Abstract
Purpose:
Investigate whether adjuvant everolimus, an mTOR inhibitor, improves progression-free survival (PFS) in advanced stage head and neck squamous cell carcinoma (HNSCC) and provide outcomes related to correlative biological factors associated with disease control.
Patients and Methods:
This was a prospective, randomized, double-blind phase II trial of advanced stage HNSCC patients from 13 institutions who were confirmed disease-free post-definitive therapy and enrolled between December 2010 and March 2015. Patients received adjuvant everolimus or placebo daily (10mg, oral) for a maximum of 1 year. p16 IHC as a surrogate marker for HPV infection and whole exome sequencing were performed. Cox proportional hazard models estimated hazard rates. Log-rank tests evaluated differences in survival. The primary endpoint was PFS. Secondary endpoints and objectives included overall survival (OS) and toxicity assessment.
Results:
52 patients (median [range] age, 58, [37-76] years; 43 men [83%], 9 women [17%]) were randomized to placebo (n=24) or everolimus (n=28). PFS favored everolimus, but was not significant (log-rank P=0.093; HR=0.44, 95% CI: 0.17-1.17). There was no difference in OS (P=0.29; HR=0.57, 95% CI: 0.20-16.2). Everolimus resulted in significant improvement in PFS for p16-negative patients (n=31) (P=0.031; HR=0.26, 95% CI: 0.07-0.97), although subgroup analysis showed no difference for p16-positive patients (n=21) (P=0.93). Further, PFS was significantly higher in TP53 mutated (TP53mut) patients treated with everolimus compared to placebo (Log-Rank P=0.027; HR=0.24, 95% CI: 0.06-0.95). No treatment difference was seen in patients with TP53 wild-type (TP53wt) tumors (p=0.79).
Conclusions:
p16-negative and TP53mut patients may benefit from adjuvant treatment with everolimus.
Trial Registration:
ClinicalTrials.gov Identifier: NCT01111058
Keywords: Everolimus, head and neck cancer, adjuvant therapy, advanced stage, mTOR inhibitor
Statement of Translational Relevance:
Advanced stage head and neck squamous cell carcinoma (HNSCC) patients are at a high risk of recurrent disease. Due to dismal 5-year survival rates, such patients are in dire need of effective adjuvant therapy. Everolimus, an mTOR inhibitor, has documented activity in HNSCC and is well tolerated with minimal long-term toxicity. This placebo-controlled phase II trial is the first to show promising results using everolimus as adjuvant therapy after complete response to definitive treatment in a subset of HPV-negative patients with advanced stage disease. In particular, HPV-negative TP53 mutated tumors appear to yield the best benefit. Thus, subsequent trials using everolimus in this patient population are warranted.
Introduction
Approximately two-thirds of tobacco-related HNSCC patients present with local-regional advanced disease, frequently due to late diagnosis. The current approach for curative intent includes chemoradiotherapy or surgery plus adjuvant radiotherapy ±chemotherapy, with immunotherapy, targeted agents and chemotherapy reserved for recurrent disease.1
There are no adjuvant protocols to reduce the high risk of recurrence in HNSCC after definitive therapy as commonly used in colon and breast cancer.2, 3 Efforts to improve outcome have been attempted, but have proven ineffective, resulted in unacceptable toxicity and/or adverse events, or both.4 A recent meta-analysis of randomized trials from 1965-2016 robustly documented no survival benefit for adjuvant chemotherapy in non-metastatic HNSCC.5 Hence, agents with low toxicity profiles, such as targeted agents, should be explored.
Many pathways altered by mutation/amplification in HNSCC converge on downstream activation of the Akt/mTOR pathway, making it a rational target for tertiary prevention.6, 7 Expression of eukaryotic initiation factor 4E (eIF4E) is functionally active through activation of the Akt/mTOR signaling pathway; moreover, overexpression of eIF4E in histologically tumor-free surgical margins of HNSCC patients was an independent predictor of recurrence.8, 9 An exploratory biomarker trial with temsirolimus, an mTOR inhibitor, in newly diagnosed advanced stage HNSCC patients showed inhibition of the Akt/mTOR pathway in tumors and PBMCs (surrogate markers) and proapoptotic activity of temsirolimus.10 Similar results were noted in a window of opportunity trial with rapamycin, in which objective clinical responses were observed in 25% of the patients enrolled.11 A phase I trial with everolimus, cisplatin and radiotherapy showed HNSCC patients tolerated everolimus at therapeutic doses (up to 10 mg/day) and that the regimen merits further evaluation, especially among patients who are status post resection harboring eIF4E in histologically negative surgical margins.12 Given these preclinical and clinical data in HNSCC, the role of mTOR inhibitors as adjuvant therapy was explored.
Everolimus is an mTOR inhibitor that has been used as an immunosuppressant in solid organ transplantation since 1996 and more recently as an anti-cancer agent.13-16 Everolimus is being investigated in cancers based on its potential to act directly on tumor cells by inhibiting cell proliferation, as well as indirectly by inhibiting angiogenesis leading to reduced tumor vascularity.17 Oral formulations (5 mg, 10 mg) are approved for patients with advanced renal cell carcinoma.
The purpose of this trial was to assess whether adjuvant therapy with everolimus could significantly improve two-year and overall progression-free survival (PFS) in patients at high risk of cancer recurrence who were free of disease after definitive local therapy for advanced stage HNSCC.
Methods
Study Design and Objectives
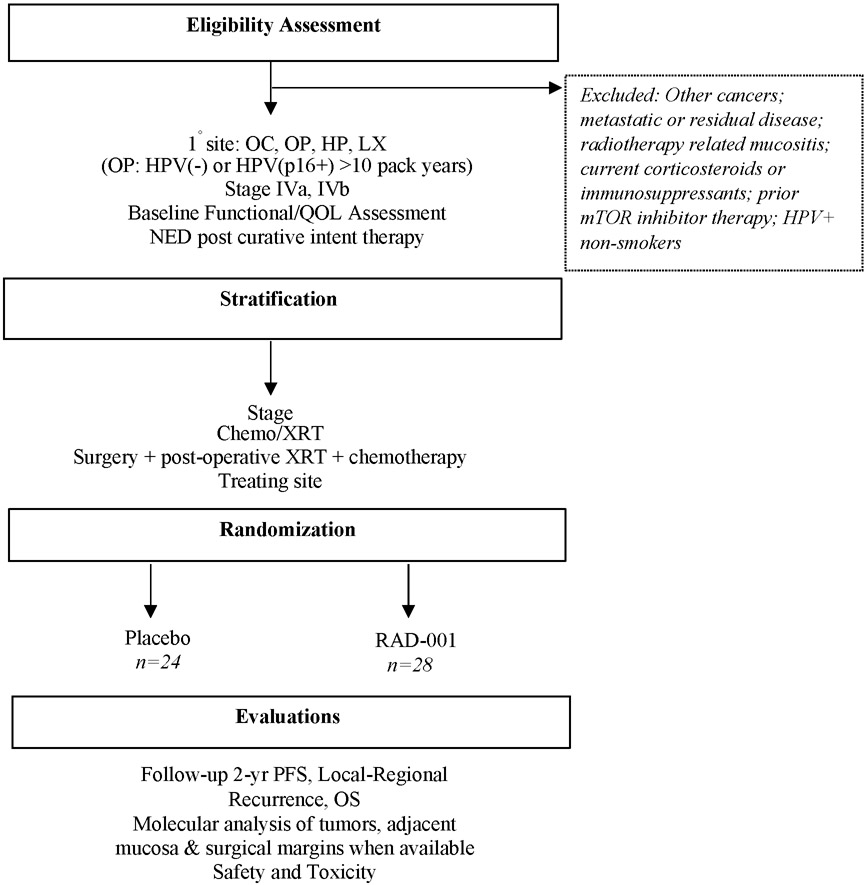
The trial was coordinated and funded through the University of Chicago Personal Cancer Care Consortium. Novartis Pharmaceuticals sponsored site participation in this investigator-initiated trial (NCT01111058). This was an IRB approved multi-institutional randomized double-blind phase II clinical trial of everolimus (intervention) versus placebo. The study was compliant with Good Clinical Practice guidelines, US 21 Code of Federal Regulations, and the Declaration of Helsinki. Written informed consent was obtained by all participants. A summary of study design is presented in Figure 1.
Figure 1. Summary of study design.
Abbreviations: OC=Oral Cavity, OP=Oropharynx, HP=Hypopharynx, LX=Larynx, HPV=Human Papillomavirus, QOL=Quality of Life, NED=No evidence of disease, XRT: Radiotherapy
Key eligibility criteria included TNM stage IVa or IVb (AJCC 6th edition at the time of enrollment; patients were later restaged and reported as AJCC 7th) HNSCC of the oral cavity, oropharynx, hypopharynx or larynx, Karnofsky performance status score >70%, adequate bone marrow, hepatic and renal function, and no evidence of disease within 16 weeks (to allow adequate time for PET confirmation and enrollment) after curative intent therapy. Oropharynx patients were required to be p16/HPV-negative or p16/HPV-positive with a minimum tobacco exposure history of 10 pack years. Exclusion criteria were patients who received anticancer therapies within 4 weeks of study drug initiation (including chemotherapy, radiation therapy, antibody-based therapy, etc.), systemic treatment with corticosteroids or immunosuppressors, acute radiotherapy related mucositis or dermatitis, metastatic disease, other malignancies in the past three years, previous treatment with mTOR inhibitors, or other severe medical conditions that could have affected participation.
Randomization was stratified for disease stage, local therapy type (IVa surgical vs. IVa non-surgical vs. IVb) and treating institution. Within 16 weeks after completion of definitive, curative-intent therapy for locally advanced HNSCC, patients received either 10 mg daily of oral everolimus or placebo for a maximum of 1 year. As PET is typically obtained at 12 weeks post-therapy to accurately determine complete response, the study allowed up to 16 weeks to account for any delay. Gastrostomy tube administration was allowed. The targeted sample size was 160 patients.
PET scans confirmed complete response to primary treatment prior to starting therapy and labs, clinical examination and/or scans were conducted at 4, 16, 32 and 52 weeks after initiating therapy. Patients were followed up for a minimum of 2 years. Disease progression was evaluated by clinical and radiographic methods and by clinical pathology if necessary. The primary endpoint was progression-free survival (PFS). Secondary endpoints and objectives included overall survival (OS), toxicity assessment and site of disease progression. We also wanted to determine whether genetic aberrations in PIK3/Akt/mTOR pathway are associated with recurrence rates and efficacy of everolimus. Next Generation Sequencing (NGS) was used to detect somatic mutations in primary pre-treatment tumor samples of patients to evaluate whether an association exists between cancer-associated mutations and favorable outcomes to mTOR-targeted therapy.
Procedures
Everolimus Administration
Everolimus and placebo were supplied by Novartis. Treatment compliance was maintained through individual drug diaries and drug accountability noted by the return of study medication. Dosing was self-administered orally as 10 mg (two 5 mg tablets) once daily from study day 1 until disease progression, unacceptable toxicity or once 1 year was reached. Instructions were given to take doses in the morning at the same time each day.
For patients unable to tolerate the dosing schedule or if unacceptable toxicity occurred as defined by grade, dose adjustments or interruptions were permitted. Patients were kept at the initial dose level (10 mg daily) when toxicity was tolerable. If dosing became intolerable to the patient or if grade-defined unacceptable toxicity occurred, everolimus was interrupted until recovery to grade ≤1, then reintroduced at the same or lower dose (5 mg daily or every other day), depending on event type and severity. Grade 4 events resulted in discontinuation. Toxicity was assessed using the NIH-NCI Common Terminology Criteria for Adverse Events, version 4.0.
Patients whose treatment was interrupted or discontinued due to an adverse event suspected to be related to everolimus were followed at least weekly until the adverse event returned to grade 1. If a dose delay of > 21 days was required, treatment was discontinued.
Research Testing
Specimens
Tissue obtained prior to curative intent therapy from the diagnostic biopsy and/or during surgical resection, serum and whole blood samples were used for the correlative studies. Serum and whole blood were collected at baseline and at weeks 4, 16, 52, then stored at −80° C.
Immunohistochemistry
For patients with available tissue, p16INK4a immunohistochemistry was performed using a mouse monoclonal anti-p16 antibody clone E6H4 on the BenchMark platform (CINtec Histology, Ventana). Interpretation was performed by a certified pathologist and results given as either negative or positive.
Next-Generation Sequencing
Clinical grade targeted next generation sequencing was performed on HNSCC DNA from formalin-fixed paraffin embedded (FFPE) tumor sections of 44/52 patients. Potential germline variants were eliminated (dbSNP 141) unless they belonged to the catalog of somatic mutation in cancer (COSMIC v82) and were rare in the population (ExAC v0.3 MAF<1E-3). Mutations were then annotated using ref Gene and filtered for non-silent effects. Raw variants were further filtered and annotated using variant-tools. Under this framework, variants were annotated in regard to RefSeq reference annotation using ANNOVAR. Only non-silent variants, including variants in splice sites affecting the open reading frame of the genes were retained. Potential germline variants included matching variants in dbSNP (version 141) were eliminated unless they were also present in the catalog of somatic mutation in cancer (COSMIC v82). Next, variants present in the ExAC (v0.3) at a population minor allelic frequency of 0.001 or greater were further excluded. Furthermore, variants identified 7 or more tumors were eliminated after confirming they did not correspond to known cancer mutational hotspots in oncogenes. Finally, the variants remaining and affecting known cancer genes from the Cancer Gene Census were manually curated by subject matter experts to eliminate rare unambiguous germline variants and alignment artifacts.
The mutations located in known HNSCC mutated genes were manually curated to eliminate technical artifacts. A total of 25 mutations in TP53 were identified in 23 patients and were further used as biomarkers for the association, where only non-silent mutations were included.
Statistical Analysis
Participants were randomized to two arms: placebo and everolimus (intervention) using permuted blocks. Patient demographics and tumor characteristics were obtained. Continuous variables are presented as mean (SD or range), whereas categorical variables are presented as number (percentage) of patients. PFS and OS were estimated by the Kaplan-Meier (1958) method and Cox (1972) proportional hazard models estimated hazard ratios (HR). Log-rank tests evaluated differences in survival. PFS was defined as the time from randomization to disease progression or death from any cause; however, if death occurred over one year after the patient’s last negative exam, the patient was censored as of the date last known progression free. Data analyses were performed with STATA statistical software, version 16 (Stata Corp).
Sample Size
The target sample size was 160 subjects (80 per arm). This sample size was chosen in order to provide 80% power at two-sided α=0.05 to detect a hazard ratio of 1.94, corresponding to a 70% vs. 50% difference in the PFS rate at two years under exponential survival distribution assumptions, using a log-rank test and allowing for a 25% loss to follow-up rate. It also assumed three years of accrual and two years of further follow-up. The HR was based on a 2-year PFS that would be clinically meaningful – 50% to 70%, the preclinical animal data, and the hazard ratio associated with activated mTOR in patient samples from previous studies.
Data Availability Statement
Sequencing data are available under NIH dbGaP Accession: phs002986.v1.p1. Additional study data are not publicly available due to information that could compromise patient privacy, but are available upon reasonable request from the corresponding author.
Results
The trial was terminated prior to achieving the accrual goal due to slow accrual rates. Of the 59 patients consented and screened, 7 were ineligible based on study criteria. A total of 52 patients from 13 institutions participated from 2010 to 2015 (mean age, 58 [range 37-76]) and randomized to receive either placebo (n=24) or everolimus (n=28). Distributions of baseline variables by treatment arm are detailed in Table 1.
Table 1.
Patient sociodemographic and clinical characteristics, post randomization.
| Variable | Placebo (n = 24) |
Everolimus (n = 28) |
|---|---|---|
| Age (years), mean (SD) | 57.3 (8.4) | 58.8 (7.3) |
| Gender | ||
| Male | 20 (83%) | 23 (82%) |
| Female | 4 (17%) | 5 (18%) |
| Race | ||
| African-American | 3 (12%) | 3 (11%) |
| Caucasian | 21 (88%) | 25 (89%) |
| Tumor primary site | ||
| Oral Cavity | 5 (21%) | 6 (21%) |
| Oropharynx | 14 (58%) | 14 (50%) |
| Larynx | 1 (4%) | 7 (25%) |
| Hypopharynx | 4 (17%) | 1 (4%) |
| Tumor stage | ||
| T1 | 0 (0%) | 5 (18%) |
| T2 | 10 (42%) | 3 (11%) |
| T3 | 6 (25%) | 4 (14%) |
| T4A or 4B | 8 (33%) | 16 (57%) |
| Nodal stage | ||
| N0 | 2 (8%) | 3 (11%) |
| N1 | 1 (4%) | 1 (4%) |
| N2 | 4 (17%) | 4 (14%) |
| N2B | 9 (38%) | 6 (21%) |
| N2C | 7 (29%) | 11 (39%) |
| N3 | 1 (4%) | 3 (11%) |
| Karnofsky Performance Score | ||
| 100 | 5 (21%) | 5 (18%) |
| 90 | 9 (38%) | 13 (46%) |
| 80 | 9 (38%) | 9 (32%) |
| Unknown | 1 (4%) | 1 (4%) |
| HPV (p16 or ISH) result | ||
| Negative | 14 (58%) | 16 (57%) |
| Positive | 10 (42%) | 12 (43%) |
| TP53 mutation status | ||
| Wild type | 13 (54%) | 15 (54%) |
| Mutated | 10 (42%) | 13 (46%) |
| Days from end of definitive treatment to study drug initiation, mean (SD) | 93 (30) | 115 (97) |
| Pack-years smoking, mean (SD) | 31.9 (24.8)1 | 48.5 (32.4)2 |
| Treatments administered | ||
| Surgery | 10 (42%) | 15 (54%) |
| RT | 23 (96%) | 23 (82%) |
| Induction chemotherapy | 4 (17%) | 5 (18%) |
12 missing observations
16 missing observations
There was equal distribution between all variables except T stage. Advanced T stage T4a and T4b were skewed towards the everolimus group (57%) v/s placebo group (33%). With regard to nodal status and margins, 4 of the 25 patients who underwent surgery had extranodal extension (2 in each arm) while 4 had positive margins (1 in the placebo group and 3 in the everolimus arm). In terms of adjuvant treatment, 17 received both cisplatin plus RT (9 in the placebo arm and 8 in the everolimus group). Eight patients received either cisplatin or RT (1 placebo patient, 7 everolimus patients). In the intent-to-treat analysis, all 52 patients contributed data to the longitudinal analysis of survival. During and after the one year of assigned treatment both groups were followed for progression, during which these participants were considered to be at risk of tumor relapse.
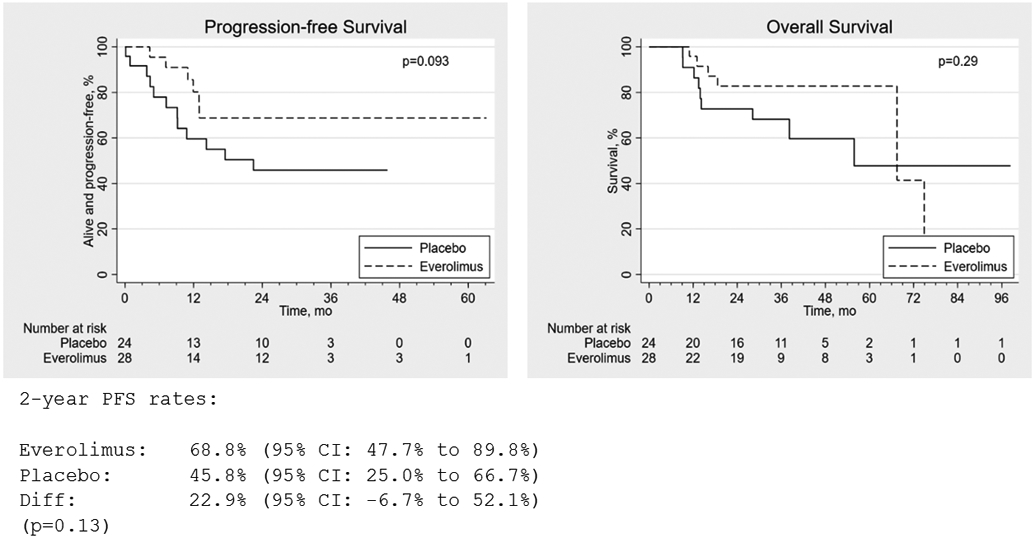
PFS favored everolimus, but the difference was not statistically significant (2-year absolute difference 22.9% (95% CI −6.7 to 52.1%), P=0.13; log-rank test for equality of survival functions, P=0.093; HR=0.44, (95% CI: 0.17-1.17)). Eighteen patients experienced recurrence or died within one year of the last known progression-free date (6 everolimus; 12 placebo). Two patients died more than one year after the last known progression-free interval date, one in each arm, and were censored in PFS analysis. Adjusting for other treatments received (surgery, RT, induction chemotherapy) had little effect on the estimated everolimus effect (adjHRs of 0.45, 0.46, and 0.46, respectively). Adjusting for pack-years of smoking also did not materially alter the treatment effect estimate (adjHR=0.51, 95% CI: 0.12, 2.18), although this analysis included only 24 observations due to missing data. There was no significant difference in OS (log-rank P=0.29; HR=0.57, 95% CI: 0.20-16.2) (Figure 2). Adjusting for other treatments received yielded similar results (adjHR=0.55, 0.55, and 0.57 for surgery, RT, and induction chemotherapy, respectively. Adjusting for pack-years of smoking also gave similar results (adjHR=0.84, 95% CI: 0.21-3.39) among those with smoking history available.
Figure 2. Progression-free and overall survival in patients receiving everolimus (E) (n=28) versus placebo (P) (n=24).
As shown in Figure 3A, everolimus treatment was significantly associated with longer PFS for p16-negative patients (log-rank P=0.031; HR=0.26, 95% CI: 0.07-0.9) while no difference was observed in p16-positive patients (log-rank P=0.93; HR=0.93, 95% CI: 0.18-4.64), although the latter is based on few events.
Figure 3. Progression-free survival in patients by p16 and TP53 status.
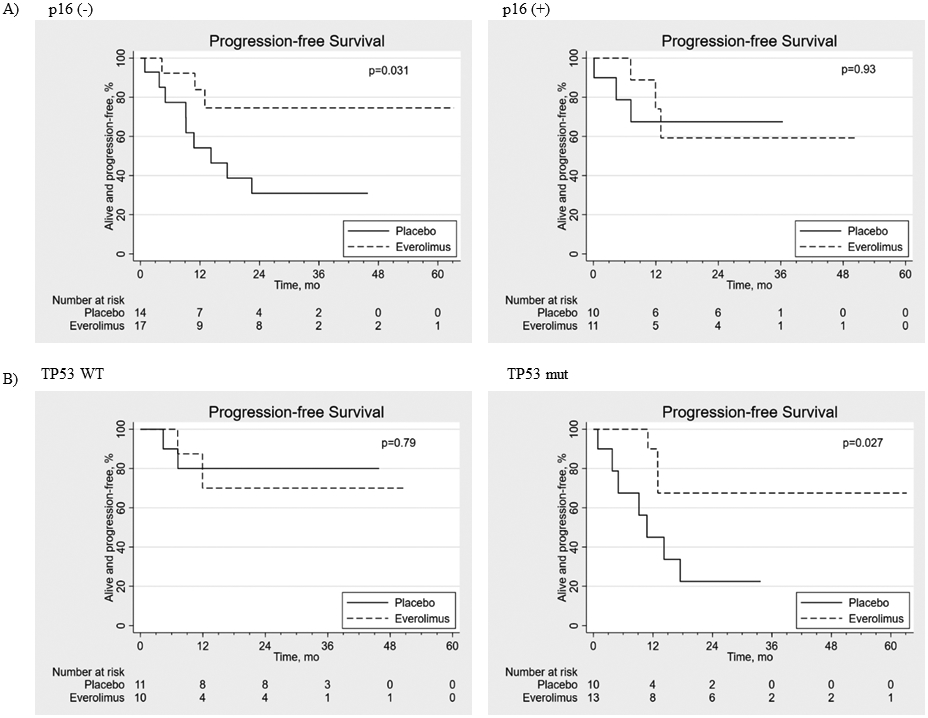
(A) Progression-free survival in p16 (−) (n=31) and p16 (+) (n=21) patients receiving everolimus (E) versus placebo (P). (B) Progression-free survival in TP53 wild-type (WT) (n=21) and TP53 mutant (mut) (n=23) patients receiving everolimus (E) versus placebo (P).
Next Generation Sequencing
After extensive filtering to eliminate potential artifacts and likely germline variants (“Methods”), we identified a total of 796 non-silent somatic mutations (693 SNP, 103 indels) affecting 335 genes in 44 available tumors. TP53 was the most frequently mutated gene, affecting 23/44 tumors. As expected, HPV-negative tumors were more likely to be TP53mut (21/25, 84% compared to 2/19, 11% in HPV-positive tumors, p<0.001). Subgroup analysis of TP53 mutational status was associated with significantly higher PFS rates in TP53mut patients treated with everolimus compared to placebo (log-rank P=0.027; HR=0.24, 95% CI: 0.06-0.95). As a result of the small sample size, the type of TP53mut that benefitted from everolimus could not be deciphered. Remarkably, this difference between everolimus vs. placebo was not seen in the TP53wt group (P=0.79; HR=1.30, 95% CI: 0.18-9.29) as shown in Figure 3B, although limited by few events. No statistically significant difference was observed for OS in either TP53wt (P=0.69; HR=1.50, 95% CI: 0.20-11.1) or TP53mut (P=0.13; HR=0.30, 95% CI: 0.06-1.55) patients. Everolimus treatment did not significantly affect OS in either p16-positive or p16-negative patients (log-rank P=0.82; HR=1.26, 95% CI: 0.17-9.50 and P=0.10; HR=0.34, 95% CI: 0.09-1.32 respectively).
TP53 Subset Analysis
A total of 25 mutations in TP53 were identified in 23 patients and were further used as a biomarker for the association with endpoints. The type of mutations observed were: Nonsense (6), frameshift (5), splicing (1), missense (12) and inframe deletion (1) (Supplementary Table S1). In the placebo arm, 46% (n=11) of patients were TP53wt, 42% (n=10) were TP53mut and 12% (n=3) unknown. In the everolimus arm, 36% (n=10) were TP53wt, 46% (n=13) were TP53mut and 18% (n=5) unknown. We observed no significant difference in TP53 mutational status between treatment groups (Pearson chi2=0.35, P=0.56). To assess the possibility of TP53 status being disproportionate with regards to initial standard of care therapies, we determined that the increase in PFS comparing everolimus to placebo in TP53mut patients occurred despite similar definitive treatment modalities (Supplementary Table S2).
Toxicity
Adverse events at least possibly related to study drug are summarized in Table 2 (full version Supplementary Table S3). Everolimus was generally well tolerated. Thirteen patients required a dose modification. Twelve patients (43%) experienced a grade 3 or higher adverse event attributed to study drug. The most frequent (>20%) adverse events from baseline were stomatitis/pharyngitis, decreased lymphocyte and platelet counts, anorexia and fatigue. A single grade 4 hyperbilirubinemia event resulted in discontinuation of everolimus for probable attribution to the drug in one patient. Two patients experienced serious adverse events possibly related to study drug: One patient was hospitalized for a skin infection and one patient experienced a thromboembolic event.
Table 2.
Adverse events at least possibly related to study drug occurring in at least 5% of patients, by NIH-NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
| Adverse Event by Grade Everolimus | ||||
|---|---|---|---|---|
| Adverse Event | Worst Grade (No, %) | |||
| 1 | 2 | 3 | 4 | |
| Anorexia | 4 (14%) | 2 (7%) | 0 | 0 |
| Cholesterol high | 4 (14%) | 1 (4%) | 0 | 0 |
| Creatinine increased | 1 (4%) | 1 (4%) | 0 | 0 |
| Diarrhea | 2 (7%) | 0 | 1 (4%) | 0 |
| Dysgeusia | 1 (4%) | 1 (4%) | 0 | 0 |
| Fatigue | 2 (7%) | 3 (11%) | 1 (4%) | 0 |
| Headache | 1 (4%) | 0 | 1 (4%) | 0 |
| Hypertriglyceridemia | 0 | 2 (7%) | 2 (7%) | 0 |
| Lymphocyte count decreased | 2 (7%) | 4 (14%) | 1 (4%) | 0 |
| Nausea | 2 (7%) | 1 (4%) | 0 | 0 |
| Neutrophil count decreased | 0 | 1 (4%) | 1 (4%) | 0 |
| Papulopustular rash | 3 (11%) | 0 | 0 | 0 |
| Platelet count decreased | 4 (14%) | 2 (7%) | 0 | 0 |
| Rash acneiform | 1 (4%) | 0 | 1 (4%) | 0 |
| Rash maculo-papular | 1 (4%) | 1 (4%) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 1 (4%) | 1 (4%) | 0 | 0 |
| Skin infection | 0 | 1 (4%) | *1 (4%) | 0 |
| Stomatitis/pharyngitis | 3 (11%) | 7 (25%) | 2 (7%) | 0 |
| White blood cell decreased | 4 (14%) | 3 (11%) | 1 (4%) | 0 |
Serious adverse event requiring hospitalization
DISCUSSION
Many targeted agents in the adjuvant setting after definitive therapy have been tested in HNSCC, but have failed. Afatinib, an ERBB blocker, has shown efficacy in both recurrent and metastatic HNSCC.18 However, treatment with Afatinib after CRT did not improve disease-free survival and was associated with more adverse events than placebo in patients with primary, unresected, clinically high- to intermediate-risk HNSCC.19 Anti-EGFR targeted therapies initially held high expectations, but the only FDA-approved targeted agent for HNSCC, Cetuximab, has shown a poor response rate and likely intrinsic resistance.20, 21 The EGFR/Erb2 inhibitor lapatinib proved unsuccessful as concomitant treatment, followed by 12 month maintenance therapy.22 Trials exploring immunotherapy as adjuvant therapy and in combination with RT or CRT after surgery are ongoing23, 24 however, recent data have been negative25.
Although HPV-associated HNSCC are the most rapidly growing tumors in the USA, worldwide smoking is still the leading cause of HNSCC. HPV-positive oropharynx patients with a >10 pack year smoking history and advanced stage HPV-negative patients have a 5-year survival of 65% and <30%, respectively.26, 27 Approximately 80% of HPV-negative tumors have TP53 mutations and this group of patients has the worst prognosis as noted in both the RTOG 0522 and ECOG 2399 trials.21, 28 Our dataset showed similar results where 84% (21/25) of p16-negative patients in our cohort had TP53mut compared to 11% (2/19) in p16-positive patients. One limitation of the trial was the later downstaging of HPV/p16+ patients in the updated AJCC 8th edition. As study design predated current intermediate risk groups, Ang et al’s NEJM report describing HPV+ patients with >10 pack years was used to make this determination.26 Interestingly, although the study enrolled a final small sample size of 52 patients, everolimus showed statistically significant improvement in PFS among p16-negative (58%) patients, with no benefit over placebo for p16-positive (42%) tumors. Given the small number of p16-positive patients and that they have a significantly better disease-free survival, the difference between everolimus and placebo may not have been seen. However, these patients do well and may not benefit from adjuvant therapy.
Furthermore, the patient subset whose tumors harbored a TP53 mutation (52%) demonstrated a significant difference in PFS. TP53 is the most frequently mutated gene in HNSCC, whose alterations significantly affect tumor progression and resistance to treatment.29-31 The Cancer Genome Atlas consortium showed that TP53mut HNSCC have worse prognosis and survival outcomes compared to TP53wt tumors.32 Given the PFS benefit observed with everolimus based on TP53 status, we then analyzed the treatment modalities received in both the TP53mut and TP53wt group and found no significant differences.
The reason for the enhanced clinical benefit of everolimus in TP53mut patients is at the present not fully understood. In general, a consistent biomarker in HNSCC associated with prognosis is p16, a surrogate for HPV infection.26, 33 As such, most p16-positive tumors are TP53wt, consistent with the low frequency of TP53mut in HPV+ HNSCC lesions, even when only p16-positive patients with a 10-pack year smoking history were enrolled.34-36 As HPV-positive patients exhibit a better prognosis, 37 which we also observed, everolimus may not be able to display further clinical benefit in this group. Alternatively, mutations in TP53 may sensitize HNSCC to mTOR inhibition. Preliminary studies from our lab have shown that mTOR inhibitors induced autophagy dependent cell death in HPV-negative TP53mut HNSCC cell lines, providing a novel mechanism of action.38 Recent studies have revealed that WT p53 regulates protein translation through the induction of 4E-BP1.39 Hence, one possibility is that TP53mut HNSCC lesions may have lost this translational control mechanism and therefore become vulnerable to the tumor suppressive effect of 4E-BP1 when unleashed downstream from mTOR inhibition.40 These, and other possibilities are under current investigation. As TP53mut patients are noted in 80% of HPV-negative tumors and have extremely poor outcomes, everolimus holds significant promise in this group of patients who are arguably most in need of more effective options.
Patients received everolimus or placebo for one year. Future directions for everolimus as adjuvant therapy should consider extending the length of time on drug, as it was very well tolerated in our study and prolonged use is standard of care in renal cell carcinoma; 41, 42 or possibly in combination with immuno-oncologic agents once safety profiles are established.
The Akt/mTOR pathway is active in HNSCC; mTOR inhibitors display biologic activity in preclinical and clinical HNSCC models, and currently available oral mTOR inhibitors are considered well-tolerated for prolonged periods up to four years.43, 44 Moreover, no increased incidence of immunosuppression has been observed in multiple trials of single-agent rapamycin or rapamycin analogues in cancer patients.45, 46 Clinical trials have shown that mTOR inhibitors have clinical activity in HNSCC.11, 12, 47 We have evidence that mTOR inhibitors hold promise for minimal residual disease in preclinical models.48-50 mTOR inhibitors have been safely used in transplant patients for years without significant side effects. This study may represent evidence that p16-negative patients and those patients with TP53mut could potentially benefit from mTOR inhibitors as adjuvant therapy. The 50% of advanced stage patients that recur will do so in the first two years of current standard treatment. Molecular analysis of TP53 in surgical margins has shown it to be a likely predictor of recurrence.51 Many studies have shown that mutant TP53 promotes sustained activation of the PI3K/Akt/mTOR pathway in cancer and results in autophagy inhibition.6, 52, 53 A possible mechanism of action for everolimus may be its ability to inhibit this activation, resulting in a cell death response. A Phase II trial randomizing p16-negative and/or TP53mut patients to everolimus and placebo for at least two years will determine if this drug can change survival in a group of patients that have the worst outcomes. Finally, given the growing cost of health care, rapamycin, which has been shown to have the same pharmacodynamic activity as sirolimus and temsirolimus54-56, is an inexpensive drug that is safe and could improve PFS in patients who recur within the first two years.
LIMITATIONS:
A key limitation of this trial is that it was underpowered due to study closure prior to complete accrual. Feasibility challenges included engaging patients in maintenance therapy after definitive treatment, randomization to placebo, and factors such as PI change of institution, which impeded routine study support and function. Although investigators were unlikely to have unconscious bias due to the drug administration structure, the risk exists for inadvertent unblinding due to recognizable side effects during follow up. There was a chance baseline difference in T staging among groups where the most common stage in the everolimus group was T4A or 4B (57%) compared to mostly T2 patients who received placebo (42%); yet, despite this imbalance we were still able to observe a survival benefit with everolimus in p16-negative patients. The data strongly support conducting a large trial randomizing high-risk p16-negative patients with TP53mut to everolimus or placebo as adjuvant therapy. Additionally, as patients were permitted to enroll up to 16 weeks after definitive treatment, the possibility of selection bias for high-risk relapse exists. Moreover, extending adjuvant therapy with everolimus administration beyond one year has the potential to further improve PFS for HNSCC patients with the historically most unfavorable prognosis.
Supplementary Material
ACKNOWLEDGEMENTS
The trial was coordinated and funded through the University of Chicago Personal Cancer Care Consortium. We would like to thank Novartis Pharmaceuticals for sponsoring site participation. We wish to also thank Bernadette Libao and Jacqueline Ansted for being the lead clinical coordinators on this trial. Lastly, we thank the patients who participated in this trial and the investigators, nurses, and clinical research staff for their support.
Financial Support:
The trial was coordinated and funded through the University of Chicago Personal Cancer Care Consortium. Novartis Pharmaceuticals sponsored site participation in this investigator-initiated trial (NCT01111058). Correlative studies were supported by an R01 grant from the National Cancer Institute (NIH/NCI 2R01CA102363) to Cherie-Ann O. Nathan.
Footnotes
Conflict of Interest Disclosures: Novartis Pharmaceuticals sponsored site participation and provided everolimus (RAD001) in this investigator-initiated trial. Everett E. Vokes has a consultant/advisory role for Novartis Pharmaceuticals. J. Silvio Gutkind was an advisory board member for Domain Therapeutics. No other relevant disclosures are reported.
REFERENCES
- 1.Oosting SF, Haddad RI. Best practice in systemic therapy for head and neck squamous cell carcinoma. Front Oncol. 2019;9:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse MA. Adjuvant therapy of colon cancer: current status and future developments. Clin Colon Rectal Surg. 2005;18:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chew HK. Adjuvant therapy for breast cancer: who should get what? West J Med. 2001;174:284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–84. [DOI] [PubMed] [Google Scholar]
- 5.Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 2021;156:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinolo AA, Hewitt SM, Amornphimoltham P, Keelawat S, Rangdaeng S, Garcia AM, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–73. [DOI] [PubMed] [Google Scholar]
- 7.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–61. [DOI] [PubMed] [Google Scholar]
- 8.Nathan CO, Franklin S, Abreo FW, Nassar R, De Benedetti A, Glass J. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17:2909–14. [DOI] [PubMed] [Google Scholar]
- 9.Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, et al. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10:5820–7. [DOI] [PubMed] [Google Scholar]
- 10.Ekshyyan O, Mills GM, Lian T, Amirghahari N, Rong X, Lowery-Nordberg M, et al. Pharmacodynamic evaluation of temsirolimus in patients with newly diagnosed advanced-stage head and neck squamous cell carcinoma. Head Neck. 2010;32:1619–28. [DOI] [PubMed] [Google Scholar]
- 11.Day TA, Shirai K, O'Brien PE, Matheus MG, Godwin K, Sood AJ, et al. Inhibition of mTOR signaling and clinical activity of rapamycin in head and neck cancer in a window of opportunity trial. Clin Cancer Res. 2019;25:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fury MG, Sherman E, Haque S, Korte S, Lisa D, Shen R, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69:591–8. [DOI] [PubMed] [Google Scholar]
- 13.Lebwohl D, Anak O, Sahmoud T, Klimovsky J, Elmroth I, Haas T, et al. Development of everolimus, a novel oral mTOR inhibitor, across a spectrum of diseases. Ann N Y Acad Sci. 2013;1291:14–32. [DOI] [PubMed] [Google Scholar]
- 14.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Onc. 2008;26:1603–10. [DOI] [PubMed] [Google Scholar]
- 15.Jac J, Amato RJ, Giessinger S, Saxena S, Willis JP. A phase II study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic renal cell carcinoma which has progressed on tyrosine kinase inhibition therapy. J Clin Oncol. 2008;26:5113.18794540 [Google Scholar]
- 16.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. [DOI] [PubMed] [Google Scholar]
- 17.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiwert TY, Fayette J, Cupissol D, Del Campo JM, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burtness B, Haddad R, Dinis J, Trigo J, Yokota T, de Souza Viana L, et al. Afatinib vs placebo as adjuvant therapy after chemoradiotherapy in squamous cell carcinoma of the head and neck: a randomized clinical trial. JAMA Oncol. 2019;5:1170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byeon HK, Ku M, Yang J. Beyond EGFR inhibition: multilateral combat strategies to stop the progression of head and neck cancer. Exp Mol Med. 2019;51:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington K, Temam S, Mehanna H, D’Cruz A, Jain M, D’Onofrio I, et al. Postoperative adjuvant Lapatinib and concurrent chemoradiotherapy followed by maintenance Lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: A phase III, randomized, double-blind, placebo-controlled study. J Clin Onc. 2015: 33(35), 4202–4209. [DOI] [PubMed] [Google Scholar]
- 23.Roche Hoffman-La. A study of atezolizumab (anti-PD-L1 antibody) as adjuvant therapy after definitive local therapy in patients with high-risk locally advanced squamous cell carcinoma of the head and neck. https://clinicaltrials.gov/ct2/show/study/NCT03452137. First posted March 2, 2018. [Google Scholar]
- 24.Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y and Lee NY. JAVELIN head and neck 100: a phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. 2019;15:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal Cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rettig E and D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am. 2015;24:379–396. [DOI] [PubMed] [Google Scholar]
- 28.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 29.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. AJCC Cancer Staging Manual (8th Edition). Springer International Publishing: American Joint Commission on Cancer. 2017. [Google Scholar]
- 34.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–369. [DOI] [PubMed] [Google Scholar]
- 35.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson BJ. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam MD, Marin Fermin J, Spiller PT, Burnett C, Rong X, Moore-Medlin T, et al. Abstract 1015: mTOR inhibitor induces non-apoptotic, autophagy dependent cell death (ADCD) in TP53 mutant HNSCC. Cancer Res. 2021;81(13 Supplement) 1015. [Google Scholar]
- 39.Tiu GC, Kerr CH, Forester CM, Krishnarao PS, Rosenblatt HD, Raj N, et al. A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies. Dev Cell. 2021;56(14):2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Feng X, Molinolo AA, Martin D, Vitale-Cross L, Nohata N, et al. 4E-BP1 is a tumor suppressor protein reactivated by mTOR inhibition in head and neck cancer. Cancer Res. 2019;79(7):1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ljungberg B, Cowan N, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Euro Urol. 2010;58(3):398–406. [DOI] [PubMed] [Google Scholar]
- 42.Meskawi M, Valdivieso R, Dell’Oglio P, Trudeau V, Larcher A, Karakiewicz. The role of everolimus in renal cell carcinoma. J Kidney Cancer VHL. 2015;2(4):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franz DN, Belousova E, Sparagana S, Martina Bebin E, Frost MD, Kuperman R, et al. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLoS One. 2016;11(6):e0158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parada MT, Alba A, Sepúlveda C, Melo J. Long-term use of everolimus in lung transplant patients. Transplant Proc. 2011;43:2313–15. [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell A, Faivre S, Burris HA 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. [DOI] [PubMed] [Google Scholar]
- 46.Hidalgo M, Buckner JC, Erlichman C, Pollack MS, Boni JP, Dukart G, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–63. [DOI] [PubMed] [Google Scholar]
- 47.Vander Broek R, Mohan S, Eytan DF, Chen Z, Van Waes C. The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk and therapies. Oral Dis. 2015;21:815–825. [DOI] [PubMed] [Google Scholar]
- 48.Nathan CO, Amirghahari N, Rong X, Giordano T, Sibley D, Nordberg M, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67:2160–8. [DOI] [PubMed] [Google Scholar]
- 49.Ekshyyan O, Moore-Medlin TN, Raley MC, Sonavane K, Rong X, Brodt M, et al. Anti-lymphangiogenic properties of mTOR inhibitors in head and neck squamous cell carcinoma experimental models. BMC Cancer. 2013;13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekshyyan O, Khandelwal A, Rong X, Moore-Medlin T, Ma X, Alexander JS, et al. Rapamycin targets interleukin 6 (IL6) expression and suppresses endothelial cell invasion stimulated by tumor cells. Am J Trans Res. 2016;8:4822–30. [PMC free article] [PubMed] [Google Scholar]
- 51.Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. [DOI] [PubMed] [Google Scholar]
- 52.Cordani M, Oppici E, Dando I, Butturini E, Dalla Pozza E, Nadal-Serrano M, et al. Mutant p53 proteins counteract autophagic mechanism sensitizing cancer cells to mTOR inhibition. Mol Oncol. 2016;10:1008–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Norberg E, Vakifahmetoglu H. Mutant p53 as a regulator and target of autophagy. Front Oncol. 2021;10:607149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen S, Walker D, Gillespie MB, Gutkind JS, Day TA. mTOR inhibitors and its role in the treatment of head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2021;13:71–81. [DOI] [PubMed] [Google Scholar]
- 55.Hu M, Ekshyyan O, Ferdinandez LH, Rong X, Caldito G, Nathan CO. Efficacy and comparative effectiveness of sirolimus as an anticancer drug. Laryngoscope. 2011;121:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen EEW, Wu K, Hartford C, Kocherginsky M, Napoli Eaton K, Zha Y, et al. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin Cancer Res. 2012;18(17):4785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data are available under NIH dbGaP Accession: phs002986.v1.p1. Additional study data are not publicly available due to information that could compromise patient privacy, but are available upon reasonable request from the corresponding author.