Abstract
Background & Aims:
Computed tomography (CT) scans can measure quantity and distribution of adipose tissue, which are associated with breast cancer prognosis. As a novel prognostic marker, radiodensity of adipose tissue has been examined in multiple cancer types, but never in breast cancer. Lower density indicates larger adipocytes with greater lipid content, whereas higher density can reflect inflammation, fibrosis, vascularity, or even metabolic changes; and both may impact breast cancer prognosis.
Methods:
We included 2,868 nonmetastatic patients with breast cancer diagnosed between January 2005 and December 2013 at Kaiser Permanente Northern California, an integrated healthcare system. From CT scans at diagnosis, we assessed the radiodensity of subcutaneous (SAT) and visceral adipose tissue (VAT) at the third lumbar vertebra and categorized their radiodensity into three levels: low (<1 standard deviation [SD] below the mean), middle (mean ± 1 SD), and high (>1 SD above the mean). Using multivariable Cox proportional hazards regression with adjustment for clinicopathological characteristics including body mass index, we calculated hazard ratios (HRs [95% confidence intervals]) for the associations of adipose tissue radiodensity with overall mortality and breast-cancer-specific mortality.
Results:
Median age at diagnosis of breast cancer was 56.0 years, most (63.3%) were non-Hispanic White and nearly half (45.6%) were stage II. Compared to middle SAT radiodensity, high SAT radiodensity was significantly associated with increased risk of overall mortality (HR: 1.45 [1.15–1.81]), non-significantly with breast-cancer-specific mortality (HR: 1.32 [0.95–1.84]). Neither low SAT radiodensity nor high or low VAT radiodensity was significantly associated with overall or breast-cancer-specific mortality.
Conclusions:
High radiodensity of SAT at diagnosis of nonmetastatic breast cancer was associated with increased risk of overall mortality, independent of adiposity and other prognostic factors. Considering both radiodensity and quantity of adipose tissue at different locations could deepen understanding of the role of adiposity in breast cancer survival.
Keywords: Breast Cancer, Subcutaneous Adipose Tissue, Visceral Adipose Tissue, Body Composition, Radiodensity, Mortality
INTRODUCTION
Obesity, an excess of adipose tissue, is implicated in the incidence and progression of breast cancer.1 Computed tomography (CT) scans performed for breast cancer diagnosis and surveillance can provide direct measures of quantity and distribution of adipose tissue that have been investigated for breast cancer survival.2–7 However, these studies did not investigate radiodensity of adipose tissue available from CT scans, although prior research has directly compared adipose tissue biopsies with their radiodensity supporting that radiodensity can act as an marker of adipose tissue quality and may provide prognostic information in breast cancer patients.8,9 For example, higher radiodensity of adipose tissue at any deposit may reflect increased levels of local and systemic inflammation and other alterations such as increased vascularity,10,11 which may impact breast tumor progression.12
Adipose tissue radiodensity (frequently referred as ‘quality’) is indicative of adipocyte size, lipid content, inflammation, oxygenation, and angiogenesis.13,14 Compared to the middle range of adipose tissue radiodensity (sometimes referred as ‘normal’ radiodensity)15, lower radiodensity is indicative of adipocyte hypertrophy and decreased vascularity,15,16 which was reported to be associated with adverse cardiometabolic risk and biomarkers.13,17 In contrast, higher radiodensity may suggest lipid depletion, higher vascularity, extracellular matrix deposition, and stronger inflammation response.15,18 Thus, alterations in adipose tissue radiodensity may reflect the changes of microenvironment and macroenvironment that may contribute to tumor progression.19 As a novel prognosis marker, adipose tissue radiodensity has been examined for mortality among many cancer types (including colorectal,15 esophageal,20 hepatocellular carcinoma,21,22 pancreatic,23 and sarcoma24,25), but not in breast cancer yet.
Independent of quantity and location, adipose tissue radiodensity can help define new body composition phenotypes and may provide novel insights into the relationship of adiposity with mortality among patients with breast cancer. Thus, we assessed the associations of radiodensity of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) with overall mortality and breast-cancer-specific mortality among patients with breast cancer.
MATERIAL & METHODS
We used data from a retrospective cohort, the Breast Cancer, Sarcopenia and Near-term Survival (B-SCANS) study, which included 3,139 female patients diagnosed with stage I-III invasive breast cancer at Kaiser Permanente Northern California (KPNC) between January 2005 and December 2013.2,3 These patients did not have a previous history of other invasive cancers and underwent an abdominal or pelvic CT scan for diagnostic purposes within 6 months of breast cancer diagnosis but prior to any systemic therapy. We excluded 271 patients due to CT artifacts likely to influence radiodensity, such as noise, beam hardening, ring, and metal artifacts.26 Our final analytic sample included 2,868 patients, of whom 2,726 (95.0%) underwent CT scans with intravenous contrast. The institutional review board at KPNC approved this study with waiver of informed consent.
Body Composition Assessment for Adipose Tissue Radiodensity
CT scans were performed at a median of 1.1 (interquartile: 0.5–1.8) months after diagnosis. SAT and VAT were assessed on a single axial CT image at the third lumbar vertebra by two centrally trained researchers using SliceOmatic Software, version 5.0 (TomoVision Inc). Areas (cm2) of SAT and VAT were demarcated using anatomic knowledge and tissue-specific Hounsfield unit (HU) ranges: (−190 HU, −30 HU) for SAT and (−150 HU, −50 HU) for VAT.27 The coefficients of variation between two staff were 0.7% for SAT and 6.7% for VAT.2,3 The radiodensity of SAT and VAT were calculated as the average HU of the area of tissue tagged as SAT and VAT, respectively.
SAT radiodensity and VAT radiodensity followed an approximately normal distribution, with means (standard deviation [SD]) of −99.1 (7.8) and −87.7 (8.2), respectively. Radiodensity of SAT and VAT were analyzed as categorical variables with three levels corresponding to low radiodensity (<mean minus 1 SD), middle radiodensity (mean ± 1 SD), and high radiodensity (>mean plus 1 SD).
Mortality Outcomes
The primary outcome was overall mortality, and the secondary outcome was breast-cancer-specific (BC-specific) mortality. Vital status and causes of death were identified through California Department of Public Health Vital Records, the National Death Index, and KPNC mortality files. Follow-up time for overall mortality was calculated as the time from CT scan to death from any cause or the end of follow-up (December 31, 2018), whichever came first. As for BC-specific mortality, the follow-up times were censored if they died from other diseases rather than breast cancer.
Patient Characteristics
We extracted patient characteristics from clinical data recorded prospectively in the electronic medical record from the KPNC tumor registry, including age at diagnosis (years), self-reported race and ethnicity (non-Hispanic White, Black, Hispanic, Asian/Pacific Islander, Others), tumor stage (I, II, III), estrogen receptor (ER; ER+, ER−), progesterone receptor (PR; PR+, PR−), human epidermal growth factor receptor 2 (HER2; HER+, HER−, unknown), smoking (current, former, never), and neutrophil-to-lymphocyte ratio (NLR). We computed Charlson comorbidity index (0, 1–2, ≥3) from diagnosis codes (excluding cancer),28 and body mass index (BMI) from clinically measured height and weight closest to the CT scan (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2) within 6-months but prior to any systemic therapy.
Statistical Analysis
Patients’ characteristics were compared using the χ2 test for categorical variables and the Kruskal Wallis test for continuous variables by SAT and VAT radiodensity groups. Differences in overall mortality by SAT and VAT radiodensity groups were assessed using the Kaplan-Meier method and the log-rank test.29,30 Spearman correlations (rs) were calculated between SAT radiodensity, VAT radiodensity, SAT area, and VAT area.31
The hazard ratios (HRs) for the associations of SAT radiodensity and VAT radiodensity with mortality were estimated in separate models using Cox proportional hazards regression.32 We considered the following potential confounders as covariates: Model 1 included age, race and ethnicity, stage, ER status, PR status, HER2 status, Charlson comorbidity index, and smoking. Model 2 included all covariates in Model 1 plus the covariate BMI, which reflected body size as a proxy measure of total adiposity. Given that CT scans were preformed prior to treatment, adipose tissue radiodensity would not be impacted by treatment. Thus, for this analysis, treatment related factors (such as chemotherapy and radiation) were not considered as confounders. There were no missing data for continuous covariates, and missing data for categorical covariates were substituted with most frequent values.33 The proportional hazards assumption was tested using the Schoenfeld residuals method and no violation was detected.34 To flexibly model non-linear associations of adipose tissue radiodensity with overall mortality, we also conducted restricted cubic spline regression (knots = 4) using the median values as the reference levels.35
For sensitivity analysis, we 1) mutually adjusted for SAT- and VAT-related variables including SAT radiodensity, VAT radiodensity, SAT area, and VAT area into the same Cox proportional hazards regression model (Model 2) to assess whether associations were independent of each other (i.e., independence among quantity, distribution, and radiodensity of adipose tissue); 2) excluded patients without contrast CT scans, since there are known minor to mild differences in adipose tissue radiodensity when contrast materials were administered36,37 and most (95.0%) patients in our study underwent CT with contrast; and 3) further adjusted for treatment-related factors (chemotherapy and radiation) that may additionally explain mortality after breast cancer diagnosis. Additionally, we conducted subgroup analysis whether the associations of adipose tissue radiodensity with overall mortality differed by covariates of interest selected a priori based on biological plausibility,38 including age, stage, ER status, Charlson comorbidity index, smoking and BMI.
All statistical analyses were two-sided and conducted using SAS statistical software, version 9.4 (SAS Institute, Inc) and R, version 4.1.2 (R Foundation for Statistical Computing) from September 9, 2021 to May 10, 2022. P < 0.05 was considered as statistically significant and point estimates were presented with 95% confidence intervals (CIs). Multiple comparisons were not adjusted.
RESULTS
The median age at diagnosis of invasive breast cancer was 56.0 (interquartile: 48.0–65.0) years among the 2,868 women in our study. A majority were non-Hispanic White (1,816 [63.3%]), and nearly half were stage II (1,307 [45.6%]). Over a median follow-up of 7.7 years (interquartile range: 5.9–9.9] years), 626 (21.8%) patients died, 336 (11.7%) from breast cancer. Adipose tissue radiodensity was negatively correlated with adipose tissue area (rs ranging from −0.77 [for VAT radiodensity and VAT area] to −0.38 [for SAT radiodensity and VAT area]), whereas SAT radiodensity was positively correlated with VAT radiodensity (rs = 0.62). More details are presented in Table S1.
Compared to patients with middle SAT radiodensity, patients at both extremes of SAT radiodensity (>1 SD above or below the cohort mean) were more likely to be older, non-Hispanic White, have a greater number of comorbidities and have a higher NLR (Table 1). In addition, compared to patients with middle SAT radiodensity, patients with low SAT radiodensity were more likely to have higher BMI and greater areas of SAT and VAT, whereas patients with high SAT radiodensity had lower BMI and lower areas of SAT and VAT. Compared to patients with middle VAT radiodensity, patients at both extremes of VAT radiodensity tended to be stage I (Table S2). In addition, patients with low VAT radiodensity tended to be older and have a greater number of comorbidities as well as higher BMI and larger areas of SAT and VAT than those with middle VAT radiodensity, whereas these characteristics were less frequent among patients with high VAT radiodensity than those with middle VAT radiodensity.
Table 1.
Patient Characteristics by SAT Radiodensity Groups
| SAT Radiodensity | ||||
|---|---|---|---|---|
| Characteristicsa | Low (N = 318) | Middle (N = 2,183) | High (N = 367) | |
| Age (years) | 58.0 (28.0–65.0) | 56.0 (48.0–64.0) | 58.0 (45.0–69.0) | 0.03 |
| Race and Ethnicity | 0.05 | |||
| Non-Hispanic White | 220 (69.2) | 1,351 (61.9) | 245 (66.8) | |
| Black | 18 (5.7) | 164 (7.5) | 32 (8.7) | |
| Hispanic | 38 (11.9) | 259 (11.9) | 32 (8.7) | |
| Asian/Pacific Island | 42 (13.2) | 393 (18.0) | 57 (15.5) | |
| Others | 0 (0) | 16 (0.7) | 1 (0.3) | |
| Stage | 0.09 | |||
| I | 76 (23.9) | 448 (20.5) | 95 (25.9) | |
| II | 135 (42.5) | 1,005 (46.0) | 167 (45.5) | |
| III | 107 (33.6) | 730 (33.4) | 105 (28.6) | |
| ER | 0.06 | |||
| ER+ | 233 (73.3) | 1,653 (75.2) | 296 (80.7) | |
| ER− | 85 (26.7) | 530 (24.3) | 71 (19.3) | |
| PR | 0.21 | |||
| PR+ | 162 (50.9) | 1,207 (55.3) | 211 (57.5) | |
| PR− | 156 (49.1) | 976 (44.7) | 156 (42.5) | |
| HER2 | 0.24 | |||
| HER2+ | 53 (16.7) | 398 (18.2) | 58 (15.8) | |
| HER2− | 246 (77.4) | 1,612 (73.8) | 288 (78.5) | |
| Unknown | 19 (6.0) | 173 (7.9) | 21 (5.7) | |
| Charlson Comorbidity Index | <0.001 | |||
| 0 | 228 (71.7) | 1,621 (74.3) | 252 (68.7) | |
| 1–2 | 74 (23.3) | 471 (21.6) | 78 (21.3) | |
| ≥3 | 16 (5.0) | 91 (4.2) | 37 (10.1) | |
| Smoking | 0.09 | |||
| Current | 26 (8.2) | 211 (9.7) | 51 (13.9) | |
| Former | 93 (29.3) | 608 (27.9) | 103 (28.1) | |
| Never | 199 (62.6) | 1364 (62.5) | 213 (58.0) | |
| BMI (kg/m2) | <0.001 | |||
| <18.5 | 0 (0) | 12 (0.5) | 32 (8.7) | |
| 18.5–24.9 | 61 (19.2) | 693 (31.7) | 222 (60.5) | |
| 25–29.9 | 114 (35.9) | 743 (34.0) | 59 (16.1) | |
| ≥30 | 143 (45.0) | 735 (33.7) | 54 (14.7) | |
| Neutrophil-to-lymphocyte Ratio | 2.3 (1.7–3.0) | 2.1 (1.6–3.0) | 2.4 (1.7–3.5) | <0.001 |
| SAT Areas (cm2) | 291.8 (223.6–386.6) | 232.4 (170.3–314.7) | 109.4 (71.4–191.8) | <0.001 |
| VAT Areas (cm2) | 125.8 (83.1–181.4) | 91.4 (46.2–148.9) | 17.3 (7.3–65.7) | <0.001 |
Abbreviations: BMI, body mass index; CT, computed tomography; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PET/CT, Positron emission tomography/computed tomography; PR, progesterone receptor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Continuous variables were presented as median (IQR) and categorical variables as Number (%). Percentages may not add up to 100% because of rounding.
P values were calculated using the χ2 test for categorical variables and the Kruskal Wallis test for continuous variable.
Adipose Tissue Radiodensity and Mortality
Patients with middle SAT radiodensity had the lowest overall mortality (P <0.001; Figure 1A), but no clear patterns or differences in mortality rates were observed for VAT radiodensity (P = 0.41; Figure 1B).
Figure 1.
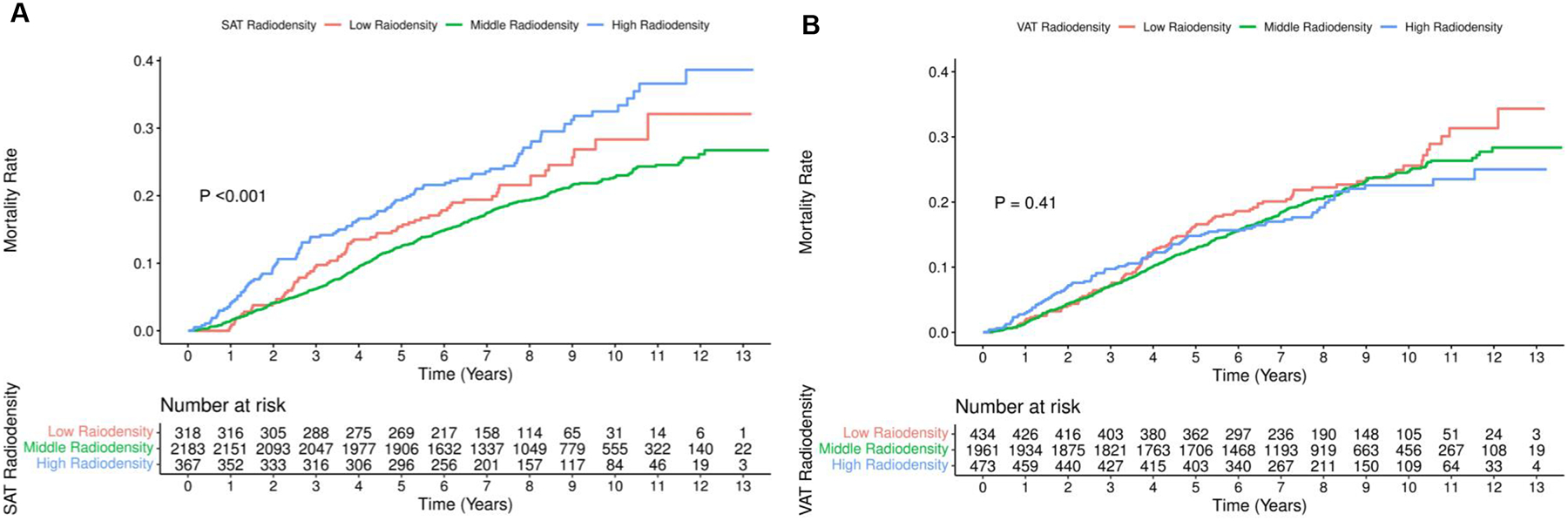
Cumulative Incidence of Mortalitya in Patients with Breast Cancer by SAT Radiodensity and VAT Radiodensity
Abbreviations: SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
a Figures 1A and 1B refer to SAT radiodensity and VAT radiodensity, respectively.
Compared to patients with middle SAT radiodensity, high SAT radiodensity was significantly associated with higher overall mortality (HR from Model 2: 1.45 [1.15–1.81]; Table 2). Associations for BC-specific mortality were in the same direction but did not achieve statistical significance (HR from Model 2: 1.32 [0.95–1.84]). Low SAT radiodensity was not significantly associated with increased risk for overall (HR from Model 2: 1.23 [0.95–1.58]) or BC-specific mortality (HR from Model 2: 1.09 [0.77–1.53]). Compared to patients with middle VAT radiodensity, high VAT radiodensity was not significantly associated with overall mortality (HR from Model 2: 1.16 [0.90–1.50]) or BC-specific mortality (HR: 1.16 [0.82–1.64]). Neither was low VAT radiodensity: the HRs from Model 2 were 1.06 (0.85–1.32) for overall mortality and 1.02 (0.75–1.39) for BC-specific mortality.
Table 2.
Hazard Ratios (With 95% CIs) of Adipose Tissue Radiodensity and Mortality among Patients with Breast Cancer
| Overall Mortality | ||
|---|---|---|
| Radiodensity | Model 1a | Model 2b |
| SAT Radiodensity, HU | ||
| Low | 1.22 (0.95–1.56) | 1.23 (0.95–1.58) |
| Middle | 1.00 | 1.00 |
| High | 1.50 (1.21–1.86) | 1.45 (1.15–1.81) |
| VAT Radiodensity, HU | ||
| Low | 1.06 (0.85–1.31) | 1.06 (0.85–1.32) |
| Middle | 1.00 | 1.00 |
| High | 1.24 (0.99–1.55) | 1.16 (0.90–1.50) |
| Radiodensity | Model 1a | Model 2b |
| SAT Radiodensity, HU | ||
| Low | 1.09 (0.78–1.53) | 1.09 (0.77–1.53) |
| Middle | 1.00 | 1.00 |
| High | 1.29 (0.94–1.76) | 1.32 (0.95–1.84) |
| VAT Radiodensity, HU | ||
| Low | 1.02 (0.75–1.38) | 1.02 (0.75–1.39) |
| Middle | 1.00 | 1.00 |
| High | 1.14 (0.84–1.56) | 1.16 (0.82–1.64) |
Abbreviations: BC-Specific, breast-cancer-specific; CI, confidence interval; HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Model 1 included the following covariates: age at diagnosis, race and ethnicity (Non-Hispanic White, Black, Hispanic, Asian/Pacific Island, Others), smoking (never, former, current), Charlson comorbidity index (0, 1–2, 3+), stage (I, II, III), ER (ER+, ER−), PR (PR+, PR−), and HER2 (HER2+, HER2−, unknown).
Model 2: Model 1 + BMI at CT scan (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2).
From restricted cubic spline regression plots (Figure 2), we observed a significant, curvilinear association for overall mortality with SAT radiodensity (P for Curvature = 0.004; P for Significance = 0.003) but not for VAT radiodensity (P for Curvature = 0.57; P for Significance = 0.66). Mirroring our results from categorical analysis, we found higher SAT radiodensity to be significantly associated with increased risk overall mortality, whereas lower SAT radiodensity was not significantly associated with increased risk of overall mortality (Figure 2A). Neither high nor low VAT radiodensity was significantly associated with increased risk of overall mortality (Figure 2B).
Figure 2.
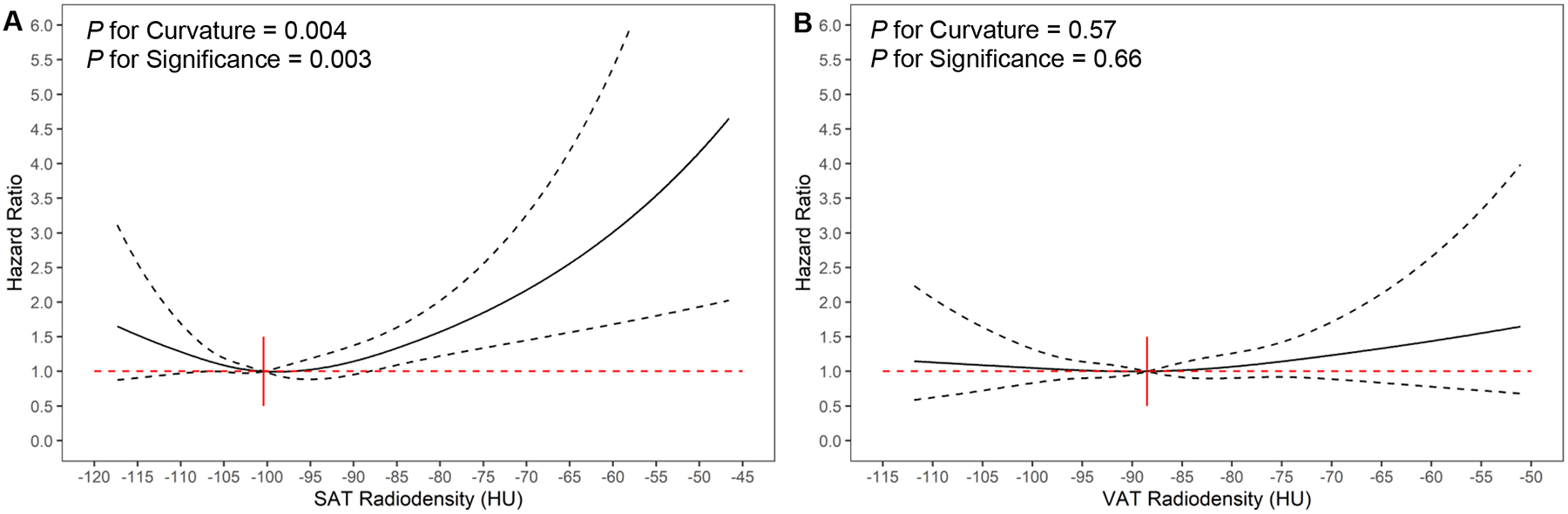
Adjusted Associationsa of Adipose Tissue Radiodensity with Overall Mortalityb among Patients with Breast Cancer Using Restricted Cubic Spline Regression
Abbreviations: HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
a The adjusted hazard ratios of adipose tissue radiodensity were calculated using restricted cubic spline regression with the median value as the reference level and all covariates included in Model 2. Point estimates of hazards ratios for adipose tissue radiodensity were plotted as the solid line and 95% confidence intervals were displayed as dotted lines. The red dotted line referred to hazard ratio equal to 1.
b Figures 2A and 2B refer to SAT radiodensity and VAT radiodensity, respectively.
Sensitivity Analysis
After mutual adjustment for SAT- and VAT-related areas and radiodensity, the findings remained similar (Table S3): only high SAT radiodensity was significantly associated with increased risk of overall mortality. Likewise, excluding patients without contrast CT scans and adjusting for treatment-related factors made little impact on the adjusted HRs (Tables S4 and S5).
Subgroup Analysis
We observed two significant interactions for 1) SAT radiodensity and Charlson Comorbidity Index and 2) VAT radiodensity and smoking (Tables S6 and S7). High SAT radiodensity was significantly associated with increased risk of overall mortality among patients with comorbidity (Charlson comorbidity index ≥1), but not for patients without comorbidity (Charlson comorbidity index = 0). Also, high VAT radiodensity was significantly associated with increased risk of overall mortality among current or former smokers, but not for never smokers.
DISCUSSION
In this cohort of 2,868 patients with nonmetastatic breast cancer, we found that compared to middle SAT radiodensity, high SAT radiodensity was significantly associated with increased risk of overall mortality. However, neither low SAT radiodensity nor high or low VAT radiodensity was significantly associated with overall or breast-cancer-specific mortality. To our knowledge, this is the first study to investigate adipose tissue radiodensity and mortality among patients with breast cancer.
To date, three studies have investigated the associations of adipose tissue radiodensity with recurrence and metastasis among breast cancer survivors,39–41 and only one study reported adjusted HRs.40 Although their primary outcomes were not mortality, recurrence and metastasis are still important landmarks for cancer progression.42 Among Korean patients with breast cancer (N = 336), increased adipose tissue radiodensities (per HU) were not significantly associated with recurrence (unadjusted HRs for SAT radiodensity: 1.04 [0.97–1.12]; VAT radiodensity: 1.00 [0.95–1.06]).39 Among Japanese patients with breast cancer (N = 271), increased VAT radiodensity (per HU) was reported to be significantly associated with metastasis (adjusted HR: 1.20 [1.01–1.43]).40 Among Belgian patients with breast cancer (N = 50), high adipose tissue radiodensity was not significantly associated with progression compared to low radiodensity (unadjusted HRs for SAT radiodensity: 1.29 [0.55–3.02]; VAT radiodensity: 0.41 [0.16–1.01]).41 In summary, the sample size of the above studies was small, and the estimates were 1) frequently unadjusted or 2) assuming a linear relationship between radiodensity and outcomes by modeling radiodensity as a continuous or binary (high vs. low) variable. This ignores the potentially different mechanisms underlying associations of lower radiodensity (lipid-rich) adipose tissue and higher radiodensity (lipid-poor or inflamed) adipose tissue with cancer outcomes. The similar scenarios apply to studies in other cancer types (including colorectal,15 esophageal,20 hepatocellular carcinoma,21,22 pancreatic,23 and sarcoma24,25), which also reported inconsistent associations of SAT radiodensity and VAT radiodensity with mortality after cancer diagnosis. Most had small sample sizes (N < 250), dichotomized radiodensity, or assumed a linear relationship, which may not fully unravel the complex biological impact of fat quality in cancer progression.15,16,18 Thus, larger studies using robust and flexible modeling strategies (like ours) are needed for breast cancer and other cancer types, which may provide more insight into the prognostic role of adipose tissue radiodensity among cancer survivors.
Our findings suggest that abdominal SAT radiodensity, rather than VAT radiodensity, is a novel characteristic defining body composition phenotype associated with mortality in breast cancer patients. Previous studies reported that abdominal SAT was more strongly correlated with breast adipose tissue (i.e. breast SAT) than VAT,43,44 thus abdominal SAT radiodensity may act as a surrogate for breast adipose tissue quality whereas VAT radiodensity may not. Since much of the breast is adipose tissue and its quality may impact the tumor microenvironment,45 abdominal SAT radiodensity may capture changes occurring throughout the body (including the breast) and provide additional information to predict prognosis after breast cancer diagnosis. This aligns with our finding that high SAT radiodensity was significantly associated with 45% higher risk of overall mortality, and was associated with 32% higher risk (although not significant) of BC-specific mortality. Possible biological explanations may be that higher SAT radiodensity indicates lipid depletion (a precursor of weight loss and cachexia),15 higher vascularity (correlated with tumor malignancy),46 extracellular matrix deposition (associated with tumor aggression and treatment failure),47 and stronger inflammatory response (a critical component in tumor progression).11 Thus, their combined contributions may result in worse progression and poor survival after breast cancer diagnosis. Supporting this, higher SAT radiodensity was significantly associated with higher NLR in our study, an important indicator for systematic inflammation which is an established prognostic marker in breast cancer.48 By contrast, lower SAT radiodensity was not significantly associated with overall and BC-specific mortality. This may be explained by the fact that lower SAT radiodensity is associated with adipocyte hypertrophy and higher SAT quantity, which was reported not to be consistently associated with breast cancer survival.2–7 Although VAT is highly metabolically active and associated with a constellation of metabolic abnormalities,12 VAT quantity together with radiodensity in this study were not significantly associated with mortality. Although the mechanism is largely unknown, one possible explanation may be that VAT in the abdomen may not directly impact tumors in the breast due to anatomical distance. By contrast, colorectal tumors are exposed to VAT: among colorectal cancer patients, higher VAT radiodensity was significantly associated with increased risk of overall mortality (HR: 1.21 [1.11–1.32]) and colorectal-cancer-specific mortality (1.22 [1.08–1.37]).15
In this study, we observed significant interactions for 1) SAT radiodensity and comorbidity and 2) VAT radiodensity and smoking. Given that comorbidity is a confirmed prognostic factor in breast cancer,49 the combined effects of high SAT radiodensity and comorbidity may impose greater risk of mortality on patients, which aligns with the magnitude differences reported in this study: 2.17 (1.58–2.98) for high SAT radiodensity and comorbidity vs. 1.45 (1.15–1.81) for high SAT radiodensity regardless of comorbidity status. The mechanism for VAT radiodensity and smoking is unclear, but one possible explanation may be that smoking induces inflammation,50 increases visceral adiposity,51 and alters adipose tissue quality.52 Thus, abnormal VAT radiodensity among these smokers may reflect their smoking intensity and duration that are known to impact breast cancer prognosis.53 However, these interaction results should be interpreted cautiously given that multiple comparisons were not adjusted.
Limitations
Our study has a large sample size and is the first to investigate adipose tissue radiodensity and mortality among patients with breast cancer. Furthermore, CT scans are commonly obtained for cancer diagnosis and surveillance, future CT imaging studies can easily assess adipose tissue radiodensity (quality), which can be obtained simultaneously with adipose tissue quantity when analyzing CT images for body composition and cancer prognosis. However, several limitations should be noted. First, abdominal adipose tissue biopsies were not performed, thus we could not verify biological effects of adipose tissue radiodensity through comparisons with biopsy-based measures of cellular fat quality. Notably, some investigators have compared adipose tissue biopsies and their radiodensity supporting that radiodensity can act as an marker of quality,8,9 and a growing number of studies have been using adipose tissue radiodensity for prognosis inference in cancer and non-cancer patients.54 Second, we used a single CT image to estimate the average of adipose tissue radiodensity like prior studies, but radiodensity values may vary across abdominal slices and further studies are needed to investigate other metrics of adipose tissue quality. However, from the perspective of clinical research, the third lumbar vertebra is the most commonly used vertebral landmark for body composition studies. Thus, our findings are still clinically meaningful for clinicians and researchers to identify patients with unfavorable adipose tissue quality at higher risk of mortality. Third, our large sample size was enabled by using real-world data of electronic medical records and imaging. Thus, we did not have all technical information about CT scans, such as contrast phase and other parameters that may introduce small variations in adipose tissue radiodensity.55 However, we found that the associations remained similar after excluding non-contrast CT scans.
CONCLUSIONS
In this large cohort, we found that higher SAT radiodensity was significantly associated with increased risk of overall mortality among patients with nonmetastatic breast cancer. Our findings suggest that adipose tissue radiodensity can provide additional information beyond adipose tissue quantity and distribution to predict breast cancer prognosis. Thus, adipose tissue radiodensity is a powerful prognostic indicator that deserves to be further investigated in clinical practice and research.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institutes of Health: K01CA226155 and R01CA251589.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Melinda L. Irwin was supported in part by Breast Cancer Research Foundation. Carla M. Prado reported honoraria from Abbott Nutrition, Nestle Health Science, and Fresenius Kabi; and paid consultancy from Nutricia and Pfizer. Others did not claim conflict of interest.
REFERENCES
- 1.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: a review of the recent literature. Current nutrition reports 2014;3(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018;4(6):798–804. (In eng). DOI: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cespedes Feliciano EM, Chen WY, Bradshaw PT, et al. Adipose Tissue Distribution and Cardiovascular Disease Risk Among Breast Cancer Survivors. J Clin Oncol 2019;37(28):2528–2536. (In eng). DOI: 10.1200/jco.19.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw PT, Cespedes Feliciano EM, Prado CM, et al. Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity 2019;27(6):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwase T, Parikh A, Dibaj SS, et al. The Prognostic Impact of Body Composition for Locally Advanced Breast Cancer Patients Who Received Neoadjuvant Chemotherapy. Cancers (Basel) 2021;13(4). DOI: 10.3390/cancers13040608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 2018;26(3):861–868. DOI: 10.1007/s00520-017-3902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwase T, Sangai T, Nagashima T, et al. Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med 2016;5(1):41–8. DOI: 10.1002/cam4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi N, Hajsadeghi F, Conneely M, et al. Accurate detection of metabolically active “brown” and “white” adipose tissues with computed tomography. Acad Radiol 2013;20(11):1443–7. DOI: 10.1016/j.acra.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Ebadi M, Dunichand-Hoedl AR, Rider E, et al. Higher subcutaneous adipose tissue radiodensity is associated with increased mortality in patients with cirrhosis. JHEP Reports 2022:100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016;11:421–49. (In eng). DOI: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–7. DOI: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 2020;7:22. DOI: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenquist KJ, Pedley A, Massaro JM, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging 2013;6(7):762–71. DOI: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RV, Allison MA, Lima JA, et al. Abdominal fat radiodensity, quantity and cardiometabolic risk: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis 2016;26(2):114–22. DOI: 10.1016/j.numecd.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cespedes Feliciano EM, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr 2021;114(6):1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torriani M, Oliveira AL, Azevedo DC, Bredella MA, Yu EW. Effects of Roux-en-Y gastric bypass surgery on visceral and subcutaneous fat density by computed tomography. Obes Surg 2015;25(2):381–5. DOI: 10.1007/s11695-014-1485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Côté JA, Nazare J-A, Nadeau M, et al. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte 2016;5(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010;59(11):2817–25. DOI: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brock CK, Hebert KL, Artiles M, et al. A Role for Adipocytes and Adipose Stem Cells in the Breast Tumor Microenvironment and Regenerative Medicine. Front Physiol 2021;12:751239. DOI: 10.3389/fphys.2021.751239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anciaux M, Van Gossum A, Wenglinski C, et al. Fat density is a novel prognostic marker in patients with esophageal cancer. Clinical nutrition ESPEN 2020;39:124–130. [DOI] [PubMed] [Google Scholar]
- 21.Ebadi M, Moctezuma-Velazquez C, Meza-Junco J, et al. Visceral adipose tissue radiodensity is linked to prognosis in hepatocellular carcinoma patients treated with selective internal radiation therapy. Cancers 2020;12(2):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Hessen L, Roumet M, Maurer MH, et al. High subcutaneous adipose tissue density correlates negatively with survival in patients with hepatocellular carcinoma. Liver Int 2021;41(4):828–836. DOI: 10.1111/liv.14755. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Lee SM, Chung YA. Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pancreatic adenocarcinoma. Clin Radiol 2018;73(12):1056 e1–1056 e10. DOI: 10.1016/j.crad.2018.07.094. [DOI] [PubMed] [Google Scholar]
- 24.Veld J, Vossen JA, De Amorim Bernstein K, Halpern EF, Torriani M, Bredella MA. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. European Radiology 2016;26(12):4649–4655. [DOI] [PubMed] [Google Scholar]
- 25.Boutin RD, Katz JR, Chaudhari AJ, et al. Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: An opportunistic study using computed tomography. Quantitative Imaging in Medicine and Surgery 2020;10(8):1580–1589. DOI: 10.21037/QIMS.2020.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics 2004;24(6):1679–91. DOI: 10.1148/rg.246045065. [DOI] [PubMed] [Google Scholar]
- 27.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc 2016;75(2):188–98. DOI: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. DOI: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53(282):457–481. (In English). DOI: Doi 10.2307/2281868. [DOI] [Google Scholar]
- 30.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. Journal of the Royal Statistical Society: Series A (General) 1972;135(2):185–198. (In English). DOI: Doi 10.2307/2344317. [DOI] [Google Scholar]
- 31.Spearman C The proof and measurement of association between two things. International journal of epidemiology 2010;39(5):1137–1150. [DOI] [PubMed] [Google Scholar]
- 32.Cox DR. Regression Models and Life-Tables. J R Stat Soc B 1972;34(2):187–220. [Google Scholar]
- 33.Little RJ, Rubin DB. Statistical analysis with missing data: John Wiley & Sons, 2019. [Google Scholar]
- 34.Schoenfeld D Partial Residuals for the Proportional Hazards Regression-Model. Biometrika 1982;69(1):239–241. (In English). DOI: Doi 10.2307/2335876. [DOI] [Google Scholar]
- 35.Smith PL. Splines as a Useful and Convenient Statistical Tool. Am Stat 1979;33(2):57–62. (In English). DOI: Doi 10.2307/2683222. [DOI] [Google Scholar]
- 36.Derstine BA, Holcombe SA, Ross BE, Wang NC, Wang SC, Su GL. Healthy US population reference values for CT visceral fat measurements and the impact of IV contrast, HU range, and spinal levels. Sci Rep 2022;12(1):2374. DOI: 10.1038/s41598-022-06232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paris MT, Furberg HF, Petruzella S, Akin O, Hotker AM, Mourtzakis M. Influence of Contrast Administration on Computed Tomography-Based Analysis of Visceral Adipose and Skeletal Muscle Tissue in Clear Cell Renal Cell Carcinoma. JPEN J Parenter Enteral Nutr 2018;42(7):1148–1155. DOI: 10.1002/jpen.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbeck N, Gnant M. Breast cancer. Lancet 2017;389(10074):1134–1150. DOI: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 39.Lee JW, Kim SY, Lee HJ, Han SW, Lee JE, Lee SM. Prognostic Significance of Abdominal-to-Gluteofemoral Adipose Tissue Distribution in Patients with Breast Cancer. J Clin Med 2019;8(9). DOI: 10.3390/jcm8091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwase T, Sangai T, Fujimoto H, et al. Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Res Treat 2020;179(2):435–443. DOI: 10.1007/s10549-019-05467-7. [DOI] [PubMed] [Google Scholar]
- 41.Franzoi MA, Vandeputte C, Eiger D, et al. Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast cancer research and treatment 2020;181(1):199–209. [DOI] [PubMed] [Google Scholar]
- 42.Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer 2021;124(1):13–26. DOI: 10.1038/s41416-020-01161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janiszewski PM, Saunders TJ, Ross R. Breast volume is an independent predictor of visceral and ectopic fat in premenopausal women. Obesity 2010;18(6):1183–1187. [DOI] [PubMed] [Google Scholar]
- 44.Schautz B, Later W, Heller M, Müller M, Bosy-Westphal A. Associations between breast adipose tissue, body fat distribution and cardiometabolic risk in women: cross-sectional data and weight-loss intervention. European journal of clinical nutrition 2011;65(7):784–790. [DOI] [PubMed] [Google Scholar]
- 45.Kothari C, Diorio C, Durocher F. The Importance of Breast Adipose Tissue in Breast Cancer. Int J Mol Sci 2020;21(16). DOI: 10.3390/ijms21165760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozzo AJ, Fuller AM, Makowski L. Contribution of Adipose Tissue to Development of Cancer. Compr Physiol 2017;8(1):237–282. DOI: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deligne C, Midwood KS. Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies? Front Oncol 2021;11:620773. DOI: 10.3389/fonc.2021.620773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106(6):dju124. DOI: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 49.Land LH, Dalton SO, Jorgensen TL, Ewertz M. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol 2012;81(2):196–205. DOI: 10.1016/j.critrevonc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Berkowitz L, Schultz BM, Salazar GA, et al. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front Immunol 2018;9:74. DOI: 10.3389/fimmu.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 2008;87(4):801–9. DOI: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 52.Tsai PC, Glastonbury CA, Eliot MN, et al. Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin Epigenetics 2018;10(1):126. DOI: 10.1186/s13148-018-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan W, Li S, Meng X, Sun Y, Jia C. Smoking and survival of breast cancer patients: A meta-analysis of cohort studies. Breast 2017;33:117–124. DOI: 10.1016/j.breast.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Monirujjaman M, Martin L, Stretch C, Mazurak VC. Adipose Tissue Radiodensity in Chronic Diseases: A Literature Review of the Applied Methodologies. Immunometabolism 2021;3(4). [Google Scholar]
- 55.Troschel AS, Troschel FM, Fuchs G, et al. Significance of Acquisition Parameters for Adipose Tissue Segmentation on CT Images. AJR Am J Roentgenol 2021;217(1):177–185. DOI: 10.2214/AJR.20.23280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


