Abstract
Oxygen (O2) plays a key role in cellular homeostasis. O2 levels are tightly regulated in vivo such that each tissue receives an optimal amount to maintain physiologic status. Physiologic O2 levels in various organs range between 2–9% in vivo, with the highest levels of 9% in the kidneys and the lowest of 0.5% in parts of the brain. This physiologic range of O2 tensions is disrupted in pathologic conditions such as cancer, where it can reach as low as 0.5%. Regardless of the state, O2 tension in vivo is maintained at significantly lower levels than ambient O2, which is approximately 21%. Yet, routine in vitro cellular manipulations are carried out in ambient air, regardless of whether or not they are eventually transferred to hypoxic conditions for subsequent studies. Even brief exposure of hematopoietic stem cells to ambient air can cause detrimental effects through a mechanism termed extraphysiologic oxygen shock/stress (EPHOSS), leading to reduced engraftment capabilities. Here, we provide an overview of the effects of ambient air exposure on stem and non-stem cell subtypes, with a focus on recent findings that reveal the impact of EPHOSS on cancer cells.
Keywords: Physioxia, Breast Cancer, Ovarian Cancer, Cancer Stem Cells, NRF2
INTRODUCTION
The emergence of cyanobacteria symbolizes the transition from anoxic to oxic atmosphere on earth (1). This increasing availability of O2 is intricately linked to the evolution of major life forms on the planet, with more complex multicellular life forms beginning to evolve as O2 levels began to rise to levels that support cellular bioenergetics and survival (2). The important roles that O2 plays in the maintenance of life makes it a potentially effective biosignature in the identification of exoplanets that could support life (3, 4).
The present level of atmospheric O2 is 21% (5), which ironically does not mirror tissue O2 levels that are typically lower and ranges between 2–9% in vivo (6). In addition, distribution of O2 in various parts within the same organ is heterogenous. Similarly, variations in O2 distribution are characteristic of pathologic conditions such as tissue hypoperfusion, respiratory impairment, and anemia (7). Solid tumors are also characterized by a heterogenous distribution of blood supply and O2. In general, the periphery of the tumors has better blood supply and thus, more access to O2, while the core of the tumors, which is typically poorly perfused can be severely hypoxic (8, 9), a feature that contributes significantly to tumor cell heterogeneity (10–12).
In essence, the physiologic and pathologic O2 conditions that are characteristic of normal or diseased states play important roles in mediating homeostasis and disease pathogenesis. This calls into question the current approaches to studying mammalian cells in vitro. Despite the fact that healthy and cancerous cells reside in significantly lower O2 tensions, in vitro manipulation of many different cell types is carried out under ambient (~19–21%) O2 conditions (6). The purpose of this review is to provide an overview of findings related to the beneficial effects of studying healthy and cancer cell types under physiologically/pathologically relevant O2 conditions. We discuss the impact of ambient O2 conditions on cancer cell behavior with a focus on findings from our recent work which demonstrated effects of ambient air on signaling pathways, specific tumor cell populations, and response to therapy (13).
OXYGEN CONSIDERATIONS FOR IN VITRO STUDIES
The definition of various O2 levels, especially with regards to how it relates to multicellular organisms is somewhat arbitrary. This is particularly true for the definition of normoxia. While one would imagine that the term “normoxia” describes “normal” physiologic O2 levels in tissues and organs, it is used to describe ambient O2 conditions to which tissues and cultured cells are exposed (14). A substantial number of comparative O2 studies involve analyses of different cell types under normoxic and other O2 conditions to observe for O2-dependent differences (15–18). One important O2 condition to which cells or tissues under normoxia are compared is hypoxia. Hypoxia is the condition whereby tissue O2 levels are decreased either due to impaired blood supply or decreased blood O2 levels (19). In the context of in vitro and ex vivo experimental systems, the hypoxic O2 level is typically set at conditions <2% O2 (20) although, some studies have considered O2 levels ranging between 3% and 7% as hypoxic, depending on experimental design (21–23). Thus, further emphasizing the arbitrary nature of the terms used to describe different O2 levels.
Physioxia on the other hand describes physiologic O2 concentrations within tissues and organs and is also referred to as “tissue normoxia” (24). In general, O2 levels in each tissue or organ are determined by the amount of blood supply to that organ (8). Therefore, in highly perfused organs, O2 levels are typically higher than in less perfused organs and tissues (25). The bone marrow (BM), however, appears to be an exception to this rule, with significant heterogeneity in O2 distribution. The highly vascular peri-sinusoidal regions have the lowest O2 levels (1.3%) while the endosteal regions have slightly higher O2 concentrations (1.8%). The outer periosteal layer is relatively non-hypoxic, with O2 levels as high as 7%, due to the relatively lower cellularity and reduced metabolic activity, compared to the BM (9). This is in line with the fact that the amount of blood (and O2) supply to tissues is governed also by their metabolic demands (10). This gradient of O2 tensions has been described in other organs. In the brain, the mean pO2 decreases with increasing depth from the dura mater (11, 12). In contrast to the brain tissue, the pO2 in the skin tissues increases with depth from the epidermis towards the sub-papillary plexus (26). In the kidney, higher cortical O2 levels, relative to the medulla, has been reported (27, 28). Furthermore, hepatocytes that make up the liver parenchyma are subjected to a gradient of O2 tensions. Hepatocytes in the periportal zone of the hepatic lobule are exposed to higher amounts of O2 compared to those located in the perivenous regions. This influences the heterogeneity and plasticity of these cell types with significant impact on differentiation and maturation of hepatocytes and subsequent metabolic zonation (29–32).
Based on these highly regulated variations in the distribution of O2 among and within tissues/organs, there is a consensus that mammalian cells of diverse types are significantly impacted by exposure to ambient O2 tension as it exceeds O2 levels within the in vivo microenvironment (33). For this reason, several studies have yielded important data on how culturing and propagating cells under physioxia may be beneficial to understanding cell behavior in the context of their in vivo microenvironment. Since physiologic O2 levels are known to range between 2–9%, experimental physioxic O2 levels are typically set at 5%, with 2–3% O2 representing the lower limit (34). Numerous studies have demonstrated the impact of physioxia in preserving important stem cell types such as induced pluripotent, embryonic, and adult stem cells. For example, mesenchymal adipose-derived stem cells (ASCs) grown under hypoxic (2–3% O2) conditions have been shown to exhibit stemness maintenance, improved proliferative capacity and effective differentiation following osteogenic stimuli (35). Such beneficial effects have also been reported for physioxia on the chondrogenic potential of BM derived mesenchymal stem cells (MSCs) (36–39). Further studies into the differential effects of physioxia on ASCs by Chen and colleagues (40) revealed positive effects of physioxia on their proliferation, migration, angiogenesis, and survival. Data from studies involving four human MSC lines showed beneficial impact of physiologic O2 tensions on the genetic stability, decreased oxidative stress and resultant survival of mesenchymal stem cells (41). Similar beneficial effects have been demonstrated with central nervous system stem cells (42), neural crest stem cells (43), lacrimal gland derived MSCs (44), nasal olfactory mucosal MSCs (45), umbilical cord MSCs (46), skeletal muscle-derived stem cells (47) and hematopoietic stem cells (48–54). The impact of physioxia on various stem cells has been reviewed extensively (25).
With regards to non-stem cell subtypes, early studies revealed a potential for an increase in the proliferative lifespan of normal diploid cells if they are cultured in O2 conditions lower than 10% (55). Numerous findings have also been reported with regards to the effects of physioxia on mechanistic processes in non-stem cells (56). Nishikawa and colleagues (57) explored the effects of changes in in vivo O2 tensions on osteoclastogenesis. They determined 5% and 2% O2 levels correspond to physioxia and physiologic hypoxia, respectively, in osteoclasts. O2 perturbation involving minimal changes within the physiologic range of O2 tensions significantly impacted osteoclastogenesis via mechanisms presumed to be associated with O2 sensing roles for ten-eleven translocation (TET) enzymes (57). Other studies have demonstrated the differential effects of ambient and physioxic O2 levels on cellular redox homeostasis. The underlying hypothesis of these studies being that exposure of cultured cells to ambient air induces development of reactive O2 species (ROS) that influences cell behavior. Ferguson et al (58) evaluated the effects of photodynamic irradiation therapy on A431 human epidermoid carcinoma cells which have either been exposed to ambient O2 conditions for extended periods or maintained under physioxic conditions. The A431 cells cultured under hyperoxic conditions responded to photodynamic irradiation through increased production of mitochondrial ROS and subsequent upregulation of genes associated with maintenance of redox homeostasis, relative to the cells under physioxia. These differences, however, did not translate into differences in the viability of the treated cells under both O2 tensions, suggesting that the antioxidant response of the cells treated under hyperoxic O2 conditions protects the cells from oxidative damage. Ambient air induced increases in cellular ROS may also be associated with the induction of O2–consuming cellular enzymes including nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (Nox4), nitric oxide synthases (NOS) and monoamine oxidase (MAO) (59). This increase in ROS production may cause cells to senesce, die or adapt to these oxidative products. However, cells that adapt to such conditions may experience changes to their phenotypes, which may confound experimental findings (60, 61). A review of the effects of O2 induced ROS production in in vitro cell culture is provided by Jagannathan et al (6).
Therefore, it can be concluded that in vitro manipulation of both stem and non-stem cell subtypes should be carried out under O2 conditions that are optimal for their growth and maintenance of their in vivo phenotypes. Indeed, multiple cell types were demonstrated to benefit from O2 tensions that range between 5–8%, which mirrors physioxic O2 conditions in tissues and organs in vivo. The 5–8% range in O2 levels was shown to be not too low to cause anoxia and not high enough to induce O2 induced oxidative injury, but just right – the so called “Goldiloxygen” zone (62).
EXTRAPHYSIOLOGIC OXYGEN SHOCK/STRESS
Hematopoietic stem cells (HSCs) reside in environments with reduced O2 tension in vivo (63, 64). Studies into EPHOSS evaluated the effects of even brief exposure of HSCs (< 1 hour) collected from mouse BM to room O2, regardless of eventual studies under physioxia or room O2 (48, 65). The collection and processing of HSCs from BM and cord blood (CB) in ambient air significantly decreased HSC yield due to rapid differentiation into hematopoietic progenitor cells (HPCs). Collection and processing of the BM and CB cells under physioxia (3% O2) led to increased yield of BM HSCs and BM engraftment capacity as determined via analyses of various phenotypic markers and competitive BM transplantation experiments. The differentiation of HSCs into HPCs occurred through a phenomenon that was referred to as EPHOSS. These studies revealed that the mechanisms responsible for EPHOSS may be associated with the increased production of mitochondrial ROS and an associated induction of mitochondrial transition permeability pore (MPTP) opening via regulatory mediation by cyclophilin D. In addition, p53 was implicated in EPHOSS due to positive effects of p53 deletion on HSC yield. Furthermore, hypoxia inducible factor 1 alpha (HIF-1α) and miR210 were linked to EPHOSS although mechanism(s) of this linkage is not well understood (48). Similar effects were observed for the preservation of HSC populations and improved BM engraftment capacity when mobilized mouse peripheral blood was collected at 3% O2 (53).
In subsequent studies, it was discovered that the collection and processing of aged mouse BM hematopoietic cells at 3% O2 enhanced the number and engraftment capability of HSCs that were otherwise presumed to be limited in their capacity for long-term repopulation (54). Similarly, despite known deficiency of HSCs and HPCs in the BM of Fanca and Fancc knockout mice, collection and processing of BM cells from these mice under physioxia led to significant increases in the yield of long-term HSCs (50). Newer mechanistic insights suggest roles for Calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and TET2 in HSC to HPC differentiation fate determination and HSC self-renewal respectively (51, 52).
Although these findings are more directly linked to the hematopoietic system, they provide compelling insights as to how other cell types can be influenced by even short-term exposure to ambient air. Cancer cells have been studied extensively under hypoxia. However, the findings of studies involving HSCs (48) beg the question of whether the initial propagation of cancer cells in air, regardless of eventual transfer to low O2, influences experimental findings and thus, suggests the need to reevaluate cancer cells in the context of EPHOSS. We explored the impact of EPHOSS on cancer cells utilizing animal models of breast cancer and human cancer cells (13).
EXTRAPHYSIOLOGIC OXYGEN SHOCK/STRESS IN CANCER
Most solid tumors are highly hypoxic (19, 66). However, O2 concentration in solid tumors is anisotropic (12, 34, 67). Specifically, solid tumors are characterized by regions of physioxia (~8% O2), hypoxia (~1% O2) and anoxia (0% O2) (12). This suggests that cancer cells within solid tumors are not necessarily subjected to a fixed amount of O2 supply. This notion is supported by reports that solid tumors can undergo cycling hypoxia, which describes spatio-temporal fluctuations in O2 tension, that leads to alternating levels of hypoxia within the same tumor (68–72). While O2 concentrations in healthy breast tissues range between 6.8% to 9% or higher, the median O2 concentration in about 40% of breast tumors is about 3.9% and can be as low as 1.3% (34, 73).
The effects of O2 levels on tumor cell behavior have been studied extensively, with the vast majority involving comparative studies between ambient O2 and hypoxia (0.5%−1%), an O2 concentration required to induce activity of the HIF proteins (74–78). However, majority of these studies were carried out only after the cells have already been exposed to ambient O2 conditions. Furthermore, majority of preclinical cancer studies, including evaluation of cancer cell response to target drugs were done in room O2. Interestingly, very few studies have explored the toxic effects of ambient O2 on cancer cells. Early studies showed that FS19 murine sarcoma cells processed from growing tumors and propagated in vitro under culture conditions in atmospheric O2 failed to proliferate, compared to those grown at 5% O2 (79). More recently, comparative studies involving four patient-derived breast cancer cell lines grown at 5% O2 and other established, routinely used breast cancer cell lines grown in ambient air revealed increased expression of the HIF-1 target gene, carbonic anhydrase 9 and decreased ROS production at 5% O2, relative to ambient air (80). Signaling pathway differences and response to targeted drugs were, however, cell line dependent with the conclusion of this study being that breast cancer cells grown at 5% O2 are mostly similar to those in ambient air. It is, however, important to note that both studies involved the use of cancer cells initially processed in ambient air prior to culture at 5% O2.
In our recent work (13), we asked whether observations made in HSCs with respect to initial exposure to ambient air also applies to tumor cells. We utilized tumor cells from transgenic mammary tumor mouse models (MMTV-PyMT and MMTV-Her2) and metastatic cells in ascites fluid collected from ovarian cancer patients. Tumor tissues were harvested under physioxia (3% O2), minced, and split into two parts which were processed into single cells under physioxia and ambient air (21%). The cells were either stained directly for analyses of CSC marker profiles, prepared for proteome analyses, re-transplanted into animals or cultured at 5% O2 and 21% O2. To ensure that observed differences were associated only with the differential O2 tensions and not intertumoral variability, the tumor cells were processed from fractions of the same tumor tissue. The ascites fluid was collected using uniquely designed syringes/needles that had been calibrated to physioxia and ambient air, respectively. The samples were then transferred immediately to the respective O2 workstations. An important aim of our experimental approach was to ensure that the tumor cells being collected, processed and evaluated under physioxia were never exposed to room O2 (Fig. 1; A and B). In a combination of flow cytometric, proteomics and sequencing approaches, we demonstrated variations in CSC marker profiles, relevant cell signaling pathways, ROS generation, alternative splicing, and response to targeted drugs due to experimental physioxic or ambient O2 conditions (Fig. 2) (13).
Figure 1.
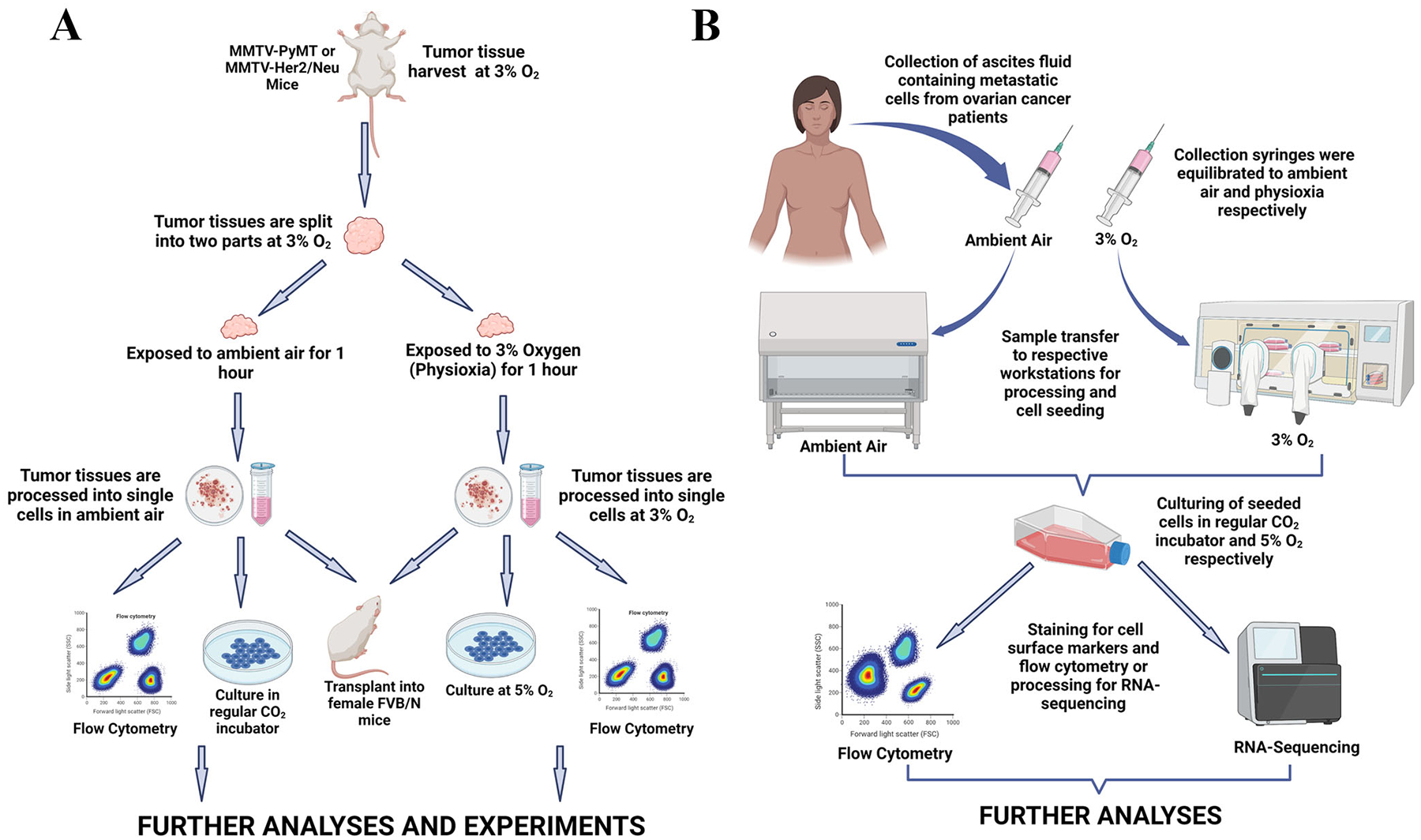
Schematic representation of experimental workflow. A. Mouse mammary tumor tissues were harvested under physioxia, split into two parts and processed into single cells at respective oxygen tensions. Processed cells were either stained for flow cytometry, cultured for 5 days or transplanted into female FVB/N mice for further studies. B. Ascites fluid was collected from ovarian cancer patients with uniquely designed syringes previously equilibrated to ambient air and physioxia. Collected samples were transferred immediately to respective workstations, processed and cultured for 5 days prior to flow cytometric and sequencing analyses. Figures were created with BioRender.com.
Figure 2.
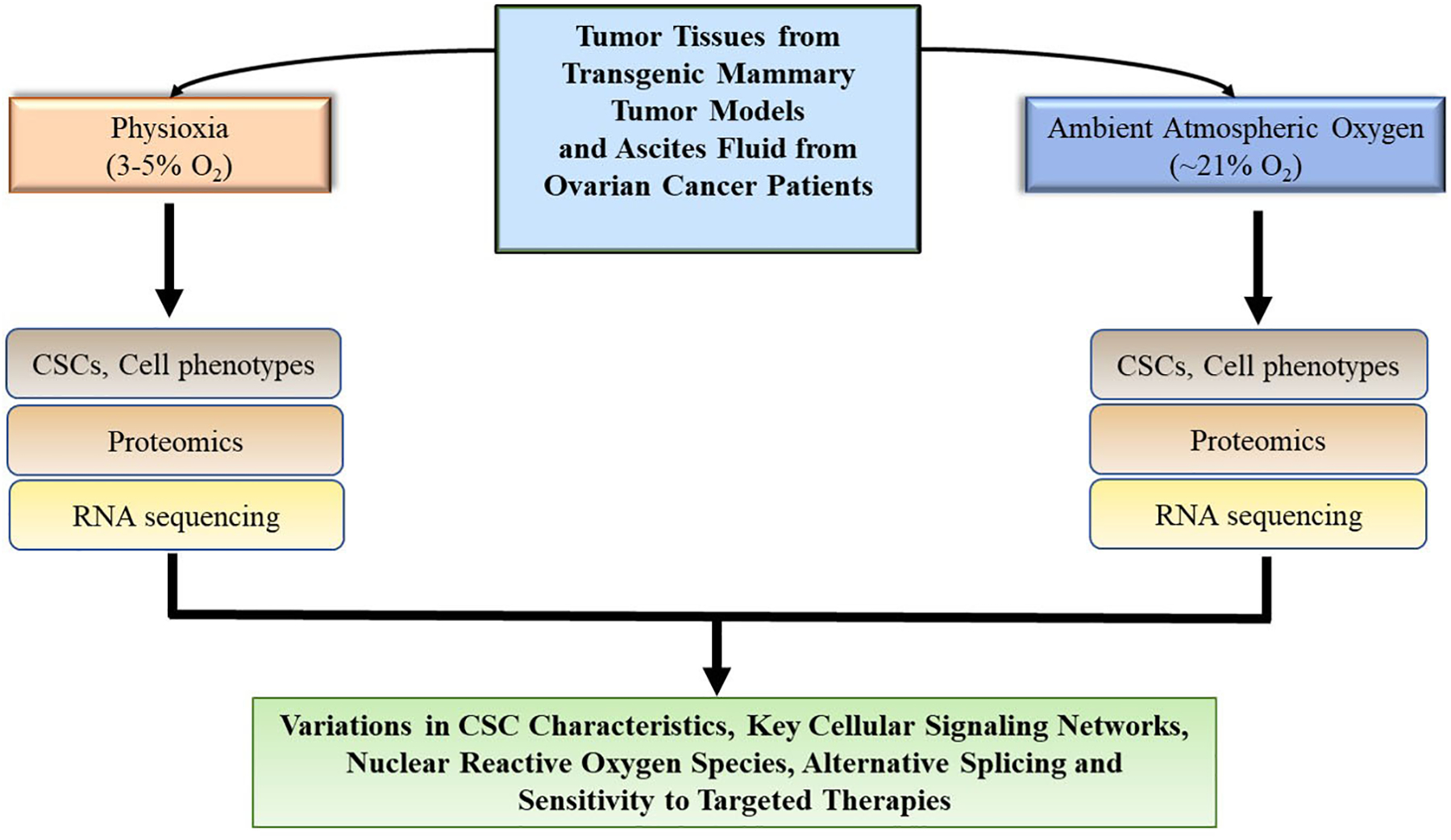
Summary of experimental findings. Flow cytometric analyses of cell phenotypes, proteomics and sequencing analyses of tumor cells in ambient air and physioxia revealed differences in relevant signaling pathways, generation of reactive oxygen species, alternative splicing and response to targeted drugs.
CSCs are important for cancer progression (81–84). To evaluate the impact of ambient air exposure to CSC marker profiles, we selected LGR5 and TSPAN8 as markers of mammary tumor stem cells. LGR5 is a biomarker of CSCs (85) which contributes to breast cancer progression via the Wnt/beta-catenin signaling axis (86). TSPAN8 is upregulated in breast CSCs and maintains stemness via expression of genes such as NANOG and OCT4 (87). Flow cytometric analyses revealed an enrichment of LGR5+ cells in both PyMT and Her2/Neu tumor models under physioxia, relative to ambient air. However, no significant changes were observed in the number of TSPAN8 positive cells in both models. Importantly, we demonstrated that the observed increase in number of LGR5+ cells is not transient, as similar observations were made following re-transplantation of the collected tumor cells into syngeneic female FVB/N mice. 2D culture conditions were however found to deplete LGR5+ cell populations under both physioxia and ambient air. We circumvented this effect via mammosphere culture conditions, which showed an enrichment of LGR5+/TSPAN8+ tumor cells. Other stem cell markers, including CD61, CD49f and EpCAM also exhibited O2 dependent changes, but observations varied based on tumor model, culture or transplant conditions. To further confirm enrichment of CSCs under physioxia, we demonstrated an increase in the expression of stemness associated genes, including Bmp6, Zeb1 and Gli2 compared to ambient air.
ROS are produced as by-products of O2 metabolism in a regulated fashion to maintain cellular homeostasis (88). The oxido-reductive processes that regulate cellular ROS production are regulated in a spatial and temporal fashion (89–91). We evaluated the impact of the differential O2 tensions on ROS production in various cellular compartments using membrane-permeant redox-sensitive fluorescent probes that were analyzed via flow cytometry. We observed increased levels of nuclear ROS production in the tumor cells under physioxia. However, cytoplasmic and mitochondrial ROS production showed no significant difference. We then proceeded to ask whether there is a correlation between LGR5 and/or TSPAN8 positivity and nuclear ROS production. LGR5+ cells were enriched within the nuclear ROS producing cell population under physioxia and ambient air. This enrichment was, however, more significant under physioxia. Thus, LGR5+ cells likely generate ROS, which probably influences their activity. Surprisingly, the increase in nuclear ROS in tumor cells under physioxia did not translate into activation of NRF2 in these cells. NRF2 is associated with oxidative stress and mediates activation of antioxidant genes (92–94). The collection/processing of tumor cells in ambient O2 led to a stabilization of NRF2. On the other hand, KEAP1, which is a negative regulator of NRF2 (95, 96) was differentially expressed under physioxia. This differential activity of NRF2 in ambient air likely occurred due to stresses induced by exposure of the cells to room O2. We speculate that the increased expression of NRF2 in ambient air may have contributed to the observed decrease in nuclear ROS. The adaptation of routinely used tumor cell lines to continuous propagation in ambient O2 is likely due to sustained development of an antioxidant response to O2 exposure and subsequent protection from oxidative stress. In support of this notion, FS19 murine sarcoma cells already adapted to growth in ambient air became more sensitive to O2 following inhibition of glutathione (79). Indeed, glutathione has been reported to be elevated in multiple forms of cancer (97) and novel therapies targeted against glutathione are being explored as potential anticancer agents (98, 99). It would be interesting to determine how much of the elevated levels of glutathione and antioxidant enzymes such as superoxide dismutase (100) in various cancers is influenced by exposure of tissues/cell to ambient air. It is likely that some of the observed anticancer effects of glutathione inhibitors (101) are due at least in part to the loss of antioxidant protection from O2 associated oxidative stress. In addition, immortalization and transformation of certain cell lines for cancer studies may protect the cells from the detrimental effects of excessive O2, possibly due to a decreased potential for replicative senescence (6). Furthermore, mechanisms associated with enhanced glycolytic processes in immortalized and cancer cells may confer adaptative properties that allow them to grow in high O2 levels for extended periods (6, 102–104). While the role of NRF2 in the mediation of oxidative processes in cancer cells has been studied extensively (105–107), it may be necessary to reevaluate some of its functions under physioxia, while concurrently eliminating ambient air as a confounding factor.
In addition to NRF2 signaling, maintenance of tumor tissues/cells under physioxia impacted several other signaling pathways including YAP signaling, Cyclin D1/cell proliferation, alternative splicing machinery, and response to targeted therapies. For example, cells under physioxia expressed lower levels of cyclin D1, which correlated with lower proliferation rate compared to cells maintained in ambient air. This is in line with previous observations that spheroids derived from HCT116 colon adenocarcinoma cells under physioxia exhibited significantly decreased growth rates, compared to those grown in ambient air (108). Additionally, cells under physioxia were less sensitive to targeted therapies. This is consistent with the notion that cancer cells with stem cell properties are slow proliferating and slow proliferating cells are less sensitive to therapies (109–112). These findings raise the question of ideal experimental conditions for cancer tissue collection, processing, and culturing for drug screening studies. Furthermore, since the epigenome significantly affects sensitivity to targeted therapies, our published report on the effects of O2 tension on the levels of the epigenetic regulator BRD4 (13) and other studies showing O2 sensing roles for epigenetic regulators TET2, KDM5A and KDM6B (51, 113, 114) suggests that observed differences in drug sensitivity under physioxia and ambient O2 may be partially influenced by differences in epigenome. However, most other studies on the influence of O2 tension on epigenome compared the epigenome under hypoxia with ambient air without considerations for initial exposure of the cells in hypoxia to ambient air. Studies involving fate-mapping of hypoxic cells revealed that tumor cells that experienced intratumoral hypoxia exhibited gene expression patterns distinct from those that were exposed to hypoxia in vitro (115). Although many different factors may have contributed to this finding, there is a likelihood that in vivo hypoxic conditions diminish ambient air induced cellular changes.
CONCLUSION AND PERSPECTIVES
Numerous studies have evaluated the impact of physiologically and pathologically relevant O2 on different cell types, including stem, adult and cancer cells of diverse types. However, these prior studies were done using tissues and cells that were initially collected, processed and propagated under ambient air before eventual transfer to workstations with low O2 levels. We demonstrated the impact of ambient air on stemness phenotype, intracellular signaling and response to therapy. Our studies examined the effect of exposing tumor tissues to ambient O2 for one hour compared to maintenance throughout at physiologic O2. Rationale for considering one hour exposure to ambient air is that, in our experience, it typically takes one hour for tumor tissues to arrive to research labs after surgery if the process of collection and distribution is well coordinated. These studies are of importance, considering the limited translatability of preclinical findings into clinical findings in cancer research. The observed differences in the response of the studied tumor cells to therapy based on differences in O2 levels suggests a need for the consideration of O2 in preclinical cancer studies. More importantly, the differential impact of ambient air and physioxia on relevant signaling pathways and cellular biomarkers provide a compelling basis for the evaluation of cancer cells in the context of EPHOSS. For example, the development of effective therapies against NRF2, based on its presumed importance for cancer progression has been met with inconsistencies (116–118). Data from our recent study adds another layer of complexity to this inconsistency, considering the sensitivity of NRF2 signaling to differential O2 tensions.
Cancer cell quiescence is associated with resistance to therapy and a quiescent state is characteristic of cancer cells with stemness properties (111, 112). We demonstrated the impact of the differential O2 tensions on the expression of stemness associated biomarkers. In line with these findings, signaling pathways associated with cell cycle progression were found to be less active under physioxia, with the tumor cells under physioxia being less proliferative than those in ambient air. We speculate that the combined differences in stemness marker profile and signaling pathway differences culminate in the development of a quiescent state that contributes to the development of resistance to therapy under physioxia. The perturbation of this intricate network perhaps contributes in part to the efficacy of hyperbaric cancer treatment in sensitizing cancer cells to some chemotherapeutic drugs (119–121), considering the impact of high dose O2 treatment on cancer cell metabolism and their redox state (121).
Metastasis is the primary cause of cancer death (122, 123). There has been limited progress in developing metastasis-targeted therapies. Paired genome analysis of primary tumors and metastasis have revealed differences in metastasis compared to primary tumors but none as new therapeutic targets (124, 125). Other studies showed very minimal differences in the genome of primary and metastatic tumors (126, 127). A recent study that analyzed treatment-naïve, primary and metastatic breast cancer identified potentially druggable alterations in genes associated with cancer epigenetics, stemness and drug resistance in metastasis although the bulk of the genomic changes observed were similar in both the primary and metastatic tumors (128). It is possible that epigenome/transcriptome of metastatic cells undergo metastasis organ-site specific changes based on tissue O2 levels, which genomic sequencing cannot identify. Therefore, studies that compare signaling in metastasis with signaling in primary tumor may need to consider O2 levels in organ site of metastasis while modeling their studies. This is especially relevant for studies on brain metastasis where O2 levels are quite low compared to other organs.
The findings of our study provide new insights into the impact of initial and prolonged exposure of tumor cells to ambient air. Surprisingly, we detected no increases in HIF signaling in tumor cells under physioxia, suggesting that some of the signaling differences observed under physioxia may be independent of HIF activation. This is, however, in contrast to other reports that both normal and cancer cells cultured at 5% O2 exhibited an increase in the levels of transcriptionally active HIF-1α, with resulting impact on proliferative capacity (129, 130), perhaps, indicating cell line to cell line differences. Most studies that examined HIF-independent hypoxia signaling compared signaling under hypoxic state with ambient air. Few of these HIF-independent signaling under hypoxia may be active under physioxia as well. In this regard, multiple differentially abundant proteins were found to overlap in three B-cell lymphoma cell lines under physioxia and hypoxia, suggesting that distinct mechanistic processes may govern tumor cell response to physioxic and hypoxic conditions (131). Therefore, it may be necessary to reevaluate few of these signaling pathways in a gradient of O2 levels instead of the fixed 1% to 21% O2 comparison.
Ambient air induced changes in stem cell marker profiles, transcriptome and signaling networks suggest the need for preclinical cancer studies to be carried out in the context of EPHOSS. An additional factor to consider in such studies would be creating an in vitro system that more closely mimics the tumor microenvironment in vivo. This is especially important for studying tumor cells in the context of their interactions with other cells that are present within a microenvironment that is characterized by decreased O2 levels (132–134). Considering the fact that 2D culture systems are limited by factors such as a lack of a tumor microenvironment, perturbation of gene expression and changes in tumor cell phenotype (135, 136), 3D co-culture systems that allow for cancer cell interaction with other relevant cell types (137–140) in O2 controlled experimental settings may aid better understanding of cancer cell behavior. While O2 considerations alone by no means address all the challenges associated with ensuring translatability of cancer research, we propose that it should be considered one more step forward in ongoing efforts to improve the status quo.
Funding source:
Falk Medical Trust Catalyst and Transformative Award, Department of Defense and the Susan G Komen for the Cure (to HN).
Footnotes
Dedication: This article is dedicated to the memory of Dr. Hal E. Broxmeyer for his contributions to science, his mentorship and unwavering support. He will be sorely missed.
Authors have no conflict of interest to declare
REFERENCES
- 1.Stamati K, Mudera V, Cheema U. Evolution of oxygen utilization in multicellular organisms and implications for cell signalling in tissue engineering. J Tissue Eng. 2011;2(1):2041731411432365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedges SB, Blair JE, Venturi ML, Shoe JL. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol Biol. 2004;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meadows VS, Reinhard CT, Arney GN, Parenteau MN, Schwieterman EW, Domagal-Goldman SD, et al. Exoplanet Biosignatures: Understanding Oxygen as a Biosignature in the Context of Its Environment. Astrobiology. 2018;18(6):630–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt TD, Spiegel DS. Prospects for detecting oxygen, water, and chlorophyll on an exo-Earth. Proc Natl Acad Sci U S A. 2014;111(37):13278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kump LR. The rise of atmospheric oxygen. Nature. 2008;451(7176):277–8. [DOI] [PubMed] [Google Scholar]
- 6.Jagannathan L, Cuddapah S, Costa M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr Pharmacol Rep. 2016;2(2):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittman RN. Regulation of Tissue Oxygenation. Integrated Systems Physiology: From Molecule to Function to Disease. San Rafael (CA)2011. [Google Scholar]
- 8.Klein D The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front Oncol. 2018;8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–65. [PubMed] [Google Scholar]
- 11.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakotomalala A, Escande A, Furlan A, Meignan S, Lartigau E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front Endocrinol (Lausanne). 2021;12:742215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar B, Adebayo AK, Prasad M, Capitano ML, Wang R, Bhat-Nakshatri P, et al. Tumor collection/processing under physioxia uncovers highly relevant signaling networks and drug sensitivity. Sci Adv. 2022;8(2):eabh3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med. 2017;113:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Liu B, Deng T, Zhong J, Feng Z, Zeng Q, et al. Normoxia is not favorable for maintaining stemness of human endothelial progenitor cells. Stem Cell Res. 2019;38:101464. [DOI] [PubMed] [Google Scholar]
- 16.Angeli-Terzidou AE, Gkotinakou IM, Pazaitou-Panayiotou K, Tsakalof A. Inhibition of calcitriol inactivating enzyme CYP24A1 gene expression by flavonoids in hepatocellular carcinoma cells under normoxia and hypoxia. Arch Biochem Biophys. 2021;704:108889. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Huang Q, Zhao J, Dong Y, Zhang L, Fang X, et al. The Impacts of Different Types of Radiation on the CRT and PDL1 Expression in Tumor Cells Under Normoxia and Hypoxia. Front Oncol. 2020;10:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Wang C, Chen X, Takada M, Fan C, Zheng X, et al. EglN2 associates with the NRF1-PGC1alpha complex and controls mitochondrial function in breast cancer. EMBO J. 2015;34(23):2953–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–76. [DOI] [PubMed] [Google Scholar]
- 20.Chen PS, Chiu WT, Hsu PL, Lin SC, Peng IC, Wang CY, et al. Pathophysiological implications of hypoxia in human diseases. J Biomed Sci. 2020;27(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed NE, Murakami M, Kaneko S, Nakashima M. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci Rep. 2016;6:35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Wang JH. Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS One. 2013;8(4):e61424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347(1):12–21. [DOI] [PubMed] [Google Scholar]
- 24.Hammond EM, Asselin MC, Forster D, O’Connor JP, Senra JM, Williams KJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol). 2014;26(5):277–88. [DOI] [PubMed] [Google Scholar]
- 25.Mas-Bargues C, Sanz-Ros J, Roman-Dominguez A, Ingles M, Gimeno-Mallench L, El Alami M, et al. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int J Mol Sci. 2019;20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandkar MR, Dhamdhere SG, Shukla S. Oxygen gradient and tumor heterogeneity: The chronicle of a toxic relationship. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188553. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Hu D, Ying W. A fast numerical method for oxygen supply in tissue with complex blood vessel network. PLoS One. 2021;16(2):e0247641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69(3):708–64. [DOI] [PubMed] [Google Scholar]
- 30.Kang YBA, Eo J, Mert S, Yarmush ML, Usta OB. Metabolic Patterning on a Chip: Towards in vitro Liver Zonation of Primary Rat and Human Hepatocytes. Sci Rep. 2018;8(1):8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87(8):1315–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonon F, Giobbe GG, Zambon A, Luni C, Gagliano O, Floreani A, et al. In vitro metabolic zonation through oxygen gradient on a chip. Sci Rep. 2019;9(1):13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ast T, Mootha VK. Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat Metab. 2019;1(9):858–60. [DOI] [PubMed] [Google Scholar]
- 34.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fotia C, Massa A, Boriani F, Baldini N, Granchi D. Prolonged exposure to hypoxic milieu improves the osteogenic potential of adipose derived stem cells. J Cell Biochem. 2015;116(7):1442–53. [DOI] [PubMed] [Google Scholar]
- 36.Pattappa G, Johnstone B, Zellner J, Docheva D, Angele P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattappa G, Schewior R, Hofmeister I, Seja J, Zellner J, Johnstone B, et al. Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis. Cells. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattappa G, Krueckel J, Schewior R, Franke D, Mench A, Koch M, et al. Physioxia Expanded Bone Marrow Derived Mesenchymal Stem Cells Have Improved Cartilage Repair in an Early Osteoarthritic Focal Defect Model. Biology (Basel). 2020;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan Safwani WKZ, Choi JR, Yong KW, Ting I, Mat Adenan NA, Pingguan-Murphy B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology. 2017;75:91–9. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Tang Q, Zhang Y, Yu M, Jing W, Tian W. Physioxia: a more effective approach for culturing human adipose-derived stem cells for cell transplantation. Stem Cell Res Ther. 2018;9(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estrada JC, Albo C, Benguria A, Dopazo A, Lopez-Romero P, Carrera-Quintanar L, et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19(5):743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20(19):7377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth M, Spaniol K, Kordes C, Schwarz S, Mertsch S, Haussinger D, et al. The Influence of Oxygen on the Proliferative Capacity and Differentiation Potential of Lacrimal Gland-Derived Mesenchymal Stem Cells. Invest Ophthalmol Vis Sci. 2015;56(8):4741–52. [DOI] [PubMed] [Google Scholar]
- 45.Zhuo Y, Wang L, Ge L, Li X, Duan D, Teng X, et al. Hypoxic Culture Promotes Dopaminergic-Neuronal Differentiation of Nasal Olfactory Mucosa Mesenchymal Stem Cells via Upregulation of Hypoxia-Inducible Factor-1alpha. Cell Transplant. 2017;26(8):1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhilai Z, Biling M, Sujun Q, Chao D, Benchao S, Shuai H, et al. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–35. [DOI] [PubMed] [Google Scholar]
- 47.Elashry MI, Kinde M, Klymiuk MC, Eldaey A, Wenisch S, Arnhold S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res Ther. 2022;13(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161(7):1553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin DY, Huang X, Gil CH, Aljoufi A, Ropa J, Broxmeyer HE. Physioxia enhances T-cell development ex vivo from human hematopoietic stem and progenitor cells. Stem Cells. 2020;38(11):1454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broxmeyer HE, Capitano ML, Cooper S, Potchanant ES, Clapp DW. Numbers of long-term hematopoietic stem cells from bone marrow of fanca and fancc knockout mice can be greatly enhanced by their collection and processing in physioxia conditions. Blood Cells Mol Dis. 2021;86:102492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aljoufi A, Zhang C, Ropa J, Chang W, Palam LR, Cooper S, et al. Physioxia induced Downregulation of Tet2 in Hematopoietic Stem Cells contributes to Enhanced Self-renewal. Blood. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broxmeyer HE, Ropa J, Capitano ML, Cooper S, Racioppi L, Sankar U. CaMKK2 Knockout Bone Marrow Cells Collected/Processed in Low Oxygen (Physioxia) Suggests CaMKK2 as a Hematopoietic Stem to Progenitor Differentiation Fate Determinant. Stem Cell Rev Rep. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aljoufi A, Cooper S, Broxmeyer HE. Collection and Processing of Mobilized Mouse Peripheral Blood at Lowered Oxygen Tension Yields Enhanced Numbers of Hematopoietic Stem Cells. Stem Cell Rev Rep. 2020;16(5):946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capitano ML, Mohamad SF, Cooper S, Guo B, Huang X, Gunawan AM, et al. Mitigating oxygen stress enhances aged mouse hematopoietic stem cell numbers and function. J Clin Invest. 2021;131(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267(5610):423–5. [DOI] [PubMed] [Google Scholar]
- 56.Keeley TP, Mann GE. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol Rev. 2019;99(1):161–234. [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa K, Seno S, Yoshihara T, Narazaki A, Sugiura Y, Shimizu R, et al. Osteoclasts adapt to physioxia perturbation through DNA demethylation. EMBO Rep. 2021;22(12):e53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson DCJ, Smerdon GR, Harries LW, Dodd NJF, Murphy MP, Curnow A, et al. Altered cellular redox homeostasis and redox responses under standard oxygen cell culture conditions versus physioxia. Free Radic Biol Med. 2018;126:322–33. [DOI] [PubMed] [Google Scholar]
- 59.Stuart JA, Fonseca J, Moradi F, Cunningham C, Seliman B, Worsfold CR, et al. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid Med Cell Longev. 2018;2018:8238459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halliwell B Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540(1–3):3–6. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37(3):99–105. [DOI] [PubMed] [Google Scholar]
- 62.Timpano S, Guild BD, Specker EJ, Melanson G, Medeiros PJ, Sproul SLJ, et al. Physioxic human cell culture improves viability, metabolism, and mitochondrial morphology while reducing DNA damage. FASEB J. 2019;33(4):5716–28. [DOI] [PubMed] [Google Scholar]
- 63.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. [DOI] [PubMed] [Google Scholar]
- 64.Morikawa T, Takubo K. Hypoxia regulates the hematopoietic stem cell niche. Pflugers Arch. 2016;468(1):13–22. [DOI] [PubMed] [Google Scholar]
- 65.Broxmeyer HE, O’Leary HA, Huang X, Mantel C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22(4):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-Modified Cancer Cell Metabolism. Front Cell Dev Biol. 2019;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hompland T, Fjeldbo CS, Lyng H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers (Basel). 2021;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bader SB, Dewhirst MW, Hammond EM. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers (Basel). 2020;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bader SB, Ma TS, Simpson CJ, Liang J, Maezono SEB, Olcina MM, et al. Replication catastrophe induced by cyclic hypoxia leads to increased APOBEC3B activity. Nucleic Acids Res. 2021;49(13):7492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res. 2010;70(24):10019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat Res. 2009;172(6):653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chun YS, Adusumilli PS, Fong Y. Employing tumor hypoxia for oncolytic therapy in breast cancer. J Mammary Gland Biol Neoplasia. 2005;10(4):311–8. [DOI] [PubMed] [Google Scholar]
- 74.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13(2):167–71. [DOI] [PubMed] [Google Scholar]
- 75.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32. [DOI] [PubMed] [Google Scholar]
- 76.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–7. [DOI] [PubMed] [Google Scholar]
- 77.Wu Z, Zuo M, Zeng L, Cui K, Liu B, Yan C, et al. OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 2021;22(1):e50827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi R, Liao C, Zhang Q. Hypoxia-Driven Effects in Cancer: Characterization, Mechanisms, and Therapeutic Implications. Cells. 2021;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alexander P, Senior PV. Toxicity of oxygen at atmospheric concentration for newly explanted cancer cells. Biochem Pharmacol. 1986;35(1):91–2. [DOI] [PubMed] [Google Scholar]
- 80.Leung EY, Askarian-Amiri ME, Singleton DC, Ferraro-Peyret C, Joseph WR, Finlay GJ, et al. Derivation of Breast Cancer Cell Lines Under Physiological (5%) Oxygen Concentrations. Front Oncol. 2018;8:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayat H, Hayat H, Dwan BF, Gudi M, Bishop JO, Wang P. A Concise Review: The Role of Stem Cells in Cancer Progression and Therapy. Onco Targets Ther. 2021;14:2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauss A, et al. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front Immunol. 2020;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajaj J, Diaz E, Reya T. Stem cells in cancer initiation and progression. J Cell Biol. 2020;219(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu L, Lin W, Wen L, Li G. Lgr5 in cancer biology: functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy. Stem Cell Res Ther. 2019;10(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang L, Tang H, Kong Y, Xie X, Chen J, Song C, et al. LGR5 Promotes Breast Cancer Progression and Maintains Stem-Like Cells Through Activation of Wnt/beta-Catenin Signaling. Stem Cells. 2015;33(10):2913–24. [DOI] [PubMed] [Google Scholar]
- 87.Zhu R, Gires O, Zhu L, Liu J, Li J, Yang H, et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat Commun. 2019;10(1):2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorlach A, Dimova EY, Petry A, Martinez-Ruiz A, Hernansanz-Agustin P, Rolo AP, et al. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015;6:372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chio IIC, Tuveson DA. ROS in Cancer: The Burning Question. Trends Mol Med. 2017;23(5):411–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780(11):1273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaludercic N, Deshwal S, Di Lisa F. Reactive oxygen species and redox compartmentalization. Front Physiol. 2014;5:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu S, Lu H, Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8(5):2252–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278(24):21592–600. [DOI] [PubMed] [Google Scholar]
- 96.Qin JJ, Cheng XD, Zhang J, Zhang WD. Dual roles and therapeutic potential of Keap1-Nrf2 pathway in pancreatic cancer: a systematic review. Cell Commun Signal. 2019;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gamcsik MP, Kasibhatla MS, Teeter SD, Colvin OM. Glutathione levels in human tumors. Biomarkers. 2012;17(8):671–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules. 2020;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desideri E, Ciccarone F, Ciriolo MR. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients. 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hileman EA, Achanta G, Huang P. Superoxide dismutase: an emerging target for cancer therapeutics. Expert Opin Ther Targets. 2001;5(6):697–710. [DOI] [PubMed] [Google Scholar]
- 101.Yoo D, Jung E, Noh J, Hyun H, Seon S, Hong S, et al. Glutathione-Depleting Pro-Oxidant as a Selective Anticancer Therapeutic Agent. ACS Omega. 2019;4(6):10070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65(1):177–85. [PubMed] [Google Scholar]
- 103.Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22(1):85–90. [DOI] [PubMed] [Google Scholar]
- 104.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9(3):293–9. [DOI] [PubMed] [Google Scholar]
- 105.Zimta AA, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, et al. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers (Basel). 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Telkoparan-Akillilar P, Panieri E, Cevik D, Suzen S, Saso L. Therapeutic Targeting of the NRF2 Signaling Pathway in Cancer. Molecules. 2021;26(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panieri E, Saso L. Potential Applications of NRF2 Inhibitors in Cancer Therapy. Oxid Med Cell Longev. 2019;2019:8592348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomes A, Guillaume L, Grimes DR, Fehrenbach J, Lobjois V, Ducommun B. Oxygen Partial Pressure Is a Rate-Limiting Parameter for Cell Proliferation in 3D Spheroids Grown in Physioxic Culture Condition. PLoS One. 2016;11(8):e0161239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahn A, Chatterjee A, Eccles MR. The Slow Cycling Phenotype: A Growing Problem for Treatment Resistance in Melanoma. Mol Cancer Ther. 2017;16(6):1002–9. [DOI] [PubMed] [Google Scholar]
- 110.Puig I, Tenbaum SP, Chicote I, Arques O, Martinez-Quintanilla J, Cuesta-Borras E, et al. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest. 2018;128(9):3887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016;2016:1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Angelis ML, Francescangeli F, La Torre F, Zeuner A. Stem Cell Plasticity and Dormancy in the Development of Cancer Therapy Resistance. Front Oncol. 2019;9:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batie M, Frost J, Frost M, Wilson JW, Schofield P, Rocha S. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science. 2019;363(6432):1222–6. [DOI] [PubMed] [Google Scholar]
- 114.Chakraborty AA, Laukka T, Myllykoski M, Ringel AE, Booker MA, Tolstorukov MY, et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363(6432):1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Godet I, Shin YJ, Ju JA, Ye IC, Wang G, Gilkes DM. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun. 2019;10(1):4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung BJ, Yoo HS, Shin S, Park YJ, Jeon SM. Dysregulation of NRF2 in Cancer: from Molecular Mechanisms to Therapeutic Opportunities. Biomol Ther (Seoul). 2018;26(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34(1):21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawasoe Y, Yokouchi M, Ueno Y, Iwaya H, Yoshida H, Komiya S. Hyperbaric oxygen as a chemotherapy adjuvant in the treatment of osteosarcoma. Oncol Rep. 2009;22(5):1045–50. [DOI] [PubMed] [Google Scholar]
- 120.Selvendiran K, Kuppusamy ML, Ahmed S, Bratasz A, Meenakshisundaram G, Rivera BK, et al. Oxygenation inhibits ovarian tumor growth by downregulating STAT3 and cyclin-D1 expressions. Cancer Biol Ther. 2010;10(4):386–90. [DOI] [PubMed] [Google Scholar]
- 121.Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer--a review. Target Oncol. 2012;7(4):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. [DOI] [PubMed] [Google Scholar]
- 123.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18(1–2):43–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomasini P, Barlesi F, Gilles S, Nanni-Metellus I, Soffietti R, Denicolai E, et al. Comparative genomic analysis of primary tumors and paired brain metastases in lung cancer patients by whole exome sequencing: a pilot study. Oncotarget. 2020;11(50):4648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30(24):2956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brannon AR, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15(8):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Turajlic S, Furney SJ, Lambros MB, Mitsopoulos C, Kozarewa I, Geyer FC, et al. Whole genome sequencing of matched primary and metastatic acral melanomas. Genome Res. 2012;22(2):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aftimos P, Oliveira M, Irrthum A, Fumagalli D, Sotiriou C, Gal-Yam EN, et al. Genomic and Transcriptomic Analyses of Breast Cancer Primaries and Matched Metastases in AURORA, the Breast International Group (BIG) Molecular Screening Initiative. Cancer Discov. 2021;11(11):2796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carrera S, Senra J, Acosta MI, Althubiti M, Hammond EM, de Verdier PJ, et al. The role of the HIF-1alpha transcription factor in increased cell division at physiological oxygen tensions. PLoS One. 2014;9(5):e97938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dus-Szachniewicz K, Drobczynski S, Ziolkowski P, Kolodziej P, Walaszek KM, Korzeniewska AK, et al. Physiological Hypoxia (Physioxia) Impairs the Early Adhesion of Single Lymphoma Cell to Marrow Stromal Cell and Extracellular Matrix. Optical Tweezers Study. Int J Mol Sci. 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dus-Szachniewicz K, Gdesz-Birula K, Zduniak K, Wisniewski JR. Proteomic-Based Analysis of Hypoxia- and Physioxia-Responsive Proteins and Pathways in Diffuse Large B-Cell Lymphoma. Cells. 2021;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roy S, Kumaravel S, Sharma A, Duran CL, Bayless KJ, Chakraborty S. Hypoxic tumor microenvironment: Implications for cancer therapy. Exp Biol Med (Maywood). 2020;245(13):1073–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863(3):382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kapalczynska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jensen C, Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci. 2020;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saraiva DP, Matias AT, Braga S, Jacinto A, Cabral MG. Establishment of a 3D Co-culture With MDA-MB-231 Breast Cancer Cell Line and Patient-Derived Immune Cells for Application in the Development of Immunotherapies. Front Oncol. 2020;10:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Betriu N, Semino CE. Development of a 3D Co-Culture System as a Cancer Model Using a Self-Assembling Peptide Scaffold. Gels. 2018;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zoetemelk M, Rausch M, Colin DJ, Dormond O, Nowak-Sliwinska P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep. 2019;9(1):7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dwyer AR, Ellies LG, Holme AL, Pixley FJ. A three-dimensional co-culture system to investigate macrophage-dependent tumor cell invasion. J Biol Methods. 2016;3(3):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]


