Abstract
The incidence of aggressive B-cell lymphomas increases with age, but for elderly or frail patients not eligible for doxorubicin-containing treatment standard therapy remains to be defined. In this prospective, multicenter, phase-2 B-R-ENDA trial, we investigated the feasibility, toxicity, and efficacy of 8 cycles rituximab combined with 6 cycles bendamustine (BR) in elderly or frail aggressive B-cell lymphoma patients: 39 patients aged >80 years and 29 patients aged 61–80 years with elevated Cumulative Illness Rating Scalescore >6 were included. Progression-free survival (PFS) and overall survival (OS) at 2 years were 45% (95% confidence interval [CI], 28%-61%) and 46% (28%-63%) for the patients age >80, as well 32% (13%-51%) and 37% (17%-57%) for frail patients age 64–80, respectively. In a preplanned retrospective analysis, we found no significant differences in PFS and OS comparing the outcome of the 39 patients age >80 years with 40 patients aged 76–80 years treated with 6xR-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and 2 x rituximab in the RICOVER-60 trial (DSHNHL 1999-1, NCT00052936, EU-20243), yet we detected lower rates of infections and treatment-related deaths in the BR-treated patients. We demonstrate that older and frail patients with aggressive B-cell lymphoma who are not able to receive standard CHOP-based therapy can benefit from anthracycline-free therapy as a feasible and effective therapeutic option.
This manuscript is dedicated to the memory of Prof Michael Pfreundschuh, founder and president of the DSHNHL, who contributed to the design of this study and passed away much too early in March, 2018.
INTRODUCTION
The incidence of aggressive B-cell lymphomas is increasing with age.1–5 More than 50% of patients are older than 60 years at the time of initial diagnosis. Patients treated with curative intent receive 6 cycles of immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) as standard of care.6–8 Toxicity and treatment-related mortality (TRM) increase with age.9 In a prospective multicenter phase-II-trial for patients aged 80 years and older with initial diagnosis of aggressive B-cell lymphoma (DLBCL), a dose-reduced protocol of R-miniCHOP-21 every 3 weeks was feasible with a 2-year overall survival (OS) of 59%, 2-year progression-free survival (PFS) of 47%, and a median OS of 29 months.10 Most common grades 3–4 adverse events (AE) were neutropenia (40%), anemia (9%), thrombocytopenia (7%), and infections (27%). Another phase-2 trial combined ofatumumab with mini-CHOP as first-line treatment in elderly DLBCL patients aged ≥80 years and showed 2-year OS of 64.7% with grades 3–4 neutropenia in 21% and febrile neutropenia in 6% of patients.11 A phase-2 study with ofatumumab and bendamustine as first-line treatment in 21 elderly DLBCL patients aged ≥70 years showed an overall response rates (ORR) of 90.5% and a median PFS and OS of 8.6 and 12.0 months, respectively. The study was closed due to low enrollment.12
For older adults or frail patients not eligible for CHOP(-like) therapy standard treatment has not been defined. Bendamustine has been frequently used, however, data from larger prospective trials have not been published. Horn et al13 showed in a retrospective trial with 20 patients that rituximab and bendamustine (BR) as first- or second-line treatment is feasible with ORR of 55% and a median OS of 19.4 months. Walter et al14 reported in a retrospective analysis of 13 evaluable elderly DLBCL patients an ORR of 62% with a median OS of 9 months. Weidmann et al15 demonstrated in a prospective phase-2 trial of 14 patients with aggressive B-cell lymphoma with a median age of 85 years who received BR as first-line treatment an ORR of 69% and an OS of 7.7 months. Bendamustine was given on 2 consecutive days with a dose of 120 mg/m2. Most common grade 3 AE were neutropenia, thrombocytopenia, anemia, decreasing renal function, and fatigue.15 Recently data of 2 prospective trials with bendamustine in elderly lymphoma patients have been published. Park et al16 described an ORR of 78% and a median OS of 10.2 months in 23 previously untreated patients with DLBCL stage II–IV and a median age of 80 years treated with BR using a bendamustine dose of 120 mg/m2 on day 1 and 2 of each cycle. Storti et al17 reported a prospective phase-II study with BR as front-line therapy in 49 frail patients with a median age of 81 years who achieved an ORR of 62% and a median OS of 30 months using 90 mg/m2 bendamustine on days 1–2. The most common grades 3–4 AE was neutropenia (37.8%), 58% of patients received granulocyte-colony stimulating factor (G-CSF).17
In older adults and frail patients, a geriatric assessment is among other reasons recommended to evaluate if he or she is fit enough to receive standard treatment. Assessment tools record the performance status, cognitive ability, nutrition status, comorbidities, and mental health.1,18–28
To answer the question whether BR is feasible, safe, and effective as first-line treatment in very old or elderly comorbid patients with aggressive B-cell lymphoma, the DSHNHL (Deutsche Studiengruppe für hochmaligne Non-Hodgkin Lymphome; German Study Group for aggressive Non-Hodgkin Lymphoma, now German Lymphoma Alliance, GLA) initiated a prospective multicenter phase-2 trial “Subcutaneous Rituximab and Intravenous Bendamustine in very Elderly Patients or Elderly Medically Non Fit Patients (‘Slow Go’) with Aggressive CD20-positive B-cell Lymphoma” (B-R-ENDA, DSHNHL 2010-1, EudraCT 2010-024004-98, NCT01686321). Geriatric assessment and quality of life (QoL) evaluation was performed using Cumulative Illness Rating Scale (CIRS)-Score,20,21 instrumental activities of daily life (IADL),23,24 Geriatric Screening Scale G827 and European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire (version 3.0, 1995).
Here we present final results.
MATERIALS AND METHODS
Study design
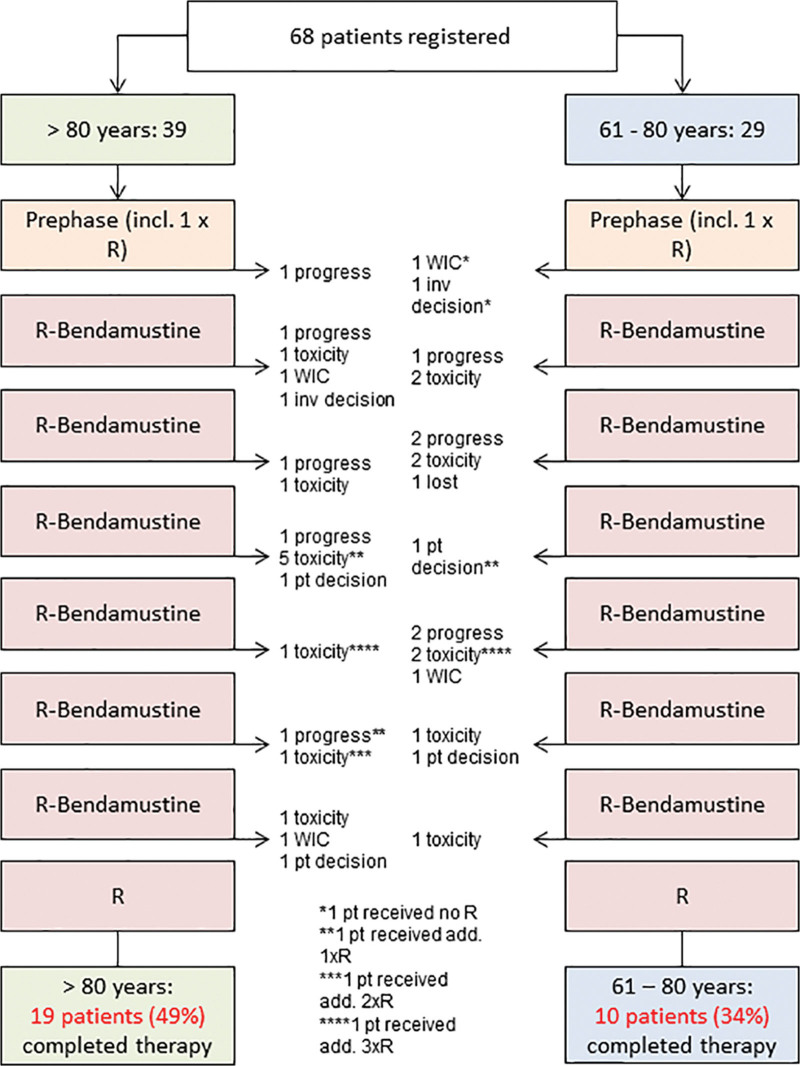
The B-R-ENDA trial was an open-label, multicenter, prospective, nonrandomized phase-2 trial including patients aged >80 years or 61–80 years with elevated CIRS score >6 and with histologically confirmed CD20+ aggressive B-cell lymphoma of any Ann-Arbor stage, any international prognostic index (IPI) score29,30 and Eastern Cooperative Oncology Group performance score (ECOG PS) <4 determined during prephase treatment, not qualifying for CHOP-like therapy according to exclusion criteria and physician’s opinion. Inclusion and exclusion criteria are given in Suppl. Table S1; consort diagram and flow chart are shown in Figure 1 and Suppl. Figure S1.
Figure 1.
Consort diagram. add. = additional; inv = investigator; pt = patient; R = rituximab; WIC = withdraw of informed consent.
Patients received a prephase treatment of prednisolone 100 mg orally on day −7 to −1, followed by rituximab 375 mg/m² intravenously (i.v.) on day −3. The trial treatment consisted of 7 further cycles of rituximab (375 mg/m² i.v. or 1400 mg subcutaneously [s.c.]) on day 1 every 3 weeks and 6 cycles of bendamustine 90 mg/m² i.v. on day 1 and 2 every 3 weeks. During the run-in-phase, 20 patients received rituximab 375 mg/m² i.v. After safety analysis by the Data Safety Monitoring Board, allpatients received an absolute dose of 1400 mg rituximab s.c. G-CSF was recommended following guidelines of the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) in cases of prolonged neutropenia or infections, a prophylaxis against pneumocystis carinii pneumonia was recommended within the protocol. An interim restaging was performed after 3 cycles (RE1: restaging-1 after 3 cycles). In case of progressive disease (PD) at that time point study treatment ended. Response evaluation was done by computed tomography (CT). In case of complete response (CR), partial response (PR) or stable disease (SD) patients continued therapy. In case of PR or SD in the final restaging (RE2) after 6 cycles of BR treatment, radiation therapy with 39.6 Gray to residual lesions of initial bulky disease (≥7.5 cm lymphoma mass) was performed. These patients received a further restaging after the end of radiotherapy (restaging RE3). Follow-up (FU) observation (3-monthly in years 1 and 2, 6-monthly afterward) within the study ended for all patients 2 years after the end of therapy of the last patient enrolled in the study. The FU period was 2 years minimum and 4.5 years at maximum. The geriatric assessment included 4 elements: CIRS Score,20,21 social situation, IADL,23,24 G8 (geriatric assessment screening tool).27 For QoL evaluation, the EORTC QLQ-C30 questionnaire (version 3.0, 1995) was used.
This study was approved by central and all local ethics committees. All persons participating in the conduct of the trial committed themselves to observe the Declaration of Helsinki and all its revisions and amendments (incl. the Edinburgh Amendment from October 2000), as well as all pertinent national laws and the ICH guidelines for Good Clinical Practice. All patients gave their written informed consent before enrollment.
Statistical analysis
The aim of the B-R-ENDA trial was to investigate the feasibility, toxicity, and efficacy of BR. The planned sample size of 50 patients in the age group >80 years (target cohort) allowed to establish a robust Kaplan-Meier estimator for the 2-year PFS rate between 30% and 50% with a 95% CI of about ±14% and ±15%, respectively. Patients in the age group ≤80 years represented the exploratory group. Thirty-nine finally included patients aged >80 years enabled the 95% CI of 2-year PFS rate to be estimated with a precision of approximately 15% and 17%. Further primary endpoints for feasibility and toxicity included the rate of treatment-related deaths, AEs, and protocol adherence. Secondary endpoints included CR rate, PR rate, rate of primary progression, relapse rate, OS, EFS, as well as geriatric assessment and QoL evaluation. Curves of duration and absolute dose of bendamustine were estimated according to the Kaplan-Meier technique, and patients with early termination due to insufficient response were censored.31 EFS was calculated as time from start of treatment to disease progression, start of salvage treatment, start of any additional, unplanned treatment, SD or unknown response, relapse, or death from any cause. PFS was defined as time from start of treatment to progression, relapse, or death from any cause. OS was defined as time from start of treatment to death from any cause. Patients with no event reported at the time of analysis were censored at the most recent assessment date. Kaplan-Meier curves were presented and Kaplan-Meier estimates at 2 years, with 95% CIs, were calculated for EFS, PFS, and OS. Scoring of the QLQ-C30 was performed according to MANUAL FOR THE USE OF EORTC MEASURES IN DAILY CLINICAL PRACTICE,32 with the means of the raw scores for the domains being transformed to lie between 0 and 100. G8, IADL, and QLQ-C30 were descriptively analyzed using mean and range. In a preplanned analysis, the EFS, PFS, and OS of patients from B-R-ENDA trial aged >80 years treated with BR were compared retrospectively with patients from the RICOVER-60 trial (six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell Lymphomas: A randomised controlled trial [DSHNHL 1999-1, NCT00052936, EU-20243]) aged 76–80 years6 treated with 6 cycles of CHOP-14 and 8 applications of rituximab using the log-rank test; Kaplan-Meier curves are presented.33 A Cox multivariable regression model was used to test whether therapeutic effects emerging from univariate analyses remained stable after adjustment for the factors of the IPI (ie, age >60 years, lactate dehydrogenase [LDH] > normal, ECOG PS >1, stage III/IV, and extralymphatic involvement >1).29 Estimates are given as hazard ratios with 95% CI and corresponding P values. Subgroup analyses were done according to the number of IPI factors (1, 2 vs 3–5), according to CIRS (≤6 vs >6), G8 (≥14 vs <14), IADL (=8 vs <8), and Quality of Life (EORTC) (≥50 vs <50, Global Score). Patient characteristics were analyzed by use of χ2 test and, if necessary, by Fisher exact test. Significance level was 0.050. Statistical analyses were done with IBM SPSS 25 and 26 software.
Data sharing statement
Individual patient information underlying the data presented in this article can be shared after deidentification. Researchers have to provide a proposal for an approval from an independent review committee to access these data. The protocol and informed consent forms will be available on request beginning 3 months and ending 5 years after publication. Requests can be addressed to the corresponding author.
RESULTS
Patients
Between July 2012 and February 2016, 68 patients from 24 German centers were included in the trial (Table 1): 39 patients aged >80 years, and 29 patients aged 61–80 years. The geriatric assessments by CIRS/G8/IADL were performed by physicians, so they were available at initial staging for all patients. QoL was evaluated by paper-based questionnaires according to EORTC QLQ-C30 questionnaire (version 3.0, 1995)32 and was available at staging for 46 of 68 patients. In patients >80 years, 72% were female, LDH was elevated in 67% of patients, 23% of patients had an ECOG >1, 51% of patients were at an advanced stage III/IV according to Ann-Arbor classification, 54% showed extralymphatic involvement, 10% in more than one localization resulting in higher IPI scores 3–5 in 46% of patients. The median CIRS score for patients >80 years was 7 (range 1–17), 69% of patients had a G8 score <14, 43% had an IADL score of 8, and the median score of Quality of Life (EORTC) was 58 (8–100). Patients aged 61–80 years were medically nonfit according to the protocol criteria. Patients >80 years were fitter than patients aged 61–80 years at initial screening. The rates of elevated LDH (76%), ECOG >1 (52%), and stage III/IV (66%) were higher for patients aged 61–80 years and the values for geriatric assessment were poorer. The median CIRS score was 10 (2–22) including 2 protocol violations regarding CIRS >6, 93% of patients had a G8 score <14, 14% had an IADL score of 8, and the median of Quality of Life (EORTC) was 33 (0–83). At initial staging, 17 patients (25%) lived at home alone, 47 (69%) lived at home with someone else, and 4 patients (6%) stayed in institutional care. Central reference pathology according to protocol was available in 91% of patients (62/68) using World Health Organization (WHO) classification of 2008.34 Histologic subtypes were diffuse large B-cell lymphoma (DLBCL) not otherwise specified, follicular lymphoma grade IIIb, and other in decreasing frequency (Table 1). For 1 patient >80 years, the diagnosis changed to chronic lymphatic leukemia. Two further patients violated the inclusion criteria—1 patient aged 61–80 years received prior chemotherapy or radiotherapy and another patient >80 years had a concomitant solid tumor disease and/or tumor disease in the past 5 years (Table 1).
Table 1.
Demographics and Disease Characteristics for Patients at the Time of Initial Staging
| >80 y (n = 39) | 61–80 y (n = 29) | Total (n = 68) | |
|---|---|---|---|
| Male | 11 (28%) | 11 (38%) | 22 (32%) |
| Female | 28 (72%) | 18 (62%) | 46 (68%) |
| Age, median (range) | 84 (81–95) | 77 (64–80) | 81 (64–95) |
| Age groups | |||
| 61–75 y | - | 8 (28%) | 8 (12%) |
| 76–80 y | - | 21 (72%) | 21 (31%) |
| 81–85 y | 25 (64%) | - | 25 (37%) |
| >85 y | 14 (36%) | - | 14 (21%) |
| LDH > normal | 26 (67%) | 22 (76%) | 48 (71%) |
| ECOG > 1 | 9 (23%) | 15 (52%) | 24 (35%) |
| Stage III/IV | 20 (51%) | 19 (66%) | 39 (57%) |
| Extralymphathic involvementa | 21 (54%) | 19 (66%) | 40 (59%) |
| Extralymphathic involvement >1 | 4 (10%) | 7 (24%) | 11 (16%) |
| IPI | |||
| 1 | 6 (15%) | 2 (7%) | 8 (12%) |
| 2 | 15 (38%) | 5 (17%) | 20 (29%) |
| 3 | 11 (28%) | 12 (41%) | 23 (34%) |
| 4, 5 | 7 (18%) | 10 (34%) | 17 (25%) |
| Bulky disease | 8 (21%) | 11 (38%) | 19 (28%) |
| B symptoms | 10 (26%) | 14 (48%) | 24 (35%) |
| Bone marrow involvement | 0 (0%) | 2 (7%) | 2 (3%) |
| Reference pathological diagnosis | |||
| Reviewed | 36 | 26 | 62 |
| Diffuse large B-cell lymphoma (DLBCL) | |||
| Not otherwise specified | 28 (78%) | 23 (88%) | 51 (82%) |
| Rare morphologic variants | 0 (0%) | 1 (4%) | 1 (2%) |
| T-cell/histiocyte-rich B-cell lymphoma | 0 (0%) | 1 (4%) | 1 (2%) |
| Primary cutaneous DLBCL, leg type | 1 (3%) | 1 (4%) | 2 (3%) |
| EBV-positive DLBCL of the elderly | 1 (3%) | 0 (0%) | 1 (2%) |
| Primary mediastinal (thymic) large B-cell lymphoma | 1 (3%) | 0 (0%) | 1 (2%) |
| Follicular lymphoma grade IIIb | 3 (8%) | 0 (0%) | 3 (5%) |
| ALK-positive large B-cell lymphoma | 1 (3%) | 0 (0%) | 1 (2%) |
| No lymphoma (CLL) | 1 (3%) | 0 (0%) | 1 (2%) |
| CIRS, median (range) | 7 (1–17) | 10 (2–22) | 8 (1–22) |
| CIRS | |||
| ≤6 | 17 (44%) | 2b (7%) | 19 (28%) |
| >6 | 22 (56%) | 27 (93%) | 49 (72%) |
| Geriatric assessment G8, median (range) | 12 (4–16) | 11 (6–15) | 11 (4–16) |
| G8 | |||
| ≥14 | 12 (31%) | 2 (7%) | 14 (21%) |
| <14 | 27 (69%) | 27 (93%) | 54 (79%) |
| IADLc | |||
| =8 | 16 (43%) | 4 (14%) | 20 (30%) |
| <8 | 21 (57%) | 25 (86%) | 46 (70%) |
| EORTCd | |||
| Quality of lifee, median (range) | 58 (8–100) | 33 (0–83) | 50 (0–100) |
| Social situation | |||
| At home alone | 12 (31%) | 5 (17%) | 17 (25%) |
| At home with someone | 25 (64%) | 22 (76%) | 47 (69%) |
| In institutional care | 2 (5%) | 2 (7%) | 4 (6%) |
Data are expressed as number of patients (percentage of total group).
aBone marrow involvement is counted as extralymphatic involvement, spleen and Waldeyer Ring are counted as lymphatic involvement.
bTwo patients with violation of inclusion criterion.
cTwo missing values for patients >80 y.
d22 (10/12) missing questionnaires.
eOne missing value for patients >80 y.
Bulky disease = .7.5 cm lymphoma mass; CIRS = Cumulative Illness Rating Scale; CLL = chronic lymphatic leukemia; ECOG = Eastern Cooperative Oncology Group performance score; EBV = Epstein-Barr virus; EORTC = European Organization for Research and Treatment of Cancer; G8 = Geriatric Screening Scale G827; IADL = instrumental activities of daily life; IPI = international prognostic index; LDH = lactate dehydrogenase.
Adherence to protocol
All patients received prephase treatment. Fifty-six percent of patients aged >80 years (22/39) and 38% (11/29) of patients aged 61–80 years completed 6 cycles of treatment per protocol (Figure 1). Early termination was due to PD (>80 years: n = 5; 61–80 years: n = 5), toxicity (n = 9; n = 7), withdrawal of consent (n = 1; n = 2), investigator’s decision (n = 1; n = 1), lost to FU (n = 0; n = 1), and patient’s wish (n = 1; n = 2). Four further patients did not receive the eighth rituximab application due to toxicity (n = 1; n = 1), withdrawal of consent (n = 1; n = 0), or patient’s wish (n = 1; n = 0). The duration of therapy according to study protocol ranged between 20 and 22 weeks for patients receiving immunochemotherapy only and 24–26 weeks for those with additional radiotherapy. For both age groups, the median total duration of bendamustine is as planned, but treatment delays increased with the duration of therapy (Suppl. Figure S2A). The median absolute dose of bendamustine was slightly smaller than the planned dose of 1080 mg/m² (>80 years: 1026 mg/m²; 61–80 years: 1021 mg/m²) due to the high number of patients with early termination of the treatment (Suppl. Figure S2B). Interestingly, neither the total duration nor the absolute dose for bendamustine or rituximab differs among the 2 age groups. Radiotherapy was planned for all patients with bulky lesions who do not show a CR after 6 cycles of bendamustine and a total of 8 applications of rituximab. Three patients received radiotherapy: 2 patients with bulky disease and either SD or partial remission after immunochemotherapy, and another patient without bulky disease and CR after immunochemotherapy for extranodal involvement. Only 2 patients (1 PR, 1 SD), both aged <80 years, did not receive consolidation radiation therapy due to physician’s choice and patient wish.
Safety
AE and deaths occurring during the study were continuously monitored by the trial office and documented in an annual safety report. The benefit–risk evaluation was considered favorable. AE were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE V4.03). In total, 849 AE and 118 serious AE (SAE) of any grade were documented (Table 2): most common AE of any grade were hematological toxicities (11%), gastrointestinal disorders (18%), general disorders (16%), and infections (10%). For grades 3–5 AE (n = 181) hematological toxicities (25%), infections (15%), metabolism and nutrition (13%), and gastrointestinal disorders (8%) were the most frequent. The rate of patients affected aged 61–80 years compared to patients >80 years was higher for infections (38% vs 23%), gastrointestinal disorders (24% vs 15%), and respiratory disorders (14% vs 8%). In Table 2, all AE are listed, which occurred in at least 5% of patients within the 2 cohorts. Secondary neoplasia was detected in 4 patients (6%): 2 carcinoma (bronchial and renal cell carcinoma) and 1 acute myeloid leukemia and 1 myelodysplastic syndrome, both with a median of 19.5 months after end of treatment (Suppl. Table S2). One Suspected Unexpected Serious Adverse Reaction (SUSAR) was reported that has emerged from an event of pulmonary embolism possibly related to the application of bendamustine. Patient information was changed; the risk profile of the whole trial had not changed. All nonlymphoma-related deaths during therapy until 60 days after treatment termination were counted as study treatment-related deaths. There were 5 treatment-related deaths within patients >80 years (13%) and 5 treatment-related deaths within patients aged 61–80 years (17%), most of them infections (70%), followed by cardiovascular events (30%).
Table 2.
AE of Any Grade (≥5% of All AEs in at Least One of the Cohorts, Listed), AEs Grades 3–5 and SAEs According to NCI-CTCAE V4.03
| >80 y (n = 39) | 61–80 y (n = 29) | Total (n = 68) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Events | Serious Adverse Events | Adverse Events | Serious Adverse Events | Adverse Events | Serious Adverse Events | ||||||||||
| Any Grade (n = 454) | Grades 3–5 (n = 102) | Any Grade (n = 56) | Grades 3–5 (n = 38) | Any Grade (n = 395) | Grades 3–5 (n = 79) | Any Grade (n = 62) | Grades 3–5 (n = 39) | Any Grade (n = 849) | Grades 3–5 (n = 181) | Any Grade (n = 118) | Grades 3–5 (n = 77) | ||||
| Event | n (%) | n (%) | Affected Patients n (%) | n (%) | n (%) | n (%) | n (%) | Affected Patients n (%) | n (%) | n (%) | n (%) | n (%) | Affected Patients n (%) | n (%) | n (%) |
| Blood and lymphatic system disorders | 58 (13) | 28 (28) | 17 (44) | 4 (7) | 4 (11) | 33 (8) | 17 (22) | 10 (35) | 2 (3) | 2 (5) | 91 (11) | 45 (25) | 27 (40) | 6 (5) | 6 (8) |
| Cardiac disorders | 16 (4) | 6 (6) | 5 (13) | 4 (7) | 4 (11) | 18 (5) | 5 (6) | 4 (14) | 6 (10) | 5 (13) | 34 (4) | 11 (6) | 9 (13) | 10 (8) | 9 (12) |
| Gastrointestinal disorders | 75 (17) | 7 (7) | 6 (15) | 8 (14) | 4 (11) | 75 (19) | 8 (10) | 7 (24) | 8 (13) | 4 (10) | 150 (18) | 15 (8) | 13 (19) | 16 (14) | 8 (10) |
| General disorders and administration site conditions | 77 (17) | 8 (8) | 4 (10) | 7 (12) | 3 (8) | 60 (15) | 4 (5) | 4 (14) | 7 (11) | 3 (8) | 137 (16) | 12 (7) | 8 (12) | 14 (12) | 6 (8) |
| Infections and infestations | 39 (9) | 11 (11) | 9 (23) | 12 (21) | 10 (26) | 42 (11) | 16 (20) | 11 (38) | 18 (29) | 12 (31) | 81 (10) | 27 (15) | 20 (29) | 30 (25) | 22 (29) |
| Metabolism and nutrition disorders | 37 (8) | 12 (12) | 10 (26) | 1 (2) | 1 (3) | 42 (11) | 11 (14) | 9 (31) | 4 (6) | 2 (5) | 79 (9) | 23 (13) | 19 (28) | 5 (4) | 3 (4) |
| Nervous system disorders | 19 (4) | 4 (4) | 3 (8) | 3 (5) | 2 (5) | 18 (5) | 2 (3) | 2 (7) | 2 (3) | 2 (5) | 37 (4) | 6 (3) | 7 (7) | 5 (4) | 4 (5) |
| Respiratory, thoracic and mediastinal disorders | 26 (6) | 3 (3) | 3 (8) | 4 (7) | 2 (5) | 24 (6) | 4 (5) | 4 (14) | 3 (5) | 2 (5) | 50 (6) | 7 (4) | 7 (10) | 7 (6) | 4 (5) |
AE = adverse events; NCI = National Cancer Institute (US National Institute of Health); CTCAE = common toxicity criteria for adverse events; SAE = serious AE; V = version.
Efficacy
The planned sample size was addressed to patients aged >80 years: 20 of 39 patients >80 years had an overall response (51%; 95% CI: 35%-68%). CR was achieved in 18 of 39 (46%; 30%–63%) and PR in 2 of 39 (5%) patients. There were 2 (5%) patients with SD, 5 (13%) with PD, and 7 (18%) patients with unknown response (Table 3). Relapse after CR occurred in 2 of 18 patients (11%).
Table 3.
Treatment Response
| >80 y (n = 39) | 61–80 y (n = 29) | Total (n = 68) | |
|---|---|---|---|
| Treatment response n (%) | |||
| CR (95% CI) | 18 (46) (30-63) | 3 (10) (2-27) | 21 (31) (20-43) |
| CR and additional treatment | 0 (0) | 2a (7) | 2 (3) |
| PR (95% CI) | 2 (5) (1-17) | 5 (17) (6-36) | 7 (10) (4-20) |
| SD | 2 (5) | 1 (3) | 3 (4) |
| PD (95% CI) | 5 (13) (4-27) | 9 (31) (15-51) | 14 (21) (12-32) |
| Unknown | 7 (18) | 4 (14) | 11 (16) |
| Study treatment-related death | 5 (13) | 5 (17) | 10 (15) |
| Relapse after CR (95% CI) | 2/18 (11) (1-35) | 0/3 (0) (0-71) | 2/21 (10) (1-30) |
| EFS, PFS, OS rates with 95% CI | |||
| 2-y EFS | 33 (18-48) | 10 (0-21) | 23 (13-33) |
| 2-y PFS | 45 (28-61) | 32 (13-51) | 40 (27-52) |
| 2-y OS | 46 (28-63) | 37 (17-57) | 42 (29-55) |
| PFS event n (%) | 21/39 (54) | 18/29 (62) | 39/68 (57) |
| PD | 5 (24) | 9 (50) | 14 (36) |
| Progression after PR, SD, unknown | 3 (14) | 1 (6) | 4 (10) |
| Relapse after CR/CR and additional treatment | 2 (10) | 1 (6) | 3 (8) |
| Death as earliest event | 11 (52) | 7 (39) | 18 (46) |
| Cause of death n (%) | 19/39 (49) | 16/29 (55) | 35/68 (51) |
| Lymphoma related | 6 (32) | 7 (44) | 13 (37) |
| Study treatment related | 5 (26) | 5 (31) | 10 (29) |
| Secondary neoplasia | 0 (0) | 1 (6) | 1 (3) |
| Concomitant disease | 1 (5) | 1 (6) | 2 (6) |
| Unknown | 7 (37) | 2 (12) | 9 (26) |
aOne patient received additional radiotherapy, 1 patient received additional rituximab.
CI = confidence interval; CR = complete remission; EFS = event-free survival; OS = overall survival; PD = progressive disease; PFS = progression-free survival; PR = partial remission; SD = stable disease.
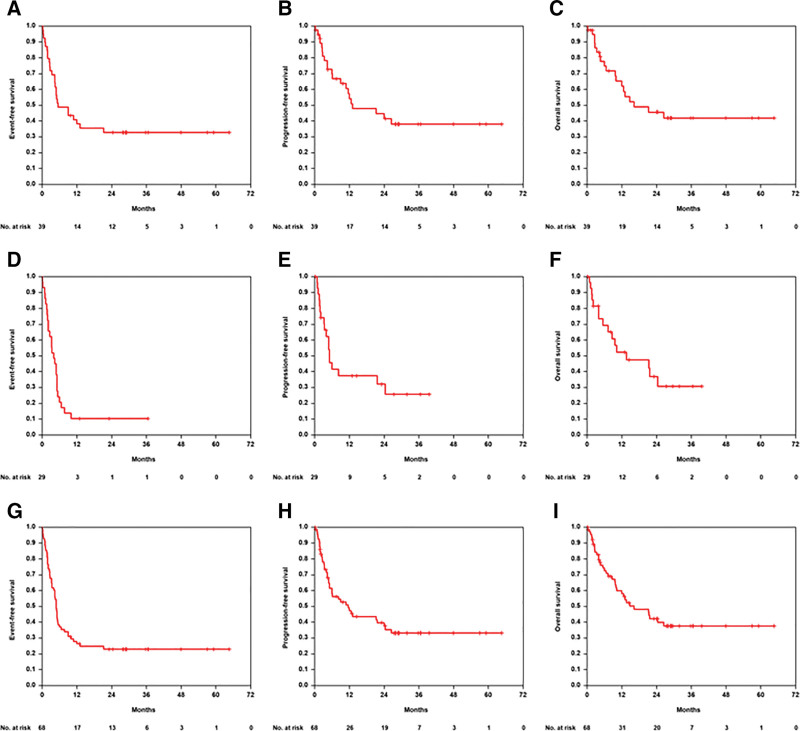
With a median observation time for OS of 29 months (range 0–65) for patients aged >80 years median EFS, PFS, and OS were 5, 13, and 16 months, respectively (Figure 2). Two-year EFS, 2-year PFS, and 2-year OS were 33% (95% CI, 18%-48%), 45% (28%-61%), 46% (28%-63%), respectively. The EFS, PFS, and OS curves show better (but not significant) outcome for patients with IPI 1, 2 versus IPI 3–5 (Suppl. Figure S3). There were no significant differences for EFS, PFS, and OS between male and female patients (data not shown). For EFS, there was no difference between the group with lower (≤6, n = 17) and higher (>6, n = 22) CIRS score (P = 0.399) in patients >80 years, but for PFS (P = 0.165) and OS (P = 0.049) patients with CIRS >6 had improved outcome (Suppl. Figure S4), possibly due to an imbalance of risk factors in this small subgroup. There were no significant differences in EFS, PFS, and OS according to G8 ≥14 (n = 12) versus G8 <14 (n = 27) in patients >80 years with a trend toward improved OS in patients with higher G8 scores (P = 0.068, Suppl. Figure S5). According to IADL, there was a significant better PFS (P = 0.038) and OS (P = 0.035) in patients aged >80 years and higher IADL score (Suppl. Figure S6). EFS, PFS, and OS according to QoL (EORTC) differed not significantly in patients aged >80 years between QoL Scores ≥50 (n = 19) versus QoL score <50 (n = 9, Suppl. Figure S7). In a multivariable analysis adjusted for IPI factors elevated LDH, ECOG >1 and >1 extralymphatic involvement constituted the most relevant risk factors for poor OS (Table 4). In total, 19 of 39 patients >80 years (49%) died: 6 of 19 (32%) tumor related, 5 of 19 (26%) treatment related, 1 of 19 (5%) due to concomitant disease and in 7 of 19 patients (37%), the cause of death remains unknown (Table 3).
Figure 2.
EFS, PFS and OS in B-R-ENDA treated patients. Event-free-survival (A, D, G), progression-free survival (B, E, H), and overall survival (C, F, I) in 39 patients aged >80 y (A–C), 29 patients aged 61–80 y (D–F), and for the 68 patients of the full analysis set (G–I).
Table 4.
Multivariable Analysis for EFS, PFS, and OS for Patients >80 y
| EFS HR (95% CI) | P | PFS HR (95% CI) | P | OS HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| LDH > normal | 2.6 (1.0-6.5) | 0.049 | 4.2 (1.2-14.8) | 0.024 | 4.3 (1.2-15.8) | 0.026 |
| ECOG > 1 | 2.1 (0.8-5.6) | 0.116 | 2.4 (0.8-7.2) | 0.136 | 3.8 (1.2-12.2) | 0.028 |
| Extralymphathic involvement > 1 | 3.2 (0.9-11.3) | 0.075 | 3.5 (0.9-13.1) | 0.067 | 6.4 (1.5-27.4) | 0.013 |
| Stage III/IV | 0.9 (0.4-2.1) | 0.759 | 0.7 (0.2-2.0) | 0.492 | 0.4 (0.1-1.4) | 0.147 |
CI = confidence interval; ECOG = Eastern Cooperative Oncology Group performance score; EFS = event-free survival; HR = hazard ratio; LDH = lactate dehydrogenase; OS = overall survival; PFS = progression-free survival.
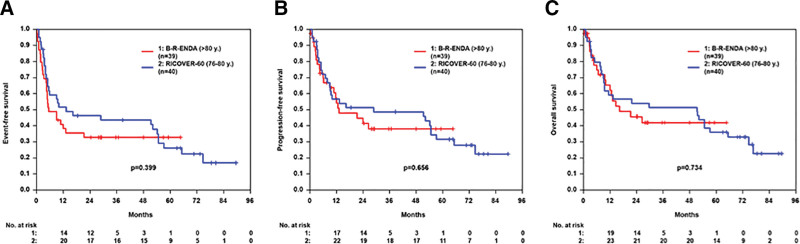
In a preplanned additional analysis, we compared the outcome of the 39 patients >80 years with 40 patients aged 76–80 years treated within the RICOVER-60 trial with 6xCHOP and 8 applications rituximab. There were no significant differences according to patient characteristic, except sex (52% male patients within RICOVER-60 vs 28% within B-R-ENDA, P = 0.028), bulky disease (42% within RICOVER-60 vs 21% within B-R-ENDA, P = 0.036), and age (Suppl. Table S3). Twenty-three percent of patients >80 years treated within the B-R-ENDA trial terminated the study early due to toxicity compared to 33% within the RICOVER-60 trial. The number of patients with an infection grades 3–5 was 23% within the B-R-ENDA trial (patients >80 years) and 44% within RICOVER-60 (patients aged 76–80 years). The TRM was 13% within the B-R-ENDA trial and 20% within RICOVER-60. EFS, PFS, and OS were not significantly different between these 2 cohorts for all patients (Figure 3) and for patients with IPI 1, 2, or 3–5 (Suppl. Figure S8). Two-year EFS, 2-year PFS, and 2-year OS for the 40 RICOVER-60 patients were 46% (95% CI, 31%-62%), 51% (36%-67%), 54% (38%-70%), respectively.
Figure 3.
EFS, PFS and OS in elderly B-R-ENDA and RICOVER-60 patients. Event-free-survival (A), progression-free survival (B), and overall survival (C) of B-R-ENDA patients aged >80 y (n = 39) and RICOVER-60 patients (6xCHOP 14 + 8xR) aged 76–80 y (n = 40).
As expected based on the inclusion criteria for this age group, the outcome of patients aged 61–80 years within the B-R-ENDA trial (n = 29) was inferior in comparison to the patients aged >80 years. Three patients achieved CR and 5 PR, the ORR was 28% (13%–47%). Two further patients achieved a CR, but the investigators decided to treat the patient additionally with radiotherapy or rituximab. There was 1 (3%) patient with SD, 9 (31%) patients with PD, and 4 (14%) patients with unknown response (Table 3). No relapse was reported. Five patients (17%) deceased during treatment due to toxicity. With a median observation time of 27 months for OS (range 0–40) for patients aged 61–80 years median EFS, PFS, and OS were 4, 5, and 14 months, respectively (Figure 2). Two-year EFS, PFS, and OS were 10% (0%–21%), 32% (13%–51%), 37% (17%–57%), respectively. In total, 16 of 29 patients aged 61–80 years (55%) have died: 7 of 16 (44%) lymphoma related, 5 of 16 (31%) treatment related, 1 of 16 (6%) due to secondary neoplasia, 1 of 16 (6%) due to concomitant disease, and in 2 of 16 patients (12%), the cause of death remains unknown (Table 3).
DISCUSSION
Although the incidence of aggressive B-cell lymphoma is increasing with age, patients older than 80 years or frail patients are excluded from most clinical trials. We sought to investigate the feasibility, toxicity, and efficacy of BR in such patients. Due to slow recruitment, the B-R-ENDA trial had to be terminated before the planned target was reached once more illustrating the difficulty of performing trials in this frail patient population.12,15 Nevertheless, this study represents the largest prospective cohort of elderly and frail patients with aggressive B-cell lymphoma and BR as first-line treatment reported so far.
According to protocol, patients >80 years were fitter than younger patients aged 61–80 years who also presented with higher LDH rates, more advanced stage and poorer performance status, higher CIRS and lower QoL scores. All patients received prephase treatment, but only half of the patients completed treatment per protocol, most of them (56%) were >80 years. Early termination of therapy was more frequent in patients ≤80 years, the major reasons being toxicity and PD.
Geriatric assessment was performed at initial screening and during FU and will be presented in further analyses in detail. Only for IADL screening the curves separated significantly for improved PFS and OS in patients aged > 80 years and higher IADL scores.
Only 2 patients with initial bulky disease and PR and SD as best response after immunochemotherapy did not receive consolidative radiation therapy, as it is recommended in Germany, based on physician’s choice and patient’s wish. Today, a [18F]fluorodeoxyglucose–positron emission tomography (PET) CT would be recommended to decide about consolidation radiation therapy in bulky disease DLBCL patients.35
Blood and lymphatic system disorders were the most common grades 3–5 AE (25%, 45/181) followed by infections (15%, 27/181), 38% of patients aged ≤80 years and 23% >80 years developed grades 3–5 infections. In total, 29% (22/77, Table 2) of all grades 3–5 SAE in B-R-ENDA treated elderly and frail patients were infections. In comparison, 27% (19/70) of all grades 3–5 SAE in R-miniCHOP treated elderly patients described by Peyrade et al10 were infections and infestations. We could demonstrate that there were 23% of B-R-ENDA treated patients aged >80 years with severe infections (grades 3–5) compared with 44% of RICOVER-60 treated patients aged 76–80 years. In both trials, the use of G-CSF was recommended according to ASCO/ESMO guidelines. In B-R-ENDA–treated patients, G-CSF was administered in 38% of patients aged >80 years and 31% of patients aged 61–80 years. Storti et al17 reported on only 4.4% grades 3–4 AE with infections in an elderly patient cohort comparable to our B-R-ENDA trial. Out of these patients 58% received G-CSF.17
The assessment of TRM was performed very conservatively, since all events until 60 days after treatment termination were counted as treatment-related. In total, 5 patients aged >80 years (13%) and 5 patients aged 61–80 years (17%) died of treatment-related causes. This is a higher rate than described by Storti et al17 for elderly frail patients treated with front-line BR (6%) or by Peyrade et al10 for R-miniCHOP–treated elderly patients (12/150 deaths, 8%). For patients treated within the RICOVER-60 trial, TRM was higher (20%) in patients aged 76 to 80 years.9
In former studies,30 low-risk-IPI patients showed significantly better EFS, PFS, and OS. In our cohort, multivariable analyses adjusted for IPI factors showed that even in older adults aged >80 years elevated LDH, ECOG >1 and more than 1 extralymphatic involvement remain the most relevant risk factors for poor outcome in contrast to stage. We could demonstrate that IPI allows a good risk stratification of lymphoma patients, while the CIRS score was not an appropriate tool for differentiation of patients at risk. Thus, the predictive power of the established IPI factors stays the same even in old patients.
For all B-R-ENDA trial patients ORR was 41%. Patients aged >80 years, who completed therapy more often than patients aged 61–80 years with higher CIRS score, showed an ORR of 51%. Prior trials using BR as first-line treatment in elderly patients with aggressive lymphoma described slightly higher ORR of 62%,17 69%,15 and 78%.16
Age alone does not seem to be the most important factor. Our data show, that older but fitter patients with a lower CIRS score had higher adherence to therapy, achieved higher response rates and less relapses. The 2-year PFS of 45% in B-R-ENDA–treated patients aged >80 years was comparable to 47% 2-year PFS in R-miniCHOP–treated elderly patients described by Peyrade et al.10 With a median OS of 16 months, the combination of BR as first-line treatment seems to be less effective than rituximab combined with miniCHOP with a median OS of 29 months10 but associated with a comparable rate of infections. The cohort was too small for further subgroup analyses except IPI factors. BR should not replace R-miniCHOP regimes, but our data show that older or frail DLBCL patients not able to receive standard CHOP-based therapy can benefit from that anthracycline-free therapeutic option.
At the time of planning the B-R-ENDA trial, the results of the R-miniCHOP trial were not yet available. Meanwhile, also miniCHOP together with ofatumumab11 or lenalidomide36 and new antibody-drug conjugates have shown promising results for elderly lymphoma patients either as monotherapy37 or in combination with BR38 or modified R-CHOP39 regimen. Again, infections and infestations remain a serious challenge for patients and physicians.36–39 Polatuzumab vedodtin combined with BR40 might be an interesting option to study for elderly and frail DLBCL patients even for up-front treatment. Further prospective and randomized trials are needed to identify new treatment approaches to better manage the dilemma between increased toxicity and reduced efficiency in elderly and frail DLBCL patients. For future trials with old and comorbid patients, there should be clear recommendations for optimal supportive care including antiinfectious prophylaxis, the use of G-CSF, and PET-CT driven decision-making.35
ACKNOWLEDGMENTS
The authors would like to thank all patients and their families for participating in this trial and all colleagues at each clinical trial site for supporting this study. All authors like to thank IOMEDICO AG, Freiburg, for trial organization, as well as Elke Stitz for trial office management at the University Medicine of Goettingen, and Beate Mann and Katja Rillich for technical assistance at the University of Leipzig.
AUTHOR CONTRIBUTIONS
LT, MZ, FZ, FB, UW did study conception and design. All the authors collected and assembled the data; provision of study material and patient care. BA, MZ, FB, FZ, LT did data analysis and interpretation. FB, BA, MZ, FZ, LT drafted or revised the manuscript. FB, FZ, MZ, AV, CK, GPK, AK, MD, AB, UW, DR, MdW, FH, VP, NS, MWH, WK, AR, GW, BA, LT reviewed and approved the final version of the manuscript. All contributing centers are listed in Suppl. Table S4.
DISCLOSURES
AV was part of an advisory Board and lectures for Novartis, BMS, Kite/Gilead, Roche and Amgen. GP-K was part of an advisory board for Janssen and Abbvie. AK Employment at Lilly Deutschland GmbH since 2018. MD received Research support (institution) by Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche, and received speakers honoraria by Amgen, Astra Zeneca, BMS/Celgene, Gilead/Kite, Incyte, Janssen, Novartis, Roche; was part of an scientific advisory board for Astra Zeneca, Beigene, BMS/Celgene, Genmab, Gilead/Kite, Incyte, Janssen, Lilly/Loxo, Morphosys, Novartis, Roche. AB was part of an advisory board for Incyte, received travel support by BMS, Novartis and Roche. VP received travel support by Abbvie, Amgen, BMS, Gilead and Roche. GW was part of an advisory board and performed lectures for Novartis, Kite/Gilead, Roche, Amgen, Takeda, Clinigen. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This trial was supported by Astellas Pharma GmbH, Munich, Germany (trial medication bendamustine) and Roche AG, Basel, Swiss (trial medication rituximab i.v. and s.c.). They provided free trial medication and an unrestricted grant for trial conduct.
Supplementary Material
Footnotes
Friederike Braulke and Florian Zettl contributed equally and Bettina Altmann and Lorenz Trümper contributed equally.
The trial is registered at www.clinicaltrials.gov as #NCT01686321.
Previous presentation: Presented as preliminary results at the annual meetings of the German, Swiss, Austrian Association of Hematology and Oncology (DGHO) in 2014 in Hamburg, Germany as poster presentation by F. Zettl (Oncol Res Treat 2014;37(suppl 5):1–313), in 2019 in Berlin as oral presentation by F. Braulke (Oncol Res Treat 2019;42(suppl 4):1–336), and as poster presentation by F. Zettl at the annual meeting of the American Society of Hematology (ASH) 2019 in Orlando, Florida, USA (Blood (2019) 134 (Supplement_1):4073).
Supplemental digital content is available for this article.
REFERENCES
- 1.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(suppl 5):vv116116–v125 [DOI] [PubMed] [Google Scholar]
- 2.Sarkozy C, Salles G, Falandry C. The biology of aging and lymphoma: a complex interplay. Curr Oncol Rep. 2015;17:32. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M. Age and sex in non-Hodgkin lymphoma therapy: it’s not all created equal, or is it? Am Soc Clin Oncol Educ Book. 2017;37:505–511. [DOI] [PubMed] [Google Scholar]
- 4.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krebs in Deutschland für 2015/2016.12. Ausgabe. Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). [In German.] Berlin, 2019. [Google Scholar]
- 6.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell Lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105–116. [DOI] [PubMed] [Google Scholar]
- 7.Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. [DOI] [PubMed] [Google Scholar]
- 8.Pfreundschuh M, Poeschel V, Zeynalova S, et al. Optimization of rituximab for the treatment of diffuse large B-cell lymphoma (II): extended rituximab exposure time in the SMARTE-R-CHOP-14 trial of the German high-grade non-Hodgkin lymphoma study group. J Clin Oncol. 2014;32:4127–4133. [DOI] [PubMed] [Google Scholar]
- 9.Zettl F, Ziepert M, Altmann B, et al. Age-dependent increase of treatment-related mortality in older patients with aggressive B cell lymphoma: analysis of outcome, treatment feasibility, and toxicity in 1171 elderly patients with aggressive B cell lymphoma—data from phase II and III trials of the DSHNHL (German High-Grade Non-Hodgkin’s Lymphoma Study Group). Ann Hematol. 2021;100:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicenter, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. [DOI] [PubMed] [Google Scholar]
- 11.Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017;4:e46–e55. [DOI] [PubMed] [Google Scholar]
- 12.Flinn IW, Erter J, Daniel DB, et al. Phase II study of bendamustine and ofatumumab in elderly patients with newly diagnosed diffuse large b-cell lymphoma who are poor candidates for R-CHOP chemotherapy. Oncologist. 2019;24:10351–1e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn J, Kleber M, Hieke S, et al. Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol. 2012;91:1579–1586. [DOI] [PubMed] [Google Scholar]
- 14.Walter E, Schmitt T, Dietrich S, et al. Rituximab and bendamustine in patients with CD20+ diffuse large B-cell lymphoma not eligible for cyclophosphamide, doxorubicin, vincristine and prednisone-like chemotherapy. Leuk Lymphoma. 2012;53:2290–2292. [DOI] [PubMed] [Google Scholar]
- 15.Weidmann E, Neumann A, Fauth F, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22:1839–1844. [DOI] [PubMed] [Google Scholar]
- 16.Park SI, Grover NS, Olajide O, et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untretated diffuse large B-cell lymphoma. Br J Haematol. 2016;175:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storti S, Spina M, Pesce EA, et al. Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: a phase II multicenter study of the Fonazione Italiana Linfomi. Haematologica. 2018;103:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. [DOI] [PubMed] [Google Scholar]
- 20.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. [DOI] [PubMed] [Google Scholar]
- 21.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Ford AB, Moscowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:94–99. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 24.Winkelmann N, Petersen I, Kiehntopf M, et al. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma. J Cancer Res Clin Oncol. 2011;137:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading cognitive status of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 26.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;39:37–49. [DOI] [PubMed] [Google Scholar]
- 27.Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. [DOI] [PubMed] [Google Scholar]
- 28.Merli F, Luminari S, Tucci A, et al. Simplified geriatric assessment in older patients with diffuse large B-cell lymphoma: the prospective elderly project of the Fondazione Italiana Linfomi. J Clin Oncol. 2021;39:1214–1222. [DOI] [PubMed] [Google Scholar]
- 29.A predictive model for aggressive non-Hodgkin lymphoma. The international non-hodgkin lymphoma prognostic factors project. N Engl J Med. 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 30.Ziepert M, Hasenclever D, Kuhnt E, et al. Standard international prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:2373–2380. [DOI] [PubMed] [Google Scholar]
- 31.Wunderlich A, Kloess M, Reiser M, et al. Practicability and acute haematological toxicity of 2- and 3-weekly CHOP and CHOEP chemotherapy for aggressive non Hodgkin’s lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol. 2003;14:881–893. [DOI] [PubMed] [Google Scholar]
- 32.Fayers PM, Aaronson NK, Bjordal K, et al. (Eds). EORTC QLQ-C30 Scoring Manual (3rd edition). Brussels: EORTC, 2001. [Google Scholar]
- 33.Gross A, Ziepert M, Scholz M. KMWin – a convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS One. 2012;7:e38960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffe ET. 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009;523:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Held G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive lymphoma. J Clin Oncol. 2014;32:1112–1118. [DOI] [PubMed] [Google Scholar]
- 36.Oberic L, Peyrade F, Puyade M, et al. Subcutaneous rituximab-miniCHOP compared with subcutaneous rituximab-MiniCHOP plus lenalidomide in diffuse large B-cell lymphoma for patients age 80 years or older. J Clin Oncol. 2021;39:1203–1213. [DOI] [PubMed] [Google Scholar]
- 37.Hamadani M, Radford J, Carlo-Stella C, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137:2634–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.