Abstract
Anxiety that occurs in association with on-off dopamine medication fluctuations is a major cause of distress, dysfunction, and lower quality of life in people with Parkinson’s disease (PD). However, the association between anxiety and on-off fluctuations is poorly understood and it is difficult to predict which patients will suffer from this atypical form of anxiety. To understand whether fluctuating anxiety in PD exists as part of an endophenotype that is associated with other signs or symptoms, we prospectively assessed the change in anxiety and a battery of clinical variables when transitioning from the off-dopamine medication state to the on state in 200 people with PD. We performed latent profile analysis with observed variables as latent profile indicators measuring the on-off-state difference in anxiety, depression, motor function, daily functioning, and the wearing off questionnaire 19 item scale (WOQ-19) in order to model unobserved (i.e., latent) profiles. A two-class model produced the best fit. The majority of participants, 69%, were categorized as having a ‘typical on-off response’ compared to a second profile constituting 31% of the sample who experienced a worsening in anxiety in the off state that was three times that of other participants. This profile referred to as “anxious fluctuators” had a Hamilton Anxiety Rating Scale change between the off and on medication state of 10.22(32.85) compared to 3.27 (7.62), higher depression scores, greater disability and was less likely to improve on select WOQ-19 items when in the on-state. Anxious fluctuators were more likely to be male and have a family history of anxiety disorder. Given the adverse impact of this profile we believe it may be important to distinguish patients with a typical on-off response from those with this more problematic course of fluctuations.
Keywords: Parkinson’s disease, anxiety, depression, fluctuations, nonmotor, dopamine
Introduction
Anxiety disorders occur in at least a third of people with Parkinson’s disease (PD), worsen quality of life, and increase the burden on family members and caregivers.1–3 Anxiety disorders described in the Diagnostic and Statistical Manual 5 (DSM 5) such as generalized anxiety disorder, panic disorder, and social phobia, are broadly represented in PD. However, atypical anxiety disorders that don’t fulfill specific DSM 5 criteria are equally common and, in some patients, occur as episodes of high anxiety associated with on-off dopamine medication fluctuations.3,4 This specific set of symptoms might fall under the diagnostic label of “Anxiety Disorder Due to Another Medical Condition”. The principal objective for this study was to gain an understanding of the clinical phenomenology of on-off dopamine medication anxiety syndromes in PD with the hope of using that knowledge to better target and test pharmacologic treatments. Using a provocative design in which dopaminergic therapy was withdrawn, we provide further clinical characterization of anxiety syndromes in PD with respect to their association with the on- and off- motor state, comorbid psychiatric symptoms, and other non-motor features of PD. The resulting analysis describes an empirically valid subtype of anxiety associated with PD-specific features.
Motor fluctuations are a nearly inevitable complication of dopaminergic therapy in Parkinson’s disease (PD) occurring in greater than 50% of patients within five years of diagnosis and nearly 100% at 10 years.5,6 Motor fluctuations are not just a complication of advanced disease, they can occur as early as 6 months after dopaminergic therapy is initiated and can be associated with either levodopa or dopamine agonists.7 Non-motor fluctuations, such as changes in neuropsychiatric, autonomic, or sensory (pain) symptoms, most often occur in patients with motor fluctuations and frequently several types of non-motor symptoms occur in the same patient.8 The exact prevalence and time of onset of non-motor fluctuations in PD is uncertain, but is likely similar to that of motor fluctuations.8–11 The largest estimate of non-motor fluctuations to date using a validated scale found a prevalence of 41% in 402 persons with PD using the Movement Disorders Society – Non-Motor Rating Scale (MDS-NMS).12
While most non-motor fluctuation symptoms, including anxiety, are reported to occur during the off-medication state there is some uncertainty, as a few studies found some non-motor symptoms are more likely to occur in the on-state.9–14 In addition, the degree of change may vary by type of non-motor symptom. For example, in a study examining 10 different non-motor symptoms the degree of change from the on- to the off- state ranged from no fluctuations for bladder urgency, dysphagia, and excessive sweating to a 230% increase in anxiety in the off-state.10 Among non-motor fluctuation symptoms anxiety stands out as the most frequent and disabling. In addition, non-fluctuating patients with prominent anxiety have an increased risk of future fluctuations.8,15,16 The relationship between anxiety and fluctuations suggests the possibility of a latent shared mechanism or a subgroup of individuals with PD predisposed toward anxiety and a complex clustering of motor and non-motor fluctuations. We believe that characterizing this latent profile defined by symptomatic response to dopaminergic therapy will have important therapeutic and mechanistic implications.
Methods
Participants
The study sample included adults, ages 21 or older, with a diagnosis of idiopathic Parkinson’s disease based on the MDS clinical diagnostic criteria.17 Participants with less than one year of PD or a diagnosis of dementia (by DSM-IV-TR) were excluded to reduce the chance of enrolling individuals with Lewy body dementia. We avoided a cognitive cut-off using a global cognitive screening measure like the mini-mental status exam or Montreal cognitive assessment because there may be other factors contributing to poor screening measure performance that do not impact participant’s independence or ADL function to a disabling degree. Further, using this definition rather than a screening measure cut-off allowed a more representative sample of patients with advanced PD who are still able to function independently. Participants were recruited from the Morris K. Udall Parkinson’s Disease Research Center at Johns Hopkins, the Johns Hopkins Parkinson’s Disease and Movement Disorders Center, and the clinics of three community-based movement disorder neurologists with adjunct faculty positions. Participants were, on average, 65 years old (SD 7.71). Most participants were men (61.0%) and white (92.5%). Additional demographic information for this sample can be found in Table 1.
Table 1.
Sample demographic and clinical information (n=200)
| Measure | Average (SD) or Count (%) | Range |
|---|---|---|
| Age (years) | 65.21 (7.7) | 36 – 85 |
| Education (years) | 16.97 (2.46) | 12 – 23 |
| Sex (% Male) | 122 (61.0%) | NA |
| Other | 5 (2.5%) | |
| Unemployed | 2 (1%) | |
| Levodopa Equivalent Daily Dosage (mg/day) | 805.24 (498.74) | 100 – 2733 |
| Montreal Cognitive Assessment Total Score | 26.74 (2.90) | 14 – 30 |
| Disease Duration (years) | 9.09 (5.81) | 1 – 29 |
| Generalized Anxiety Disorder (% Diagnosed) | 38 (19%) | NA |
| Panic Disorder (% Diagnosed) | 19 (9.6%) | NA |
| Major Depressive Disorder (% Diagnosed) | 16 (8%) | NA |
| Hamilton Anxiety Rating Scale Total Score | 9.15 (5.45) | 0 – 32 |
| Hamilton Depression Rating Scale Total Score | 4.91 (4.59) | 0 – 36 |
| Schwab & England Score | 90.38 (11.37) | 35 – 100 |
| UPDRS part 3 | 25.49 (11.96) | 4 – 66 |
| Wearing Off Questionnaire | 2.56 (3.04) | 0 – 14 |
| Parkinson Anxiety Scale Total Score | 10.11 (7.35) | 0 – 34 |
| Symbol Digit Modality Test | 43.24 (12.88) | 2 – 75 |
| Stroop Color-Word T-Score | 44.69 (10.37) | 15 – 70 |
Procedures
The study used a provocative design to evaluate whether a hypodopaminergic state is associated with a distinct syndrome characterized by high anxiety and other nonmotor symptoms among people with PD. Participants were assessed in both the “on” and “off” medication state according to the clinical protocol, Core Assessment for Surgical Interventional Therapies in Parkinson’s disease.18 In line with this protocol, participants were asked to temporarily stop all dopaminergic medications at least 12 hours prior to their study visit. The first battery of measures was administered while participants were in this “off” medication state. Then, participants took their usual morning dopaminergic medications and completed assessments recommended by the National Institute for Neurological Disorders and Stroke-Common Data Elements for Parkinson’s disease clinical features.19 After participants indicated they were in their best “on” medication state (mean (SD)=72.28 (22.72) minutes), we re-administered the same battery used previously during the “off” state assessment. All assessments were conducted by MDS trained personnel in a research office setting. The Johns Hopkins University Institutional Review Board approved the study protocol. The research team obtained written consent from all participants prior to their participation.
Measures
On-and off-state testing battery
Seven measures composed the “on/off” battery. These measures evaluated mood, wearing-off, motor, and cognitive symptoms for all study participants.
Mood Symptoms.
The Hamilton Anxiety Rating Scale (HAM-A) and the Hamilton Depression Rating Scale (HAM-D) are self-report measures of severity of anxiety and depressive symptoms, respectively.20,21 The HAM-A is a 14-item questionnaire, with each item assigned a score ranging from 0 (“not present”) to 4 (“severe”). The total score of this scale ranges from 0 to 56, with scores greater that 17 indicating mild severity, scores between 18 and 24 indicating mild to moderate severity and scores greater than 25 indicating moderate to severe severity. The HAM-D is a 17-item questionnaire, with each item assigned a score ranging from 0 (“not present”) to 4 (“severe”). The total score of this scale ranges from 0 to 68, with scores greater than 7 indicating mild depressive symptoms. Both the HAM-A and the HAM-D have been recommended for use in Parkinson’s disease populations. The inter-rater reliability of the HAM-A is 0.87, the Cronbach’s alpha is 0.86, the inter-item correlation and convergent validity are satisfactory.22 The HAM-D has a specificity of 0.92 and a sensitivity of 0.99 for depression in PD.23 We modified the rater administered version of the HAM-A and HAM-D to be answered for the current state, on- or off-dopamine medication, such that changes in appetite, sleep, etc., were asked based on state rather than time frame.
Wearing-Off Symptoms.
We evaluated common motor and non-motor symptoms of Parkinson’s disease using a 19-symptom wearing-off questionnaire (WOQ-19).24 This questionnaire is a “present/not present” checklist of 19 items representing a wide range of Parkinson’s disease-related motor and non-motor symptoms. A total score was generated for each subject, with higher scores indicating a greater number of symptoms experienced in the off-state. This scale has a sensitivity of 0.88 and a specificity of 0.80 for detecting wearing off symptoms.25
Physical Disability and Motor Function.
We measured physical disability using the Schwab and England Activities of Daily Living Scale.26 This scale assesses a participant’s level of independence. Scores range from 0 to 100, with 0 indicating “bedridden and vegetative functions” and 100 indicating “completely independent”. We assessed motor functioning of all participants with the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III. The MDS-UPDRS part III assesses motor symptom severity on a scale of 0–108, with higher scores indicating greater motor impairment. The MDS-UPDRS part III was developed for use in Parkinson’s disease, with a Cronbach’s alpha of 0.93.27
Neuropsychological Measures.
Trained test administrators conducted the neuropsychological testing. Participants completed the Symbol Digit Modalities Test and the Stroop Color and Word Test.28,29 These tests evaluate the executive function cognitive domain, a domain often affected by PD. Both neuropsychological tests were administered according to recommended procedures. Scores from these tests were standardized based on age and educational attainment.
NINDS-CDE Recommended Assessments for Parkinson’s disease Specific Clinical Features.
After participants completed the “off” state assessment, we collected demographic and clinical information as recommended by the NINDS-CDE. These included personal medical history, family medical history, a medication log, and several scales assessing PD clinical features.
Medical History.
All participants provided their history of PD, including their age at disease diagnosis, side of disease onset, and the age they first noticed PD related symptoms. Participants also reported family history of PD or family history of any psychiatric disorders. The remaining sections of the MDS-UPDRS I, II, IV were completed. Additionally, all participants supplied a list of their current medications (dopaminergic and others). In order to standardize dopaminergic content of medications across different medication types, we calculated the levodopa equivalent daily dose (LEDD) contributed by each medication for each subject in accordance with convention.30 The 8-item Parkinson’s Disease Questionnaire (PDQ-8) was completed to assess quality of life.31
Psychiatric Disorders.
The structured clinical interview for DSM-IV-TR (SCID) evaluated the presence or history of any axis I disorders. The Parkinson Anxiety Scale (PAS) was administered outside of the fluctuation paradigm above to provide a measure of anxiety independent of the change in dopamine medication status.32 The PAS is a 12-item scale containing three subscales that assess specific anxiety subtypes (persistent anxiety, episodic anxiety, and avoidance behavior). PAS scores range from 0 to 48, with higher scores indicating more severe anxiety. The Montreal Cognitive Assessment (MoCA) was used to evaluate cognitive impairment. The MoCA assesses cognition on a scale of 0–30, with lower scores indicating greater cognitive impairment and has been validated in a PD population. The test-retest reliability of the MoCA is 0.79 and the convergent validity is 0.72.33
Analytic Plan
Descriptive statistics were conducted using Stata Version 16. Latent Profile Analyses (LPA) (similar to latent class analysis, but allowing continuous indicators) were conducted in MPlus Version 8.3.34,35 The following observed variables were used to model unobserved (i.e., latent) profiles: on-off-state difference in HAM-A total score, HAM-D total score, WOQ-19 individual items, Schwab and England score, and UPDRS part III. The default is to assume latent profile indicators are conditionally uncorrelated, but we allowed correlation between HAM-D and HAM-A because depression and anxiety are highly comorbid in PD.
Models for the LPA were fit iteratively, starting with one class and then adding additional classes until fit no longer improved. Several model fit statistics contributed to decisions about which model best fit the data, including Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), the Lo-Mendell-Rubin Likelihood Ratio test (LMR LRT), and entropy. We also considered class size and avoided profile prevalences less than 5%. Finally, we assessed each profile for clinical meaningfulness. From the final LPA model, we determined (1) the proportion of the sample that belongs in each profile (class prevalence), and (2) the means of each latent profile, conditioned upon membership in each class. We then tested whether specific participant characteristics such as age, sex, variability of symptoms between on- and off-states (as change scores), disease duration, and motor fluctuations predicted membership in the identified latent profiles. As profile membership is a latent variable, it would be inappropriate to assign individual profiles as if they were observed and to regress membership on predictors or to calculate profile-specific means. Instead we used Vermunt’s corrected three-step method to account for uncertainty in profile membership.36
Results
Sample characteristics
Table 1 displays the descriptive statistics for relevant demographic and clinical variables for the 200 people in this sample. The sample was typical for PD, being primarily male (61 %), Caucasian (92.5%), and retired (58%). The average age for participants was 65.2 years and most had at least a 4-year college degree (average, 17 years). The average disease duration was 9.1 years, with most participants in the Hoehn and Yahr 2 stage of disease severity (76.5%).
Latent Profile Model Fit
Fit statistics for models with two, three, four, or five profiles are reported in Table 2. Compared to a one-class model, the two-class model had lower BIC and fit the data significantly better based on LMR likelihood ratio test (−2LL difference: 25.92; df: 15; p=.0389). Three- and four-class models were rejected as neither fit the data significantly better than a more parsimonious model with a profile prevalence of at least 5% in each class.
Table 2.
Latent Profile Model Fit Statistics
| Number of Profiles | Entropy | Parameters | Bayesian Information Criterion (BIC) | Log Likelihood | Lo-Mendell-Rubin Likelihood Ratio p-value |
|---|---|---|---|---|---|
| 2 | .827 | 29 | 5505.714 | −2676.032 | 0.0389 |
| 3 | .865 | 44 | 5434.523 | −2600.699 | 0.002 |
| 4 | .846 | 59 | 5480.86 | −2584.131 | 0.6213 |
Lo-Mendell Rubin Likelihood Ratio test compares the difference in −2LL values for a k-class model to a model with k-1 classes to a chi-square distribution with degrees of freedom equal to the difference in number of parameters between the two models.
Change profiles
Figure 1 shows profiles from the LPA for the two-class model. The parameter estimates for this figure were transformed to z-scores by subtracting the overall variable mean and dividing by the overall variable standard deviation, allowing for an easier comparison of magnitudes for each variable for each profile. In Figure 1, each plotted point represents a difference in scores between the off- and on-dopamine medication state for each variable. The blue line represents patients with a ‘typical’ on-off response, while the red line represents ‘anxious fluctuators’.
Figure 1.
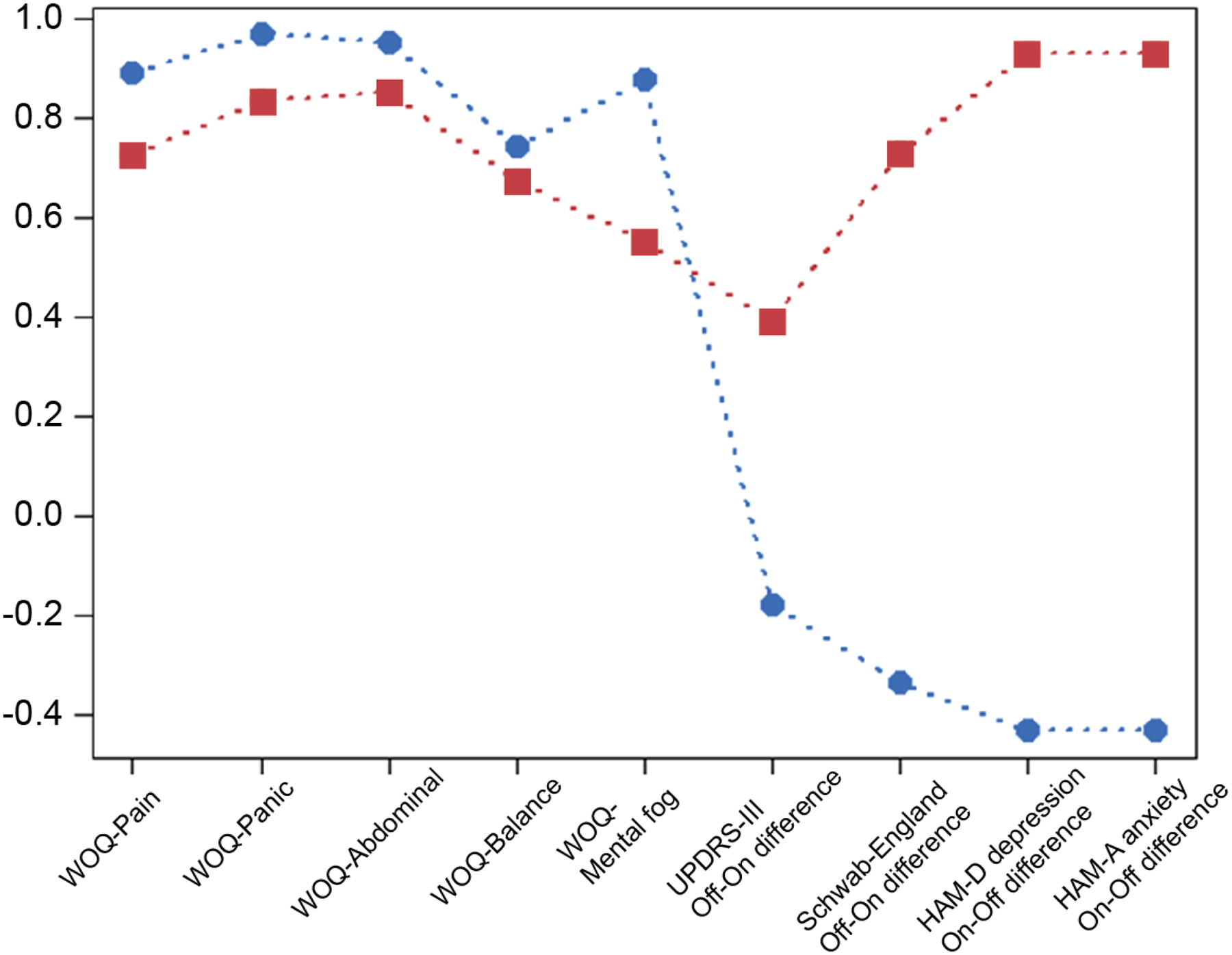
Latent profiles comparing on state versus off state for included indicators noted on the horizontal axis. Each plotted point represents a difference in scores between on state and off state. Circles represent patients with typical on off response, while the squares represents anxious fluctuators. Anxious fluctuators had large fluctuations in clinician-rated anxiety, depression, daily functioning, and motor impairment, but smaller responses in other self-rated non-motor symptoms like mental cloudiness, pain, and abdominal discomfort than patients with typical on-off response.
Table 3 compares estimates of differences in scores between on and off medication for participants with a ‘typical on-off response’ (69% of the sample) to others who exhibited worsening anxiety in the off state (31% of the sample; ‘anxious fluctuators’). The change in anxiety from off state to on state was three times greater for anxious fluctuators compared to patients with a typical on-off response (HAM-A change between the off and on medication state of 10.22 (32.85) compared to 3.27 (7.62)). In addition, anxious fluctuators had larger changes in depression (HAM-D), motor function (UPDRS part III), and daily functioning (S&E).
Table 3.
Estimated differences between medication states in two-class model. Data from 200 PD patients.
| Variable | Anxious Fluctuators (31%) | Typical “on-off” Response (69%) |
|---|---|---|
| Hamilton Anxiety Rating Scale Total Score | 10.22 (32.85) | 3.271 (7.62) |
| Hamilton Depression Rating Scale Total Score | 4.365 (10.2) | 0.89 (.96) |
| Schwab & England Score | 14.733 (13.26) | 4.721 (4.07) |
| UPDRS part 3 | 16.59 (10.42) | 12.057 (5.94) |
| Wearing Off Questionnaire – balance | .68 | .74 |
| Wearing Off Questionnaire – panic attacks | .83 | .97 |
| Wearing Off Questionnaire – mental cloudiness | .55 | .88 |
| Wearing Off Questionnaire – pain | .73 | .89 |
| Wearing Off Questionnaire – abdominal discomfort | .85 | .95 |
Estimates are reported as mean(SD) for continuous variables or prevalence for WOQ categorical variables which represent change from on to off state (WOQ 1 = symptom is present in the off state, 0 = not present)
One participant had missing data for this scale and was excluded from the analysis.
Individual items from the WOQ are scored “1” if symptoms are present in the off-state, such that a lower score in Figure 1 and Table 3 indicates greater symptom burden. Overall, the anxious fluctuators had less favorable clinical outcomes when transitioning between on-off medication states with greater worsening of HAM-A anxiety, HAM-D depression, UPDRS part III motor impairment, and S&E disability in the off state and more balance problems, panic attacks, mental cloudiness, pain, and abdominal discomfort on the WOQ-19 items.
For comparison, participants scores on the UPDRS part IV (n=200) motor fluctuations items were: 4.3 Time spent in the off state normal 69 (34.5%), slight 103 (51.5%), mild 21 (10.5%), moderate 5 (2.5%), severe 2 (1.0%); 4.4 Functional impact of fluctuations normal 89 (44.5%), slight 58 (29.0%), mild 25 (12.5%), moderate 19 (9.5%), severe 9 (4.5%); 4.5 Complexity of motor fluctuations normal 71 (35.5%), slight 96 (48.0%), mild 2 (1.0%), moderate 10 (5.0%), severe 21 (10.5%).
Characteristics associated with anxious fluctuations
Table 4 shows 3-step corrected profile specific means and frequencies of potential predictors of profile membership, along with odds ratios for membership in the anxious fluctuator profile vs. typical on-off response. Anxious fluctuators were more anxious outside of the provocative on-off protocol on the PAS and SCID, more likely to be male, with a younger age of Parkinson’s disease onset, longer duration of PD, and more than twice as likely to have a family history of anxiety and depression. Anxious fluctuators were less likely to be employed, had higher scores on the Schwab and England and UPDRS parts I and II indicating greater impairment of motor and non-motor activities of daily living.
Table 4.
Univariate Predictors of Latent Profile Membership, Odds ratios comparing typical on-off response to anxious fluctuators
| Variable | Anxious Fluctuators (31%) | Typical “on-off” Response (69%) | OR (95% CI) p-value |
|---|---|---|---|
| DBS (%) | .20 (.05) | .08 (.03) | .918 (.865, 973) .062 |
| Family Hx Depression | .43 (.07) | .21 (.04) | .352 (.169, .736) .007 |
| Family Hx Anxiety | .42 (.07) | .17 (.03) | .279 (.129, .604) .002 |
| Family Hx PD | .46 (.07) | .30 (.04) | .516 (.256, 1.04) .067 |
| Male | .54 (.07) | .32(.04) | .397 (.197, .80) .009 |
| Race (Caucasian) | .92 (.04) | .93 (.02) | .64 (.20, 2.05) .876 |
| Age | 63.06 (1.12) | 66.19 (.65) | 1.06 (1.01, 1.11) .43 |
| Education | 16.73 (.31) | 17.07 (.23) | 1.06 (.93, 1.20) .53 |
| Antidepressant Use | .52 (.07) | .41 (.04) | .656 (.332, 3.158) .225 |
| Antipsychotic Use | .05 (.03) | .03 (.02) | .563 (.10, 290.42) .545 |
| Anxiolytic Use | .26 (.06) | .18 (.04) | .62 (.277, 1.39) .264 |
| Parkinson Anxiety Scale | 14.96 (1.19) | 9.31 (.63) | .013 (.871, 957) <.001 |
| MDS UPDRS II | 15.54 (.94) | 10.58 (.66) | .911 (.861, .964) <.001 |
| MDS UPDRSI | 15.49 (.91) | 10.41 (.53) | .871 (.804, .944) <.001 |
| PD disease duration | 11.12 (.80) | 8.16 (.50) | .918 (.865, .973) <.001 |
| Age at PD onset | 51.94 (1.35) | 58.04 (0.79) | 1.08 (1.04, 1.12) <.001 |
| Levodopa Equivalent Daily Dosage (mg/day) | 972.36 (84.70) | 728.38 (37.34) | .999 (.999, .999) <.001 |
| Any SCID anxiety | .76 (.06) | .40 (.04) | .213 (.097, .468) <.001 |
| Montreal Cognitive Assessment Total Score | 27.02 (0.42) | 26.61 (0.25) | .948 (.828, 1.09) <.453 |
Profile-specific means and counts are calculated via Vermunt’s 3-step corrected method Odds Ratios are for membership in Profile 2 (Typical) vs Profile 1 (Anxious Fluctuators).
Table 5 shows odds ratios from a multivariate model which includes age PD onset, disease duration, sex, DBS, use of antipsychotic medication, and family history of anxiety. As a class anxious fluctuators had higher odds of being male and having a family history of anxiety compared to participants with a typical on-off response. The other variables, age of onset PD, PD duration, DBS and use of antipsychotic medication, were not statistically significantly associated with class.
Table 5.
Multivariate Predictors of Latent Profile Membership
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| PD Duration | 0.968 | (.907, 1.032) .319 |
| Age PD Onset | 1.053 | (.997, 1.113) .068 |
| Male Sex | 0.443 | (.202, .969) .041 |
| DBS | 0.802 | (.226, 2.844) .733 |
| Use of Antipsychotics | 0.658 | (.104, 4.176) .657 |
| Family History of Anxiety | 0.302 | (.13, .704) .006 |
Discussion
In a cohort of 200 people with PD assessed in both the on- and off- dopamine medication state we identified a latent profile representing 31% of patients with precipitous worsening of anxiety, depression, motor function, and ADL functioning in the off-medication state compared to the on-medication state. A greater proportion of patients in this profile also reported the persistence of mental cloudiness, poor balance, pain, abdominal discomfort, and panic attacks, even after transitioning from the off-state to the on-state. This profile was associated with an overall higher score on the UPDRS parts I and II indicating greater impairment of motor and non-motor activities of daily living. In this manuscript we refer to the identified latent profile as “anxious fluctuators” as they are distinguished by higher anxiety at baseline and more than a threefold increase in anxiety severity between the on an off-medication state. Anxious fluctuators may represent a subgroup of PD patients that experience a greater magnitude of change between the off- and on-medication state in anxiety, depression, and motor function, but paradoxically had less-medication associated relief of other non-motor symptoms. Given the adverse impact of this profile we believe it may be important to distinguish patients with a typical on-off response from those with this more problematic course of fluctuations.
Identifying anxious fluctuators without a reliable biomarker will be challenging, however in our multivariate model (Table 5) two variables were associated with greater odds of being in the ‘anxious fluctuator’ profile: family history of anxiety and male sex. Assuming a family history of anxiety disorders may be genetic, rather than shared behavioral patterns (e.g., maladaptive coping to adverse events), it is possible that certain anxiety disorders and the pathophysiology of PD overlap in some patients. The possibility of a shared genetic risk between PD and anxiety is supported by numerous epidemiologic and family studies showing an increased risk of PD in anxious individuals and families.37 We also found a greater than 2 fold increased odds of being an anxious fluctuator associated with male sex. However, a study in 100 PWP using a similar on-off design found no sex differences in association with the frequency or severity of non-motor fluctuations while a small observational study with just 16 women using the WOQ-19 (administered in the on-state only) reported that female sex was a risk factor for non-motor fluctuations.38 Although our sample is the largest by a wide margin, we advise caution when interpreting this finding as there is no consensus regarding sex as a risk factor for non-motor fluctuations and our finding is associated with a specific anxious latent class that may not be applicable to non-motor fluctuations in general.
Comparing our univariate findings to those of a case-control study of 70 “mood fluctuators” identified using retrospective chart review there were many similarities, but also striking differences. The main findings of Racette et al. were “mood fluctuators had significantly younger age at PD onset, and longer disease duration and were more likely to have dementia, psychosis, clinical depression, and motor complications”.9 Similar to our anxious fluctuators, anxious mood was the most common fluctuation in the off-period occurring in 81% of mood fluctuators. Further, younger age of PD onset, longer disease duration, and more motor complications occurred in both cohorts and mood fluctuators had a trend toward worse postural reflex which is similar to our finding of poor balance on the WOQ in anxious fluctuators. Unlike Racette et al. we excluded participants with dementia and found anxious fluctuators had similar MoCA scores compared to those of the control group. Finally, the prevalence of ‘clinically significant mood fluctuations’ estimated at 7% in the retrospective study was much lower than the latent profile of 31% identified in our prospective study. The prevalence difference could be due to the use of the provocative design in our study (e.g., provoking the levodopa off state), which might unmask latent symptoms that would otherwise only emerge with further disease progression.
Motor complications are known to be associated with disease duration and higher doses of dopamine replacement therapy rather than duration of exposure to levodopa therapy, with disease duration now recognized as the more important factor.7,39 Anxious fluctuators had both a 250mg/day greater LEDD and a longer disease duration compared to those with a typical on-off response. However, neither variable was significant in the multivariate model suggesting other latent factors may be more important. To this point, the timing of the onset of fluctuations varies widely from six months to 10 years, which suggests the occurrence of non-motor fluctuations has more to do with disease heterogeneity rather than a simple linear association with disease duration.9,10,12,40 While it is widely accepted that levodopa responsiveness varies in atypical Parkinsonian disorders, it may also be that the variation in levodopa responsiveness within idiopathic PD is more extensive than accounted for in our current approach to treatment. Indeed, the heterogeneity within idiopathic PD likely has significant implications for prognosis and treatment responsiveness41 and we suspect the latent profiles identified by the current model could represent one or more subtypes.
We do not think anxious fluctuations are simply due to lower responsiveness to levodopa because this group had a greater change in UPDRS part III motor symptoms due to symptomatic therapy compared to those with a more typical on-off response. Similarly, anxious fluctuators also had higher prescribed LEDD on average, suggesting that they were not simply under-dosed. While idiosyncratic factors such as protein consumption, compliance with medication dosing and timing, or stress may contribute to fluctuations it seems more likely that anxious fluctuators have some combination of a reduced ability to store and transport dopamine (e.g., VMAT2) or altered post-synaptic sensitivity to stimulation by dopamine, resulting in a more transient and less consistent response to interval dosing. This concept describing the discrepancies between the time course of pharmacological effect and plasma drug concentrations has been demonstrated in a series of levodopa infusion studies and is considered one of the main causes of motor fluctuations.42,43 For example, Nutt et al. demonstrated that for levodopa “Teq1/2” can be thought of as “the time course of the drug passing from the plasma to the striatum, conversion to dopamine, diffusion to dopamine receptors, development of clinical effect, and dissipation of clinical effect”.44 We hypothesize that the latent profile we identify in this study is influenced by mechanisms that reside in this pathway.
Regardless of whether the latent profile with anxious fluctuations is due to different subtypes of disease or more idiosyncratic variables, there is a need for better strategies to address fluctuations in PD. New extended release levodopa therapies and many dopamine agonists have demonstrated efficacy for both treating and delaying onset of motor fluctuations and may be beneficial for patients with this anxious fluctuation profile.45 On-demand-therapy is an emerging modality and is offered in a variety of routes of administration, which might offer rescue to patients once an off episode begins.46 Although it is possible that non-dopaminergic pathways may contribute to this latent profile, our model and levodopa infusion studies demonstrate that mood and anxiety often change in direct response to levels of exogenous dopamine.13 Therefore early identification of patients with this latent profile could be used as a signal to proactively start therapies shown to delay the onset of motor fluctuations in order to mitigate adverse outcomes.
Several limitations of this study should be considered when drawing conclusions about our findings. First, the variables assessed in the on-off battery were limited due to sample size constraints to prevent oversaturation of the model. Second, due to the nature of the statistical methods profile membership for any individual is a matter of Bayesian probability and was determined cross sectionally, therefore we cannot be certain that profile membership is static within an individual. However, the possibility that one could move from a state of anxious fluctuations to a more favorable state is a therapeutically optimistic concept. Levodopa withdrawal is being used as a proxy for studying spontaneous on-off fluctuations in PD, which likely does not account for anxiety produced due to the uncertainty of the more spontaneous off-state and may lead to a more pronounced difference between states than is seen with normal therapy where the goal is to prevent severe “off” states. Further, motor- and non-motor fluctuations can be temporally dissociated but without an objective marker of non-motor fluctuations to define the non-motor “off” or “on” state, this design is the closest experimental approximation to spontaneous fluctuations. Despite this, we believe levodopa withdrawal is a reasonable substitute as it can establish whether anxiety and other nonmotor symptoms change in response to dopamine. Finally, although we modified the HAM-A and HAM-D to assess state based changes in anxiety and depression there is still the possibility that the validity of some of the questions was diminished due to this change, however overall both of these instruments have demonstrated sensitivity to change in PD.
In summary, anxious fluctuations occurred in 31% of participants and were characterized by a precipitous worsening of anxiety, depression, motor function, and ADL functioning in the off-medication state compared to the on-medication state. We believe that this more problematic difference in response to the on- vs off- medication state represents a latent characteristic in a subgroup of PWP. Regardless, given that our intentional manipulation of dopaminergic medication status, on- vs off-state provoked these differences it is likely that interventions that facilitate a more stable and consistent response to dopamine replacement will not only improve motor impairment but also non-motor symptoms such as anxiety and depression in these individuals. Future studies should try to establish a biomarker e.g., PET or fMRI imaging, to enhance the objective sensitivity of the profile.
Anxiety associated with on-off dopaminergic medication fluctuations is a major cause of distress and lower quality of life in Parkinson’s
Up to one third of patients in this study had high anxiety in the off state compared to the on-dopamine medication state
Patients with high anxiety in the off-state also had higher depression and greater disability
‘Anxious fluctuators’ were more likely to be male and to have a family history of anxiety disorders
Acknowledgements:
Dr. Greg Pontone and the study described in this manuscript was funded by the NIH/NIA as part of a K23 award (K23 AG044441-01A1). Jared T Hinkle Receives tuition and stipend support through the Medical Scientist Training Program at the Johns Hopkins School of Medicine (NIH/NIGMS 5 T32 GM007309) and the National Institute on Aging (F30AG067643).
Financial Disclosure/Conflict of Interest Statement:
The other authors report no conflicts of interest, financial disclosures, or funding pertinent to this manuscript. Greg Pontone and Zoltan Mari have consulted for Acadia Pharmaceuticals Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2016;31(8):1125–1133. doi: 10.1002/mds.26643 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Pontone GM, Dissanayka N, Apostolova L, et al. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinsons Dis. 2019;5:30–8. eCollection 2019. doi: 10.1038/s41531-019-0102-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pontone GM, Williams JR, Anderson KE, et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Mov Disord. 2009;24(9):1333–1338. doi: 10.1002/mds.22611 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pontone GM, Williams JR, Anderson KE, et al. Anxiety and self-perceived health status in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(4):249–254. doi: 10.1016/j.parkreldis.2011.01.005 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ, Mason S, Foltynie T, Winder-Rhodes S, Barker RA, Williams-Gray CH. Motor complications in Parkinson’s disease: 13-year follow-up of the CamPaIGN cohort. Mov Disord. 2020;35(1):185–190. doi: 10.1002/mds.27882 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–2508. doi: 351/24/2498 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: Frequent and disabling. Neurology. 2002;59(3):408–413. doi: 10.1212/wnl.59.3.408 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Racette BA, Hartlein JM, Hershey T, Mink JW, Perlmutter JS, Black KJ. Clinical features and comorbidity of mood fluctuations in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(4):438–442. doi: 10.1176/jnp.14.4.438 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology. 2013;80(9):800–809. doi: 10.1212/WNL.0b013e318285c0ed [doi]. [DOI] [PubMed] [Google Scholar]
- 11.van der Velden RMJ, Broen MPG, Kuijf ML, Leentjens AFG. Frequency of mood and anxiety fluctuations in Parkinson’s disease patients with motor fluctuations: A systematic review. Mov Disord. 2018;33(10):1521–1527. doi: 10.1002/mds.27465 [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Blazquez C, Schrag A, Rizos A, Chaudhuri KR, Martinez-Martin P, Weintraub D. Prevalence of non-motor symptoms and non-motor fluctuations in Parkinson’s disease using the MDS-NMS. Mov Disord Clin Pract. 2020;8(2):231–239. doi: 10.1002/mdc3.13122 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maricle RA, Nutt JG, Carter JH. Mood and anxiety fluctuation in Parkinson’s disease associated with levodopa infusion: preliminary findings. Mov Disord. 1995. May;10(3):329–32. doi: 10.1002/mds.870100316. [DOI] [PubMed] [Google Scholar]
- 14.Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE. Anxiety and motor fluctuations in Parkinson’s disease: A cross-sectional observational study. Parkinsonism Relat Disord. 2012;18(10):1084–1088. doi: 10.1016/j.parkreldis.2012.06.007 [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle JT, Perepezko K, Gonzalez LL, Mills KA, Pontone GM. Apathy and Anxiety in De Novo Parkinson’s Disease Predict the Severity of Motor Complications. Mov Disord Clin Pract. 2020. Dec 4;8(1):76–84. doi: 10.1002/mdc3.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly MJ, Lawton MA, Baig F, Ruffmann C, Barber TR, Lo C, Klein JC, Ben-Shlomo Y, Hu MT. Predictors of motor complications in early Parkinson’s disease: A prospective cohort study. Mov Disord. 2019. Aug;34(8):1174–1183. doi: 10.1002/mds.27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 18.Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord. 1999. July 01;14(4):572–84. [DOI] [PubMed] [Google Scholar]
- 19.Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, et al. National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clin Trials. 2012. June 01;9(3):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M, White JM. Clinical syndromes in depressive states. J Ment Sci. 1959;105:985–998. doi: 10.1192/bjp.105.441.985 [doi]. [DOI] [PubMed] [Google Scholar]
- 22.Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Anxiety rating scales in Parkinson’s disease: A validation study of the Hamilton anxiety rating scale, the beck anxiety inventory, and the hospital anxiety and depression scale. Mov Disord. 2011;26(3):407–415. doi: 10.1002/mds.23184 [doi]. [DOI] [PubMed] [Google Scholar]
- 23.Naarding P, Leentjens AF, van Kooten F, Verhey FR. Disease-specific properties of the rating scale for depression in patients with stroke, Alzheimer’s dementia, and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(3):329–334. doi: 10.1176/jnp.14.3.329 [doi]. [DOI] [PubMed] [Google Scholar]
- 24.Stacy M The wearing-off phenomenon and the use of questionnaires to facilitate its recognition in Parkinson’s disease. J Neural Transm (Vienna). 2010;117(7):837–846. doi: 10.1007/s00702-010-0424-5 [doi]. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Martin P, Tolosa E, Hernandez B, Badia X, ValidQUICK Study Group. Validation of the “QUICK” questionnaire--a tool for diagnosis of “wearing-off” in patients with Parkinson’s disease. Mov Disord. 2008;23(6):830–836. doi: 10.1002/mds.21944 [doi]. [DOI] [PubMed] [Google Scholar]
- 26.McRae C, Diem G, Vo A, O’Brien C, Seeberger L. Schwab & England: Standardization of administration. Mov Disord. 2000;15(2):335–336. doi: [doi]. [DOI] [PubMed] [Google Scholar]
- 27.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340 [doi]. [DOI] [PubMed] [Google Scholar]
- 28.Smith A Symbol Digit Modalities Test. Western Psychological Services; Los Angeles, CA; 1982 [Google Scholar]
- 29.Golden CJ, Freshwater SM. Stroop color and word test. 1978 [Google Scholar]
- 30.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429 [doi]. [DOI] [PubMed] [Google Scholar]
- 31.Tan LC, Lau PN, Au WL, Luo N. Validation of PDQ-8 as an independent instrument in English and Chinese. J Neurol Sci. 2007;255(1–2):77–80. doi: S0022–510X(07)00101–3 [pii]. [DOI] [PubMed] [Google Scholar]
- 32.Leentjens AF, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, Martinez-Martin P. The Parkinson anxiety scale (PAS): Development and validation of a new anxiety scale. Mov Disord. 2014;29(8):1035–1043. doi: 10.1002/mds.25919 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043–1046. doi: 10.1002/mds.22017 [doi]. [DOI] [PubMed] [Google Scholar]
- 34.Gibson WA Psychometrika, 24, 229–252 (1959) [Google Scholar]
- 35.Muthén LK, & Muthén BO (1998-2017). Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- 36.Asparouhov T & Muthén B (2014). Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Structural Equation Modeling: A Multidisciplinary Journal, 21:3, 329–341. DOI: 10.1080/10705511.2014.915181 [DOI] [Google Scholar]
- 37.Lin CH, Lin JW, Liu YC, Chang CH, Wu RM. Risk of Parkinson’s disease following anxiety disorders: a nationwide population-based cohort study. Eur J Neurol. 2015. September 01;22(9):1280–7. [DOI] [PubMed] [Google Scholar]
- 38.Picillo M, Palladino R, Moccia M, Erro R, Amboni M, Vitale C, Barone P, Pellecchia MT. Gender and non motor fluctuations in Parkinson’s disease: A prospective study. Parkinsonism Relat Disord. 2016. Jun;27:89–92. doi: 10.1016/j.parkreldis.2016.04.001. Epub 2016 Apr 4. [DOI] [PubMed] [Google Scholar]
- 39.Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: Insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731–2742. doi: 10.1093/brain/awu195 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stocchi F, Antonini A, Barone P, Tinazzi M, Zappia M, Onofrj M, Ruggieri S, Morgante L, Bonuccelli U, Lopiano L, Pramstaller P, Albanese A, Attar M, Posocco V, Colombo D, Abbruzzese G; DEEP study group. Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord. 2014. Feb;20(2):204–11. doi: 10.1016/j.parkreldis.2013.10.027. Epub 2013 Nov 5. [DOI] [PubMed] [Google Scholar]
- 41.Espay AJ, Kalia LV, Gan-Or Z, Williams-Gray CH, Bedard PL, Rowe SM, Morgante F, Fasano A, Stecher B, Kauffman MA, Farrer MJ, Coffey CS, Schwarzschild MA, Sherer T, Postuma RB, Strafella AP, Singleton AB, Barker RA, Kieburtz K, Olanow CW, Lozano A, Kordower JH, Cedarbaum JM, Brundin P, Standaert DG, Lang AE. Disease modification and biomarker development in Parkinson disease: Revision or reconstruction? Neurology. 2020. Mar 17;94(11):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holford N, Nutt JG. Disease progression, drug action and Parkinson’s disease: why time cannot be ignored. Eur J Clin Pharmacol. 2008. February 01;64(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. The “on-off” phenomenon in Parkinson’s disease. Relation to levodopa absorption and transport. N Engl J Med. 1984. February 23;310(8):483–8. [DOI] [PubMed] [Google Scholar]
- 44.Nutt JG, Holford NH. The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol. 1996. May 01;39(5):561–73. [DOI] [PubMed] [Google Scholar]
- 45.Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018. Aug;33(8):1248–1266. doi: 10.1002/mds.27372. Epub 2018 Mar 23. [DOI] [PubMed] [Google Scholar]
- 46.Olanow CW, Factor SA, Espay AJ, Hauser RA, Shill HA, Isaacson S, Pahwa R, Leinonen M, Bhargava P, Sciarappa K, Navia B, Blum D; CTH-300 Study investigators. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 2020. Feb;19(2):135–144. doi: 10.1016/S1474-4422(19)30396-5. Epub 2019 Dec 7. [DOI] [PubMed] [Google Scholar]


