Abstract
Objective
The purpose of this study was to elaborate the characteristics of paraspinal muscles in lower lumbar, to compare the differences of paraspinal muscle between patients with lumbar spinal stenosis and normal people and to explore the influencing factors of paraspinal muscle degeneration in patients with lumbar spinal stenosis.
Method
The 39 pairs of patients and normal people were selected by propensity score matching. The differences of multifidus muscle and erection spine muscle parameters between the two groups were compared by independent-samples t-test and the relationship between age, paraspinal muscle degeneration and other factors in patients with lumbar spinal stenosis was analyzed by Pearson or Spearman correlation analysis.
Result
The general conditions of the two groups (patients with lumbar spinal stenosis and normal people) were well matched. There were significant differences in the relative fatty cross sectional area, fatty infiltration and relative signal intensity of multifidus muscle at L3 level. The fatty infiltration and relative signal intensity of multifidus muscle at L4 level and the relative signal intensity of multifidus muscle at L5 level were also significantly different. For male, the relative fatty cross sectional area, the fatty infiltration and relative signal intensity of multifidus muscle in patients were higher than those in healthy peers. For female, the relative signal intensity of multifidus muscle in patients was higher, too. In patients group, age was significantly correlated with the relative fatty cross sectional area, fatty infiltration and relative signal intensity of multifidus muscle and erector spinae muscle. Weight and BMI were significantly correlated with the relative total cross-sectional area of erector spinae muscle. The fatty infiltration increased more significantly with age in patients than that in normal people.
Conclusion
The change rules of paraspinal muscles in patients with lumbar spinal stenosis are similar to those in normal people. The degeneration of paraspinal muscle in patients with lumbar spinal stenosis was more severe than that in normal people, mostly in multifidus muscle. The paraspinal muscle degeneration was related to age in patients, and the effect of age on atrophy of paraspinal muscle was greater than that of normal people.
Keywords: multifidus, erector spinae muscle, paraspinal muscle degeneration, normal people, lumbar spinal stenosis
Introduction
With the aging of the population, the incidence of lumbar degenerative diseases is gradually increasing. Recently, many studies focused on the degeneration of paraspinal muscle in lumbar degenerative diseases (1–6), because paraspinal muscle plays an important role in maintaining stability. Ogon studied the degeneration of paraspinal muscles in 40 pairs of patients with chronic nonspecific low back pain and lumbar spinal stenosis (7). Other studies also investigated the degeneration of paraspinal muscle in patients with low back pain (8, 9) and lumbar degenerative kyphosis (3, 10).
Lumbar spinal stenosis (LSS) is one of the most common lumbar degenerative diseases (11), which is associated with high social and economic burden (12). Many researches also explored the degeneration of paraspinal muscle in patients with LSS (5, 13, 14). Yagi investigated the degeneration of paraspinal muscle in patients with both simple LSS and degenerative scoliosis combined with LSS. By analyzing the data of 60 pairs of female patients, they found that the cross-sectional area of multifidus muscle was significantly smaller in patients with degenerative scoliosis combined with LSS than that in patients with simple LSS (15). Another study found that the decrease of cross-sectional area and atrophy in multifidus muscle was associated with poorer outcome in patients with LSS (16), while it only measured the parameters of multifidus muscle. Although these researches investigated the degeneration of paraspinal muscle in patients with LSS, the difference of paraspinal muscle between normal people and patients with lumbar spinal stenosis was unclear.
Previous studies focused on the degeneration of paraspinal muscles, especially in lower lumbar, while their results were not comparable due to the differences of measurements (17–20). Some studies measured paraspinal muscle parameters at single level of lumbar (13, 14, 21), while others measured at multi-levels (4, 10, 17). The parameters they measured were also different from each other. There lacked a study detailing paraspinal muscle parameters.
So the purpose of this study was to elaborate the characteristics of paraspinal muscles in lower lumbar, to compare the differences of paraspinal muscle between patients with LSS and normal people and to explore the influencing factors of paraspinal muscle degeneration in patients with LSS.
Method
General information
This study was approved by the Ethics Committee of Peking University Third Hospital. There were 93 patients in our study, who were diagnosed as lumbar spinal stenosis and underwent posterior lumbar decompression and fusion surgery from October 2018 to June 2019. The inclusion criteria were (i) age was from 50 to 80 years old, (ii) diagnosed as lumbar spinal stenosis, (iii) undertook lumbar MRI test. The exclusion criteria were (i) with other spinal diseases, (ii) with a history of spinal surgery, (iii) with neuromuscular diseases, (iiii) lumbar MRI was uncomplete. Control group included 45 normal middle-aged and elderly people, who were prospectively recruited from February 2020 to November 2020. The inclusion criteria were (i) age was from 50 to 80 years old, (ii) without spinal diseases, (iii) without spinal surgery, (iiii) without low back pain and trauma in past 3 months. The exclusion criteria were (i) with neuromuscular diseases, (ii) with MRI contraindications. All the normal people signed the informed consent forms.
Clinical measurements
The data of both patients and normal people was recorded, including the age, gender, height, weight and history of hypertension or diabetes. All patients underwent lumbar magnetic resonance imaging (MRI) within 1 month before surgery. All normal people underwent lumbar MRI within 1 month before we measured the parameters.
Measurements of the multifidus (MF) and erector spinae muscle (ES) were obtained from T2-weighted images by Image J software. MRIs were required with Signa HDxt 3.0T (General Electric Company, USA). Patients were placed in the supine position, with their legs straight and the lumbar spine in a neutral posture. Axial MRI was parallel to the inferior endplate of the vertebral body. All muscles were measured bilaterally at the inferior vertebral endplate of L3 to L5. The mean value of left and right paraspinal muscle was calculated. Region of interest was used to measure muscular parameters, including: total cross-sectional area (tCSA), fatty cross-sectional area (fCSA), fatty infiltration (FI) and signal intensity (SI). The fCSA was defined as the area of fatty tissue in muscle, which was measured by the thresholding technique. The FI was defined as the ratio of fCSA to tCSA. They reflected the degeneration of paraspinal muscles.
In order to reduce the influence of height, weight and body size on paraspinal muscle parameters, we calculated the relative cross-sectional area (rCSA) and relative signal intensity (rSI). The relative total cross-sectional area (rtCSA) was defined as the ratio of cross-sectional area of paraspinal muscle to cross-sectional area of vertebral body. The relative fatty cross-sectional area (rfCSA) was defined as the ratio of cross-sectional area of fatty tissue to cross-sectional area of vertebral body. The relative signal intensity (rSI) was defined as the ratio of signal intensity of paraspinal muscle to signal intensity of subcutaneous fat.
Statistical analysis
SPSS version 22.0 (IBM company, USA) was used to analyze the collected data. By using propensity score matching, we matched 93 patients with LSS and 45 normal people at a ratio of 1:1. The matching model we used was logistic regression model. To get a good matching score, the influencing factors such as age, gender, height, weight and body mass index (BMI) were used as matching indexes. The values were expressed as mean ± standard deviation. Age, BMI and paraspinal muscle parameters were continuous variable while gender was categorical variable. We measured the paraspinal muscle parameters at a level of L3 to L5 and analyzed the change rule of paraspinal muscle parameters. The differences of paraspinal muscle parameters between patients with LSS and normal people were compared. The independent sample t-test or rank sum test were used to explore the difference of continuous variables between the two groups, while the chi-square test was used to analyze the difference of categorical variables. Correlations between measurements of paraspinal muscle and other factors were investigated by Pearson correlation analysis or Spearman correlation analysis. Statistical significance was set at p-value < 0.05.
Results
By using propensity score matching, we matched 39 pairs of patients with LSS and normal middle-age and elderly people well. As showed in Table 1 , there were 18 males and 21 females in patients group. The average age of patients was 62.9 ± 7.8 years. 10 patients were diagnosed as hypertension and 4 patients were diagnosed as diabetes. In normal middle-age and elderly people group (normal group), there were 16 males and 23 females. The average age of normal people was 62.1 ± 7.3 years. 10 patients were diagnosed as hypertension and 6 patients were diagnosed as diabetes. There was no significant difference in age, gender, height, weight, BMI and comorbidities between the two groups, indicating that the two groups were matched well.
Table 1.
The basic information of two groups.
| Parameters | Patients group | Normal group | P-value |
|---|---|---|---|
| Age (years) | 62.9 ± 7.8 | 62.1 ± 7.3 | 0.666 |
| Gender (M/F) | 18/21 | 16/23 | 0.648 |
| Height (cm) | 163.7 ± 8.7 | 163.2 ± 9.1 | 0.767 |
| Weight (kg) | 68.2 ± 10.3 | 66.1 ± 11.8 | 0.502 |
| BMI (kg/m2) | 25.4 ± 2.9 | 24.7 ± 2.9 | 0.298 |
| Hypertension (Y/N) | 10/29 | 10/29 | 1.00 |
| Diabetes (Y/N) | 4/35 | 6/33 | 0.735 |
BMI, body mass index.
To explore the differences in paraspinal muscles between patients with LSS and normal middle-aged and elderly people, we measured the praspinal muscle parameters, including rtCSA, rfCSA, FI and rSI. The results were recorded in Table 2 . Compared with the normal group, the change rules of paraspinal muscle in the patient group were similar. From top to bottom of the spinal axis, the relative cross-sectional area of MF increases, while that of ES decreases. The FI and rSI of MF and ES increased gradually.
Table 2.
The comparison of paraspinal muscle parameters at L3 to L5 levels.
| Parameters | Patients group | Normal group | P-value |
|---|---|---|---|
| L3 | |||
| MF rtCSA | 0.96 ± 0.30 | 0.94 ± 0.17 | 0.731 |
| MF rfCSA | 0.32 ± 0.17 | 0.27 ± 0.15 | 0.042* |
| ES rtCSA | 2.60 ± 0.62 | 2.71 ± 0.43 | 0.385 |
| ES rfCSA | 0.56 ± 0.24 | 0.59 ± 0.22 | 0.415 |
| MF FI | 0.34 ± 0.14 | 0.28 ± 0.11 | 0.013* |
| ES FI | 0.22 ± 0.08 | 0.22 ± 0.08 | 0.948 |
| MF rSI | 0.49 ± 0.13 | 0.40 ± 0.09 | <0.01** |
| ES rSI | 0.41 ± 0.09 | 0.37 ± 0.08 | 0.030* |
| L4 | |||
| MF rtCSA | 1.29 ± 0.32 | 1.29 ± 0.24 | 0.946 |
| MF rfCSA | 0.48 ± 0.23 | 0.41 ± 0.19 | 0.062 |
| ES rtCSA | 2.20 ± 0.52 | 2.38 ± 0.40 | 0.083 |
| ES rfCSA | 0.66 ± 0.29 | 0.71 ± 0.23 | 0.412 |
| MF FI | 0.37 ± 0.15 | 0.31 ± 0.11 | 0.044* |
| ES FI | 0.29 ± 0.10 | 0.30 ± 0.09 | 0.964 |
| MF rSI | 0.53 ± 0.13 | 0.44 ± 0.11 | 0.002** |
| ES rSI | 0.47 ± 0.11 | 0.43 ± 0.10 | 0.116 |
| L5 | |||
| MF rtCSA | 1.52 ± 0.36 | 1.55 ± 0.32 | 0.630 |
| MF rfCSA | 0.55 ± 0.20 | 0.50 ± 0.23 | 0.102 |
| ES rtCSA | 1.39 ± 0.41 | 1.55 ± 0.42 | 0.088 |
| ES rfCSA | 0.57 ± 0.26 | 0.63 ± 0.25 | 0.143 |
| MF FI | 0.37 ± 0.15 | 0.32 ± 0.11 | 0.087 |
| ES FI | 0.42 ± 0.14 | 0.40 ± 0.12 | 0.653 |
| MF rSI | 0.54 ± 0.13 | 0.47 ± 0.11 | 0.014* |
| ES rSI | 0.56 ± 0.11 | 0.51 ± 0.10 | 0.070 |
MF, multifidus; ES, erector spinae; FI, fatty infiltration; rtCSA, relative total cross sectional area; rfCSA, relative fatty cross sectional area; rSI, relative signal intensity *means P value < 0.05; **means P value < 0.01.
For paraspinal muscle parameters at L3 level, there were significant differences in the rfCSA, FI, rSI of MF and rSI of ES (P<0.05). The FI and rSI of MF at L4 level and the rSI of MF at L5 level were also significantly different (P<0.05). Although there was no significant difference in rfCSA of MF and rSI of ES at L4 level and rfCSA, FI of MF, and rSI of ES at L5 level, those parameters of patients were higher than those of normal people. The results reflected that the overall degeneration of paraspinal muscle in patients with LSS was worse than that in normal people.
We further compared the mean values of paraspinal muscle parameters from L3 to L5 level between the two groups (The mean value was used in the following analyses) and found that the rfCSA, FI, rSI of MF and rSI of ES in patients group were significantly higher than those in normal group ( Table 3 ). The degeneration of paraspinal muscle in patients with LSS was significantly severer than that in normal people, which was mainly manifested in multifidus muscle ( Figures 1 , 2 ).
Table 3.
The mean values of paraspinal muscle parameters.
| Parameters | Patients group | Normal group | P-value |
|---|---|---|---|
| MF rtCSA | 1.25 ± 0.28 | 1.26 ± 0.22 | 0.897 |
| MF rfCSA | 0.45 ± 0.18 | 0.39 ± 0.18 | 0.013* |
| ES rtCSA | 2.06 ± 0.38 | 2.21 ± 0.35 | 0.072 |
| ES rfCSA | 0.60 ± 0.20 | 0.64 ± 0.21 | 0.404 |
| MF FI | 0.36 ± 0.14 | 0.30 ± 0.10 | 0.014* |
| ES FI | 0.31 ± 0.09 | 0.31 ± 0.09 | 0.671 |
| MF rSI | 0.52 ± 0.12 | 0.44 ± 0.10 | 0.001** |
| ES rSI | 0.48 ± 0.09 | 0.44 ± 0.09 | 0.038* |
MF, multifidus; ES, erector spinae; FI, fatty infiltration; rtCSA, relative total cross sectional area; rfCSA, relative fatty cross sectional area; rSI, relative signal intensity *means P value < 0.05; **means P value < 0.01.
Figure 1.
The images of paraspinal muscle in a 71 years old normal female. (A, D) were at L3 level. (B, E) were at L4 level. (C, F) were at L5 level. The FI of MF was 36.8% and the FI of ES was 48.2%.
Figure 2.
The images of paraspinal muscle in a 70 years old female patient. (A, D) were at L3 level. (B, E) were at L4 level. (C, F) were at L5 level. The FI of MF was 57.6% and the FI of ES was 43.0%.
These patients were divided into two groups according to the gender. There was no significant difference in paraspinal muscle parameters between the two groups. But compared with males, the rfCSA and FI of MF and ES were higher in females ( Table 4 ).
Table 4.
Comparison of paraspinal muscle parameters between genders in patients.
| Parameter | Male | Female | P-value |
|---|---|---|---|
| Age (yrs) | 62.2 ± 8.0 | 63.5 ± 7.9 | 0.610 |
| Height (cm) | 170.1 ± 7.3 | 158.3 ± 5.7 | <0.01** |
| Weight (kg) | 72.6 ± 10.0 | 64.4 ± 9.1 | 0.011* |
| BMI (kg/m2) | 25.0 ± 2.6 | 25.7 ± 3.1 | 0.568 |
| MF rtCSA | 1.22 ± 0.30 | 1.29 ± 0.27 | 0.452 |
| MF rfCSA | 0.43 ± 0.16 | 0.47 ± 0.19 | 0.477 |
| ES rtCSA | 2.16 ± 0.38 | 1.98 ± 0.36 | 0.122 |
| ES rfCSA | 0.59 ± 0.18 | 0.61 ± 0.21 | 0.786 |
| MF FI | 0.36 ± 0.16 | 0.37 ± 0.12 | 0.832 |
| ES FI | 0.30 ± 0.11 | 0.32 ± 0.08 | 0.692 |
| MF rSI | 0.52 ± 0.15 | 0.52 ± 0.09 | 0.957 |
| ES rSI | 0.48 ± 0.10 | 0.48 ± 0.07 | 0.796 |
BMI, body mass index; MF, multifidus; ES, erector spinae; FI, fatty infiltration; rtCSA, relative total cross sectional area; rfCSA, relative fatty cross sectional area; rSI, relative signal intensity *means P value < 0.05; **means P value < 0.01.
Then we compared the differences of paraspinal muscle parameters between patients and normal people under different gender. As the results showed in Table 5 , for males, the rfCSA, FI and rSI of MF were higher in patients than those in normal peers (p<0.05). For females, the rSI of MF was higher in patients than that in normal peers (p<0.05). Although there was no significant difference in the rfCSA and FI of MF between female patients and normal peers, the rfCSA and FI of MF were also higher in female patients.
Table 5.
Comparison of paraspinal muscle parameters between patients and normal peers under different gender.
| Parameter | Male | Female | ||||
|---|---|---|---|---|---|---|
| Patients | Normal peers | P-value | Patients | Normal peers | P-value | |
| Age (yrs) | 62.2 ± 8.0 | 60.8 ± 7.2 | 0.592 | 63.5 ± 7.9 | 63.1 ± 7.4 | 0.991 |
| Height (cm) | 170.1 ± 7.3 | 171.6 ± 5.7 | 0.508 | 158.3 ± 5.7 | 157.4 ± 5.8 | 0.579 |
| Weight (kg) | 72.6 ± 10.0 | 74.6 ± 10.7 | 0.577 | 64.4 ± 9.1 | 60.3 ± 8.5 | 0.128 |
| BMI(kg/m2) | 25.0 ± 2.6 | 25.2 ± 2.5 | 0.817 | 25.7 ± 3.1 | 24.3 ± 3.1 | 0.154 |
| MF rtCSA | 1.22 ± 0.30 | 1.25 ± 0.21 | 0.721 | 1.29 ± 0.27 | 1.27 ± 0.23 | 0.835 |
| MF rfCSA | 0.43 ± 0.16 | 0.32 ± 0.09 | 0.020* | 0.47 ± 0.19 | 0.44 ± 0.21 | 0.184 |
| ES rtCSA | 2.16 ± 0.38 | 2.31 ± 0.41 | 0.273 | 1.98 ± 0.36 | 2.14 ± 0.30 | 0.098 |
| ES rfCSA | 0.59 ± 0.18 | 0.56 ± 0.12 | 0.623 | 0.61 ± 0.21 | 0.70 ± 0.24 | 0.192 |
| MF FI | 0.36 ± 0.16 | 0.25 ± 0.05 | 0.025* | 0.37 ± 0.12 | 0.34 ± 0.12 | 0.275 |
| ES FI | 0.30 ± 0.11 | 0.27 ± 0.06 | 0.198 | 0.32 ± 0.08 | 0.34 ± 0.10 | 0.613 |
| MF rSI | 0.52 ± 0.15 | 0.40 ± 0.10 | 0.009** | 0.52 ± 0.09 | 0.46 ± 0.10 | 0.045* |
| ES rSI | 0.48 ± 0.10 | 0.41 ± 0.09 | 0.059 | 0.48 ± 0.07 | 0.46 ± 0.09 | 0.273 |
BMI, body mass index; MF, multifidus; ES, erector spinae; FI, fatty infiltration; rtCSA, relative total cross sectional area; rfCSA, relative fatty cross sectional area; rSI, relative signal intensity *means P value < 0.05; **means P value < 0.01.
We used correlation analysis to explore the relationship between paraspinal muscle parameters and other factors, such as age, height, weight and BMI in patients with LSS and the results were recorded in Table 6 . Age was significantly correlated with the rfCSA, FI and rSI of both MF and ES (p<0.05). Weight and BMI were significantly correlated with the rtCSA of ES (p<0.05).
Table 6.
Relationships between paraspinal muscle parameters and other factors in patients.
| Parameter | MF rtCSA | MF rfCSA | ES rtCSA | ES rfCSA | MF FI | ES FI | MF rSI | ES rSI |
|---|---|---|---|---|---|---|---|---|
| Age | -0.056 | 0.427** | -0.053 | 0.354* | 0.499** | 0.415** | 0.498** | 0.390* |
| Height | -0.100 | -0.195 | 0.183 | -0.100 | -0.181 | -0.216 | -0.217 | -0.228 |
| Weight | 0.150 | 0.094 | 0.378* | 0.055 | -0.148 | -0.108 | -0.171 | -0.122 |
| BMI | 0.246 | 0.283 | 0.364* | 0.289 | 0.038 | 0.145 | 0.077 | 0.118 |
BMI, body mass index; MF, multifidus; ES, erector spinae; FI, fatty infiltration; rtCSA, relative total cross sectional area; rfCSA, relative fatty cross sectional area; rSI, relative signal intensity *means P value < 0.05; **means P value < 0.01.
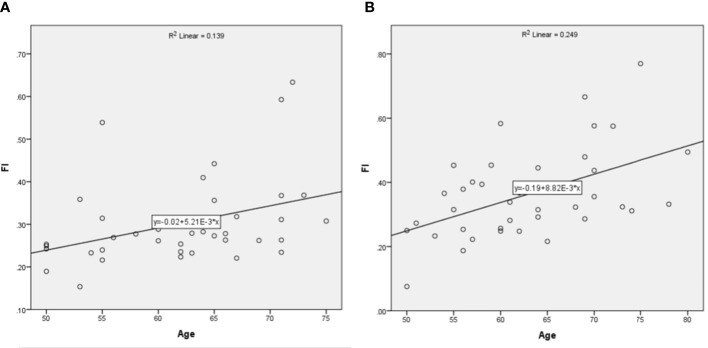
The linear regression analysis was used to evaluate the relationship between age and the FI of MF. As showed in Figure 3 , the slope of fitted linear was higher in patients than that in normal people, indicating that with the increase of age, the fatty infiltration increased more significantly in patients than that in normal people.
Figure 3.
The relationship between age and FI of MF. (A) was in normal people and (B) was in patients.
Discussion
With the aging of population, the incidence of lumbar degenerative diseases is gradually increasing. Lumbar spinal stenosis is one of the common lumbar diseases and many researches focused on the degeneration of paraspinal muscle in lumbar spinal stenosis. Zotti found that the decrease of cross-sectional area of MF was correlated to the outcome in patients with LSS (16). But this study only measured the MF and lacked the data of normal people. In this study, we found that the degeneration of paraspinal muscle was worse in patients with LSS compared with that in normal people, especially in the multifidus.
Previous studies found that age, gender and BMI may influence the degeneration of paraspinal muscle (2, 18, 21). In order to increase the comparability, we used propensity score matching to minimize the differences in general conditions between the two groups. As the results showed in Table 1 , there was no significant difference in general conditions, indicating the two groups were matched well.
The change rule of paraspinal muscle in patients with LSS was similar to that in normal people. From top to bottom of the spinal axis, the relative cross-sectional area of MF increased, while that of ES decreased. The FI and the rSI of MF and ES increased gradually. Another research measured the volume of paraspinal muscle in patients with LSS and found that the volume of paraspinal muscle was the largest at L3-4 level and gradually decreased toward the caudal end (22). They measured the volume of paraspinal muscle rather than parameters of both MF and ES, which was different with our study, but the change rule of paraspinal muscle was similar to our finding.
Recently many studies focused on the degeneration of paraspinal muscle in patients with chronic nonspecific low back pain and lumbar degenerative diseases. Ogon found that intracellular lipid content in multifidus muscle cells was higher in patients with chronic nonspecific low back pain than that in patients with LSS (7). Yagi found that the cross-sectional area of multiuse muscle was significantly smaller in patients with degenerative scoliosis combined with LSS than that in patients with single LSS (15). Liu found that FI in multifidus muscle at L5-S1 could be a predictor of functional improvement after surgery in patients with L4-5 single-segment LSS (14). But few studies investigated the differences of paraspinal muscle between patients with LSS and normal people.
By comparing the paraspinal muscle parameters between the two groups, the degeneration of paraspinal muscle was worse in patients with LSS than that in normal people. For parameters at L3 level, the rfCSA, FI, rSI of MF and rSI of ES were higher in patients group than those in normal group. Besides, the FI and rSI of MF at L4 level and the rSI of MF at L5 level were also higher in patients group. Moreover, the mean value of parameters from L3 to L5 level, such as rfCSA, FI, rSI of MF and rSI of ES were also significantly higher in patients group than those in normal group. Paraspinal muscle is important to maintain the spinal stability. The atrophy of paraspinal muscle led to the increase of fatty cross-sectional area and decrease of functional cross-sectional area, which was associated with the decrease in muscle strength (23), resulting in weakness in maintaining spinal stability. So we should pay attention to the degeneration of paraspinal muscle in LSS.
The degeneration of MF was significantly worse in patients than that in normal people, which may relate to the denervation of muscle fibers and differences in muscle stress. Yoshihara found that the denervation caused by compression of nerve root may lead to the degeneration of type I and type II fibers, causing structural changes of multifidus muscle (24). So nerve compression in patients with LSS may cause more degeneration of multifidus muscle.
In addition, the degeneration of MF was significantly different between the two groups, while there was no significant difference in degeneration of ES. While maintaining the spinal stability, the paraspinal muscle also bears the stress from the body or activities. The stress within physiological range is help to the exercise of muscle, but the overlord stress will cause muscle injury or degeneration. Previous studies reported that the MF bear more stress than the ES (25, 26), which may explain the degeneration of MF was worse rather than the ES. However, there also existed different conclusions. Lee measured the paraspinal muscle parameters of 650 patients from CT test and found that the atrophy of ES appeared earlier and more severe than the MF, which may relate to the difference of anatomical structure (27). Our conclusion was different with Lee’s, which may be associated with the difference of paraspinal muscle measurements and characteristics of population. In this study, we measured paraspinal muscle parameters from T2-weighted MRI, while Lee measured those from CT. All patients in this study were diagnosed as LSS, while the subject in Lee’ study was patient without spinal surgery, deformity and neuromuscular diseases. The inclusion criteria were broader than those in this study. The differences of paraspinal muscle degeneration need to be further explored.
In normal people, we found that the rfCSA and FI of MF, rfCSA and FI of ES and rSI of MF were significantly greater in females than those in males. In patients with lumbar disease, the FI of MF was also higher in females than that in males (18, 28). Compared to above results, there was no significant difference in paraspinal muscle parameter between different genders in patients with LSS, but the atrophy of paraspinal muscle was more severe in female than that in male. In addition, compared with normal people, the degeneration of multifidus muscle was higher in patients with lumbar spinal stenosis under different genders.
Age was an important factor for the degeneration of paraspinal muscle in patients with LSS and it was positively correlated with the rfCSA, FI and rSI of MF and ES, indicating that the degeneration of paraspinal muscle increased gradually with age. This result was consistent with previous studies (16, 18, 28, 29). We used the linear regression analysis to evaluate the relationship between age and FI of MF. The slope of fitted linear was higher in patients than that in normal people, indicating that with the increase of age, the degeneration of MF increased more significantly in patients than that in normal people. Shahidi analyzed the data of 199 patients who were between 18 and 80 years old. They found that the FI of MF and ES increased with age. They further compared paraspinal muscle parameters with the data of healthy people from Crawford’s study and found that the age-fat infiltration rate fitted line had a higher slope in female patients (18). Their results were consistent with this study. Except for age, weight and BMI were significantly correlated with the rtCSA of ES rather than the rtCSA of MF, suggesting that weight mainly affected the quantity of erector spinae.
In this study, propensity score matching was used to ensure the comparability between patients with lumbar spinal stenosis and normal people and reduce the influence of individual factors on the results. Paraspinal muscle parameters from L3 to L5 and the mean value of parameters were compared between the two groups and the differences in paraspinal muscle parameters were comprehensively analyzed. Patients with lumbar spinal stenosis are mainly middle-aged and elderly people, so this study focused on middle-aged and elderly people over 50 years old. However, there are some shortcomings in this study. Firstly, this study is a single-center study, which may have selection bias. Secondly, the number of people included in this study was small and the study with a larger sample size is needed to verify our results in the future.
Conclusion
The change rules of paraspinal muscle in patients with lumbar spinal stenosis were similar to those in normal people. The degeneration of paraspinal muscle was more severe in patients with lumbar spinal stenosis than that in normal people, especially in multifidus. The degeneration of paraspinal muscle in patients with lumbar spinal stenosis was mainly related to age and the effect of age on atrophy of paraspinal muscle was greater than that of normal people.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University Third Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
WW conceived the project and analyzed the data. All authors contributed towards the interpretation and the collection of the data. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-026A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Stanuszek A, Jedrzejek A, Gancarczyk-Urlik E, Kolodziej I, Pisarska-Adamczyk M, Milczarek O, et al. Preoperative paraspinal and psoas major muscle atrophy and paraspinal muscle fatty degeneration as factors influencing the results of surgical treatment of lumbar disc disease. Arch Orthop Trauma Surg (2022) 142(7):1375–84. doi: 10.1007/s00402-021-03754-x [DOI] [PubMed] [Google Scholar]
- 2. Hida T, Eastlack RK, Kanemura T, Mundis GJ, Imagama S, Akbarnia BA, et al. Effect of race, age, and gender on lumbar muscle volume and fat infiltration in the degenerative spine. J Orthop Sci (2021) 26(1):69–74. doi: 10.1016/j.jos.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 3. Ding JZ, Kong C, Li XY, Sun XY, Lu SB, Zhao GG, et al. Different degeneration patterns of paraspinal muscles in degenerative lumbar diseases: a MRI analysis of 154 patients. Eur Spine J (2022) 31(3):764–73. doi: 10.1007/s00586-021-07053-2 [DOI] [PubMed] [Google Scholar]
- 4. Leng J, Han G, Zeng Y, Chen Z, Li W. The effect of paraspinal muscle degeneration on distal pedicle screw loosening following corrective surgery for degenerative lumbar scoliosis. Spine (Phila Pa 1976) (2020) 45(9):590–8. doi: 10.1097/BRS.0000000000003336 [DOI] [PubMed] [Google Scholar]
- 5. Hiyama A, Katoh H, Sakai D, Tanaka M, Sato M, Watanabe M. The correlation analysis between sagittal alignment and cross-sectional area of paraspinal muscle in patients with lumbar spinal stenosis and degenerative spondylolisthesis. BMC Musculoskelet Disord (2019) 20(1):352. doi: 10.1186/s12891-019-2733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xia W, Fu H, Zhu Z, Liu C, Wang K, Xu S, et al. Association between back muscle degeneration and spinal-pelvic parameters in patients with degenerative spinal kyphosis. BMC Musculoskelet Disord (2019) 20(1):454. doi: 10.1186/s12891-019-2837-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogon I, Takebayashi T, Takashima H, Morita T, Yoshimoto M, Terashima Y, et al. Magnetic resonance spectroscopic analysis of multifidus muscles lipid content and association with spinopelvic malalignment in chronic low back pain. Br J Radiol (2017) 90(1073), 20160753. doi: 10.1259/bjr.20160753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalichman L, Hodges P, Li L, Guermazi A, Hunter DJ. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J (2010) 19(7):1136–44. doi: 10.1007/s00586-009-1257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jermy JE, Copley PC, Poon M, Demetriades AK. Does pre-operative multifidus morphology on MRI predict clinical outcomes in adults following surgical treatment for degenerative lumbar spine disease? A systematic review. Eur Spine J (2020) 29(6):1318–27. doi: 10.1007/s00586-020-06423-6 [DOI] [PubMed] [Google Scholar]
- 10. Hyun SJ, Bae CW, Lee SH, Rhim SC. Fatty degeneration of the paraspinal muscle in patients with degenerative lumbar kyphosis: A new evaluation method of quantitative digital analysis using MRI and CT scan. Clin Spine Surg (2016) 29(10):441–7. doi: 10.1097/BSD.0b013e3182aa28b0 [DOI] [PubMed] [Google Scholar]
- 11. Kim CH, Chung CK, Park CS, Choi B, Hahn S, Kim MJ. Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: a nationwide cohort study. Spine J (2013) 13(10):1230–7. doi: 10.1016/j.spinee.2013.06.069 [DOI] [PubMed] [Google Scholar]
- 12. Waldrop R, Cheng J, Devin C, McGirt M, Fehlings M, Berven S. The burden of spinal disorders in the elderly. Neurosurgery (2015) 77 Suppl 4:S46–50. doi: 10.1227/NEU.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 13. Miki T, Naoki F, Takashima H, Takebayashi T. Associations between paraspinal muscle morphology, disc degeneration, and clinical features in patients with lumbar spinal stenosis. Prog Rehabil Med (2020) 5:20200015. doi: 10.2490/prm.20200015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Liu Y, Hai Y, Liu T, Guan L, Chen X. Fat infiltration in the multifidus muscle as a predictor of prognosis after decompression and fusion in patients with single-segment degenerative lumbar spinal stenosis: An ambispective cohort study based on propensity score matching. World Neurosurg (2019) 128:e989–e1001. doi: 10.1016/j.wneu.2019.05.055 [DOI] [PubMed] [Google Scholar]
- 15. Yagi M, Hosogane N, Watanabe K, Asazuma T, Matsumoto M. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J (2016) 16(4):451–8. doi: 10.1016/j.spinee.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 16. Zotti M, Boas FV, Clifton T, Piche M, Yoon WW, Freeman B. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis? Eur Spine J (2017) 26(10):2589–97. doi: 10.1007/s00586-017-4986-x [DOI] [PubMed] [Google Scholar]
- 17. Sun D, Liu P, Cheng J, Ma Z, Liu J, Qin T. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord (2017) 18(1):167. doi: 10.1186/s12891-017-1522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, et al. Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976) (2017) 42(8):616–23. doi: 10.1097/BRS.0000000000001848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW. An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine (Phila Pa 1976) (2011) 36(13):E868–74. doi: 10.1097/BRS.0b013e3181ef6b51 [DOI] [PubMed] [Google Scholar]
- 20. Ranson CA, Burnett AF, Kerslake R, Batt ME, O'Sullivan PB. An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur Spine J (2006) 15(6):764–73. doi: 10.1007/s00586-005-0909-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng X, Li X, Xu Z, Wang L, Cai W, Yang S, et al. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg (2020) 10(8):1590–601. doi: 10.21037/qims-19-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boissiere L, Moal B, Gille O, De-Roquefeuil E, Durieux M, Obeid I, et al. Lumbar spinal muscles and spinal canal study by MRI three-dimensional reconstruction in adult lumbar spinal stenosis. Orthop Traumatol Surg Res (2017) 103(2):279–83. doi: 10.1016/j.otsr.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 23. Fortin M, Wilk N, Dobrescu O, Martel P, Santaguida C, Weber MH. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract (2018) 38:1–7. doi: 10.1016/j.msksp.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 24. Okada Y. Histochemical study on the atrophy of the quadriceps femoris muscle caused by knee joint injuries of rats. Hiroshima J Med Sci (1989) 38(1):13–21. [PubMed] [Google Scholar]
- 25. Mayer JM, Graves JE, Clark BC, Formikell M, Ploutz-Snyder LL. The use of magnetic resonance imaging to evaluate lumbar muscle activity during trunk extension exercise at varying intensities. Spine (Phila Pa 1976) (2005) 30(22):2556–63. doi: 10.1097/01.brs.0000186321.24370.4b [DOI] [PubMed] [Google Scholar]
- 26. Ng J, Richardson C. EMG study of erector spinae and multifidus in two isometric back extension exercises. Aust J Physiother (1994) 40(2):115–21. doi: 10.1016/S0004-9514(14)60458-X [DOI] [PubMed] [Google Scholar]
- 27. Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J (2017) 17(1):81–7. doi: 10.1016/j.spinee.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 28. Takayama K, Kita T, Nakamura H, Kanematsu F, Yasunami T, Sakanaka H, et al. New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine (Phila Pa 1976) (2016) 41(2):E84–90. doi: 10.1097/BRS.0000000000001154 [DOI] [PubMed] [Google Scholar]
- 29. Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, et al. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol (2016) 37(4):742–8. doi: 10.3174/ajnr.A4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.