Abstract
Previously we identified B6.EDA+/+ mice as a novel mouse model that presents with elevated IOP and trabecular meshwork damage. Here, we expand on our previous findings by measuring aqueous humor outflow facility and analyzing the integrity of the inner wall of Schlemm’s canal. As expected, intraocular pressure (IOP) was increased, and outflow facility was decreased compared to C57BL/6J controls. B6.EDA+/+ mice had significantly increased expression of the adherens junction protein, VE-cadherin by the inner wall endothelium of Schlemm’s canal. These data suggest that in addition to trabecular meshwork damage, there are changes in Schlemm’s canal in B6.EDA+/+ mice that lead to aqueous outflow dysfunction and ocular hypertension.
Keywords: FN-EDA, Outflow facility, IOP, VE-Cadherin, Ocular hypertension
Mouse models of ocular hypertension are an invaluable resource to study aqueous humor outflow, as well as trabecular meshwork and Schlemm’s canal dysfunction, due to the many anatomical, physiological, and pharmacological similarities between the anterior segments of rodent and human eyes. The major portion of aqueous humor flows through the conventional outflow pathway from the trabecular meshwork into Schlemm’s canal, from there to be returned to the venous system via scleral collector channels. Recently, a consensus recommendation described the importance of measuring outflow dysfunction in mouse models of ocular hypertension (McDowell et al., 2022). Outflow facility is calculated from pressure-dependent flow measurements, equal to the reciprocal total resistance to aqueous humor outflow from the ocular anterior segment.
Here, we studied aqueous humor outflow and changes to Schlemm’s canal endothelium in B6.EDA+/+ mice. Generation of B6.EDA+/+ mice, which express only FN containing EDA, has previously been described (Muro et al., 2003). We previously reported that B6.EDA+/+ mice have significantly elevated IOP by 3.5 months of age (Roberts et al., 2020), which persists to at least one year of age (Mavlyutov et al., 2022). B6. EDA+/+ mice also develop increased ECM production and expression of TLR4, a known receptor which is activated by FN-EDA, in the trabecular meshwork leading to downstream fibro-inflammatory responses (Hernandez et al., 2020; Mavlyutov et al., 2022; Roberts et al., 2020). Additionally, we have demonstrated glaucomatous phenotypes in B6. EDA+/+ mice in the retina, optic nerve (ON), and optic nerve head (ONH) including increased microgliosis and increased Tlr4 expression in Iba1 positive ONH cells at 9 months of age, as well as ON damage and RGC loss by one year of age compared to C57BL/6J controls (Mavlyutov et al., 2022). This goal of this study was to complete the characterization of changes in the anterior segment tissues responsible for elevated IOP and outflow dysfunction.
All experiments were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC) Guidelines and Regulations. B6. EDA+/+ mice were bred and maintained in house. C57BL/6J mice were purchased from the Jackson Laboratories. All mice were housed in the UW-Madison vivarium; maintained on a normal 12-h light/dark cycle and a 4% fat diet (Harkland Teklad, Madison, WI, USA) with food and water available ad libitum.
IOP was measured in five-month-old B6.EDA+/+ mice and C57BL/6J control mice with a rebound tonometer (TonoLab tonometer, Colonial Medical Supply, Franconia, NH) as we previously described (Hernandez et al., 2017; Roberts et al., 2020). IOP’s are shown as means (± SD) and statistical significance determined by Student’s t-test, n = 22 eyes/-strain. Immediately following IOP measurements, animals were sacrificed by CO2 inhalation and eyes enucleated, de-identified, and shipped overnight to Duke University in low glucose DMEM on ice for ex vivo outflow facility measurements. To measure the outflow facility, the iPerfusion system was used (Sherwood et al., 2016). Degassed DBG was perfused through mouse eyes and after an acclimation phase of 30 min at 12 mmHg, the eyes underwent a sequence of 9 pressure steps, starting at 5 mmHg in steps of 1.5 mmHg to a maximum of 17 mmHg, then decreasing to 8 mmHg for the final step. Data analysis was completed as previously described using a non-linear flow-pressure model to account for pressure dependence of outflow facility (Sherwood et al., 2016). Traces that were asymmetric between contralateral eyes, showed signs of clogs in the needles, air bubble in the line, or leakage as judged by three masked observers were not included in final data set.
As expected, B6.EDA+/+ mice had significantly elevated IOP (17.8 ± 2.3 mmHg, n = 22 eyes, p < 0.0001) compared to C57BL/6J control mice (13.1 ± 1.0 mmHg, n = 22 eyes), Fig. 1A. In addition, outflow facility significantly decreased by 26.9% (p = 0.032) in B6.EDA+/+ mice (3.17 ± 1.62 nl/min/mmHg, n = 11 eyes) compared to C57BL/6J control mice (4.34 ± 1.48 nl/min/mmHg, n = 16 eyes), Fig. 1B. These data verify the ocular hypertension phenotype is due to dysfunction of aqueous humor outflow.
Fig. 1. B6.EDA+/+ mice have decreased outflow facility and elevated IOP.
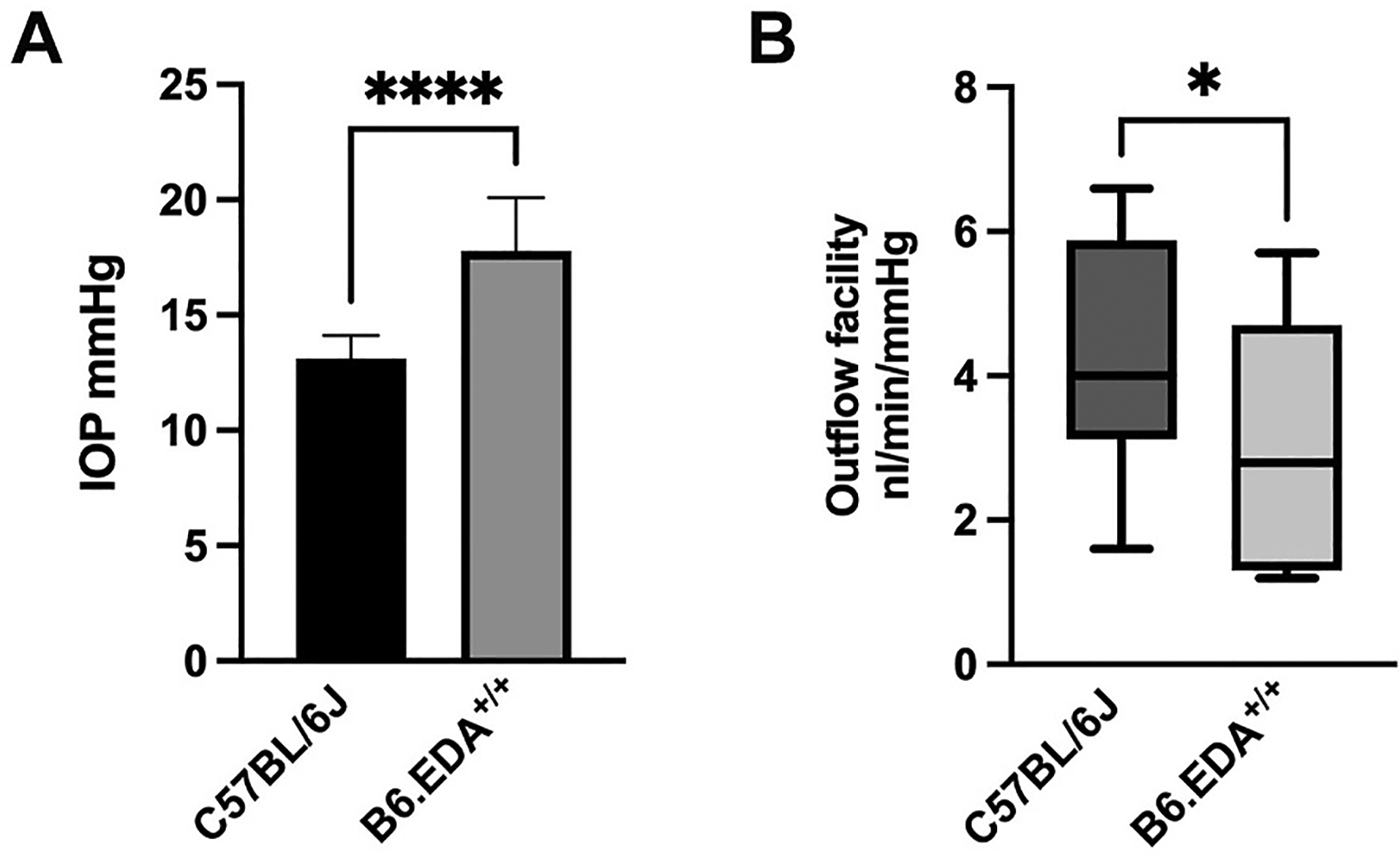
(A) B6.EDA+/+ mice (n = 22 eyes) have elevated IOP compared to C57BL/6J controls (n = 22 eyes), ****p < 0.0001. (B) B6.EDA+/+ mice (n = 11 eyes) have decreased outflow facility compared to C57BL/6J controls (n = 16 eyes); p = 0.032. Statistical significance determined by Student’s t-test.
To further analyze the structure and integrity of Schlemm’s canal, we performed immunohistochemistry and immunofluorescence microscopy to visualize the expression of VE-cadherin and a Schlemm’s canal marker PECAM-1. VE-cadherin associates as dimers between adjacent endothelial cells to form adherens junctions. Importantly, disruption of VE-cadherin function can lead to increased cell and tissue permeability (Corada et al., 1999). VE-cadherin is known to be expressed in primary human Schlemm’s canal endothelial cells in culture as well as increase the trans-endothelial resistance values in culture (Gonzalez et al., 2004; Heimark et al., 2002; Perkumas and Stamer, 2012). In addition, VE-cadherin was recently shown to be increased in glaucomatous Schlemm’s canal endothelial cells in culture (Kelly et al., 2021), suggesting that the glaucomatous Schlemm’s canal endothelial cells may have decreased paracellular permeability which could affect aqueous humor outflow in vivo.
Here, we utilized B6.EDA+/+ mice and C57BL/6J control mice (11–12 months old, n = 4–5 mice/strain) to evaluate the expression of VE-cadherin in the inner wall of Schlemm’s canal. We previously showed B6.EDA+/+ mice at 12 months of age have elevated IOP, increased ECM deposition in the TM and JCT region, thickened and more continuous Schlemm’s canal basement membrane, and more smaller giant vacuoles consistent with human primary open angle glaucoma (POAG) phenotypes (Mavlyutov et al., 2022). Immunohistochemistry was performed as previously described (Mavlyutov et al., 2022). Primary antibodies against VE-cadherin 1/250 (AbCam #ab205336) and PECAM1 1/100 (Millipore/Sigma #MAB1398Z) and secondary antibodies Donkey anti Guinea pig Cy3 conjugated and Donkey anti Rabbit Cy2 conjugated, (Jackson Immunoresearch #706545148 and #711225152) were used in the staining protocol. Two sections from each eye were analyzed. Images were acquired and analyzed as previously described (Mavlyutov et al., 2022). The inner wall of Schlemm’s canal endothelium (detected by red channel, PECAM1 labeling) was selected by thresholding (equal settings for threshold selection were applied for all images), and intensities for VE-cadherin (detected by green channel) were quantified in selected areas. VE-cadherin expression was significantly increased in the inner wall endothelium of Schlemm’s canal of B6.EDA+/+ mice compared to C57BL/6J controls (Fig. 2A and B). There was no statistical difference in the total area of Schlemm’s canal in sections from B6.EDA+/+ mice compared to C57BL/6J controls as measured by ImageJ (Fig. 2C). These data suggest that less permeable adherens junctions in the inner wall endothelium of Schlemm’s canal may be contributing to the decreased outflow facility in B6.EDA+/+ mice. However, it is well known that segmental outflow occurs through high flow and low flow regions of the TM/SC and future work will be necessary to determine the permeability of the adherens junctions in each of these flow regions in B6.EDA+/+ mice. Previous work demonstrated that the inner wall of Schlemm’s canal and the JCT region are the main location for aqueous humor outflow resistance (Lutjen-Drecoll, 1999; Vahabikashi et al., 2019). We previously reported ultrastructural changes to both the trabecular meshwork as well as Schlemm’s canal endothelium in B6.EDA+/+ mice (Mavlyutov et al., 2022). Here we show that junctions between Schlemm’s canal endothelium are affected as well, demonstrating increased VE-cadherin expression in B6.EDA+/+ mice.
Fig. 2. B6.EDA+/+ mice express increased amount of VE-Cadherin in the endothelium of the inner wall of Schlemm’s canal.
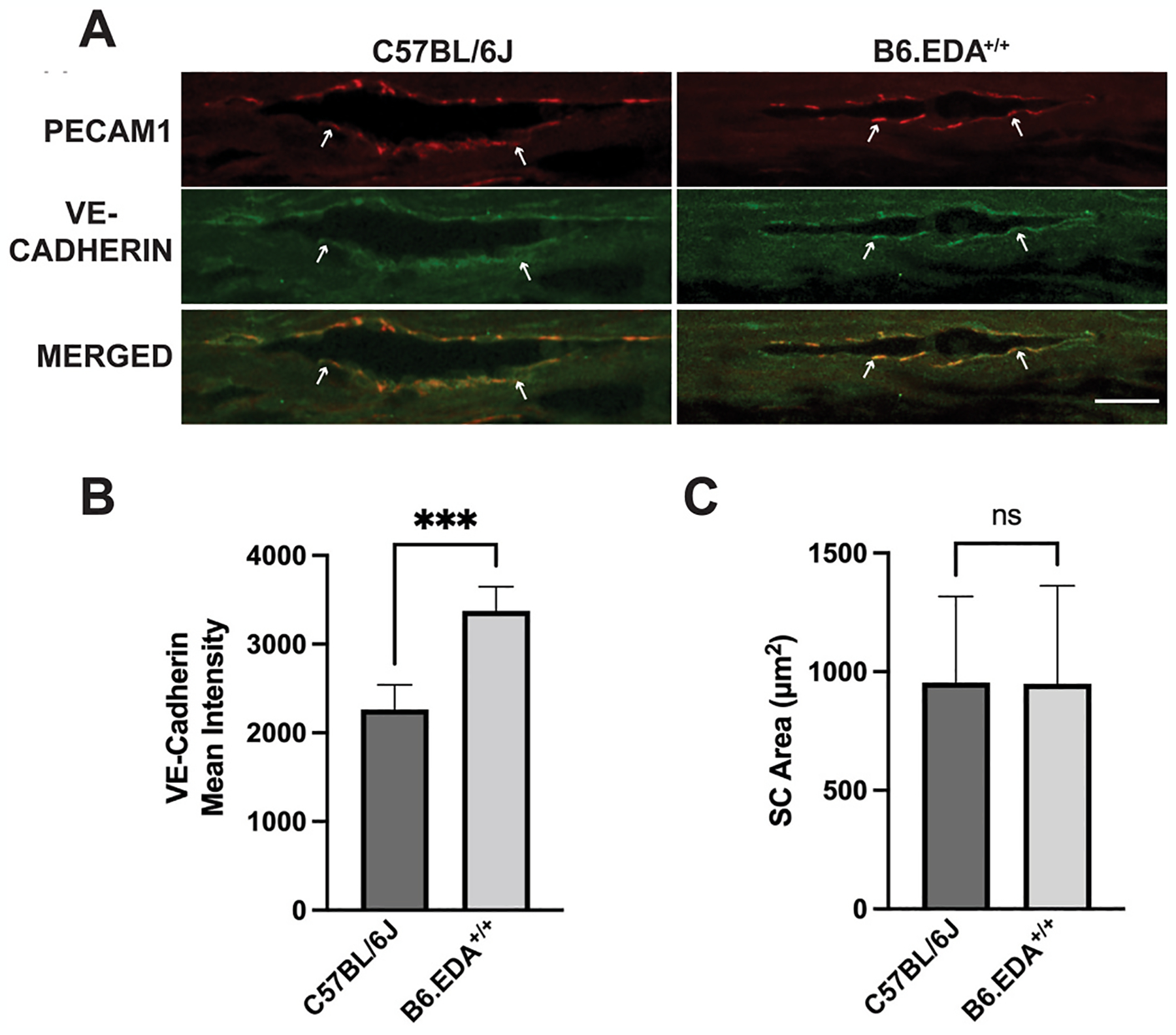
(A) Cryosections from eyes of C57BL/6J and B6.EDA+/+ mice were immunolabeled for endothelial marker PECAM1 (red) and VE-Cadherin (green), scale bar 20 μm. (B) Average intensity of VE-Cadherin in the inner wall of Schlemm’s canal was quantified by ImageJ analysis. (C) Schlemm’s canal area quantified by ImageJ analysis. Significance determined by Student’s t-test, n = 4–5 mice/strain, **p < 0.01.
In conclusion, here we have extended our previous findings by establishing that B6.EDA+/+ mice are a bonafide mouse model of ocular hypertension due to aqueous humor outflow dysfunction. Decreased outflow facility is consistent with our prior work showing elevated IOP and ultrastructural changes to the TM and Schlemm’s canal (Mavlyutov et al., 2022; Roberts et al., 2020). Importantly, such changes are observed in POAG patients (McDowell et al., 2022). As we previously reported, these mice also demonstrate glaucomatous damage in the retina and optic nerve (Mavlyutov et al., 2022), making B6.EDA+/+ mice a valuable resource to evaluate the molecular pathology of ocular hypertension and glaucoma in a slowly progressive chronic mouse model system.
Acknowledgements
The authors acknowledge Kelsey Dail for help with maintenance of the animal colonies. This work was supported by National Institute of Health R01EY02652 (CMM). This work was also supported in part by Unrestricted Grants from Research to Prevent Blindness to the UW-Madison Department of Ophthalmology and Visual Sciences and to Department of Ophthalmology at Duke University, and the Core Grant for Vision Research from the NIH to the University of Wisconsin-Madison (P30 EY016665). In addition, this study was supported by National Institutes of Health Grants R01EY028608, P30EY005722, R01EY022359 (WDS and Duke University).
Footnotes
Declarations of competing interest
None.
Data availability
Data will be made available on request.
References
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E, 1999. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. In: Proceedings of the National Academy of Sciences of the United States of America, vol. 96, pp. 9815–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Caballero M, Liton PB, Stamer WD, Epstein DL, 2004. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest Ophthalmol. Visual Sci 45, 1389–1395. [DOI] [PubMed] [Google Scholar]
- Heimark RL, Kaochar S, Stamer WD, 2002. Human Schlemm’s canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr. Eye Res 25, 299–308. [DOI] [PubMed] [Google Scholar]
- Hernandez H, Medina-Ortiz WE, Luan T, Clark AF, McDowell CM, 2017. Crosstalk between transforming growth factor beta-2 and toll-like receptor 4 in the trabecular meshwork. Invest Ophthalmol. Visual Sci 58, 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez H, Roberts AL, McDowell CM, 2020. Nuclear factor-kappa beta signaling is required for transforming growth factor Beta-2 induced ocular hypertension. Exp. Eye Res 191, 107920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RA, Perkumas KM, Campbell M, Farrar GJ, Stamer WD, Humphries P, O’Callaghan J, O’Brien CJ, 2021. Fibrotic changes to schlemm’s canal endothelial cells in glaucoma. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, 1999. Functional morphology of the trabecular meshwork in primate eyes. Prog. Retin. Eye Res 18, 91–119. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Myrah JJ, Chauhan AK, Liu Y, McDowell CM, 2022. Fibronectin extra domain A (FN-EDA) causes glaucomatous trabecular meshwork, retina, and optic nerve damage in mice. Cell Biosci. 12, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CM, Kizhatil K, Elliott MH, Overby DR, van Batenburg-Sherwood J, Millar JC, Kuehn MH, Zode G, Acott TS, Anderson MG, Bhattacharya SK, Bertrand JA, Borras T, Bovenkamp DE, Cheng L, Danias J, De Ieso ML, Du Y, Faralli JA, Fuchshofer R, Ganapathy PS, Gong H, Herberg S, Hernandez H, Humphries P, John SWM, Kaufman PL, Keller KE, Kelley MJ, Kelly RA, Krizaj D, Kumar A, Leonard BC, Lieberman RL, Liton P, Liu Y, Liu KC, Lopez NN, Mao W, Mavlyutov T, McDonnell F, McLellan GJ, Mzyk P, Nartey A, Pasquale LR, Patel GC, Pattabiraman PP, Peters DM, Raghunathan V, Rao PV, Rayana N, Raychaudhuri U, Reina-Torres E, Ren R, Rhee D, Chowdhury UR, Samples JR, Samples EG, Sharif N, Schuman JS, Sheffield VC, Stevenson CH, Soundararajan A, Subramanian P, Sugali CK, Sun Y, Toris CB, Torrejon KY, Vahabikashi A, Vranka JA, Wang T, Willoughby CE, Xin C, Yun H, Zhang HF, Fautsch MP, Tamm ER, Clark AF, Ethier CR, Stamer WD, 2022. Consensus recommendation for mouse models of ocular hypertension to study aqueous humor outflow and its mechanisms. Invest Ophthalmol. Visual Sci 63, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE, 2003. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol 162, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkumas KM, Stamer WD, 2012. Protein markers and differentiation in culture for Schlemm’s canal endothelial cells. Exp. Eye Res 96, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Mavlyutov TA, Perlmutter TE, Curry SM, Harris SL, Chauhan AK, McDowell CM, 2020. Fibronectin extra domain A (FN-EDA) elevates intraocular pressure through Toll-like receptor 4 signaling. Sci. Rep 10, 9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR, 2016. Measurement of outflow facility using iPerfusion. PLoS One 11, e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabikashi A, Gelman A, Dong B, Gong L, Cha EDK, Schimmel M, Tamm ER, Perkumas K, Stamer WD, Sun C, Zhang HF, Gong H, Johnson M, 2019. Increased Stiffness and Flow Resistance of the Inner Wall of Schlemm’s Canal in Glaucomatous Human Eyes. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


