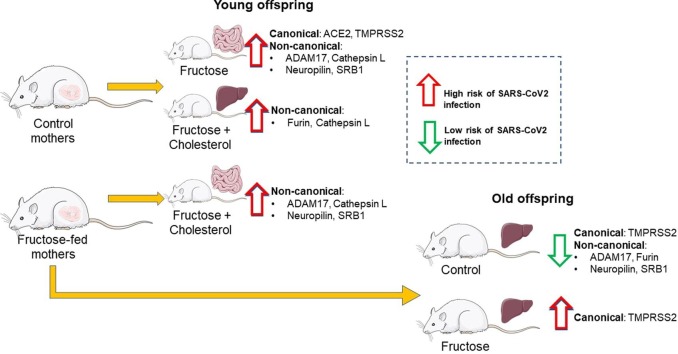
Graphical abstract
Keywords: Fructose, Fetal programming, SARS-CoV-2, Liver, Ileum, Cholesterol
Abstract
Fructose-rich beverages and foods consumption correlates with the epidemic rise in cardiovascular disease, diabetes and obesity. Severity of COVID-19 has been related to these metabolic diseases. Fructose-rich foods could place people at an increased risk for severe COVID-19. We investigated whether maternal fructose intake in offspring affects hepatic and ileal gene expression of proteins that permit SARS-CoV2 entry to the cell. Carbohydrates were supplied to pregnant rats in drinking water. Adult and young male descendants subjected to water, liquid fructose alone or as a part of a Western diet, were studied. Maternal fructose reduced hepatic SARS-CoV2 entry factors expression in older offspring. On the contrary, maternal fructose boosted the Western diet-induced increase in viral entry factors expression in ileum of young descendants. Maternal fructose intake produced a fetal programming that increases hepatic viral protection and, in contrast, exacerbates fructose plus cholesterol-induced diminution in SARS-CoV2 protection in small intestine of progeny.
1. Introduction
Food patterns and diet have greatly changed during recent decades in both industrialized and developing countries together with a sedentary lifestyle resulting in dramatic increases of obesity, metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD), and type 2 diabetes (T2DM) (Taskinen et al., 2019). MetS increases the risk of developing hypertension, cardiovascular diseases (CVD), T2DM, NAFLD, hyperuricemia, gout and chronic kidney disease (CKD) (Zhang et al., 2017).
Fructose is a monosaccharide found naturally in fruits, vegetables and honey. Fructose is also used as added sugar in the form of sucrose or high fructose corn syrup (HFCS) to sweeten a wide variety of processed foods and sugary drinks. High fructose diets and extensive commercial use of HFCS have been associated with the rising prevalence of MetS worldwide (Zhang et al., 2017). Experimental studies have shown that fructose can induce many features of MetS in rats, whereas glucose intake does not (Johnson et al., 2009). Thus, diets containing 10 % wt/vol fructose in drinking water cause hypertriglyceridemia and fatty liver (Roglans et al., 2002). In fact, both obesity, MetS and T2DM are often called “diet-related diseases”. The term “processed food-related disease” refers to diseases where diet is one of the essential causative factors, and includes T2DM, hypertension, heart disease, obesity, dementia, fatty liver disease, and cancer. Thus, the healthcare community is increasingly aware that the global pandemic of these non-communicable diseases has its origins in our Western processed food diet, which should be extensively and urgently regulated (Lustig, 2020).
Interestingly, it has recently been found that 88 % of adults in the USA are metabolically unhealthy. This means that only 12 % of Americans, even those at “normal weight”, have safe levels of blood sugar, triglycerides, high-density lipoprotein (HDL), and blood pressure. The prevalence of metabolic health in adults from the USA and probably most Western countries is alarmingly low, and this situation has serious implications for public health (Araújo et al., 2019). From the 1970s, there has been both an increase in overall sugar consumption in the United States and several other westernized countries and the replacement of sucrose with HFCS in beverages and other processed foods. Although the consumption of HFCS has been rising in parallel to the increase in obesity and diabetes, there is currently no conclusive scientific information demonstrating a clear association between consumption of fructose and metabolic diseases. In fact, a paradox arises when HFCS intake has been declining over the last two decades, whereas rates of overweight among adults and diabetes have continued to increase in the same timeframe (Laughlin et al., 2014). Therefore, since it is well-established that metabolic events during pre- and postnatal development modulate metabolic disease risk in later life (Koletzko et al., 2005), the maternal diet being the most important event (Vickers et al., 2005) and fructose has frequently been linked to obesity, MetS and CVD (Johnson et al., 2009), maternal fructose intake could serve to explain the paradox. In fact, we and others have previously shown that maternal fructose intake provokes many features of MetS in adult male offspring (Alzamendi et al., 2016, Rodríguez et al., 2016, Saad et al., 2016). Moreover, maternal fructose intake can modulate how male progeny respond to a liquid fructose supplementation when adults (Fauste et al., 2020). However, although a connection between a high maternal consumption of fructose-containing beverages and the global epidemic of obesity and MetS could exist (Rodríguez et al., 2013, Vilà et al., 2011), ingestion of these beverages and fruit juices is still permitted and not regulated during gestation.
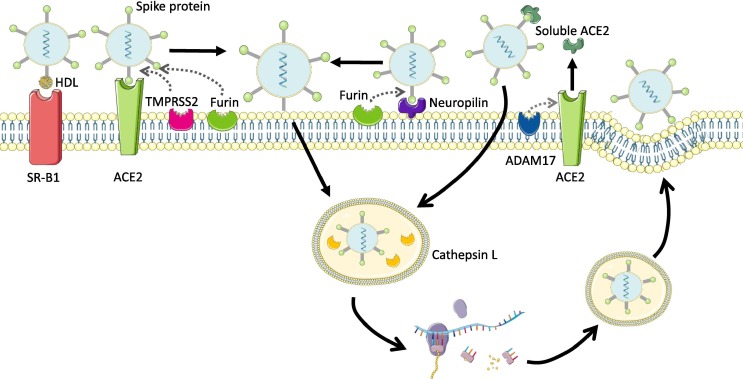
COVID-19 is a respiratory disease caused by the novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which has reached pandemic status. Canonical SARS-CoV-2 host cell entry occurs by binding to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) and then, the transmembrane protease serine 2 (TMPRSS2) cleaves the viral spike (S) protein, allowing fusion of cellular and viral membranes (Fig. 1 ). However, since the expression of these two molecules is negligible in many tissues, the possibility of viral entry via non-canonical pathways cannot be discarded. Thus, these alternative pathways would involve both other proteases, such as Cathepsin L, ADAM metallopeptidase domain 17 (ADAM17) or furin (Coate et al., 2020, Yeung et al., 2021) as well as other putative receptors, such as neuropilin-1 (Daly et al., 2020) or HDL-scavenger receptor B type 1 (SRB1) (Wei et al., 2020) (Fig. 1).
Fig. 1.
Cell surface receptors and cofactors facilitating SARS-CoV-2 entry. Canonical pathway occurs by binding to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) and then, the transmembrane protease serine 2 (TMPRSS2) cleaves the viral spike protein, allowing fusion of cellular and viral membranes. Non-canonical pathways would involve other proteases: Cathepsin L, ADAM metallopeptidase domain 17 (ADAM17) or furin; and receptors: neuropilin-1 or HDL-scavenger receptor B type 1 (SRB1). SRB1 alone or in collaboration with HDL augments SARS-CoV-2 attachment and then, host cell entry is completed through ACE2 interaction. The neuropilin and furin collaboration would be an alternative entry to SARS-CoV-2 to that made by the ACE2 and TMPRSS2 cooperation. ADAM17 does cleave ACE2, and releases a soluble form of ACE2 that can interact with SARS-CoV-2 and mediate its entry to the cell. This image has been created using Servier Medical Art (https://smart.servier.com).
While COVID-19 affects all population groups, severe pathology and mortality is disproportionately highest in the elderly and/or in those patients with underlying conditions, such as T2DM, obesity and other chronic diseases (Coate et al., 2020). Moreover, since the Western-style diet is known to contribute to the prevalence of these metabolic diseases, fructose-rich processed foods and sugary drinks and/or high-fat diets could place these populations at an increased risk for severe COVID-19 pathology and mortality (Butler & Barrientos, 2020).
Nevertheless, controversy exists about whether pre-existing abnormalities related to metabolic diseases determine the severity of COVID-19. Thus, one study showed that patients with metabolic-associated fatty liver disease (MAFLD) have a higher risk of COVID-19 disease progression and liver blood test abnormalities than patients without MAFLD (Ji et al., 2020). In contrast, another study did not find a higher susceptibility of fatty liver to SARS-CoV-2 infection. None of the genes necessary for SARS-CoV-2 infection was differentially expressed between lean or obese controls and patients with simple steatosis or non-alcoholic steatohepatitis (NASH). Moreover, no increase in liver gene expression of SARS-CoV-2 critical entry proteins was found between MAFLD and control mice (Biquard et al., 2020). Nevertheless, a more recent study indicated that SARS-CoV-2 entry factors in liver are differently affected by T2DM and NAFLD in obese patients. While obese women with T2D have unexpectedly lower levels of ACE2 and TMPRSS2 than obese normoglycemic women, obese patients with NASH showed a markedly higher expression of these genes, suggesting that advanced stages of NAFLD might predispose individuals to COVID-19 (Fondevila et al., 2021).
In the present study, we examined in rats if maternal fructose, in comparison to glucose, affects the hepatic gene expression of SARS-CoV-2 critical cell entry factors in adult male descendants. Then, we studied if maternal fructose determined how liquid fructose affects the gene expression of key factors of SARS-CoV-2 entry to the hepatocyte in adult male rats. Finally, we determined in young males how maternal fructose is able to modulate the effects of liquid fructose, tagatose (an epimer of fructose) and fructose plus cholesterol (as an example of a Western-style diet) on hepatic and ileal gene expression of SARS-CoV-2 entry-dependent factors.
2. Materials and methods
2.1. Animals and experimental design
An animal model of maternal liquid fructose intake was developed as previously described (Fauste et al., 2020, Rodrigo et al., 2018, Rodríguez et al., 2013). Female Sprague-Dawley rats weighing 200–240 g were fed ad libitum, a standard rat chow diet (Teklad Global 14 % Protein Rodent Maintenance Diet, Envigo, USA), and housed under controlled light and temperature conditions (12-h light–dark cycle; 22 ± 1 °C). The experimental protocol was approved by the Animal Research Committee of the University San Pablo-CEU, Madrid, Spain (ref. numbers 10/206458.9/13 and 10/042445.9/19). The experimental design was separated into two protocols.
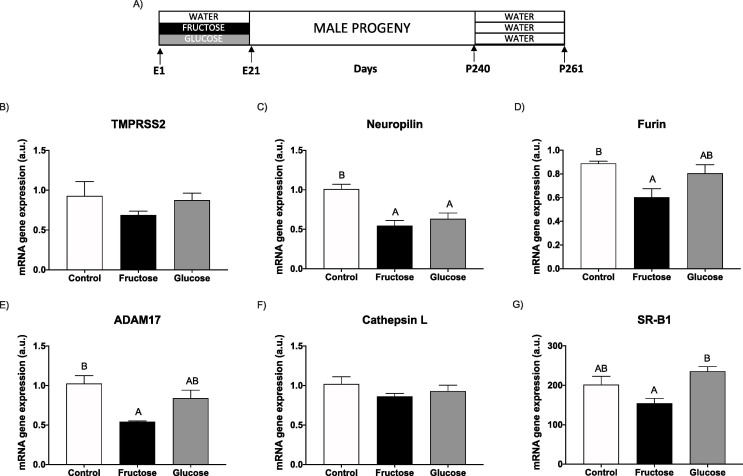
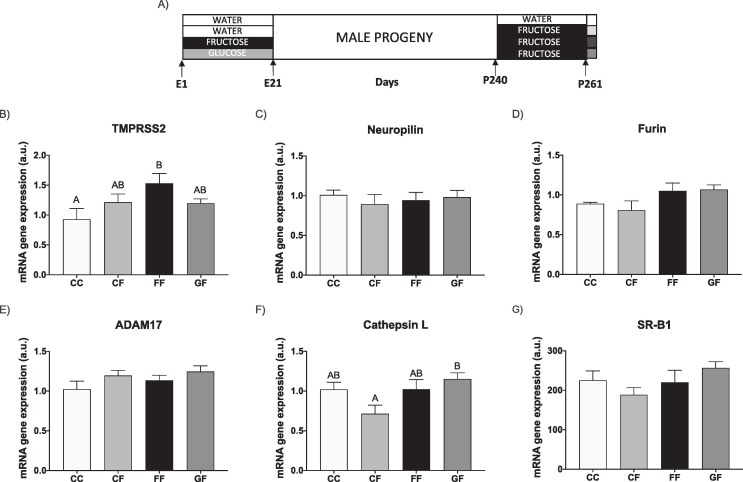
In the first protocol, pregnant animals were randomly separated into a control group, a fructose-supplemented group (Fructose), and a glucose-supplemented group (Glucose) (five to six rats per group) (Rodríguez et al., 2013). Fructose and glucose were supplied as a 10 % (wt/vol) solution in drinking water throughout gestation. The concentration used here (10 % wt/vol) is very close to that of sugar-sweetened beverages (SSB). Control animals received no supplementary sugar. Pregnant rats were allowed to deliver and on the day of birth, each suckling litter was reduced to nine pups per mother. After delivery, both mothers and their pups were maintained with water and food ad libitum. At 21 days of age, pups were separated by gender and male progeny were kept fed on a standard rat chow diet (Teklad Global 14 % Protein Rodent Maintenance Diet, Envigo, USA) and water without additives. Animals within each experimental group were born to different dams to minimize the “litter effect”. In order to know the effects in adult progeny at 240 days of age, one half of the male progeny were randomly separated. When the progeny were 261-days-old, they were sacrificed and blood and livers were collected. Remarkably, these animals had received no subsequent additive in the drinking water for their entire lives (Rodrigo et al., 2018) (Fig. 2 A). The other half of the male progeny were subjected to the next protocol: independently from the experimental group of mothers to which they had been born, they were maintained on solid pellets and supplied with drinking water containing 10 % (wt/vol) fructose. Thus, three experimental groups were formed: C/F, F/F, G/F, the first letter indicating whether the mothers had been supplied with tap water during pregnancy (C: control), or water containing a carbohydrate (F: fructose; G: glucose); and the second letter indicating the period with fructose (F), when they were adults. When the progeny were 261-days-old, they were sacrificed and livers were immediately removed, placed in liquid nitrogen and kept at −80 °C until analysis. In parallel, a fourth experimental group was used, C/C: male progeny from control mothers supplied with water without any additives when adult. The period with fructose was selected to last 21 days (from 240 to 261 days of age) (Fauste et al., 2020) (Fig. 3 A).
Fig. 2.
Fructose in pregnancy affects hepatic cell surface receptors and cofactors facilitating SARS-CoV-2 entry in adult male progeny. (A) Experimental design. Hepatic levels of specific mRNA for (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17, (F) cathepsin L, and (G) SRB1 genes of 261-day-old male progeny from control (empty bar), fructose-fed (black bar) and glucose-fed (grey bar) pregnant rats. Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 5 to 6 litters. Values not sharing a common letter are significantly different (P < 0.05). TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1. E: embryonic/fetal days (E21: delivery); P: postnatal days.
Fig. 3.
Liquid fructose in gestation exacerbates fructose-induced augmentation of hepatic TMPRSS2 expression in adult male progeny. (A) Experimental design. Hepatic levels of specific mRNA for (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17, (F) cathepsin L, and (G) SRB1 genes. Liver (mRNA) expression of fructose-fed male adult progeny from control (C/F, light grey bar), fructose- (F/F, black bar), and glucose-supplemented (G/F, dark grey bar) mothers. C/C: Control 261-day-old male offspring from control pregnant rats (empty bar, C/C). Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 5 to 6 litters. Values not sharing a common letter are significantly different (P < 0.05). TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1. E: embryonic/fetal days (E21: delivery); P: postnatal days.
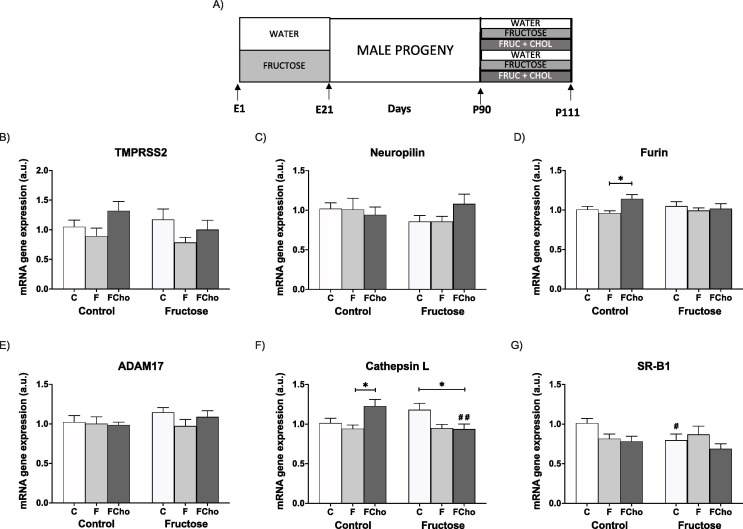
In the second protocol, pregnant rats were randomly separated into a control group (no supplementary sugar) and a fructose-supplemented group (fructose 10 % wt/vol in drinking water) (seven to eight rats per group) throughout gestation (Rodríguez et al., 2013). Pregnant rats were allowed to deliver and on the day of birth, each suckling litter was reduced to nine pups per mother. After delivery, both mothers and their pups were maintained with water and food ad libitum. At 21 days of age, pups were separated by gender and male progeny were kept fed on a standard rat chow diet (Teklad Global 14 % Protein Rodent Maintenance Diet, Envigo, USA) and water without additives. When the offspring were 3 months old, they were subjected to a new treatment for 21 days regardless of the group of mothers they were born. Male progeny from Control or Fructose-fed mothers were randomly separated into four experimental groups (animals within each experimental group were born to different dams to minimize the “litter effect”): control (C, tap water), fructose (F, fructose 10 % wt/vol in drinking water), fructose and cholesterol diet (FCho, fructose 10 % wt/vol in drinking water and solid food with 2 % added cholesterol; Tecklad Custome Diets TD.07841, Envigo, USA) and tagatose (T, tagatose 10 % wt/vol in drinking water). After 21 days, they were sacrificed and liver and ileum were immediately removed, placed in liquid nitrogen and kept at −80 °C until analysis (Fig. 4 A and 6A).
Fig. 4.
Fructose and fructose plus cholesterol affect hepatic cell surface receptors and cofactors facilitating SARS-CoV-2 entry in young male progeny. (A) Experimental design. Hepatic levels of specific mRNA for (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17, (F) cathepsin L, and (G) SRB1 genes. Liver (mRNA) expression from control (C, empty bar), fructose- (F, light grey bar), and fructose plus cholesterol-supplemented (FCho, dark grey bar) young male progeny from Control (left panel) or Fructose-fed (right panel) mothers. Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 7 to 8 litters. Asterisks denote a significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between the groups under the crossbar (groups with a different diet but the same motheŕs diet). Hash symbols denote a significant difference (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) as compared to the Control mothers (groups with the same diet but different motheŕs diet). TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1. E: embryonic/fetal days (E21: delivery); P: postnatal days.
2.2. RNA extraction and gene expression by qPCR
Total RNA was isolated from liver or ileum using Ribopure (Invitrogen, ThermoFisher Scientific, USA). Total RNA was subjected to DNase I treatment using Turbo DNA-free (Invitrogen, ThermoFisher Scientific, USA), and RNA integrity was confirmed by agarose gel electrophoresis. Afterwards, cDNA was synthesized by oligo(dT)-primed reverse transcription with Superscript II (Invitrogen, ThermoFisher Scientific, USA). qPCRs were performed using a CXF96® Touch (Bio-Rad, California, USA). The reaction solution was carried out in a volume of 20 μl, containing 10 pmol of both forward and reverse primers, 10x SYBR Premix Ex Taq (Takara Bio Inc., Japan) and the appropriate nanograms of the cDNA stock. Rps29 was used as a reference gene for qPCR. The primer sequences were obtained either from the Atlas RT-PCR Primer Sequences (Clontech, CA, USA) or designed using Primer3 software (University of Massachusetts Medical School, MA, USA) (Rozen & Skaletsky, 2000).
Samples were analysed in duplicate on each assay. Amplification of non-specific targets was discarded using the melting curve analysis method for each amplicon. qPCR efficiency and linearity were assessed by optimization of the standard curves for each target. The transcription was quantified with CFX Maestro 2.0 software (Bio-Rad, California, USA) using the efficiency correction method (Pfaffl, 2001).
2.3. Statistical analysis
Results were expressed as means ± S.E. On the first protocol, treatment effects were analyzed by one-way analysis of variance (ANOVA). When treatment effects were significantly different (P < 0.05), means were tested by Tukeýs multiple range test. When the variance was not homogeneous, a post hoc Tamhane test was performed. Significant differences were indicated with different letters.
On the second protocol, treatment effects were analyzed by two-way analysis of variance (ANOVA). Data that were not normally distributed were log transformed to achieve data normality. Then, the Bonferroni test was used for post hoc analysis to identify the source of significant variance. Significant differences (P < 0.05) were indicated either with asterisks (*) between groups of animals receiving different treatments but belonging to the same dietary group of mothers or hash symbols (#) between groups of rats with the same treatment but coming from different dietary group of mothers. All statistical valuation was performed using SPSS version 25 computer program.
3. Results
3.1. Maternal fructose decreases hepatic SARS-CoV-2 cell entry factors in adult male progeny
We have taken advantage of our well-stablished animal model of MetS in which fetal programming is achieved by maternal fructose intake (Fauste et al., 2020, Rodrigo et al., 2018, Rodríguez et al., 2013). Thus, male rats born of fructose-fed mothers exhibited impaired insulin signalling and hyperinsulinemia (Rodríguez et al., 2016). However, surprisingly, hepatic cell surface receptors and cofactors facilitating SARS-CoV-2 entry showed a reduced gene expression (Fig. 2). Although ACE2 gene expression was not detected in liver and TMPRSS2 mRNA hepatic levels (Fig. 2B) showed no differences between descendants from control, fructose-fed and glucose-fed mothers, furin and neuropilin 1 (protease and receptor, respectively, known to collaborate together in the virus entry (Drucker, 2021)) gene expression displayed a reduction in progeny born of carbohydrate-fed mothers, the fructose group being more deeply affected (Fig. 2C and 2D). ADAM17 (another factor belonging to the non-canonical pathway viral entry, as are furin and cathepsin) produced the same results (Fig. 2E) as those described above for furin (Fig. 2D). However, cathepsin L showed no differences between the three groups (Fig. 2F).
Cholesterol is critical for viral entry and replication. In fact, SARS-CoV-2 spike protein interacts with HDL, indirectly facilitating viral entry through the SR-B1 cell surface receptor (Drucker, 2021). In accordance with findings here observed for other receptors and cofactors involved in the SARS-CoV-2 cell access, SR-B1 gene expression was diminished in progeny from fructose-fed dams becoming significant in comparison to males born of glucose-fed mothers (Fig. 2G).
Therefore, maternal fructose intake seems to provoke a clear reduction in the hepatic gene expression of viral entry factors in adult male progeny and, possibly, this would protect the organ from SARS-CoV-2 cell infection.
3.2. Maternal fructose exacerbates fructose-induced augmentation of hepatic TMPRSS2 gene expression in adult male progeny
Bearing in mind the unexpected changes observed in the gene expression of male progeny born from fructose-supplemented mothers and in order to discover if this phenotype was conserved or reversed by a short liquid fructose-feeding period (3 weeks), we subjected male progeny from control, fructose- and glucose-fed mothers to a fructose liquid solution and determined if the maternal fructose intake influences the response of the adult offspring to fructose on hepatic receptors and cofactors that enable SARS-CoV-2 entry to the cell (Fig. 3A).
However, as shown in Fig. 3, whereas hepatic gene expression of the majority of key factors determined here for permitting SARS-CoV-2 entrance to the cell was not affected by fructose ingestion (Fig. 3C-3G), TMPRSS2 expression was induced in fructose-fed rats and this augmentation was clearly maternal-intake dependent (Fig. 3B). Thus, fructose-induced increase in TMPRSS2 expression was more pronounced in progeny from fructose-fed mothers, the effect becoming significantly different in comparison to rats from the control (C/C) group. Unfortunately, ACE2 gene expression, the necessary collaborator of this protease for the virus to enter to the cell, was not detected.
3.3. Maternal fructose exacerbates Western-type diet-induced increase of SARS-CoV-2 cell entry factors in ileum but not in liver of young male offspring
Once we found that liquid fructose could influence the gene expression of a key molecule in SARS-CoV-2 entry to the cell, TMPRSS2, in adult rats and, moreover, that this effect was dependent of the motheŕs diet, we checked if this situation could be also found in liver of younger rats. We were also interested in studying the effect of a Western-style diet and extend the analysis to another interesting tissue that has also been involved in SARS-CoV-2 infection, that is, ileum.
First, in liver, few changes were observed. Thus, gene expression of TMPRSS2 (Fig. 4B), neuropilin (Fig. 4C), and ADAM17 (Fig. 4E) was affected neither by maternal fructose nor by the diet consumed when descendants were adolescent. Furin and cathepsin L mRNA levels were increased by fructose plus cholesterol diet (FCho) (but not by fructose alone, F) in progeny from Control mothers but, curiously, this effect was lost for furin (Fig. 4D), or even reverted for cathepsin (Fig. 4F), in descendants from Fructose-fed mothers. For SRB1, in accordance with the effects observed in adult rats (Fig. 2G), maternal fructose significantly decreased the mRNA levels of this gene (Fig. 4G).
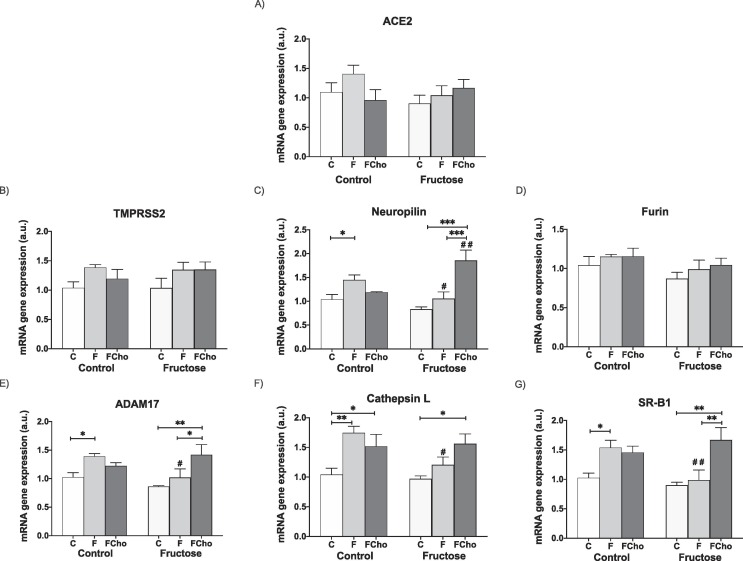
Interestingly, the effects found in ileum were more evident than in liver. Thus, liquid fructose did seem to generate less protection against viral infection in ileum since ACE2 and TMPRSS2 (Fig. 5 A and 5B) trended to increase and neuropilin (Fig. 5C), ADAM17 (Fig. 5E), cathepsin (Fig. 5F) and SRB1 (Fig. 5G) gene expression were significantly augmented in descendants from Control mothers after fructose intake (F) in comparison to those rats that ingested only water (C). Furin gene expression was not affected (Fig. 5D). Surprisingly, all these differences in gene expression between fructose (F) and controls (C) disappeared when descendants were born from Fructose-fed mothers.
Fig. 5.
Maternal fructose exacerbates Western-type diet-induced increase of SARS-CoV-2 cell entry factors in ileum of young male offspring. Experimental design shown in Fig. 4. Ileal levels of specific mRNA for (A) ACE2, (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17, (F) cathepsin L, and (G) SRB1 genes. Ileum (mRNA) expression from control (C, empty bar), fructose- (F, light grey bar), and fructose plus cholesterol-supplemented (FCho, dark grey bar) young male progeny from Control (left panel) or Fructose-fed (right panel) mothers. Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 7 to 8 litters. Asterisks denote a significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between the groups under the crossbar (groups with a different diet but the same motheŕs diet). Hash symbols denote a significant difference (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) as compared to the Control mothers (groups with the same diet but different motheŕs diet). ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1.
However, this protection against SARS-CoV-2 infection that apparently maternal fructose provoked in ileum when progeny ingested liquid fructose, was the opposite when descendants received a Western-style diet. Thus, whereas the fructose plus cholesterol (FCho) diet hardly produced any increase in gene expression (except for cathepsin (Fig. 5F) and SRB1 (Fig. 5G)) in progeny from Control mothers, descendants from Fructose-fed mothers were markedly affected. Although there were no observed differences for ACE2, TMPRSS2 and furin, gene expression was significantly increased for neuropilin (Fig. 5C), ADAM17, cathepsin L, and SRB1 (Fig. 5E-5G) when descendants from Fructose-fed mothers had ingested a Western-type diet (FCho) (in comparison to those that received fructose alone, F, or only water, C), indicating that these animals would be more prone to a SARS-CoV-2 infection, at least, at the level of small intestine.
3.4. Maternal fructose modulates how the sweetener tagatose affects SARS-CoV-2 cell entry factors in young male offspring
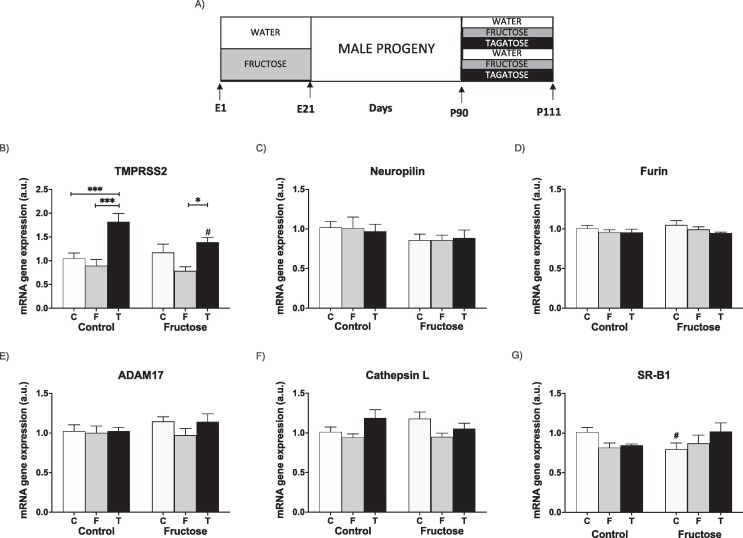
In order to demonstrate that the effects here observed for direct liquid fructose intake were specific for this carbohydrate, tagatose was used instead (Fig. 6 A).
Fig. 6.
Tagatose affects hepatic cell surface receptors and cofactors facilitating SARS-CoV-2 entry in liver of young male progeny. (A) Experimental design. Hepatic levels of specific mRNA for (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17, (F) cathepsin L, and (G) SRB1 genes. Liver (mRNA) expression from control (C, empty bar), fructose- (F, light grey bar), and tagatose-supplemented (T, dark bar) young male progeny from Control (left panel) or Fructose-fed (right panel) mothers. Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 7 to 8 litters. Asterisks denote a significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between the groups under the crossbar (groups with a different diet but the same motheŕs diet). Hash symbols denote a significant difference (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) as compared to the Control mothers (groups with the same diet but different motheŕs diet). TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1. E: embryonic/fetal days (E21: delivery); P: postnatal days.
Curiously, although fructose intake did not have any effect in the liver gene expression of adolescent descendants regardless of the motheŕs diet (Fig. 4B), TMPRSS2 gene expression (Fig. 6B) was significantly increased by tagatose consumption, although this effect was less evident when progeny were born from fructose-fed mothers. The other genes here measured were not affected by tagatose consumption (Fig. 6C-6G).
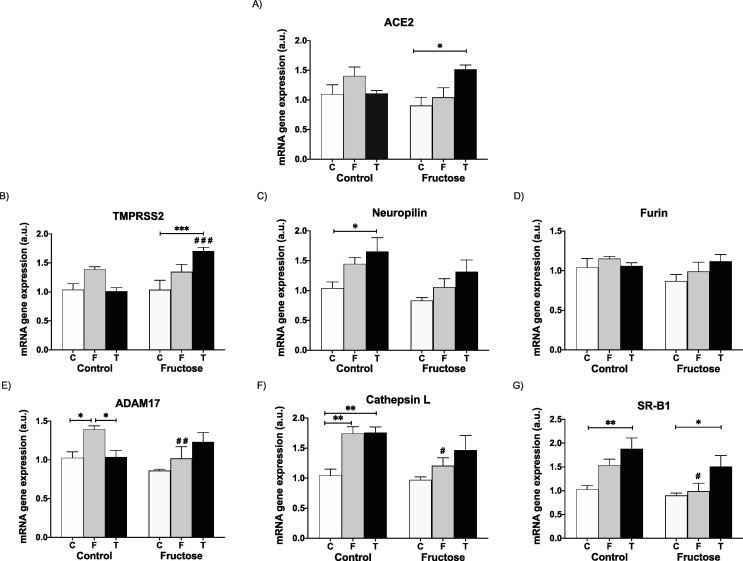
Again, the effects observed in ileum were more marked than in liver. Thus, whereas the effects that liquid fructose (F) provoked in ACE2, TMPRSS2 and ADAM17 in descendants from Control mothers were not observed after tagatose consumption (T) (Fig. 7 A, 7B and 7E, respectively), the effects of fructose intake in neuropilin (Fig. 7C), cathepsin (Fig. 7F) and SRB1 (Fig. 7G) gene expression not only were resembled by tagatose intake, but were even more pronounced, in comparison to those rats that ingested only water (C). Furin gene expression was not affected (Fig. 7D). Once again, as already observed for fructose, all these differences in gene expression (except for SRB1, Fig. 7G) between tagatose (T) and controls (C) found in the progeny from Control mothers, were mitigated when descendants were born from Fructose-fed mothers (Fig. 7C and 7F). On the contrary, it was precisely in the progeny of Fructose-fed mothers where tagatose intake (T) provoked clear increases in ACE2 and TMPRSS2 (Fig. 7A and 7B) gene expression which became significant versus those rats that consumed water without additives (Control, C).
Fig. 7.
Maternal fructose modulates how the sweetener tagatose affects SARS-CoV-2 cell entry factors in ileum of young male offspring. Experimental design shown in Fig. 6. Ileal levels of specific mRNA for (A) ACE2, (B) TMPRSS2, (C) neuropilin-1, (D) furin, (E) ADAM17 (F) cathepsin L and (G) SRB1 genes. Ileum (mRNA) expression from control (C, empty bar), fructose- (F, light grey bar), and tagatose-supplemented (T, dark bar) young male progeny from Control (left panel) or Fructose-fed (right panel) mothers. Relative target gene mRNA levels were measured by Real Time PCR as explained in Materials and Methods, normalized to Rps29 levels and expressed in arbitrary units (a.u.). Data are means ± S.E. from 7 to 8 litters. Asterisks denote a significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between the groups under the crossbar (groups with a different diet but the same motheŕs diet). Hash symbols denote a significant difference (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) as compared to the Control mothers (groups with the same diet but different motheŕs diet). ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease serine 2; ADAM17: ADAM metallopeptidase domain 17; SRB1: HDL-scavenger receptor B type 1.
4. Discussion
Whether people with metabolic diseases such as T2DM, CVD or obesity do exhibit increased susceptibility to SARS-CoV-2 infection continues to be debated and uncertain. However, COVID-19 infection clearly results in increased rates of hospitalization, greater severity of illness and mortality in patients with diabetes, CVD or obesity. Thus, knowing if the ACE2 expression and its cofactors facilitating SARS-CoV-2 infectivity is dysregulated in cells and tissues of patients with diabetes, CVD or obesity, and contributes to the pathophysiology of SARS-CoV-2 infection, seems to be crucial to understanding and preventing COVID-19 disease (Drucker, 2021).
Since a Western-style diet is known to contribute to the prevalence of these metabolic diseases, and therefore all of them have been encompassed within the term “processed food-related diseases”, the possibility that fructose-enriched foods, sugary drinks, high-fat and/or high-cholesterol diets could place these patients at an increased risk for severe COVID-19 pathology and mortality should not be discarded (Butler & Barrientos, 2020).
Animal models are critical for understanding viral pathogenesis, vaccine development, and drug screening. Small animal models are essential for research and antiviral therapeutic development. Rodent models are popular because of their affordability, availability, and clear genetic backgrounds and they have been widely used for studying the pathogenesis of human coronaviruses (Jiang et al., 2020).
To elucidate these mechanisms, we have used our rat model of fetal programming provoked by maternal fructose intake in which typical features of MetS appear, directly(Rodrigo et al., 2018, Rodríguez et al., 2016) or after fructose supplementation, in adult male progeny (Fauste et al., 2020). This animal model of MetS has been previously used by us and others and has been useful in demonstrating that maternal fructose intake causes clear dysregulations in progeny with possible clinical implications (Alzamendi et al., 2016). Interestingly, although male descendants of fructose-fed mothers exhibited impaired insulin signalling and hyperinsulinemia (Rodríguez et al., 2016), viral entry to the hepatocyte via non-canonical pathways did not seem to be facilitated. Thus, gene expression both of proteases such as ADAM17 and furin, and receptors such as neuropilin and SRB1, was decreased in males from fructose-fed mothers in comparison to those from control dams. These findings are interesting since neuropilin has been described as an alternative receptor to ACE2 for the viral entry (Daly et al., 2020), and moreover, has been reported to be assisted by furin. Furthermore, the likelihood that SARS-CoV-2 can bind and enter liver cells via the canonical pathway involving ACE2 and TMPRSS2 seems to be reduced since ACE2, in our hands, was not detected. Nevertheless, it could possibly be due to the heterogeneity of the liver cell composition. In fact, a similar situation has already been demonstrated in human pancreas where ACE2 and its protease are present in pancreatic ducts but not in β cells (Coate et al., 2020).
Surprisingly, although maternal fructose intake did seem to protect the liver from SARS-CoV-2 cell infection in adult male progeny, the findings turned out to be different when these descendants from control, fructose- or glucose-fed mothers, were themselves subjected to liquid fructose. Whereas most of the molecules here determined did not show any differences between the four groups studied, TMPRSS2 gene expression was induced by fructose intake and the effect became significant in male born of fructose-fed mothers versus control males. Interestingly, this protease has been proposed as one of the molecules responsible for the relative protection of infants and children against severe COVID19 illness (Schuler et al., 2021). In fact, TMPRSS2 expression has been demonstrated to increase with aging in mice and humans.
On the other hand, reports studying the pathophysiology of SARS-CoV-2 infection in people with diabetes, ECV and obesity, have found that gastrointestinal tract, liver, islets and adipose tissue are also affected, raising uncertainty about their implication in the severity of COVID-19 (Drucker, 2021). For example, ACE2 receptors are abundant in the small intestine and they have been related to the abdominal pain and diarrhea that COVID 19 patients frequently report (Ji et al., 2020).
Interestingly, it has been demonstrated that younger rats can be more greatly affected than older ones by the introduction of sugary drinks and, therefore, it is logical to think that more marked effects of the mothers’ diets could be detected if the offspring were introduced to sugar at a younger age (Kendig et al., 2015). Although young people seems to be less affected by COVID19 infection and also with less severity than older individuals, adolescents have a more frequent ingestion of processed food and beverages containing fructose than older people.
Therefore, we determine if maternal fructose in young males is able to modulate the effects of liquid fructose alone or as a part of a Western-style diet (along with a cholesterol-rich food) on hepatic and ileal gene expression of SARS-CoV-2 entry-dependent factors. In contrast to adult rats, both maternal fructose intake and direct fructose consumption in adolescent rats did not affect the hepatic gene expression of viral entry factors. The only effect that was coincident to those found in older rats was that maternal fructose intake diminished SRB1 mRNA expression in the progeny in comparison to descendants from control dams. These findings would indicate that young animals seem to be refractory to show changes in the expression of viral entrance molecules in response to diet modifications. It was only when cholesterol was included in the diet along with liquid fructose, that an increase in the expression of two proteases of the non-canonical pathway (furin and cathepsin) was observed in the progeny from control dams which, however, was not observed in descendants from fructose-fed mothers.
Whereas direct intake of liquid fructose by young males did not produce any effect in hepatic viral entry factors, fructose-induced changes were clearly observed in ileum. Although the canonical pathway (ACE2 and TMPRSS2) only showed a trend to increase, alternative proteases such as ADAM17 and cathepsin L gene expression were significantly induced by fructose intake. Moreover, the other receptor that has been demonstrated to collaborate with ACE2 to facilitate SARS-CoV-2 passage to the cell, SRB1 (Wei et al., 2020), also displayed a fructose-induced increase. Further, the alternative receptor to ACE2, neuropilin-1, was also increased in descendants from control mothers that had consumed liquid fructose. Curiously, it was surprising not to observe these fructose-provoked changes in descendants from fructose-fed mothers. These findings, in consonance to those found in liver, would again indicate that maternal fructose intake could be protecting against the harmful effects that direct liquid fructose intake produces in the viral entry factors to the cell in these adolescent rats.
Interestingly, the opposite was found when the progenies received a Western-style diet. Maternal fructose intake exacerbated the detrimental fructose plus cholesterol-induced effects in SARS-CoV-2 cell entry molecules found in descendants from control mothers. Although ACE2 and TMPRSS2 were not affected, the non-canonical pathway (cathepsin and ADAM17) gene expression was augmented in males from fructose-fed mothers after receiving the Western-type diet versus descendants consuming water or liquid fructose. Furthermore, the alternative ACE2 receptor, neuropilin, and the ACE2 collaborating receptor, SRB1, also exhibited a Western-style diet-induced increase. Thus, as Coate and col have proposed (Coate et al., 2020) for human β cells, although gene expression findings found here for ACE2 and TMPRSS2 do not suggest that SARS-CoV-2 can enter ileal cells via the canonical pathway, they do not exclude the possibility of viral entry via non-canonical pathways involving other suggested effector proteases and receptors of SARS-CoV2. Furthermore, these findings would reinforce those studies in humans showing that patients with MAFLD or advanced stages of NAFLD displayed higher expression of SARS-CoV-2 entry factors and, therefore, higher risk of COVID-19 disease progression than patients without these metabolic diseases (Fondevila et al., 2021, Ji et al., 2020).
Moreover, though ACE2 has been reported not to participate in SARS-CoV-2 cell entry in rats, it has been demonstrated in rodents expressing human recombinant ACE2 (hACE2) that the other factors facilitating the entrance of SARS-CoV-2 to the cell must be implicated (Jiang et al., 2020). It remains to be elucidated in humans (or in the hACE2 mice) whether ACE2 receptor gene expression is also affected by a Western-diet intake and, moreover, if the motheŕs diet could be involved.
Interestingly, during the preparation of the present report, an interesting study showing that some human cell lines were unable to support SARS-CoV-2 infection despite displaying a strong expression of ACE2 has been published (Yeung et al., 2021). Moreover, since it was observed that inhibition of ADAM17 diminished SARS-CoV-2 infection, the authors proposed that complexes formed by SARS-CoV-2 and soluble ACE2 (released by ADAM17 protease activity from the surface of the cells), might be able to attach and enter the tissues where ACE2 is poorly expressed (Yeung et al., 2021) (Fig. 1).
Our previous reports reveal the importance of studying the effects of fructose in comparison to other sweeteners, in order to be sure that the effects provoked by fructose are specific to this carbohydrate. For this reason, in the present study we used an epimer of fructose called tagatose to compare to fructose. This epimer is increasingly used as a low-caloric sugar alternative and modestly improves glycemic control in individuals with and without diabetes (Noronha et al., 2018). In fact, it has been shown that chronic overconsumption of tagatose does not exert the same deleterious metabolic derangements observed after fructose administration (Collotta et al., 2018). In the present study, hepatic gene expression of most of the factors showed no response to tagatose, as had already been observed with fructose, except for TMPRSS2. The expression of TMPRSS2 was significantly increased after receiving tagatose in comparison to progeny having drunk fructose or water. This finding is important since this protease has been proposed as one of the molecules responsible for the severity of COVID19 (Schuler et al., 2021). Interestingly, it was observed in ileum that those effects found in descendants from control mothers after fructose intake were specific to this carbohydrate in ACE2, TMPRSS2 and ADAM17, but not for neuropilin, cathepsin or SRB1 gene expression since they were also observed after tagatose intake. Curiously, again, these effects disappeared or were mitigated by maternal fructose consumption. Interestingly, the specific effects of tagatose, ileal ACE2 and TMPRSS2 gene expression were significantly augmented in young males from fructose-fed mothers. These results found here with fructose and tagatose should make us reconsider the possibility of reducing or avoiding the wide and enormous use of carbohydrates as components of a great variety of foods as sweeteners, preservatives and other functions.
As limitations of the present study, apart from difficulties in extrapolating results from experimental animals to humans: a) although Bellamine et al (Bellamine et al., 2021) have shown that protein levels of key factors for viral entry mimic the mRNA levels and, moreover, parallel to the susceptibility of cells to SARS-CoV-2 infection, we have not directly measured SARS-CoV-2 binding or entry into the cells but have instead assessed (mRNA) gene expression of receptors and proteases required for the entry of this virus in liver or ileum; b) we have used simple sugar solutions instead of sucrose (table sugar) or high fructose corn syrup (HFCS), and thus more evident effects could have been observed since it is known that fructose absorption is improved by the presence of glucose; c) we have used fructose plus cholesterol instead other Western-type diets that also include saturated fat (Rajcic et al., 2021) and thus, possibly, more harmful effects could have been observed; d) determinations have not been carried out in other tissues susceptible to SARS-CoV-2 infection, such as lung, serum and adipose tissue. Thus, to confirm the applicability of our findings it would be necessary to address in the future the limitations of the present study.
In summary, we have demonstrated that maternal fructose intake provided some protection against SARS-CoV-2 infection to descendants. Curiously, however, one of the most prominent results found here is that maternal fructose intake exacerbates the fructose plus cholesterol-induced augmentation in the ileal SARS-CoV-2 cell entry factors gene expression in young male progeny.. Thus, bearing in mind the potential influence of processed foods and fructose-rich beverages in the development of many common non-communicable diseases such as atherosclerosis, metabolic syndrome, etc, and the more and more evident relationship of these metabolic diseases with the COVID disease, we would like to suggest that a reduction in the consumption of fructose-sweetened beverages, especially during gestation, all over the world could be beneficial.
Grant Support: Ministerio de Ciencia e Innovación (MICIN): SAF2017-89537-R/MCIN/AEI/https://doi.org/10.13039/501100011033 and ERDF “A way of making Europe”, and PID2020-118054RB-I00/MCIN/AEI /https://doi.org/10.13039/501100011033.
Disclosure
None of the authors have any conflicts of interest to report.
Data Transparency
All data will be made available to other researchers under request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Jose M. Garrido and his team for their help in handling the rats, and Brian Crilly for his editorial help. This work was supported by grants from Ministerio de Ciencia e Innovación (MICIN): SAF2017-89537-R/MCIN/AEI/10.13039/501100011033 and ERDF “A way of making Europe”, and PID2020-118054RB-I00/MCIN/AEI /10.13039/501100011033. Silvia Rodrigo was supported with a FUSP-CEU fellowship. Elena Fauste was supported with a FPU fellowship from MICIN. C.B. conceived and designed the study. E.F., C.D., M.P., P.O., S.R., and M.I.P. contributed reagents/materials/analysis tools for gene expression studies and parameter analysis. L.R., E.F., and M.I.P. handled the animals. M.I.P. and J.J.A-M analyzed the data. C.B. wrote the paper. None of the authors have any conflicts of interest to report.
Data availability
Data will be made available on request.
References
- Alzamendi A., Zubiría G., Moreno G., Portales A., Spinedi E., Giovambattista A. High risk of metabolic and adipose tissue dysfunctions in adult male progeny, due to prenatal and adulthood malnutrition induced by fructose rich diet. Nutrients. 2016;8(3):178. doi: 10.3390/nu8030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo J., Cai J., Stevens J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metabolic Syndrome and Related Disorders. 2019;17(1):46–52. doi: 10.1089/met.2018.0105. [DOI] [PubMed] [Google Scholar]
- Bellamine A., Pham T.N.Q., Jain J., Wilson J., Sahin K., Dallaire F.…Cohen É. L-Carnitine tartrate downregulates the ACE2 receptor and limits SARS-CoV-2 infection. Nutrients. 2021;13(4) doi: 10.3390/nu13041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biquard L., Valla D., Rautou P.E. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease. Journal of Hepatology. 2020;73(3):717–718. doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain, Behavior, and Immunity. 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate K.C., Cha J., Shrestha S., Wang W., Gonçalves L.M., Almaça J.…Dai C. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell metabolism. 2020;32(6):1028–1040. doi: 10.1016/j.cmet.2020.11.006. e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collotta D., Lucarini L., Chiazza F., Cento A.S., Durante M., Sgambellone S.…Mastrocola R. Reduced susceptibility to sugar-induced metabolic derangements and impairments of myocardial redox signaling in mice chronically fed with D-tagatose when compared to fructose. Oxidative medicine and cellular longevity. 2018;2018 doi: 10.1155/2018/5042428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Antón-Plágaro C.…Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metabolism. 2021;33(3):479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauste E., Rodrigo S., Rodríguez L., Donis C., García A., Barbas C.…Bocos C. FGF21-protection against fructose-induced lipid accretion and oxidative stress is influenced by maternal nutrition in male progeny. Journal of Functional Foods. 2020;64 [Google Scholar]
- Fondevila M.F., Mercado-Gómez M., Rodríguez A., Gonzalez-Rellan M.J., Iruzubieta P., Valentí V.…Nogueiras R. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. Journal of Hepatology. 2021;74(2):469–471. doi: 10.1016/j.jhep.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., Lau G. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: A preliminary analysis. Journal of Hepatology. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R.D., Liu M.Q., Chen Y., Shan C., Zhou Y.W., Shen X.R.…Shi Z.L. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 2020;182(1):50–58.e58. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.J., Perez-Pozo S.E., Sautin Y.Y., Manitius J., Sanchez-Lozada L.G., Feig D.I.…Nakagawa T. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocrine Reviews. 2009;30(1):96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig M.D., Ekayanti W., Stewart H., Boakes R.A., Rooney K. Metabolic effects of access to sucrose drink in female rats and transmission of some effects to their offspring. PLoS One1. 2015;10(7):e0131107. doi: 10.1371/journal.pone.0131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B., Broekaert I., Demmelmair H., Franke J., Hannibal I., Oberle D.…Project E.C.O. Protein intake in the first year of life: a risk factor for later obesity? The E.U. childhood obesity project. Advances in Experimental Medicine and Biology. 2005;569:69–79. doi: 10.1007/1-4020-3535-7_12. [DOI] [PubMed] [Google Scholar]
- Laughlin M.R., Bantle J.P., Havel P.J., Parks E., Klurfeld D.M., Teff K., Maruvada P. Clinical research strategies for fructose metabolism. Advances in Nutrition. 2014;5(3):248–259. doi: 10.3945/an.113.005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig R.H. Ultraprocessed food: Addictive, toxic, and ready for regulation. Nutrients. 2020;12(11) doi: 10.3390/nu12113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha J.C., Braunstein C.R., Blanco Mejia S., Khan T.A., Kendall C.W.C., Wolever T.M.S.…Sievenpiper J.L. The effect of small doses of fructose and its epimers on glycemic control: A systematic review and meta-analysis of controlled feeding trials. Nutrients. 2018;10(11) doi: 10.3390/nu10111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic acids Research. 2001;29(9):e45–e. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcic D., Baumann A., Hernández-Arriaga A., Brandt A., Nier A., Jin C.J.…Bergheim I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biology. 2021;41 doi: 10.1016/j.redox.2021.101879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S., Fauste E., de la Cuesta M., Rodríguez L., Álvarez-Millán J.J., Panadero M.I.…Bocos C. Maternal fructose induces gender-dependent changes in both LXRα promoter methylation and cholesterol metabolism in progeny. The Journal of Nutritional Biochemistry. 2018;61:163–172. doi: 10.1016/j.jnutbio.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Rodríguez L., Panadero M.I., Roglans N., Otero P., Alvarez-Millán J.J., Laguna J.C., Bocos C. Fructose during pregnancy affects maternal and fetal leptin signaling. The Journal of Nutritional Biochemistry. 2013;24(10):1709–1716. doi: 10.1016/j.jnutbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Rodríguez L., Panadero M.I., Roglans N., Otero P., Rodrigo S., Álvarez-Millán J.J.…Bocos C. Fructose only in pregnancy provokes hyperinsulinemia, hypoadiponectinemia, and impaired insulin signaling in adult male, but not female, progeny. European Journal of Nutrition. 2016;55(2):665–674. doi: 10.1007/s00394-015-0886-1. [DOI] [PubMed] [Google Scholar]
- Roglans N., Sanguino E., Peris C., Alegret M., Vázquez M., Adzet T.…Sánchez R.M. Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. The Journal of Pharmacology and Experimental Therapeutics. 2002;302(1):232–239. doi: 10.1124/jpet.302.1.232. [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Saad A.F., Dickerson J., Kechichian T.B., Yin H., Gamble P., Salazar A., Costantine M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. American Journal of Obstetrics and Gynecology. 2016;215(3):e371–e376. doi: 10.1016/j.ajog.2016.03.038. [DOI] [PubMed] [Google Scholar]
- Schuler B.A., Habermann A.C., Plosa E.J., Taylor C.J., Jetter C., Negretti N.M.…Network H.C.A.B. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. The Journal of Clinical Investigation. 2021;131(1) doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen M.R., Packard C.J., Borén J. Dietary fructose and the metabolic syndrome. Nutrients. 2019;11(9) doi: 10.3390/nu11091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M.H., Gluckman P.D., Coveny A.H., Hofman P.L., Cutfield W.S., Gertler A.…Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10):4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Vilà L., Roglans N., Perna V., Sánchez R.M., Vázquez-Carrera M., Alegret M., Laguna J.C. Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose. The Journal of Nutritional Biochemistry. 2011;22(8):741–751. doi: 10.1016/j.jnutbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Wei C., Wan L., Yan Q., Wang X., Zhang J., Yang X.…Zhong H. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2(12):1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- Yeung M.L., Teng J.L.L., Jia L., Zhang C., Huang C., Cai J.P.…Yuen K.Y. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021 doi: 10.1016/j.cell.2021.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.M., Jiao R.Q., Kong L.D. High dietary fructose: Direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients. 2017;9(4) doi: 10.3390/nu9040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.