Abstract
Yersinia enterocolitica serotype O:3 and O:8 urease-negative mutants unable to express the 19-kDa β subunit of urease were constructed and tested for virulence and arthritogenicity. Our results indicate that urease is needed for full virulence in oral infections and that it is not an arthritogenic factor in the rat model.
Reactive arthritis (ReA) is an acute, nonpurulent arthritis that occurs following an infection elsewhere in the body and is triggered by a variety of microbes (2). The diagnosis of ReA is based on both clinical findings and laboratory evidence of the triggering infection. Although several studies have addressed the disease mechanisms behind ReA, the problem is largely unresolved. The similar clinical manifestations induced by a variety of different microorganisms have raised the question of whether conserved immunodominant antigens are involved. We have tried to tackle the problem of the pathogenesis of ReA by establishing an animal model in which ReA is induced by injection of live Yersinia enterocolitica O:8 into rats (14, 27). The animal model was used to show that the Yersinia adhesin YadA and its collagen-binding ability play a role in the induction of ReA (8, 9).
In the search for arthritogenetic molecules from arthritis-causing bacteria, the urease molecule of Yersinia is of interest because Mertz et al. induced arthritis in preimmunized male Wistar rats by intra-articular injection of the 19-kDa β subunit of Y. enterocolitica urease (15, 25). Furthermore, the urease β subunit was shown to be a target for the synovial T-cell response of patients with Y. enterocolitica-caused ReA (21). Western blot and enzyme-linked immunosorbent assay analysis of sera from patients suffering from Y. enterocolitica infection revealed antibodies to this antigen (15). Hermann et al. found that the β subunit of Yersinia urease is an immunodominant antigen both for proliferative CD4+ T cells and for synovial CD8+ T-cell clones (1, 11). In the present work, urease-negative mutants of Y. enterocolitica unable to express the 19-kDa β subunit were constructed and studied for virulence and arthritogenicity.
Construction of urease-negative mutants of Y. enterocolitica O:3 and O:8.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were stored and cultured as described previously (9). The presence of the virulence plasmid in Y. enterocolitica strains was confirmed by the expression of YadA using the autoagglutination test (13), and urease activity was tested using the phenol red urease test.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Comments | Source or reference |
|---|---|---|
| Y. enterocolitica | ||
| 8081 | Serotype O:8 wild-type strain, carries restriction modification system expressing restriction endonuclease YenI (12, 29); urease positive | 20 |
| 8081-R−M+ | Serotype O:8 pYV+YenI restriction endonuclease-deficient derivative of 8081; urease positive | 28 |
| 8081-c | pYV-cured derivative of 8081; urease positive | 20 |
| 8081-U-GB | 8081-r− m+yeuA::Km-GB; urease negative | This work |
| 6471/76 (=YeO3) | Wild-type serotype O:3 strain; pYV+ patient isolate; urease positive | 24 |
| 6471/76-c (=YeO3-c) | pYV-cured derivative of 6471/76; urease positive | 24 |
| YeO3-U | YeO3 yeuA::Km-GB; urease negative | This work |
| YeO3-U::pCGL1 | YeO3-U complemented by chromosomally integrated pCGL1; urease positive | This work |
| E. coli | ||
| C600 | thi thr leu tonA lacY supE | 3 |
| Sy327λpir | (lac pro) argE(Am) rif nalA recA56(λpir) | 17 |
| Sm10λpir | thi thr leu tonA lacY supE recA::RP4-2Tc::Mu-Km (λpir) | 17 |
| Plasmids | ||
| pRV1 | Suicide vector containing cat gene of pACYC184 cloned into PstI site of pJM703.1 (17); contains R6K oriR RP4 mob and must be replicated in λpir hosts | 26 |
| pCGL1 | 4.5-kb ClaI fragment of p19kd-107 carrying yeuABC genes cloned into ClaI site of pRV1 | This work |
| pUC-4K | Origin of Km-GB | Pharmacia-LKB, Uppsala, Sweden |
| p19kd-107 | yeuA, yeuB, and yeuC genes of YeO3 cloned into pBR322 | 25 |
| p14 | Derivative of p19kd-107 with upstream region of yeuA deleted and occupied by two tandem Km-GB fragments | This work |
| pPA14 | 1.3-kb EcoRI fragment of p19kd-107 upstream of yeuA cloned into EcoRI site of p14 | This work |
| pPA7 | 7.0-kb StyI-ScaI fragment of pPA14 cloned into EcoRV site of pRV1 | This work |
| pPL3 | pPA7 with other Km-GB deleted | This work |
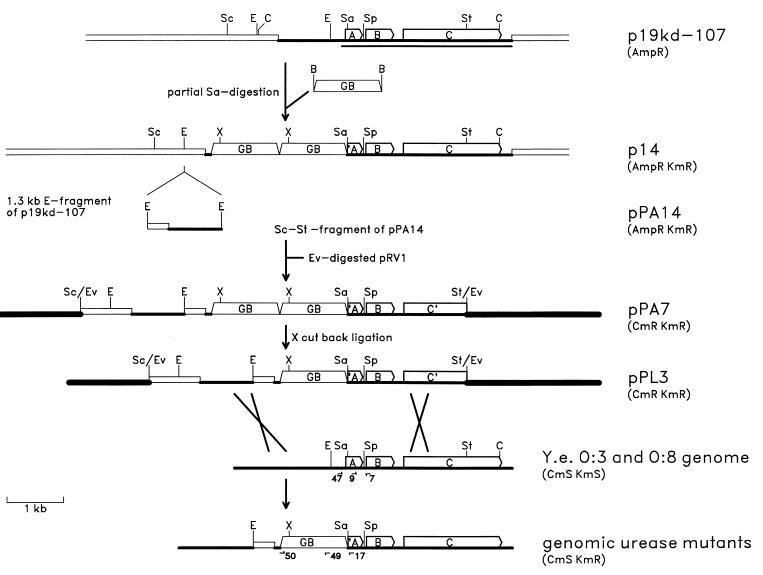
The mutant strains were constructed using the marker exchange method, and the construction strategy is outlined in Fig. 1. The kanamycin resistance gene block cassette (Km-GB) of pUC-4K was cloned into plasmid p19kd-107 (carrying the yeuABC genes; Fig. 1 and reference 25) that was partially digested with Sau3AI. Restriction digestion analysis showed that one recombinant plasmid, designated p14, carried two copies of Km-GB in succession inserted in the beginning or upstream of the yeuA gene. During the partial Sau3AI digestion and ligation, p14 had lost a fragment of about 1.5 kb of Y. enterocolitica DNA just upstream of the Km-GB insertion site. To reintroduce this fragment, which is necessary for homologous recombination, p19kd-107 was digested with EcoRI and the purified 1.3-kb EcoRI fragment was cloned into the EcoRI site of p14. The resulting plasmid was designated pPA14. A 7.0-kb StyI-ScaI fragment of pPA14 was cloned into EcoRV-digested suicide vector pRV1 to obtain pPA7. The extra copy of Km-GB was removed by digestion with XhoI, followed by religation; the resulting plasmid, pPL3, was mobilized into Y. enterocolitica strains YeO3 and 8081-R−M+ (Table 1); and Cmr Kmr Yersinia transconjugants were selected on yersinia-selective CIN agar to obtain derivatives that had pPL3 integrated into the chromosome via homologous recombination. To select derivatives with a second recombination event to eliminate the suicide vector, cycloserine enrichment was used as previously described (8, 18). Cms Kmr urease-negative clones were obtained for both serotypes; the serotype O:3 mutant was designated YeO3-U, and the serotype O:8 mutant was designated 8081-U-GB.
FIG. 1.
Construction strategy used for urease-negative mutants of Y. enterocolitica serotypes O:3 and O:8. The linearized restriction map of plasmid p19kd-107 is shown at the top. DNAs from different origins are indicated by different types of lines. The open bars indicate pBR322 DNA, medium-thick lines indicate Y. enterocolitica genomic DNA, thick lines indicate the suicide vector pRV1 DNA, and the open trapezoids indicate Km-GB DNA. Part of the genomic fragment cloned into the BamHI site of pBR322 has been sequenced (25), and the location of the 2,680-bp nucleotide sequence (accession no. Z18865) is shown by the thin line under the p19kd-107 map. The yeuABC genes are indicated by open arrows and the letters A, B, and C, respectively, and truncated forms of the genes are indicated by ′A and C′. Restriction endonuclease recognition sites are indicated above the maps, and the following abbreviations are used: C, ClaI; E, EcoRI; Ev, EcoRV; Sa, Sau3AI (only one site of many is indicated); Sc, ScaI; Sp, SphI; St, StyI; X, XhoI. The antibiotic resistance(s) mediated by each construct is indicated in parentheses on the right. The approximate locations of the crossovers between the pPL3 and the Y. enterocolitica serotype O:3 and O:8 genomic DNAs are indicated by crossed lines. The oligonucleotide primers used for PCRs and sequencing are indicated by the arrows and numbers under the genomic maps at the bottom.
Characterization of YeO3-U and 8081-U-GB.
The exact location of the Km-GB insertion in the mutants was determined by PCR and sequencing. The Y. enterocolitica O:3 urease operon (GenBank accession no. Z18865)-specific oligonucleotide primers used (Fig. 1, bottom) were Pr7 (nucleotides 487 to 468 of the sequence with accession no. Z18865; 5′ to 3′ primer sequence direction), Pr9 (nucleotides 168 to 187), MS47 (nucleotides 1 to 18), and Pr17 (nucleotides 194 to 175). The primers MS49 (nucleotides 718 to 735) and MS50 (nucleotides 1645 to 1628) are specific for Km-GB in pUC4-k (GenBank accession no. X06404). To exactly locate the Km-GB insertion site, primer Pr17 was used for sequencing of the PCR product obtained using primers MS47 and Pr7. In PCRs, chromosomal DNAs both from the two mutants and from the parental wild-type strains were used as templates. Standard PCR amplification was performed as previously described (23) and as suggested by the supplier of the thermostable DNA polymerase DynaZyme (Finnzymes Oy, Espoo, Finland) using 50 pmol of each primer per reaction mixture.
PCR using the primers MS47 and Pr7 gave the expected 487-bp PCR products with the wild-type templates but not with the urease mutant templates, while primers Pr9 and Pr7 gave 320-bp fragments with templates from all four strains (data not shown), indicating that the region upstream of yeuA was missing from the mutants. Pr7 was used in combination with Km-GB-specific primers MS49 and MS50, and 1.6-kb fragments were obtained with primers Pr7 and MS50 with the templates from the mutants but not with those from the wild-type bacteria (data not shown). These results indicated that the Km-GB insertion had taken place about 400 bp upstream of Pr7. This was confirmed by sequencing of the PCR products using Pr17 as a primer, and the sequence showed that Km-GB was in the Sau3AI site at position 95 of the sequence with accession no. Z18865. Taken together, these results indicated that the genetic organization of the urease operon in the mutants was as shown at the bottom of Fig. 1; i.e., the region upstream of the urease operon until the EcoRI site was intact. The about 250-bp fragment between the EcoRI site and the Sau3AI site at position 95 of the sequenced region was occupied by the fragment containing the 375-bp pBR322 fragment derived from p19kd-107 and the 1.2-kb Km-GB sequence.
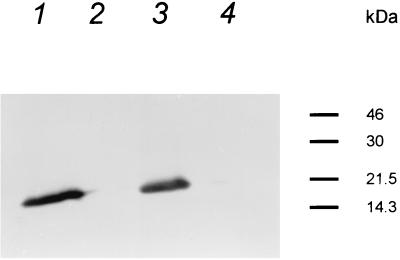
The sequence of the urease operon of Y. enterocolitica O:8 (6) (GenBank accession no. L24101) is almost identical to that of serotype O:3. The O:8 sequence starts at the EcoRI site and shows that the 250-bp fragment mentioned above contains a promoter motif. In the mutants constructed in this work, the promoter would be missing and any transcription coming from upstream genes would be stopped by Km-GB. Thus, it was very likely that there was no transcription through the truncated yeuA gene to the intact yeuB and downstream genes and that the mutants most likely would not express any of the urease subunit polypeptides. This conclusion was supported by Western blot analysis, which showed that the wild-type bacteria (Fig. 2, lanes 1 and 3), but not the urease mutants (Fig. 2, lanes 2 and 4), expressed the β subunit.
FIG. 2.
Western blot analysis of expression of the urease β subunit by Y. enterocolitica wild-type and urease mutant strains. Whole-cell lysates of Y. enterocolitica strains were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described by Ausubel et al. (4) using an acrylamide concentration of 12% in the separating gel and electroblotted onto nitrocellulose filters. The urease β subunit was detected by monoclonal antibody I2cl20, which is specific for the Y. enterocolitica O:3 β subunit (a kind gift of S. Batsford, Freiburg, Germany) diluted 1:500. Lanes: 1, 8081; 2, 8081-U-GB; 3, YeO3; 4, YeO3-U. Molecular masses are indicated on the right.
The stability of the constructed urease mutants in vivo was assessed in bacteria recovered from feces of rats several weeks after the initial challenge. All of the recovered colonies were Kmr and urease negative, and in addition, the bacteria maintained the virulence plasmid, as shown by positive autoagglutination test results. These results demonstrated that the mutation was stable in vivo at least for several weeks.
Construction of YeO3-U::pCGL1.
The 4.5-kb ClaI fragment of p19kd-107 carrying the yeuABC genes was cloned into the ClaI site of suicide vector pRV1 to obtain pCGL1. pCGL1 was mobilized into YeO3-U, and one urease-positive Kmr Cmr clone designated YeO3-U::pCGL1 was chosen for cycloserine enrichment to select for derivatives with a second recombination event to eliminate the suicide vector and Km-GB. However, no Kms Cms clones were obtained from several enriched cultures.
PCR using genomic DNA from YeO3-U::pCGL1 and primers MS47 and Pr7 (Fig. 1) gave the expected 487-bp product that was not obtained when YeO3-U DNA was used as the template (data not shown). YeO3-U::pCGL1 was Kmr, indicating that Km-GB was still present in the genome, and since it was urease positive (the yeuABC genes alone do not confer urease positivity on bacteria [25]), these results indicated that pCGL1 had integrated into the defective urease operon of YeO3-U by a single crossing over at the yeuABC region, thereby restoring a functional urease operon.
Virulence in mice.
Female 6- to 8-week-old DBA/2 mice were used for virulence studies and were acquired from Bomholtgård Breeding Research Center Ltd. The mice were found to be healthy by the breeder and were kept under conventional conditions throughout the experiments. Virulence experiments with intragastrically infected DBA/2 mice were performed with wild-type strains 8081 and YeO3 and with the respective urease mutants 8081-U-GB and YeO3-U and complemented strain YeO3-U::pCGL1. For animal injections, bacteria were grown at room temperature on an orbital shaker (180 rpm) in 1 liter of tryptic soy broth in a 2-liter Erlenmeyer bottle until early log phase. The bacterial cells were collected, washed, and finally diluted to appropriate concentrations in 0.9% NaCl as described earlier (9). For mouse infections, the bacterial suspensions were adjusted to 1010 CFU/ml. From this suspension, serial 10-fold dilutions were prepared and 100-μl volumes of appropriate dilutions were used for animal inoculations. The actual concentrations of viable bacteria were determined by colony counts on agar plates. Mice that were inoculated intragastrically were kept without solid food for 4 to 12 h prior to bacterial challenge. The bacterial suspension (100 μl) was administered intragastrically directly to the stomachs of the mice using a 20-gauge stainless steel ball-tipped catheter. Mice injected with the serotype O:3 strains were given 10 mg of desferrioxamine mesylate (Desferal; Ciba-Geigy AG, Basel, Switzerland) intraperitoneally 1 day before the Yersinia injection. Animals were observed for 40 days after infection.
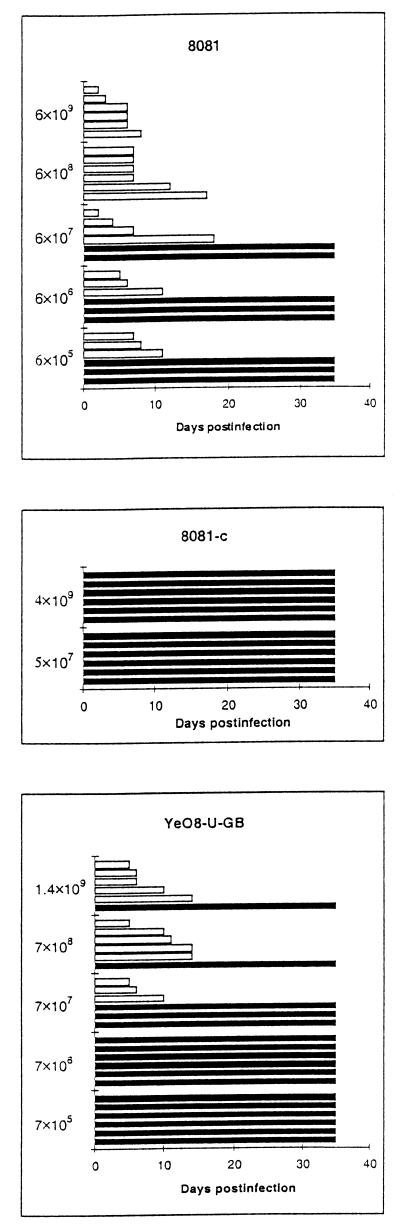
The lack of urease activity in 8081-U-GB reduced the virulence of the bacteria, as shown in Fig. 3. The 50% lethal dose (LD50) of 8081 in these experiments was about 106 bacteria per mouse, whereas the LD50 of 8081-U-GB was almost 108 bacteria per mouse, i.e., about 100-fold higher (P = 0.000152, calculated with Student's t test). It should be noted that 8081 and 8081R−M+ do not differ in virulence (7).
FIG. 3.
Survival of DBA/2 mice after intragastric inoculation with different doses of Y. enterocolitica O:8 strains 8081, YeO8-U-GB, and 8081-c. Bars represent the survival of individual mice, and open bars indicate mice that died during the experiment. Bacterial doses are indicated on the left.
Experiment-to-experiment variation prevented reliable assessment of possible differences in virulence among YeO3, YeO3-U, and YeO3-U::pCGL1. Therefore, mice were coinfected with an equal mixture of either 109 YeO3 and 109 YeO3-U bacteria or 109 YeO3 and 109 YeO3-U::CGL1 bacteria and bacterial counts in different organs of the mice were determined after defined time points (Table 2). One hundred colonies were patched on kanamycin-containing plates to determine the percentages of YeO3-U bacteria in the total counts. At 2 and 4 days after infection, two or three mice were killed and their spleens, livers, and Peyer's patches were aseptically prepared and homogenized in 1, 3, and 0.5 ml of sterile phosphate-buffered saline, respectively. Y. enterocolitica from the homogenates and serial dilutions thereof were recovered on CIN agar without antibiotics. The percentage of YeO3-U in the recovered colonies was determined by patching 50 or all of the recovered colonies (if fewer than 50) onto kanamycin-containing plates. Another coinfection experiment was performed with strains YeO3 and YeO3-U::pCGL1, and the percentage screening was done with chloramphenicol-containing plates. At 2 days after the inoculation, high numbers of yersiniae were recovered from the Peyer's patches and spleens. Clearly fewer bacteria were recovered from the livers. At later time points, the number of bacteria increased in livers but the spleens were what most of the bacteria colonized.
TABLE 2.
Virulence of Y. enterocolitica O:3 strains for coinfected mice
| Strains and day postinfection | No. of mice | Mean no. of CFU/mg ± SEM (Kmr/Cmr %)a in:
|
||
|---|---|---|---|---|
| Peyer's patches | Spleen | Liver | ||
| YeO3 + YeO3-U Kmr | ||||
| 2 | 6 | 59,600 ± 46,900 (49 ± 21) | 75,700 ± 129,200 (31 ± 35) | 900 ± 1,540 (43 ± 37) |
| 4 | 8 | 78,900 ± 143,500 (35 ± 27) | 130,200 ± 176,600 (20 ± 24)b | 21,900 ± 55,900 (21 ± 24)c |
| YeO3 + YeO3-U::pCGL1 Cmr | ||||
| 2 | 8 | 70,600 ± 93,800 (62 ± 31) | 31,500 ± 69,800 (62 ± 34) | 4,500 ± 12,700 (56 ± 41) |
| 4 | 6 | 2,000 ± 2,600 (75 ± 25) | 25,500 ± 29,900 (69 ± 39) | 15,200 ± 21,100 (68 ± 43) |
The bacterial counts in mouse organs at different time points are means of three separate experiments in which the following bacterial mixtures were injected: experiment 1, a dose of 2.74 × 109 CFU/mouse consisting of 51% YeO3 and 49% YeO3-U; experiment 2, a dose of 2.6 × 109 CFU/mouse consisting of 40% YeO3 and 60% YeO3-U; experiment 3, a dose of 1.45 × 109 CFU/mouse consisting of 46% YeO3 and 54% YeO3-U. For YeO3 and YeO3-U::pCGL1, the corresponding bacterial mixtures were as follows: experiment 1, a dose of 3.8 × 109 CFU/mouse consisting of 42% YeO3 and 58% YeO3-U::pCGL1; experiment 2, a dose of 4.6 × 109 CFU/mouse consisting of 45% YeO3 and 55% YeO3-U::pCGL1; experiment 3, a dose of 2.6 × 109 bact/mouse consisting of 45% YeO3 and 55% YeO3-U::pCGL1.
P = 0.050 calculated by the Mann-Whitney test for a comparison of the percentage of antibiotic resistance at the time of sacrifice with the initial percentage.
P = 0.039.
The percentages of Kmr (YeO3-U) and Cmr (YeO3-U::pCGL1) bacteria in the different organs were determined from recovered colonies (Table 2). Two days after inoculation, YeO3-U and YeO3-U::pCGL1 were present in proportions approximately similar to that in the inoculum in all of the organs tested. The wild-type YeO3 bacteria had outcompeted the YeO3-U bacteria in spleens and livers on day 4 after inoculation, because the percentage of Kmr colonies was clearly diminished in spleens (P = 0.050) and livers (P = 0.039) compared to the percentages in the inoculum (statistics were calculated with the Mann-Whitney test). These results showed that urease activity is of importance for virulence when the bacteria are given intragastrically. The data also suggested that YeO3-U bacteria were defective in the ability to spread from the Peyer's patches into deeper tissues.
In the percentages of YeO3- and YeO3-U::pCGL1-infected mice, there were, on the other hand, no statistical differences between the 4-day samples and the inoculum, indicating that the YeO3-U::pCGL1 bacteria were as virulent as the wild-type YeO3 bacteria when administered orally to the mice. The plasmid-cured YeO3-c strain was, on the other hand, completely avirulent, as expected (data not shown).
Virulence in rats.
Adult male Lewis/SsNHsd rats weighing 225 to 275 g were purchased from Harlan Sprague-Dawley, Inc., Indianapolis, Ind. Since the rat model absolutely relies on pathogen-free rats (9, 10), the animals' health was monitored by both the breeder and the Microbiology Laboratories, North Harrow, Middlesex, England, and only rats free of the most common rat pathogens were accepted for the experiments. The rats were also free of Bacillus piliformis when examined by diagnostic provocation. The rats were kept on autoclaved bedding of aspen wood in Macrolon cages under filter tops. The cages were in laminar-flow hoods, and the rats were fed a standard autoclaved diet and water ad libitum.
Increasing doses of 8081-U-GB and 8081 bacteria were administered in a total volume of 100 to 200 μl into the tail veins of rats. During the injections, the rats were lightly anesthetized with methoxyflurane (Metofane; Pitman-Moore, Washington Crossing, N.J.). Virulence was estimated on the basis of the number of dead rats in each group after bacterial inoculation. The estimated LD50 of YeO8-U-GB is 3 × 108 bacteria (Table 3), while that of 8081 is about 6 × 107 bacteria (9), i.e., a difference of about fivefold.
TABLE 3.
Virulence and arthritogenicity of Y. enterocolitica O:8 strains in i.v. injected rats
| Bacterial strain and no. of bacteria injected | No. of rats injected | Virulence
|
Arthritogenicity
|
|||
|---|---|---|---|---|---|---|
| No. of rats dead | Days of death | No. of arthritic rats | Day of onseta | Severitya | ||
| 8081-U-GB | ||||||
| 3 × 109 | 3 | 3 | 1, 1, 1 | 0 | ||
| 6 × 108 | 4 | 2 | 1, 1 | 2 | 8.5 | 5.25 |
| 3 × 108 | 6 | 3 | 1, 2, 12 | 1 | 11 | 3.5 |
| 6 × 107 | 5 | 0 | 3 | 9 | 3.2 | |
| 5 × 107 | 5 | 0 | 5 | 9.4 | 3.3 | |
| 4 × 107 | 5 | 0 | 3 | 14 | 1.8 | |
| 9 × 106 | 5 | 0 | 2 | 16 | 3.25 | |
| 5 × 106 | 5 | 0 | 1 | 24 | 1 | |
| 8081 | ||||||
| 4 × 107 | 10 | 0 | 9 | 6.7 | 4.72 | |
| 2 × 107 | 5 | 0 | 3 | 12.3 | 2.1 | |
The mean arithmetic score was calculated from the maximum score observed for each arthritic animal (see the text for details). Mean values are shown. Only rats with arthritis were included.
Arthritogenicity in rats.
To assess arthritogenicity, rats were examined for the onset and severity of arthritis by two independent observers as described earlier (9). Briefly, each limb was examined every day at the beginning of the experiment and later every 2 days. Scores of 0 to 4 were assigned for each limb, 0 for negative and 4 for gross distortion with severe arthritic changes. Even though the virulence of 8081-U-GB was slightly decreased in rats after intravenous (i.v.) bacterial injection, we noted that the arthritogenicity of YeO8-U-GB was almost the same as that of 8081, without any statistically significant differences when calculated using Fisher's exact test (Table 3). YeO8-U-GB bacteria, at a dose range of 4 × 107 to 6 × 107 bacteria per rat, induced an arthritis incidence of 73% (11 of 15), which is very close to the 80% (12 of 15) caused by 2 × 107 to 4 × 107 8081 bacteria in this work or to the 75% (9 of 12) caused by 5 × 107 8081 bacteria reported in a previous study (9). The onset of arthritis was slightly but not significantly delayed among the rats injected with 8081-U-GB compared with the 8081-injected rats (mean onset, 10.6 ± 3.6 versus 9.6 ± 6.2 days). The severity of arthritis, on the other hand, was to some degree milder in YeO8-U-GB-injected rats than in 8081-injected rats (Table 3), with mean severities of 2.9 ± 1.7 and 4.1 ± 2.1, respectively.
In this study, we examined the role of urease in the virulence and arthritogenicity of Y. enterocolitica. The results demonstrated that urease seems to play a role in the virulence of orally infected mice or i.v. infected rats (Fig. 3 and Table 2). On the other hand, our results also showed that urease and the β subunit of urease are not needed for the induction of arthritis in the rat model.
The decrease in virulence after intragastric inoculation of the mutated strains indicates that the main role of urease is during the initial stage of the bacterial infection, when the bacteria reach the stomach and small intestine. This was detected not only by regular LD50 virulence tests of animals but also in the coinfection experiments by the ability of the Y. enterocolitica O:3 wild-type strain but not of the mutant to spread from Peyer's patches into deeper tissues. This is understandable when the fact that Y. enterocolitica is an invasive pathogen which enters the body via the gastrointestinal tract is taken into account.
Urease has been claimed to be a virulence factor in Y. enterocolitica primarily by enhancing the survival of bacteria during passage through the stomach and not by altering the pH of the macroenvironment but rather by generating enough ammonia from the hydrolysis of urea to maintain a suitable intracellular pH, helping the bacteria to tolerate acidic conditions (5). In a recent study, however, Riot et al. (22) showed that urease is not involved in the virulence of Y. pseudotuberculosis. Because there are several differences between Y. enterocolitica and Y. pseudotuberculosis, the results obtained by Riot et al. do not exclude the possibility that urease is involved in the virulence of Y. enterocolitica.
Y. enterocolitica O:3-injected mice behaved clearly differently from Y. enterocolitica O:8-injected mice after intragastric inoculation. Whether this is a question of differences generally seen in animal studies after Y. enterocolitica O:3 and O:8 injections remains unknown. Human-pathogenic Y. enterocolitica O:3 induces no fatal disease in experimental animals unless the animals are pretreated with desferrioxamine mesylate, in contrast to Y. enterocolitica O:8, which induces a dramatic disease picture, including joint disorders, in rats. Y. enterocolitica O:8, on the other hand, very seldom causes arthritis in humans. The Yersinia 19-kDa urease β subunit has, however, been used to study synovial T-cell response, indicating that this particular antigen still is considered to be of arthritogenic interest (16). Our experimental arthritis resembles human Yersinia-triggered ReA considerably more than does the model used by Mertz et al. (15). They induced arthritis by injecting the 19-kDa urease peptide intra-articularly into presensitized rats. It should be noted that similar arthritis can be induced by using a variety of cationic antigens, including methylated bovine serum albumin (19). In contradiction to what has earlier been speculated on the arthritogenicity of the urease β subunit, our results do not support the idea that the β subunit is responsible for the arthritogenic disorder that occurs in rats after Yersinia infection. Yersinia strain 8081-U-GB frequently induced arthritis after injection at several different bacterial doses. In addition, the joint swellings observed were of about the same severity as those seen after 8081 infection and the swelling tended to last for weeks. The primary conclusion of this study is that the Y. enterocolitica O:8 urease β subunit, however, does not play any role in the induction of arthritis after bacterial i.v. injection of rats.
Acknowledgments
This work was supported by grants from the Foundation for Swedish Culture in Finland, the Academy of Finland, the Sigrid Juselius Foundation, and the University of Turku Foundation.
REFERENCES
- 1.Ackermann B, Gohlke F, Herr W, Yu D T Y, Raybourne R, Meyer zum Büschenfelde K-H, Märker-Hermann E. Quantification of TNF-α and IFN-γ producing Yersinia urease β-subunit specific CD8+ and CD4+ T cells using an Elispot assay—a study in reactive arthritis. Arthritis Rheum. 1996;39:S163. [Google Scholar]
- 2.Ahvonen P, Sievers K, Aho K. Arthritis associated with Yersinia enterocolitica infection. Acta Rheumatol Scand. 1969;15:232–253. doi: 10.3109/rhe1.1969.15.issue-1-4.32. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard R K. Segregation of new lysogenic types during growth of doubly lysogenic strain derived from Escherichia coli K12. Genetics. 1954;39:440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 5.de Koning-Ward T F, Robins-Browne R M. Contribution of urease to acid tolerance in Yersinia enterocolitica. Infect Immun. 1995;63:3790–3795. doi: 10.1128/iai.63.10.3790-3795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning-Ward T F, Ward A C, Robins-Browne R M. Characterisation of the urease-encoding gene complex of Yersinia enterocolitica. Gene. 1994;145:25–32. doi: 10.1016/0378-1119(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 7.Gripenberg-Lerche, C., and M. Skurnik. Unpublished data.
- 8.Gripenberg-Lerche C, Skurnik M, Toivanen P. Role of YadA-mediated collagen binding in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun. 1995;63:3222–3226. doi: 10.1128/iai.63.8.3222-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gripenberg-Lerche C, Skurnik M, Zhang L, Söderström K-O, Toivanen P. Role of YadA in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun. 1994;62:5568–5575. doi: 10.1128/iai.62.12.5568-5575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gripenberg-Lerche C, Toivanen P. Yersinia associated arthritis in SHR rats: effect of the microbial status of the host. Ann Rheum Dis. 1993;52:223–228. doi: 10.1136/ard.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann E. Enterobacterial antigens with tropism for joint structures and HLA-B27-restricted cytotoxic T-cells in reactive arthritis. Scand J Rheumatol. 1995;24:S203–S206. doi: 10.3109/03009749509100929. [DOI] [PubMed] [Google Scholar]
- 12.Kinder S A, Badger J L, Bryant G O, Pepe J C, Miller V L. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R−M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- 13.Laird W J, Cavanaugh D C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980;11:430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merilahti-Palo R, Gripenberg-Lerche C, Söderström K-O, Toivanen P. Long term follow up of SHR rats with experimental yersinia associated arthritis. Ann Rheum Dis. 1992;51:91–96. doi: 10.1136/ard.51.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertz A K H, Batsford S R, Curschellas E, Kist M J, Gondolf K B. Cationic Yersinia antigen-induced chronic allergic arthritis in rats. A model for reactive arthritis in humans. J Clin Investig. 1991;88:632–642. doi: 10.1172/JCI115348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertz A K H, Ugrinovic S, Lauster R, Wu P, Grolms M, Böttcher U, Appel H, Yin Z, Schiltz E, Batsford S, Schauer-Petrowski C, Braun J, Distler A, Sieper J. Characterization of the synovial T cell response to various recombinant Yersinia antigens in Yersinia enterocolitica-triggered reactive arthritis. Heat-shock protein 60 drives a major immune response. Arthritis Rheum. 1998;41:315–326. doi: 10.1002/1529-0131(199802)41:2<315::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepe J C, Badger J L, Miller V L. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol. 1994;11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 19.Pettipher E R, Blake S. Antigen-induced arthritis. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press Limited; 1995. pp. 457–470. [Google Scholar]
- 20.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Probst P, Hermann E, Meyer zum Büschenfelde K-H, Fleischer B. Identification of the Yersinia enterocolitica urease β subunit as target antigen for human synovial T lymphocytes in reactive arthritis. Infect Immun. 1993;61:4507–4509. doi: 10.1128/iai.61.10.4507-4509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riot B, Berche P, Simonet M. Urease is not involved in the virulence of Yersinia pseudotuberculosis in mice. Infect Immun. 1997;65:1985–1990. doi: 10.1128/iai.65.5.1985-1990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 24.Skurnik M. Lack of correlation between the presence of plasmids and fimbriae in Yersinia enterocolitica and Yersinia pseudotuberculosis. J Appl Bacteriol. 1984;56:355–363. doi: 10.1111/j.1365-2672.1984.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 25.Skurnik M, Batsford S, Mertz A, Schiltz E, Toivanen P. The putative arthritogenic cationic 19-kilodalton antigen of Yersinia enterocolitica is a urease β-subunit. Infect Immun. 1993;61:2498–2504. doi: 10.1128/iai.61.6.2498-2504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skurnik M, Venho R, Toivanen P, Al-Hendy A. A novel locus of Yersinia enterocolitica serotype O:3 involved in lipopolysaccharide outer core biosynthesis. Mol Microbiol. 1995;17:575–594. doi: 10.1111/j.1365-2958.1995.mmi_17030575.x. [DOI] [PubMed] [Google Scholar]
- 27.Toivanen A, Merilahti-Palo R, Gripenberg C, Lahesmaa-Rantala R, Söderström K-O, Jaakkola U-M. Yersinia-associated arthritis in the rat: experimental model for human reactive arthritis? Acta Pathol Microbiol Immunol Scand Sect C Immunol. 1986;94:261–269. doi: 10.1111/j.1699-0463.1986.tb02121.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Radziejewska-Lebrecht J, Krajewska-Pietrasik D, Toivanen P, Skurnik M. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol Microbiol. 1997;23:63–76. doi: 10.1046/j.1365-2958.1997.1871558.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Skurnik M. Isolation of an R− M+ mutant of Yersinia enterocolitica serotype O:8 and its application in construction of rough mutants utilizing mini-Tn5 derivatives and lipopolysaccharide-specific phage. J Bacteriol. 1994;176:1756–1760. doi: 10.1128/jb.176.6.1756-1760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]