Abstract
Background:
Deficits in executive functions (EFs), cognitive processes that control goal-directed behaviors, are associated with psychopathology and neurological disorders. Little is known about the molecular bases of EF individual differences. Prior candidate gene studies have been underpowered in their search for dopaminergic processes involved in cognitive functioning, and existing EF genome-wide association studies (GWASs) used small sample sizes and/or focused on individual tasks that are imprecise measures of EF.
Methods:
We conducted a GWAS of a Common EF (cEF) factor score based on multiple tasks in the UK Biobank (N=427,037 European-descent individuals).
Results:
We found 129 independent genome-wide significant lead variants in 112 distinct loci and that cEF was associated with fast synaptic transmission processes (synaptic, potassium channel, and GABA pathways) in gene-based analyses. cEF was genetically correlated with measures of intelligence (IQ) and cognitive processing speed, but cEF and IQ showed differential genetic associations with psychiatric disorders and educational attainment.
Conclusions:
Results suggest that cEF is a genetically distinct cognitive construct that is particularly relevant to understanding the genetic variance in psychiatric disorders.
Keywords: Cognitive control, Executive functioning, Genetic correlations, Genome-wide association analysis, Latent variable measurement, Neurocognitive functioning
Deficits in executive functions (EFs), cognitive control processes that regulate thoughts and actions during goal-directed behavior (1), characterize many brain disorders. They are associated with almost all psychiatric disorders, leading some to suggest that EF deficits are a transdiagnostic risk factor for psychopathology (2–5). Recent work using single nucleotide polymorphism (SNP) effects from large genome-wide associations studies (GWASs) to estimate genetic correlations suggests that cognition–psychopathology associations may be partially genetic in origin (6–8). These studies have primarily focused on general cognitive ability (g) or intelligence quotient (IQ), the cognitive construct with the largest GWAS sample sizes. However, adult phenotypic and twin studies suggest that a Common EF (cEF) factor capturing variance shared across diverse EF tasks is distinguishable from IQ at the phenotypic and genetic levels, and predicts behavior over and above IQ (1,9,10). Here, we conduct a GWAS of a cEF factor score generated from UK Biobank (UKB) data (11) to discover cEF’s molecular underpinnings. We then test the hypotheses that cEF is genetically separable from IQ and cognitive processing speed and is the cognitive dimension most relevant for understanding genetic variation underlying psychopathology.
EFs are a family of cognitive functions (12) that include response inhibition, interference control, working memory updating and capacity, and mental set shifting (1). Because EFs are control processes, EF tasks involve processes that are being controlled (e.g., visual processing) in addition to the control processes of interest (e.g., biasing attention towards task-relevant information) (13). These non-control processes contribute to individual differences in performance on specific tasks, leading to the “task impurity problem” (13). Thus, GWAS loci and molecular processes associated with individual EF tasks may capture cognitive processes other than EF. Individual EF tasks can also show low reliability (13), decreasing power for association tests. The task impurity and reliability problems can be reduced by extracting common variance across multiple EF tasks with a cEF factor (9,14,15).
Five independent twin studies showed that across samples and ages, cEF is moderately to highly heritable (14–17) (46%-100%) and highly phenotypically and genetically stable across time (10,18). However, little is known about cEF’s molecular underpinnings. Most historical perspectives from the candidate gene (19) and animal (20) literatures argued that neurocognitive function is supported by metabotropic processes, particularly the slow neuromodulator effects of the dopaminergic systems, although candidate gene associations often fail to replicate in large well-powered GWAS (21). Work in humans and monkeys suggests that fast ionotropic processes influence EF, particularly the excitatory neurotransmitter glutamate (via activation of Anti-N-methyl-D-aspartate [NMDA] receptors) (22). Fast inhibitory GABAergic processes have also been studied in relation to EFs, particularly tasks that require response inhibition, interference control, and selection (23). Existing GWASs of EF have had insufficient power to test hypotheses regarding these molecular mechanisms. To date, the largest GWASs of EFs and processing speed (24,25) focused on individual neurocognitive tasks (Ns=1,311-32,070) and collectively identified only two genome-wide significant variants.
In contrast, GWASs of IQ have reached large sample sizes and yielded numerous associations (6–8). These associations may improve understanding of cEF, which correlates moderately with IQ (9,26); however, cEF and IQ are not genetically identical, at least in adults. In young adult and middle-aged twin samples (9,14), cEF’s phenotypic and genetic correlations with IQ are moderate (rs=.53–.68; rGs=.57–.59) and significantly lower than 1.0. Importantly, IQ genetically correlates with variance specific to working memory processes in addition to cEF (9,14), suggesting that IQ variation is supported by both cEF and working memory-specific abilities in adults. Phenotypic literature also suggests that EFs show discriminant predictive validity of behavioral problems when controlling for IQ (27). Genetic correlations derived from GWAS provide an opportunity to evaluate whether cEF may capture distinct genetic variance from IQ and show stronger relations to psychopathology.
Here, we report a GWAS of a phenotypic cEF factor score based on the commonality of five EF tasks assessed at multiple occasions in the UKB (N=427,037). We also conducted GWAS of factor scores for IQ (verbal-numerical reasoning; N=216,381) and cognitive processing speed (N=432,297) for comparison. We validated the factors by demonstrating that polygenic scores (PGSs) for cEF and IQ based on these GWASs differentially predicted multiple EF latent variables and IQ in deeply phenotyped young adult samples. We hypothesized that the genetic correlation of cEF with IQ would be substantial but significantly less than 1.0, and that cEF would be genetically associated with psychopathology when controlling for IQ and speed.
Methods
Participants
Participants were 501,826 individuals in the UK Biobank (UKB) study (11,28) who had completed at least one cognitive assessment at the time that the data were released to us (Supplementary Table S1). We restricted genetic analyses to 427,037 individuals of European ancestry as determined by principle components (PC) analysis (mean age=56.849, SD=8.009, 54% female) whose genotypes were imputed to the Haplotype Reference Consortium (29), 1000 Genomes, and UK10K reference panels by the UKB (28).
Measures
The cognitive measures (Figure 1 and Supplementary Methods) included one classic neuropsychological EF task, the trail-making task, and four other cognitive tasks, symbol-digit substitution, backward digit span, prospective memory, and pairs matching. A validation study of the UKB cognitive measures (30) showed that these tasks correlate with reference measures in ways that suggest they include executive components, as discussed in detail in the Supplementary Methods. We reasoned that a common factor extracting shared variance across these tasks and the trail-making task would be similar to the Common EF factors examined in smaller studies (10,15,16,18). We also included measures of IQ (the fluid intelligence/reasoning test) and speed (the “Snap” game reaction time [RT]).
Figure 1. Descriptions of cognitive measures used to obtain factor scores.
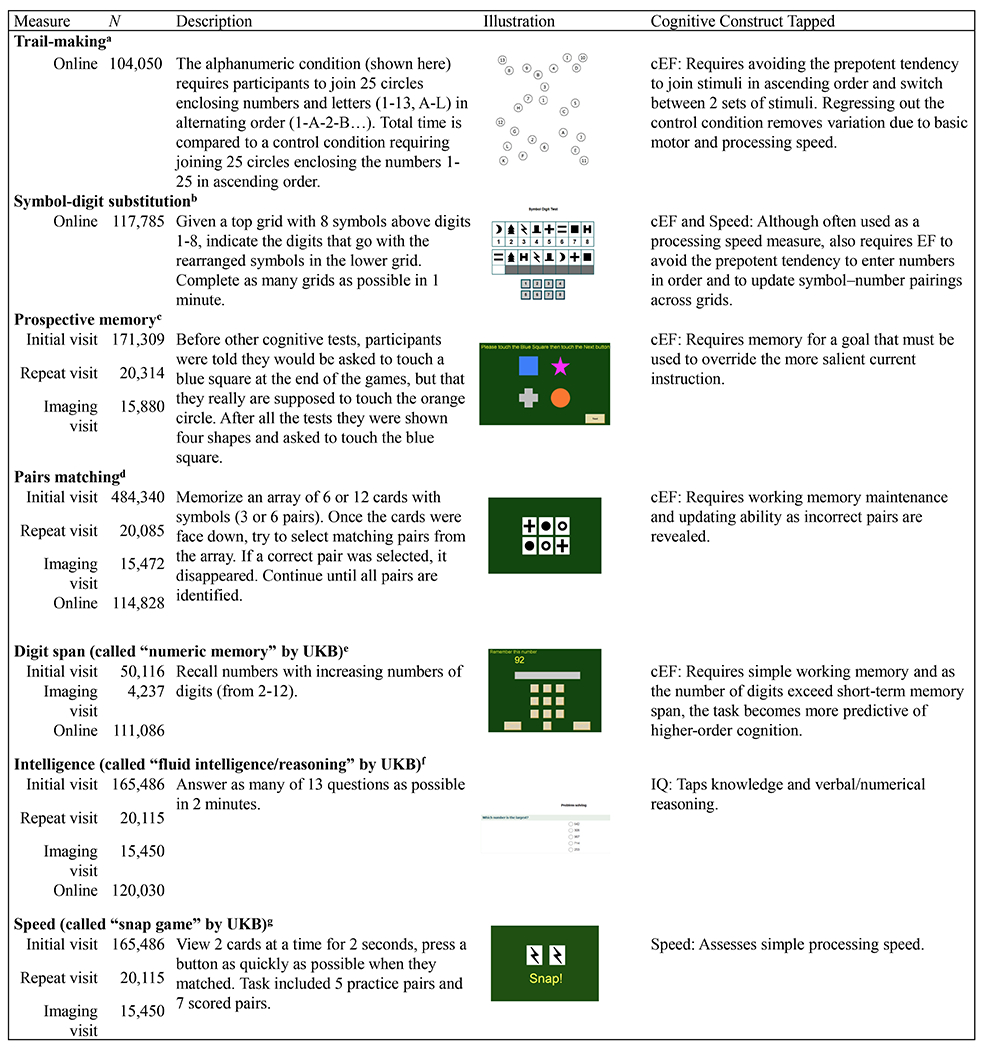
See Supplementary Methods for additional details and Supplementary Table S1 for descriptive statistics. cEF = common executive functioning; UKB = UK Biobank; IQ = intelligence. aDependent measure was the unstandardized residual of the log10-transformed time in seconds to correctly complete the alphanumeric set after regressing out the log10-transformed numeric path time; bDependent measure was the number of symbol-digit matches made correctly in 1 min; cDependent measure was a categorical variable coded as 1 for correct and 0 for incorrect on first try; dDependent measure was the sum of the log10-transformed number of incorrect matches +1 in the 6- and 12-card rounds; eDependent measure was the maximum number of digits remembered correctly; fDependent measure was the number of correct answers; gDependent measure was the log10-transformed mean time in ms to correctly identify matches across 7 pairs, excluding pairs with times < 50ms (anticipatory responses) and >2,000ms (responses that occurred after cards had disappeared). Scores were reversed in models so higher numbers indicated faster speed.
Analysis Procedures
Factor scores.
We focused on a phenotypic cEF factor score, which was possible because the tasks used in our model were all from the same UKB sample. Using a phenotypic factor score (vs. a genetic factor with GenomicSEM) has the advantages that 1) its interpretation is consistent with similar factors estimated in the phenotypic literature; and 2) the result is a score that we can return to UKB for use in other phenotypic and genetic studies. Figure 2A presents the correlations among the cognitive measures (see Table 1 for genetic correlations). We used Mplus for the confirmatory factor analysis used to obtain the cEF scores (Figure 2B). Model fit was good, CFI=.980, RMSEA=.009 (see Supplementary Table S2 for fit statistics of all structural equation models). We also calculated IQ and Speed factor scores using relevant measures from UKB (see Supplementary Methods).
Figure 2. Development of a Common Executive Functioning (cEF) factor across cognitive tasks in the UK Biobank.
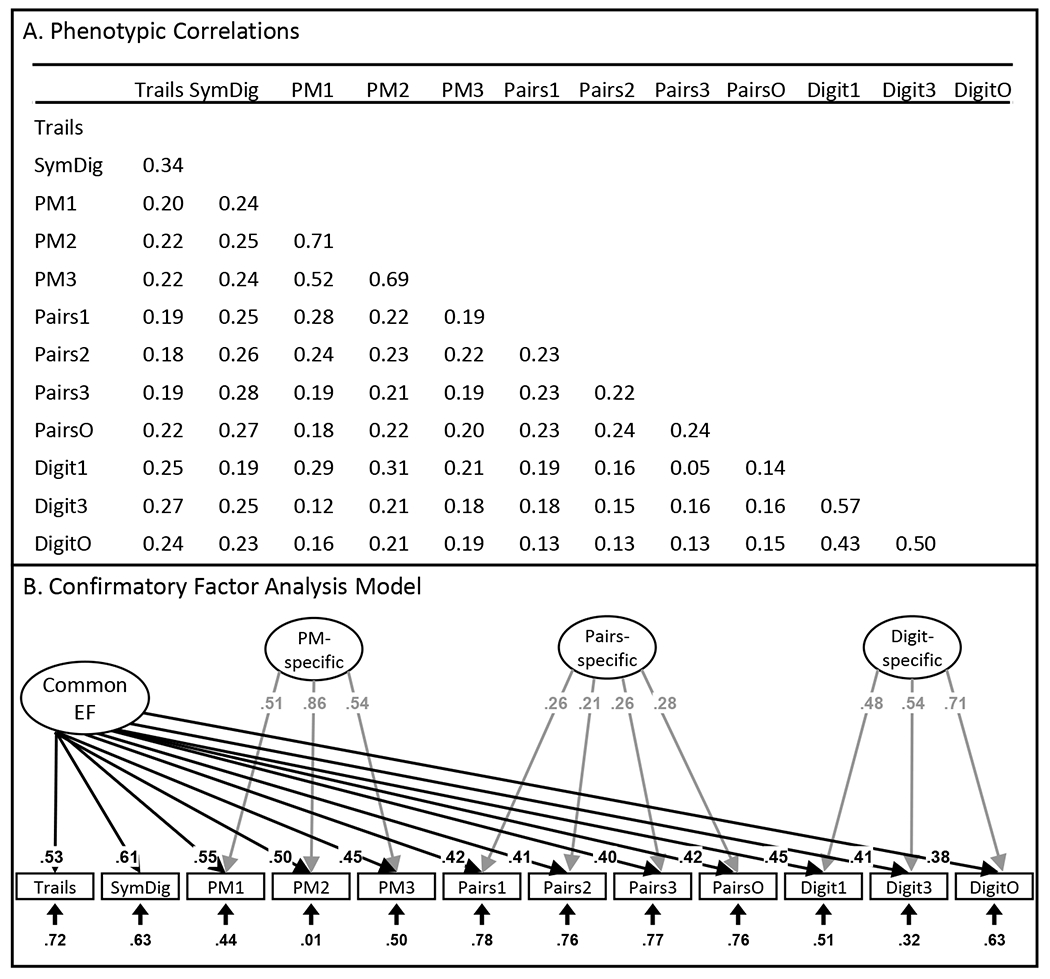
(a) Correlations taken from Mplus; (b) Confirmatory factor analysis model used to extract factor scores. Ellipses indicate latent variables; rectangles indicate observed variables. Numbers on arrows are standardized factor loadings, and numbers at the end of arrows are residual variances. All parameters were statistically significant (p<.05). Trails= trail-making (online); SymDig= symbol-digit substitution (online); PM=prospective memory; Pairs=pairs matching; Digit=digit span. Task names with 1=first assessment; with 2=repeat assessment; with 3=imaging visit assessment; with O=online follow-up. Directionality was reversed for some variables so that for all variables, higher scores indicate better performance.
Table 1.
Heritability (diagonal) and genetic correlations (off-diagonal) between Common Executive Functioning (cEF) indicators and cEF factor scores
| Measure | Symbol Digit | Pairs Matching | Digit Span | Prospect. Memory | Trail-making | Dense cEF | Sparse cEF | Full cEF |
|---|---|---|---|---|---|---|---|---|
| Symbol–Digit | 0.1245 (0.0079) | |||||||
| Pairs Matching | 0.6603 (0.0271) | 0.0713 (0.003) | ||||||
| Digit Span | 0.3226 (0.0345) | 0.4420 (0.0263) | 0.1337 (0.0069) | |||||
| Prospective Memory | 0.4479 (0.0414) | 0.5982 (0.0348) | 0.4539 (0.0355) | 0.0527 (0.0039) | ||||
| Trail-making | 0.7126 (0.0322) | 0.7085 (0.0317) | 0.6530 (0.0293) | 0.5927 (0.0463) | 0.1136 (0.0084) | |||
| Dense sample cEF | 0.8428 (0.0138) | 0.8580 (0.0207) | 0.6653 (0.0214) | 0.6416 (0.0365) | 0.9274 (0.0133) | 0.1894 (0.0105) | ||
| Sparse sample cEF | 0.7031 (0.0307) | 0.9831 (0.0074) | 0.5580 (0.0259) | 0.7052 (0.0308) | 0.7771 (0.0381) | 0.9230 (0.0286) | 0.0696 (0.0038) | |
| Full sample cEF | 0.7683 (0.0178) | 0.9527 (0.0047) | 0.6164 (0.0178) | 0.7046 (0.0255) | 0.8452 (0.0215) | 0.9629 (0.0106) | 0.9892 (0.0073) | 0.0906 (0.0038) |
Note. The heritability of each measure (standard error) is shown on the diagonal in bold-face type. The lower diagonal contains the genetic correlations of each indicator and common executive functioning (cEF) factor scores in the densely phenotyped (dense), sparsely phenotyped (sparse), and full samples, as estimated by linkage-disequilibrium score regression. When there were multiple assessments of the same task (pairs matching, digit span), the measure is the average of the z-scores for all assessments, except for the categorical prospective memory task, for which the measure used for this table is the first assessment.
Genetic analyses.
We followed the same procedure for GWASs of the cEF, Speed, and IQ factor scores. We ran a test of association using BOLT-LMM (31), controlling for age, age2, sex, first 10 European principal components (PCs), first 10 global PCs, batch, and site. We tested consistency of the cEF results by conducting GWASs in two UKB subsamples: The “densely” assessed sample (n=93,024) completed at least the trail-making task, a classic neuropsychological EF task that has been used to tap cEF factors in prior studies (10,15). The “sparsely” phenotyped sample consisted of the remaining individuals who completed at least one neurocognitive task and were unrelated to people in the densely phenotyped sample (n=256,135).
Genome-wide results were entered in the Functional Mapping and Annotation (FUMA)/Multi-marker Analysis of GenoMic Annotation (MAGMA) (32) pipeline (33), linkage disequilibrium score (LDSC) regression (34), and PrediXcan (35) to characterize the results. We calculated genetic correlations of cEF with psychiatric, personality, neurological, and health-related outcomes via LD Hub (36) with the GWAS summary statistics from the full sample.
We used multi-trait-based conditional & joint analysis (mtCOJO, within the GCTA-GSMR family of methods) (37) using GWAS summary data to discover SNP effects that were related to cEF above and beyond IQ and vice versa (per SNP). We then ran the same FUMA/MAGMA pipeline on the resulting summary statistics to discover what biological pathways remain after accounting for the other cognitive ability.
We used GenomicSEM (38) to evaluate genetic multiple regression models using our factor scores to predict individual outcomes and psychopathology factors. We also used GenomicSEM to run a CFA and then GWAS using the individual cEF task GWAS summary statistics then evaluated the similarity to our cEF factor GWAS by assessing the genetic correlation, overlapping genome-wide signal, and consistency of SNP effects. We did not compute a GWAS for IQ or Speed in GenomicSEM, given that they included fewer indicators and are not the primary focus of this study.
PGS analyses.
We used the UKB GWAS summary statistics to calculate PGSs for cEF and IQ in two twin samples (39) (see Supplementary Methods). PGSs were generated with summary best linear unbiased predictor (SBLUP) analyses, using all SNPs with the --score function in PLINK (40) (version 1.90b4.4).
Results
GWAS of cEF Factor Score
We found 129 lead (r2<0.1) and 299 independent (r2<0.6) SNPs in 112 distinct loci that were significantly associated with cEF in the full sample (Figure 3, Supplementary Figures S1–S2, and Supplementary Tables S3–S9). The SNP with the lowest p-value (rs12707117, β= −0.012, p=2.1e-26) is an expression quantitative trait locus (eQTL) in cerebellar tissue mapped to EXOC4. Q-Q plots (Supplementary Figure S1) showed departure from expected p-values under the null hypothesis for the full sample and subsamples (λfull=1.6946, λdense=1.311, λsparse=1.3101), but the low LDSC intercepts (full=1.0381, dense=1.0128, sparse=1.0238) suggest that this inflation reflects polygenicity rather than confounding stratification.
Figure 3. Manhattan plots for GWAS of Common Executive Functioning (cEF) factor score.
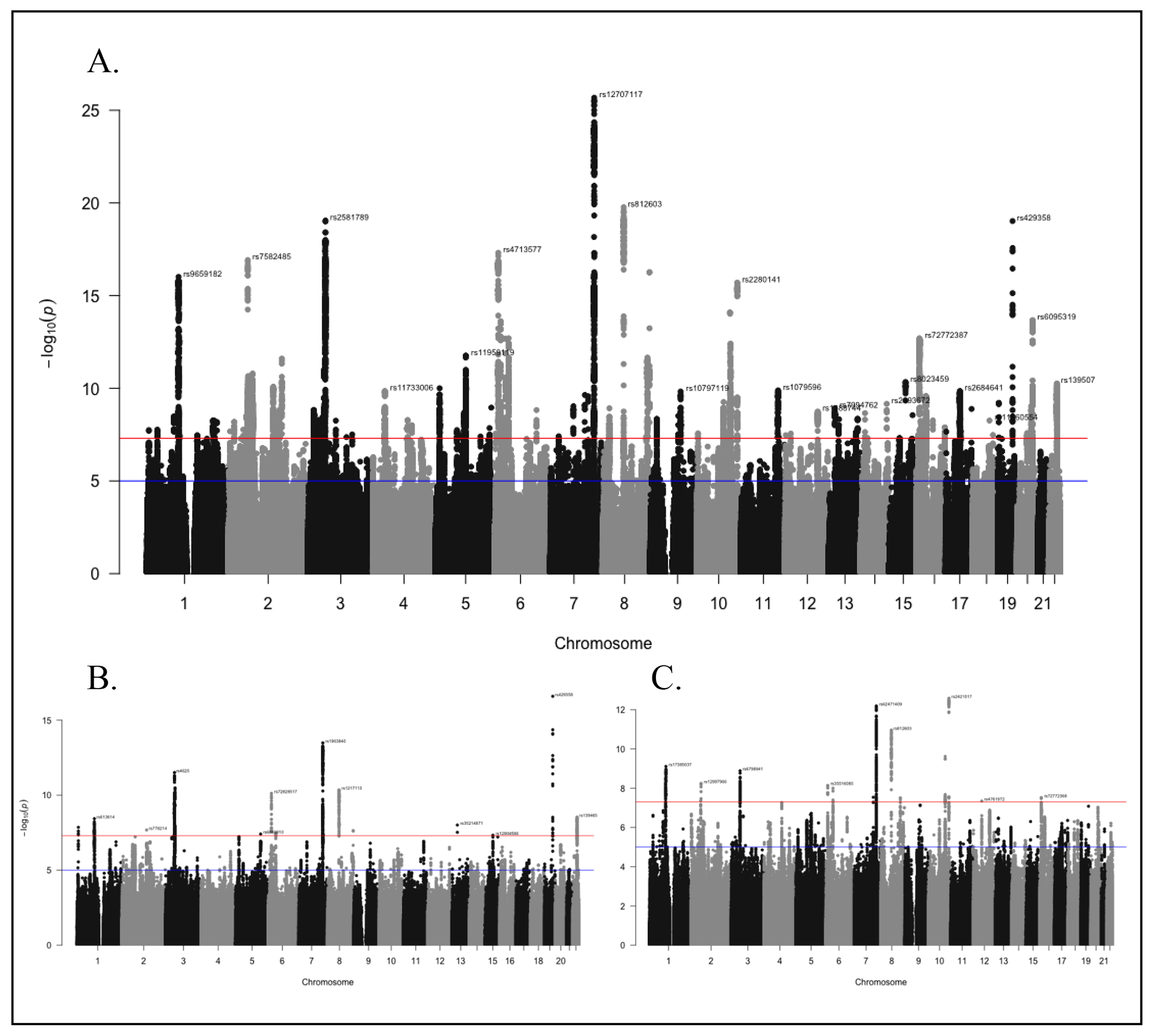
(a) Results in the full sample, (b) results in the densely phenotyped sample, and (c) results in the sparsely phenotyped sample. Each dot is a single nucleotide polymorphism (SNP), chromosomes are organized on the x-axis, and the y-axis represents the negative log10 of the p-value for each SNP.
SNP-heritability (SNP-h2) of cEF estimated via BOLT-REML was 0.104 (se=0.002) and via LDSC was 0.091 (se=0.0038) (see Table 1). Although the LDSC SNP-h2 for the densely and sparsely phenotyped subsamples differed as expected (see Supplementary Methods), their genetic correlation (rG=0.923) confirmed they measured substantially overlapping constructs (see Supplementary Results for further comparisons). Therefore, the following analyses use the full sample.
Comparison to GenomicSEM Analysis
To evaluate the similarity of our results across methods, we used GenomicSEM to run a genetic confirmatory factor analysis and GWAS using the five individual cEF task GWAS summary statistics (see Supplementary Results). The genetic correlation between the factor score cEF and GenomicSEM cEF was 0.996 (se=0.0009), suggesting very high overlap in the genetic signal across the two approaches.
Of the 299 independent (r2<0.6) genome-wide significant SNPs for our phenotypic factor score, only 3 showed evidence that they were not mediated by the GenomicSEM cEF factor (i.e., they were task-specific variants; see Supplementary Results). These results confirm that our GWAS of the phenotypic factor score is appropriate, thus, we only present the results of our cEF phenotypic factor GWAS.
Genetic Separability of cEF and IQ
cEF factor scores phenotypically correlated with IQ factor scores (r=0.35, p<.001) and Speed factor scores (r=0.28, p<.001). IQ and Speed factor scores weakly correlated with each other (r=0.17, p<.001), demonstrating divergence at the phenotypic level. SNP-h2 estimated via BOLT-REML for IQ was .242 (se=0.003), and for Speed was 0.094 (se=0.002). LDSC correlations indicated that the IQ factor scores were highly genetically correlated with IQ measures used in prior GWAS (7,6): rG=0.9639 (se=.0046) to rG=.9817 (se=.0043).
Genetic correlation.
The LDSC genetic correlation between cEF and IQ was 0.743 (se=0.013, p=1.00e-221), which was significantly lower than 1.0 (p=1.4e-59). Similarly, the BOLT-REML genetic correlation was 0.766 (se=0.007), p<1e-300); the 95% confidence interval (0.752 to 0.778) did not include 1.0. These SNP-based genetic correlations reflect the genetic separability of cEF and IQ and are similar to those from twin-based rG estimates of IQ and cEF (rG=0.69) for this age range (14).
GWAS of cEF conditioned on IQ.
Due to the moderate to high genetic correlation for cEF and IQ, we anticipated that statistical power would be lower for conditional GWAS from mt-COJO. Consistent with this expectation, we identified 41 lead SNPs significantly associated with cEF when conditioned on IQ (Supplementary Figure S3, Supplementary Table S10). Notably, the EXOC4 variant remained significantly associated with cEF, as did APOE. We identified 17 lead SNPs significantly associated with IQ, conditioning on cEF (Supplementary Table S11). These results indicate that there are specific genetic effects for cEF and IQ.
PGS analyses.
We created PGSs of cEF and IQ in two young adult twin samples that were deeply phenotyped on multiple EF latent variables (cEF, Updating-specific, and Shifting-specific factors) and full-scale IQ. To maximize power and minimize the number of tests, we created the model shown in Figure 4, which integrates full-scale IQ data and the multiple waves of EF data in line with our previously published twin models of these data (16,18). We restricted the PGS analysis to individuals of European ancestry (based the first 3 PCs), resulting in a final N of 916 (Supplementary Table S12 provides results for less conservative ancestry restrictions).
Figure 4. Analysis model of polygenic scores (PGSs) predicting executive functioning (EF) latent variables and full-scale intelligence scores (IQ) in Colorado twin data.
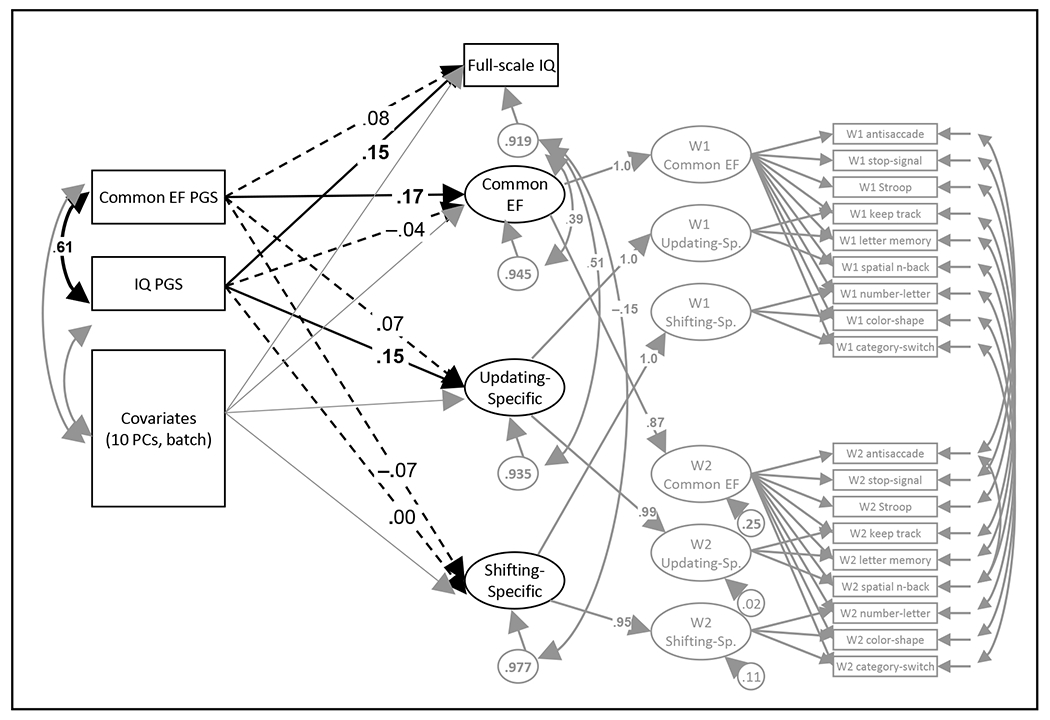
Paths of primary interest are shown in black with thicker lines. Solid lines and boldface type indicate p<.05; dashed lines indicate p>.05. Analyses were limited to twins with European ancestry based on the first three principal components (N=916 with genetic data). The three EF latent variables were based on 9 laboratory tasks at Wave 1 (W1; Longitudinal Twin Study [LTS] age 17 n=571, Community Twin Sample [CTS] age 21 n=298), and on 9 tasks at Wave 2 (W2; LTS only at age 23, n=555). Full-scale IQ was based on 11 Wechsler Adult Intelligence Scale subtests in the LTS (age 16, n=584), and 4 Wechsler Abbreviated Scale of Intelligence subtests in the CTS (age 21, n=297). Age, sex, and age*sex were regressed out of each measure within each sample and wave prior to analysis.
Controlling for its shared variance with the IQ PGS (r=0.607, se=0.027), the cEF PGS predicted the cEF latent variable (standardized β=0.171, p=.014, partial r=.136), but not the Updating-specific and Shifting-specific factors or full-scale IQ (βs= −0.068-0.078, ps>.101, partial rs= −0.053-.050). The standardized beta for predicting the cEF latent variable in the twin samples was similar to those we found for predicting the cEF factor scores across the independent UKB subsamples (βs=0.095-0.145; see Supplementary Materials). Thus, the cEF PGS shows a similar association with the deeply phenotyped cEF latent factor as it does with the UKB cEF factor score from which it was derived, supporting the conclusion that they tap similar constructs.
Conversely, controlling for its shared variance with the cEF PGS, the IQ PGS predicted full-scale IQ (β=0.149, p=.003, partial r=0.121) as well as the Updating-specific latent variable (β=0.147, p=.046, partial r=0.119), but not the cEF or Shifting-specific latent variables (βs= −0.037-0.003, ps>.591, partial rs= −0.030-0.007). The association of the IQ PGS with the Updating-specific factor is consistent with prior adult twin studies showing that IQ is genetically related to working-memory-specific variables over and above its association with cEF (9,14). These results further support the conclusion that the cEF and IQ factors in UKB are tapping similar constructs as those assessed in these carefully phenotyped young adult twin samples.
Genetic Separability of cEF and IQ is Key for Psychiatric Dysfunction
LDSC correlations using published GWAS summary statistics indicated that the cEF factor score significantly negatively genetically correlated (with Bonferroni correction α=.0012 for 41 traits) with all psychiatric disorders except autism spectrum disorder, anxiety, and obsessive-compulsive disorder (Figure 5A, Supplementary Table S13). 95% confidence intervals of cEF and IQ rG did not overlap for 5 of the 11 psychiatric traits, but did overlap for neuropsychiatric symptoms, personality, sleep, biometric traits, and most substance use measures.
Figure 5. Genetic associations of Common Executive Functioning (cEF) and Intelligence (IQ) factor scores in the UK Biobank (UKB) with psychiatric, behavioral, and health traits.
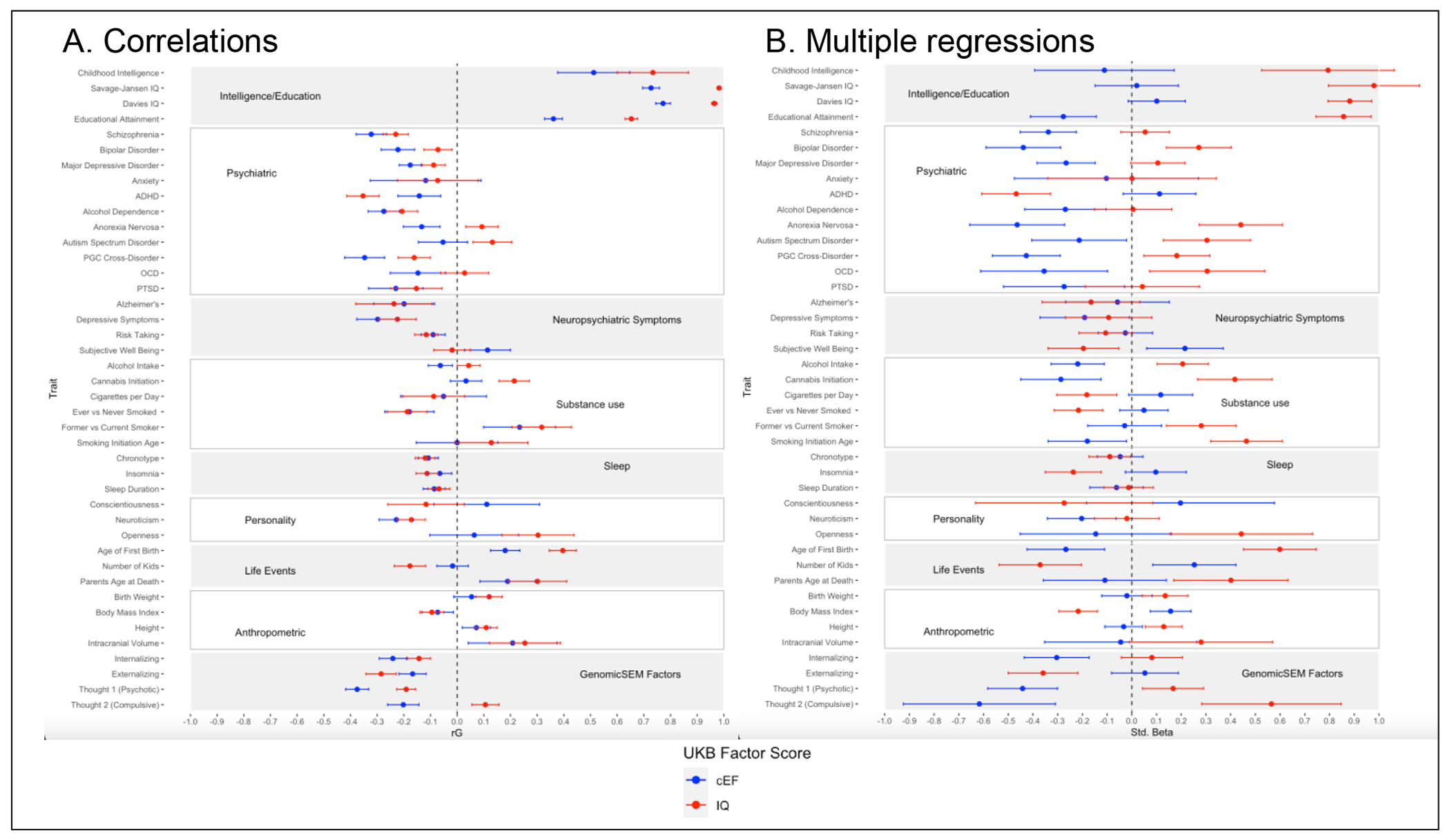
(a) Genetic correlations, estimated with linkage-disequilibrium score regression; (b) standardized partial regression coefficients from genomic structural equation models for cEF controlling for the genetics of IQ and Speed, and for IQ controlling for the genetics of cEF and Speed. Bars indicate 95% confidence intervals.
Multiple regressions using GenomicSEM (Figure 5B, Supplementary Table S14) indicated that after controlling for Speed and IQ, cEF remained significantly negatively associated with most psychopathologies, except ADHD, but was no longer positively associated with educational attainment. After controlling for Speed and cEF, IQ had a significant negative association only with ADHD and had positive associations with anorexia nervosa, autism spectrum disorder, bipolar disorder, obsessive-compulsive disorder, and PGC cross-disorder. Together, these results suggest that the genes specific to cEF and those specific to IQ have different influences on the pathogenesis of psychiatric traits.
To formally test the hypothesis that common psychiatric disorders are more genetically related to cEF than IQ, we estimated a GenomicSEM model in which the cEF, IQ, and Speed factor scores predicted four genetic psychopathology factors: Internalizing, Externalizing, and two Thought Disorder factors (Psychosis and Compulsive Disorders; Figure 6A, χ2(59)=397.333, CFI=0.955, SRMR=0.077); see Supplementary Methods for details on this model. cEF was significantly negatively associated with the Internalizing and both Psychosis and Compulsive Thought Disorder factors (βs= −0.304 to −0.617), but not the Externalizing factor (β=0.053), controlling for IQ and Speed. In contrast, controlling for cEF and Speed, IQ was significantly negatively related to the Externalizing factor (β= −0.359), but was not significantly associated with the Internalizing factor (β=0.081) and was positively related to both Psychosis and Compulsive Thought Disorder factors (βs=0.167-0.565).
Figure 6. Genomic structural equation models (GenomicSEMs).
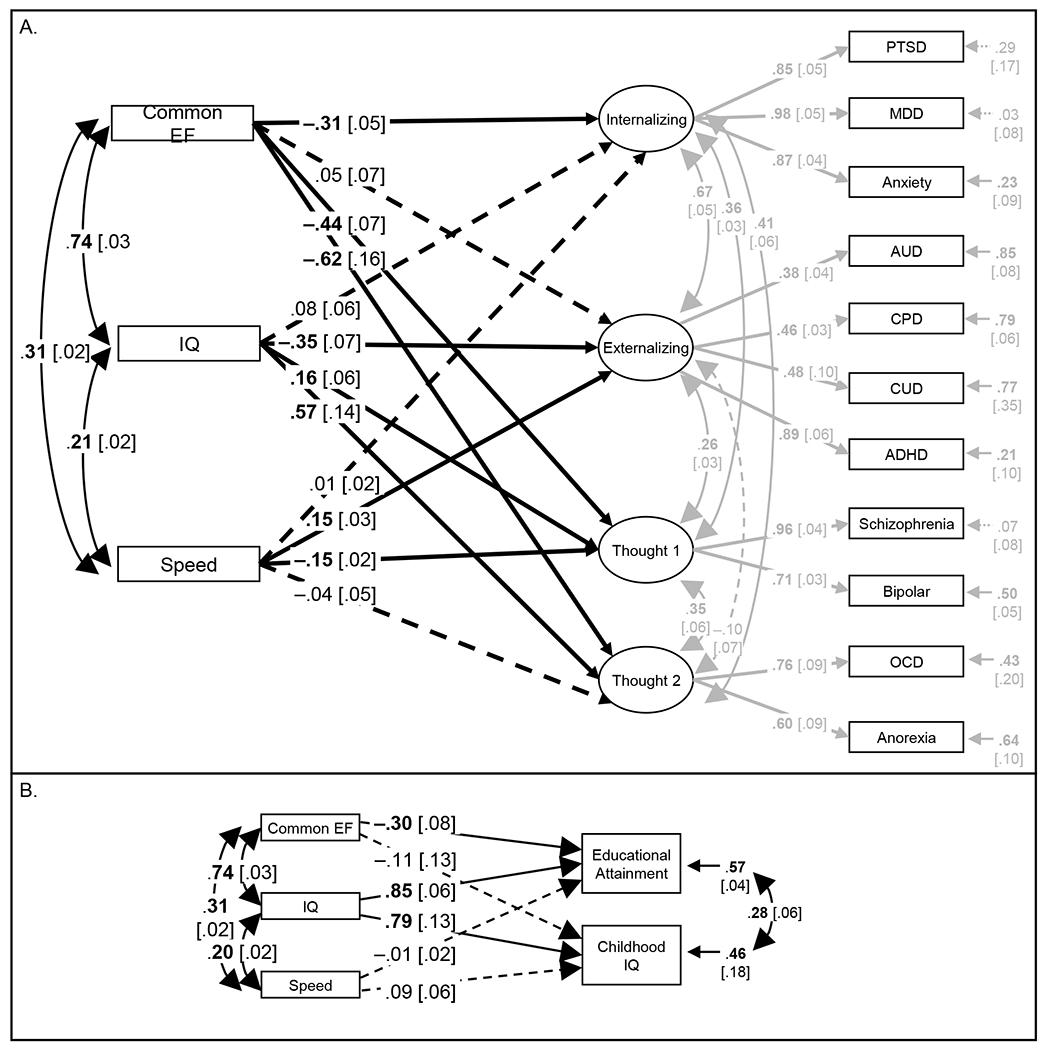
(a) Common Executive Functioning (cEF), Intelligence (IQ), and Speed factor scores predict four correlated psychopathology factors; (b) cEF, IQ, and Speed factor scores predict IQ-related traits. Ellipses indicate latent variables; rectangles indicate observed variables. PTSD=post-traumatic stress disorder MDD=major depressive disorder; AUD=alcohol use disorder; CPD=cigarettes per day; CUD=cannabis use disorder; ADHD=attention-deficit/hyperactivity disorder; Bipolar=bipolar disorder; OCD=obsessive-compulsive disorder; Anorexia=anorexia nervosa. Numbers on single-headed arrows are fully standardized factor loadings or regression coefficients, numbers on curved double-headed arrows are correlations, and numbers at the ends of arrows are residual variances. Boldface type and solid lines indicate p<.05; dashed lines indicate p>.05.
Figure 6B highlights results for traits that show the opposite pattern (individual models also shown in Figure 5 and Supplementary Table S14): IQ was significantly positively related to educational attainment and childhood IQ, controlling for cEF and Speed (βs=0.79 to 0.86, p<7.4e-9); there was a weaker (educational attainment β= −0.27, p=3.0e-4) or a null (childhood IQ β= −0.11, p=.411) association with cEF controlling for IQ and Speed. Interestingly, the cEF genetic association with educational attainment changed from positive to significantly negative after controlling for IQ. cEF showed negative genetic correlations with several disorders that are positively genetically correlated with IQ and educational attainment, such as anorexia nervosa, autism spectrum disorder, and bipolar disorder (37); it may be that the genetic variance unique to lower cEF reflects part of this genetic risk for these disorders that is positively associated with education, leading to this negative partial genetic correlation with higher cEF.
Genetic Associations with cEF Implicate GABAergic and Synaptic Molecular Pathways
In MAGMA we identified 319 genes significantly associated with cEF in the full sample (Bonferroni α= 0.05/18597=2.689e-6), 21 of which were consistent across the smaller densely and sparsely phenotyped subsamples. The strongest association again was EXOC4 (Supplementary Figure S4, Supplementary Table S16).
Using gene-set analyses of this gene list, we found 12 associated gene sets (post-Bonferroni correction), all of which could be summarized under three broad pathways: potassium channel activity, synaptic structure, or GABA receptor activity (Figure 7A, Supplementary Table S17). Suggestive associations of additional pathways (corrected p<0.1), also implicated synaptic, potassium channel, and ionotropic pathways. To account for some genes appearing in multiple associated pathways, we conducted a conditional gene-set analysis accounting for overlap in genes amongst the top pathways (41), excluding the Gene Ontology (GO) (42,43) terms “synapse,” “GABAA gene,” and “voltage-gated potassium channel” pathways due to multicollinearity. Results indicated that the GO “GABA receptor complex” and “regulation of synapse structure or activity” pathways were associated with cEF over and above other discovered pathways. See the Supplementary Results for genetic pathway analyses of the cEF mt-COJO GWAS and gene expression analyses.
Figure 7. Associated gene-set categories from MAGMA gene-set analysis.
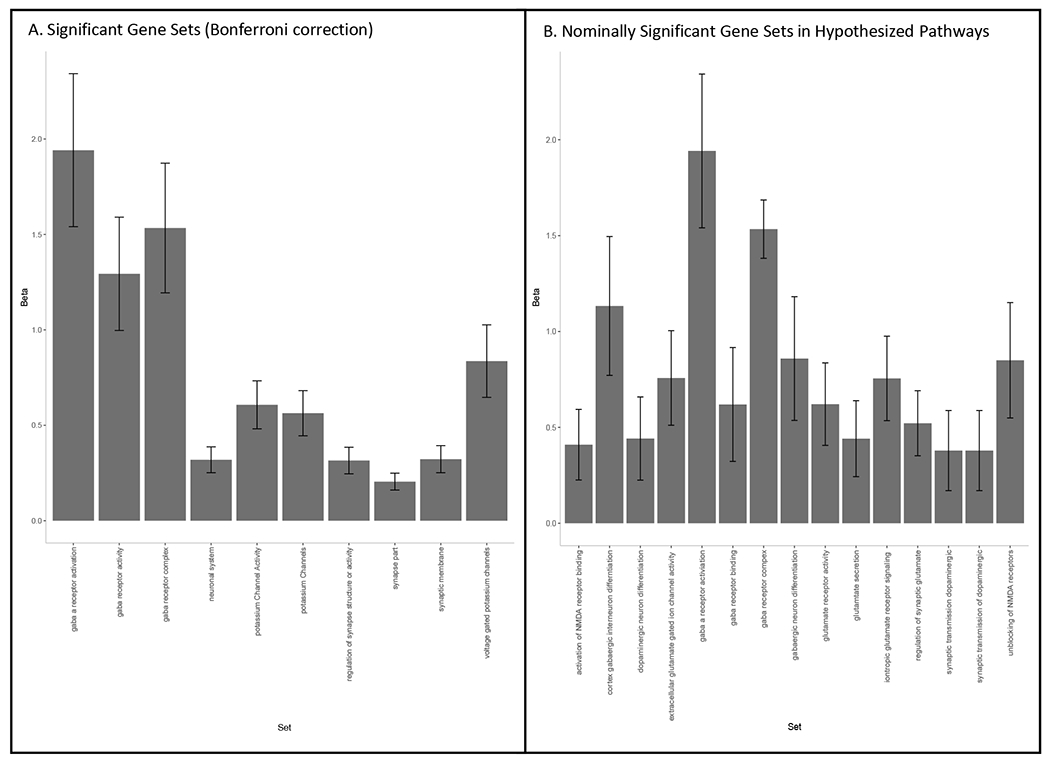
Signal GO term and curated gene set enrichment for SNPs influencing Common Executive Functioning (cEF) factor score as the MAGMA gene enrichment beta and standard error. (a) Gene-sets significantly associated after Bonferroni correction for 10,651 tests (α = 4.7E-06); (b) Gene-sets in hypothesized pathways that were nominally significant. VG = voltage-gated. Bars indicate standard errors.
Genetic Associations with cEF Do Not Strongly Implicate Dopaminergic Pathways or Replicate Candidate Genes
Test of hypothesized/popular pathways.
While there were 10 nominally significant pathways from a priori hypothesized categories (dopaminergic, glutaminergic, and GABA pathways), the effect sizes were highest for GABA (Figure 7B). For glutaminergic pathways, the strongest association was NMDA receptor activation, which is previously supported (22). Dopaminergic genes showed the weakest evidence for association among pre-hypothesized pathways.
Candidate gene analysis.
We found little evidence that the most popular candidate gene polymorphisms (19) were related to cEF at levels above chance. COMT val/met (rs4680), the most-studied candidate gene polymorphism for cEF, was not significant at the genome-wide level (β= −.002, p=.021). Previously studied polymorphisms of DRD2 (rs1079596: β=0.010, p=1.3e-10; rs2075654: β=0.010, p=1.4e-100) were genome-wide significant; however, the effects sizes are much smaller than previously reported (44). Using MAGMA to determine the degree of association of historical cEF candidate genes themselves as opposed to the most-studied specific polymorphisms within them (19), only DRD2 was associated with cEF (p=1.15E-12, all gene-wise summary statistics available in Supplementary Table S16).
Discussion
We conducted a GWAS of a cEF factor score in the UKB that minimized the task impurity problem and incorporated existing knowledge of the factor structure of EFs. Our results suggest that genetic influences on cEF involve variation within fast ionotropic and synaptic pathways, in particular GABAergic pathways, rather than the commonly studied metabotropic and dopaminergic pathways. We demonstrated cEF’s genetic overlap with IQ but found important differences between them, as shown through differential associations with education and psychiatric disorders.
In line with twin literature (9,14), this study supports the importance of cEF as a cognitive dimension that is partially genetically related to IQ and Speed in adulthood. Although there was a high genetic correlation between cEF and IQ (LDSC rG=.743), this correlation was significantly lower than 1.0, indicating some specific variance. This separability has important implications for understanding cognitive aspects of psychopathology. Controlling for IQ and Speed, cEF remained significantly negatively genetically associated with the Internalizing Disorder and Compulsive and Psychotic Thought Disorder factors, whereas IQ did not. Contrastingly, controlling for their genetic overlap, IQ remained strongly positively associated with education and childhood IQ, while cEF did not.
Although cEF and IQ showed genetic separability in their associations with these outcomes when controlling for one another, it is important to remember that they show more similar patterns when considered separately. Consistent with the hypothesis that low cEF is a transdiagnostic risk factor for psychopathology (the p-factor) (2–5), cEF negatively correlated with all four psychopathology factors. EF also positively correlated with education attainment. Similarly, IQ negatively correlated with all but the Compulsive Thought Disorder factor and positively correlated with educational attainment. In some multiple regressions, relationships became non-significant, which suggests that the variance unique to cEF or IQ is not related to the outcome; e.g., it appears that the genetic variance in Externalizing Disorders that is related to cEF overlaps entirely with IQ, whereas the variance unique to cEF is related to the other psychopathology factors. This particular result was unexpected, but may be consistent with prior findings that externalizing psychopathology is particularly associated with working memory (45), which includes Updating-specific abilities that are related to IQ but not cEF (see Figure 4). In other cases, relationships with cEF or IQ even slightly reversed (e.g., educational attainment and cEF) in the multiple regressions compared to the correlational models. Such suppression effects suggest that although the variance shared with IQ is positively related to educational attainment, the variance unique to cEF or IQ actually is negatively related. Again, this result was not expected but is intriguing if replicated, as are similar reversals in IQ’s relation to some psychiatric disorders such as bipolar disorder.
The current results extend those of a recently published GenomicSEM GWAS on a genetic g factor (46), which focused on a singular dimension of cognitive ability that included EF, IQ, and speed tasks. Our follow-up analyses of this genetic g model (see Supplementary Results) suggest that some of their reported relationships with educational and mental health outcomes were not fully mediated by the genetic g factor. Our results characterize the heterogeneity of these relationships with EF, IQ, and Speed, providing a different and complementary perspective to the focus on commonality (46). Both commonality and uniqueness of cognitive abilities are important to consider in relation to psychopathology (8).
Multiple lines of evidence suggested the importance of GABA to cEF variation. We found little evidence that dopaminergic processes genetically relate to individual differences in cEF, outside the DRD2 gene; other monoamine (dopamine and serotonin) candidate genes were not associated with cEF, despite very high power to detect previously reported associations. Together, our findings strongly implicate a key role of fast synaptic communication mechanisms underlying the inheritance of cEF, rather than the slow neuromodulatory processes that are often hypothesized in the literature.
Altered GABAergic functioning is also associated with cognitive deficits in psychiatric illnesses (47–49), consistent with our finding that cEF was genetically correlated with nearly all psychiatric disorders. These results are in line with past literature, suggesting cEF is broadly genetically associated with psychopathology (2). Disruption to the excitatory/inhibitory neurotransmission balance related to GABAergic processes may explain such transdiagnostic associations with cognitive deficits, particularly EFs (47,48,50).
These results should be interpreted in context of several limitations. First, attaining the large sample size needed for GWAS necessitates minimal phenotyping that might be both shorter and less detailed than gold-standard measures of a construct (21). The UKB cognitive battery was not designed to tap cEF. This battery contained one classic neuropsychological EF task, the trail-making task; the other cognitive measures are not commonly used to assess EFs. However, as described in Figure 1, these tasks do have EF demands. Indeed, a validation study (30) suggested that these bespoke UKB tests correlated similarly or more strongly with reference EF tests (e.g., a tower test) as they did with reference tests for the intended constructs (e.g., memory) (see Supplemental Methods for more discussion). Our factor analytic approach enabled us to extract this shared EF variance. We reasoned that a common factor extracting shared variance across these tasks and the trail-making task would be closely related to the Common EF factors examined in smaller studies (10,15,16,18), two of which also used the trail-making task (10,15). Indeed, PGSs based on the cEF and IQ factors differentially predicted gold-standard EF latent variables and full-scale IQ in independent, deeply phenotyped young adult samples. However, keeping in mind that different conceptualizations of similar constructs can lead to different results, our findings may be interpreted with caution until there are bigger samples with a more complete set of gold-standard EF tasks.
Second, our IQ measure was a factor score based on repeated administrations of the UKB’s “fluid intelligence/reasoning” task. This test, which has also been included in recent GWAS of general cognitive ability (7), includes items that require reasoning and is genetically strongly correlated (rG=.87) (46) with matrix pattern recognition, a classic fluid IQ measure. In the UKB it shows a different pattern of association with age compared to other UKB cognitive measures, leading some to suggest that it may tap crystallized IQ instead of, or in addition to, fluid IQ (51,52). Yet in an independent sample (30) it showed a similar association with cross-sectional age as other UKB cognitive measures and also correlated most strongly with test of working memory and non-verbal reasoning, leading the authors to suggest that it “may be more fluid than was suggested by Hagenaars et al. [2016]” (p. 18). Given these mixed patterns, this measure’s genetic associations with cEF and other outcomes might be most appropriately compared to the literature on general IQ (indeed, we examined its relation to full-scale IQ in our PGS analysis).
Although we would have preferred to use multiple IQ measures just as we used multiple EF measures, there were not additional IQ measures with sufficient sample sizes. The potential concern that our comparisons of IQ to cEF may be unbalanced is allayed by the facts that 1) our IQ score was genetically highly genetically correlated (rGs=.96-.98) with prior large GWASs of IQ (6,7) that assessed IQ with factor scores as well as the same UKB measure we used; 2) cEF was not uniformly more genetically related to outcomes compared to IQ, but these two traits showed differential prediction of psychopathology and educational outcomes; and 3) the PGSs for these factors showed comparable effect sizes (β=.17 vs .15) when predicting their gold-standard counterparts in the Colorado sample, and the IQ PGS also predicted the Updating-specific factor (β=.15) in this sample, in line with prior findings that the Updating-specific factor is genetically related to IQ (9,14).
Finally, because the UKB is overwhelmingly European ancestry, we restricted our analysis to European samples to avoid confounds due to population stratification. Although it is possible and perhaps likely that the molecular underpinnings of cEF generalize to non-European populations, further work is needed to replicate these observations in diverse populations of sufficient sizes and similar phenotypes.
Conclusion
cEF is heritable and highly polygenic, with clear indication for a role of synaptic, GABAergic, and ionotropic pathways. cEF is genetically related to, but separable from, IQ, and cEF is robustly related to genetic risk for general psychopathology even controlling for its genetic overlap with general IQ and Speed.
Supplementary Material
Key Resource Table
Acknowledgements
This research was supported by grants from the National Institutes of Health: MH063207, MH016880, MH001865, MH100141, AG046938, DA042742, and DA046064. We thank Mengzhen Liu, Marie Banich, Marissa Ehringer, and Hannah Snyder for their advice. We also thank Cold Spring Harbor Laboratory for hosting this manuscript on their preprint server, bioRxiv. This research has been completed using the UK Biobank under application number 24795.
Footnotes
Competing Interests
The authors declare no competing interests.
Data Access
Summary statistics for all GWASs (cEF, IQ, RT, and all indicators) will be available upon publication of this work through the FriedmanLab github or available upon request. All results run on FUMA have been made publicly available through that platform. cEF results can be accessed via FUMA (cEF full sample: https://fuma.ctglab.nl/browse/65, cEF densely phenotyped sample: https://fuma.ctglab.nl/browse/66, cEF sparsely phenotyped sample: https://fuma.ctglab.nl/browse/67). Full results for the IQ and Speed GWAS are also available on FUMA (IQ: https://fuma.ctglab.nl/browse/114, Speed: https://fuma.ctglab.nl/browse/118). All biological results for cEF-specific and IQ-specific GWAS can be downloaded here (cEF-specific: https://fuma.ctglab.nl/browse/116, IQ-specific: https://fuma.ctglab.nl/browse/117).
References
- 1.Friedman NP, Miyake A (2017): Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex 86: 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder HR, Miyake A, Hankin BL (2015): Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Front Psychol 6: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. (2014): The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2: 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatoum AS, Rhee SH, Corley RP, Hewitt JK, Friedman NP (2018): Do executive functions explain the covariance between internalizing and externalizing behaviors? Dev Psychopathol 30: 1371–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martel MM, Pan PM, Hoffmann MS, Gadelha A, do Rosário MC, Mari JJ, et al. (2017): A general psychopathology factor (p factor) in children: Structural model analysis and external validation through familial risk and child global executive function. J Abnorm Psychol 126: 137–148. [DOI] [PubMed] [Google Scholar]
- 6.Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. (2018): Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 50: 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. (2018): Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9: 2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey C, Strong R, Huang Y, Aslibekyan S, Gentleman R, Smoller J, et al. (2021): Shared and distinct genetic influences between cognitive domains and psychiatric disorder risk based on genome-wide data. Biol Psychiatry 89: S45–S46. [Google Scholar]
- 9.Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK (2008): Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen 137: 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavson DE, Panizzon MS, Elman JA, Franz CE, Reynolds CA, Jacobson KC, et al. (2018): Stability of genetic and environmental influences on executive functions in midlife. Psychol Aging 33: 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. (2015): UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12: e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond A (2013): Executive functions. Annu Rev Psychol 64: 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol 41: 49–100. [DOI] [PubMed] [Google Scholar]
- 14.Gustavson DE, Panizzon MS, Franz CE, Friedman NP, Reynolds CA, Jacobson KC, et al. (2018): Genetic and environmental architecture of executive functions in midlife. Neuropsychology 32: 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelhardt LE, Briley DA, Mann FD, Harden KP, Tucker-Drob EM (2015): Genes unite executive functions in childhood. Psychol Sci 26: 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman NP, Hatoum AS, Gustavson DE, Corley RP, Hewitt JK, Young SE (2020): Executive functions and impulsivity are genetically distinct and independently predict psychopathology: Results from two adult twin studies. Clin Psychol Sci 8: 519–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freis SM, Morrison CL, Lessem JM, Hewitt JK, Friedman NP (2022): Genetic and environmental influences on executive functions and intelligence in middle childhood. Dev Sci 25: e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK (2016): Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Dev Psychol 52: 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes JJM, Dean AJ, Nandam LS, O’Connell RG, Bellgrove MA (2011): The molecular genetics of executive function: Role of monoamine system genes. Biol Psychiatry 69: e127–e143. [DOI] [PubMed] [Google Scholar]
- 20.Robbins TW (2000): Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res 133: 130–138. [DOI] [PubMed] [Google Scholar]
- 21.Friedman NP, Banich MT, Keller MC (2021): Twin studies to GWAS: there and back again. Trends Cogn Sci 0. 10.1016/j.tics.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoet G, Snyder LH (2006): Effects of the NMDA antagonist ketamine on task-switching performance: Evidence for specific impairments of executive control. Neuropsychopharmacology 31: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Heise K-F, Chalavi S, Puts NAJ, Edden RAE, Swinnen SP (2022): The role of MRS-assessed GABA in human behavioral performance. Prog Neurobiol 212: 102247. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim-Verbaas CA, Bressler J, Debette S, Schuur M, Smith AV, Bis JC, et al. (2016): GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol Psychiatry 21: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagenaars SP, Cox SR, Hill WD, Davies G, Liewald DCM, Harris SE, et al. (2018): Genetic contributions to Trail Making Test performance in UK Biobank. Mol Psychiatry 23: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 26.Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK (2006): Not all executive functions are related to intelligence. Psychol Sci 17: 172–179. [DOI] [PubMed] [Google Scholar]
- 27.Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK (2007): Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychol Sci 18: 893–900. [DOI] [PubMed] [Google Scholar]
- 28.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. (2018): The UK Biobank resource with deep phenotyping and genomic data. Nature 562: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. (2016): A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48: 1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fawns-Ritchie C, Deary IJ (2020): Reliability and validity of the UK Biobank cognitive tests. PLOS ONE 15: e0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh P-R, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM, et al. (2015): Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet 47: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeuw CA, Mooij JM, Heskes T, Posthuma D (2015): MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput Biol 11: e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe K, Taskesen E, Bochoven A van, Posthuma D (2017): Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. (2015): LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47: 291–295B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. (2015): A gene-based association method for mapping traits using reference transcriptome data. Nat Genet 47: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. (2017): LD Hub: A centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, et al. (2018): Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun 9: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grotzinger AD, Mijke R, Ronald de V, Ritchie SJ, Mallard TT, David HW, et al. (2019): Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav 3: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corley RP, Reynolds CA, Wadsworth SJ, Rhea S-A, Hewitt JK (2019): The Colorado Twin Registry: 2019 update. Twin Res Hum Genet 22: 707–715. [DOI] [PubMed] [Google Scholar]
- 40.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. (2007): PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Leeuw CA, Stringer S, Dekkers IA, Heskes T, Posthuma D (2018): Conditional and interaction gene-set analysis reveals novel functional pathways for blood pressure. Nat Commun 9: 3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. (2000): Gene Ontology: Tool for the unification of biology. Nat Genet 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Gene Ontology Resource: 20 years and still GOing strong (2019): Nucleic Acids Res 47: D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kollins SH, Anastopoulos AD, Lachiewicz AM, FitzGerald D, Morrissey-Kane E, Garrett ME, et al. (2008): SNPs in dopamine D2 receptor gene (DRD2) and norepinephrine transporter gene (NET) are associated with continuous performance task (CPT) phenotypes in ADHD children and their families. Am J Med Genet B Neuropsychiatr Genet 147B: 1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang-Pollock C, Shapiro Z, Galloway-Long H, Weigard A (2017): Is poor working memory a transdiagnostic risk factor for psychopathology? J Abnorm Child Psychol 45: 1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de la Fuente J, Davies G, Grotzinger AD, Tucker-Drob EM, Deary IJ (2021): A general dimension of genetic sharing across diverse cognitive traits inferred from molecular data. Nat Hum Behav 5: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marín O (2012): Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13: 107–120. [DOI] [PubMed] [Google Scholar]
- 48.Prévot T, Sibille E (2021): Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry 26: 151–167. [DOI] [PubMed] [Google Scholar]
- 49.Reddy-Thootkur M, Kraguljac NV, Lahti AC (2020): The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders – A systematic review of magnetic resonance spectroscopy studies. Schizophr Res. 10.1016/j.schres.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson BR, Gao W-J (2018): PV interneurons: Critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornelis MC, Wang Y, Holland T, Agarwal P, Weintraub S, Morris MC (2019): Age and cognitive decline in the UK Biobank. PLOS ONE 14: e0213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ, et al. (2016): Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry 21: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


