Abstract
Ageing is accompanied by decrements in the size and function of skeletal muscle that compromises independence and quality of life in older adults. Developing therapeutic strategies to ameliorate these changes is critical but requires an in-depth mechanistic understanding of the underlying physiology. Over the past 25 years, studies on the contractile mechanics of isolated human muscle fibres have been instrumental in facilitating our understanding of the cellular mechanisms contributing to age-related skeletal muscle dysfunction. The purpose of this review is to characterize the changes that occur in single muscle fibre size and contractile function with ageing and identify key areas for future research. Surprisingly, most studies observe that the size and contractile function of fibres expressing slow myosin heavy chain (MHC) I are well-preserved with ageing. In contrast, there are profound age-related decrements in the size and contractile function of the fibres expressing the MHC II isoforms. Notably, lifelong aerobic exercise training is unable to prevent most of the decrements in fast fibre contractile function, which have been implicated as a primary mechanism for the age-related loss in whole-muscle power output. These findings reveal a critical need to investigate the effectiveness of other nutritional, pharmaceutical, or exercise strategies, such as lifelong resistance training, to preserve fast fibre size and function with ageing. Moreover, integrating single fibre contractile mechanics with the molecular profile and other parameters important to contractile function (e.g., phosphorylation of regulatory proteins, innervation status, mitochondrial function, fibre economy) is necessary to comprehensively understand the ageing skeletal muscle phenotype.
Keywords: skeletal muscle, older adult, myosin heavy chain, muscle power, cross-bridge cycle, shortening velocity, muscle quality
Graphical Abstract
Advancing age is accompanied by decrements in whole-muscle strength and power that exceed the losses in muscle mass. The single fibre preparation has been instrumental in facilitating our understanding of the cellular mechanisms contributing to this phenomenon. Surprisingly, and at odds with some of the earlier findings, both size and contractile function (force, power, shortening velocity, Ca2+ sensitivity, and rates of force development [RFD]) of the MHC I fibres appear well-preserved with ageing. In contrast, several studies observe profound age-related decrements in function – namely force and power – of the MHC II fibres. The decrements in MHC II fibre function are primarily attributable to fibre atrophy, rather than age-related alterations in the intrinsic contractile mechanics, per se. Since MHC II fibres can generate greater force and power than MHC I fibres, these findings implicate fast fibre atrophy as an important therapeutic target for attenuating age-related decrements in whole-muscle strength and power.
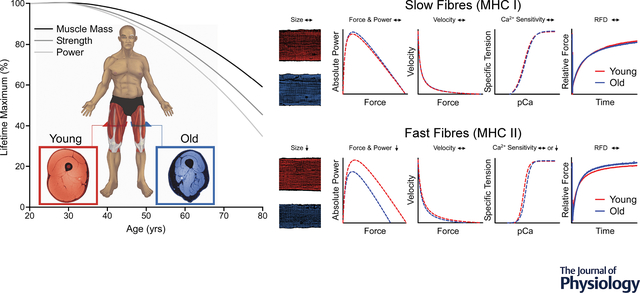
Introduction
Advancing age is accompanied by a progressive loss of skeletal muscle mass and function (i.e., strength and power) that generally begins around the fourth to fifth decade of life and accelerates thereafter (Metter et al., 1997; Janssen et al., 2000; Alcazar et al., 2020). The age-related decline in muscle function is critically important because it results in reduced mobility and can ultimately lead to physical frailty, which is characterized by a greater vulnerability for increased dependency and all-cause mortality (Morley et al., 2013). Undoubtedly, the loss in muscle function is attributed, at least in part, to the age-related loss of muscle mass that occurs from a reduction in both the number and the size of the individual muscle fibres (Lexell et al., 1988; Doherty, 2003). However, it is well-known that the loss in total muscle mass is unable to account for all the age-related decrements in muscle function. For example, the longitudinal declines in knee extensor strength observed in the Health, Aging and Body Composition study were ~3x greater than reductions in leg lean mass (Goodpaster et al., 2006), and findings from the Baltimore Longitudinal Study of Aging suggest that muscle power output declines even more precipitously than strength (Metter et al., 1997). These studies, and several others (Skelton et al., 1994; Frontera et al., 2000a; Janssen et al., 2000; Hughes et al., 2002; Delmonico et al., 2009; Reid et al., 2014; Alcazar et al., 2020), have led to the consensus that age-related declines in muscle mass (~0.5–1% per year) are exceeded by reductions in maximal strength (~1–3% per year) and power (~3–4% per year). Despite the widespread recognition of this phenomenon, the mechanisms for the accelerated loss in maximal strength and power with ageing remains elusive and are imperative to identify in order to develop targeted therapeutic strategies to preserve mobility and quality of life in older adults.
Multiple mechanisms have been proposed to explain the greater loss in maximal strength and power relative to muscle mass with ageing, including decreased voluntary neural activation (Harridge et al., 1999; Russ et al., 2012), infiltration of intermuscular adipose and fibrotic tissue (Alnaqeeb et al., 1984; Lexell, 1995; Kent-Braun et al., 2000; Beavers et al., 2013; Straight et al., 2019), motor unit remodeling and instability of the neuromuscular junction (Hepple & Rice, 2016), impaired cross-bridge mechanics and Ca2+ handling (Larsson et al., 1997; Miller & Toth, 2013; Lamboley et al., 2015), and/or the selective atrophy of fibres expressing the myosin heavy chain (MHC) II isoforms (Sundberg et al., 2018). A recent meta-analysis summarizing findings from 54 studies revealed that the majority of ageing literature reports no differences in voluntary neural activation between healthy younger and older adults, and even when all studies were analyzed together, there was only a modest reduction in voluntary activation with ageing (Rozand et al., 2020). This finding provides compelling evidence that the age-related loss in maximal strength, at least for healthy older adults, is determined primarily by mechanisms within the muscle.
A unique and powerful experimental approach to investigate the cellular mechanisms of age-related skeletal muscle dysfunction in humans is the skinned fibre preparation, in which the cell membrane of biopsied muscle fibres is mechanically peeled or chemically permeabilized. This preparation has the advantage of allowing precise control of the intracellular milieu surrounding the contractile and regulatory proteins, which enables mechanistic investigation of how individual molecules or ions such as fatigue-inducing metabolites (Sundberg et al., 2018) or Ca2+ (Teigen et al., 2020) may contribute to age-related skeletal muscle dysfunction. The data generated from these in vitro experiments are also often analogous to more well-known in vivo measures of muscle performance, such as maximal isometric force, power, and rates of force development, and thus, provide an attractive lens for a translational evaluation of skeletal muscle function. Furthermore, when paired with MHC determination, the single fibre technique can provide valuable insight into the effects of acute and chronic perturbations (e.g., exercise and ageing) on myocellular size and function in a fibre type-specific manner.
The overarching purpose of this narrative review is to describe the current state of knowledge regarding the changes in human single muscle fibre size and contractile function that occur with ageing. Specifically, we will compare and discuss findings from a vast body of international research spanning ~25 years that has sought to characterize the effects of ageing on single muscle fibre size, isometric force (Po), shortening velocity (Vo), power, rates of force development (ktr), and calcium sensitivity in MHC I and MHC II fibres. Particular attention will be paid to factors that may account for heterogenous findings and inter-laboratory discrepancies, including characteristics of the human subjects, such as biological age, physical activity levels and exercise training status, as well as key differences in the methodology of measuring single fibre size and contractile function. Lastly, we will conclude with a discussion of gaps in knowledge and future directions, which highlights that despite considerable advancements in our understanding, there is still much to learn about the cellular mechanisms contributing to skeletal muscle dysfunction with ageing.
Ageing Single Muscle Fibre Size and Contractile Function
Size – Cross-Sectional Area (CSA)
A critical determinant for understanding the age-related changes in single fibre contractile function is the fibre cross-sectional area (CSA), as this will determine, in part, the number of cross-bridges available to be bound in parallel. Of the 27 studies that compared single muscle fibres from younger and older adults (Table A1), 22 reported statistical comparisons between the age groups for the MHC I (Figure 1) and 21 for the MHC II fibres (Figure 2). Notably, 16 of these studies provided data from men, whereas only 9 studies provided comparisons in women (4 of which only included women) and 2 studies combined the data from the men and women. Given the greater CSA in single muscle fibres from men compared with women (Teigen et al., 2020), particularly in the fast fibres (Figure 2), there is a clear need to consider sex as a biologically relevant variable in single muscle fibre analyses.
Figure 1. MHC I fibre size, absolute Po, and specific Po from younger and older adults.
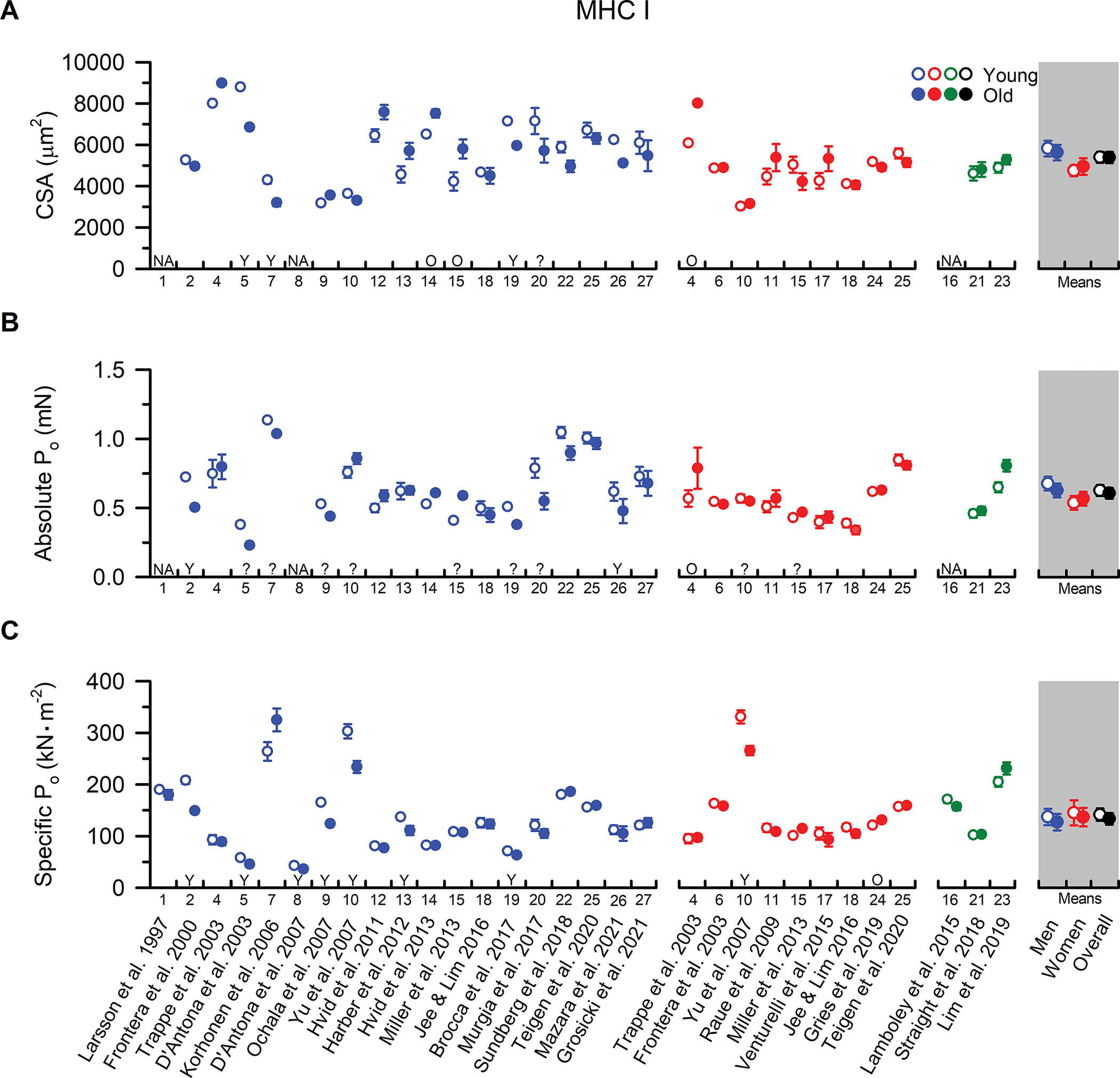
Individual study data and collective means for (A) cross-sectional area (CSA), (B) absolute peak force (Po), and (C) specific tension (Po /CSA) of MHC I fibres. Symbols represent the means from men (blue), women (red), and when the sexes were combined (green). The collective means (shaded grey regions) for men, women, and all studies combined (black symbols) were calculated by pooling the results from all the studies. Unpaired t-tests were performed on the collective means to test for age differences (young vs. old) using the pooled data. If assumptions of normality or homogeneity of variance were violated, then a non-parametric Mann-Whitney U test was used. To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression, with the caveat that not all studies could be included in this calculation because the sample sizes were not reported. None of the conclusions differed between the weighted and unweighted analyses, and thus, we report the collective means and p-values based on the unweighted analyses to allow inclusion of all the studies in the pooled results. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. If the numerical data were unavailable in the tables or text, values were extracted from figures or obtained from the authors. For studies with interventions, the pre-intervention values are presented, and when data from multiple cohorts or muscle groups were available, the data are reported from the vastus lateralis of the mobile, independently living cohort. If absolute Po was not provided, it was calculated from CSA and Po/CSA. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. N/A = No value was reported or was unable to be determined. ? = statistical comparisons were not provided. Values are mean ± SE. If SE was not provided, it was calculated based on the SD and fibre sample size, if available.
Figure 2. MHC II fibre size, absolute Po, and specific Po from younger and older adults.
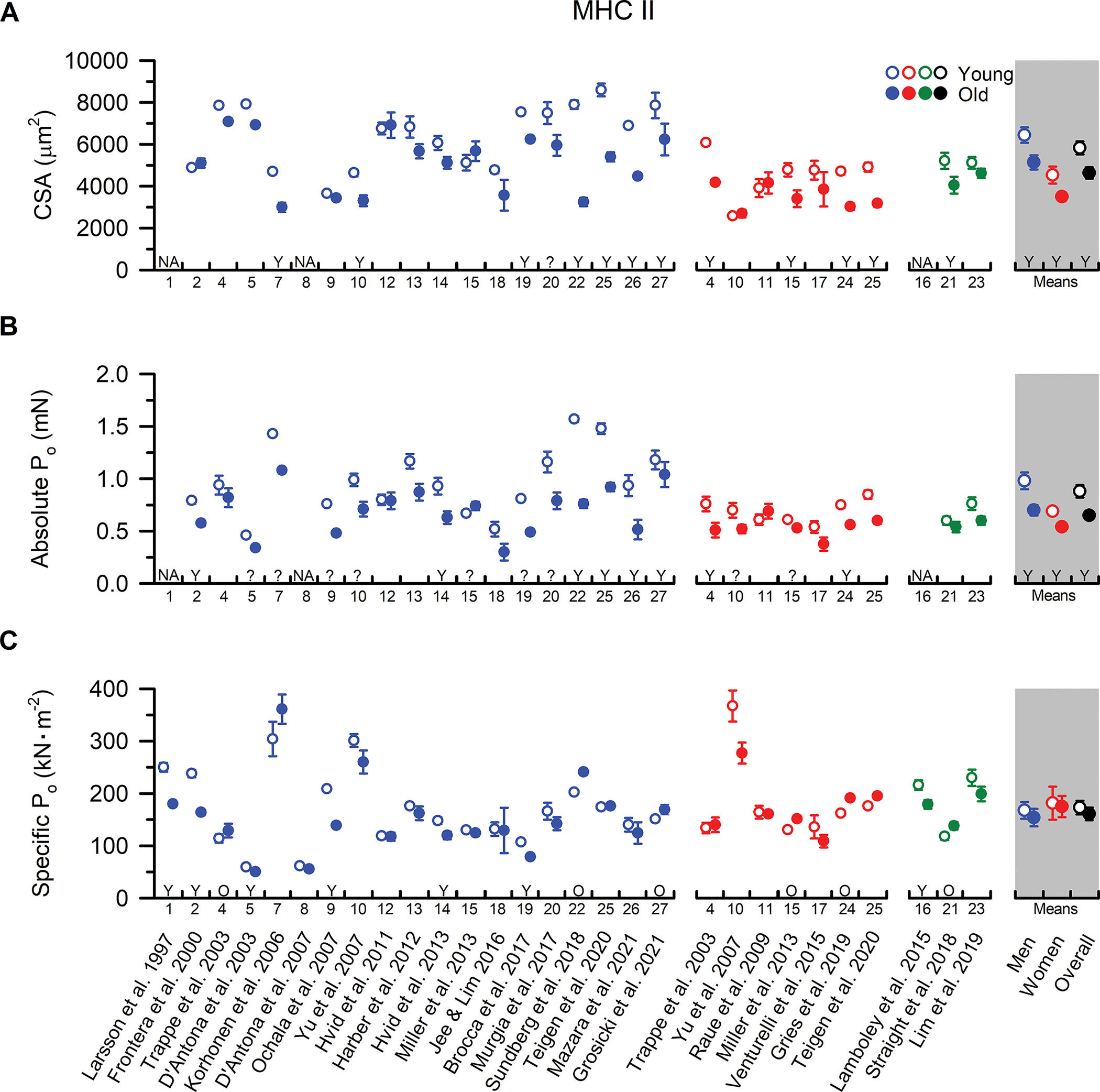
Individual study data and collective means for (A) cross-sectional area (CSA), (B) absolute peak force (Po), and (C) specific tension (Po /CSA) of MHC II fibres. Symbols represent the means from men (blue), women (red), and when the sexes were combined (green). The collective means (shaded grey regions) for men, women, and all studies combined (black symbols) were calculated by pooling the results from all studies. Unpaired t-tests were performed on the collective means to test for age differences (young vs. old) using the pooled data. If assumptions of normality or homogeneity of variance were violated, then a non-parametric Mann-Whitney U test was used. To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression, with the caveat that not all studies could be included in this calculation because the sample sizes were not reported. None of the conclusions differed between the analyses, and thus, we report the collective means and p-values based on the unweighted analyses to allow inclusion of all the studies in the pooled results. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. In instances where the numerical data were unavailable in the tables or text, values were extracted from figures or obtained from the authors. Data shown from individual studies were restricted to MHC IIa fibres if the MHC IIx and hybrid IIa/IIx were also reported; otherwise, the data represent the MHC II fibres which may include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. For studies with interventions, the pre-intervention values are presented, and when data from several cohorts or muscle groups were available, the data are reported from the vastus lateralis of the mobile, independently living cohort. If absolute Po was not provided, it was calculated from CSA and Po/CSA. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. N/A = no value was reported or was unable to be determined. ? = statistical comparisons were not provided. Values are mean ± SE. In instances where SE was not provided, it was calculated based on the SD and fibre sample size, if available.
Notwithstanding the apparent sex-specific differences in muscle fibre size, notable agreement is observed in studies comparing MHC I fibre CSA between younger and older men and women (Figure 1). In men, collective means indicate no difference in MHC I fibre CSA between younger and older adults (P=0.713), and this finding is corroborated by 11 of the 16 studies that provided statistical comparisons. Meanwhile, three studies observed larger MHC I fibres in younger men while two reported larger MHC I fibres in older men. The significantly greater MHC I fibre CSA in younger men reported by Korhonen et al. (2006) may be explained by the inclusion of only sprint-trained athletes, as physical activity and training status are well-known to alter fibre size. Alternatively, the relatively small number of fibres studied per participant (~4) as well as the wide age range in the older cohort (i.e., 53–77 yrs) provide additional potential explanations for this finding (Table A1). Regarding the other two studies that reported larger MHC I fibres in younger men (D’Antona et al., 2003; Brocca et al., 2017), no clear explanation is apparent. However, it is noteworthy that both of these studies were performed by the same research group as inter-laboratory methodological differences in measuring/estimating fibre size (Table A2), such as accounting for fibre swelling in solution and/or assuming a circular or elliptical cross-sectional shape, may contribute to this disparity (Kalakoutis et al., 2021). Despite these inter-laboratory differences, the collective literature aligns in supporting no effect of ageing on MHC I fibre CSA in men.
In women, the case for a lack of an age-related change in MHC I fibre size is strengthened, with eight of the nine studies reporting no difference in CSA between younger and older women (collective mean P=0.618). Importantly, the observation that MHC I fibre size does not differ with age is also commonly reported by studies using immunohistochemistry (IHC) of muscle cross-sections for the measurement of fibre size (Nilwik et al., 2013; Callahan et al., 2014; Verdijk et al., 2014; Murgia et al., 2017; Kelly et al., 2018). Deviating from this theme, however, Trappe et al. (2003) reported greater MHC I fibre CSA in older compared with younger women, and larger MHC I fibres have also been observed in older compared with younger men (Hvid et al., 2013; Miller et al., 2013). Though highly-speculative, this observation may be explained by a compensatory hypertrophy of the slow muscle fibres in an effort to offset well-documented declines in fast fibre size (Lexell et al., 1988). Intriguingly, cross-sectional comparisons of MHC I fibre size in endurance trained older and younger individuals suggest that habitual physical activity and lifelong aerobic exercise may augment this hypertrophic response (Coggan et al., 1990; Grosicki et al., 2021), and significant increases in slow muscle fibre size following aerobic exercise training in older adults have been observed (Aniansson et al., 1992; Harber et al., 2012). Taken together, the data allude to a preservation of ageing MHC I fibre size, and also perhaps the hypertrophic potential, in older men and women.
Distinct from the MHC I fibres, evidence for age-related atrophy of the MHC II fibres is apparent in both men (P=0.014) and women (P=0.044) (Figure 2). This finding is consistent with the seminal cadaveric work of Lexell and colleagues, who attributed ageing muscle atrophy to both a loss of muscle fibres as well as a reduction in fast fibre size (Lexell et al., 1988). Moreover, studies using IHC of muscle cross-sections have also reported age-related atrophy of the MHC II fibres in groups of lifelong recreationally active adults (Soendenbroe et al., 2022), sprint-trained athletes (Korhonen et al., 2006), and world-class masters athletes (Sonjak et al., 2019), suggesting that the fast fibre atrophy is not solely due to age differences in physical activity levels or training status. In this context, the lack of an age-related difference in MHC II fibre size reported by nearly half of the single fibre studies is unanticipated. In fact, of the studies that provided statistical comparisons, only 7 of 16 in men and 4 of 7 in women reported reduced MHC II fibre size in the older cohorts. This unexpected observation is likely explained by an unintentional ‘selection bias’ whereby small and atrophied fast muscle fibres from older adults are less likely to be studied, and those that are studied are more likely to fail single muscle fibre physiology experimentation. Indeed, muscle fibre experimental failure is more common in fast versus slow muscle fibres (Yu et al., 2007), and a greater percentage of these failures are observed in fibres from older adults (Trappe et al., 2003; Grosicki et al., 2021). However, it is worth noting that even in circumstances where no age-related differences in MHC II fibre size were observed, a limited capacity for growth in the MHC II fibres from older compared with younger muscle fibres has been documented (Raue et al., 2009). We speculate that the limited myocellular growth potential may contribute, at least in part, to the lack of an apparent benefit for MHC II fibre size observed with lifelong aerobic exercise in older men (Grosicki et al., 2021) and women (Gries et al., 2019). Alternatively, aerobic exercise training may not provide a sufficient stimulus for the fast fibres and a more robust anabolic stimulus, such as lifelong resistance training, may afford superior protection to fast fibre size with ageing and is worth further investigation. In support of this hypothesis, prolonged resistance exercise training (i.e., 24-weeks) has been shown to increase MHC II fibre size by an average of 24–29% in healthy older men and women when measured with IHC (Leenders et al., 2013; Nilwik et al., 2013).
Peak Isometric Force (Po)
Although correlations between muscle fibre CSA and isometric force (Po) vary considerably between research groups (r = 0.26 – 0.82) (Kalakoutis et al., 2021), the comparisons of absolute peak isometric force (Po) in MHC I (Figure 1) and MHC II fibres (Figure 2) between younger and older adults generally mirrored the trends in muscle fibre size. Specifically, of the studies reporting statistical comparisons, 8 of 10 in men (collective mean P=0.512) and 6 of 7 in women (collective mean P=0.713) found no difference in slow fibre absolute Po between younger and older adults, and the collective mean supports this majority (P=0.954). Deviating from this theme, Trappe et al. (2003) reported greater MHC I fibre absolute Po in older compared with younger women, which is consistent with, and likely explained by, the greater slow fibre size observed in the older adults. Meanwhile, two studies reported greater MHC I fibre absolute Po in younger compared with older men in the absence of any age-related difference in muscle fibre size (Frontera et al., 2000b; Mazara et al., 2021). This observation alludes to the possibility of an age-related reduction in MHC I fibre specific tension, defined as isometric force normalized to fibre cross-sectional area (Po/CSA). However, only 7 of 19 studies in men and 1 of 9 studies in women support this possibility (Figure 1), and the collective means show no age-related difference in MHC I fibre specific tension in men (P=0.559) or women (P=0.791). Thus, interpreting all the data together provides compelling evidence that ageing seems to have minimal, if any, influence on MHC I fibre isometric force, be it absolute or normalized to cell size.
Of the studies reporting statistical comparisons for MHC II fibre absolute Po, 6 of 10 in men and 2 of 5 in women reported reduced Po in older compared with younger adults. Meanwhile, the collective means indicate a reduction in absolute Po that may be slightly more pronounced in men (−28%, P=0.006) than women (−21%, P=0.019). Notably, the four most recent studies in men, which came from three different research groups, reported a reduction in MHC II fibre absolute Po with advancing age (Figure 2), and lifelong aerobic exercise training appears unable to attenuate the deficits in absolute Po in older men or women (Gries et al., 2019; Grosicki et al., 2021). Furthermore, in several of the studies where no age-related differences were detected, MHC II fibre absolute Po values were numerically higher, albeit not statistically different, in the younger compared with older adults (Figure 2). Conversely, in women, the collective means hint at less of a decrement in MHC II fibre absolute Po with advancing age, and there is less agreement among the more recent studies. This potential sex-related difference aligns with findings from Teigen et al. (2020) showing a reduction in MHC II fibre absolute Po in older men but not women. This finding is most likely explained by a more pronounced ‘selection bias’ in fast fibres from older women during single fibre experimentation. Indeed, MHC II fibres from older women are ~20–25% smaller than those from older men and are more likely to fail during experimentation (Trappe et al., 2003). However, while limitations with the single fibre physiology procedure likely explain at least a portion of the sex-specific differences in MHC II fibre size and contractile function with ageing, biological mechanisms should not be overlooked and are worth further investigation.
Unlike the age-related decrements in fast fibre size and absolute Po, intrinsic contractile function, determined by comparing the specific tension, appears to be relatively well-preserved in both older men (P=0.457) and women (P=0.798). However, it should be highlighted that age-related differences in MHC II fibre specific tension were reported in nearly half of the studies, but the directionality of the findings was mixed (i.e., seven studies showed greater Po/CSA in younger adults and six showed the opposite). More detailed comparison of the experimental procedures and participant characteristics between these studies revealed that 6 of the 7 reporting reduced specific tension with ageing pre-treated the fibres with a permeabilization detergent (i.e., Brij or Triton-X100), which may negatively affect force production (Kalakoutis et al., 2021). However, several other inter-laboratory differences also existed including, but not limited to, the duration of fibre storage, experimental temperature, and/or the techniques for measuring/estimating fibre CSA (Table A2). Given the array of inconsistent findings, there is a clear need to identify whether the discrepancies in the literature are due to biological variability or differences in methodology.
Unloaded Shortening Velocity (Vo)
We identified 18 studies comparing single muscle fibre unloaded shortening velocity (Vo) between younger and older men and women (Figure 3). In the MHC I fibres, collective means indicated no age-related difference in Vo for men (P=0.544) or women (P=0.993). Furthermore, 9 of 15 studies in men and 5 of 7 studies in women reported no difference in MHC I fibre velocity in younger and older participants. A similar trend was observed in the MHC II fibres, with 7 of 15 studies in men and 5 of 6 studies in women indicating no difference in shortening velocity between younger and older adults (collective means: men, P=0.803; women, P=0.895). The reason for the discrepancies between studies is unclear, but notably, most of the studies reporting an age-related reduction in velocity were published before 2010. In addition to the inter-laboratory differences in methodology discussed above (Table A2), another potential explanation for these discrepancies is a difference in statistical approach between the earlier and later studies. More specifically, contemporary best-practice recommends the use of a nested analysis whereby a mean for each single fibre parameter within a subject is determined. This subject mean is then weighted by the number of fibres studied to compute a group mean that is used to compare between cohorts. Though this nested approach diminishes statistical power when compared to earlier studies that counted each single fibre experiment as an independent observation (Krivickas et al., 2001; D’Antona et al., 2003; Korhonen et al., 2006), it is less prone to type I error and properly accounts for the hierarchical structure of the data from single fibre studies. However, biological factors such as differences in lifestyle (e.g., physical activity, diet, etc.) may also be contributing to the discrepancies in the literature. For example, lifelong participation in aerobic exercise training may increase single fibre shortening velocity in older adults (Gries et al., 2019; Grosicki et al., 2021), leaving open the possibility that different physical activity levels and/or training status among the older cohorts may explain some of the inter-study differences (D’Antona et al., 2007). Nonetheless, the collective literature suggests that contrary to the slowed contractile velocity observed at the whole muscle level (Lanza et al., 2003; Thom et al., 2007; Dalton et al., 2012), ageing has little to no effect on MHC I or II fibre unloaded shortening velocity in both men and women. It is important to recognize that despite the lack of an age difference at the single fibre level because the shortening velocities of fast fibres are ~3-fold greater than slow fibres, a selective atrophy and/or loss of fast fibres with ageing would likely manifest as a pronounced slowing of velocity of the whole-muscle in vivo.
Figure 3. MHC I and MHC II fibre Vo from younger and older adults.
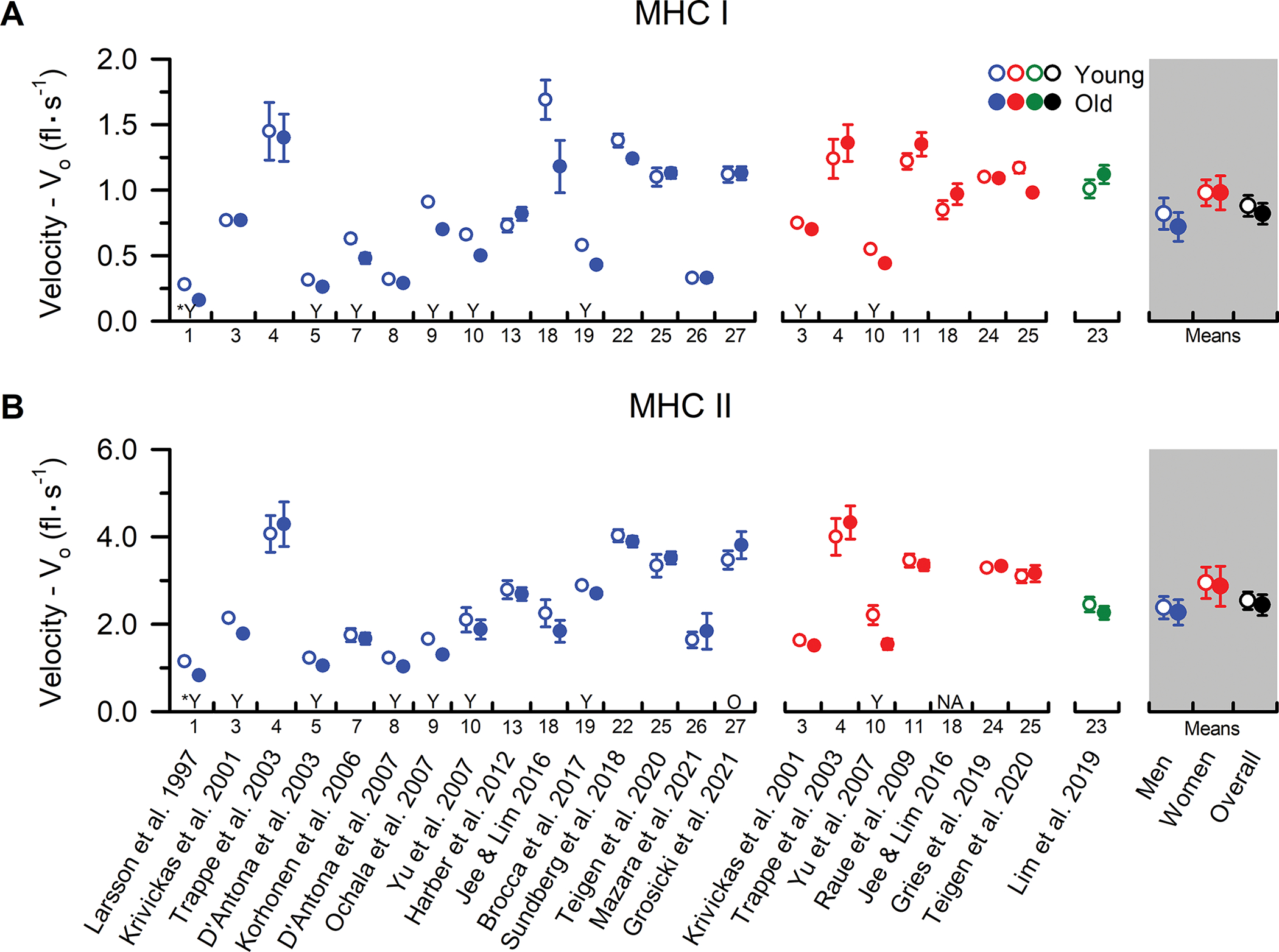
Individual study data and collective means for unloaded shortening velocity (Vo) of (A) MHC I and (B) MHC II fibres. Symbols represent the means from men (blue) and women (red). The collective means (shaded grey regions) for men, women, and all studies combined (black symbols) were calculated by pooling the results from all studies. Unpaired t-tests were performed on the collective means to test for age differences (young vs. old) using the pooled data. If assumptions of normality or homogeneity of variance were violated, then a non-parametric Mann-Whitney U test was used. To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression, with the caveat that not all studies could be included in this calculation because the sample sizes were not reported. None of the conclusions differed between the analyses, and thus, we report the collective means and p-values based on the unweighted analyses to allow inclusion of all the studies in the pooled results. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. In instances where the numerical data were unavailable in the tables or text, values were extracted from figures or obtained from the authors. Data shown from individual studies were restricted to MHC IIa fibres if the MHC IIx and hybrid IIa/IIx were also reported; otherwise, the data represent the MHC II fibres which may include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. For studies with interventions, the pre-intervention values are presented, and when data from several cohorts or muscle groups were available, the data are reported from the vastus lateralis of the mobile, independently living cohort. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. *Y = Statistical comparisons for chemically skinned fibres were not provided, thus data from the combined chemically skinned and freeze-dried fibres are shown. N/A = no value was reported or was unable to be determined. Values are mean ± SE. In instances where SE was not provided, it was calculated based on the SD and fibre sample size, if available.
Peak Power
To date, the majority of studies investigating single fibre contractile function with ageing have focused on peak isometric force (Po) and unloaded shortening velocity (Vo) in saturating Ca2+ conditions. While these parameters provide important insight into single fibre contractile function and cross-bridge kinetics, they represent two extremes of the force-velocity relationship of skeletal muscle that are rarely, if ever, encountered during voluntary muscle contractions in vivo. For example, shortening contractions in vivo are always loaded with at least the mass of the limb, and therefore, the fibres never shorten under fully unloaded conditions. In addition, in vivo studies have found that the age-related decrements in physical function, including losses in ambulation and responding to unexpected perturbations to prevent falls, are more closely associated with the ability to generate power than isometric strength (Bassey et al., 1992; Skelton et al., 2002; Bean et al., 2003; Cuoco et al., 2004). Thus, from a translational perspective, perhaps the most important single fibre functional parameter is the ability of muscle fibres to shorten rapidly under submaximal loads and generate power.
Despite the growing recognition of the importance of power output to physical function in older adults (Reid & Fielding, 2012), only six studies have compared fibre power between younger and older adults and only a single study has included a group of older men and women within the same study (Figure 4). Based on the findings for CSA, Po, and Vo of the MHC I fibres, it is perhaps not surprising that five out of the six studies have also found no differences in absolute peak power between younger and older adults. The only divergent finding comes from Trappe et al. (2003) who observed greater absolute power in older compared with younger women. The power data from this study are in close agreement with the Po data and can be attributed to the larger MHC I fibre size in the older women as indicated by the lack of an age difference in power when normalized to fibre CSA. Nevertheless, the collective data, albeit from a small number of studies, provide compelling evidence that ageing has no effect on absolute power (P=0.831) or power normalized to fibre size (P=1.000) in MHC I fibres. An important observation, however, is that the absolute power of the MHC I fibres from older adults who participated in lifelong aerobic exercise training were greater than that of healthy active younger men and women (Gries et al., 2019; Grosicki et al., 2021), providing further evidence that MHC I fibres of older adults likely have a preserved ability to adapt to aerobic exercise training.
Figure 4. MHC I and MHC II fibre absolute and normalized power from younger and older adults.
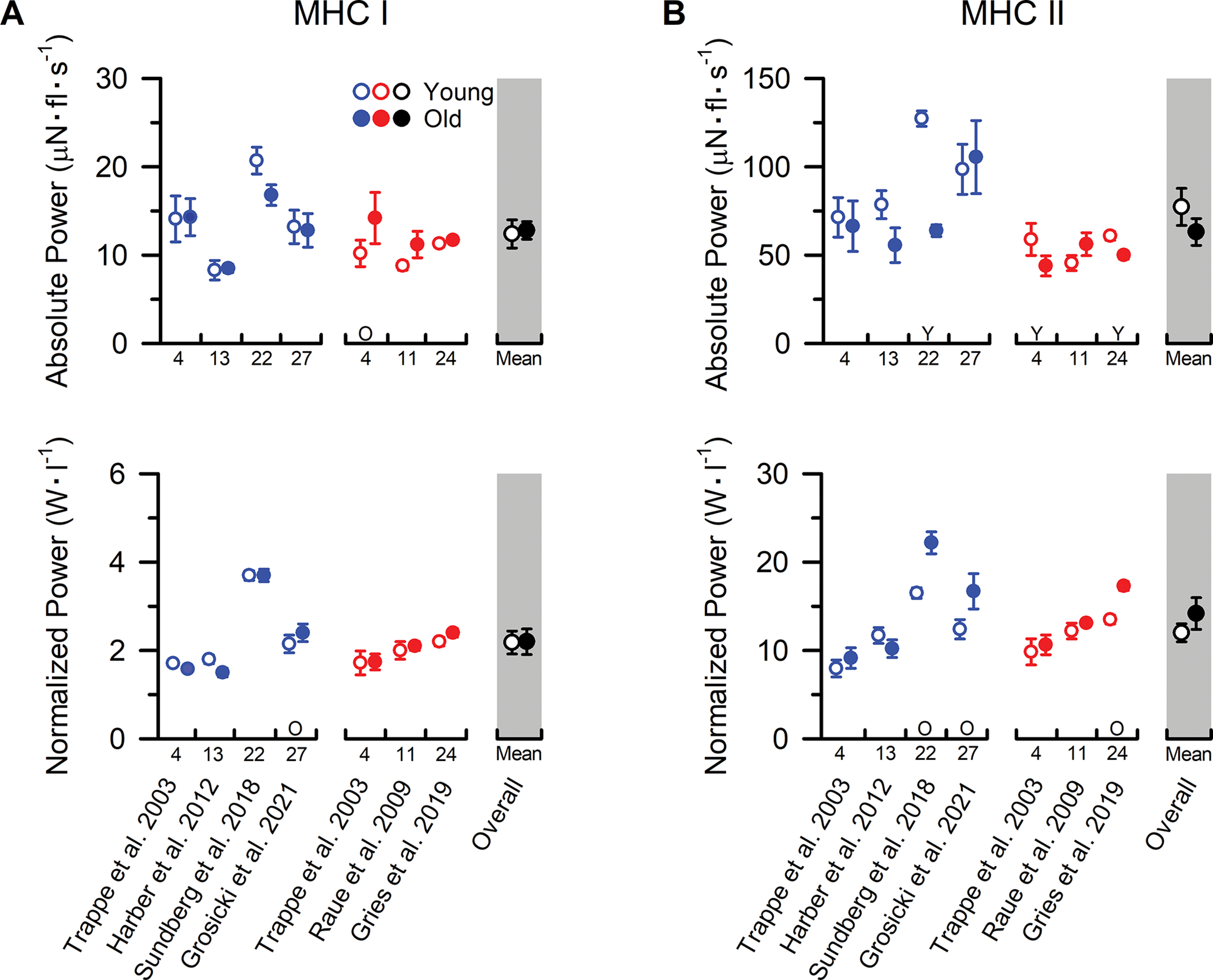
Individual study data and collective means for absolute and normalized power of (A) MHC I and (B) MHC II fibres. Symbols represent the means from men (blue) and women (red). The collective means (shaded grey regions) for all studies combined (black symbols) were calculated by pooling the results from all the studies. Due to the limited number of studies comparing absolute and normalized power in single fibres from younger and older adults, the group mean statistical analysis was conducted using an unpaired t-test only with the sexes combined (overall). If assumptions of normality or homogeneity of variance were violated, then a non-parametric Mann-Whitney U test was used. To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression, with the caveat that not all studies could be included in this calculation because the sample sizes were not reported. None of the conclusions differed between the analyses, and thus, we report the collective means and p-values based on the unweighted analyses to allow inclusion of all the studies in the pooled results. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. Data shown from individual studies were restricted to MHC IIa fibres if the MHC IIx and hybrid IIa/IIx were also reported; otherwise, the data represent the MHC II fibres which include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. For studies with interventions, the pre-intervention values are presented. If numerical data from tables or text were unavailable, values were extracted from figures. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. Values are mean ± SE. In instances where SE was not provided, it was calculated based on the SD and fibre sample size, if available.
In contrast to the findings from the slow fibres, the data on absolute peak power of the MHC II fibres are less clear with three out of six studies observing lower absolute power in older adults (Figure 4), and the remaining studies observing no age differences (collective mean P=0.250). Interestingly, five of the six studies were conducted in the same laboratory, which minimizes the likelihood that the discrepancies are from inter-laboratory differences in methodology. This does not, however, eliminate the possibility of an inadvertent ‘selection bias’ (discussed above) being more prevalent in one study versus another, and single fibre experiments may have been performed by different investigators. The between study discrepancies may also be explained by the interplay of natural biological variability and 1) the inherent limitations of employing cross-sectional study designs in ageing research and/or 2) the relatively modest number of fibres and participants that can logistically be studied in single fibre physiology experiments (Table A1). Irrespective of the potential explanation, it is important to note that the three studies observing lower absolute power in MHC II fibres of older adults also reported smaller CSA (Trappe et a. 2003 [data from women but not men], Sundberg et al. 2018, Gries et al. 2019). In addition, the study with the largest age difference in MHC II fibre size and absolute power also observed a close association between the age-related decrements in whole-muscle power output and estimates of the amount of lean tissue composed of MHC II fibres (Sundberg et al. 2018). Collectively, these data provide preliminary evidence that the accelerated age-related decrements in whole-muscle power output relative to the loss in total muscle mass might be explained, at least in part, by the selective atrophy of the fast fibres. Given the importance of whole-muscle absolute power output to mobility and physical function (Reid & Fielding, 2012), additional studies are clearly needed to further investigate this theory.
Similar to the findings on absolute power of the MHC II fibres, the data on peak power normalized to fibre size are unclear with three out of six studies actually observing higher normalized power in older adults (Figure 4) and the remaining studies observing no age differences (collective mean P=0.314). The explanation for the discrepancies between studies is unknown, but it is interesting to note that the three studies observing higher normalized power also reported smaller CSA of the MHC II fibres from both healthy older adults and those who performed lifelong aerobic exercise training (Sundberg et al. 2018, Gries et al. 2019, Grosicki et al. 2021). The only data deviating from this relationship was the older women from Trappe et al. (2003), who showed no age difference in normalized power, but had both a lower absolute power and MHC II fibre CSA compared with the younger women. Nevertheless, the possibility that atrophied fast fibres from older adults may have greater normalized power is intriguing and has been suggested to be a compensatory response to help offset the age-related decrements in whole muscle function (Grosicki et al., 2016). It should be acknowledged, however, that an equally plausible explanation is that large fibres, irrespective of age, may be geometrically constrained and required to devote more space within the cell to structures other than the contractile apparatus. Indeed, this theory is supported by the observation that size-specific Po in fibres from healthy young adults decreases with increasing fibre CSA (Gilliver et al., 2009), which is accompanied by a decrease in fibre stiffness (Miller et al., 2015). Regardless of the potential explanation, normalized MHC II fibre power clearly does not decrease with ageing, indicating that the intrinsic contractile function of the fibres is preserved. Thus, these data provide further support that the age-related decrease in absolute power observed in half the studies can be explained entirely by the fast fibre atrophy. This observation is critically important to whole-muscle function because absolute power is a major determinant for an individual to perform daily activities and MHC II fibres generate ~5–6-fold greater power than MHC I fibres (Figure 4). Future studies should determine whether fast fibre atrophy is an obligatory consequence of healthy ageing and identify the most effective strategies to prevent or slow the atrophy to help preserve whole-muscle function.
Rates of Force Development – Low- to High-Force Transition of the Cross-Bridge Cycle (ktr)
Another important factor for physical function, in addition to the force and power generating capacity of the muscle, is the rate that force can be developed. This functional parameter may be particularly important for older adults in order to prevent falls during an unexpected perturbation (Bento et al., 2010). At the single fibre level, rates of force development are thought to be limited by the kinetics of the low- to high-force state of the cross-bridge cycle and can be assessed by measuring the rate of force redevelopment (ktr) following a rapid slack re-extension maneuver of a Ca2+ activated fibre (Metzger & Moss, 1990a, b). Despite the potential functional relevance, only three studies have compared ktr in MHC I and MHC II fibres from younger and older adults and only a single study has included a group of older men and women (Figure 5). Surprisingly, all three studies did not observe a difference in ktr between the MHC I or MHC II fibres from younger and older adults, nor was there a difference between fibres from older men and women. It should be noted that one additional study compared ktr in fibres from a group of older masters athletes and nonathletes versus younger adults and reported age-related decrements in ktr that were approximately two-fold lower in both older adult cohorts compared to the younger adults (Power et al., 2016). However, the investigators were unable to determine the MHC isoforms of the fibres from this study, and instead categorized the fibres as ‘slow type’ fibres based on Vo. With this approach, the authors also reported an ~53–57% lower absolute Po, ~43–54% lower specific Po, and ~46–67% slower Vo in the ‘slow type’ fibres from both the older adult cohorts compared with the younger adults. As shown in Figures 1, 3, and 5, these findings do not align with the prevailing single fibre literature, which highlights the necessity of single fibre studies comparing between age groups based on the MHC isoforms. Notwithstanding the discrepancies from this study, the data suggest that the rate of force development assessed with ktr, and therefore, the kinetics of the low- to high-force state of the cross-bridge cycle, are well-preserved in both MHC I and MHC II fibres with ageing. It is important to recognize, however, that similar to Vo, the ktr of MHC II fibres is ~3-fold greater than MHC I fibres; as a result, a selective loss and/or atrophy of fast fibres with ageing would likely manifest as a pronounced slowing of the rates of force development of the whole-muscle in vivo.
Figure 5. MHC I and MHC II fibre ktr from younger and older adults.
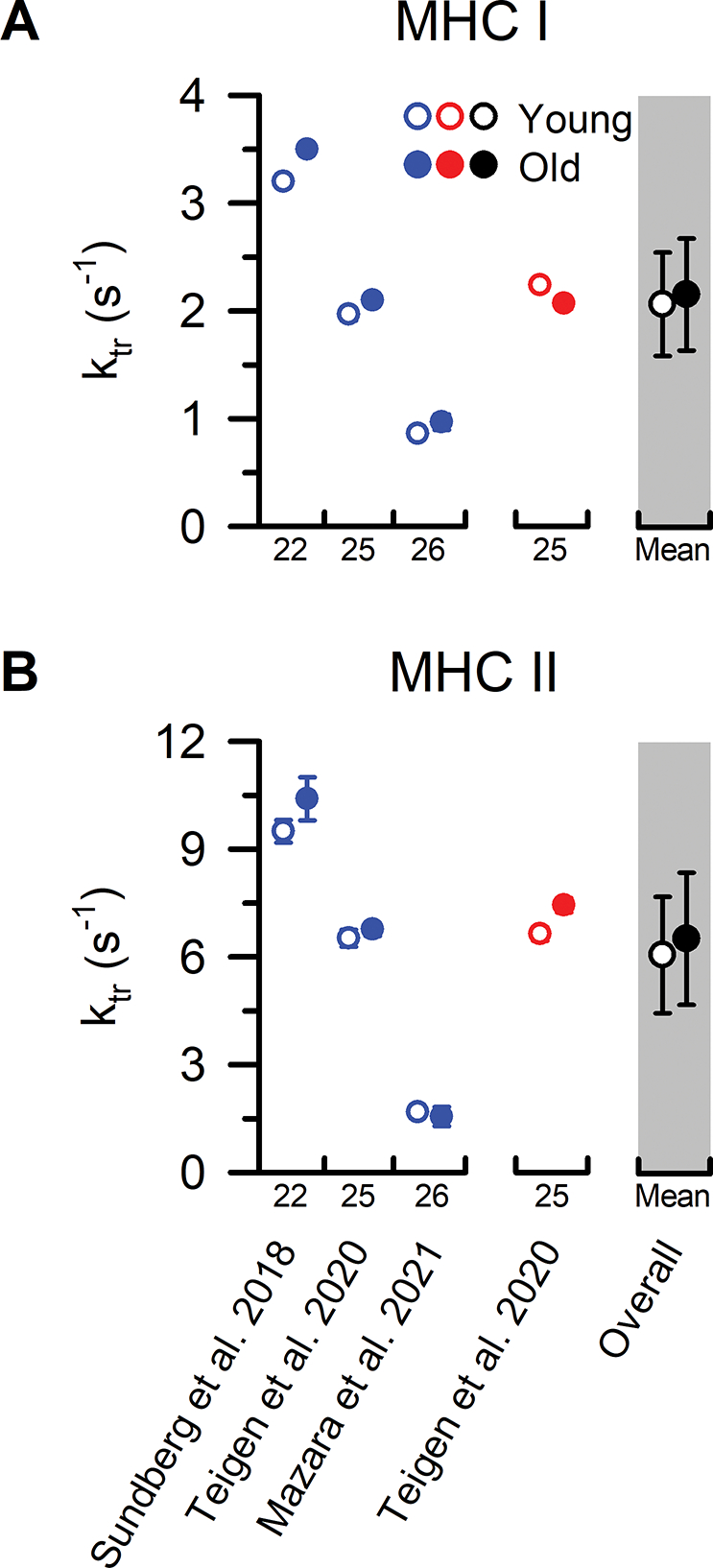
Individual study data and collective means for rate of force redevelopment (ktr) of (A) MHC I and (B) MHC II fibres. Symbols represent the means from men (blue) and women (red). The collective means (shaded grey regions) for all studies combined (black symbols) were calculated by pooling the results from all the studies. Due to the limited number of studies comparing ktr in single fibres from younger and older adults, statistical analysis was conducted using a non-parametric Mann-Whitney U test with only the sexes combined (overall). To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression. None of the conclusions differed between the analyses, and thus, we report the collective means and p-values based on the unweighted analyses. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. Data shown from individual studies were restricted to MHC IIa fibres if the MHC IIx and hybrid IIa/IIx were also reported; otherwise, the data represent the MHC II fibres which include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. Values are mean ± SE. In instances where SE was not provided, it was calculated based on the SD and fibre sample size, if available.
Calcium Sensitivity (pCa50)
As mentioned above, the majority of studies investigating single fibre contractile function with ageing have been conducted in saturating Ca2+ conditions, which precludes assessment of potential age-related differences in the sensitivity of the myofilaments to Ca2+. These studies are important because an age-related reduction in Ca2+ sensitivity may be contributing to the more precipitous loss in whole-muscle strength and power relative to the loss in total muscle mass with ageing. The isolated fibre preparation provides an ideal experimental approach to address this question, because it allows precise control over the intracellular concentrations of Ca2+ ([Ca2+]) surrounding the regulatory and contractile proteins. The approach involves activating the fibre in a range of [Ca2+], measuring and plotting the isometric force relative to the [Ca2+] in pCa units (where pCa = -log10 [Ca2+]), and fitting the sigmoidal curve with the Hill equation to obtain the [Ca2+] that elicits 50% peak isometric force (Po), or more commonly known as pCa50. Accordingly, a decrement in myofilament Ca2+ sensitivity would manifest as a rightward shift in the force-pCa curve and a lower pCa50 value (i.e., higher [Ca2+]).
Only six studies have investigated Ca2+ sensitivity in MHC I and II fibres from younger and older adults and only one has reported the data from both older men and women (Figure 6). In agreement with the other previously discussed contractile function parameters from the MHC I fibres, five of the six studies observed no age-related differences in Ca2+ sensitivity. The only disparate finding is from Straight et al. (2018) who observed a lower Ca2+ sensitivity in MHC I fibres from older compared with younger adults (~0.14 pCa units). The explanation for this discrepancy is unclear, but it is noteworthy that the experimental approach in this study was well-designed including measurement and control of sarcomere spacing and temperature, determination of the pure and hybrid fibres based on the MHC isoforms with SDS-PAGE, and studying a relatively large, and nearly equal (45 vs. 50 fibres), number of MHC I fibres from both age cohorts (Tables A1 and A2). The physical activity levels measured with triaxial accelerometry also did not differ between the age cohorts, which is important because physical activity and disuse have been shown to alter Ca2+ sensitivity irrespective of age (Widrick et al., 1998; Godard et al., 2002; Mounier et al., 2009; Hvid et al., 2011; Hvid et al., 2013). The only apparent difference is the pCa50 of the older and younger adults in this study were ~0.19–0.31 lower compared to the mean from the other five studies (i.e., ~54–104% higher [Ca2+] at pCa50), leaving open the possibility that experimental solution composition and/or fibre handling may be responsible for these unique findings (Straight et al., 2018). Regardless of the explanation, the collective data, albeit from a small number of studies, provides compelling evidence that ageing has little to no effect on Ca2+ sensitivity in MHC I fibres (P=0.590).
Figure 6. MHC I and MHC II fibre pCa50 from younger and older adults.
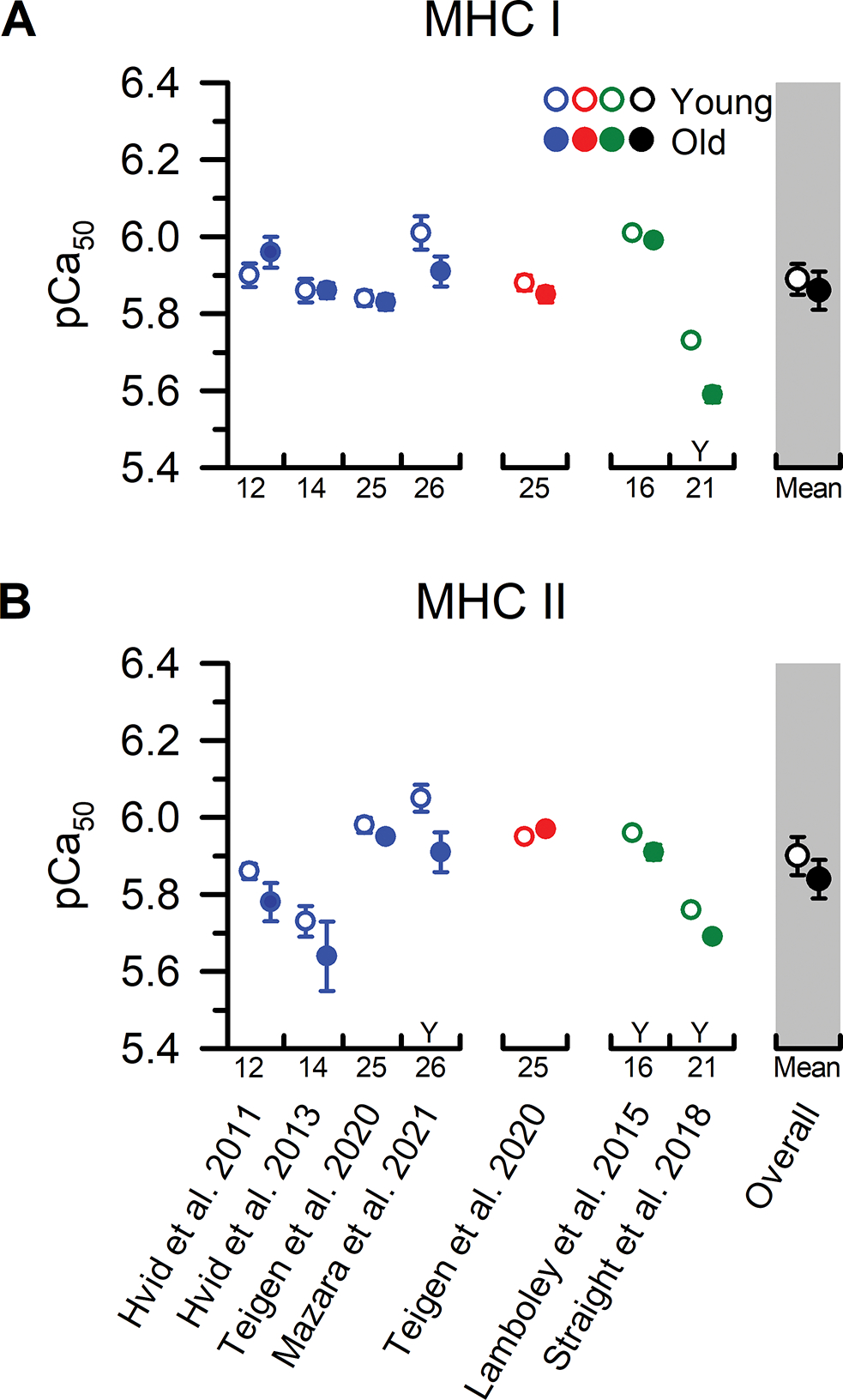
Individual study data and collective means for calcium sensitivity (pCa50) of (A) MHC I and (B) MHC II fibres. Symbols represent the means from men (blue), women (red), and when the sexes were combined (green). The collective means (shaded grey regions) for all studies combined (black symbols) were calculated by pooling the results from all the studies. Due to the limited number of studies comparing pCa50 in single fibres from younger and older adults, statistical analysis was conducted using an unpaired t-test with only the sexes combined (overall). To evaluate whether differences in the sample sizes between studies influenced the pooled results, the collective means were also compared between age groups using a weighted linear regression. None of the conclusions differed between the analyses, and thus, we report the collective means and p-values based on the unweighted analyses. For ease of identifying the data from each study both within and between figures, all studies were arranged in chronological order and assigned a numerical value that is located beneath the x-axes. Data shown from individual studies were restricted to MHC IIa fibres if the MHC IIx and hybrid IIa/IIx were also reported; otherwise, the data represent the MHC II fibres which include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. Statistical significance (P < 0.05) was extracted from the results of the individual studies. Y = Young > Old. O = Old > Young. Values are the mean ± SE.
In contrast to the findings from the MHC I fibres, the findings on Ca2+ sensitivity of the MHC II fibres are less clear with three out of six studies observing lower Ca2+ sensitivity in older adults (Figure 6), and the remaining observing no age differences (collective mean P=0.368). The explanation for the discrepancies between studies is unclear, but one interesting observation is even in the three studies that observed an age difference in Ca2+ sensitivity, the differences were modest, ranging from as small as 0.05–0.07 pCa units (Lamboley et al., 2015; Straight et al., 2018) up to 0.14 pCa units (Mazara et al., 2021). This is particularly notable because the increments that the [Ca2+] were added were larger than the observed age differences in Ca2+ sensitivity [i.e., increments ranged from 0.1–0.13 pCa units (Lamboley et al., 2015; Teigen et al., 2020) up to 0.2–0.25 pCa units (Hvid et al., 2011; Hvid et al., 2013; Straight et al., 2018; Mazara et al., 2021)]. This methodological limitation makes it difficult to reliably resolve differences in Ca2+ sensitivity at the levels reported, especially in the MHC II fibres which have a steeper force-pCa curve than MHC I fibres. Other potential explanations for the discrepancies, as discussed in detail previously (Straight et al., 2018; Teigen et al., 2020), are that some studies did not measure and control sarcomere spacing and temperature, the number of MHC II fibres studied was modest, physical activity levels of the participants were not measured or reported, and the MHC isoforms were all examined together (combined IIa, hybrid IIa/IIx, and IIx). It is important to resolve these discrepancies, because there is some evidence suggesting that there is less stored Ca2+ in the sarcoplasmic reticulum (Lamboley et al., 2015) and a lower amplitude of the intracellular Ca2+ transient in fibres from older compared with younger adults (Delbono et al., 1995), which may cause even modest changes in ageing muscle Ca2+ sensitivity to become functionally relevant.
Gaps in Knowledge and Future Directions
The most readily apparent need in the ageing single fibre field is for studies to systematically work through resolving some of the discrepancies in the prevailing literature. For example, decreased MHC II fibre size appears to be the primary contributor to age-related decrements in absolute force and power (Figures 2 & 4); yet, only ~50% of single fibre studies have observed an age-related difference in fast fibre size. It is important to identify whether this inconsistency is due to true biological variability or is a methodological limitation of single fibre physiology experiments. We posit that inter-laboratory differences in methodologies for preparing and measuring fibre size (Table A2) in conjunction with the inherent limitations of the single fibre physiology technique (e.g., ‘selection bias’ and low fibre n) combine to yield these inconsistent findings. Detailed attention to rectifying these discrepancies along with continued advances in single fibre technology will permit a more definitive understanding of the effects of ageing on MHC II fibre specific tension and normalized power. This is critical for determining whether altered intrinsic contractile mechanics is contributing to age-related decrements in muscle function (Lim & Frontera, 2022).
Beyond resolving the discrepancies in the literature, examination of single fibre contractile mechanics in a range of experimental conditions that more closely mimics the intracellular environments in vivo should increase the translational impact of the discoveries from this technique. For example, the vast majority of single fibre studies have been conducted at, or less than, room temperature (Table A2) and in an activating solution that mimics near quiescent skeletal muscle (pH 7.0 + 0 mM inorganic phosphate [Pi]). However, the actual concentration of Pi in quiescent human skeletal muscle is ~3–5 mM (Kemp et al., 2007), and intracellular concentrations of H+, Pi, and other metabolites rapidly change at the onset of contractile activity in both younger and older adults (Sundberg et al., 2019). This is important because H+ and Pi both individually and collectively disrupt cross-bridge function (Debold et al., 2016; Sundberg & Fitts, 2019), and only a single study has investigated the effects of these metabolites in muscle fibres from younger and older adults (Sundberg et al., 2018). In addition, several regulatory proteins, such as myosin binding protein-C and myosin regulatory light chain (RLC), are phosphorylated during contractile activity and can modulate cross-bridge kinetics (Vandenboom, 2016; McNamara & Sadayappan, 2018). Whether ageing affects the phosphorylation state of these regulatory proteins has received limited attention (Miller et al., 2013; Brocca et al., 2017; Teigen et al., 2020), and to our knowledge no studies have examined how the phosphorylation of these proteins alters contractile function in human skeletal muscle. Importantly, these experiments should be conducted in a range of elevated [H+] and [Pi] because evidence from rat fibres suggests that the metabolite-induced decrements in fibre shortening velocity and peak power are exacerbated when RLC is phosphorylated at near physiological temperatures (Karatzaferi et al., 2008). Thus, as single fibre physiology techniques continue to advance, it will be important to conduct ageing single fibre experiments closer to physiological temperatures (Miller et al., 2013; Sundberg et al., 2018) and in a range of conditions that more closely resemble the intracellular milieu during in vivo skeletal muscle contraction.
Another area essential to advancing our understanding of the ageing skeletal muscle phenotype is to begin integrating single fibre contractile mechanics with the molecular profile and other parameters important to contractile function (e.g., mitochondrial respiration). For example, advancements in single fibre proteomics analyses revealed an unexpected increase in glycolytic proteins in MHC I but a decrease in MHC IIa muscle fibres from older compared to younger men (Murgia et al., 2017). This apparent divergence in changing the metabolic protein profile without a concomitant change in the MHC isoform expression could have significant effects on contractile function. Indeed, while skeletal muscle has the remarkable ability to increase its energetic demand over 100-fold from rest to high-intensity contractile activity, accommodating this demand requires a tight coupling between the rates of ATP hydrolysis and resynthesis through the synchronized activation of the creatine kinase and adenylate kinase reactions, glycolysis, and oxidative phosphorylation (Hochachka & McClelland, 1997; Sahlin et al., 1998; Westerblad et al., 2010). Any age-related changes that may cause a disruption in the coupling of the ATP hydrolysis rates and mechanical outputs of the muscle, such as altered mitochondrial function (Conley et al., 2000), disrupted neural activation, innervation status and neuromuscular transmission (Hepple & Rice, 2016; Hunter et al., 2016), or decreased fibre economy via inefficiencies in myofibrillar and/or ion transport ATPases, could be contributing to the age-related skeletal muscle dysfunction and warrants investigation.
Concluding Remarks
Since the seminal study by Larsson and colleagues ~25 years ago, investigations on the contractile mechanics of isolated human muscle fibres have been instrumental in facilitating our understanding of the cellular mechanisms underlying age-related skeletal muscle dysfunction. The collective literature provides compelling evidence that ageing differentially effects fibres expressing the MHC I versus the MHC II isoforms. Surprisingly, and at odds with some of the earlier findings in the field, both the size and contractile function of the MHC I fibres appear to be well-preserved with ageing. In contrast, there are several studies observing profound age-related decrements in contractile function – primarily absolute force and power – of the fibres expressing the MHC II isoforms. Notably, most of the decrements in MHC II fibre function appear attributable to fibre atrophy, rather than age-related alterations in the intrinsic contractile mechanics, per se. Lifelong aerobic exercise training seems unable to prevent, or even attenuate, most of the age-related decrements in MHC II fibre size and contractile function, revealing a critical need to identify other nutritional, pharmaceutical, or exercise strategies, such as lifelong resistance training, to protect the fast fibres from ageing. In addition, few longitudinal studies have been conducted (Frontera et al., 2000a; Reid et al., 2014), and both the cross-sectional and longitudinal studies have been limited to a narrow range of intracellular environments and generally lack an integration of single fibre mechanical measures with the molecular profile or other parameters important to contractile function. Thus, while the past 25 years have provided clarity regarding the effects of ageing on single muscle fibre size and contractile function, there is still much to learn in this evolving field.
Supplementary Material
Acknowledgements
We thank Dr. Mehdi Maadooliat for assistance with the statistical analyses on the pooled data, and Ethan Claunch for the illustration in the figure abstract. We also thank Laura Teigen and Drs. Robert Fitts and Kevin Murach for their valuable comments on an earlier draft of the manuscript.
Funding
This work was supported by a National Institute of Aging R01 (AG048262) to CWS.
Biographies

Gregory Grosicki (left) is an Associate Professor of Kinesiology at Georgia Southern University (Savannah, GA, USA). As part of his PhD studies in the Human Performance Laboratory at Ball State University, he investigated single fibre contractile function with ageing and lifelong endurance exercise. His current research examines the acute and chronic cardiovascular responses to various exercise and nutritional interventions.

Carlos Zepeda (middle) is a PhD student at Marquette University (Milwaukee, WI, USA) working under the tutelage of Dr. Sundberg. His dissertation work is focused on the mechanisms of fiber-type specific atrophy and loss in contractile function with ageing.

Christopher Sundberg (right) is an Assistant Professor at Marquette University. His current research aims to develop a comprehensive understanding of the mechanisms responsible for the age-related loss in muscle power and increased fatigability by integrating techniques to study these phenomena in the intact neuromuscular system down to the cellular and molecular levels.
APPENDIX
Table A1.
Table 1: Number of MHC I and II fibres studied from the young and old adults.
| Study No. | Year | Author | Subjects | Physical Activity | Biopsy Site(s) |
MHC I (n) |
MHC II (n) |
||
|---|---|---|---|---|---|---|---|---|---|
| Young | Old | Young | Old | ||||||
|
| |||||||||
| 1 | 1997 | Larsson et al. | 4 YM (25–31y) 4 OM (73–81y) |
YM: Inactive OM: 2 Inactive, 2 Active |
VL | 47 *71 |
41 *66 |
23 *77 |
23 *30 |
| 2 | 2000 | Frontera et al. | 7 YM (37y) 12 OM (74y) 12 OW (72y) |
YM: 657 kcal/wk OM: 1,431 kcal/wk OW: 820 kcal/wk |
VL | 91 | 221 | 92 | 82 |
| 3 | 2001 | Krivickas et al. | 7 YM (37y) 7 YW (27y) 12 OM (74y) 12 OW (72y) |
Not Reported | VL | YM: 91 YW: 117 |
OM: 221 OW: 240 |
YM: 92 YW: 17 |
OM: 82 OW: 56 |
| 4 | 2003 | Trappe et al. | 6 YM (25y) 6 YW (25y) 6 OM (80y) 6 OW (78y) |
Healthy inactive | VL | YM: 29 YW: 52 |
OM: 69 OW: 80 |
YM: 20 YW: 21 |
OM: 17 OW: 7 |
| 5 | 2003 | D’Antona et al. | 7 YM (30y) 7 OM (73y) |
Healthy inactive | VL | 143 | 128 | 75 | 64 |
| 6 | 2003 | Frontera et al. | 7 YW (27y) 14 OW (74y) |
Healthy inactive | VL | 126 | 109 | N/A | N/A |
| 7 | 2006 | Korhonen et al. | 8 YM (18–33y) 9 OM (53–77y) |
Sprint Athletes | VL | 35 | 36 | 24 | 29 |
| 8 | 2007 | D’Antona et al. | 5 YM (30y) 10 OM (73y) |
YM: Recreationally active OM: Active |
VL | Not Reported | Not Reported | Not Reported | Not Reported |
| 9 | 2007 | Ochala et al. | 6 YM (32y) 6 OM (66y) |
YM: Healthy inactive OM: < 1 hr walking/d |
VL | 62 | 56 | 55 | 62 |
| 10 | 2007 | Yu et al. | 7 YM (20–43y) 6 YW (20–43y) 10 OM (65–85y) 12 OW (65–85y) |
Not Reported | VL | YM: 54 YW: 60 |
OM: 64 OW: 150 |
YM: 11 YW: 19 |
OM: 12 OW: 26 |
| 11 | 2009 | Raue et al. | 9 YW (21y) 6 OW (85y) |
Healthy inactive | VL | Not Reported | Not Reported | Not Reported | Not Reported |
| 12 | 2011 | Hvid et al. | 9 YM (24y) 8 OM (67y) |
YM: 4.5 h/wk OM: 4.8 h/wk |
VL | CE: 53 pCa: 40 |
CE: 60 pCa: 44 |
CE: 35 pCa: 40 |
CE: 19 pCa: 15 |
| 13 | 2012 | Harber et al. | 7 YM (20y) 6 OM (74y) |
Healthy inactive | VL | Young + Old: 145 | Young + Old: 127 | ||
| 14 | 2013 | Hvid et al. | 11 YM (24y) 11 OM (67y) |
YM: 4.3 h/wk OM: 4.4 h/wk |
VL | CE: 83 pCa: 42 |
CE: 111 pCa:62 |
CE: 29 pCa: 16 |
CE: 17 pCa: 11 |
| 15 | 2013 | Miller et al. | 5 YM (25y) 7 YW (24y) 5 OM (70y) 7 OW (68y) |
YM, YW: Minimally active OM, OW: Moderately active |
VL | YM: 70 YW: 84 |
OM: 59 OW: 97 |
YM: 53 YW: 69 |
OM: 52 OW: 50 |
| 16 | 2015 | Lamboley et al. | 10 YM + 6 YW (22y) 13 OM + 7 OW (70y) |
Recreationally active | VL | CE: 47 pCa: 26 |
CE: 62 pCa: 46 |
CE: 45 pCa: 21 |
CE:27 pCa: 25 |
| 17 | 2015 | Venturelli et al. | 8 YW (25y) 8 OW (87y) |
YW: physically active OW: healthy inactive |
VL BB |
27 | 22 | 20 | 11 |
| 18 | 2016 | Jee & Lim | 16 YM (29y) 11 YW (30y) 11 OM (71y) 7 OW (67y) |
Healthy inactive | VL | YM: 65 YW: 25 |
OM: 21 OW: 31 |
YM: 27 YW: 4 |
OM: 7 OW: 0 |
| 19 | 2017 | Brocca et al. | 10 YM (23y) 10 OM (71y) |
Physically active | VL | 37 | 37 | 30 | 45 |
| 20 | 2017 | Murgia et al. | 4 YM (24y) 4 OM (70y) |
Physically active | VL | 20 | 27 | 20 | 23 |
|
| |||||||||
| 21 | 2018 | Straight et al. | 5 YM + 6 YW (24y) 3 OM + 7 OW (69y) |
YM + YW: 415 kcal/d OM + OW: 415 kcal/d |
VL | 45 | 50 | 42 | 38 |
| 22 | 2018 | Sundberg et al. | 6 YM (23y) 6 OM (82y) |
YM: 9,175 steps/d OM: 7,977 steps/d |
VL | 56 | 59 | 60 | 53 |
| 23 | 2019 | Lim et al. | 6 YM + 4 YW (26y) 5 OM + 2 OW (79y) |
YM + YW: physically active OM + OW: healthy inactive |
VL | 66 | 86 | 41 | 47 |
| 24 | 2019 | Gries et al. | 10 YE (25y) 10 OH (75y) 7 LLE (72y) |
YE: 11,518 steps/d OH: 6,801 steps/d LLE: 7,463 steps/d |
VL | 114 | OH:110 LLE: 83 |
87 | OH: 99 LLE: 65 |
| 25 | 2020 | Teigen et al. | 11 YM (25y) 8 YW (23y) 14 OM (76y) 7 OW (77y) |
YM: 8,760 steps/d YW: 10,151 steps/d OM: 9,275 steps/d OW: 5,925 steps/d |
VL | YMCE: 40 YWCE: 45 YMpCa: 37 YWpCa: 31 |
OMCE: 97 OWCE: 59 OMpCa: 38 OWpCa: 35 |
YMCE: 37 YWCE: 50 OMpCa: 33 OWpCa: 34 |
OMCE: 87 OWCE: 59 OMpCa: 30 OWpCa: 31 |
| 26 | 2021 | Mazara et al. | 8 YM (22–35y) 8 OM (60–81y) |
YM: 105 kcal/wk OM 105 kcal/wk |
VL | 27 | 42 | 40 | 13 |
| 27 | 2021 | Grosicki et al. | 10 YE (25y) 10 OH (75y) 21 LLE (74y) |
YE: 9,404 steps/d OH: 5,813 steps/d LLE: 9,560 steps/d |
VL | 109 | OH: 102 LLE: 228 |
110 | OH: 84 LLE: 199 |
Twenty-seven studies were identified that compared single muscle fibre contractile function between a young and older adult cohort. Studies were included in the tables and figures if they identified the myosin heavy chain (MHC) isoforms of the fibres using SDS-PAGE or western blotting. The values presented for the number of MHC II fibres were restricted to MHC IIa fibres if MHC IIx and hybrid IIa/IIx fibres were also reported; otherwise, the values shown represent the MHC II fibres which include a mixture of MHC IIa, hybrid IIa/IIx, and IIx isoforms. For studies with interventions, the pre-intervention values are presented, and when data from several cohorts were available, the data are reported from the vastus lateralis of the mobile, independently living cohort. For ease of identification within and between figures and tables, studies were arranged in chronological order and each assigned a numerical value. YM, young men; YW, young women; OM, old men; OW, old women; YE, young exercisers; LLE; lifelong exercisers; OH; old healthy nonexercisers; VL, vastus lateralis; BB, biceps brachii; MHC, myosin heavy chain
combined number of chemically skinned and freeze-dried fibres; CE, number of fibers studied for contractile mechanics experiments; pCa, number of fibers studied for calcium sensitivity experiments.
Table A2.
Table 2: Experimental conditions that affect measurements of size and contractile function of single muscle fibres.
| Study No. | Year | Author | Temp. (°C) | pH | Pi (mM) | Sarcomere Length (µm) | Ionic Strength (mM) | Pre-experimental Treatment | Storage Conditions | Storage Duration | CSA Measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1 | 1997 | Larsson et al. | 12 | 7 | 0 | 2.79 ± 0.04 (2.69–2.85) | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning freeze-dried | ≤3 wks | Diameter+depth in solution (ellipse) |
| 2 | 2000 | Frontera et al. | 15 | 7 | 0 | 2.75–2.85 | 180 | 0.5% Brij-58 pre-activating | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse −20%) |
| 3 | 2001 | Krivickas et al. | 15 | 7 | 0 | 2.75–2.85 | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning | Not Reported | Not Reported |
| 4 | 2003 | Trappe et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 5 | 2003 | D'Antona et al. | 12 | 7 | 0 | 2.5 | 200 | 1% Triton X100 1 hr pre-activating | −20°C skinning | ≤3 wks | Diameter in solution (cylinder) |
| 6 | 2003 | Frontera et al. | 15 | 7 | 0 | 2.75–2.85 | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse −20%) |
| 7 | 2006 | Korhonen et al. | 15 | 7 | 0 | 2.76 ± 0.04 (2.66–2.85) | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning −80°C long-term sucrose | ≤2–3 wks in skinning | Diameter+depth in solution (ellipse −20%) |
| 8 | 2007 | D'Antona et al. | 12 | 7 | 0 | 2.5 | 200 | 1 % Triton X100 1 hr pre-activating | −20°C skinning | ≤3 wks | Diameter in solution (cylinder) |
| 9 | 2007 | Ochala et al. | 15 | 7 | 0 | 2.75–2.85 | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse −20%) |
| 10 | 2007 | Yu et al. | 12 | 7 | 0 | 2.75 (2.70–2.85) | 180 | 0.5% Brij-58 30 min. pre-activating | −20°C skinning | ≤3 wks | Diameter+depth in solution (ellipse −20%) |
| 11 | 2009 | Raue et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 12 | 2011 | Hvid et al. | 22.1 ± 0.0 room temp. | 7.1 ± 0.01 | 0 | 120% slack length | Not Reported | 1% Triton X100 60s | −20°C skinning | Not Reported | Diameter in solution (cylinder) |
| 13 | 2012 | Harber et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 14 | 2013 | Hvid et al. | 22.1 ± 0.0 room temp. | 7.1 ± 0.01 | 0 | 120% slack length | Not Reported | 1% Triton X100 60s | −20°C skinning | ≤4 mos | Diameter in solution (cylinder) |
| 15 | 2013 | Miller et al. | 15, 25 | 7 | 0, 5 | 2.65 | 175 | 1% Triton X100 30 min 4°C pre-activating | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse) |
| 16 | 2015 | Lamboley et al. | 23 ± 2 room temp. | 7.1 | 0 | 120% slack length | Not Reported | Mechanically Skinned | ~10°C paraffin oil | Not Reported | Diameter in solution (ellipse) |
| 17 | 2015 | Venturelli et al. | 12 | 7 | 0 | 120% slack length | 200 | 1% Triton X100 | −20°C skinning | ≤2 wks | Diameter in solution (cylinder) |
| 18 | 2016 | Jee & Lim | 15.3 | 7 | 0 | 2.6–2.7 | Not Reported | None | −20°C skinning | ≤2 wks | Diameter+depth in solution (ellipse −20%) |
| 19 | 2017 | Brocca et al. | 12 | 7 | 0 | 2.5 | Not Reported | 0.1% Triton X100 1 hr | −20°C skinning | Not Reported | Diameter+depth in solution (ellipse) |
| 20 | 2017 | Murgia et al. | 12 | 7 | 0 | 120% slack length | 200 | 1% Triton X100 | −20°C skinning | ≤2 wks | Diameter in solution (cylinder) |
| 21 | 2018 | Straight et al. | 15 | 7 | 0 | 2.65 | 175 | 1% Triton X100 20 min. 4°C pre-activating | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse) |
| 22 | 2018 | Sundberg et al. | 15, 30 | 7, 6.2 | 0, *4, 30 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 23 | 2019 | Lim et al. | 15 | 7 | 0 | 2.75–2.85 | 180 | 0.5% Brij-58 30 min. | −20°C skinning | ≤4 wks | Diameter+depth in solution (ellipse −20%) |
| 24 | 2019 | Gries et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 25 | 2020 | Teigen et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
| 26 | 2021 | Mazara et al. | 16 | 7 | 0 | ~2.8 | Not Reported | pre-activating | −20°C skinning | ≤4 wks | Diameter in solution (cylinder) |
| 27 | 2021 | Grosicki et al. | 15 | 7 | 0 | 2.5 | 180 | None | −20°C skinning | ≤4 wks | Diameter in air (cylinder) |
Studies were included in the tables and figures if they identified the myosin heavy chain (MHC) isoforms of the fibres using SDS-PAGE or western blotting. For ease of identification within and between figures and tables, studies were arranged in chronological order and each assigned a numerical value. In the studies with two experimental temperatures, data from 15°C were included in the figures. In studies with multiple [Pi] and pH, the 0 mM Pi and pH 7 condition was included in the figures unless otherwise noted by *.
Values reported in Figure 4 for absolute and normalized power of MHC I and II fibres were obtained from pH 7 and 4 mM Pi. Pre-activating solutions had reduced Ca2+-EGTA buffering capacity. −20%, indicates cross-sectional area (CSA) of single fibres was corrected for the 20% swelling that occurs during skinning.
Footnotes
Conflicts of Interest
No conflicts of interests, financial or otherwise, are declared by the authors.
References
- Alcazar J, Aagaard P, Haddock B, Kamper RS, Hansen SK, Prescott E, Alegre LM, Frandsen U & Suetta C. (2020). Age- and Sex-Specific Changes in Lower-Limb Muscle Power Throughout the Lifespan. J Gerontol A Biol Sci Med Sci 75, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Al Zaid NS & Goldspink G. (1984). Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139, 677–689. [PMC free article] [PubMed] [Google Scholar]
- Aniansson A, Grimby G & Hedberg M. (1992). Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol 73, 812–816. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ & Lipsitz LA. (1992). Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82, 321–327. [DOI] [PubMed] [Google Scholar]
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM & Ferrucci L. (2003). A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci 58, 728–733. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ & Kritchevsky SB. (2013). Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr 97, 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento PC, Pereira G, Ugrinowitsch C & Rodacki AL. (2010). Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech (Bristol, Avon) 25, 450–454. [DOI] [PubMed] [Google Scholar]
- Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M & Bottinelli R. (2017). Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595, 4823–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Bedrin NG, Subramanian M, Berking J, Ades PA, Toth MJ & Miller MS. (2014). Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol 116, 1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, Rogers MA, King DS, Brown M, Nemeth PM & Holloszy JO. (1990). Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol 68, 1896–1901. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA & Esselman PC. (2000). Oxidative capacity and ageing in human muscle. J Physiol 526 Pt 1, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J & Fielding RA. (2004). Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci 59, 1200–1206. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B & Bottinelli R. (2003). The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Carlizzi CN & Bottinelli R. (2007). Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol 100, 603–611. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA & Rice CL. (2012). The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47, 85–92. [DOI] [PubMed] [Google Scholar]
- Debold EP, Fitts RH, Sundberg CW & Nosek TM. (2016). Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc 48, 2270–2280. [DOI] [PubMed] [Google Scholar]
- Delbono O, O’Rourke KS & Ettinger WH. (1995). Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol 148, 211–222. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB & Goodpaster BH. (2009). Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90, 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. (2003). Invited review: Aging and sarcopenia. J Appl Physiol 95, 1717–1727. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ & Roubenoff R. (2000a). Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88, 1321–1326. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. (2003). Strength training in older women: early and late changes in whole muscle and single cells.. Muscle Nerve 27, 601–608. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R & Roubenoff R. (2000b). Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279, C611–618. [DOI] [PubMed] [Google Scholar]
- Gilliver SF, Degens H, Rittweger J, Sargeant AJ & Jones DA. (2009). Variation in the determinants of power of chemically skinned human muscle fibres. Exp Physiol 94, 1070–1078. [DOI] [PubMed] [Google Scholar]
- Godard MP, Gallagher PM, Raue U & Trappe SW. (2002). Alterations in single muscle fiber calcium sensitivity with resistance training in older women. Pflugers Arch 444, 419–425. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M & Newman AB. (2006). The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Gries KJ, Minchev K, Raue U, Grosicki GJ, Begue G, Finch WH, Graham B, Trappe TA & Trappe S. (2019). Single-muscle fiber contractile properties in lifelong aerobic exercising women. J Appl Physiol 127, 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosicki GJ, Gries K, Minchev K, Raue U, Chambers T, Begue G, Finch HW, Graham B, Trappe T & Trappe S. (2021). Single Muscle Fiber Contractile Characteristics with Lifelong Endurance Exercise. J Physiol 599, 3549–3565. [DOI] [PubMed] [Google Scholar]
- Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, Newman AB, Cummings S, Harris T, Kritchevsky S, Goodpaster BH & Trappe S. (2016). Improved single muscle fiber quality in the oldest-old. J Appl Physiol 121, 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA & Trappe S. (2012). Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol 113, 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harridge SD, Kryger A & Stensgaard A. (1999). Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve 22, 831–839. [DOI] [PubMed] [Google Scholar]
- Hepple RT & Rice CL. (2016). Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594, 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW & McClelland GB. (1997). Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol 200, 381–386. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ & Singh MA. (2002). Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76, 473–481. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Pereira HM & Keenan KG. (2016). The aging neuromuscular system and motor performance. J Appl Physiol 121, 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M & Suetta C. (2011). Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol 589, 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid LG, Suetta C, Aagaard P, Kjaer M, Frandsen U & Ortenblad N. (2013). Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp Gerontol 48, 154–161. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM & Ross R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89, 81–88. [DOI] [PubMed] [Google Scholar]
- Jee H & Lim JY. (2016). Discrepancies between Skinned Single Muscle Fibres and Whole Thigh Muscle Function Characteristics in Young and Elderly Human Subjects. Biomed Res Int 2016, 6206959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalakoutis M, Di Giulio I, Douiri A, Ochala J, Harridge SDR & Woledge RC. (2021). Methodological considerations in measuring specific force in human single skinned muscle fibres. Acta Physiol (Oxf) 233, e13719. [DOI] [PubMed] [Google Scholar]
- Karatzaferi C, Franks-Skiba K & Cooke R. (2008). Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294, R948–955. [DOI] [PubMed] [Google Scholar]
- Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC & Bamman MM. (2018). Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57, E52–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Meyerspeer M & Moser E. (2007). Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed 20, 555–565. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV & Young K. (2000). Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88, 662–668. [DOI] [PubMed] [Google Scholar]
- Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipilä S, Mero A, Viitasalo JT, Larsson L & Suominen H. (2006). Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101, 906–917. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R & Frontera WR. (2001). Age- and Gender-Related Differences in Maximum Shortening Velocity of Skeletal Muscle Fibers. Am J Phys Med Rehabil 80, 447–455. [DOI] [PubMed] [Google Scholar]
- Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM & Lamb GD. (2015). Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593, 2499–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Towse TF, Caldwell GE, Wigmore DM & Kent-Braun JA. (2003). Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol 95, 2361–2369. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X & Frontera WR. (1997). Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272, C638–649. [DOI] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R & van Loon LJ. (2013). Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci 68, 769–779. [DOI] [PubMed] [Google Scholar]
- Lexell J (1995). Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No, 11–16. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor C & Sjoström M. (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year old men. J Neurol Sci 84, 275–294. [DOI] [PubMed] [Google Scholar]
- Lim JY, Choi SJ, Widrick JJ, Phillips EM, Frontera WR (2019) Passive force and viscoelastic properties of single fibers in human aging muscles. Eur J Appl Physiol 119, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Lim JY & Frontera WR. (2022). Single skeletal muscle fiber mechanical properties: a muscle quality biomarker of human aging. Eur J Appl Physiol 122, 1383–1395. [DOI] [PubMed] [Google Scholar]
- Mazara N, Zwambag DP, Noonan AM, Weersink E, Brown SHM & Power GA. (2021). Rate of force development is Ca2+-dependent and influenced by Ca2+-sensitivity in human single muscle fibres from older adults. Exp Gerontol 150, 111348. [DOI] [PubMed] [Google Scholar]
- McNamara JW & Sadayappan S. (2018). Skeletal myosin binding protein-C: An increasingly important regulator of striated muscle physiology. Arch Biochem Biophys 660, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter EJ, Conwit R & Tobin J. (1997). Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 52, B267–B276. [DOI] [PubMed] [Google Scholar]
- Metzger JM & Moss RL. (1990a). Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 247, 1088–1090. [DOI] [PubMed] [Google Scholar]
- Metzger JM & Moss RL. (1990b). pH modulation of the kinetics of a Ca2(+)-sensitive cross-bridge state transition in mammalian single skeletal muscle fibres. J Physiol 428, 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Ades PA, Palmer BM & Toth MJ. (2015). Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am J Physiol Cell Physiol 308, C473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM & Toth MJ. (2013). Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol 115, 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS & Toth MJ. (2013). Myofilament protein alterations promote physical disability in aging and disease. Exerc Sport Sci Rev 41, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF & Walston J. (2013). Frailty consensus: a call to action. J Am Med Dir Assoc 14, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier Y, Tiffreau V, Montel V, Bastide B & Stevens L. (2009). Phenotypical transitions and Ca2+ activation properties in human muscle fibers: effects of a 60-day bed rest and countermeasures. J Appl Physiol 106, 1086–1099. [DOI] [PubMed] [Google Scholar]
- Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C & Mann M. (2017). Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep 19, 2396–2409. [DOI] [PubMed] [Google Scholar]
- Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB & van Loon LJ. (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48, 492–498. [DOI] [PubMed] [Google Scholar]
- Ochala J, Frontera WR, Dorer DJ, Van Hoecke J & Krivickas LS. (2007). Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62, 375–381. [DOI] [PubMed] [Google Scholar]
- Power GA, Minozzo FC, Spendiff S, Filion ME, Konokhova Y, Purves-Smith MF, Pion C, Aubertin-Leheudre M, Morais JA, Herzog W, Hepple RT, Taivassalo T & Rassier DE. (2016). Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol 310, C318–327. [DOI] [PubMed] [Google Scholar]
- Raue U, Slivka D, Minchev K & Trappe S. (2009). Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol 106, 1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF & Fielding RA. (2012). Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR & Fielding RA. (2014). Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozand V, Sundberg CW, Hunter SK & Smith AE. (2020). Age-related Deficits in Voluntary Activation: A Systematic Review and Meta-analysis. Med Sci Sports Exerc 52, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Gregg-Cornell K, Conaway MJ & Clark BC. (2012). Evolving concepts on the age-related changes in “muscle quality”. J Cachexia Sarcopenia Muscle 3, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M & Soderlund K. (1998). Energy supply and muscle fatigue in humans. Acta Physiol Scand 162, 261–266. [DOI] [PubMed] [Google Scholar]
- Skelton D, Greig C, Davies J & Young A. (1994). Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23, 371–377. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Kennedy J & Rutherford OM. (2002). Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing 31, 119–125. [DOI] [PubMed] [Google Scholar]
- Soendenbroe C, Dahl CL, Meulengracht C, Tamáš M, Svensson RB, Schjerling P, Kjaer M, Andersen JL & Mackey AL. (2022). Preserved stem cell content and innervation profile of elderly human skeletal muscle with lifelong recreational exercise. J Physiol 600, 1969–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonjak V, Jacob K, Morais JA, Rivera-Zengotita M, Spendiff S, Spake C, Taivassalo T, Chevalier S & Hepple RT. (2019). Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 597, 5009–5023. [DOI] [PubMed] [Google Scholar]
- Straight CR, Ades PA, Toth MJ & Miller MS. (2018). Age-related reduction in single muscle fiber calcium sensitivity is associated with decreased muscle power in men and women. Exp Gerontol 102, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight CR, Voigt TB, Jala AV, Chase JD, Ringham OR, Ades PA, Toth MJ & Miller MS. (2019). Quadriceps Lipid Content Has Sex-Specific Associations With Whole-Muscle, Cellular, and Molecular Contractile Function in Older Adults. J Gerontol A Biol Sci Med Sci 74, 1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW & Fitts RH. (2019). Bioenergetic basis of skeletal muscle fatigue. Curr Opin Physiol 10, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW, Hunter SK, Trappe SW, Smith CS & Fitts RH. (2018). Effects of elevated H(+) and P(i) on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596, 3993–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW, Prost RW, Fitts RH & Hunter SK. (2019). Bioenergetic basis for the increased fatigability with ageing. J Physiol 597, 4943–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teigen LE, Sundberg CW, Kelly LJ, Hunter SK & Fitts RH. (2020). Ca2+ dependency of limb muscle fiber contractile mechanics in young and older adults. Am J Physiol Cell Physiol 318, C1238–c1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom JM, Morse CI, Birch KM & Narici MV. (2007). Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100, 613–619. [DOI] [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J & Trappe T. (2003). Single muscle fibre contractile properties in young and old men and women. J Physiol 552, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenboom R (2016). Modulation of Skeletal Muscle Contraction by Myosin Phosphorylation. Compr Physiol 7, 171–212. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Saggin P, Muti E, Naro F, Cancellara L, Toniolo L, Tarperi C, Calabria E, Richardson RS, Reggiani C & Schena F. (2015). In vivo and in vitro evidence that intrinsic upper- and lower-limb skeletal muscle function is unaffected by ageing and disuse in oldest-old humans. Acta Physiol (Oxf) 215, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F & van Loon LJ. (2014). Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 36, 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD & Katz A. (2010). Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res 316, 3093–3099. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL & Fitts RH. (1998). Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol 85, 1949–1956. [DOI] [PubMed] [Google Scholar]
- Yu F, Hedström M, Cristea A, Dalén N & Larsson L. (2007). Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 190, 229–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


