho IF. Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. Acta Paediatr Scand. 1990;79:322–327. [DOI] [PubMed] [Google Scholar]
- 31.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly DA, Bucuvalas JC, Alonso EM, et al. ; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:798–825. [DOI] [PubMed] [Google Scholar]
- 33.McDiarmid SV, Anand R, Martz K, et al. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254:145–154. [DOI] [PubMed] [Google Scholar]
- 34.Alonso EM, Ng VL, Anand R, et al. ; Studies of Pediatric Liver Transplantation (SPLIT) Research Group. The SPLIT research agenda 2013. Pediatr Transplant. 2013;17:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong SS, Anokute CC. The effect of consanguinity on pregnancy outcome in Saudi Arabia. J R Soc Health. 1990;110:146–147. [DOI] [PubMed] [Google Scholar]
- 36.Shagrani M, Burkholder J, Broering D, et al. Genetic profiling of children with advanced cholestatic liver disease. Clin Genet. 2017;92:52–61. [DOI] [PubMed] [Google Scholar]
- 37.Francavilla R, Hadzic N, Underhill J, et al. Role of HLA compatibility in pediatric liver transplantation. Transplantation. 1998;66:53–58. [DOI] [PubMed] [Google Scholar]