Abstract
Ethiopia is one of the countries in sub-Saharan Africa with the highest burden of childhood undernutrition. Despite the high burden of this scourge, little is known about the magnitude and contributing determinants to anthropometric failure among children aged 0–23 months, a period regarded as the best window of opportunity for interventions against undernutrition. This study examined factors associated with undernutrition (stunting, wasting, and underweight) among Ethiopian children aged 0–23 months. This study used a total weighted sample of 2146 children aged 0–23 months from the 2019 Ethiopian Mini Demographic and Health Survey. The data were cleaned and weighted using STATA version 14.0. Height-for-age (HFA), weight-for-height (WFH), and weight-for-age (WFA) z-scores < − 2 SD were calculated and classified as stunted, wasting, and underweight, respectively. Multilevel mixed-effects logistic regression models adjusted for cluster and survey weights were used. Adjusted odds ratio (AOR) and 95% confidence interval (CI) were estimated. Statistical significance was declared at p < 0.05. The overall weighted prevalence of stunting, wasting, and underweight respectively were 27.21% [95% CI (25.32–29.18)], 7.80% [95% CI (6.71–9.03)], and 16.44% [95% CI (14.90–18.09)] among children aged 0–23 months in Ethiopia. Female children were less likely to be associated with stunting [AOR: 0.68, 95% CI (0.54–0.86)], wasting [AOR: 0.70, 95% CI (0.51, 0.98)], and underweight [AOR: 0.64, 95% CI (0.49, 0.83)] than their male counterparts. Conversely, older children aged 12–17 months [AOR: 2.22, 95% CI (1.52, 3.23)] and 18–23 months [AOR: 4.16, 95% CI (2.75, 6.27)] were significantly at an increased odds of becoming stunted. Similarly, the likelihood of being underweight was higher in older age groups: 6–11 months [AOR: 1.74, 95% CI (1.15, 2.63)], 12–17 months [AOR: 2.13, 95% CI (1.40, 3.24)], and 18–23 months [AOR: 4.08, 95% CI (2.58, 6.44)] compared with the children younger than 6 months. Lower wealth quintile was one of the other significant determinants of stunting and underweight. The study’s findings indicated that the most consistent significant risk factors for undernutrition among children aged 0–23 months are: male sex, older age groups and lower wealth quintile. These findings emphasize the importance of strengthening nutrition-specific and sensitive interventions that address the immediate and underlying drivers of childhood undernutrition in early life, as well as targeting low-income households with male children, in order for Ethiopia to meet the Sustainable Development Goals (SDGs) 1,2 and 3 by 2030.
Subject terms: Diseases, Health care, Risk factors
Introduction
Undernutrition, which includes stunting, wasting, and underweight is a major public health concern, especially in many low and middle-income countries1. Low height-for-age is known as stunting, and it can be caused by poor nutrition in utero and/or early childhood. Wasting refers to a condition where a child is thin for their height. A child who is underweight (low weight-for-age), may be stunted, wasted, or both2,3. Any form of undernutrition in children aged 0 to 23 months is a serious threat because its consequences including physical, psychological and socioeconomic impacts can last into adulthood and are irreversible4.
Globally in 2020, about 149 million children under the age of five were stunted, and 45 million were estimated to be wasted3. Notably, Sub-Saharan Africa is home to the majority of children who are undernourished in the world, and the east African region where Ethiopia is located has been reported to have the highest prevalence of undernutrition5–7.
The first 1000 days of a child's life (0–23 months) are a critical period of rapid physical and mental development. The damage caused by malnutrition during the first 1,000 days has a negative impact on the child's health, cognitive development, physical growth, and school and work performance later in life8. A closer look of existing evidence reveal that children aged 0 to 23 months have been reported to be disproportionately affected by various types of malnutrition4,9–12.
In Ethiopia, malnutrition contributes more than 50 percent of all infant and child deaths8, and according to the recent 2019 Ethiopian mini Demographic and Health Survey (EMDHS), the prevalence of stunting (37%), wasting (7%) and underweight (21%) was unacceptably high13. Moreover, existing primary studies in different parts of the country have reported high burden of undernutrition in children aged 0–23 months- with prevalence of stunting (21.8%-42⋅7%)11,14–16, wasting (9.9%-11.6%)16, and underweight (27.7%)16. Ethiopia as a nation is still dealing with a number of underlying vulnerabilities for childhood malnutrition, including food insecurity17,18, caused by multifactorial determinants such as prolonged drought and famine, poverty and civil unrest19–21.
According to the 2018 Global Nutrition Report, country progress toward global targets can be tracked using determinants such as child wasting, stunting, and exclusive breastfeeding, making undernutrition a significant indicator of a nation’s socioeconomic developmental status. Ethiopia is one of 100 countries on track to achieve none of these targets22. In Ethiopia, undernutrition remains a major concern, and the country suffers from a substantial burden of acute and chronic malnutrition in early childhood23–25. In order to address undernutrition, the Ethiopian government is implementing several initiatives to combat childhood malnutrition including the National Nutritional Plans I and II and the Seqota Declaration. For instance, the recent Seqota Declaration has made statement to end stunting in Ethiopia for children under two years old through effective coordination and collaboration of sectors, leading to the broader vision "to end stunting in children under two years in Ethiopia by 203025.
Undernutrition has been reported to be associated with multiple factors: sex of the child26, age of the child26–28, birth size29,30, birth order28, short birth interval31, lack of exclusive breastfeeding32, having repeated diarrheal episodes32, lack of improved sanitation facilities31,33, maternal body mass index (BMI)27,31,32, maternal education29,30, and wealth status29,30,34 are among the factors associated with childhood stunting. Similarly, different studies have elicited the predictors of wasting in children, such as sex of the child27,35, birth size36, cough27, fever37, maternal BMI36, maternal education27,38, diarrheal morbidity35,38,39, complementary food starting before 6 months38, and unimproved drinking water40. Furthermore, sex of the child41,42, child age42,43, diarrheal morbidity in the last two weeks41–43, paternal education41,43, preceding birth interval43, birth order42, type of birth42, maternal body mass index42, birth size of child42, duration of breastfeeding42, and wealth index42,43 had a significant effect on underweight.
Although childhood malnutrition in Ethiopia has been widely reported, evidence on the relative strength of its determinants focusing on early in life (0–23 months) has not been adequately investigated, as previous studies have mainly examined the problem among children 0–59 months generally30,37,40,42,44,45. Also, early studies have put more emphasis on socioeconomic disparity46, stunting29,30,37, wasting40, underweight42, or spatial variations31,40.
Previous studies have concentrated on specific types of undernutrition in children aged 0–56 or 6–59 months29–31,37,40,47. Furthermore, existing evidence in Ethiopia that shows problem of malnutrition during the first 0–23 months was limited to local areas10,16,48–50, with some focusing on anaemia51,52, and levels of dietary diversity53. As far as our knowledge, there have been no studies conducted exclusively among children aged 0 to 23 months, using nationally representative data and rigorous analysis. Therefore, this study aimed to assess the determinants of undernutrition (stunting, wasting, and underweight) among children aged 0–23 months in Ethiopia. Identifying the determinants in this “critical window of opportunity” period is important for interventions against undernutrition and to meet the Sustainable Development Goals (SDGs) by 2030, in particular SDG 2, which aims to end hunger, achieve food security and improve nutrition.
Methods
Study design and setting
The current study used the 2019 Ethiopia Mini Demographic and Health Survey (EMDHS). The EMDHS) used a cross-sectional design and is the latest survey addressing childhood health issues in Ethiopia. Ethiopia is located in the horn of Africa. Its geographical coordinates are 9.145° N latitude and 40.4897° East longitude. The country covers an area of 1.1 million square Kilometers. Administratively, Ethiopia is divided into eleven geographical regions and two administrative cities (namely, Addis Ababa, Afar, Amhara, Benishangul-Gumuz, Dire Dawa, Gambella, Harari, Oromia, Somali, Southern Nations and Nationalities and People [SNNP], Tigray, Sidama, and South West Ethiopia Peoples' Region). In this analysis, Sidama and South West Ethiopia Peoples' regions were under South Nations and Nationalities Peoples region. Oromia, Amhara, and SNNP are highly populous states that account for 37·9%, 21·6%, and 21·3% of the country’s population, respectively54.
Sampling and population
The 2019 Ethiopia Mini Demographic and Health Survey (EMDHS) is the second Mini Demographic and Health Survey conducted in Ethiopia. Data collection took place from 21 March 2019 to 28 June 201913. The data was based on the nationally representative sample that provided estimates at the national and regional levels and for urban and rural areas. The sampling frame used for the 2019 EMDHS is a frame of all census enumeration areas (EAs) created for the 2019 Ethiopia Population and Housing Census (EPHC). The census frame is a complete list of the 149,093 EAs created for the 2019 EPHC. An EA is a geographic area covering an average of 131 households. The 2019 EMDHS sample was stratified and selected in two stages. In the first stage, a total of 305 EAs (93 in urban areas and 212 in rural areas) were selected proportionally, considering the EA size. In the second stage of selection, a fixed number of 30 households per cluster were selected with an equal probability systematic selection from the newly created household listing. In all selected households, height and weight measurements were collected from children age 0–59 months13. The 2019 EMDHS gathered anthropometric data from children under five (n = 5,279 for stunting, n = 5,408 for wasting, and n = 5,338 for underweight)13. For the analysis of the current study, a total of 2,146 children aged 0–23 months who had valid and complete anthropometric measurements were included.
Data collection
In the anthropometry questionnaire, height and weight measurements were recorded for eligible children aged 0–59 months in all interviewed households. Weight measurements were obtained using lightweight, electronic SECA 874 scales with a digital screen and the mother and child function. Height measurements were performed using measuring boards. Children younger than age 24 months were measured lying down (recumbent) on the board. Health professionals were trained to measure children’s height and weight. Training on child height measurement included standardization exercises13.
Variables and measurement
Stunting, wasting, and underweight were taken separately as dependent variables with binary categories. All anthropometric failure outcomes were constructed based on the 2006 World Health Organization (WHO) child growth standards1. Stunting was defined as a height-for-age z score less than − 2 standard deviations (SDs) of the median, wasting as a weight-for-height z score less than − 2 SDs, and underweight as a weight-for-age z score of less than − 2 SDs1.
Individual, household and community-level factors were considered as the potential determinants variables. The individual-level factors included were child sex, child age, number of under-five children, birth order, birth interval, dietary diversity score, meal frequency, breastfeeding status, received vitamin A in last 6 months, maternal age (years), maternal education, antenatal care, place of delivery. Household-level factors include household wealth, household size, household toilet facility, and household’s source of drinking water. Community-level factors include place of residence and region.
Minimum dietary diversity is a proxy for adequate micronutrient density of foods. Minimum dietary diversity defined as the proportion of children age 6–23 months who received a minimum of five out of eight food groups during the previous day. The five groups should come from a list of eight food groups: breast milk; grains, roots, and tubers; legumes and nuts; dairy products (milk, yogurt, and cheese); flesh foods (meat, fish, poultry, and liver/organ meat); eggs; vitamin A-rich fruits and vegetables; and other fruits and vegetables. Minimum meal frequency was defined as proportion of children age 6–23 months who received solid, semisolid, or soft food (including milk feeds for non-breastfed children) the minimum number of times or more during the previous day.
Minimum meal frequency is a proxy for meeting energy requirements. Breastfed children aged 6–8 months are considered to be fed with a minimum meal frequency if they receive solid, semisolid, or soft foods at least twice a day. Breastfed children aged 6–23 months are considered to be fed with a minimum meal frequency if they receive solid, semisolid, or soft foods at least three times a day. Non-breastfed children aged 6–23 months are considered to be fed with a minimum meal frequency if they receive solid, semisolid, or soft foods or milk feeds at least four times a day and if at least one of the feeds is a solid, semisolid, or soft food55.
Toilet facility was categorized as “Improved", "Unimproved" or "Open defecation. Facilities would be considered improved if any of the following occurred: flush/pour flush toilets to piped sewer systems, septic tanks, and pit latrines; ventilated improved pit (VIP) latrines; pit latrines with slabs; and composting toilets. Unimproved facilities included: flush or pour-flush to elsewhere; pit latrine without a slab or open pit; bucket, hanging toilet or hanging latrine. Other facilities, including households with no facility or use of bush/field, were considered as open defecation56. Source of drinking water was categorized as “Improved”, or “Unimproved”. Improved sources of drinking water included piped water, public taps, standpipes, tube wells, boreholes, protected dug wells and springs, and rainwater. Other sources of drinking water were regarded as unimproved56. The principal components statistical procedure was used to determine the weights for the wealth index based on the number and kinds of consumer goods they own, ranging from a television to a bicycle or car, and housing characteristics such as source of drinking water, toilet facilities, and flooring materials. This index was divided into quintiles categories, and the bottom 40% of household wealth index factor score were classified as the poorest households, the next 20% as the middle-class households and the top 40% as the rich households, as used in the past studies6.
Data extraction and analysis for the current study
Data extraction
The EMDHS, 2019 household member recode (PR) file, a nationally representative large-scale dataset, served as the data source for this analysis. To gain access to the EMDHS-2019 dataset, we used the study title and significance to download the data from the Measure DHS website at www.measuredhs.com after receiving permission for registration. This was followed by the extraction of a wide range of information about potential individual and community level factors.
Data analysis
Analyses were performed using STATA version 14.0 Statistical software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Given the complexity of the two-stage sampling design of EMDHS, sample weighting was used to account for stratification and clustering for precision. For the complex sample design, it is necessary to consider three types of information: (i) the primary sampling unit or cluster variable, (ii) the stratification variable, and (iii) the weight variable to ensure that the estimates are representative at the national level. Data cleaning was performed prior to the analysis to ensure that our findings were consistent with the number of young children categorized in the final Ethiopia Mini-DHS 2019 report and according to the three anthropometric indices of nutritional status: height-for-age, weight-for-height, and weight-for-age. Descriptive analysis was conducted to describe the prevalence of undernutrition according to the independent variables. Multilevel mixed effect models were particularly suitable for our analysis where data for participants were organized at more than one level due to the hierarchical nature of DHS data (i.e., nested data). With such cluster data, children within a cluster may be more similar to each other than children in the rest of the cluster. As a result, we applied a multilevel logistic regression model to assess the association between determinants and undernutrition (stunting, wasting, and underweight). All possible covariates with a p-value of less than 0.25 in the multilevel bi-variable analysis were included in the multilevel multivariable logistic regression models. To avoid or minimize statistical error, multicollinearity was tested. In this study, the variance inflation factor was less than 10, so there was no multicollinearity collinearity between independent variables.
For the analysis, four models were used. The primary model (empty or null model) was fitted without explanatory variables. The second model (individual level factors), third model (community-level factors), and fourth model (final or full model) were adjusted for individual and community-level factors simultaneously. Adjusted odds ratio (AOR), along with their corresponding 95% confidence intervals (CIs), were used to estimate the strength and direction of the association between the determinants and outcome variables (stunting, wasting, and underweight). Statistical significance was defined as a p‐value of less than 0.05.
For model comparison, we used the log-likelihood ratio (LL) and the Deviance Information Criterion (DIC). The DIC was used as a measure of how well our different models fitted the data with a lower value on DIC indicating a better fit of the model. Moreover, Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) were used as diagnostics to determine the goodness of fit. After the values for each model of AIC and BIC were compared, the lowest one was thought to be a better explanatory model. Intra-cluster correlation coefficient (ICC), median odds ratio (MOR), and proportional change in variance (PCV) statistics were calculated to measure the variation between clusters57,58. ICC is the measure of variation attributed to contextual neighborhood factors (such as residential level factors) and is often used to operationalize the concept of contextual phenomena59. MOR is used to measure unexplained cluster heterogeneity and it is the median value of the odds ratio between the clusters at low and high risk of outcome variables. PCV is used to measure the total variation of each outcome explained by the final model57,59.
Ethical considerations
The data were downloaded and analyzed after the purpose of the analysis was communicated and approved by MEASURE DHS. After the approval, we downloaded this study data from http://www.dhsprogram.com. Ethics approval and participants consent were not required, as this is publicly available data.
Ethics approval and consent to participate
The data were obtained via online registration to measure the DHS program and downloaded after the purpose of the analysis was communicated and approved. An approval letter for the use of the EDHS data set was gained from MEASURE DHS. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Socio demographic characteristics of the study's participants
A total of 2,146 (weighted sample) children aged 0–23 months were included in this study. Almost half (50.5%) of these children were males. One in four sample children were infants (6–11 months old). Most of the children (82.1%) had a preceding birth interval of 24 months and above. The majority of children were from rural areas (73.1%). Of all the study participants, 86.0% were currently breastfeeding. About 21.5% of the children were from the poorest households while 21.2% were from the richest households. The overall prevalence of minimum meal frequency and minimum dietary diversity practice was 46.8% and 10.2%, respectively (Table 1).
Table 1.
Distribution of stunting, wasting, and underweight by categories of selected variables among children aged 0–23 months in Ethiopia, EMDHS 2019.
| Characteristics | Total Weight Freq. N (%) | Prevalence of Stunting, n (%) | Prevalence of Wasting, n (%) | Prevalence of Underweight, n (%) |
|---|---|---|---|---|
| Child-related factors | ||||
| Age (months) | ||||
| < 6 | 561 (26.1) | 89 (16.0) | 48 (30.2) | 52 (15.3) |
| 6–11 | 517 (24.1) | 132 (23.6) | 31 (19.4) | 86 (25.3) |
| 12–17 | 583 (27.2) | 170 (30.5) | 49 (30.5) | 119 (34.9) |
| 18–23 | 484 (22.6) | 167 (29.9) | 31 (19.8) | 84 (24.6) |
| Sex | ||||
| Male | 1,021 (50.5) | 316 (58.3) | 101 (64.4) | 204 (61.4) |
| Female | 1,001 (49.5) | 226 (41.7) | 56 (35.6) | 128 (38.6) |
| Number of under-five children | ||||
| 1 | 806 (39.9) | 195 (36.1) | 46 (29.8) | 118 (35.5) |
| 2+ | 1,216 (60.1) | 346 (63.9) | 110 (70.2) | 214 (64.5) |
| Birth order | ||||
| 1 | 468 (23.1) | 123 (22.8) | 14 (8.9) | 63 (19.1) |
| 2 | 421 (20.8) | 110 (20.4) | 32 (20.9) | 65 (29.7) |
| 3 | 285 (14.1) | 77 (14.3) | 22 (14.4) | 42 (12.7) |
| 4 | 230 (11.4) | 59 (10.8) | 16 (10.4) | 38 (11.5) |
| 5+ | 616 (30.5) | 171 (31.6) | 71 (45.3) | 128 (36.9) |
| Birth interval (n = 1,513) | ||||
| < 24 months | 277 (17.9) | 84 (21.4) | 25 (17.5) | 53 (19.7) |
| ≥ 24 months | 1,270 (82.1) | 327 (79.6) | 118 (82.5) | 215 (80.3) |
| Vitamin A in last 6 months (n = 1,991) | ||||
| Yes | 1,324 (66.5) | 340 (63.7) | 122 (79.3) | 219 (67.1) |
| No | 667 (33.5) | 194 (36.3) | 32 (20.6) | 107 (32.9) |
| Currently breastfeeding | ||||
| Yes | 1,739 (86.0) | 457 (84.4) | 130 (83.3) | 272 (81.8) |
| No | 283 (14.0) | 84 (15.6) | 26 (16.7) | 60 (18.2) |
| Minimum meal frequency | ||||
| Yes | 1,006 (46.8) | 285 (51.1) | 51 (41.5) | 154 (45.0) |
| No | 1,141 (53.2) | 273 (48.9) | 110 (68.5) | 188 (55.0) |
| Minimum dietary diversity | ||||
| Yes | 205 (10.2) | 53 (9.8) | 7 (4.4) | 28 (8.4) |
| No | 1,817 (89.8) | 489 (90.2) | 150 (95.6) | 304 (91.6) |
| Maternal factors | ||||
| Age (years) | ||||
| < 20 | 159 (7.9) | 54 (10.1) | 8 (5.1) | 40 (12.1) |
| 20–34 | 1,486 (73.5) | 400 (73.9) | 111 (71.1) | 216 (65.1) |
| 35–49 | 377 (18.6) | 86 (16.0) | 37 (23.8) | 76 (22.8) |
| Educational level | ||||
| No education | 937 (46.3) | 286 (52.9) | 93 (59.6) | 189 (56.8) |
| Primary | 805 (39.8) | 212 (39.3) | 51 (32.5) | 130 (39.1) |
| Secondary | 179 (8.8) | 28 (5.2) | 10 (6.5) | 9 (2.7) |
| Higher | 101 (5.0) | 14 (2.6) | 2 (1.3) | 5 (1.4) |
| ANC visit | ||||
| No ANC | 499 (25.3) | 137 (26.2) | 53 (35.8) | 100 (31.2) |
| 1–3 | 598 (30.3) | 149 (28.5) | 53 (35.9) | 111 (34.7) |
| 4+ | 877 (44.4) | 237 (45.3) | 42 (28.3) | 109 (34.1) |
| Place of delivery | ||||
| Home | 897 (44.9) | 266 (49.3) | 89 (57.3) | 184 (55.4) |
| Health facility | 1,099 (55.1) | 274 (50.1) | 67 (42.6) | 148 (44.6) |
| Household-level factors | ||||
| Household size | ||||
| 1–4 | 675 (33.4) | 168 (31.0) | 32 (20.8) | 103 (31.1) |
| 5+ | 1,347 (66.6) | 373 (69.0) | 124 (79.2) | 229 (68.9) |
| Wealth index | ||||
| Poorest | 435 (21.5) | 121 (22.3) | 50 (32.2) | 95 (28.6) |
| Poorer | 434 (21.5) | 125 (23.0) | 37 (23.9) | 76 (20.9) |
| Middle | 378 (18.7) | 125 (23.1) | 16 (10.4) | 63 (18.9) |
| Richer | 344 (17.0) | 99 (18.3) | 29 (19.0) | 58 (17.5) |
| Richest | 429 (21.2) | 72 (13.2) | 22 (14.4) | 40 (12.1) |
| Toilet facility | ||||
| Improved | 337 (17.0) | 63 (12.0) | 23 (14.5) | 53 (16.1) |
| Unimproved | 1,048 (52.8) | 193 (36.4) | 68 (44.1) | 148 (44.9) |
| Open defecation | 600 (30.2) | 273 (51.6) | 65 (41.4) | 128 (38.9) |
| Source of drinking water | ||||
| Improved | 1,282 (64.1) | 351 (65.8) | 108 (69.5) | 207 (62.9) |
| Surface water | 314 (15.7) | 69 (12.9) | 26 (16.8) | 63 (19.1) |
| Unimproved | 405 (20.2) | 114 (21.3) | 21 (13.7) | 59 (18.0) |
| Community-level factors | ||||
| Residence | ||||
| Urban | 543 (26.9) | 105 (19.6) | 37 (23.8) | 82 (24.9) |
| Rural | 1,478 (73.1) | 436 (80.4) | 119 (76.2) | 249 (75.1) |
| Region | ||||
| Tigray | 149 (7.4) | 46 (8.5) | 20 (12.8) | 39 (11.8) |
| Afar | 31 (1.5) | 9 (1.7) | 5 (2.9) | 7 (2.0) |
| Amhara | 414 (20.5) | 152 (28.0) | 37 (23.7) | 110 (33.3) |
| Oromia | 786 (38.9) | 189 (34.9) | 41 (26.3) | 78 (23.6) |
| Somali | 124 (6.2) | 20 (3.7) | 20 (13.1) | 21 (6.4) |
| Benishangul | 23 (1.2) | 7 (1.3) | 1 (1.1) | 5 (1.6) |
| SNNP | 404 (20.0) | 107 (19.8) | 28 (17.9) | 65 (19.5) |
| Gambela | 9 (0.4) | 1 (0.2) | 2 (1.1) | 1.8 (0.5) |
| Harari | 5 (0.3) | 1 (0.2) | 1 (0.1) | 1 (0.2) |
| Addis Ababa | 61 (3.0) | 6 (1.1) | 1 (0.3) | 2 (0.5) |
| Dire Dawa | 12 (0.6) | 2 (0.3) | 1 (0.4) | 1 (0.4) |
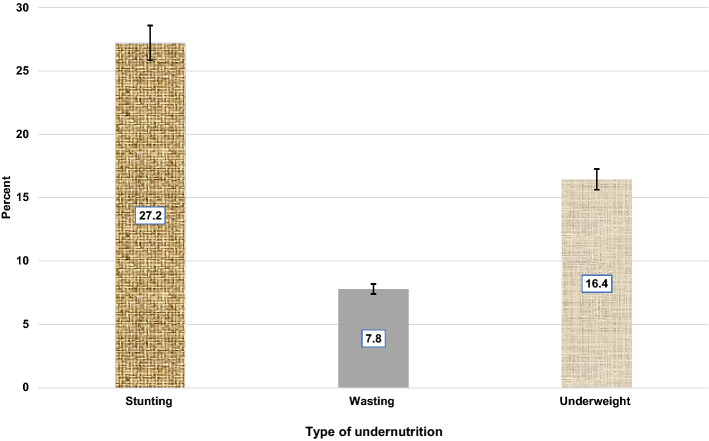
Prevalence of stunting
The prevalence of stunting was found to be 27.21% [95% CI (25.32–29.18)]. The prevalence of stunting generally differed by sex with 58.3% of stunted children were males. The proportion of stunted children was higher among uneducated mothers (52.9%) and rural residents (80.4%). Children in rural areas were more likely to be stunted compared to those in urban areas (80.4% versus 19.6%) (Fig. 1, Table 1).
Figure 1.
Undernutrition among children 0–23 months of age in Ethiopia, EMDHS-2019.
Prevalence of wasting
The prevalence of wasting was found to be 7.80% [95% CI (6.71–9.03)]. Almost two-thirds (64.4%) of wasted children were males and 76.2% lived in rural areas. Moreover, 68.5% and 95.6% of children that lacked minimum meal frequency and dietary diversity were wasted, respectively (Fig. 1, Table 1).
Prevalence of underweight
The prevalence of underweight was found to be 16.44% [95% CI (14.90–18.09)]. About 61.4% of underweight children were males, 34.9% were between the age of 12–17 months, and 75.1% of underweight children lived in rural areas (Fig. 1, Table 1).
Factors associated with stunting, wasting, and underweight
The result of the multilevel bivariable logistic regression analysis for stunting, wasting, and underweight is shown in Table 2 and Supplementary File 1.
Table 2.
Bivariable multilevel logistic regression analysis of factors associated with childhood stunting, wasting, and underweight among children aged 0–23 months in Ethiopia, EMDHS 2019.
| Characteristics | Stunted | Wasting | Underweight | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion, 95% CI | Unadjusted OR (95% CI) | p-value | Proportion, 95% CI | Unadjusted OR (95% CI) | p-value | Proportion, 95% CI | Unadjusted OR (95% CI) | p-value | |
| Child-related factors | |||||||||
| Age (months) | |||||||||
| < 6 | 17.2 (14.3–20.6) | Ref | 30.9 (24.9–37.5) | Ref | 18.1 (14.5–22.3) | Ref | |||
| 6–11 | 20.8 (17.7–24.4) | 1.46 (1.06–2.02) | 0.021 | 22.2 (17.0–28.4) | 0.70 (0.46–1.08) | 0.111 | 22.8 (18.9–27.4) | 1.41 (0.97–2.03) | 0.069 |
| 12–17 | 30.6 (26.9–34.5) | 2.18 (1.60–2.97) | p < 0.001 | 26.1 (20.5–32.5) | 0.77 (0.51–1.16) | 0.223 | 28.9 (24.6–33.8) | 1.68 (1.18–2.39) | 0.004 |
| 18–23 | 31.3 (27.6–35.3) | 3.68 (2.67–5.05) | p < 0.001 | 20.8 (15.8–26.8) | 0.82 (0.53–1.26) | 0.367 | 30.0 (25.6–34.9) | 2.80 (1.96–4.01) | p < 0.001 |
| Sex | |||||||||
| Male | 56.3 (52.1–60.4) | Ref | 58.9 (52.1–65.4) | Ref | 59.6 (54.5–64.4) | Ref | |||
| Female | 43.7 (39.6–47.8) | 0.72 (0.59–0.89) | 0.003 | 41.1 (34.5–47.9) | 0.65 (0.48–0.89) | 0.008 | 40.4 (35.6–45.5) | 0.62 (0.49–0.79) | p < 0.001 |
| Number of under-five children | |||||||||
| 1 | 35.7 (31.7–39.8) | Ref | 32.8 (26.6–39.7) | Ref | 36.8 (31.9–41.9) | Ref | |||
| 2+ | 64.3 (60.1–68.2) | 1.13 (0.91–1.41) | 0.268 | 67.2 (60.3–73.4) | 1.22 (0.87–1.69) | 0.239 | 63.2 (58.1–68.1) | 1.01 (0.78–1.31) | 0.913 |
| Birth order | |||||||||
| 1 | 20.4 (17.2–24.1) | Ref | 17.2 (12.5–23.1) | Ref | 20.3 (16.5–24.8) | Ref | |||
| 2–4 | 48.7 (44.5–52.9) | 1.17 (0.88–1.54) | 0.269 | 41.4 (34.7–48.4) | 1.14 (0.74–1.75) | 0.561 | 43.7 (38.7–48.9) | 1.03 (0.74–1.41) | 0.873 |
| 4+ | 30.9 (27.1–34.9) | 1.11 (0.82–1.51) | 0.485 | 41.4 (34.7–48.4) | 1.92 (1.22–2.99) | 0.004 | 35.9 (31.1–41.0) | 1.38 (0.97–1.94) | 0.065 |
| Birth interval | |||||||||
| < 24 months | 20.0 (16.4–24.0) | Ref | 17.2 (12.1–23.7) | Ref | 17.9 (13.8–22.7) | Ref | |||
| ≥ 24 months | 80.0 (75.9–83.5) | 0.84 (0.62–1.14) | 0.261 | 82.8 (76.2–87.9) | 1.11 (0.71–1.74) | 0.651 | 82.1 (77.2–86.1) | 1.01 (0.71–1.44) | 0.951 |
| Vitamin A in last 6 months | |||||||||
| Yes | 59.7 (55.4–63.8) | Ref | 74.7 (68.1–80.4) | Ref | 61.5 (56.3–66.4) | Ref | |||
| No | 40.3 (36.1–44.5) | 1.43 (1.14–1.17) | 0.002 | 25.3 (19.6–31.8) | 0.60 (0.42–0.86) | 0.006 | 38.5 (33.6–43.7) | 1.21 (0.93–1.56) | 0.146 |
| Currently breastfeeding | |||||||||
| Yes | 17.5 (14.5–20.9) | Ref | 16.2 (11.6–21.9) | Ref | 19.2 (15.5–23.6) | Ref | |||
| No | 82.5 (79.1–85.5) | 1.28 (0.96–1.71) | 0.088 | 83.8 (78.0–88.3) | 1.06 (0.69–1.62) | 0.780 | 80.8 (76.4–84.5) | 1.53 (1.11–2.11) | 0.010 |
| Minimum meal frequency | |||||||||
| Yes | 53.2 (49.1–57.3) | Ref | 39.6 (33.1–46.4) | Ref | 48.1 (43.1–53.2) | Ref | |||
| No | 46.8 (42.6–50.9) | 0.64 (0.52–0.80) | p < 0.001 | 60.4 (53.5–66.8) | 1.40 (1.02–1.93) | 0.037 | 51.9 (46.7–56.9) | 0.93 (0.73–1.19) | 0.558 |
| Minimum dietary diversity | |||||||||
| Yes | 7.6 (5.6–10.2) | Ref | 3.5 (1.7–7.2) | Ref | 5.8 (3.8–8.8) | Ref | |||
| No | 92.4 (89.8–94.3) | 1.13 (0.76–1.67) | 0.542 | 96.5 (92.7–98.3) | 2.75 (1.24–6.07) | 0.012 | 94.1 (91.2–96.1) | 1.57 (0.95–2.58) | 0.076 |
| Maternal factors | |||||||||
| Age (years) | |||||||||
| < 20 | 8.9 (6.8–11.6) | Ref | 10.6 (7.0–15.7) | Ref | 10.6 (7.8–14.2) | Ref | |||
| 20–34 | 75.6 (71.8–79.1) | 0.91 (0.62–1.34) | 0.654 | 70.7 (63.9–76.6) | 0.72 (0.43–1.20) | 0.209 | 71.0 (66.1–75.5) | 0.76 (0.50–1.15) | 0.198 |
| 35–49 | 15.4 (12.6–18.7) | 0.84 (0.54–1.32) | 0.449 | 18.7 (13.8–24.7) | 0.98 (0.53–1.79) | 0.942 | 18.4 (14.7–22.7) | 0.97 (0.59–1.58) | 0.910 |
| Educational level | |||||||||
| No education | 55.5 (51.3–59.7) | 1.35 (1.08–1.68) | 0.008 | 64.1 (57.2–70.5) | 1.91 (1.37–2.65) | 0.002 | 59.6 (54.4–64.6) | 1.67 (1.29–2.15) | p < 0.001 |
| Primary and above | 44.4 (40.3–48.6) | Ref | 35.8 (29.4–42.7) | Ref | 40.4 (35.4–45.6) | Ref | |||
| ANC visit | |||||||||
| Yes | 71.8 (67.7–75.4) | Ref | 60.2 (53.1–66.9) | Ref | 67.5 (62.4–72.2) | Ref | |||
| No | 28.2 (24.5–32.2) | 1.26 (0.98–1.62) | 0.075 | 39.8 (33.1–46.9) | 1.98 (1.42–2.78) | p < 0.001 | 32.5 (27.7–37.6) | 1.58 (1.19–2.10) | 0.001 |
| Place of delivery | |||||||||
| Home | 49.2 (45.0–53.5) | 1.32 (1.05–1.66) | 0.019 | 54.6 (47.5–61.4) | 1.52 (1.09–2.12) | 0.013 | 54.5 (49.3–59.6) | 1.72 (1.31–2.24) | p < 0.001 |
| Health facility | 50.8 (46.5–54.9) | Ref | 45.4 (38.5–52.4) | Ref | 45.5 (40.3–50.7) | Ref | |||
| Household-level factors | |||||||||
| Household size | |||||||||
| 1–4 | 30.3 (26.5–34.3) | Ref | 25.3 (19.7–31.7) | Ref | 27.9 (23.4–32.7) | Ref | |||
| 5+ | 69.7 (65.7–73.4) | 1.08 (0.85–1.36) | 0.527 | 74.7 (68.2–80.3) | 1.39 (0.97–1.98) | 0.065 | 72.1 (67.3–76.5) | 1.21 (0.93–1.59) | 0.160 |
| Wealth index | |||||||||
| Poorest | 34.4 (30.5–38.5) | 2.40 (1.69–3.41) | p < 0.001 | 50.5 (43.5–57.4) | 3.09 (1.89–5.03) | p < 0.001 | 42.1 (37.0–47.2) | 2.98 (1.99–4.47) | p < 0.001 |
| Poorer | 19.9 (16.7–23.5) | 2.64 (1.81–3.86) | p < 0.001 | 16.2 (11.6–21.9) | 1.80 (1.02–3.19) | 0.043 | 19.7 (15.9–24.2) | 2.55 (1.63–3.98) | p < 0.001 |
| Middle | 16.5 (13.6–19.9) | 2.63 (1.78–3.88) | p < 0.001 | 8.1 (5.0–12.8) | 1.02 (0.52–1.99) | 0.945 | 12.2 (9.2–16.1) | 1.76 (1.09–2.85) | 0.021 |
| Richer | 15.2 (12.4–18.5) | 2.41 (1.63–3.57) | p < 0.001 | 10.6 (7.0–15.7) | 1.39 (0.75–2.58) | 0.298 | 12.2 (9.2–16.0) | 1.81 (1.13–2.92) | 0.014 |
| Richest | 13.9 (11.2–17.1) | Ref | 14.6 (10.4–20.3) | Ref | 13.6 (10.4–17.6) | Ref | |||
| Toilet facility | |||||||||
| Improved | 14.8 (12.0–18.1) | Ref | 16.3 (11.8–22.2) | Ref | 15.8 (12.3–19.9) | Ref | |||
| Unimproved | 39.4 (35.4–43.7) | 1.68 (1.21–2.32) | 0.002 | 23.9 (18.5–30.4) | 0.84 (0.51–1.38) | 0.490 | 32.4 (27.7–37.4) | 1.17 (0.80–1.71) | 0.411 |
| Open defecation | 45.7 (41.5–50.0) | 1.95 (1.41–2.71) | p < 0.001 | 59.7 (52.6–66.3) | 2.09 (1.34–3.28) | 0.001 | 51.8 (46.6–57.0) | 1.98 (1.37–2.85) | p < 0.001 |
| Source of drinking water | |||||||||
| Improved | 14.8 (12.0–18.1) | Ref | 16.3 (11.8–22.2) | Ref | 15.8 (12.3–19.9) | Ref | |||
| Unimproved | 39.4 (35.4–43.7) | 1.04 (0.76–1.44) | 0.781 | 23.9 (18.5–30.5) | 1.67 (1.10–2.53) | 0.015 | 32.3 (27.8–37.4) | 1.09 (0.78–1.54) | 0.590 |
| Surface water | 45.7 (41.5–50.0) | 1.15 (0.85–1.54) | 0.360 | 59.7 (52.6–66.3) | 0.86 (0.55–1.36) | 0.524 | 51.8 (46.6–57.0) | 1.57 (1.12–2.21) | 0.010 |
| Community-level factors | |||||||||
| Residence | |||||||||
| Urban | 15.6 (12.8–18.9) | Ref | 21.2 (16.0–27.5) | Ref | 19.2 (15.4–23.6) | Ref | |||
| Rural | 84.4 (81.1–87.2) | 2.31 (1.68–3.17) | p < 0.001 | 78.8 (72.5–83.9) | 1.25 (0.82–1.93) | 0.293 | 80.8 (76.4––84.5) | 1.62 (1.13–2.32) | 0.009 |
| Region | |||||||||
| City administration | 14.8 (12.1–18.1) | 0.53 (0.38–0.75) | p < 0.001 | 9.5 (6.2–14.5) | 0.48 (0.28–0.84) | 0.009 | 12.2 (9.2–16.7) | 0.47 (0.31–0.71) | p < 0.001 |
| Pastoralist | 19.5 (16.4–23.1) | 0.79 (0.56–1.11) | 0.181 | 35.8 (29.4–42.8) | 2.27 (1.52–3.39) | p < 0.001 | 22.8 (18.7–27.5) | 1.07 (0.74–1.56) | 0.696 |
| Agrarian | 65.6 (61.4–69.5) | Ref | 54.5 (47.5–61.4) | Ref | 64.9 (59.8–69.7) | Ref | |||
Determinants of stunting among children aged 0–23 months
The odds of being stunted were lower in female children compared with male children [AOR: 0.68, 95% CI (0.54–0.86)]. The odds of stunting were also less likely among children born of mothers aged 35–49 years old [AOR: 0.57, 95% CI (0.27, 0.97)] than children born of younger mothers. The likelihood of being stunted among children aged 12–17 months [AOR: 2.22, 95% CI (1.52, 3.23)] and 18–23 months old [AOR: 4.16, 95% CI (2.75, 6.27)] were 2.22 and 4.16 times higher compared with children in age grouped less than 6 months. Likewise, the probability of being stunted was higher in children from the poorest [AOR: 2.06, 95% CI (1.12, 3.79)], poorer [AOR: 2.25, 95% CI (1.27, 3.97)], middle wealth quintile [AOR: 2.08, 95% CI (1.19, 3.65)], and richer households [AOR: 2.02, 95% CI (1.19, 3.42)] compared to those children richest households (Table 3).
Table 3.
Multivariable multilevel logistic regression analysis of factors associated with childhood stunting among children aged 0–23 months in Ethiopia, EMDHS 2019.
| Characteristics | Null model (Model 0) | Individual level model (Model 1), AOR (95% CI) | Community level model (Model 2), AOR (95% CI) | Full model (Model 3), AOR (95% CI) |
|---|---|---|---|---|
| Child related factors | ||||
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.68 (0.54–0.86)* | 0.68 (0.54–0.86)* | ||
| Age (months) | ||||
| < 6 | Ref | Ref | ||
| 6–11 | 1.36 (0.94–1.98) | 1.36 (0.93–1.97) | ||
| 12–17 | 2.23 (1.54–3.25)** | 2.22 (1.52–3.23)** | ||
| 18–23 | 4.25 (2.82–6.40)** | 4.16 (2.75–6.27)** | ||
| Number of under-five children | ||||
| 1 | Ref | Ref | ||
| 2+ | 1.19 (0.92–1.56) | 1.22 (0.93–1.59) | ||
| Vitamin A in last 6 months | ||||
| Yes | Ref | Ref | ||
| No | 1.14 (0.88–1.49) | 1.13 (0.87–1.47) | ||
| Currently breastfeeding | ||||
| Yes | Ref | Ref | ||
| No | 0.82 (0.58–1.17) | 0.85 (0.59–1.21) | ||
| Minimum meal frequency | ||||
| Yes | Ref | Ref | ||
| No | 0.89 (0.66–1.18) | 0.88 (0.65–1.17) | ||
| Minimum dietary diversity | ||||
| Yes | Ref | Ref | ||
| No | 1.29 (0.84–2.00) | 1.30 (0.84–2.01) | ||
| Maternal factors | ||||
| Age (years) | ||||
| < 20 | Ref | Ref | ||
| 20–34 | 0.75 (0.49–1.15) | 0.74 (0.48–1.14) | ||
| 35–49 | 0.59 (0.35–1.01) | 0.57 (0.27–0.97)* | ||
| Educational level | ||||
| No education | 1.26 (0.95–1.65) | 1.29 (0.98–1.70) | ||
| Primary and above | Ref | Ref | ||
| ANC visit | ||||
| Yes | 0.95 (0.70–1.29) | 0.93 (0.68–1.26) | ||
| No | Ref | Ref | ||
| Place of delivery | ||||
| Home | 0.98 (0.73–1.31) | 0.97 (0.72–1.30) | ||
| Health facility | Ref | Ref | ||
| Household-level factors | ||||
| Household size | ||||
| 1–4 | Ref | Ref | ||
| 5+ | 0.95 (0.72–1.25) | 0.94 (0.72–1.24) | ||
| Wealth index | ||||
| Poorest | 2.32 (1.37–3.93)* | 2.06 (1.12–3.79)* | ||
| Poorer | 2.73 (1.68–4.42)** | 2.25 (1.27–3.97)* | ||
| Middle | 2.52 (1.57–4.03)** | 2.08 (1.19–3.65)* | ||
| Richer | 2.36 (1.49–3.73)** | 2.02 (1.19–3.42)* | ||
| Richest | Ref | Ref | ||
| Toilet facility | ||||
| Improved | Ref | Ref | ||
| Unimproved | 1.11 (0.76–1.63) | 1.12 (0.73–1.73) | ||
| Open defecation | 1.19 (0.78–1.82) | 1.01 (0.68–1.51) | ||
| Source of drinking water | ||||
| Improved | Ref | Ref | ||
| Unimproved | 1.06 (0.77–1.46) | 0.82 (0.57–1.18) | ||
| Surface water | 0.80 (0.56–1.15) | 1.08 (0.78–1.48) | ||
| Community-level factors | ||||
| Residence | ||||
| Urban | Ref | Ref | ||
| Rural | 2.09 (1.48–2.95)** | 1.19 (0.75–1.89) | ||
| Region | ||||
| City administration | 0.75 (0.52–1.09) | 0.82 (0.54–1.24) | ||
| Pastoralist | 0.81 (0.58–1.13) | 0.73 (0.48–1.11) | ||
| Agrarian | Ref | Ref | ||
| Random effect | ||||
| Community variance (SE) | 0.4200 (0.008) | 0.3102 (0.0103) | 0.3333 (0.0081) | 0.3092 (0.0103) |
| ICC (%) | 11.32 | 8.62 | 9.19 | 8.59 |
| MOR | 1.85 | 1.69 | 1.73 | 1.69 |
| PCV (%) | Ref | 26.14 | 20.64 | 26.38 |
| Model fit statistics | ||||
| LL | − 1157.2118 | − 990.348 | − 1141.2625 | − 988.614 |
| DIC(-2Log-likelihood) | 2,314.42 | 1,980.69 | 2,282.52 | 1,977.22 |
| AIC | 2318.42 | 2030.698 | 2292.525 | 2033.229 |
| BIC | 2329.64 | 2168.906 | 2320.582 | 2188.022 |
**p-value < 0.001; *p-value < 0.05; SE Standard Error; ICC Intra-class Correlation Coefficient; MOR Median Odds Ratio; PCV Proportional Change in Variance; AIC Akaike’s Information Criterion; BIC Bayesian information Criteria; DIC Deviance Information Criterion; LL Log-likelihood.
Determinants of wasting among children aged 0–23 months
Being a female child [AOR: 0.70, 95% CI (0.51, 0.98)], a child residing in the rural area [AOR: 0.49, 95% CI (0.92, 0.92)], and a child living in city administrations [AOR: 0.49, 95% CI (0.26, 0.92) were associated with lower odds of being wasted (Table 4).
Table 4.
Multivariable multilevel logistic regression analysis of factors associated with childhood wasting among children aged 0–23 months in Ethiopia, EMDHS 2019.
| Characteristics | Null model (Model 0) | Individual level model (Model 1), AOR (95% CI) | Community level model (Model 2), AOR (95% CI) | Full model (Model 3), AOR (95% CI) |
|---|---|---|---|---|
| Child related factors | ||||
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.69 (0.49–0.96)* | 0.70 (0.51–0.98)* | ||
| Age (months) | ||||
| < 6 | Ref | Ref | ||
| 6–11 | 0.83 (0.51–1.37) | 0.81 (0.49–1.34) | ||
| 12–17 | 1.07 (0.65–1.76) | 1.03 (0.63–1.70) | ||
| 18–23 | 1.28 (0.73–2.26) | 1.30 (0.74–2.29) | ||
| Number of under-five children | ||||
| 1 | Ref | Ref | ||
| 2+ | 0.88 (0.59–1.30) | 0.88 (0.59–1.29) | ||
| Vitamin A in last 6 months | ||||
| Yes | Ref | Ref | ||
| No | 0.77 (0.51–1.15) | 0.79 (0.52–1.19) | ||
| Currently breastfeeding | ||||
| Yes | Ref | Ref | ||
| No | 0.93 (0.56–1.57) | 0.89 (0.53–1.52) | ||
| Minimum meal frequency | ||||
| Yes | Ref | Ref | ||
| No | 1.17 (0.76–1.80) | 1.16 (0.75–1.78) | ||
| Minimum dietary diversity | ||||
| Yes | Ref | Ref | ||
| No | 2.26 (0.94–5.46) | 2.21 (0.91–5.34) | ||
| Maternal factors | ||||
| Age (years) | ||||
| < 20 | Ref | Ref | ||
| 20–34 | 0.76 (0.43–1.37) | 0.78 (0.44–1.40) | ||
| 35–49 | 0.78 (0.38–1.59) | 0.81 (0.40–1.66) | ||
| Educational level | ||||
| No education | 1.32 (0.88–1.97) | 1.32 (0.87–1.98) | ||
| Primary and above | Ref | Ref | ||
| ANC visit | ||||
| Yes | 0.65 (0.43–0.99) | 0.68 (0.45–1.04) | ||
| No | Ref | Ref | ||
| Place of delivery | ||||
| Home | 0.81 (0.52–1.24) | 0.83 (0.53–1.28) | ||
| Health facility | Ref | Ref | ||
| Household-level factors | ||||
| Household size | ||||
| 1–4 | Ref | Ref | ||
| 5+ | 1.44 (0.95–2.20) | 0.94 (0.72–1.24) | ||
| Wealth index | ||||
| Poorest | 1.61 (0.77–3.36) | 1.76 (0.74–4.18) | ||
| Poorer | 1.41 (0.69–2.85) | 1.79 (0.78–4.10) | ||
| Middle | 0.95 (0.44–2.03) | 1.14 (0.47–2.71) | ||
| Richer | 1.25 (0.62–2.49) | 1.48 (0.68–3.24) | ||
| Richest | Ref | Ref | ||
| Toilet facility | ||||
| Improved | Ref | Ref | ||
| Unimproved | 0.66 (0.37–1.16) | 0.68 (0.38–1.22) | ||
| Open defecation | 1.26 (0.71–2.25) | 1.29 (0.72–2.32) | ||
| Source of drinking water | ||||
| Improved | Ref | Ref | ||
| Unimproved | 0.99 (0.62–1.58) | 0.67 (0.41–1.09) | ||
| Surface water | 0.67 (0.41–1.09) | 0.96 (0.60–1.52) | ||
| Community-level factors | ||||
| Residence | ||||
| Urban | Ref | Ref | ||
| Rural | 0.88 (0.56–1.38) | 0.49 (0.92–0.92)* | ||
| Region | ||||
| City administration | 0.45 (0.25–0.82)* | 0.49 (0.26–0.92)* | ||
| Pastoralist | 2.26 (1.51–3.38)** | 1.43 (0.87–2.36) | ||
| Agrarian | Ref | Ref | ||
| Random effect | ||||
| Community variance (SE) | 0.5722 (0.0178) | 0.3587 (0.0263) | 0.3999 (0.0192) | 0.2989 (0.0279) |
| ICC (%) | 14.81 | 9.83 | 10.84 | 8.33 |
| MOR | 2.05 | 1.76 | 1.82 | 1.68 |
| PCV (%) | Ref | 37.31 | 30.11 | 48.28 |
| Model fit statistics | ||||
| LL | − 642.33 | − 561.01 | − 627.05 | − 553.79 |
| DIC(-2Log-likelihood) | 1,284.66 | 1,122.02 | 1,254.1 | 1,107.58 |
| AIC | 1288.66 | 1172.003 | 1264.09 | 1163.583 |
| BIC | 1299.91 | 1310.479 | 1292.20 | 1318.676 |
**p-value < 0.001; *p-value < 0.05; SE Standard Error; ICC Intra-class Correlation Coefficient; MOR Median Odds Ratio; PCV Proportional Change in Variance; AIC Akaike’s Information Criterion; BIC Bayesian information Criteria; DIC Deviance Information Criterion; LL Log-likelihood.
Determinants of underweight among children aged 0–23 months
The odds of being underweight were lower in female children compared with male children [AOR: 0.64, 95% CI (0.49, 0.83)]. The likelihood of being underweight was higher in older age groups: 6–11 months [AOR: 1.74, 95% CI (1.15, 2.63)], 12–17 months [AOR: 2.13, 95% CI (1.40, 3.24)], and 18–23 months [AOR: 4.08, 95% CI (2.58, 6.44)] compared with the ages group of less than 6 months. Also, a child from households from the poorest [AOR: 2.28, 95% CI (1.14, 4.54)] and poorer wealth quartiles [AOR: 2.42, 95% CI (1.26, 4.63)] were higher odds of being underweight. Moreover, children living in city administrations [AOR: 0.57, 95% CI (0.35, 0.93)] were significantly associated with lowers odds of being underweight (Table 5).
Table 5.
Multivariable multilevel logistic regression analysis of factors associated with childhood underweight among children aged 0–23 months in Ethiopia, EMDHS 2019.
| Characteristics | Null model (Model 0) | Individual level model (Model 1), AOR (95% CI) | Community level model (Model 2), AOR (95% CI) | Full model (Model 3), AOR (95% CI) |
|---|---|---|---|---|
| Child related factors | ||||
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.64 (0.49–0.83)* | 0.64 (0.49–0.83)* | ||
| Age (months) | ||||
| < 6 | Ref | Ref | ||
| 6–11 | 1.78 (1.18–2.70)* | 1.74 (1.15–2.63)* | ||
| 12–17 | 2.19 (1.44–3.34)** | 2.13 (1.40–3.24)** | ||
| 18–23 | 4.19 (2.65–6.64)** | 4.08 (2.58–6.44)** | ||
| Number of under-five children | ||||
| 1 | Ref | Ref | ||
| 2+ | 0.86 (0.64–1.17) | 0.89 (0.66–1.20) | ||
| Vitamin A in last 6 months | ||||
| Yes | Ref | Ref | ||
| No | 1.10 (0.81–1.48) | 1.09 (0.81–1.47) | ||
| Currently breastfeeding | ||||
| Yes | Ref | Ref | ||
| No | 1.13 (0.76–1.67) | 1.15 (0.78–1.71) | ||
| Minimum meal frequency | ||||
| Yes | Ref | Ref | ||
| No | 1.40 (1.02–1.94)* | 1.36 (0.99–1.88) | ||
| Minimum dietary diversity | ||||
| Yes | Ref | Ref | ||
| No | 1.46 (0.85–2.52) | 1.49 (0.87–2.55) | ||
| Maternal factors | ||||
| Age (years) | ||||
| < 20 | Ref | Ref | ||
| 20–34 | 0.71 (0.44–1.13) | 0.70 (0.44–1.12) | ||
| 35–49 | 0.75 (0.43–1.32) | 0.72 (0.41–1.28) | ||
| Educational level | ||||
| No education | 1.22 (0.89–1.66) | 1.28 (0.94–1.76) | ||
| Primary and above | Ref | Ref | ||
| ANC visit | ||||
| Yes | 0.91 (0.65–1.27) | 0.90 (0.64–1.26) | ||
| No | Ref | Ref | ||
| Place of delivery | ||||
| Home | 1.16 (0.83–1.62) | 1.19 (0.85–1.67) | ||
| Health facility | Ref | Ref | ||
| Household-level factors | ||||
| Household size | ||||
| 1–4 | Ref | Ref | ||
| 5+ | 1.18 (0.86–1.63) | 1.16 (0.84–1.59) | ||
| Wealth index | ||||
| Poorest | 1.95 (1.08–3.51)* | 2.28 (1.14–4.54)* | ||
| Poorer | 2.17 (1.25–3.77)* | 2.42 (1.26–4.63)* | ||
| Middle | 1.57 (0.89–2.74) | 1.72 (0.89–3.32) | ||
| Richer | 1.55 (0.91–2.68) | 1.72 (0.93–3.18) | ||
| Richest | Ref | Ref | ||
| Toilet facility | ||||
| Improved | Ref | Ref | ||
| Unimproved | 0.81 (0.52–1.26) | 0.76 (0.48–1.20) | ||
| Open defecation | 1.06 (0.66–1.70) | 1.05 (0.65–1.68) | ||
| Source of drinking water | ||||
| Improved | Ref | Ref | ||
| Unimproved | 0.94 (0.65–1.37) | 0.98 (0.68–1.41) | ||
| Surface water | 1.04 (0.70–1.54) | 1.07 (0.73–1.57) | ||
| Community-level factors | ||||
| Residence | ||||
| Urban | Ref | Ref | ||
| Rural | 1.26 (0.85–1.85) | 0.60 (0.36–1.01) | ||
| Region | ||||
| City administration | 0.53 (0.33–0.82)* | 0.57 (0.35–0.93)* | ||
| Pastoralist | 1.08 (0.74–1.57) | 0.75 (0.48–1.16) | ||
| Agrarian | Ref | Ref | ||
| Random effect | ||||
| Community-level variance (SE) | 0.5049 (0.0093) | 0.3971 (0.0119) | 0.4588 (0.0094) | 0.3330 (0.0125) |
| ICC (%) | 13.30 | 10.77 | 12.23 | 9.19 |
| MOR | 1.96 | 1.81 | 1.90 | 1.73 |
| PCV (%) | Ref | 21.35 | 9.11 | 34.04 |
| Model fit statistics | ||||
| LL | − 935.70 | − 818.23 | − 927.47 | − 813.99 |
| DIC(-2Log-likelihood) | 1,871.40 | 1,636.46 | 1,854.94 | 1,627.98 |
| AIC | 1875.41 | 1686.456 | 1864.937 | 1683.986 |
| BIC | 1886.66 | 1825.051 | 1893.067 | 1839.212 |
**p-value < 0.001; *p-value < 0.05; SE Standard Error; ICC Intra-class Correlation Coefficient; MOR Median Odds Ratio; PCV Proportional Change in Variance; AIC Akaike’s Information Criterion; BIC Bayesian information Criteria; DIC Deviance Information Criterion; LL Log-likelihood.
Random effect analysis
The Intraclass Correlation Coefficient (ICC) in the null model for stunting, wasting and underweight was 11.32%, 14.81%, and 13.30%, respectively, which shows the variability of the conditions attributed to the clustering effect. The median odds ratio (MOR) value of the null models for stunting, wasting and underweight were 1.85, 2.05, and 1.96, respectively, which also indicates the presence of variation in the prevalence of stunting, wasting and underweight between clusters. The MOR measures the unexplained cluster heterogeneity (the variation between clusters) by comparing two individuals from two randomly chosen different clusters. In addition, the proportional change in variance (PCV) of the final models for stunting, wasting and underweight were 26.38%, 48.28% and 34.04%, respectively, indicating that the full model best explains the variability. The smallest values of Log-likelihood, AIC, and BIC were observed in model 3 and this implies that the full model for childhood stunting, wasting and underweight was a better explanatory model. As a result, interpretations and reports were made based on model 3 (full model) (Tables 3, 4 and 5).
Discussion
Undernutrition is unacceptably high in Ethiopia, especially among young children10,16,48,50. When undernutrition occurs in the first 1000 days of life (0–23 months) it continues to the age of five and has long-term effects including physically, psychologically, culturally and socioeconomically60. In Ethiopia, evidence on the relative strengths of determinants of undernutrition in children aged 0 to 23 months is lacking at the national level, which is essential for understanding its underlying mechanisms. This study assessed the determinants of stunting (low height-for-age), wasting (low weight-for-height), and underweight (low weight-for-age) among children aged 0–23 months. The prevalence of stunting, wasting, and underweight was found to be 27.21%, 7.80%, and 16.44%, respectively. Multilevel multivariate analysis revealed that the most consistent significant risk factors for stunting, wasting, and underweight among children aged 0–23 months are: sex of the child, child’s age, maternal age, household-wealth status, and the type of place of residence.
The prevalence of stunting in the current study at 27.21% indicates that stunting continues to be major public health problem in the country. The observed prevalence of stunting in Ethiopia should be categorized as high as per international standards at a range between 20 to 30%. This level of stunting should be-a trigger-level as a basis of public health decisions61. This finding was consistent with studies carried out among infants between the ages of 0 and 23 months in Nigeria 29%62, Bangladesh 30.9%63, and Indonesia 28.4%64. However, although the prevalence of stunting in Ethiopia was higher than the international standards, was relatively lower than that of Indian infants aged 0–23 months (33%)65.
The observed prevalence of wasting and underweight was also in agreement with a study report from Indonesia66. However, higher prevalence of wasting (17%) was reported from the Dabat Health and Demographic Surveillance System (HDSS) site, northwest Ethiopia39, and Sahel region of Burkina Faso 25%67, among children 6–23 months. On the other hand, our finding was higher than the prevalence of wasting (2.0%) and underweight (2.1%) that was reported in China68. The current study findings were also higher than the prevalence of wasting (15.3%) and underweight (21.8%) reported in India69, and underweight prevalence reported in Bangladesh 24.9%63. The discrepancies in the results could be attributed to differences in sample size, study design, and other cultural, environmental and social determinants like dietary habits70–72.
In the current study, female children had reduced odds of being stunted, wasted and underweight than male counterparts. This finding concurred with previous observations in studies carried out in three East Africa countries (i.e. Rwanda, Uganda and Tanzania)6, and in similar studies conducted in Rwanda73, Kenya74, Nigeria62,Ghana75, Senegal76, and Indonesia64. Similar to the current study findings, all the above studies reported undernutrition to be higher in boys than in girls. In line with our findings, several studies in Ethiopia have also reported high odds of under-nutrition among male under-five children27,41,47,77, an indication that male children are more vulnerable to these conditions. Likewise, a study in Indonesia reported that the prevalence was higher in boys compare to girls (32.6 vs.14.2%, 25.9 vs 9.4% and 27.0 vs 18.0%), respectively for undernutrition, stunting and wasting78. Evidence suggests that this gender-based health disparity may be due to men being more vulnerable than women to various infections79–81.
In this study, an increase in the age of the child was positively associated with childhood stunting and being underweight, which is consistent with several other related studies conducted elsewhere6,37,82–85. The undernutrition attributed to the older can be explained by two major factors: 1) A lack of adequate and balanced food intake to meet the metabolic demand needed for childhood growth as they age; and 2) older child's frequent interactions with their surroundings, which may increase the risks of infections and exposure to childhood diseases, needing more nutrients for both growth and to fight infections6,86. For example, older children under five are vulnerable to diarrheal diseases, parasitic infections, and other acute illnesses, which potentiate the likelihood of stunting and underweight status. Furthermore, as the child grows and after the first six months, the breast milk becomes insufficient to meet nutritional needs, and if complementary foods are not introduced as needed, this change from exclusively breastfeeding to complementary family food can leave a child vulnerable to undernutrition. Therefore, in order to reduce childhood undernutrition, interventions aimed at improving child nutrition may need to focus on children that are starting complimentary feeding.
Our findings revealed that the odds of being stunted was also less likely among children born of middle age adults (35–49 years old) than children born of younger mothers. We hypothesis that this result could be explained by the fact that adult and well-mature mothers could have adequate knowledge about issues related to undermatron and may have significant experience towards infant and effective child feeding practices. Additionally, it is plausible to argue that this age group of mothers is possibly better educated than young Ethiopian girls (15–19 years old) and may be aware of their children's dietary demands and requirements. Among the well-known links between education and child health are that mothers' formal education directly provides an opportunity for them to understand health knowledge, improving their ability to identify illness and seek treatment for their children87. In support of this, several studies across various settings have shown that maternal education is one of the significant factors in providing protective effects against all childhood undernutrition indicators31,42,47,88–90.
In addition, the likelihood of having childhood growth retardation decreased as the wealth index increased. For example, children from richer families were less likely to be stunted or underweight. Several previous studies conducted in Ethiopia47, Tanzania88, Ghana75, Rwanda89, and Indonesia64 reported a positive association between higher wealth status and reduced odds of undernutrition. This relationship could reflect that higher wealth ensures adequate nutritious food supply and other required variables for effective growth in the households, while children from low-socioeconomic status families are less likely to have access to optimal nutrition, hence more likely to suffer from poor nutritional status.
According to the findings of this study, children who lived in rural areas had a decreased odds of being wasted. The notable difference in the rate of childhood wasting among urban and rural children could be explained satisfactorily by differences in the mothers' lifestyles from the Ethiopian context as follows: (i) rural-dwelling mothers spend much more time with their infants and breastfed regularly. (ii) The majority of rural mothers were both dependent on agriculture or raising livestock. With these backgrounds, it is likely that their children would have better access to animal dairy products such as milk, better supporting the rural children’s nutritional status. (iii) Mothers in rural areas currently receive home-visiting assistance and counseling from health extension workers. Furthermore, most urban mothers are employed and have limited time to care for their children, limiting regular breastfeeding, which in the absence of proper supplementary feeds can potentially lead to infection and undernutrition.
Furthermore, this study found that, compared to children living in agrarian regions, those born to mothers who resided in cities (such as Addis Ababa, the capital city) were less likely to be wasted and underweight. This pattern could relate to the fact that the mothers who live in cities have higher socioeconomic status, better housing conditions, and better access to adequate water and sanitation, all of which contribute to their children's health and nutritional quality.
There are several limitations to our analysis. Firstly, we used the DHS dataset, which is the cross-sectional data. As such, the analysis of a cross sectional study could not provide evidence of a causal relationship between outcome and independent variables. Secondly, data on personal and household practices were based on the mothers’ recall, which might have been subject to recall bias. Thirdly, the analysis did not include all determinants of childhood undernutrition, such as household water quality, maternal nutritional status, childhood-related illnesses and underlying disease conditions due to lack of data detailing these variables in the DHS. Fourthly, despite the use of a comprehensive set of variables in the analysis, the influence of residual confounding as a result of unmeasured covariates (such as childhood infections) was not addressed because childhood malnutrition has multifactorial determinants. Despite these limitations, the study's data was collected across the country, making it nationally representative. Furthermore, we applied a multilevel analysis to the model, which is more appropriate for cluster data.
Conclusions
The undernutrition scourge remains a higher public health issue of significance among children aged 0–23 months in Ethiopia. Our analyses identified, female children were less likely to be predisposed to stunting, wasting, and underweight compared to the male counterparts. Children susceptibility to stunting and underweight was associated with age, with older aged children being more likely to be stunted and underweight compared to younger children. Children from the poorest household wealth index had higher odds of being stunted and underweight than children from the richest households. As a result, reducing undernutrition in early life in Ethiopia necessitates nutrition interventions tailored to at-risk populations (i.e. those who are about to begin complementary feeding (6–23 months), male children, and those living in poverty). Nutrition-sensitive conditional cash transfer interventions that target low-income households with the male children are needed for Ethiopia to meet the Sustainable Development Goals (SDGs) 1, 2 and 3 by 2030. Moreover, addressing the nutritional problem in the first 1000 days (0–23 months) ensures the best possible time frame for children in Ethiopia, with long-term benefits.
Supplementary Information
Acknowledgements
We would like to thank the Measure DHS Program for providing the DHS datasets.
Abbreviations
- AOR
Adjusted odds ratio
- ANC
Antenatal care visits
- BMI
Body mass index
- CI
Confidence interval
- EDHS
Ethiopian Demographic and Health Surveys
- SNNP
Southern Nations and Nationalities and People
- WHO
World Health Organization
Author contributions
B.S.: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft. P.P.: Visualization, Validation, Writing—review & editing. L.M.: Visualization, Validation, Writing—review & editing. A.K.: Supervision, Visualization, Methodology, Writing—review & editing. G.B., D.A., Y.T., D.Z., F.D., Z.T., D.W., K.S., D.G. and G.N.: Writing—review & editing. K.E.A.: Supervision, Visualization, Validation, Writing—review & editing. All authors have read and approved the final manuscript.
Funding
No organization funded this research.
Data availability
The datasets analysed during the current study are available in the Measure DHS website https://dhsprogram.com after formal online registration and submission of the project title and detailed project description.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25160-y.
References
- 1.WHO child growth standards: methods and development. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization; 2006. [Internet]. Available from: http://www.who.int/nutrition/publications/childgrowthstandards_technical_report_1/en/
- 2.World Health Organization (WHO). World Health Organizaiton and World Bank, levels and trends in child malnutrition: key findings of the 2020 edition. in Nutrition and Food Saftey. World Health Organization, Editor; 2020. [Internet]. Available from: https://www.unicef.org/media/69816/file/Joint-malnutrition-estimates-2020.pdf
- 3.World Health Organization (WHO), 2022. Fact sheets—Malnutrition [Internet]. [cited 2022 Jan 16]. Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition
- 4.Moshi CC, Sebastian PJ, Mushumbusi DG, Azizi KA, Meghji WP, Kitunda ME, et al. Determinants of underweight among children aged 0–23 months in Tanzania. Food Sci. Nutr. 2022;10(4):1167–1174. doi: 10.1002/fsn3.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akombi BJ, Agho KE, Merom D, Renzaho AM, Hall JJ. Child malnutrition in sub-Saharan Africa: A meta-analysis of demographic and health surveys (2006–2016) PLoS ONE. 2017;12(5):e0177338. doi: 10.1371/journal.pone.0177338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agho KE, Akombi BJ, Ferdous AJ, Mbugua I, Kamara JK. Childhood undernutrition in three disadvantaged East African Districts: A multinomial analysis. BMC Pediatr. 2019;19(1):118. doi: 10.1186/s12887-019-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Nutrition Report—Country Nutrition Profiles. [Internet]. Available from: https://globalnutritionreport.org/resources/nutrition-profiles/africa/eastern-africa/
- 8.Nutrition | Ethiopia | U.S. Agency for International Development [Internet]. 2021 [cited 2022 Sep 21]. Available from: https://www.usaid.gov/ethiopia/nutrition
- 9.Aboagye RG, Seidu AA, Ahinkorah BO, Arthur-Holmes F, Cadri A, Dadzie LK, et al. Dietary diversity and undernutrition in children aged 6–23 months in Sub-Saharan Africa. Nutrients. 2021;13(10):3431. doi: 10.3390/nu13103431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agedew E, Chane T. Prevalence of stunting among children aged 6–23 months in Kemba Woreda, Southern Ethiopia: A community based cross-sectional study. Adv. Public Health. 2015;26(2015):e164670. [Google Scholar]
- 11.Tadele TT, Gebremedhin CC, Markos MU, Fitsum EL. Stunting and associated factors among 6–23 month old children in drought vulnerable kebeles of Demba Gofa district, southern Ethiopia. BMC Nutr. 2022;8(1):9. doi: 10.1186/s40795-022-00501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Onis M, Branca F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016;12:12–26. doi: 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EPHI and ICF. EPHI ICF. Ethiopia MiniDemographic and Health Survey 2019: Key indicators. Rockville, Maryland, USA: EPHI and ICF. 2019. 2019.
- 14.Roba KT, O’Connor TP, Belachew T, O’Brien NM. Anemia and undernutrition among children aged 6–23 months in two agroecological zones of rural Ethiopia. Pediatr. Health Med. Ther. 2016;31(7):131–140. doi: 10.2147/PHMT.S109574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baye K, Guyot JP, Icard-Vernière C, Mouquet-Rivier C. Nutrient intakes from complementary foods consumed by young children (aged 12–23 months) from North Wollo, northern Ethiopia: The need for agro-ecologically adapted interventions. Public Health Nutr. 2013;16(10):1741–1750. doi: 10.1017/S1368980012005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tafese Z, Reta F, Mulugeta B, Anato A. Child undernutrition and associated factors among children 6–23 months old in largely food insecure areas of rural Ethiopia. J. Nutr. Sci. 2022;11:e63. doi: 10.1017/jns.2022.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam Birhan N, Bitew BD. Associated risk factors of underweight among under-five children in Ethiopia using multilevel ordinal logistic regression model. Afr. Health Sci. 2021;21(1):362–372. doi: 10.4314/ahs.v21i1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yirga AA, Mwambi HG, Ayele DG, Melesse SF. Factors affecting child malnutrition in Ethiopia. Afr. Health Sci. 2019;19(2):1897. doi: 10.4314/ahs.v19i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feleke S, Egata G, Mesfin F, Yilak G, Molla A. Undernutrition and associated factors in orphan children aged 6–59 months in Gambella Southwest, Ethiopia: A community-based cross-sectional study. BMJ Open. 2021;11(7):e045892. doi: 10.1136/bmjopen-2020-045892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brhanie, T. W. Undernutrition determinants among under five children in Ethiopia. Columbia Univ. J. Glob. Health. 2019 Dec 21 [cited 2022 Oct 3];9(2).
- 21.Kinfu AY. Child undernutrition in war-torn society: The Ethiopian experience. J. Biosoc. Sci. 1999;31(3):403–418. doi: 10.1017/S0021932099004034. [DOI] [PubMed] [Google Scholar]
- 22.Country progress against global targets. [Internet]. [cited 2022 Sep 22]. Available from: https://globalnutritionreport.org/reports/global-nutrition-report-2018/appendix-2-countries-track-global-nutrition-targets/
- 23.Abdulahi A, Shab-Bidar S, Rezaei S, Djafarian K. Nutritional status of under five children in Ethiopia: A systematic review and meta-analysis. Ethiop. J. Health Sci. 2017;27(2):175–188. doi: 10.4314/ejhs.v27i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Nutrition Policy. [Internet]. Federal Democratic Republic of Ethiopia. 2019.; Available from: https://www.nipn.ephi.gov.et/sites/default/files/2020-05/Food%20and%20Nutrition%20Policy.pdf
- 25.Ministry of Health (MoH) Ethiopia. Seqota Declaration [Internet]. [cited 2022 Nov 15]. Available from: https://www.moh.gov.et/site/am/node/170
- 26.Paramashanti BA, Benita S. Early introduction of complementary food and childhood stunting were linked among children aged 6–23 months. J. Gizi Klin Indones. 2020;17(1):1–8. doi: 10.22146/ijcn.53788. [DOI] [Google Scholar]
- 27.Roba AA, Assefa N, Dessie Y, Tolera A, Teji K, Elena H, et al. Prevalence and determinants of concurrent wasting and stunting and other indicators of malnutrition among children 6–59 months old in Kersa, Ethiopia. Matern. Child Nutr. 2021;17(3):e13172. doi: 10.1111/mcn.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geda NR, Feng CX, Henry CJ, Lepnurm R, Janzen B, Whiting SJ. Multiple anthropometric and nutritional deficiencies in young children in Ethiopia: A multi-level analysis based on a nationally representative data. BMC Pediatr. 2021;21(1):11. doi: 10.1186/s12887-020-02467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayelign A, Zerfu T. Household, dietary and healthcare factors predicting childhood stunting in Ethiopia. Heliyon. 2021;7(4):e06733. doi: 10.1016/j.heliyon.2021.e06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantay Gebru K, Mekonnen Haileselassie W, Haftom Temesgen A, Oumer Seid A, Afework MB. Determinants of stunting among under-five children in Ethiopia: a multilevel mixed-effects analysis of 2016 Ethiopian demographic and health survey data. BMC Pediatr. 2019;19(1):176. doi: 10.1186/s12887-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haile D, Azage M, Mola T, Rainey R. Exploring spatial variations and factors associated with childhood stunting in Ethiopia: Spatial and multilevel analysis. BMC Pediatr. 2016;16(1):49. doi: 10.1186/s12887-016-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eshete Tadesse S, Chane Mekonnen T, Adane M. Priorities for intervention of childhood stunting in northeastern Ethiopia: A matched case-control study. PLoS ONE. 2020;15(9):e0239255. doi: 10.1371/journal.pone.0239255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahiledengle B, Petrucka P, Kumie A, Mwanri L, Beressa G, Atlaw D, et al. Association between water, sanitation and hygiene (WASH) and child undernutrition in Ethiopia: A hierarchical approach. BMC Public Health. 2022;22(1):1943. doi: 10.1186/s12889-022-14309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stunting and its determinant factors among children aged 6–59 months in Ethiopia | Italian. J. Pediatr. [cited 2022 Jan 28]. 10.1186/s13052-017-0433-1
- 35.Dires S, Mareg M. The magnitude of wasting and associated factors among children aged 2–5 years in Southern Ethiopia: A cross-sectional study. BioMed Res Int. 2021;7(2021):e6645996. doi: 10.1155/2021/6645996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekele T, Rahman B, Rawstorne P. The effect of access to water, sanitation and handwashing facilities on child growth indicators: Evidence from the Ethiopia Demographic and Health Survey 2016. PLoS ONE. 2020;15(9):e0239313. doi: 10.1371/journal.pone.0239313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muche, A., Gezie, L. D., Baraki, AGegzabher, Amsalu, E. T. Predictors of stunting among children age 6–59 months in Ethiopia using Bayesian multi-level analysis. Sci Rep. 11, 3759 (2021). [DOI] [PMC free article] [PubMed]
- 38.Wasting in under five children is significantly varied between rice producing and non-producing households of Libokemkem district, Amhara region, Ethiopia. BMC Pediatrics. [cited 2022 Jan 28]. 10.1186/s12887-019-1677-2 [DOI] [PMC free article] [PubMed]
- 39.Derso T, Tariku A, Biks GA, Wassie MM. Stunting, wasting and associated factors among children aged 6–24 months in Dabat health and demographic surveillance system site: A community based cross-sectional study in Ethiopia. BMC Pediatr. 2017;17(1):96. doi: 10.1186/s12887-017-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seboka, B. T., Alene, T. D., Ngusie, H. S., Hailegebreal, S., Yehualashet, D. E., Gilano, G. et al. Spatial variations and determinants of acute malnutrition among under-five children in Ethiopia: Evidence from Ethiopian demographic health survey. Ann. Glob. Health87(1), 114. [DOI] [PMC free article] [PubMed]
- 41.Asfaw M, Wondaferash M, Taha M, Dube L. Prevalence of undernutrition and associated factors among children aged between six to fifty nine months in Bule Hora district, South Ethiopia. BMC Public Health. 2015;15(1):41. doi: 10.1186/s12889-015-1370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birhan NA, Belay DB. Associated risk factors of underweight among under-five children in Ethiopia using multilevel ordinal logistic regression model. Afr. Health Sci. 2021;21(1):362–372. doi: 10.4314/ahs.v21i1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bekele SA, Fetene MZ. Modeling non-Gaussian data analysis on determinants of underweight among under five children in rural Ethiopia: Ethiopian demographic and health survey 2016 evidences. PLoS ONE. 2021;16(5):e0251239. doi: 10.1371/journal.pone.0251239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesfaw LM, Dessie ZG. Multilevel multivariate analysis on the anthropometric indicators of under-five children in Ethiopia: EMDHS 2019. BMC Pediatr. 2022;22(1):162. doi: 10.1186/s12887-022-03172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gebreayohanes M, Dessie A. Prevalence of stunting and its associated factors among children 6–59 months of age in pastoralist community, Northeast Ethiopia: A community-based cross-sectional study. PLoS ONE. 2022;17(2):e0256722. doi: 10.1371/journal.pone.0256722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohammed SH, Muhammad F, Pakzad R, Alizadeh S. Socioeconomic inequality in stunting among under-5 children in Ethiopia: A decomposition analysis. BMC Res. Notes. 2019;12(1):184. doi: 10.1186/s13104-019-4229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muche A, Dewau R. Severe stunting and its associated factors among children aged 6–59 months in Ethiopia; Multilevel ordinal logistic regression model. Ital. J. Pediatr. 2021;47(1):161. doi: 10.1186/s13052-021-01110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sewenet, T., Selassie, M., Zenebe, Y., Yimam, W. & Woretaw, L. Undernutrition and associated factors among children aged 6–23 Months in Dessie Town, Northeastern Ethiopia, 2021: A community based cross-sectional study. Front Pediatr. 10, 916726 (2022). [DOI] [PMC free article] [PubMed]
- 49.Teji Roba K, O’Connor TP, Belachew T, O’Brien NM. Anemia and undernutrition among children aged 6–23 months in two agroecological zones of rural Ethiopia. Pediatr. Health Med. Ther. 2016;7:131–140. doi: 10.2147/PHMT.S109574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desalegn B, Lambert C, Riedel S, Negese T, Biesalski H. Feeding practices and undernutrition in 6–23-month-old children of orthodox christian mothers in rural Tigray, Ethiopia: Longitudinal study. Nutrients. 2019;11(1):138. doi: 10.3390/nu11010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alemayehu M, Meskele M, Alemayehu B, Yakob B. Prevalence and correlates of anemia among children aged 6–23 months in Wolaita Zone, Southern Ethiopia. PLoS ONE. 2019;14(3):e0206268. doi: 10.1371/journal.pone.0206268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegegne M, Abate KH, Belachew T. Anaemia and associated factors among children aged 6–23 months in agrarian community of Bale zone: a cross-sectional study. J. Nutr. Sci. 2022;11:e96. doi: 10.1017/jns.2022.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belete KT, Daba DB, Shallo SA, Yebassa MA, Danusa KT, Gadisa DA. Levels of dietary diversity and its associated factors among children aged 6–23 months in West Shoa, Ethiopia: A comparative cross-sectional study. J Nutr Sci. 2022;11:e20. doi: 10.1017/jns.2022.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Central Statistical Agency (CSA). Population Projections for Ethiopia, 2007–2037, Addis, Ethiopia [Internet]. [cited 2022 May 16]. [Internet]. Available from: http://www.csa.gov.et/census-report/population-projections/category/368-population-projection-2007-2037
- 55.World Health Organization (WHO). Indicators for Assessing Infant and Young Child Feeding Practices. Part 1: Definitions. Geneva: WHO. 2008 [Internet]. Available from: http://www.who.int/nutrition/publications/infantfeeding/9789241596664/en/
- 56.WASH in the 2030 Agenda New global indicators for drinking water, sanitation and hygiene. [Internet]. Available from: https://washdata.org/report/jmp-2017-wash-2030-agenda
- 57.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J. Epidemiol. Commun. Health. 2006;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: Integrating random and fixed effects in multilevel logistic regression. Am. J. Epidemiol. 2005;161(1):81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 59.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multi level analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J. Epidemiol. Commun. Health. 2006;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayneris-Perxachs J, Swann JR. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur. J. Nutr. 2019;58(3):909–930. doi: 10.1007/s00394-018-1679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Onis M, Borghi E, Arimond M, Webb P, Croft T, Saha K, et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2019;22(1):175–179. doi: 10.1017/S1368980018002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akombi BJ, Agho KE, Hall JJ, Merom D, Astell-Burt T, Renzaho AMN. Stunting and severe stunting among children under-5 years in Nigeria: A multilevel analysis. BMC Pediatr. 2017;17(1):15. doi: 10.1186/s12887-016-0770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choudhury N, Raihan MJ, Sultana S, Mahmud Z, Farzana FD, Haque MA, et al. Determinants of age-specific undernutrition in children aged less than 2 years-the Bangladesh context: Determinants of childhood malnutrition-the Bangladesh context. Matern. Child Nutr. 2017;13(3):e12362. doi: 10.1111/mcn.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torlesse H, Cronin AA, Sebayang SK, Nandy R. Determinants of stunting in Indonesian children: Evidence from a cross-sectional survey indicate a prominent role for the water, sanitation and hygiene sector in stunting reduction. BMC Public Health. 2016;16(1):669. doi: 10.1186/s12889-016-3339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wali N, Agho KE, Renzaho AMN. Factors associated with stunting among children under 5 years in Five South Asian Countries (2014–2018): Analysis of demographic health surveys. Nutrients. 2020;12(12):3875. doi: 10.3390/nu12123875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meiandayati R, Wulansari R, Hanifah L, Laksminingsih AE. Wasting and associated factors among infants aged 0–23 months in 13 provinces in Indonesia: Evidence from Indonesia Family Life Surveys (IFLS) 2000, 2007 and 2014. Mal. J. Nutr. 2018;24(3):323–331. [Google Scholar]
- 67.Paré, B. C., Dahourou, D. L., Kabore, A., Sana, A., Kinda, R. & Ouaro, B. et al. Prevalence of wasting and associated factors among 6 to 23 months old children in the Sahel Region of Burkina Faso. Pan. Afr. Med. J. [Internet]. 2019 Nov 26 [cited 2022 Sep 26];34. http://www.panafrican-med-journal.com/content/article/34/164/full/ [DOI] [PMC free article] [PubMed]
- 68.Liu J, Sun J, Huang J, Huo J. Prevalence of malnutrition and associated factors of stunting among 6–23-month-old infants in Central Rural China in 2019. Int. J. Environ. Res. Public Health. 2021;18(15):8165. doi: 10.3390/ijerph18158165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeyakumar A, Nikam S, Nayak S. Prevalence and risk factors of undernutrition among children less than 2 years in urban slums of Pune, Maharashtra, India. Ecol. Food Nutr. 2019;58(5):456–469. doi: 10.1080/03670244.2019.1613985. [DOI] [PubMed] [Google Scholar]
- 70.Bitew ZW, Alemu A, Ayele EG, Worku T. Dietary diversity and practice of pregnant and lactating women in Ethiopia: A systematic review and meta-analysis. Food Sci. Nutr. 2021;9(5):2686–2702. doi: 10.1002/fsn3.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melesse MB, van den Berg M. Consumer nutrition knowledge and dietary behavior in urban Ethiopia: A comprehensive study. Ecol. Food Nutr. 2021;60(2):244–256. doi: 10.1080/03670244.2020.1835655. [DOI] [PubMed] [Google Scholar]
- 72.Hiruy AF, Teshome AA, Desta YT, Zuo X, He S, Assefa EG, et al. Dietary condition and feeding practices of children aged 6–23 months in Ethiopia: Analysis of 2005–2016 demographic and health survey. Eur. J. Clin. Nutr. 2021;75(7):1047–1059. doi: 10.1038/s41430-020-00828-8. [DOI] [PubMed] [Google Scholar]
- 73.Mukabutera A, Thomson DR, Hedt-Gauthier BL, Basinga P, Nyirazinyoye L, Murray M. Risk factors associated with underweight status in children under five: An analysis of the 2010 Rwanda Demographic Health Survey (RDHS) BMC Nutr. 2016;2(1):40. doi: 10.1186/s40795-016-0078-2. [DOI] [Google Scholar]
- 74.Trends and determinants of undernutrition among young Kenyan children: Kenya Demographic and Health Survey; 1993, 1998, 2003 and 2008–2009 | Public Health Nutrition | Cambridge Core [Internet]. [cited 2022 Apr 27]. Available from: https://www.cambridge.org/core/journals/public-health-nutrition/article/trends-and-determinants-of-undernutrition-among-young-kenyan-children-kenya-demographic-and-health-survey-1993-1998-2003-and-20082009/E4B420845279F6CE2A6836C6704054F7 [DOI] [PubMed]
- 75.Darteh EKM, Acquah E, Kumi-Kyereme A. Correlates of stunting among children in Ghana. BMC Public Health. 2014;14(1):504. doi: 10.1186/1471-2458-14-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garenne M, Myatt M, Khara T, Dolan C, Briend A. Concurrent wasting and stunting among under-five children in Niakhar, Senegal. Matern. Child Nutr. 2019;15(2):e12736. doi: 10.1111/mcn.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Determinants of Wasting Among Under-Five Children in Ethiopia: (A Multilevel Logistic Regression Model Approach). Int. J. Stat. Med. Res. [Internet]. [cited 2022 Apr 27]. Available from: https://www.lifescienceglobal.com/pms/index.php/ijsmr/article/view/2466
- 78.Hadju V, Yunus R, Arundhana AI, Salmah AU, Wahyu A. Nutritional status of infants 0–23 months of age and its relationship with socioeconomic factors in Pangkep. Asian J. Clin. Nutr. 2017;9(2):71–76. doi: 10.3923/ajcn.2017.71.76. [DOI] [Google Scholar]
- 79.Green MS. The male predominance in the incidence of infectious diseases in children: A postulated explanation for disparities in the literature. Int. J. Epidemiol. 1992;21(2):381–386. doi: 10.1093/ije/21.2.381. [DOI] [PubMed] [Google Scholar]
- 80.R P. Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 2013 Apr [cited 2022 May 16];50(2). [DOI] [PubMed]
- 81.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr. Infect. Dis. J. 2008;27(4):302–308. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 82.Odei Obeng-Amoako GA, Karamagi CAS, Nangendo J, Okiring J, Kiirya Y, Aryeetey R, et al. Factors associated with concurrent wasting and stunting among children 6–59 months in Karamoja, Uganda. Matern. Child Nutr. 2021;17(1):e13074. doi: 10.1111/mcn.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.García Cruz L, González Azpeitia G, Reyes Súarez D, Santana Rodríguez A, Loro Ferrer J, Serra-Majem L. Factors associated with stunting among children aged 0 to 59 months from the Central Region of Mozambique. Nutrients. 2017;9(5):491. doi: 10.3390/nu9050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bukusuba J, Kaaya AN, Atukwase A. Predictors of stunting in children aged 6 to 59 months: A case-control study in Southwest Uganda. Food Nutr Bull. 2017;38(4):542–553. doi: 10.1177/0379572117731666. [DOI] [PubMed] [Google Scholar]
- 85.Simelane MS, Chemhaka GB, Zwane E. A multilevel analysis of individual, household and community level factors on stunting among children aged 6–59 months in Eswatini: A secondary analysis of the Eswatini 2010 and 2014 Multiple Indicator Cluster Surveys. PLoS ONE. 2020;15(10):e0241548. doi: 10.1371/journal.pone.0241548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization, Unicef. Ending preventable child deaths from pneumonia and diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). [DOI] [PubMed]
- 87.Glewwe P. Why does mother’s schooling raise child health in developing countries? Evidence from Morocco. J. Hum. Resour. 1999;34(1):124. doi: 10.2307/146305. [DOI] [Google Scholar]
- 88.Chirande L, Charwe D, Mbwana H, Victor R, Kimboka S, Issaka AI, et al. Determinants of stunting and severe stunting among under-fives in Tanzania: Evidence from the 2010 cross-sectional household survey. BMC Pediatr. 2015;15(1):165. doi: 10.1186/s12887-015-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nshimyiryo A, Hedt-Gauthier B, Mutaganzwa C, Kirk CM, Beck K, Ndayisaba A, et al. Risk factors for stunting among children under five years: a cross-sectional population-based study in Rwanda using the 2015 Demographic and Health Survey. BMC Public Health. 2019;19(1):175. doi: 10.1186/s12889-019-6504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H, Yuan S, Fang H, Huang G, Huang Q, Wang H, et al. Prevalence and associated factors for stunting, underweight and wasting among children under 6 years of age in rural Hunan Province, China: A community-based cross-sectional study. BMC Public Health. 2022;22(1):483. doi: 10.1186/s12889-022-12875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available in the Measure DHS website https://dhsprogram.com after formal online registration and submission of the project title and detailed project description.