Abstract
Due to COVID-19, the Dutch breast cancer screening program was interrupted for three months with uncertain long-term effects. The aim of this study was to estimate the long-term impact of this interruption on delay in detection, tumour size of screen-detected breast cancers, and interval cancer rate. After validation, the micro-simulation model SiMRiSc was used to calculate the effects of interruption of the breast cancer screening program for three months and for hypothetical interruptions of six and twelve months. A scenario without interruption was used as reference. Outcomes considered were tumour size of screen-detected breast cancers and interval cancer rate. Women of 55–59 and 60–64 years old at time of interruption were considered. Uncertainties were estimated using a sensitivity analysis. The three-month interruption had no clinically relevant long-term effect on the tumour size of screen-detected breast cancers. A 19% increase in interval cancer rate was found between last screening before and first screening after interruption compared to no interruption. Hypothetical interruptions of six and twelve months resulted in larger increases in interval cancer rate of 38% and 78% between last screening before and first screening after interruption, respectively, and an increase in middle-sized tumours in first screening after interruption of 26% and 47%, respectively. In conclusion, the interruption of the Dutch screening program is not expected to result in a long-term delay in detection or clinically relevant change in tumour size of screen-detected cancers, but only affects the interval cancer rate between last screening before and first screening after interruption.
Keywords: Breast neoplasms, COVID-19, Computational modelling, Mass screening, Mammography, Incidence rate, Netherlands
1. Introduction
Breast cancer is the second most occurring type of cancer worldwide and the leading cause of cancer-related death for women (Lauby-Secretan et al., 2015). To increase the efficiency of treatment and increase the life expectancy through early-stage detection, screening programs were set up in many countries (Lauby-Secretan et al., 2015; IKNL, 2022; Vanni et al., 2020). The screening is associated with an average reduction in risk of death from breast cancer of 23% in western countries (Lauby-Secretan et al., 2015). With the rapid spread of the coronavirus disease 2019 (COVID-19), health care systems were pressured worldwide (IKNL, 2022; Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium et al., 2021). To reduce the pressure on health care, mitigate the spread of COVID-19, and reallocate staff and equipment to support COVID-19 care, multiple breast screening programs were interrupted. The interruptions lasted from one month in Australia, to up to six months in the United Kingdom (Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium et al., 2021). In the Netherlands, the interruption lasted three months, from March 16 until Mid-June 2020 (IKNL, 2022). The Dutch breast cancer screening program invites all women of 50–74 years for a mammographic examination (IKNL, 2022).
The largest immediate consequence observed was a decrease in diagnosed breast cancer cases in 2020, with the largest decrease in women eligible for screening and in low-stage breast cancers (IKNL, 2022; Dinmohamed et al., 2020; Maringe et al., 2020; Eijkelboom et al., 2021a; Zhou et al., 2022). In the Netherlands, the interruption was associated with a decrease in diagnosed cases from March until June 2020 (IKNL, 2022; Eijkelboom et al., 2021a; Eijkelboom et al., 2021b). This observed decrease in breast cancer diagnosis raised the question if the interruption will also result in a long-term delay in detection and diagnosis of breast cancer (IKNL, 2022; Eijkelboom et al., 2021b). The first studies on the long-term effects of an interruption of the screening program showed a potential increase in mortality, but additional effects are unknown (Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium et al., 2021; Maringe et al., 2020; Kregting et al., 2020; Yong et al., 2020; Sharpless, 2020; Duffy et al., 2021; Joung et al., 2022). Most studies on long-term effects found an expected increase in large-sized or late-stage breast cancer cases (Vanni et al., 2020; Yong et al., 2020; Cancer Counsil NSW, 2020; Rutter et al., 2018; Degeling et al., 2021). Conversely, others found that the interruption of the breast cancer screening program did not lead to an increase in large-sized or late-stage breast cancer (Filipe et al., 2020). Only a few studies have investigated the effect on incidence of screen-detected and interval cancers after interruption of screening, where an increase in breast cancer incidence is expected at some point (Yong et al., 2020; Cancer Counsil NSW, 2020).
In daily practice, the long-term effects due to the interruption of screening will take long to show. To predict whether long-term effects will occur and, if so, when, a simulation model can be of use. A simulation model also allows for varying input parameters, such as the characteristics of the interruption (i.e., length of the interruption) which makes comparisons with other screening programs possible. The previously validated Simulation Model on Radiation Risk and breast cancer Screening (SiMRiSc) (Wang et al., 2020; de Bock et al., 2013; Greuter et al., 2010) has been developed to provide information on the short- and long-term risks and benefits of different screening scenarios and can be used to simulate the effects of the three-month interruption of the Dutch breast cancer screening program and hypothetical interruptions of six and twelve months.
This study aimed to estimate the long-term impact of the interruption of the Dutch breast cancer screening program due to COVID-19 on delay in detection, tumour size of screen-detected breast cancer, and interval cancer rate using the SiMRiSc model.
2. Methods
2.1. SiMRiSc model
The SiMRiSc model was used to estimate the impact of the interruption on long-term delay in detection, tumour size of screen-detected breast cancer, and interval cancer rate of the Dutch breast cancer screening program. SiMRiSc is a micro-simulation Markov model. In SiMRiSc, women are modelled individually, each with their own risk to develop a breast cancer at a certain age, tumour growth (in case there is a breast cancer) and related detection by screening or self-detection, and life expectancy. The values for these parameters are based on distributions which are independently derived from published data and used to specify the population. Model input consists of parameters on population incidence, tumour induction due to radiation, preclinical tumour growth, and mammography.
For this study, the input parameters were based on the Dutch population and the Dutch screening program in 2019 (Table 1 ). An additional risk of tumour induction due to the ionising radiation of mammography was also simulated (National Research Council, 2006). Screening sensitivity was dependent on tumour diameter and breast density (Table 2 ), which in turn both depend on age (Isheden and Humphreys, 2019). A participation rate of 75.7% was used. This meant that there was a probability of 75.7% for an individual woman to go to screening at a certain point in time. Furthermore, we assumed a screening interval of two years and 100% capacity after interruption. The screen-detected cancers simulated with SiMRiSc only included invasive tumours. A description of the model can be found in Appendix A.1. and more detail and previous validation of the SiMRiSc model can be found in previous studies (Wang et al., 2020; de Bock et al., 2013; Greuter et al., 2010). This study used publicly available anonymized data, thus was exempt from ethical compliance (IRB approval M22.302538 Medical Ethics Review Board Groningen, The Netherlands).
Table 1.
Input parameters and estimates for SiMRiSc with 95% confidence intervals.
| Parameter | Base value (95% CI) | Reference | |||||
|---|---|---|---|---|---|---|---|
| Population incidence |
Cumulative lifetime invasive breast cancer risk at age 70 | 0.226 (0.010) | (Wang et al., 2020) | ||||
| Mean onset age of breast cancer | 72.9 (1.1) years | ||||||
| SD onset age of breast cancer | 21.1 (0.93) years | ||||||
| Average life expectancy population | 79 years | (de Bock et al., 2013) | |||||
| Breast density distribution (/age group) |
BI-RADS density | 1 | 2 | 3 | 4 | (Wang et al., 2020) | |
| < 40 | 0.05 | 0.30 | 0.48 | 0.17 | |||
| 40–50 | 0.06 | 0.34 | 0.47 | 0.13 | |||
| 50–60 | 0.08 | 0.50 | 0.37 | 0.05 | |||
| 60–70 | 0.15 | 0.53 | 0.29 | 0.03 | |||
| > 70 | 0.18 | 0.54 | 0.26 | 0.02 | |||
| Tumour induction radiation |
Tumour induction risk due to radiation /gray | 0.51 (0.63) | (Greuter et al., 2010; National Research Council, 2006) | ||||
| Dose | 3 (2) mGy | ||||||
| Preclinical tumour growth |
Tumour doubling time (/age group) | < 50 | 80 (28) days | (Wang et al., 2020; Peer et al., 1993) | |||
| 50–70 | 157 (25) days | ||||||
| > 70 | 188 (52) days | ||||||
| Self-detection diameter | 2.92 (1.65) mm | (Greuter et al., 2010) | |||||
| Mammography | Sensitivity beta function | BI-RADS density distribution mean | 1 | 2 | 3 | 4 | (Wang et al., 2020; Isheden and Humphreys, 2019) |
| 0.061 (0.041) | 0.163 (0.088) | 0.400 (0.208) | 0.826 (0.172) | ||||
| Specificity | > 40 | 0.965 (0.005) | (de Bock et al., 2013) | ||||
| Detection threshold | 5 mm | (Hanna et al., 2020) | |||||
| Participation rate | 75.7% | (Center for Population Screening, 2020) | |||||
| Systematic error detection | 10% | (Koleva-Kolarova et al., 2018) | |||||
Table 2.
Sensitivity values for tumour diameters of 0.5, 1, and 2 cm per BIRADS category.
| Sensitivity (%) |
BIRADS density category |
|||
|---|---|---|---|---|
| Tumour size (cm) | A | B | C | D |
| 0.5 | 11.6% | 10.0% | 7.0% | 3.7% |
| 1 | 60.7% | 57.2% | 48.9% | 34.4% |
| 2 | 99.5% | 99.4% | 99.2% | 98.7% |
2.2. Validation
The model was validated by comparing the simulated results to the most recently published observed data of the Dutch screening program including the number of screenings per age group published by the National Evaluation Team for Breast cancer screening (NETB) from 2011 (Fracheboud et al., 2014). The detection rate of screen-detected breast cancers stratified by age group and tumour size observed by the national screening program was compared to the detection rate from SiMRiSc. In addition, the interval cancer rate and number of false positives simulated were compared to the number observed by the Netherlands Comprehensive Cancer Organisation (IKNL) in 2017 and 2019, respectively (IKNL, 2021).
2.3. Scenarios
To estimate the impact of the screening interruption on the outcomes of the Dutch breast cancer screening, different scenarios were modelled. The effects of the three-month interruption of screening were simulated as a delay in screening, resulting in a three-month older age for each screened woman in each following round after restart. Hypothetical interruptions of six and twelve months were modelled similarly, to show the effect of longer interruptions and to give an indication on the effect of a delayed screening uptake and a reduced capacity after restart. As a reference, the screening without interruption was used. Each scenario was simulated ten times for 100,000 women. Also, an uptake of 39.2% was simulated, to investigate the effect of the lower participation (71.2%) and lower invitation (55.1%) of the screening program in 2020 (IKNL, 2021).
2.4. Outcomes
The outcomes considered in validation were screen-detected breast cancers stratified to age and tumour size, interval cancer rate, and number of false positives (Appendix A.2.). For the long-term effects, screen-detected tumour size and interval cancer rate in detection rate were considered (Appendix A.2.). Women of 55–59- and 60–64-years-old at time of interruption from the last screening before up to third screening after interruption were considered. Interval cancers were defined as ‘non-screen-detected breast cancers diagnosed before next screening after a negative screening or false positive screening’. Delay of screening, therefore, will result in a longer period to detect interval cancers.
2.5. Sensitivity analysis
To determine the uncertainty in the outcomes of the model, a univariate sensitivity analysis was performed for screen-detected cancers, interval cancer rate, and false positives. The input parameters were changed one by one to their minimum or maximum estimates (95% confidence intervals) (Table 1) and the variation in the detection rate was recorded.
3. Results
3.1. Validation
The simulated detection rate showed no significant deviations from the observed data for all age groups, interval cancer rate, and tumour size (Table 3 ). Only the simulated false positive rate was significantly larger than observed in the validation cohort.
Table 3.
Validation input parameters in detection rate per 1000 screened women.
| Observed |
SiMRiSc4 |
||
|---|---|---|---|
| Detection rate /1000 | Detection rate /1000 (95% CI) | ||
| Age group1 | 49 | 4.7 | – |
| 50–54 | 3.5 | 3.4 (2.6–4.6) | |
| 55–59 | 3.9 | 4.0 (3.2–5.0) | |
| 60–64 | 5.3 | 4.8 (3.8–5.8) | |
| 65–69 | 6.1 | 5.9 (5.0–7.7) | |
| 70–74 | 6.3 | 5.9 (4.5–7.4) | |
| > 74 | 6.2 | – | |
| Tumour size1 | < 2 cm | 3.9 | 3.7 (3.1–4.4) |
| 2–5 cm | 0.8 | 0.93 (0.86–0.97) | |
| > 5 cm | 0.1 | 0.05 (0.04–0.05) | |
| Interval cancer rate2 | 2.2 | 2.0 (1.7–2.2) | |
| False positives3 | 15.7 | 34.7 (29.7–39.7) | |
Data NETB of 2011 (Fracheboud et al., 2014).
Data IKNL of 2017.
Data IKNL of 2019 (IKNL, 2021).
Input parameters SiMRiSc based on 2019 (reference scenario).
3.2. Interruption
3.2.1. 3 months
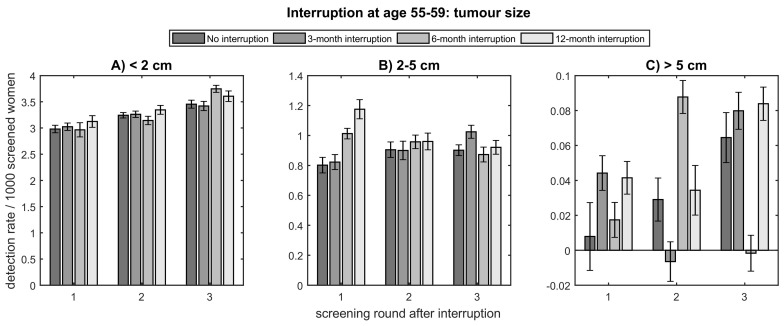
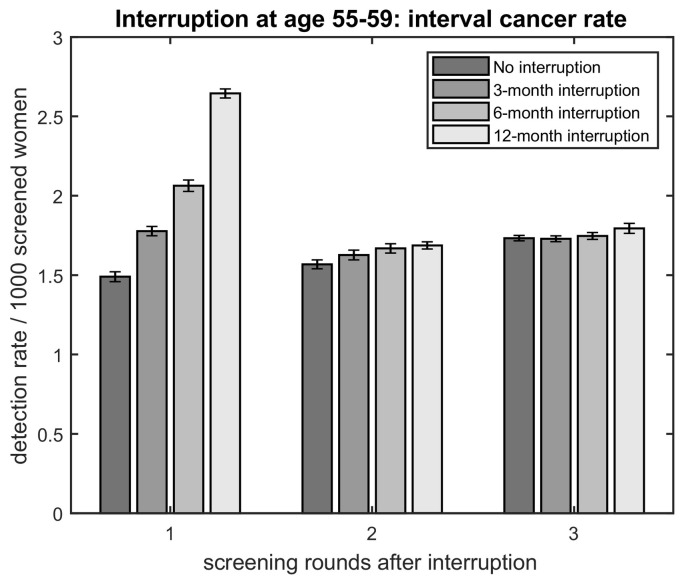
For an interruption of three months, no clinically relevant change in screen-detected tumour size was found in the first three rounds after interruption for women aged 55–59 (Fig. 1 ). Some large-sized tumours were detected one round earlier, but no change in incidence of large-sized tumours was found (Fig. 1C). The interval cancer rate increased with 0.29 (19%) before the first screening after interruption and no clinically relevant change was found between following rounds compared to a scenario without interruption (Fig. 2 , Appendix A.3.1.). For women aged 60–64, the three-month interruption had similar effects, with an increase in interval cancer rate of 20% before first screening after interruption and a fluctuation in detection rate as a function of screening round for large-sized tumours (Appendix A.3.). For this age group, a small increase in middle-sized tumours of 0.11 in detection rate in the first screening after interruption was found (Appendix A.3.1., A.3.2.).
Fig. 1.
Detection rate per 1000 screened women for no interruption, and an interruption of 3, 6, and 12 months at age 55–59 for A) small-, B) middle-, and C) large-sized tumours.
Fig. 2.
Detection rate per 1000 screened women of interval cancers for no interruption, and an interruption of 3, 6, and 12 months at age 55–59 per screening round, where each screening round includes interval cancers detected in the period between previous and this screening round.
3.2.2. 6 and 12 months
For hypothetical interruptions of six or twelve months, the incidence of small-sized tumours was not affected in the first three rounds after interruption at age 55–59 at time of interruption (Fig. 1A). The detection rate of middle-sized tumours showed an increase of in the first screening after hypothetical interruptions of six and twelve months with 0.21 and 0.37, respectively (Fig. 1B, Appendix A.3.1.). For the large-sized tumours, again a fluctuation in detection rate as a function of screening round was found for an interruption of six months, but no clinically relevant change in incidence was found (Fig. 1C). An increased interval cancer rate of 0.57 (38%) and 1.15 (78%) between last screening before and first after interruption of six and twelve months, respectively, and no clinically relevant change between the following rounds was found (Fig. 2, Appendix A.3.1.). For 60–64-year-old women, the effects on interval cancer rate and middle-sized tumours were similar, but in addition an increase in detection rate of small-sized tumours of 0.19 and 0.44 in the first screening after interruption was found for six and twelve months, respectively (Appendix A.3.).
3.3. Uptake
The lower uptake of 2020 showed an increased detection rate for middle- and large-sized tumours of 0.28 and 0.04, respectively (Table 4 ). The interval cancer rate increased with 3.1 (155%). No clinically relevant change in detection rate was found for small-sized tumours and age groups.
Table 4.
Detection rate per 1000 screened women with the 75.7% uptake of 2019 (reference scenario) and the 39.2% uptake of 2020.
| Detection rate /1000 (95% CI) |
|||
|---|---|---|---|
| Uptake 2019 (75.7%)1 | Uptake 2020 (39.2%)1 | ||
| Age group | 50–54 | 3.4 (2.6–4.6) | 3.6 (2.5–5.0) |
| 55–59 | 4.0 (3.2–5.0) | 4.4 (3.3–5.7) | |
| 60–64 | 4.8 (3.8–5.8) | 5.2 (4.0–6.8) | |
| 65–69 | 5.9 (5.0–7.7) | 6.6 (5.4–8.6) | |
| 70–74 | 5.9 (4.5–7.4) | 6.8 (4.8–9.2) | |
| Tumour size | < 2 cm | 3.7 (3.1–4.4) | 3.8 (3.1–4.7) |
| 2–5 cm | 0.93 (0.86–0.97) | 1.21 (1.05–1.41) | |
| > 5 cm | 0.05 (0.04–0.05) | 0.09 (0.08–0.11) | |
| Interval cancer rate | 2.0 (1.7–2.2) | 5.1 (4.7–5.4) | |
| False positives | 34.7 (29.7–39.7) | 34.5 (29.5–39.4) | |
Data IKNL (2021).
3.4. Sensitivity analysis
The detection rate averaged over all age groups was most sensitive to the tumour doubling time for age groups 50–70 and > 70, with an increase in detection rate of 15% for an increase in doubling time to its maximum estimate (Appendix A.3.4.). The detection rate per age also increased for an increase in lifetime risk, a decrease in mean and standard deviation in the onset age of breast cancer, a decrease in sensitivity BIRADS-B and -C, an increase in tumour induction risk/gray, and an increase in dose. The interval cancer rate depended on the same parameters as the detection rate for all age groups (Appendix A.3.5.). The number of false positives was mainly sensitive to the specificity >40 years (Appendix A.3.6.).
4. Discussion
The aim of this study was to estimate the impact of the interruption of the breast cancer screening program on delay in detection, tumour size of screen-detected breast cancers, and interval cancer rate by simulating the effects with the previously validated SiMRiSc model. After successful validation of SiMRiSc with the updated input parameters, for an interruption of three months, no clinically relevant change in screen-detected tumour size was found for a 3-month interruption, however an approximately 20% increase in interval cancer rate was found before the first screening after interruption. For hypothetical interruptions of six and twelve months, a small increase of middle-sized tumours in the first screening after interruption was found, in addition to a larger increase in interval cancer rate before the first screening after interruption. For women aged 60–64, an increase in small-sized tumours in the first screening after interruption was found.
The limited estimated effect on tumour size and increase in interval cancer rate due to the interruption is in line with results from previous studies (Yong et al., 2020; Cancer Counsil NSW, 2020; de Bock et al., 2013; IKNL, 2021). First, in our model, we found an increase in interval cancer rate between the last screening before and first screening after interruption and a sharper increase in interval cancer rate for a larger interruption time. This is in line with the used definition of interval cancers and the higher probability to develop an interval cancer within a longer interval. Furthermore, the simulation of the 3-month interruption did not show an increase in detection rate, which is in line with what was observed in the Dutch screening program (IKNL, 2022). Some screening programs have reported an increase in detection rate for a longer interval (Yong et al., 2020; Mangone et al., 2022), which is also in line with our model results. We found that a small increase in detection rate is expected for 6- and 12-month interruptions. Moreover, our results of an interruption of three months having no clinically relevant effect on screen-detected tumour size correspond to the results of other Dutch studies and an Australian study, which also found no shift in size (IKNL, 2022; Eijkelboom et al., 2021b; Cancer Counsil NSW, 2020; Filipe et al., 2020). On the other hand, multiple international studies (from Italy, the United States, Canada, and Australia) found an increase in large-sized (Vanni et al., 2020) or late-stage tumours (Vanni et al., 2020; Yong et al., 2020; Rutter et al., 2018; Degeling et al., 2021) after interruption. Also, we found that the screen-detection of some large-sized tumours is expected earlier due to screening delay. Screen-detected large-sized tumours are often fast-growing tumours. Due to the delay, tumours can continue growing and the threshold of 5 cm for large-sized tumours is reached sooner.
The different findings on the impact of an interruption on tumour size could be caused by variations in screening program and interruption lengths. Australia and Canada have comparable screening programs to the Dutch screening program, and had their programs interrupted for one and three months, respectively (Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium et al., 2021). Based on our model estimation no change in incidence of large-sized screen-detected cancer in the Dutch screening program was revealed with a three-month interruption. This would also be expected for the Canadian and Australian screening programs. The United Kingdom has a triennial screening, which was interrupted for six months (Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium et al., 2021). Our results of a hypothetical interruption of six months were based on a biennial screening and therefore could give other results than for the screening program in the United Kingdom. For example, a triennial screening is associated with more interval cancers and an increase in small-sized tumours per round (de Bock et al., 2013; Canelo-Aybar et al., 2021), but also with larger-sized screen-detected cancers (Canelo-Aybar et al., 2021). For a six-month interruption of the Dutch screening program, increased numbers of interval and middle-sized tumours were found.
This study focussed on the screening interruption, which was modelled as a delay of screening, in line with the Dutch program where all women were invited again as scheduled before the interruption. Other effects of COVID-19 were lower participation out of fear of infection and a lower capacity after restart of screening (IKNL, 2022). In the Netherlands, this resulted in a participation rate decrease of 75.7% to 71.2%, and only 55.1% of the women eligible for screening were invited (IKNL, 2021). This reduction in uptake resulted in an increased detection rate of middle- (0.28) and large-sized (0.04) tumours and an increased interval cancer rate (155%).
Our results showed that a short screening interruption does not result in a clinically relevant change in screen-detected tumour size, and, therefore, suggests that a short screening interruption can be managed by just restarting and continuing the program. It is important that all women continue to be invited, including women invited for their last screening. Furthermore, it is crucial that the screening capacity is restored as fast as possible.
4.1. Strengths and limitations
This study showed multiple strengths to be considered when interpreting the results. The consequences of the interruption of the Dutch breast cancer screening program on delay in detection, screen-detected tumour size, and interval cancer rate were successfully estimated using SiMRiSc. SiMRiSc was validated for mammography breast cancer screening related research in previous studies (Wang et al., 2020; de Bock et al., 2013; Greuter et al., 2010; Koleva-Kolarova et al., 2018; Lu et al., 2012; Brokken, 2021). Furthermore, the SiMRiSc model could easily be adjusted to the studied population by changing the input parameters. Moreover, the model was not adjusted to fit with observed data, but all input parameters were derived independently from literature. To ensure the outcomes were reliable, a validation was performed, which showed the range modelled with SiMRiSc matched the observed values from NETB and IKNL. Also, a univariate sensitivity analysis was performed to study the influence of each input parameter and determine the uncertainty in the output. The influence of each parameter was consistent with previous literature (Wang et al., 2020; de Bock et al., 2013; Greuter et al., 2010). In addition, for each simulation the average of ten iterations was presented, to ensure the standard error of the simulated outcomes was <5% of point estimate.
This study also had some limitations that should be considered. First, validation of output showed a higher false positive rate than observed in the Dutch screening. Sensitivity analysis showed this was due to an imperfect specificity, although a higher specificity would not comply with the studied population. Therefore, false positives were not used to compare scenarios, which is known to be a limitation of the model (Wang et al., 2020; Koleva-Kolarova et al., 2018). Second, validation of the input parameters of SiMRiSc depended on available literature. For this study, the most recent data on age and tumour size in detection rate was of 2011, while the simulated scenario was of 2019 (Fracheboud et al., 2014). For the interval cancer rate and false positives data from 2017 and 2019 was used, respectively (IKNL, 2021). Although the data came from different years, it is estimated that the results would be similar since the screening program has not changed. The participation rate was slightly higher in previous years, which could mean that the number of screen-detected cancers was slightly underestimated in the simulated data (IKNL, 2021). Third, this study only considered the interruption of the screening program. Other factors that could impact a delay in detection of screen-detected cancers, such as the limited capacity of the program after restarting, the Dutch screening temporarily extending the screening interval from two to a maximum of three years, or a lower participation rate, were not considered (RIVM, 2020). Fourth, a limitation of this modelling approach is that we could not provide insights on which restart strategies would work best. Finally, SiMRiSc did not include all types of screen-detected breast cancer, but only included invasive tumours, while ductal carcinoma in situ (DCIS) accounts for over 20% of screen-detected cancers (Fracheboud et al., 2014; IKNL, 2021). Therefore, the effects of the changes of the Dutch screening program on detection of DCIS could not be considered.
5. Conclusion
In conclusion, the three-month interruption of the Dutch breast cancer screening program, resulting in an observed decrease in diagnosis in early 2020, is not expected to have long-term effects on the size of screen-detected cancers and long-term delay in detection. Only an increase in interval cancer rate of approximately 20% is expected between the last screening before and first after interruption. After restarting the screening program, the number of tumours found is expected to return to comparable levels of previous years without interruption. Furthermore, no change in tumour size of screen-detected cancers is expected. If it is ever necessary to interrupt the program again, the screening program should be continued as soon as possible, since interruptions of six and twelve months showed larger impacts on tumour size and interval cancer rate. When extrapolating our results to other screening programs, differences in screening program (i.e., screening interval and age of invitation) should be considered.
Funding
K. Poelhekken is supported by the W.J. Kolff Institute (WJKI) for her PhD study. The WJKI had no involvement in the study.
CRediT authorship contribution statement
Keris Poelhekken: Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Marcel J.W. Greuter: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Linda de Munck: Validation, Resources, Writing – review & editing, Visualization. Sabine Siesling: Validation, Resources, Writing – review & editing, Visualization. Frank B. Brokken: Software, Data curation. Geertruida H. de Bock: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
None declared.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107376.
Appendix A. Supplementary data
Supplementary material
Data availability
For this study, only publically available data was used. The code of the model is also publically available.
References
- Breast Screening Working Group (WG2) of the Covid-19 and Cancer Global Modelling Consortium, Figueroa J.D., Gray E., Pashayan N., Deandrea S., Karch A., Badhra Vale D., Elder K., Procopio P., van Ravesteyn N.T., Mutabi M., Canfell K., Nickson C. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev. Med. 2021 doi: 10.1016/j.ypmed.2021.106585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokken F.B. University of Groningen; 2021. Simrisc V 14.04.00.https://fbb-git.gitlab.io/simrisc/ 2020. [Online]. Available. [Google Scholar]
- Cancer Counsil NSW . Australian Government - Department of Health; Jun. 2020. Simulated Impacts of COVID-19 Scenarios on Cancer Screening - Summary Report.https://www.health.gov.au/resources/publications/simulated-impacts-of-covid-19-scenarios-on-cancer-screening-summary-report Accessed: Jan. 21, 2022. [Online]. Available. [Google Scholar]
- Canelo-Aybar C., Posso M., Montero N., Solà I., Saz-Parkinson Z., Duffy S.W., Follmann M., Gräwingholt A., Rossi P.G., Alonso-Coello P. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission initiative on breast Cancer (ECIBC) Br. J. Cancer. 2021 doi: 10.1038/s41416-021-01521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Population Screening . RIVM; 2020. Factsheet bevolkingsonderzoek borstkanker 2020.https://www.rivm.nl/documenten/factsheet-borstkanker Accessed: Feb. 03, 2021. [Online]. Available: [Google Scholar]
- de Bock G.H., Vermeulen K.M., Jansen L., Oosterwijk J.C., Siesling S., Dorrius M.D., Feenstra T., Houssami T., Greuter M.J.W. Which screening strategy should be offered to women with BRCA1 or BRCA2 mutations? A simulation of comparative cost-effectiveness. Br. J. Cancer. 2013;108:1579–1586. doi: 10.1038/bjc.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degeling K., Baxter N.N., Emery J., Jenkins M.A., Franchini F., Gibbs P., Mann G.B., McArthur G., Solomon B.J., IJzerman M.J. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia-Pacific J. Clin. Oncol. Feb. 2021 doi: 10.1111/ajco.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed A.G., Cellamare M., Visser O., de Munck L., Elferink M.A.G., Westenend P.J., Wesseling J., Broeders M.J.M., Kuipers E.J., Merkx M.A.W., Nagtegaal I.D., Siesling S. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J. Hematol. Oncol. Nov. 2020;13:147. doi: 10.1186/s13045-020-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S.W., Tabár L., Yen A.M., Dean P.B., Smith R.A., Jonsson H., Törnberg S., Chiu S.Y., Chen S.L., Jen G.H., Ku M.M., Hsu C., Ahlgren J., Maroni R., Holmberg L., Chen T.H. Beneficial effect of consecutive screening mammography examinations on mortality from breast Cancer: a prospective study. Radiology. Mar. 2021 doi: 10.1148/radiol.2021203935. [DOI] [PubMed] [Google Scholar]
- Eijkelboom A.H., de Munck L., Lobbes M.B.I., van Gils C.H., Wesseling J., Westenend P.J., Guerrero Paez C., Pijnappel R.M., Verkooijen H.M., Broeders M.J.M., Siesling S. NABON COVID-19 consortium and the COVID and Cancer-NL consortium, ‘impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic’. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106602. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelboom A.H., de Munck L., Vrancken Peeters M.J.T.F.D., et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J. Hematol. Oncol. 2021;14(64) doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M.D., van Deukeren D., Kip M., Doeksen A., Pronk A., Verheijen P.M., Heikens J.T., Witkamp A.J., Richir M.C. Effect of the COVID-19 pandemic on surgical breast cancer care in the Netherlands: a multicenter retrospective cohort study. Clin. Breast Cancer. Dec. 2020;20(6):454–461. doi: 10.1016/j.clbc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracheboud J., van Luijt P.A., Sankatsing V.D.V., Ripping T.M., Broeders M.J.M., Otten J.D.M., van Ineveld B.M., EAM Heijnsdijk, ALM Verbeek, Holland R., den Heeten G.J., de Bruijn A.E., de Koning H.J. National Evaluation Team for Breast cancer Screening (NETB); 2014. National Evaluation of Breast Cancer Screening in the Netherlands 1990–2011/2012 (XIII)http://www.erasmusmc.nl/mgz/publicationsx/Landelijke evaluatie borstkanker [Online]. Available. [Google Scholar]
- Greuter M.J.W., Jansen-van der Weide M.C., Jacobi C.E., Oosterwijk J.C., Jansen L., Oudkerk M., de Bock G.H. The validation of a simulation model incorporating radiation risk for mammography breast cancer screening in women with a hereditary-increased breast cancer risk. Eur. J. Cancer. Feb. 2010;46(3):495–504. doi: 10.1016/j.ejca.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Hanna T.P., King W.D., Thibodeau S., Jalink M., Paulin G.A., Harvey-Jones E., O’Sullivan D.E., Booth C.M., Sullivan R., Aggarwal A. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371 doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKNL . IKNL; 2021. Monitor Bevolkingsonderzoek Borstkanker 2020/2021.http://www.iknl.nl/borstkankermonitor [Online]. Available: [Google Scholar]
- IKNL COVID-19 en Borstkanker. 2022. https://iknl.nl/covid-19/covid-19-en-borstkanker Accessed: Sep. 16, 2022. [Online]. Available.
- Isheden G., Humphreys K. Modelling breast cancer tumour growth for a stable disease population. Stat. Methods Med. Res. 2019;28(3):681–702. doi: 10.1177/0962280217734583. [DOI] [PubMed] [Google Scholar]
- Joung R.H., Nelson H., Mullett T.W., Kurtzman S.H., Shafir S., Harris J.B., Yao K.A., Brajcich B.C., Bilimoria K.Y., Cance W.G. A national quality improvement study identifying and addressing cancer screening deficits due to the COVID-19 pandemic. Cancer. 2022 doi: 10.1002/cncr.34157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva-Kolarova R.G., Daszczuk A.M., de Jonge C., Hantash M.K.A., Zhan Z.Z., Postema E.J., Feenstra T.L., Pijnappel R.M., Greuter M.J.W., de Bock G.H. A modelling study to evaluate the costs and effects of lowering the starting age of population breast cancer screening. Maturitas. 2018;109:81–88. doi: 10.1016/j.maturitas.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Kregting L., Kaljouw S., de Jonge L., Jansen E.E.L., Peterse E.F.P., Heijnsdijk E.A.M., van Ravesteyn N.T., Lansdorp-Vogelaar I., de Kok I.M.C.M. Effects of cancer screening restart strategies after COVID-19 disruption. Eur. J. Cancer. 2020;138:S16. doi: 10.1016/S0959-8049(20)30561-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B., Scoccianti C., Loomis D., Benbrahim-Tallaa L., Bouvard V., Bianchini F., Straif K. Breast-Cancer screening - viewpoint of the IARC working group. N. Engl. J. Med. Jun. 2015;372:2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- Lu W., Greuter M.J.W., Schaapveld M., Vermeulen K.M., Wiggers T., de Bock G.H. Safety and cost-effectiveness of shortening hospital follow-up after breast cancer treatment. BJS. 2012;99(9):1227–1233. doi: 10.1002/bjs.8850. [DOI] [PubMed] [Google Scholar]
- Mangone L., Mancuso P., Braghiroli M.B., Bisceglia I., Campari C., Caroli S., Marino M., Caldarella A., Giorgi Rossi P., Pinto C. Prompt resumption of screening Programme reduced the impact of COVID-19 on new breast Cancer diagnoses in northern Italy. Cancers. 2022;14(12):3029. doi: 10.3390/cancers14123029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., Rachet B., Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The Lancet Oncol. Aug. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington DC: 2006. Committee on the Biological Effects of Ionizing Radiation., ‘Health Effects of Exposure to Low Levels of Ionizing Radiation (BEIR VII Phase 2) [Google Scholar]
- Peer P.G., van Dijck J.A., Hendriks J.H., Holland R., Verbeek A.L. Age-dependent growth rate of primary breast cancer. Cancer. 1993;71:3547–3551. doi: 10.1002/1097-0142(19930601)71:11<3547::aid-cncr2820711114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- RIVM Tijdelijke Verlenging Uitnodigingsinterval Naar Maximaal 3 JAar. 2020. https://www.rivm.nl/bevolkingsonderzoek-borstkanker/mammografie/later-uitgenodigd Accessed: Jan. 21, 2022. [Online]. Available.
- Rutter C.M., Kim J.J., Meester R.G.S., Sprague B.L., Burger E.A., Zauber A.G., Ergun M.A., Campos N.G., Doubeni C.A., Trentham-Dietz A., Sy S., Alagoz O., Stout N., Lansdorp-Vogelaar I., Corley D.A., Tosteson A.N.A. Effect of time to diagnostic testing for breast, cervical, and colorectal Cancer screening abnormalities on screening efficacy: a modelling study. Cancer Epidemiol. Biomark. Prev. Feb. 2018;27(2):158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N.E. COVID-19 and cancer. Science. Jun. 2020;368(6497):1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- Vanni G., Pellicciaro M., Materazzo M., Bruno V., Oldani C., Pistolese C.A., Buonomo C., Caspi J., Gualtieri P., Chiaravalloti A., Palombi L., Piccione E., Buonomo O.C. Lockdown of breast cancer screening for COVID-19: possible scenario. In Vivo. 2020;34(5):3047–3053. doi: 10.21873/invivo.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Phi X.A., Greuter M.J.W., Daszczuk A.M., Feenstra T.L., Pijnappel R.M., Vermeulen K.M., Buls N., Houssami N., Lu W., de Bock G.H. The cost-effectiveness of digital breast tomosynthesis in a population breast cancer screening program. Eur. Radiol. 2020;30:5437–5445. doi: 10.1007/s00330-020-06812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.H.E., Mainprize J.G., Yaffe M.J., Ruan Y., Poirier A.E., Coldman A., Nadeau C., Iragorri N., Hilsden R.J., Brenner D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J. Med. Screen. 2020 doi: 10.1177/0969141320974711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Z., Kane S., Ramsey C., et al. Comparison of early- and late-stage breast and colorectal Cancer diagnoses during vs before the COVID-19 pandemic. JAMA Netw. Open. 2022;2 doi: 10.1001/jamanetworkopen.2021.48581. e2148581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
For this study, only publically available data was used. The code of the model is also publically available.