Abstract
Informed consent is a foundational ethical and legal principle in human subjects research and clinical care. Yet, there is extensive debate over how much information must be disclosed to meet ethical goals and legal requirements, especially about non‐medical risks. In this online, survey‐based experiment of a diverse sample of the US general population, we explored one aspect of this debate by testing whether the level of detail included in informed consent regarding genetic anti‐discrimination protections alters individuals' willingness to participate in a hypothetical research study and their concerns regarding genetic discrimination. Participants were randomized to receive sample informed consent language with one of three levels of disclosure regarding the protections and limitations of the Genetic Information Nondiscrimination Act (GINA). Our sample (n = 1,195) had a mean age of 45.9 (SD = 17.9) years and 40% with ≤high school education. Participants were 51.3% female and 36.7% non‐Hispanic White. On average, those who received consent language with none of GINA's limitations highlighted were more willing to participate than those who were warned about various gaps in GINA. They also had significantly lower perceived risk of discrimination than those presented with the most information about limitations. Our study found that providing more comprehensive information about GINA notably lessened willingness to participate in the hypothetical studies, highlighting the need for clinicians and researchers to thoughtfully consider how to disclose anti‐discrimination risks in informed consent.
Keywords: discrimination, Genetic Information Nondiscrimination Act, genetic research, genetic testing, informed consent
Short abstract

What is known about this topic
It is well established that informed consent is a necessary component of ethical research and clinical care. This informed consent should include disclosure of risks, including non‐medical risks.
What this paper adds to the topic
This paper digs into the difficult nuance of how and to what extent to disclose non‐medical risks. It adds significantly to the literature by bringing empirical analysis of the impact of different consent language on individuals' perceptions.
1. INTRODUCTION
Studies have shown that some individuals decline participation in clinical and research genetic testing due to fear of genetic discrimination (Allain et al., 2012; Amendola et al., 2018; Robinson et al., 2016). Most notably, they are fearful of how insurers and employers will use their genetic information for eligibility and employment decisions (Wauters & Van Hoyweghen, 2016). Much of the research and discussion of these fears focuses on lack of protections in the realms of life, disability, and long‐term care insurance (LTC) specifically (McGuire & Majumder, 2009; Prince & Roche, 2014; Rothstein, 2008). Arguably, however, this concern is simultaneously overbroad and too limited. It is too broad because there is relatively little evidence of widespread discrimination by these entities (Areheart & Roberts, 2019; Barlow‐Stewart et al., 2018; Suter, 2018), so it may be potentially against one's best interest to forego testing out of fear of an unlikely event. It is too limited because federal law, under the Genetic Information Nondiscrimination Act (GINA), and state law counterparts generally only restrict health insurers and employers from considering genetic testing, leaving a vast number of entities potentially able to engage in genetic discrimination (Anderson et al., 2021; GINA, 2008).
GINA is a federal anti‐discrimination law, passed in 2008, aimed at encouraging greater uptake of genetic testing in the clinical and research realms by assuaging concerns of genetic discrimination (GINA, 2008). It prohibits employers with 15 or more employees from considering genetic information in hiring, firing, promotion, and other employment decisions. It also prohibits health insurers from using an applicant's genetic information—defined as genetic test results and family health history—to set premiums, make coverage and other health insurance decisions. GINA does not prevent employers and health insurers from considering manifested symptoms, so it is predominately relevant only for asymptomatic individuals undergoing predictive or presymptomatic testing to determine their risk for genetic conditions.
There are some exceptions and gaps in the law. Most notably, GINA's scope only covers employers and health insurers. US anti‐discrimination law generally does not restrict insurers other than health insurers or other actors such as lenders or educational institutions from discriminating on the basis of genetic test results. Despite these broad gaps, discussion usually focuses on how GINA fails to cover life, disability, and LTC insurers (McGuire & Majumder, 2009; Prince & Roche, 2014; Rothstein, 2008).
One potential reason that the public discussion about the limits of GINA tends to focus narrowly on life, disability, and LTC insurers is because these insurances were traditionally most likely to take into account medical information when deciding whether to insure an individual. Therefore, clinicians and researchers often specifically mention these entities when patients and participants are considering genetic testing. Indeed, the Office of Human Research Protections in the US Department of Health and Human Services (HHS) recommends that informed consent documents for research include language about how federal genetic anti‐discrimination laws do not extend to life, disability, and LTC insurers (OHRP, 2009). As the guidance acknowledges, '[g]iven that GINA has implications regarding actual or perceived risks of genetic research and an individual's willingness to participate in such research, investigators and Institutional Review Boards (IRBs) should be aware of the protections provided by GINA as well as the limitations in the law's scope and effect' (OHRP, 2009). The guidance provides model language for inclusion in informed consent documents, which includes the following warning: 'Be aware that this new Federal law does not protect you against genetic discrimination by companies that sell life insurance, disability insurance, or long‐term care insurance' (OHRP, 2009). Many genetic research programs have adopted similar language in their informed consent documents (CSER, n.d.) Additionally, while this guidance is focused on the research realm, similar kinds of disclosures could be an important part of obtaining informed consent for clinical genetic testing.
There are questions, however, about the impact of such language on participants and whether this disclosure provides the appropriate level of information about gaps in GINA. In general, informed consent for research should include information that a reasonable person would want to know prior to joining a study (45 CFR §46.116(a)(4), 2018) In the clinical context, many states also use a reasonable person standard for informed consent (Canterbury v. Spence, 1972; Sawicki, 2015). On the one hand, given recognized fear of genetic discrimination, particularly in insurance and employment, it is logical to include information about the lack of coverage for life, disability, and LTC insurance. It is also foreseeable that a reasonable person might want to know about the lack of protections in other areas, like mortgage lending, education, or other insurance products, like auto and property insurance. On the other hand, we cannot presume clinicians and researchers know this information nor expect informed consent documents to list every possible specific risk of a study or clinical procedure, especially if some are likely to be very rare or unknown or if disclosure would require listing every omission in an anti‐discrimination law. Too much detail about the limits in GINA's coverage could overwhelm individuals and heighten fears of discrimination, when those risks may be quite low depending on the clinical or research testing being considered.
Determining the appropriate balance has important implications for clinicians and researchers who are consenting individuals for genetic testing. While there are many nuanced aspects to this debate, a starting question is whether and how the details regarding legal protections presented in informed consent documents affect individuals' willingness to undergo genetic testing and their understanding of the law. In this survey‐based experiment with the US public, we tested whether individuals' willingness to participate in a hypothetical genetic research study is affected by the level of detail included in an informed consent document regarding genetic anti‐discrimination protections. In our study, a diverse sample of US participants was randomized to one of three types of informed consent language. We assessed how changes in the descriptions of legal protections and gaps alter fears of genetic discrimination and willingness to participate in a hypothetical study involving genetic testing.
2. METHODS
2.1. Participants
We used Qualtrics Research Services to recruit US residents aged 18 years or older, with quotas set to ensure that gender, age, race/ethnicity, educational attainment, and household income mirrored the general US population. Responses were collected from June 29 to July 20, 2020. The first page of the survey included consent information, such as the voluntary nature of the study, contact information for the principal investigator and IRB, and assurances that no personal information would be collected. Those who consented could click to continue to the survey questions. This study was deemed as exempt human subjects research by the University of Iowa Institutional Review Board (IRB) (202002765).
2.2. Instrumentation and procedures
The survey was developed by an interdisciplinary team of researchers, based on a literature review, and included both validated and novel measures. Our primary goal was to assess whether the amount of information provided about legal protections could affect individual's willingness to participate in the study and, relatedly, their concerns about discrimination and difficulty in coming to a decision about whether to join the study. To achieve these goals, near the beginning of the survey, participants read a hypothetical scenario where they were asked to imagine being invited to participate in a genetic research study and provided with a short snippet of informed consent language regarding GINA. Participants were randomized to the amount of information provided about GINA in the informed consent language—(a) current language recommended by HHS (basic disclosure consent), (b) the basic disclosure consent, but without the reference to other insurance types not covered by GINA (minimum disclosure consent), and (c) the basic disclosure consent, but with additional examples of other types of entities not regulated by GINA (comprehensive disclosure consent) (Figure 1). The consents were similar in terms of length, reading level, and number of participants randomized to each group (Figure 1). 1
FIGURE 1.
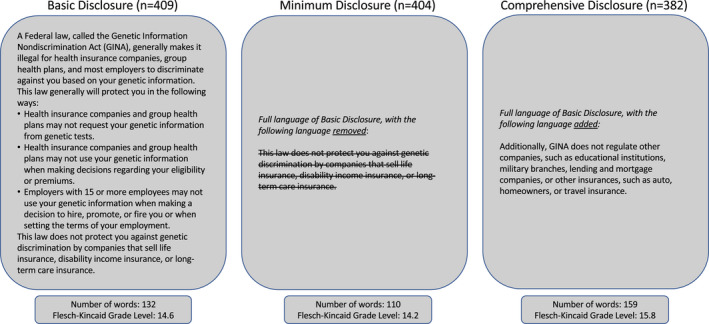
Consent randomization
2.2.1. Primary measures
After receiving the information about the research scenario and the consent, participants used 7‐point Likert scales to respond to three items that served as our primary outcome measures:
Willingness to participate in the hypothetical study, with scale anchors of Not at all likely (1) and Very likely (7) (Willingness 1).
Perceived risk of genetic discrimination based on the informed consent language, with scale anchors of Not at all at risk (1) and At high risk (7).
Ease of deciding whether to participate, with scale anchors of Very difficult (1) and Very easy (7).
Qualitative responses were collected to further assess why participants felt they were or were not at risk for discrimination based on the informed consent.
Since we were particularly interested in how informed consent language affected willingness to participate in the hypothetical study, we further interrogated the interplay between consent and willingness. Participants were asked if their willingness to participate in the genetic testing study would change if the informed consent language was different. Thus, after the first round of primary questions, participants were randomized to be shown one of the two remaining consent language options and were asked whether their willingness to participate would change (Willingness 2). Respondents who indicated their willingness would change provided their reasons via open‐ended response prompts.
2.2.2. Control variables
The remainder of the survey included questions that measured our control variables. We included factors, such as demographics, fear of genetic discrimination, knowledge of genetics, and knowledge of anti‐discrimination laws, that we hypothesized could also impact individuals' willingness to participate in a hypothetical genetic study and their concerns of genetic discrimination (Supplementary Materials). In the demographics section, we included questions about political ideology (7‐point Likert scale with scale anchors of extremely liberal [1] and extremely conservative [7]) and religiosity (7‐point Likert scale with scale anchors of not at all religious [1] to very religious [7]) given research showing links between these characteristics and beliefs about privacy and liberty (Iyer et al., 2012). We used a validated scale of self‐reported health with responses ranging from poor (1) to excellent (5) (Ware & Sherbourne, 1992).
Prior to receiving the informed consent language, participants answered questions about how familiar they were with GINA—our subjective knowledge measure—and, to contextualize this familiarity, we also asked their familiarity with the Health Insurance Portability and Accountability Act (HIPAA) and the Affordable Care Act (ACA), as these are two healthcare laws that also regulate health privacy and health insurance coverage, respectively. We also asked participants again about their subjective knowledge of the three healthcare laws after they saw the consents to examine how the language may have altered their subjective knowledge. We also asked seven questions to measure objective knowledge about GINA. The objective knowledge questions were only asked after participants received both informed consent languages.
2.3. Data analysis
We first calculated descriptive statistics for demographics and the outcome measures. Next, we used the objective and subjective knowledge of GINA questions to undertake a manipulation check to see whether participants' knowledge was altered after reading informed consent languages.
For the primary data analysis, we ran independent samples t tests and one‐way ANOVAs to test for the impact of the informed consent language on our three primary outcome measures, willingness to participate, concerns of genetic discrimination, and ease of decision to participate. We performed multivariable regression analyses to identify whether the consent language received was associated with willingness to participate and concerns of discrimination, even when controlling for demographics and knowledge of GINA and genetics. To account for multiple comparisons, the p‐value considered to be statistically significant was adjusted to p = 0.025 (0.05/2 comparisons). We also found no order effects or significant skew, kurtosis, and multicollinearity of our main outcome measures.
In two instances, we asked respondents open‐ended questions. To analyze qualitative responses, a research assistant (CAH) read through the comments and identified key themes, utilizing Microsoft Excel®. One of the authors (AERP) reviewed these themes, and the two individuals met several times to discuss themes and ensure consistent interpretation of categories. Qualitative responses were then sorted into the identified themes and exemplary quotes pulled for inclusion in the manuscript.
3. RESULTS
3.1. Demographics
At the close of the survey, 1807 responses were received from Qualtrics that met quotas and inclusion criteria. Of these, 550 were excluded for closing the browser before finishing the survey and 62 were excluded in data quality checks due to inconsistent answers. The final number of participants meeting inclusion criteria was 1,195 (completion rate = 66.1%). We conducted checks to confirm that those excluded were not disproportionately representative of one particular consent received.
Sample demographics mirrored approximate rates in the general population: 48.7% of participants identified as male, 36.7% as non‐white, and had a mean age of 45.9 (SD = 17.9) (Table 1). Respondents had a mean of 4.06 (SD = 1.76) for political ideology and a mean of 4.49 (SD = 2.03) for religiosity. Respondents' self‐reported health had a mean of 3.29 (SD = 1.02).
TABLE 1.
Participant demographics (N = 1,195) a
| n | % | |
|---|---|---|
| Age (1195) | ||
| 18–24 | 174 | 14.6 |
| 25–34 | 186 | 15.6 |
| 35–44 | 246 | 20.6 |
| 45–54 | 151 | 12.6 |
| 55–64 | 206 | 17.2 |
| 65+ | 232 | 19.4 |
| Gender (n = 1,177) | ||
| Female | 604 | 51.3 |
| Male | 573 | 48.7 |
| Race/Ethnicity (n = 1,179) | ||
| White, Non‐Hispanic | 746 | 63.3 |
| Black, Non‐Hispanic | 141 | 12.0 |
| Hispanic | 203 | 17.2 |
| Other | 89 | 7.6 |
| Education (n = 1,184) | ||
| Less than high school | 45 | 3.8 |
| High school/GED | 438 | 37.0 |
| Some college | 258 | 21.8 |
| 4‐year college degree | 261 | 22.0 |
| Graduate/professional degree | 182 | 15.4 |
| Income (in dollars) (n = 1,138) | ||
| <19,999 | 172 | 15.1 |
| 20,000–49,999 | 296 | 26.0 |
| 50,000–74,999 | 234 | 20.6 |
| 75,000–99,999 | 160 | 14.1 |
| >100,000 | 276 | 24.3 |
Missing values are refused to answer or other.
3.2. Consent and GINA knowledge
While we primarily collected information about knowledge of GINA as a control variable, we also used the data as a manipulation check to see whether the differing informed consent languages affected individuals' subjective and objective knowledge of GINA. We unearthed several interesting findings regarding the interplay between knowledge of GINA and informed consent. First, overall, participants reported less familiarity with GINA than the ACA and HIPAA (Table S5). After seeing the informed consent language (which did not address the ACA or HIPAA), we again asked the participants this series of questions about their knowledge of the laws. Here, participants reported that they felt they knew all three laws better (Table S5), not just GINA. However, it is worth noting that the change in self‐reported familiarity was higher for GINA than the change for the ACA, or HIPAA (Table S2). Second, even though the objective GINA questions were asked after the consent language was provided, there was not a statistically significant difference in objective GINA scores across the three consent groups. 2
3.3. Consent and willingness to participate
After receiving the consent language, participants indicated a general willingness to participate in the hypothetical study (Willingness 1) and found it relatively easy to make that decision (Table 2). There was, however, a statistically significant difference across the informed consent groups (p < 0.001). Bonferroni‐corrected post hoc comparisons revealed that willingness to participate for those who received the minimum disclosure consent was significantly higher than when the basic disclosure consent (p < 0.001) or comprehensive disclosure consent (p < 0.001) was presented (Table 2). There was no significant difference between the basic disclosure and the comprehensive disclosure consent (p = 1.00). Thus, on average, those who received the consent with none of GINA's limitations highlighted were more willing to participate than those who were warned either that GINA did not protect against discrimination by life, disability, and LTC insurance or against discrimination by multiple insurances, educational institutions, the military, and lending or other organizations.
TABLE 2.
Willingness to participate, discrimination, and ease across scenarios and consent
| Overall (N = 1,195) | Basic disclosure (n = 409) | Minimum disclosure (n = 404) | Comprehensive disclosure (n = 382) | One‐way ANOVA | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | Test statistic | p‐value | |
| Willingness to participate I | 4.84 (1.77) | 4.70 (1.80)a | 5.14 (1.67)a,b | 4.66 (1.79)b | F(2, 1,192) = 9.26 | <0.001 |
| Concern of discrimination | 3.62 (1.85) | 3.63 (1.88) | 3.43 (1.82)c | 3.80 (1.83)c | F(2, 1,192) = 3.39 | 0.019 |
| Ease of decision | 5.51 (1.38) | 5.48 (1.45) | 5.61 (1.32) | 5.46 (1.36) | F(2, 1,192) = 1.37 | 0.256 |
Note: Responses were provided on 7‐point Likert scales with verbal scale anchor labels where 1 = lowest willingness, concern, and ease and 7 = highest willingness, concern, and ease. Values that share the same superscript letter for Willingness to Participate I significantly different from each other at p < 0.001. Values that share the same superscript letter for Concern of Discrimination significantly different from each other at p < 0.05.
The significance of receiving the minimum disclosure consent held in our willing to participate regression model (p = 0.003) (Table 3). Additionally, subjective and objective knowledge of GINA and subjective knowledge of genetics had positive associations with willingness to participate (p < 0.001). Higher political conservatism (p = 0.002) and greater concerns of genetic discrimination (p < 0.001) were associated with decreased willingness to participate (Table 3).
TABLE 3.
Willingness to participate and fear of discrimination
| Concern of genetic discrimination | Willingness to participate I | Willingness to participate II | |
|---|---|---|---|
| B (95% CI) | B (95% CI) | Odds ratio (95% CI) | |
| Consent scenario | |||
| Basic disclosure | Ref | Ref | — |
| Minimum disclosure | −0.17 (−0.44, 0.10) | 0.34 (0.12, 0.57)** | — |
| Comprehensive disclosure | 0.10 (−0.18, 0.37) | −0.14 (−0.37, 0.09) | — |
| Willingness I | |||
| Not willing | — | — | Ref |
| No preference | — | — | 1.61 (0.79, 3.23) |
| Willing | — | — | 2.23 (1.38, 3.62)*** |
| Concern of gen. disc. | — | −0.11 (−0.16, −0.05)*** | 1.12 (1.03, 1.23)** |
| Knowledge metrics | |||
| Subj. GINA knowledge | 0.15 (0.08, 0.22)*** | 0.19 (0.13, 0.24)*** | 1.27 (1.15, 1.41)*** |
| Obj. GINA knowledge | 0.00 (−0.14, 0.13) | 0.23 (0.13, 0.24)*** | 0.87 (0.71, 1.07) |
| Subj. genetic knowledge | 0.01 (−0.12, 0.13) | 0.39 (0.29, 0.49)*** | 0.87 (0.72, 1.04) |
| Obj. genetic knowledge | 0.22 (−0.19, 0.63) | 0.39 (0.05, 0.73) | 1.77 (0.98, 3.21) |
| Age | −0.01 (−0.02, 0.00)* | 0.00 (−0.01, 0.01) | 1.01 (1.00, 1.02) |
| Education | 0.10 (−0.03, 0.23) | 0.04 (−0.06, 0.15) | 1.22 (1.01, 1.47) |
| Income | −0.01 (−0.07, 0.04) | 0.04 (−0.01, 0.08) | 0.91 (0.84, 1.00) |
| Political ideology | −0.02 (−0.09, 0.05) | −0.09 (−0.15, −0.03)** | 0.97 (0.88, 1.07) |
| Religiosity | 0.09 (0.03, 0.15)** | 0.02 (−0.03, 0.07) | 1.03 (0.95, 1.13) |
| Self‐reported health | −0.06 (−0.18, 0.06) | −0.02 (−0.12, 0.08) | 0.86 (0.72, 1.02) |
| Gender | |||
| Male | Ref | Ref | Ref |
| Female | −0.12 (−0.37, 0.12) | −0.15 (−0.35, 0.06) | 0.89 (0.62, 1.27) |
| Race/ethnicity | |||
| Non‐Hispanic White | Ref | Ref | Ref |
| Non‐Hispanic Black | 0.43 (0.04, 0.83) | 0.34 (0.01, 0.66) | 0.83 (0.48, 1.46) |
| Hispanic | 0.08 (−0.26, 0.41) | 0.08 (−0.19, 0.36) | 0.89 (0.56, 1.43) |
| Other race/ethnicity | 0.28 (−0.17, 0.73) | −0.27 (−0.64, 0.10) | 1.41 (0.73, 2.72) |
| Constant | 3.00 (2.00, 3.91)*** | 2.44 (1.63, 3.24)*** | 0.16 (0.04, 0.66)* |
Note: R 2=0.10; R 2=0.26; Pseudo R 2=0.09. — indicates measure was not included in the model.
*p < 0.025; **p < 0.01; ***p < 0.001.
3.3.1. Impact of seeing alternative informed consent language
When participants were provided alternative informed consent language, 388 participants (32.5% of total population) indicated that their willingness to participate would change (Willingness 2). Of these, 40 (10.3%) initially were less willing to participate (Likert response 1–3), 25 (6.4%) initially indicated that they were unsure about participating (Likert response 4), and 323 (83.2%) initially indicated that they were willing to participate. Participants who indicated a greater willingness to participate initially (n = 577) were associated with a higher odds of changing their mind after receiving a different consent scenario, OR = 3.22, p < 0.001. Regression analyses found that originally being willing to participate had a statistically significant positive association with change in willingness to participate when controlling for both demographic and knowledge measures (p = 0.001) (Table 3). Thus, those who were originally willing to participate were more likely to change their willingness after seeing new informed consent language.
Through qualitative responses, we could further assess the directionality of how participants' participation would change. Qualitative responses predominantly focused on concerns of discrimination. For those who were presented more information about the limits of GINA in the second consent (from the minimum disclosure consent to the comprehensive disclosure consent), many noted that they were more concerned with discrimination. For those who were presented with less information in the second consent (from the comprehensive disclosure consent to the minimum disclosure consent and the basic disclosure consent to the minimum disclosure consent), qualitative responses seemed to indicate that some believed that discrimination was no longer a concern because a particular gap in GINA was no longer highlighted. This occurred despite the fact that we did not ask them to ignore the previous consent language they had seen (Table 4).
TABLE 4.
Qualitative themes and example quotes
| Would your willingness to participate in the study change if you had been provided the following language? | ||
|---|---|---|
| Theme | Consent | Example quotes |
| Discrimination | Received more information about gaps in second Consent scenario |
|
| Received less information in second consent scenario |
|
|
| Please explain your answer to: based on the informed consent language, do you believe you are at risk of genetic discrimination? | ||
|---|---|---|
| Theme | Likert a | Example quotes |
| Family History/ Personal history of disease | Lower perceived risk |
|
| Higher perceived risk |
|
|
| Employment & insurance status | Lower perceived risk |
|
| Higher perceived risk |
|
|
| Race/racial appearance | Lower perceived risk |
|
| Higher perceived risk |
|
|
| Trust in laws and protections | Lower perceived risk |
|
| Higher perceived risk |
|
|
Note: 'Lower perceived risk' were responses of 1–3, and 'higher perceived risk' were responses of 5–7.
Scale anchors of Not at all at risk (1) and At high risk (7).
3.4. Beliefs about discrimination risk and ease of deciding to participate
Overall, participants believed that they were at low risk of genetic discrimination (M = 3.62) (Table 2). Participants in the minimum disclosure consent had significantly lower perceived risk of genetic discrimination than those in the comprehensive disclosure consent (p = 0.015), but there was no statistically significant difference between the minimum disclosure and basic disclosure (p = 0.365). There was no statistical difference in ease of making a participation decision across consent scenarios, t(1193) = 0.13, p = 0.551 (Table 2).
These bivariate findings, however, should be read cautiously, since in multivariate regression modeling the consent language was not found to be statistically significantly associated with concern of genetic discrimination. Older age was associated with a decreased perceived risk (p = 0.011), whereas greater religiosity (p = 0.004) and higher subjective knowledge of GINA (p < 0.001) were associated with increased perceived risk (Table 3).
Open‐ended responses helped identify participants' reasons for feeling they were at risk for genetic discrimination (Table 4). Three common themes arose. First, many participants contextualized their belief of genetic discrimination with their own situation. For example, qualitative responses mentioned family history, personal history, or insurance needs. The presence or absence of these factors seemed to both increase and decrease, respectively, perceived risk of discrimination. For example, those noting that they did not have a family history of disease generally expressed feeling less at risk of genetic discrimination than those with a family history (Table 4). Similarly, those feeling less at risk of genetic discrimination noted that they already have necessary insurance or are retired, whereas those feeling more at risk noted that they might not be able to get insurance or worked for a small company. Second, some of those who thought they were less at risk mentioned that they were white or light‐skinned, while some of those who thought they were at greater risk mentioned being a person of color. The question specifically asked about the risk of genetic discrimination, yet comments about race or ethnicity and skin color was a relatively common theme that arose in the qualitative comments (Table 4). It is unclear whether respondents thought that their skin color, race, or ethnicity made them at greater or lower risk of discrimination in general or of genetic discrimination specifically. Third, many comments raised themes around confidence or lack of confidence in the legal protections and privacy of the research study. Several of these quotes specifically mentioned GINA's protections or gaps. However, others seemed to imply a general trust or distrust in how information is used broadly in society (Table 4).
4. DISCUSSION
Obtaining informed consent is essential for clinicians and researchers to ensure that individuals understand the risks and benefits of the medical procedure/testing or research study they are considering (Faden & Beauchamp, 1986). It also fulfills ethical goals of respect for autonomy by allowing individuals to make informed choices about whether to participate in such activities (Belmont Report, 1979; Canterbury v. Spence, 1972).
There is a question, however, of how much information can and should be shared in consent documents to achieve informed consent. As our study finds, providing greater information about the limits in the law's anti‐discrimination protections may decrease individual's willingness to participate and affect their perception of risk for discrimination. Those participants who were first presented with the comprehensive disclosure consent were less willing to participate in the hypothetical study and had higher perceived risk of discrimination. The effects of the inclusion of additional GINA limitations regarding willingness to participate persisted when controlling for demographics and knowledge metrics.
The findings hold implications for the tension between beneficence and autonomy goals with informed consent. For clinicians, the results might push toward offering less information so as to not unnecessarily scare people away from genetic testing. For researchers, the desire to encourage participation in genomic studies could similarly push toward offering less information so as to not scare people away from enrolling. Indeed, one of the explicit goals of GINA was to increase the uptake of medically recommended genetic testing and participation in genetic research. Describing GINA's limits may undermine the goals of the law since a more fulsome description of what GINA fails to cover increases concern of discrimination and decreases willingness to participate. In the clinical context, one could even make a therapeutic argument for withholding some non‐essential information, when the belief is that patients will make better medical 'choices' with less information (Nishi v. Hartwell, 1970). However, such decisions might threaten the autonomy goals underlying informed consent and the importance of allowing individuals to make their own choices even if not seen as 'better' by the clinician or researcher. However, as is discussed further below, it is difficult to assess whether more or less information is indeed helpful for the individual in reaching the decision best for them.
To ensure that people have an appropriate understanding of the relevant risks when making autonomous decisions, the HHS Guidance on GINA indicates the importance of describing not just GINA's protections in informed consent, but also its limitations (OHRP, 2009). However, this same guidance only highlights information about limitations related to life, LTC, and disability insurance and thus does not include all information about limitations. Previous research has highlighted that individuals worry about genetic discrimination (Wauters & Van Hoyweghen, 2016), so disclosing gaps, particularly around insurance is likely relevant to the average person. The qualitative comments from our study also show the importance of disclosing more complete information, as some participants clearly contextualized their willingness to participate or belief in discrimination based on the consent language. For example, one participant specifically noted that they work for a small business not covered by GINA; thus, they had a higher perceived risk of discrimination.
However, given the lack of robust evidence of widespread genetic discrimination, providing more information about the gaps in GINA could unnecessarily add to a fear of discrimination (Joly et al., 2010). This raises a question of what the reactions would be if we could provide data about the likelihood of discrimination as a way for individuals to calibrate the risk that they may be facing, much as a consent document might list the likelihood of a medical complication from surgery. Simply telling people there is no protection against something, without telling them how great the risk is or whether the degree of risk is known, could be viewed as inadequate information that could imply risks or degree of risks that do not actually exist. However, it is difficult to quantify anti‐discrimination risks because they are not systematically reported or measured; insurance and employment decisions are often black‐box decisions; and laws, scientific understanding, and behaviors of employers and insurers could change in future.
One potential option would be to not disclose risks that are viewed as small or difficult to quantify, especially because too much information in an informed consent document could make the entire document too lengthy and difficult to process the information, and participants might view the risks as greater than they actually are. However, one important theme raised by the qualitative findings arose for those respondents who received less information about the GINA's limits in the second consent language presented to them. Several of these respondents noted that they were now less worried about genetic discrimination because the language they were concerned about was removed. The language that remained, however, only mentioned that GINA covered health insurance and employment and said nothing about whether the other areas were or were not covered. In actuality, the underlying protections remain the same. This may indicate that participants assume that researchers will raise any areas of potential concern and, if they do not raise it in consent language, it is not a concern. Further research is needed to assess whether this theme arising from qualitative responses holds across populations.
Interestingly, while providing more information about gaps in the law affected willingness to participate in the study and increased fears of discrimination, we did not find any statistically significant difference in objective knowledge of GINA across the consent scenarios. Much prior research has noted that individuals often fail to fully read or comprehend informed consent documents (Bernhardt et al., 2015). Although we did not present participants with lengthy consent documents, it is plausible that they skimmed the information and, for those who received information about gaps in GINA, generally noted that there were gaps, but did not review the information in depth. This hypothesis is bolstered by the fact that we did not find significant differences in willingness to participate or concerns of discrimination between the basic disclosure consent and the comprehensive disclosure consent, despite different levels of information about gaps presented in each. Overall, the impact of the consent language on willingness to participate remained statistically significant even when controlling for objective knowledge in regression analysis. Thus, this paper highlights that disclosure of information by no means guarantees comprehension, although comprehension would be the ideal to strive for in informed consent (Canterbury v. Spence, 1972).
Providing the informed consent language, however, did appear to increase participants' beliefs about their knowledge of GINA. When we first asked about familiarity with GINA (prior to the consent language), respondents indicated low subjective familiarity with the law. This finding replicates previous literature in this area (Lenartz et al., 2021), which also showed that subjective knowledge and objective knowledge of GINA do not always correlate. However, after receiving the informed consent language, the respondents reported higher familiarity with GINA, bringing the mean familiarity closer in line with familiarity with the ACA and HIPAA. Surprisingly, respondents also indicated that they were more familiar with these other two laws as well even though the consent language presented did not mention these other laws, suggesting that participants may have felt more confident about their knowledge of health law more generally after reading about GINA. The increase, though significant, was small.
A final important finding from this study is that no matter what language is presented in informed consent language, and indeed perhaps no matter what legal coverage exists, individuals are going to contextualize perceived risks through their situation and lived experiences. This theme arose in several different ways in the qualitative responses. Most notably, although our study never specifically asked about racial discrimination, a portion of respondents, identifying as both white and Black, mentioned their race or skin color when discussing the risk of genetic discrimination. The survey was collecting responses between June and July 2020, at the height of the Black Lives Matter marches, so this may have been at the forefront of people's minds during survey completion.
4.1. Study limitations
This study has several limitations. First, participants were responding to a hypothetical scenario. When making an actual decision about participation in a research study, the informed consent document language may have more or less impact on willingness to participate in the study because individuals are also weighing other potential risks and benefits of the study. Similarly, informed consent in research is not solely about the informed consent document. In a research study, participants have the opportunity not just to read the document, but also to talk to a study team member about questions or concerns during the informed consent process. Second, we asked participants about whether their willingness to participate in the study would change based on different consent language, but we did not capture the directionality and strength of this change other than through qualitative comments. Future research could further explore how new information alters willingness to participate.
5. CONCLUSION
Overall, this study has implications for informed consent beyond descriptions of GINA. Increasing collection of large‐scale behavioral or genomic data, blurring boundaries between research and clinical care, and preservation of data for secondary research now necessitates the growing disclosure of non‐medical information, since many foreseeable risks are privacy‐related, such as loss of confidentiality, risk of discrimination, or misuse of personal data (Daar, 1995; Sawicki, 2015; Prince, Forthcoming). As our study shows, the way these non‐medical risks are described may affect individuals' willingness to participate in research or clinical care. Researchers and clinicians, therefore, should think carefully about how best to present non‐medical risk information, including information about potential but unquantified risks, when obtaining informed consent. The goal should be to achieve sufficient disclosure without providing too much information about potentially small risks.
AUTHOR CONTRIBUTIONS
Anya Prince: Conceptualization; data curation; formal analysis; funding acquisition; project administration; writing – original draft; writing – review and editing. Sonia M. Suter: Conceptualization; writing – review and editing. Wendy R. Uhlmann: Conceptualization; writing – review and editing. Aaron M. Scherer: Conceptualization; data curation; formal analysis; methodology; writing – review and editing. Anya Prince and Aaron M. Scherer confirm that they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest
AERP declares that she has no conflict of interest. SMS declares that she has no conflict of interest. WRU declares that she has no conflict of interest. AMS declares that he has no conflict of interest.
Human studies and informed consent
This study was deemed as exempt by the University of Iowa Institutional Review Board (202002765). The first page of the survey included consent information, such as the voluntary nature of the study, contact information for the principal investigator and IRB, and assurances that no personal information would be collected. Those who consented could click to continue to the survey questions.
Animal studies
No non‐human animal studies were carried out by the authors for this article.
Data sharing and data accessibility
The survey instrument is available in the Supplementary Materials, and the survey data and Stata syntax are available upon request from the corresponding author.
Supporting information
Data S1
Tables S1–S5
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R00HG008819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Thank you to Andrea Lenartz and Colleen Anderson Hartley for research support during survey design and analysis and the Iowa Social Sciences Research Center for survey support. Dr. Melanie Myers served as Action Editor on the manuscript review process and publication decision.
Prince, A. E. R. , Suter, S. M. , Uhlmann, W. R. , & Scherer, A. M. (2022). The goldilocks conundrum: Disclosing discrimination risks in informed consent. Journal of Genetic Counseling, 31, 1383–1393. 10.1002/jgc4.1613
Endnotes
Participants were also randomized to the disease being examined in the genetic research study—diabetes or Alzheimer’s disease because we hypothesized that there could be potential differences between participants’ attitudes toward participating in a study related to a treatable (diabetes) versus non‐treatable (Alzheimer’s disease) condition (Table S1). Since we did not identify any significant findings related to the disease presented, we do not report any findings related to this hypothesis in the main text (Supplemental materials).
Other findings related to GINA knowledge are included in the supplementary materials.
REFERENCES
- Allain, D. C. , Friedman, S. , & Senter, L. (2012). Consumer awareness and attitudes about insurance discrimination post enactment of the Genetic Information Nondiscrimination Act. Familial Cancer, 11, 637–644. [DOI] [PubMed] [Google Scholar]
- Amendola, L. M. , Robinson, J. O. , Hart, R. , Biswas, S. , Lee, K. , Bernhardt, B. A. , East, K. , Gilmore, M. J. , Kauffman, T. L. , Lewis, K. L. , Roche, M. , Scollon, S. , Wynn, J. , & Blout, C. (2018). Why patients decline genomic sequencing studies: Experiences from the CSER Consortium. Journal of Genetic Counseling, 27, 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. , Lewis, A. C. F. , & Prince, A. E. R. (2021). The problems with patchwork: State approaches to regulating insurer use of genetic information. DePaul Journal of Health Care Law, 22, 1–40. [Google Scholar]
- Areheart, B. A. , & Roberts, J. L. (2019). GINA, big data, and the future of employee privacy. Yale Law Journal, 128, 710–790. [Google Scholar]
- Barlow‐Stewart, K. , Liepins, M. , Doble, A. , & Otlowski, M. (2018). How are genetic test results being used by Australian life insurers? European Journal of Human Genetics, 1, 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont Report . (1979). The national commission for the protection of human subjects of biomedical and behavioral research. https://www.hhs.gov/ohrp/regulations‐and‐policy/belmont‐report/read‐the‐belmont‐report/index.html
- Bernhardt, B. A. , Roche, M. I. , Perry, D. L. , Scollon, S. R. , Tomlinson, A. N. , & Skinner, D. (2015). Experiences with obtaining informed consent for genomic sequencing. American Journal of Medical Genetics Part A, 167, 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canterbury v. Spence, 464 F.2d 772 (D.C. Cir. 1972).
- Clinical Sequencing Evidence‐Generating Research (CSER) . (n.d.). CSER research materials. https://cser‐consortium.org/cser‐research‐materials
- Daar, J. F. (1995). Informed consent: Defining limits through therapeutic parameters. Whittier Law Review, 16, 187–209. [PubMed] [Google Scholar]
- Faden, R. R. , & Beauchamp, T. L. (1986). A history and theory of informed consent. Oxford University Press. [Google Scholar]
- Genetic Information Nondiscrimination Act (GINA), Pub Law 110–233 . (2008). https://www.govinfo.gov/content/pkg/PLAW‐110publ233/pdf/PLAW‐110publ233.pdf
- General requirements for informed consent, 45 CFR §46.116(a)(4) . (2018). https://www.hhs.gov/ohrp/regulations‐and‐policy/regulations/45‐cfr‐46/revised‐common‐rule‐regulatorytext/index.html#46.116
- Iyer, R. , Koleva, S. , Graham, J. , Ditto, P. , & Haidt, J. (2012). Understanding libertarian morality: The psychological dispositions of self‐identified libertarians. PLoS One, 7, e42366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly, Y. , Braker, M. , & Le Huynh, M. (2010). Genetic discrimination in private insurance: Global perspectives. New Genetics and Society, 29, 351–368. 10.1080/14636778.2010.528189 [DOI] [Google Scholar]
- Lenartz, A. , Scherer, A. M. , Uhlmann, W. R. , Suter, S. M. , Anderson Hartley, C. , & Prince, A. E. (2021). The persistent lack of knowledge and misunderstanding of the Genetic Information Nondiscrimination Act (GINA) more than a decade after passage. Genetics in Medicine, 23, 2324–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, A. L. , & Majumder, M. A. (2009). Two cheers for GINA. Genome Medicine, 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi v. Hartwell, 473 P.2d 116 (HI 1970).
- Office of Human Research Protections (OHRP), US Health and Human Services . (2009). Genetic Information Nondiscrimination Act (GINA): OHRP guidance. https://www.hhs.gov/ohrp/regulations‐and‐policy/guidance/guidance‐on‐genetic‐information‐nondiscrimination‐act/index.html
- Prince, A. E. R. , & Roche, M. I. (2014). Genetic information, non‐discrimination, and privacy protections in genetic counseling practice. Journal of Genetic Counseling, 23, 891–902. 10.1007/s10897-014-9743-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince, A. E. R. (forthcoming). Disclosing privacy and discrimination protectsion in informed consent. Health Matrix. [Google Scholar]
- Robinson, J. O. , Carroll, T. M. , Feuerman, L. Z. , Perry, D. L. , Hoffman‐Andrews, L. , Walsh, R. C. , Christensen, K. D. , Green, R. C. , & McGuire, A. L. (2016). Participants and study decliners' perspectives about the risks of participating in a clinical trial of whole genome sequencing. Journal of Empirical Research on Human Research Ethics, 11, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein, M. A. (2008). Is GINA worth the wait? The Journal of Law, Medicine & Ethics, 36, 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki, N. N. (2015). Modernizing informed consent: Expanding the boundaries of materiality. University of Illinois Law Review, 2016, 821–871. [Google Scholar]
- Suter, S. M. (2018). GINA at 10 years: The battle over 'genetic information' continues in court. Journal of Law and the Biosciences, 5, 495–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. E., Jr. , & Sherbourne, C. D. (1992). The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Medical Care, 30, 473–483. [PubMed] [Google Scholar]
- Wauters, A. , & Van Hoyweghen, I. (2016). Global trends on fears and concerns of genetic discrimination: A systematic literature review. Journal of Human Genetics, 61(4), 275–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Data Availability Statement
The survey instrument is available in the Supplementary Materials, and the survey data and Stata syntax are available upon request from the corresponding author.


