Abstract
A toxigenic non-O1/non-O139 strain of Vibrio cholerae (10259) was found to contain a new variant of the toxin-coregulated pilus (TCP) protein gene (tcpA) as determined by PCR and Southern hybridization experiments. Nucleotide sequence analysis data of the new tcpA gene in strain 10259 (O53) showed it to be about 74 and 72% identical to those of O1 classical and El Tor biotype strains, respectively. The predicted amino acid sequence of the 10259 TcpA protein shared about 81 and 78% identity with the corresponding sequences of classical and El Tor TcpA strains, respectively. An antiserum raised against the TCP of a classical strain, O395, although it recognized the TcpA protein of strain 10259 in an immunoblotting experiment, exhibited considerably less protection against 10259 challenge compared to that observed against the parent strain. Incidentally, the tcpA sequences of two other toxigenic non-O1/non-O139 strains (V2 and S7, both belonging to the serogroup O37) were determined to be almost identical to that of classical tcpA. Further, tcpA of another toxigenic non-O1/non-O139 strain V315-1 (O nontypeable) was closely related to that of El Tor tcpA. Analysis of these results with those already available in the literature suggests that there are at least four major variants of the tcpA gene in V. cholerae which probably evolved in parallel from a common ancestral gene. Existence of highly conserved as well as hypervariable regions within the sequence of the TcpA protein would also predict that such evolution is under the control of considerable selection pressure.
In order to establish infection, Vibrio cholerae must enter into the host by the oral route and reach the gut where it colonizes through intestinal attachment (adhesion) and a subsequent multiplication process. Intestinal colonization is believed to be mediated by colonization factors expressed by vibrios, the best characterized of which is the toxin-coregulated pilus (TCP) (27). Expression of TCP and cholera toxin (CT) is coordinately regulated by the toxR regulon. The TCP is composed of a major 20-kDa subunit protein called TcpA, the amino acid sequence of which shows homology with type 4 pilus proteins expressed by other pathogenic microorganisms (23). A considerable difference in the epitope or antigenic structure of TcpA between classical and El Tor biotype strains of V. cholerae O1 has been well documented (7), despite the fact that the amino acid sequence of TcpA from an El Tor strain shows extensive homology (about 82%) with that of a classical biotype strain (6, 20). Further, the optimum cultural condition for TCP or TcpA expression has been found to be somewhat different for classical and El Tor biotype strains (8, 27, 28). The molecular mechanism of TCP biosynthesis probably involves many of the genes present in the tcp gene cluster (11, 18), another unlinked gene, tcpG (19), and the toxR regulon. The gene cluster encoding TCP, an accessory colonization factor, the virulence gene regulator ToxT, and certain other genes are located within the V. cholerae pathogenicity island (VPI) commonly associated with the epidemic strains of V. cholerae (9) as a result of VPIΦ acquisition (10).
Epidemic-causing strains of V. cholerae O139 possess the tcpA gene (5) with sequences identical to those of El Tor biotype strains (20). Until recently, non-O1/non-O139 strains have been shown to lack the tcpA gene (26), although the majority of these contain toxR sequences and some, at least, also possess genes for CT (ctxAB) (15). Recent studies (4, 21), however, have demonstrated the presence of the tcpA gene in certain non-O1/non-O139 V. cholerae strains. These observations assume considerable significance in view of the fact that TCP acts as a receptor for filamentous bacteriophage (designated CTX φ) carrying the CTX genetic element (29).
In the present study, we have investigated the tcpA gene of certain strains of non-O1/non-O139 V. cholerae with a probe to determine its relatedness to tcpA of classical and El Tor biotype strains. In the process, we have identified and characterized a new variant of TcpA in a toxigenic non-O1/non-O139 strain.
V. cholerae strains used in this study are listed in Table 1. Strain S7, belonging to serogroup O37, was kindly provided to us by Elisabeth Bik. A multiplex PCR assay was used for the detection of tcpA (classical and El Tor biotype) and ctxA of V. cholerae strains using three primer pairs (sense and antisense) designed by Keasler and Hall (12). Briefly, all three primer pairs were added to the PCR mixture of a given strain for the simultaneous generation of amplified products of its tcpA (either of classical or El Tor biotype) and/or ctxA. Identification of amplified products was based on the determination of their sizes following electrophoresis in an agarose gel (12). A tcpA gene probe of 2 kb was prepared from the plasmid pSC18.1 (2) by digestion with HindIII. The probe contained the entire tcpA gene (classical) along with its upstream (1-kb) and downstream (0.4-kb) flanking regions (23).
TABLE 1.
V. cholerae strainsa used in this study
| Strain | Serogroup (biotype) | Reference |
|---|---|---|
| O395 | O1 (classical) | 13 |
| AD48 | O1 (El Tor) | 4 |
| SG25 | O139 | 16 |
| V2 | O37 | 3 |
| S7 | O37 | 1 |
| 10259 | O53b | 3 |
| V315-1 | ONTc | 4 |
Toxigenic clinical strains.
Earlier typed as O59 by Smith serotyping scheme (3).
ONT, O nontypeable.
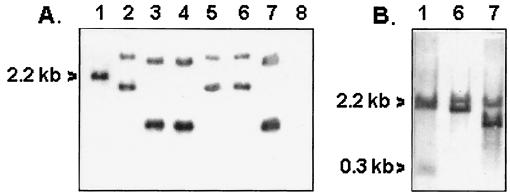
Figure 1 shows Southern hybridization band patterns of PstI-digested chromosomal DNA preparations of tcpA probe positive non-O1/non-O139 V. cholerae strains. Similar data generated with O1 and O139 strains are included in the figure for comparison. While strains V2 and S7 (both belonging to the same serogroup, O37) gave patterns similar to that of the classical strain (O395), strain V315-1 (ONT) showed a pattern identical to those of the El Tor (AD48) and O139 (SG25) strains (Fig. 1A). The other non-O1/non-O139 strain, 10259 (O53), produced only a single band of about 2.2 kb under similar experimental conditions, although an additional band of about 300 bp could be detected when the PstI-digested material was electrophoresed for a shorter period and the blot developed under less stringent conditions (Fig. 1B). However, the band pattern was quite different from those of classical and El Tor strains. The specificity of bands obtained in Southern hybridization experiments was further established by using the 0.6-kb PCR amplicon (derived from the classical strain, O395) as a tcpA probe (data not shown). It may be mentioned here that strain 10259 yielded an amplicon in the classical position with classical primers only but not in the multiplex PCR assay which was used in an earlier study (4).
FIG. 1.
Southern hybridization band patterns of PstI-digested chromosomal DNA preparations of V. cholerae strains developed with a tcpA probe. Strains used were 10259 (lane 1), V315-1 (lane 2), V2 (lane 3), S7 (lane 4), SG25 (lane 5), AD48 (lane 6), O395 (lane 7), and a negative control (lane 8). Digested material was electrophoresed for 4 h (A) or 2 h (B) and developed with the probe using high (A) or low (B) stringent conditions. Band positions of 10259 digest are indicated.
The unusual band pattern observed with strain 10259 and its failure to respond to multiplex PCR assay suggested alterations in its nucleotide sequence within and/or around the tcpA gene. Therefore, the nucleotide sequence of tcpA was determined by analyzing the tcpA amplicon (617 bp) generated from strain 10259 using the classical primer pair 5′-CACGATAAGAAAACCGGTCAAGAG-3′ (sense) and 5′-ACCAAATGCAACGCCGAATGGAGC-3′ (antisense). Amplicons derived from three different PCRs were independently purified by a QIAquick gel extraction kit (Qiagen) and sequenced by an automated DNA sequenator (Applied Biosystems) using the end primers as well as two other internal primers of the following sequences: 5′-AATGCCGCTGGTAATAAAGC-3′ (sense) and 5′-CAATGCAATAGCTGATTTC-3′ (antisense). A comparison of nucleotide sequence data shows that 10259 tcpA shares about 74 and 72% identity with those of classical and El Tor biotype strains, respectively (Fig. 2). Further, the single PstI site in this tcpA is shifted 12 bp downstream with respect to the PstI site of the classical strain. This data along with the band pattern in the Southern hybridization experiment (Fig. 1) would also predict the shifting or generation of new PstI sites in both upstream and downstream regions flanking the tcpA gene of 10259. The predicted amino acid sequence of the 10259 TcpA protein shows about 81 and 78% identity with TcpA sequences of classical and El Tor biotype strains, respectively (Fig. 3). However, some of the amino acid changes in 10259 TcpA appear to be conservative in nature compared with the corresponding TcpA sequence of either classical or El Tor biotype strains. This is also reflected in the overall similarity of the hydropathicity profile plots of the three variant proteins (data not shown). An interesting feature of the 10259 TcpA sequence is the existence of an alanine-rich hydrophobic region (e.g., residues 61 to 68) which is less pronounced in classical and El Tor TcpA proteins.
FIG. 2.
Nucleotide sequence of the tcpA gene of the toxigenic non-O1/non-O139 V. cholerae strain 10259 (II). The sequence is compared with those of O1 classical (I), El Tor (III), and 151/208 (NAG) (IV), indicating only the differences which occur in the later sequences. The PstI sites are indicated by arrow heads. GenBank accession numbers of I, II, III, and IV are M33514, AF139626, U 09807, and AF030309, respectively.
FIG. 3.
The predicted amino acid sequence of 10259 TcpA (II) and its comparison with the corresponding sequences of O1 classical (I), El Tor (III), and 151/208 (NAG) (IV) strains. Identical amino acids are shaded black, and conservative changes with respect to the 10259 sequence are shaded gray.
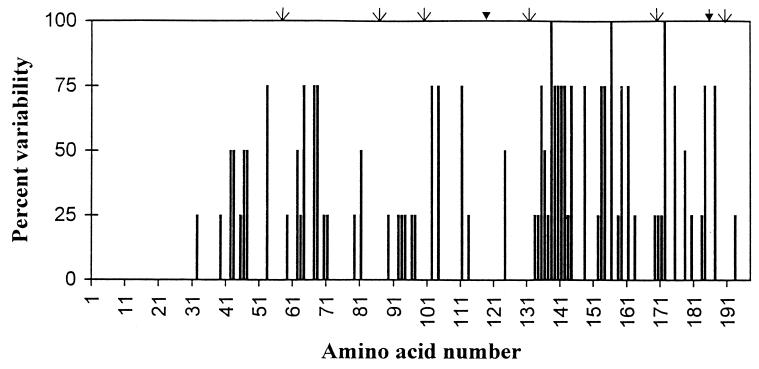
A new type of tcpA sequence from two nontoxigenic non-O1/non-O139 V. cholerae strains, 151/208 (NAG), which differs significantly from those of classical and El Tor tcpA sequences has recently been published (17). However, the 10259 tcpA gene differs considerably from the 151/208 (NAG) sequence, showing about 73% identity at the nucleotide level (Fig. 2) and 79% identity at the amino acid level (Fig. 3). Thus, the 10259 tcpA gene appears to have diverged as much from 151/208 (NAG) tcpA as from both classical and El Tor tcpA genes. The variability in the predicted amino acid sequences of the four variants of TcpA is analyzed in Fig. 4. All the variants show conservation of amino acids at their amino terminal ends as well as in certain other positions which are critically important for the mediation of the biological function(s) common to these molecules (24). Analysis of data also reveals that the sequence variations are primarily located in the C-terminal half of the protein, more particularly within or around the disulfide loop formed by conserved cysteine residues at positions 120 and 186. Further, sequence variations among these proteins are more pronounced in certain regions (e.g., between amino acids 133 and 144 and 152 and 163) than in other regions. The variability is 100% at positions 138, 156, and 172. Interestingly, all four variants have conserved proline residues at positions 58, 87, 99, 132, 169 (except 10259 TcpA, which has a threonine), and 191. All these considerations would suggest the existence of at least four major variants of tcpA genes which probably evolved in parallel, though independently, from a common ancestral gene. The existence of highly conserved as well as hypervariable regions within the sequence of the TcpA protein would also predict that such evolution is under the control of considerable selection pressure.
FIG. 4.
Diagrammatic (solid lines) representation of variability in the amino acid sequences among four major variants of TcpA. The positions of the conserved cysteine and proline residues are indicated by thick and thin arrows, respectively.
Polyclonal as well as monoclonal antibodies raised against TCP from classical vibrios established a biotype-specific epitope difference in TcpA (7, 25). Such epitopes are primarily located within or around the disulfide loop in the C-terminal half of TcpA which contain some of the variable regions described here (Fig. 4). These regions were also shown to be immunodominant in nature and possess biotype-specific protective epitopes (8, 20, 25). Interestingly, an antiserum raised against the TCP of classical strain O395, although it recognized the TcpA protein of strain 10259 in the immunoblotting experiment (data not shown), exhibited considerably less protection against 10259 challenge compared to that observed against the parent strain O395 in passive protection experiments (Table 2). All these data appear to suggest that the variability in the immunodominant C-terminal domains of TcpA leads to antigenic variation which may be significant enough to affect the level of cross-protection among strains carrying different variants of TcpA. Building up of a theoretical model of TcpA and the pilus fiber (accession numbers PDB ID 1QQZ and RCSB 001169) revealed that the majority of the hypervariable region residues are indeed located along the surface of the fiber, making these readily accessible to antibodies (R. Chattopadhyaya and A. C. Ghose, unpublished data). It may be mentioned that pili of Neisseria gonorrhoeae are known to undergo antigenic variation through genetic alterations of the hypervariable regions of its structural protein PilE, which allows the bacteria to escape recognition by the immune system of the host (14). However, it is quite possible that variations in TcpA may have other functional significance as well, particularly in light of the recent report (10) that TcpA also acts as a coat protein of the bacteriophage VPIΦ, produced by vibrios containing VPI.
TABLE 2.
Protective activity of anti-TCP seruma against challenge with V. cholerae strains in the suckling mouse model
| V. cholerae challenge strainb | Preincubated with | Dilution of antiserumc | No. of survivors/ no. challengedd (% protection) |
|---|---|---|---|
| O395 | Normal saline | 0/6 (0) | |
| Preimmune serum | 1:40 | 0/6 (0) | |
| Anti-TCP serum | 1:40* | 5/6 (83.3) | |
| 1:80** | 3/6 (50.0) | ||
| 1:160 | 2/6 (33.3) | ||
| 10259 | Normal saline | 0/6 (0) | |
| Preimmune serum | 1:20 | 0/6 (0) | |
| Anti-TCP serum | 1:20** | 3/6 (50.0) | |
| 1:40*** | 1/6 (16.6) | ||
| 1:80 | 0/6 (0) |
An antiserum was raised by immunization of a rabbit with whole cells of strain O395 grown under a TCP-expressing condition (in colonization factor antigen agar; pH 6.5; 25°C for 36 h). It was rendered specific for TCP by absorption with O395 whole cells (boiled) grown under TCP nonexpressing condition (in Tris-buffered medium; pH 7.2; 37°C for 18 h). Residual antilipopolysaccharide activity in the antiserum was removed by absorption with purified lipopolysaccharide of O395.
Each mouse was challenged with about 10 times the 50% lethal dose of strains O395 (5 × 107 CFU) or 10259 (2 × 108 CFU) in 0.1 ml with or without the antiserum.
*, P < 0.01; **, 0.05 < P < 0.1; ***, P > 0.1; when compared against corresponding preimmune serum (Fisher's exact test).
Six mice were included in each group, and the percent protection was determined as the number of survivors after 24 h of challenge over the number of mice challenged in each group.
Nucleotide sequence data of tcpA from strains V2 and S7 and their comparison with that of the classical tcpA gene showed only three nucleotide changes (data not shown). On the other hand, the partial tcpA sequence (538 bp) of V315-1 was found to be closely similar to that of the El Tor strains, with changes only in five nucleotide positions (data not shown). These results suggest that non-O1 V. cholerae strains may possess the tcpA gene with diverse sequences. Thus, some of these may contain sequences almost identical to those of classical strains (e.g., V2 and S7), while others are identical to those of El Tor strains (e.g., O139) (5, 20). Since O139 strains are likely to be evolved from an O1 El Tor strain, it would be tempting to speculate that strains V2 and S7 may have a classical origin. It may be mentioned here that strain S7, which shows similarity with V2 by belonging to the same serogroup, O37, and sharing other characteristics, was found to be closely related to O1 classical strains and caused a large cholera outbreak in Sudan in 1968 (1).
A study (29) has shown that TcpA plays an important role in phage-mediated acquisition of the CTX genetic element in V. cholerae strains. Strain 10259, carrying the new variant of tcpA, contained ctxAB, zot, ace, and the RS element (which form the CTX genetic element), as well as the regulatory element toxR (4). The strain also colonized well in the suckling mouse model (22) and produced CT both in vitro and in vivo (3, 22). Although we have yet to analyze the complete tcp gene cluster in the toxigenic non-O1/non-O139 strains harboring tcpA, the demonstration of the tcpA probe positive 5-kb fragments in the Southern hybridization experiment carried out with the XbaI-digested chromosomal DNA of all these strains (data not shown) would suggest the existence of such a cluster (26), which is essential for TCP biosynthesis.
Acknowledgments
The work is supported by grants from the Council of Scientific & Industrial Research, Government of India.
REFERENCES
- 1.Bik E M, Gouw R D, Mooi F R. DNA fingerprinting of Vibrio cholerae strains with a novel insertion sequence element: a tool to identify epidemic strains. J Clin Microbiol. 1996;34:1453–1461. doi: 10.1128/jcm.34.6.1453-1461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang S L, Taylor R K, Koomey M, Mekalanos J J. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 3.Datta-Roy K, Banerjee K, De S P, Ghose A C. Comparative study of expression of hemagglutinins, hemolysins, and enterotoxins by clinical and environmental isolates of non-O1 Vibrio cholerae in relation to their enteropathogenicity. Appl Environ Microbiol. 1986;52:875–879. doi: 10.1128/aem.52.4.875-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh C, Nandy R K, Dasgupta S K, Nair G B, Hall R H, Ghose A C. A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR and other virulence factors in non-O1/non-O139 Vibrio cholerae. Microb Pathog. 1997;22:199–208. doi: 10.1006/mpat.1996.0105. [DOI] [PubMed] [Google Scholar]
- 5.Hall R H, Khambaty F M, Kothary M, Keasler S P. Non-O1 Vibrio cholerae. Lancet. 1993;342:430. doi: 10.1016/0140-6736(93)92839-l. [DOI] [PubMed] [Google Scholar]
- 6.Iredell J R, Manning P A. Biotype-specific tcpA genes in Vibrio cholerae. FEMS Microbiol Lett. 1994;121:47–54. doi: 10.1111/j.1574-6968.1994.tb07074.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonson G, Holmgren J, Svennerholm A M. Epitope differences in toxin-coregulated pili produced by classical and El Tor Vibrio cholerae O1. Microb Pathog. 1991;11:179–188. doi: 10.1016/0882-4010(91)90048-f. [DOI] [PubMed] [Google Scholar]
- 8.Jonson G, Holmgren J, Svennerholm A M. Analysis of expression of toxin-coregulated pili in classical and El Tor Vibrio cholerae O1 in vitro and in vivo. Infect Immun. 1992;60:4278–4284. doi: 10.1128/iai.60.10.4278-4284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaolis D K R, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaolis D K R, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman M R, Shaw C E, Jones I D, Taylor R K. Biogenesis and regulation of the Vibrio cholerae toxin-coregulated pilus: analogies to other virulence factor secretory systems. Gene. 1993;126:43–49. doi: 10.1016/0378-1119(93)90588-t. [DOI] [PubMed] [Google Scholar]
- 12.Keasler S P, Hall R H. Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet. 1993;341:1661. doi: 10.1016/0140-6736(93)90792-f. [DOI] [PubMed] [Google Scholar]
- 13.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 14.Meyer T F, Gibbs C P, Hass R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- 15.Morris J G., Jr . Non-O group 1 Vibrio cholerae strains not associated with epidemic disease. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 103–115. [Google Scholar]
- 16.Nandy R K, Sengupta T K, Mukhopadhyay S, Ghose A C. A comparative study of the properties of Vibrio cholerae O139, O1 and other non-O1 strains. J Med Microbiol. 1995;42:251–257. doi: 10.1099/00222615-42-4-251. [DOI] [PubMed] [Google Scholar]
- 17.Novais R C, Coelho A, Salles C A, Vicente A C. Toxin-co-regulated pilus cluster in non-O1, non-toxigenic Vibrio cholerae: evidence of a third allele of pilin gene. FEMS Microbiol Lett. 1999;171:49–55. doi: 10.1111/j.1574-6968.1999.tb13411.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogierman M A, Zabihi S, Mourtzios L, Manning P A. Genetic organization and sequence of the promoter-distal region of the tcp gene cluster of Vibrio cholerae. Gene. 1993;126:51–60. doi: 10.1016/0378-1119(93)90589-u. [DOI] [PubMed] [Google Scholar]
- 19.Peek J A, Taylor R K. Characterization of a periplasmic thiol: disulphide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci USA. 1992;89:6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 21.Said B, Smith H R, Scotland S M, Rowe B. Detection and differentiation of the gene for toxin co-regulated pili (tcpA) in Vibrio cholerae non-O1 using the polymerase chain reaction. FEMS Microbiol Lett. 1995;125:205–210. doi: 10.1111/j.1574-6968.1995.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta T K. Identification and immunological characterisation of cell surface proteins responsible for intestinal adhesion and colonization of diarrhoeagenic non-O1 Vibrio cholerae. Ph.D. thesis. Calcutta, India: University of Calcutta; 1995. [Google Scholar]
- 23.Shaw C E, Taylor R K. Vibrio cholerae O395 tcpA pilin gene sequence and comparison of predicted protein structural features to those of type 4 pilins. Infect Immun. 1990;58:3042–3049. doi: 10.1128/iai.58.9.3042-3049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Lafferty M J, Peek J A, Taylor R K. Domains within Vibrio cholerae toxin coregulated pilin subunit that mediate bacterial colonization. Gene. 1997;192:79–85. doi: 10.1016/s0378-1119(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 25.Sun D, Seyer J M, Kovari I, Sumrada R A, Taylor R K. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect Immun. 1991;59:114–118. doi: 10.1128/iai.59.1.114-118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor R, Shaw C, Peterson K, Spears P, Mekalanos J. Safe, live Vibrio cholerae vaccines? Vaccine. 1988;6:151–154. doi: 10.1016/s0264-410x(88)80019-7. [DOI] [PubMed] [Google Scholar]
- 27.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss E, Attridge S R. In vitro production of toxin-coregulated pili by Vibrio cholerae El Tor. Microb Pathog. 1993;15:255–268. doi: 10.1006/mpat.1993.1076. [DOI] [PubMed] [Google Scholar]
- 29.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]