OBJECTIVES:
Proliferation of COVID-19 research underscored the need for improved awareness among investigators, research staff and bedside clinicians of the operational details of clinical studies. The objective was to describe the genesis, goals, participation, procedures, and outcomes of two research operations committees in an academic ICU during the COVID-19 pandemic.
DESIGN:
Two-phase, single-center multistudy cohort.
SETTING:
University-affiliated ICU in Hamilton, ON, Canada.
PATIENTS:
Adult patients in the ICU, medical stepdown unit, or COVID-19 ward.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
An interprofessional COVID Collaborative was convened at the pandemic onset within our department, to proactively coordinate studies, help navigate multiple authentic consent encounters by different research staff, and determine which studies would be suitable for coenrollment. From March 2020 to May 2021, five non-COVID trials continued, two were paused then restarted, and five were launched. Over 15 months, 161 patients were involved in 215 trial enrollments, 110 (51.1%) of which were into a COVID treatment trial. The overall informed consent rate (proportion agreed of those eligible and approached including a priori and deferred consent models) was 83% (215/259). The informed consent rate was lower for COVID-19 trials (110/142, 77.5%) than other trials (105/117, 89.7%; p = 0.01). Patients with COVID-19 were significantly more likely to be coenrolled in two or more studies (29/77, 37.7%) compared with other patients (13/84, 15.5%; p = 0.002). Review items for each new study were collated, refined, and evolved into a modifiable checklist template to set up each study for success. The COVID Collaborative expanded to a more formal Department of Critical Care Research Operations Committee in June 2021, supporting sustainable research operations during and beyond the pandemic.
CONCLUSIONS:
Structured coordination and increased communication about research operations among diverse research stakeholders cultivated a sense of shared purpose and enhanced the integrity of clinical research operations.
Keywords: clinical research, critical care research, pandemic, randomized trials
KEY POINTS
Question: How can increased coordination among investigators, research staff and bedside clinicians optimize the conduct of concurrent studies in the pandemic?
Findings: From this two-phase single-center cohort, an interprofessional Department of Critical Care Research Operations Committee emerged, to ensure fulsome review of protocols incorporating bedside staff perspectives, and to enhance communication across research groups. Informed consent rates were significantly lower for COVID-19 trials than other trials. Coenrollment was significantly more common among patients with versus without COVID-19.
Meanings: An intentional, inclusive, structured approach taken by diverse stakeholders enhanced the integrity of clinical research operations during chaotic pandemic times.
As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increased the demand for critical care around the world, research to diagnose, monitor and treat patients in the ICU became a global priority (1). Increased clinical workload and the imperative to find effective COVID-19 treatments halted many ongoing studies as investigations focused on COVID-19; however, jurisdictional research priorities varied (2). A public health ethics perspective (3–5) suggests that both research that is pandemic-focused and research that is not pandemic-focused should continue whenever possible with decisions to pause or pursue preexisting studies conditional on infection control mandates, bedside and research staff safety, consent models, and protocol complexity (6).
Matching a strong research response to strong clinical response is crucial in public health emergencies (7, 8), including generating high-quality randomized trial evidence (9). Many COVID-specific trials and observational studies underscored the need for increased awareness of the operational details of each, coordinated among research teams and bedside clinicians. Concerns emerged about inundating vulnerable persons with research information. Hospital visitor restrictions precluded many in-person consent encounters (10). SARS-CoV-2 transmission concerns (11) and virtual connections heralded more telephone consenting and innovative digitally enhanced consent processes (12). Redoubled efforts have been necessary to uphold the tenets of free, informed, and ongoing consent (13).
Literature referenced threats to protocol fidelity and safety oversight (14–16) and general substandard research (17). Remote monitoring, regulatory flexibility, and prioritized data collection occurred (18, 19). Structured reporting of modified trial methods and conduct for extenuating pandemic circumstances (20) are being published (21, 22), while large U.S. centers reported ramping down and redirecting research (23). We identified no studies on the optimal operationalization of many studies in the ICU setting.
Therefore, we developed two interprofessional committees to facilitate optimal research in the Department of Critical Care at our hospital, guided by respect for the patient and family experience. The objective of this report is to describe their genesis, goals, participation, procedures, and outcomes.
MATERIALS AND METHODS
St. Joseph’s Healthcare Hamilton is a McMaster University-affiliated Canadian hospital. Our group has led and participated in investigator-initiated ICU clinical research for 33 years. Our 24-bed medical-surgical tertiary care service expanded during the pandemic to 46 surge beds.
COVID Collaborative (March 2020 to May 2021)
Genesis
Catalysts for the COVID Collaborative were: 1) Bedside staff feedback on clinical research growth in our ICU highlighted the need for better awareness of study procedures relevant to their role. 2) When multiple COVID-specific studies were planned, the ICU leadership suggested operational review and safety oversight for each protocol, ensuring coordination with existing research.
Goal
The overall goal of the COVID Collaborative was to facilitate communication and coordination among research teams and with bedside staff regarding each study.
Participation
The COVID Collaborative was comprised of early, mid-career and senior faculty investigators and research coordinators engaged in ongoing studies and planning to conduct new pandemic studies. We met by videoconference and through email exchange to canvass ideas for planning, tracking, and coordinating studies, assembling every 1–3 months as needed.
Procedures
We formalized our approach to research operations for more intentional communication among investigative teams regarding each study’s eligibility criteria, enrollment window, consent methods, coenrollment opportunities, and follow-up schedule. Given anticipated consent burdens for patients with COVID-19 and their families, we reviewed optimal procedures to ensure integrity of the informed consent process, minimize decisional burden, and maximize timely enrollment. Acknowledging that protocol requirements for bedside staff could magnify distractions from patient care, pandemic stress underscored the imperative to meet staff training needs. We reviewed the fiduciary and medicolegal responsibility of intensivists for patients in a closed ICU, affirming the need for an ICU physician to be the local lead and liaison, responsible for staff training and addressing questions arising for studies originating in other departments.
From March 2020 to May 2021, we tracked ICU admissions, recording COVID-19 status (24). We documented academic trials in the ICU, and for COVID-specific trials, those in the ICU or the COVID ward. Interventions were pharmacologic, physical (e.g., prone positioning, rehabilitation), or fluid-related (crystalloids, blood products). The ICU admission registry and screening logs were used to record study timelines, consent encounters, enrollments, and coenrollments (25). Data were analyzed using descriptive statistics; comparisons used Fisher exact test.
Department of Critical Care Research Operations Committee (June 2021–Present)
Genesis.
Catalysts for the Department of Critical Care Research Operations Committee (DoCCROC) were: 1) Departmental, hospital, university, and research ethics board leadership suggested more formal multidisciplinary operational review of each new study before a launch. 2) Our Research Institute suggested developing terms of reference for a departmental operations review and approval process as a prototype for others.
Goal.
To advance the care and improve the outcomes of critically ill adults through research excellence, optimizing implementation through coordinated planning and execution.
Participation.
Reflecting multidisciplinary ICU scholarship, clinical representatives are from medicine, pharmacy, nursing, physiotherapy, and respiratory therapy, as well as each study lead and research team. Inclusivity is a hallmark. Research pharmacy representation reflects key roles acquiring, preparing and dispensing study drug, often placebo, and computer order entry in the hospital information system (Epic Systems, Verona, WI), facilitated by the chief information officer. The nurse manager, nurse educator, and respiratory therapist educator advise on training for their colleagues. All intensivists are members including the departmental chair, educational lead, and heads of service of the ICU, medical stepdown, and critical care response team. Convened by the academic chair, anyone interested is welcome (e.g., clinical scholars, locum intensivists, physician consultants in internal medicine, infectious diseases, and trainees). Since June 2021, we meet every 1–4 months as needed by videoconference, ideally within 2 weeks of a requested new protocol discussion.
Procedures.
The DoCCROC reviews each new protocol aiming to enroll patients in the ICU, or medical stepdown or COVID ward in anticipation of possible ICU admission. Bedside staff questions about protocol implementation guided initial discussions. Other North American research-intense ICUs were consulted to garner ideas for processes that would serve pandemic needs but be durable beyond. We developed terms of reference (Table 1) to serve the overall goal, specific objectives and core values; this living document will be updated to stay abreast of evolving needs. We drafted candidate items for operational review of each new study (Table 2), iteratively refining them.
TABLE 1.
Terms of Reference for Department of Critical Care Operations Committee
| Overall goal | To advance the care and improve the outcomes of critically ill adults through clinical research excellence, optimizing implementation through coordinated planning and execution |
| Specific objectives | To support an ongoing coordinated approach to clinical research |
| To develop and share research skills, tools, and expertise within the department | |
| To create continuing education opportunities for interdisciplinary team members | |
| To foster community among emerging and established researchers | |
| Relevant studies | Included: prospective clinical studies enrolling patients in the ICU or potentially admitted to ICU |
| Excluded: nonrandomized laboratory or diagnostic studies, retrospective audits, cohort studies, surveys, case reports | |
| Core values | Responsibility and accountability to our patients, families, colleagues, and our community |
| Collegial, respectful relationships | |
| Timely, transparent communication | |
| An interprofessional culture of inquiry | |
| Ethical principles of human subjects research | |
| Ethical research principles | Beneficence |
| Nonmaleficence | |
| Autonomy and respect for persons | |
| Informed consent | |
| Scientific integrity | |
| Confidentiality | |
| Conflict of interest |
In this table, we outline basic terms of reference for the Department of Critical Care Research Operations Committee.
TABLE 2.
Tool for Interprofessional Operations Review: Initial Domains
| DoCC leadership awareness |
| RN manager (awareness, staff training needs, staff capacity, unit resources) |
| MD director (awareness, physician training needs, coenrollment with existing studies) |
| Link to a local SJHH PI |
| Local PI identification (especially if study PI is from outside the ICU or outside SJHH) |
| Responsibilities of local PI |
| DoCC-led studies (practical, contractual) |
| Studies led from outside the DoCC (practical, contractual) |
| Trainee-led studies enrolling patients in the DoCC (practical, contractual) |
| Staff education |
| RN educator |
| RT educator |
| ICU pharmacists |
| ICU RNs |
| ICU RTs |
| ICU physiotherapists |
| MD educational lead |
| Intensivist group (by the service overall, suitability, and adaptations to our population) |
| Other ICU staff (as relevant for those implementing or affected by the protocol) |
| Patient-based communication |
| Most responsible physician (intensivist) knowledge of an enrollment (per patient assent, medicolegal responsibility, knowledge of coenrolled studies) |
| Bedside staff awareness of an enrollment (per patient protocol requirements, impact on workload) |
| Primary referring service awareness as relevant (e.g., thoracic surgery) |
| Study logistics |
| Assessing patient eligibility (enrolling patients without contraindications) |
| Consent training (for a valid consent encounter in the critical care context) |
| Consent documentation (patient’s chart vs research notes) |
| Protocol oversight (exemptions, modifications, adherence) |
| Explicit handling of (severe) adverse events (clinical notes, institutional review board disclosure) |
| Collaborative coenrollment (principles, considerations) |
| Trainee role(s) and supervisor responsibilities (training, documentation) |
| General capacity (ICU, laboratory, etc.) |
| Health informatics (computerized provider order entry for studies, research flowsheet, health information system notification of patient research enrollment, data extraction) |
DoCC = Department of Critical Care, MD = medical doctor, PI = principal investigator, RN = registered nurse, RT = respiratory therapist, SJHH = St. Joseph’s Healthcare Hamilton.
In this table, we list items addressed under operational review of each study.
Today, the scope of studies for review includes those led by department members and studies led by teams outside the department who seek to enroll ICU patients. This review does not serve as a priority-setting exercise, but DoCCROC dialogue and approval precedes the launch of any study fitting the criteria.
The lead ICU investigator typically shares a 1–2 page study summary. During the meeting, slides outline the rationale, objective, and design features to facilitate discussion about issues such as the eligibility criteria, enrollment window, and intervention. Thereafter, dialogue involves anticipated bedside staff workflow, patient and family experience, and research personnel considerations. Dimensions of operational review address clinical and research staff awareness and training, ICU capacity, site-specific logistics to ensure protocol fidelity, and strategies to uphold the ethical principles that govern human subjects research. Operational readiness is determined collaboratively. Across protocols and research groups, activities are coordinated to optimize collegial, responsive protocol implementation. Any outstanding actions are addressed and resolved by the lead investigator and associated research personnel.
No formal approval by the Hamilton Integrated Research Ethics Board was necessary for this study, as patient-level data focused on research improvement were reported in aggregate. Procedures for studies in this report were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
RESULTS
COVID Collaborative
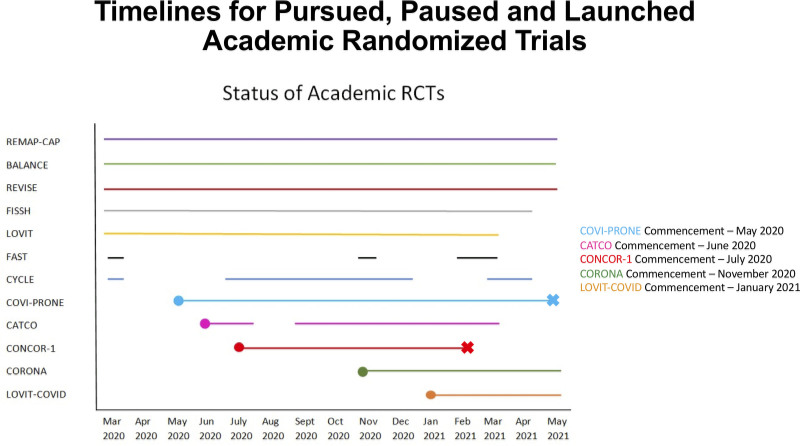
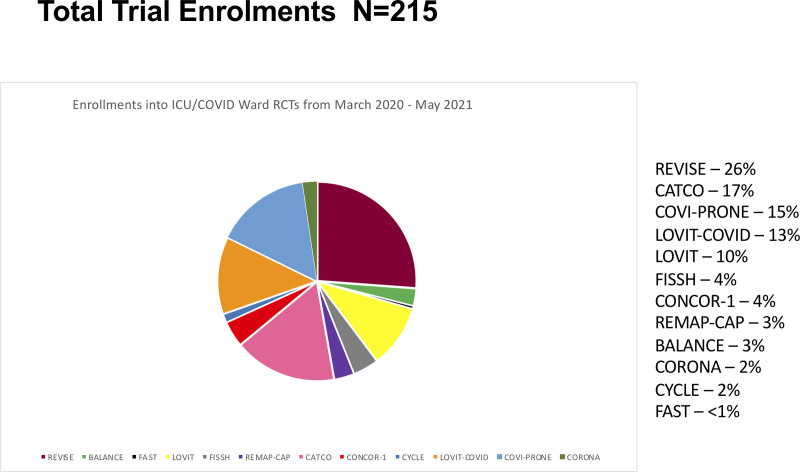
From March 2020 to May 2021, the peak number of monthly ICU admissions was 94 (January 2021); the peak monthly admissions with COVID-19 was 42 (April 2021). Figure 1 identifies the five trials that were pursued (26–30), two which were paused and resumed (31, 32), and five that were launched (33–37). Overall, 161 patients were involved in 215 trial enrollments (Fig. 2), 110 (51.1%) of which were into a COVID treatment trial (24). The proportion of enrollments in either a general or a COVID-specific trial is shown in eFigure 3 (http://links.lww.com/CCX/B99), with the number of trials operational each month.
Figure 1.
Timelines for pursued, paused, and launched academic randomized trials. In this figure, we display the periods during which these randomized trials were operational during the first three pandemic waves. BALANCE = Bacteremia Antibiotic Length Actually Needed for Clinical Effectiveness, CATCO = Canadian Treatments for COVID-19, CONCOR-1 = CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness, CORONA = COvid pRONe hypoxemiA: Prone Positioning for Hypoxemic COVID-19 Patients With Do-not-intubate Goals, COVI-PRONE = Awake Prone Position in Hypoxemic Patients With Coronavirus Disease 19 COVID-19, CYCLE = A Randomized Clinical Trial of Early In-bed Cycling for Mechanically Ventilated Patients, FAST = The Frequency of Screening and SBT Technique Trial, FISSH = Fluids in Septic Shock, LOVIT = Lessening Organ Dysfunction With VITamin C, LOVIT-COVID = Lessening Organ Dysfunction With VITamin C-COVID-19, RCT = randomized clinical trial, REMAP-CAP = Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia, REVISE = Re-EValuating the Inhibition of Stress Erosions.
Figure 2.
Total trial enrollments. In this figure, we show enrollments in these randomized trials. Several patients were enrolled in two or more trials during this period. BALANCE = Bacteremia Antibiotic Length Actually Needed for Clinical Effectiveness, CATCO = Canadian Treatments for COVID-19, CONCOR-1 = CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness, CORONA = COvid pRONe hypoxemiA: Prone Positioning for Hypoxemic COVID-19 Patients With Do-not-intubate Goals, COVI-PRONE = Awake Prone Position in Hypoxemic Patients With Coronavirus Disease 19 COVID-19, CYCLE = A Randomized Clinical Trial of Early In-bed Cycling for Mechanically Ventilated Patients, FAST = The Frequency of Screening and SBT Technique Trial, FISSH = Fluids in Septic Shock, LOVIT = Lessening Organ Dysfunction With VITamin C, LOVIT-COVID = Lessening Organ Dysfunction With VITamin C-COVID-19, RCT = randomized clinical trial, REMAP-CAP = Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia, REVISE = Re-EValuating the Inhibition of Stress Erosions.
When possible, consent encounters involved first-person a priori consent with patients or substitute decision-makers. The deferred consent model was used as necessary, if ethically approved. Commonly, informed consent was obtained from substitute decision-makers by witnessed telephone or videoconferencing due to patient illness and visitation restrictions. The overall trial consent rate (proportion agreed of those eligible and approached including a priori and deferred consent models) was 83% (215/259); the informed consent rate was 77.5% for COVID-specific trials (110/142) and 89.7% for non-COVID-specific trials (105/117; p = 0.01) (eFig. 4, http://links.lww.com/CCX/B99).
Coenrollment in two or more trials involved either concurrent or sequential consent encounters with patients or families. Out of the 161 patients enrolled in all trials, 119 patients were enrolled in a single trial, 33 enrolled in two trials, and nine enrolled in three or more trials. The corresponding number for patients with COVID-19 were 48 patients enrolled in a single trial, 21 in two trials, and eight in three or more trials. Over the first three pandemic waves, coenrollment increased (eFig. 5, http://links.lww.com/CCX/B99), loosely mirroring increased availability of COVID-19-specific trials. Patients with COVID-19 compared with without were significantly more likely to be coenrolled (29/77, 37.7% vs 13/84, 15.5%, respectively; p = 0.002). During this period, two pandemic-specific trials were completed (33, 35).
To minimize participant research burden, if a patient was coenrolled in studies with the same endpoint, we clustered follow-up contact. For example, both a COVID-19 therapeutics platform trial (34) and convalescent plasma trial (35) required 30-day vital status; one contact was made for both.
Department of Critical Care Research Operations Committee
Features of the first eight studies undergoing operational review included two pilot randomized trials, one pilot cluster randomized trial, two different domains of a platform trial, two cohort studies, and one case series of a new technology (Table 3). Discussions addressed educational features of each trial. For example, review of domains related to anticoagulant thromboprophylaxis for COVID-19 within Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia and extended duration corticosteroids within Canadian Treatments for COVID-19 (CATCO) were preceded by learnings about platform trial design (38).
TABLE 3.
New Studies for Interprofessional Operational Review
| Study and Design | Consent Model(s) | Intervention(s) | Operational Focus | Educational Focus | Status |
|---|---|---|---|---|---|
| Noninvasive ventilation and dexmedetomidine in critically ill adults: a pragmatic pilot randomized controlled trial | A priori or deferred | Dexmedetomidine vs placebo for intolerance of noninvasive ventilation | Identifying patients with sustained discomfort when noninvasive ventilation applied | Pre-prepared study drug syringes stored in ICU to minimize research pharmacy workload | Started |
| Pilot randomized trial (blinded) | |||||
| Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia | A priori | New domain: Intermediate vs low-dose heparin thromboprophylaxis | Electronic medical record build to facilitate weight-based adjustments of different drugs | Design of platform adaptive trials and selection of domains for an individual patient | Started |
| Randomized platform trial (unblinded) | |||||
| Canadian COVID-19 Prospective Cohort Study | A priori | Functional and quality of life questionnaires, healthcare resource use (no comparison) | Coenrollment opportunities with minimal consent burden | Coordinating long-term outcome assessments across several studies | Started, closed |
| Multicenter cohort study | |||||
| Sex-matched and nonsex-matched RBC transfusions | Waived | Sex-matched vs nonsex-matched RBC transfusion | Gender sensitivity during screening; patient and substitute decision-maker notification about enrollment (by whom, when, how) | Design of cluster trials and ensuring participant awareness | Started |
| Cluster randomized registry trial (blinded) | |||||
| Cuff leak and airway obstruction in mechanically ventilated ICU patients: a randomized controlled trial | A priori or deferred | Extubation management informed by cuff leak test results vs not informed by cuff leak test results | Minimizing pre-randomization cuff leak tests as part of standard practice | Disutility of cuff leak for most intubated patients pending extubation | In preparation |
| Pilot randomized trial (unblinded) | |||||
| Noninvasive ventilation Helmet Study | Waived | Feasibility of noninvasive ventilation with helmet (no comparison) | Capacity assessment and readiness to launch | Introducing health technology focused on feasibility focus prior to evaluation | In preparation |
| Multicenter case series | |||||
| Frailty, rehabilitation, and outcomes in adult and pediatric survivors of COVID-19 | A priori | Frailty, rehabilitation and outcomes of ICU COVID-19 survivors (no comparison) | Identification of optimal timing for consent approach | Data linkage to an international registry | In preparation |
| Multicenter cohort study | |||||
| Canadian Treatments for COVID-19 | A priori | New domain: Standard or extended duration corticosteroids for COVID-19 pneumonia | Identifying patients at day 10 of corticosteroid therapy, ensuring drug adherence post-ICU | Umbrella, basket, and platform trials | In preparation |
| Randomized platform trial (unblinded) |
In this table, we present the first eight studies reviewed using the Department of Critical Care Research Operations Committee framework, including design features, consent model(s), and intervention(s). Examples of discussion points for operational and educational focus.
The DoCCROC review for these eight studies was undertaken either before or after research ethics board review. Designed to facilitate smooth study implementation, share information across personnel and teams, and address departmental needs or expectations, the DoCCROC is neither an independent ethical nor scientific review body; however, it informs and responds to ethical and scientific considerations from within and outside the ICU community.
While aiming to facilitate new studies, sometimes research initiated external to the department may entail additional preparation, recognizing contextual considerations that may differ from general wards. One cluster randomized transfusion trial led by another division with research ethics board approval subsequently required operational clarification regarding a fundamental ethical issue about operationalizing the waived consent model. We averted problems by deferring the launch to discuss how, when, and by whom the informational brochure would be disseminated, thereby reliably notifying patients and families, preserving their opportunity to ask questions and opt out. Our process underscores how ethics approval of a study does not constitute operational preparedness to start it.
We created a fillable checklist for each study. The template for the extended corticosteroid domain of CATCO (34) is shown in eTable 4 (http://links.lww.com/CCX/B99). Henceforth, the checklist will be signed by the academic chair and forwarded to the departmental chair, signaling departmental operational readiness to launch.
Four trials operational during this time frame are now published (a convalescent plasma trial [39], the remdesivir domain in CATCO [40], the awake prone positioning trial [41], and vitamin C for sepsis trial [42]).
DISCUSSION
Our COVID Collaborative evolved formally into the DoCCROC, serving several functions. The lens of a single-center multistudy management approach cultivates a broader perspective than that of an individual study and ensuring coordination across studies.
Over 15 months, we examined all 12 academic trials in the ICU as well as COVID-specific trials on the COVID ward. Some preexisting trials were paused and restarted while others continued throughout each wave; meanwhile, five COVID-specific trials were launched and two COVID-specific trials finished. Patients and families remained receptive to trials that could inform care of future patients, some of which offered COVID-19-specific therapies to which they would not otherwise have had access. Provincial load adjustments during pandemic surges (43) somewhat limited enrollments, conditional on transfers and eligibility windows. Consent rates varied; lower rates for COVID-19 trials may reflect fear-induced decisional paralysis, intervention uncertainty or preference, and minimal bedside family presence. Coenrollment increased over time, and aligned with the range of relevant trials, was significantly greater for patients with COVID-19.
This novel, structured approach to research operations also offers experiential education. Acknowledging concerns about therapeutic misconception during the pandemic, ensuring an authentic consent process received special attention. We referred to 13 steps to optimize the three-phase informed consent process from Canadian research coordinators (44). We discussed which studies are suitable for coenrollment and mapped out a proactive approach to navigate complex concurrent or consecutive consent encounters, sometimes using different consent models for staff with different levels of experience (45, 46). All patients with COVID-19 in our institution were tracked; many were represented in the first wave publication (47), while the World Health Organization registry continues (48).
A common discussion topic was capacity. Sensitivity to family members’ capacity acknowledged probable angst about a COVID-19 diagnosis. Research pharmacy pressures increased dramatically as dozens of drug trials were proposed across the organization, often launched with infectious diseases and general medicine colleagues. Additional bedside nurses, pharmacists and respiratory therapists ensured clinical coverage and research support, including patient and family conversations. Each protocol’s implications for ICU workflow encouraged realistic but time-sensitive start-ups. Several locum attending physicians actively assisted with screening, increasing research capacity. A durable lesson learned is acknowledgment that despite research team readiness, the appropriate time to begin enrollment requires earnest situational awareness and external capacity assessment.
This report is limited in that we did not elicit views from patients or families. We did not analyze two activated trials on the COVID-19 ward into which no patients were enrolled, nor record eligible patients not approached when research capacity was exceeded, nor capture reasons for consent declines. Our enrollment analysis focused on randomized trials, although a nonrandomized swab study associated with self-limited bleeding in a therapeutically anticoagulated patient was paused for clinician education, and was completed on other wards, highlighting how even low-risk studies require staff training, and may warrant cautions or exemptions in high-risk populations.
Strengths of this report include broad stakeholder input from ICU staff, hospital, university, research institute, and research ethics board representatives whose observations motivated this work. Academic trials were the focus of analyzed patients over 15 months by the COVID Collaborative. We prioritized efficient, synchronous, transparent dialogue through videoconferencing rather than asynchronous email exchange when in-person or hybrid meetings were impossible. Following group discussion, we aim to help set up each study for success.
This approach to research operations intentionally elicits diverse disciplinary voices while keeping the patient-family dyad in the center of the research process. Unique features extending the remit of a conventional impact review are a focus on methodologic education and leadership development. Our early pandemic alliance transformed into the more formal DoCCROC to serve future needs, including research in the medical stepdown unit and critical care response team engagement. This roadmap for activating and sustaining several studies by different research groups facilitated by a departmental academic director may offer a framework for research operations in other departments and institutions.
CONCLUSIONS
Increased clinical pressures and research imperatives during the pandemic made an efficient, formalized, coordinated approach paramount. Harnessing interprofessional ideas and facilitating professional development, we convened two committees to carefully implement existing studies and assess research readiness for several new COVID-19 trials. Attention to research operations has been enhanced by diverse stakeholder input, cultivating a sense of teamwork and shared purpose while advancing science to improve the processes and outcomes of care for seriously ill patients.
ACKNOWLEDGMENTS
We appreciate feedback from the bedside clinical staff, which prompted the need to convene these groups and continue to offer feedback about the integrity of our research process. Suggestions from the Chair of the Department of Medicine (Dr. Mark Crowther) and Co-Chairs of the Hamilton Integrated Research Ethics Board (Dr. Fred Spencer and Dr. Mark Inman) helped to shape this document. We thank scientific colleagues for engaging in the process of enhancing research operations and biostatistician Diane Heels-Ansdell for her assistance.
Supplementary Material
APPENDIX
Department of Critical Care Research Operations Committee: Waleed Alhazzani, Matthew Bell, Kimberley Bloomfield, Zain Chagla, Dipayan Chaudhuri, Jason Cheung, France Clarke, Deborah Cook, Mary Copland, Sarah Culgin, Erick Duan, Zoe Fu, Jennifer Gain, Abby Hurd, Roman Jaeschke, Hilary Lee, Katryn Love, Laurel Kelly, Michelle Kho, Kate Kim, Kimberley Lewis, Tania Ligori, Karlo Matic, Katlynne Nelson, Heather O‘Grady, Jeffrey Overington, Dan Perri, Brenda Reeve, Anna Rozenberg, Jill Rudkowski, Lois Saunders, Joanna Semrau, Mark Soth, Christine Wallace, Lily Waugh, Angela Wright, and Nicole Zytaruk.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by Academic Critical Care Chair, McMaster University/St. Joseph’s Healthcare.
Dr Cook holds a Canada Research Chair from the Canadian Institutes for Health Research. Dr. Lewis holds the Constantine Douketis New Researcher Award from the Research Institute of St. Joseph’s Healthcare Hamilton. Dr. Kho holds a Canada Research Chair from the Canadian Institutes for Health Research. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Waleed Alhazzani, Matthew Bell, Kimberley Bloomfield, Zain Chagla, Dipayan Chaudhuri, Jason Cheung, France Clarke, Deborah Cook, Mary Copland, Sarah Culgin, Erick Duan, Zoe Fu, Jennifer Gain, Abby Hurd, Roman Jaeschke, Hilary Lee, Katryn Love, Laurel Kelly, Michelle Kho, Kate Kim, Kimberley Lewis, Tania Ligori, Karlo Matic, Katlynne Nelson, Heather O‘Grady, Jeffrey Overington, Dan Perri, Brenda Reeve, Anna Rozenberg, Jill Rudkowski, Lois Saunders, Joanna Semrau, Mark Soth, Christine Wallace, Lily Waugh, Angela Wright, and Nicole Zytaruk
REFERENCES
- 1.Rome BN, Avorn J: Drug evaluation during the Covid-19 pandemic. N Engl J Med 2020; 382:2282–2284 [DOI] [PubMed] [Google Scholar]
- 2.Duffett M, Cook DJ, Strong G, et al. : The effect of COVID-19 on critical care research: A prospective longitudinal multinational survey. medRxiv Preprint posted online October 23, 2020. doi: 10.1101/2020.10.21.20216945 [DOI] [Google Scholar]
- 3.Emanuel EJ, Persad G, Upshur R, et al. : Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; 382:2049–2055 [DOI] [PubMed] [Google Scholar]
- 4.Peckham S, Hann A: Public Health Ethics and Practice. Bristol, United Kingdom, The Policy Press, 2020 [Google Scholar]
- 5.Chong SA, Capps BJ, Subramaniam M, et al. : Clinical research in times of pandemics. Public Health Ethics 2010; 3:35–38 [Google Scholar]
- 6.Cook DJ, Kho ME, Duan E, et al. : Principles guiding non-pandemic critical care research during a pandemic. Crit Care Med 2020; 48:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurie N, Manolio T, Patterson AP, et al. : Research as a part of public health emergency response. N Engl J Med 2013; 368:1251–1255 [DOI] [PubMed] [Google Scholar]
- 8.Cook DJ, Marshall JC, Fowler RA: Critical illness in patients with COVID-19: Mounting an effective clinical and research response. JAMA 2020; 323:1559–1560 [DOI] [PubMed] [Google Scholar]
- 9.Chagla Z, Laupland KB, Schwartz IS: Desperate times call for evidence-based measures: Prioritizing science during the COVID-19 pandemic. J Assoc Med Micriobio and Infect Dis Canada 2020; 53:127–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaye DK: Navigating ethical challenges of conducting randomized clinical trials on COVID-19. Philos Ethics Humanit Med 2022; 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Doremalen N, Bushmaker T, Morris DH, et al. : Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382:1564–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaton E, Stang J, Biros M, et al. : The use of electronic consent for COVID-19 clinical trials: Lessons for emergency care research during a pandemic and beyond. Acad Emerg Med 2020:1183–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tri-Council Policy Statement: thical Conduct for Research Involving Humans – TCPS 2 (2018). EAvailable at: https://ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2018.html. Accessed November 13, 2022.
- 14.FDA Guidance on Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency. Guidance for Industry, Investigators, and Institutional Review Boards. 2021. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency. Accessed November 13, 2022
- 15.Hsu NS, Hendriks S, Ramos KM, et al. : Ethical considerations of COVID-19-related adjustments to clinical research. Nat Med 2021; 27:191–193 [DOI] [PubMed] [Google Scholar]
- 16.Fleming TR, Labriola D, Wittes J: Conducting clinical research during the COVID-19 pandemic: Protecting scientific integrity. JAMA 2020; 324:33–34 [DOI] [PubMed] [Google Scholar]
- 17.Bramstedt KA: The carnage of substandard research during the COVID-19 pandemic: A call for quality. J Med Ethics 2020; 46:803–807 [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Newman AB: Preserving clinical trial integrity during the coronavirus pandemic. JAMA 2020; 323:2135–2136 [DOI] [PubMed] [Google Scholar]
- 19.Lynch HF, Dickert NW, Zettler P, et al. : Regulatory flexibility for COVID-19 research. J Law Biosci 2020; 7:lsaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orkin A, Gill PJ, Ghersi D, et al. ; for the CONSERVE Group: Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances. The CONSERVE 2021 statement. JAMA 2021; 326:257–265 [DOI] [PubMed] [Google Scholar]
- 21.Reid JC, Molloy A, Strong G, et al. ; for the CYCLE Investigators: Research interrupted: Applying the CONSERVE 2021 Statement to a randomized trial of rehabilitation during critical illness affected by the COVID-19 pandemic. Trials 2022; 23:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finfer S, Micallef S, Hammond N, et al. ; PLUS Study Investigators; for the PLUS Study Investigators and the Australian and New Zealand intensive care society clinical trials group: Balanced multielectrolyte solution versus saline in critically Ill adults. N Engl J Med 2022; 386:815–826 [DOI] [PubMed] [Google Scholar]
- 23.Stiles DF, Ruotolo BL, Kim H, et al. : Managing human subjects research during a global pandemic at an academic center: Lessons learned from COVID-19. Acad Med 2022; 97:48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matic K, Clarke F, Culgin S, et al. : Critical care research response to COVID-19 through academic RCTs. Canadian Critical Care Forum Abstract Book 2021 [Google Scholar]
- 25.Cook DJ, Blythe D, Rischbieth A, et al. : Enrollment of intensive care unit patients into clinical studies: A trinational survey of researchers’ experiences, beliefs, and practices. Crit Care Med 2008; 36:2100–2105 [DOI] [PubMed] [Google Scholar]
- 26.REMAPCAP: A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT02735707. Accessed November 13, 2022.
- 27.BALANCE: Bacteremia Antibiotic Length Actually Needed for Clinical Effectiveness. Available at: https://clinicaltrials.gov/ct2/show/NCT03005145. Accessed November 13, 2022.
- 28.REVISE: Re-EValuating the Inhibition of Stress Erosions. Available at: https://clinicaltrials.gov/ct2/show/NCT03374800. Accessed November 13, 2022.
- 29.FISSH: Fluids in Septic Shock. Available at: https://clinicaltrials.gov/ct2/show/NCT03677102. Accessed November 13, 2022.
- 30.LOVIT: Lessening Organ Dysfunction With VITamin C. Available at: https://clinicaltrials.gov/ct2/show/NCT03680274. Accessed November 13, 2022.
- 31.CYCLE: A Randomized Clinical Trial of Early In-bed Cycling for Mechanically Ventilated Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT03471247. Accessed November 13, 2022.
- 32.FAST-NAWC: The Frequency of Screening and SBT Technique Trial; A North American Weaning Collaborative. Available at: https://clinicaltrials.gov/ct2/show/NCT02969226. Accessed November 13, 2022.
- 33.COVI-PRONE: Awake Prone Position in Hypoxemic Patients With Coronavirus Disease 19 COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04350723. Accessed November 13, 2022.
- 34.CATCO: Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO). Available at: https://clinicaltrials.gov/ct2/show/NCT04330690. Accessed November 13, 2022.
- 35.CONCOR-1: CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness. Available at: https://clinicaltrials.gov/ct2/show/NCT04348656. Accessed November 13, 2022.
- 36.CORONA: (COvid pRONe hypoxemiA): Prone Positioning for Hypoxemic COVID-19 Patients With Do-not-intubate Goals. https://www.clinicaltrials.gov/ct2/show/NCT04402879. Accessed November 13, 2022.
- 37.LOVIT-COVID: Lessening Organ Dysfunction With VITamin C - COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04401150. Accessed November 13, 2022.
- 38.Bhatt DL, Mehta C: Adaptive designs for clinical trials. N Engl J Med 2016; 375:65–74 [DOI] [PubMed] [Google Scholar]
- 39.Bégin P, Callum J, Jamula E, et al. ; for the CONCOR-1 Study Group: Convalescent plasma for hospitalized patients with COVID-19: An open-label, randomized controlled trial. Nat Med 2012; 27:2012–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali K, Azher T, Baqi M, et al. ; Canadian Treatments for COVID-19 (CATCO); Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group: Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial. CMAJ 2022; 194:E242–E251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alhazzani W, Parhar K, Weatherald J, et al. ; for the COVI-PRONE Trial investigators and the Saudi Critical Care Trials Group: Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: A randomized clinical trial. JAMA 2022; 327:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamontagne F, Masse MH, Menard J, et al. ; LOVIT Investigators and the Canadian Critical Care Trials Group: Intravenous vitamin C for adults with sepsis in the intensive care unit. N Engl J Med 2022; 386:2387–2398 [DOI] [PubMed] [Google Scholar]
- 43.Mitchell SH, Rigler J, Baum K: Regional transfer coordination and hospital load balancing during COVID-19 surges. JAMA Health Forum 2022; 3:e215048. [DOI] [PubMed] [Google Scholar]
- 44.Smith O, McDonald E, Zytaruk N, et al. ; for the North American PROTECT Research Coordinators and the Canadian Critical Care Trials Group: Enhancing the informed consent process for critical care research: Strategies from a thromboprophylaxis trial. Intensive Crit Care Nurs 2013; 29:300–309 [DOI] [PubMed] [Google Scholar]
- 45.Smith O, McDonald E, Zytaruk N, et al. ; for the PROTECT Research Coordinators, PROTECT Investigators, Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group: Rates and determinants of informed consent: A case study of an international thromboprophylaxis trial. J Crit Care 2013; 28:28–39 [DOI] [PubMed] [Google Scholar]
- 46.Cook DJ, McDonald E, Smith O, et al. ; for the PROTECT Investigators, Canadian Critical Care Trials Group and Australian and New Zealand Intensive Care Society Clinical Trials Group: Coenrolment of critically ill patients into multiple studies: Patterns, predictors and consequences. Crit Care 2013; 17:R1–R8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murthy S, Archambault PM, Atique A, et al. ; for the SPRINT-SARI Canada Investigators and the Canadian Critical Care Trials Group: Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: A national cohort study. Can Med Assoc J Open 2021; 9:E181–E188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.SPRINT-SARI: Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection. Available at: https://clinicaltrials.gov/ct2/show/NCT02498587. Accessed November 13, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.