The metabolic-associated fatty liver disease (MAFLD) criteria1 is aimed at capturing the heterogeneity of the disease with the goal of improving patient stratification and management. However, as is well-known, the metabolic factors used in the nomenclature are complex and correlated, and their nuanced contribution to the definition needs to be quantified to accurately estimate clinical relevance and stratify the population at risk2.
In a nationally representative cohort, NHANES 2017-2018, we assessed the relative prognostic importance of the seven key metabolic factors defined per the MAFLD criteria for steatosis and fibrosis outcomes, using separate models, one per metabolic factor per outcome (sample size N = 4369, see Supplementary Table S1). We defined hepatic steatosis using controlled attenuation parameter (CAP) at the higher sensitivity cut-off point (CAP ≥ 290 dB/m), and fibrosis as the median liver stiffness (LSM; LSM ≥ 8.2 kPa) both measured using vibration-controlled transient elastography3. The models were all adjusted for diabetes, overweight status, age, ethnicity, and sex (see Supplementary Methods).
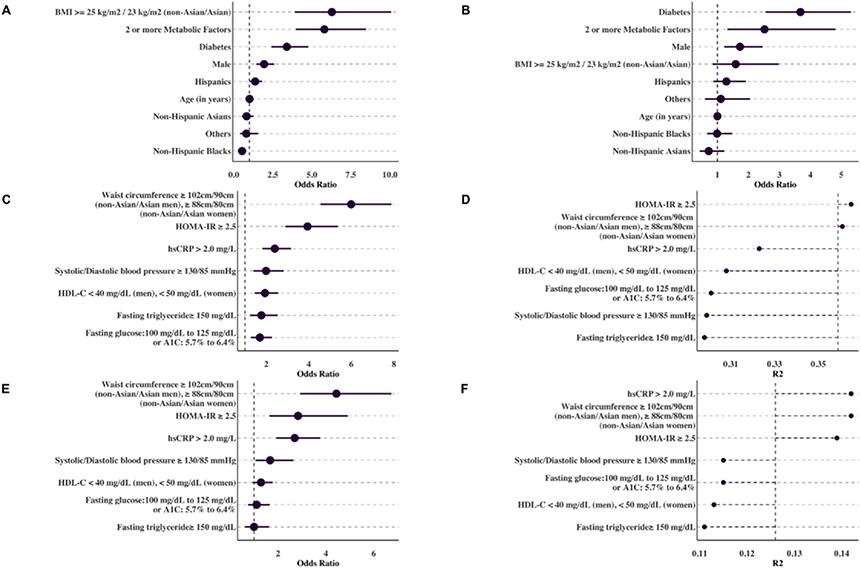
The presence of two or more metabolic factors conferred increased odds of steatosis as well as increased odds of fibrosis, independent of elevated BMI (BMI ≥ 25 kg/m2 non-Asians; 23 kg/m2 for Asians) and diabetes (Figure 1, A-B). Individuals with two or more metabolic factors had significantly higher odds of steatosis (adjusted OR (aOR): 5.79, 95% CI: 3.98, 8.43, p = 3.95 1x10−17, CAP ≥ 290 dB/m), and fibrosis (aOR: 2.5, 95% CI: 1.3, 4.81, p = 7.15 1x10−3, LSM ≥ 8.2kPa). Insulin resistance and increased central obesity as measured by elevated waist circumference were the top two metabolic factors by odds ratio and Nagelkerke R2 (Figure 1, C-F) for steatosis. For CAP ≥ 290 dB/m, elevated waist circumference (WC ≥ 102cm/90cm for non-Asian/Asian men, and WC ≥ 88cm/80cm for non-Asian/Asian women) was associated with aOR: 5.98 (95% CI: 4.54, 7.87, p < 000001) while insulin resistance, as measured by the homeostatic model assessment of insulin resistance (HOMA-IR ≥ 2.5) had aOR: 3.96 (95% CI: 2.9, 5.4, p < 0.00001). For LSM ≥ 8.2 kPa, elevated waist circumference was associated with aOR: 4.43 (95% CI: 2.9, 6.7, p < 0.000001) while insulin resistance had an aOR: 2.8, (95% CI: 1.63, 4.9, p < 0.001).
Figure 1.
(A - B): Strength of association for the MAFLD criteria for CAP ≥ 290 dB/m (A) and LSM ≥ 8.2 kPa (B), ordered by odds ratio. An elevated OR suggests that the risk factor has a strong relative importance for steatosis and fibrosis prognosis.
(C - F): Strength of association for each metabolic factor included in the MAFLD criteria for CAP ≥ 290 dB/m (C, D) and LSM ≥ 8.2 kPa (E, F). The risk factors are ordered according to odds ratio (C, E), and the estimated variance (R2) explained by each metabolic factor (D,F). On figure D and F, the dotted line indicates the variance explained for the MAFLD criteria model for CAP ≥ 290 dB/m (A) and LSM ≥ 8.2 kPa (B).
The addition of these top 2 metabolic risk factors, elevated waist circumference and insulin resistance, to the diabetes and overweight model, improved steatosis classification accuracy, with an overall continuous net reclassification improvement (NRI) of 77% (95% CI 71, 82), with 45% (95% CI 41, 50) for cases and 31% (95% CI 28, 35) for non-cases, an AUC of 0.81 (95% CI 0.8, 0.83), and a Nagelkerke R2 of 0.41 (Supplementary Table S2). In comparison, the MAFLD model, 2 or more metabolic factors, diabetes, and overweight status, improved the overall classification accuracy for hepatic steatosis with an overall continuous NRI 65% (95% CI 61, 70) with 82% (95% CI 0.79, 0.85) for cases but had a reduced NRI of −17% (95% CI −20, −13) for non-cases when compared to a diabetes and overweight model. The Top 2 model exhibited improved classification accuracy for fibrosis with an overall continuous NRI of 61% (95% CI 52, 70) with 50% (95% CI 41, 58) for cases and 12% (95% CI 8, 15) for non-cases, AUC of 0.75 (95% CI 0.73, 0.76) and Nagelkerke R2 of 0.16.
The relationship between waist circumference and the risk of developing steatosis4 has been established, with the underlying hypothesis that visceral fat is a key factor in the development of liver disease, and waist circumference (or increased central obesity) is a surrogate of visceral fat.Similarly, insulin resistance has been studied extensively in patients with NAFLD, but whether insulin resistance is a cause or consequence of NAFLD is still unclea5,6. Our findings add to these prior results in two significant ways. First, for steatosis and fibrosis, amongst the entire panel of factors that comprise metabolic dysfunction, higher waist circumference and insulin resistance are the two most important factors. Second, while waist circumference and insulin resistance are correlated, including both factors increases the classification accuracy over a model that only includes waist circumference or insulin resistance for both steatosis and fibrosis. Given that fatty liver disease remains under-diagnosed in real-world settings7,8 and the challenge of deploying a screening heuristic requires laboratory tests, our findings highlight the potential of simplifying the MAFLD criteria/definition to identify the highest yield groups for screening and risk stratification.
Our study has several strengths. To the best of our knowledge, we are the first to examine the relative and independent contribution of the different metabolic factors defined as risk factors for steatosis and the impact of the factors for fibrosis in a nationally representative sample. We highlight the role of insulin resistance and increased central obesity for both steatosis and fibrosis that is independent of diabetes and, interestingly, overweight status. Second, we leverage survey-weighted logistic regression methods to determine the independent relative importance of the metabolic factors to assess the additive and non-linear contributions of metabolic variables in a representative US population sample.
Our study has several limitations. First, in the absence of longitudinal data, it is difficult to assess the directionality of the associations, especially between insulin resistance and fatty liver6. Second, we had a high percentage of missing data in self-report use of lipid-lowering drugs and antihypertensive drugs. We can thus only evaluate the relative importance of elevated triglycerides, reduced HDL-C, and elevated blood pressure independent of medication use, and cannot assess the interactions with medications to control the same. Third, our unweighted sample size for CAP and LSM, did not allow us to fully dissect the association between ethnicity and the relative importance of metabolic factors in one comprehensive model.
Metabolic dysfunction as captured by the MAFLD criteria are key risk factors for steatosis and potential progression to fibrosis. This study shines light on the factors that dominate the association (e.g, visceral adiposity, and insulin resistance) with steatosis and fibrosis, demonstrating that factors of high prevalence in the US are also of highest risk for liver disease.
Supplementary Material
Table 1.
Definition of the seven metabolic factors as defined by Eslam et al.1
| Metabolic Factor | Definition |
|---|---|
| Waist circumference | Waist circumference ≥ 102/88 cm in men/women (or ≥ 90/80 cm in Asian men/women) |
| Blood pressure | Blood pressure ≥ 130/85 mmHg or specific drug treatment |
| Plasma triglycerides | Plasma triglycerides ≥ 150 mg/dl (≥ 1.70 mmol/L) or specific drug treatment |
| HDL-cholesterol | HDL-cholesterol < 40 mg/dl (< 1.0 mmol/L) for men and <50 mg/dl (<1.3 mmol/L) for women or specific drug treatment |
| Prediabetes | Fasting glucose levels 100 to 125 mg/dl [5.6 to 6.9 mmol/L], or 2-hour post-load glucose levels 140 to 199 mg/dl [7.8 to 11.0 mmol] or HbA1c 5.7% to 6.4% [39 to 47 mmol/mol] |
| Insulin Resistance | Homeostasis model assessment of insulin resistance (HOMA-IR)9 score ≥ 2.5 |
| Inflammation | Plasma high-sensitivity C-reactive protein level >2 mg/L |
Table 2.
Characteristics of the cohort. Cohort was imputed using multivariate imputation by chained equations10. All proportions and means are specified together with their 95% confidence interval. Table S1 provides the characteristics of the non-imputed dataset with percent missing denoted as [%]. Mean values for the metabolic factors are in Table S1.
| Characteristic | Healthy CAP < 290 dB/m N = 2732 |
Hepatic Steatosis CAP ≥ 290 dB/m LSM < 8.2 kPa, N = 1234 |
Fibrosis LSM ≥ 8.2 kPa N = 403 |
|---|---|---|---|
| Mean Age, years | 44.3 (42.9, 45.7) | 50.3 (49, 51.4) | 51.6 (49.1, 54.2) |
| Sex, % | |||
| Female | 55.9 (53.4, 58.4) | 42.9 (39.2, 46.6) | 38.3 (31.5, 45.2) |
| Male | 44.1 (41.6, 46.6) | 57.1 (53.4, 60.8) | 61.7 (54.8, 68.5) |
| Ethnicity, % | |||
| Non-hispanic Whites | 63.3 (58.2, 68.3) | 63.5 (56.7, 70.3) | 61 (52.6, 69.3) |
| Non-hispanic Asians | 5.2 (3.3, 7.1) | 4.9 (3.1, 6.6) | 3.7 (1.7, 5.6) |
| Non-hispanic Blacks | 12 (8.7, 15.3) | 7.5 (5, 10) | 10.3 (5.4, 15.2) |
| Hispanics | 14.7 (11.1, 18.2) | 19.9 (14, 25.8) | 19.6 (14, 25.1) |
| Others | 4.9 (3.5, 6.3) | 4.2 (2.4, 6) | 5.6 (2.6, 8.5) |
| Diabetes, % | 6.7 (5.5, 7.9) | 23.2 (19.9, 26.4)) | 39.5 (32.7, 46.3) |
| Lean: BMI ≤ 25 kg/m2 / 23 kg/m2 (non-Asian/Asian), % | 38.8 (34.9, 42.7) | 5 (2.8, 7.2) | 11.3 (5.9, 16.7) |
| Overweight: BMI 25-30 kg/m2 / 23-25 kg/m2 (Caucasian/Asian), % | 34 (31.6, 36.4) | 25 (20.9, 29.1) | 11.1 (7.8, 14.5) |
| Obese: BMI ≥ 30 kg/m2 / 25 kg/m2 (non-Asian/Asian), % | 27.2 (23.3, 31.2) | 70 (64.3, 75.8) | 77.5 (71.1, 84) |
| Metabolic Factors, % | |||
| 0 Metabolic Factors | 19 (15.8, 22.3) | 1.9 (0.9, 2.9) | 4.4 (−0.6, 9.3) |
| 1 Metabolic Factor | 26.2 (22.8, 29.6) | 6.3 (3.9, 8.8) | 7.6 (1.8, 13.5) |
| 2 or more Metabolic Factors | 54.8 (50.6, 59) | 91.8 (89.2, 94.4) | 88 (81.5, 94.4) |
| Waist circumference ≥ 102cm/90cm (non-Asian/Asian men), ≥ 88cm/80cm (non-Asian/Asian women), % | 46.1 (41.6, 50.5) | 86.3 (83.3, 89.2) | 85.4 (79.9, 90.8) |
| HOMA-IR ≥ 2.5, % | 33.1 (27.7, 38.6) | 74.4 (69.4, 79.4) | 78.6 (71, 86.1) |
| hsCRP > 2.0 mg/L, % | 36.4 (32.2, 40.6) | 60.5 (55.9, 65.2) | 71.6 (66.2, 76.9) |
| Fasting glucose:100 mg/dL to 125 mg/dL or A1C: 5.7% to 6.4%, % | 36.5 (32.7, 40.2) | 44.8 (40.9, 48.8) | 30.6 (23.7, 37.5) |
| HDL-C < 40 mg/dL (men), < 50 mg/dL (women), % | 20.9 (18.5, 23.2) | 39.5 (35.3, 43.8) | 38.9 (32, 45.9) |
| Fasting triglyceride ≥ 150 mg/dL, % | 7.5 (5.7, 9.3) | 18.6 (14.7, 22.4) | 16.5 (10.6, 22.4) |
| Systolic/Diastolic blood pressure ≥ 130/85 mmHg, % | 6.3 (4.6, 8.1) | 13.8 (11.2, 16.3) | 15.6 (11, 20.2) |
Table 3.
Area under the Receiver Operating Curve (AUCROC), Nagelkerke R2, and continuous Net Reclassification Improvement (NRI) for the different models. The overall NRI is the sum of the net reclassifications for cases (P[up∣case] - P[down∣case]) and non-cases (P[down∣non-case] - P(up∣non-case]). A positive NRI indicated improved reclassification. The base model for the NRI comparison includes diabetes, overweight status and is adjusted for sex, age, and ethnicity. The two-category NRI (NRI(p)) is given in Table S2. WC = Elevated Waist Circumference; IR = Insulin Resistance; BP = Elevated Blood Pressure.
| NRI Continuous | ||||||
|---|---|---|---|---|---|---|
| Model | Features* | AUC | R2** | Overall | NRI+ | NRI− |
| CAP ≥ 290 dB/m | ||||||
| Diabetes | Diabetes | 0.69 (0.67, 0.7) | 0.15 | |||
| Overweight | Overweight | 0.73 (0.71, 0.75) | 0.25 | |||
| DB+Overweight | Diabetes, Overweight | 0.76 (0.74, 0.77) | 0.29 | |||
| MAFLD | Diabetes, Overweight, 2 or more MF | 0.79 (0.77, 0.8) | 0.36 | 0.65 (0.61, 0.7) | 0.82 (0.79, 0.85) | −0.17 (−0.2, −0.13) |
| WC | Diabetes, Overweight, WC | 0.79 (0.78, 0.81) | 0.36 | 0.6 (0.55, 0.66) | 0.54 (0.49, 0.58) | 0.07 (0.03, 0.1) |
| Top 2 | Diabetes, Overweight, WC, IR | 0.81 (0.8, 0.83) | 0.41 | 0.77 (0.71, 0.82) | 0.45 (0.41, 0.5) | 0.31 (0.28, 0.35) |
| Top 4 | Diabetes, Overweight, WC, IR, BP, Inflammation | 0.82 (0.81, 0.84) | 0.42 | 0.75 (0.69, 0.8) | 0.49 (0.44, 0.53) | 0.26 (0.23, 0.3) |
| Non-Blood Markers | Diabetes, Overweight, WC, BP | 0.8 (0.78, 0.81) | 0.37 | 0.57 (0.51, 0.63) | 0.48 (0.44, 0.53) | 0.08 (0.05, 0.12) |
| LSM ≥ 8.2 kPa | ||||||
| Diabetes | Diabetes | 0.69 (0.67, 0.71) | 0.1 | |||
| Overweight | Overweight | 0.66 (0.64, 0.68) | 0.06 | |||
| DB+Overweight | Diabetes, Overweight | 0.7 (0.68, 0.72) | 0.11 | |||
| MAFLD | Diabetes, Overweight, 2 or more MF | 0.72 (0.7, 0.74) | 0.13 | 0.37 (0.3, 0.45) | 0.72 (0.65, 0.79) | −0.35 (−0.38, −0.32) |
| WC | Diabetes, Overweight, WC | 0.73 (0.71, 0.75) | 0.14 | 0.4 (0.31, 0.49) | 0.48 (0.4, 0.57) | −0.08 (−0.11, 0.05) |
| Top 2 | Diabetes, Overweight, WC, IR | 0.75 (0.73, 0.76) | 0.16 | 0.61 (0.52, 0.7) | 0.5 (0.41, 0.58) | 0.12 (0.08, 0.15) |
| Top 4 | Diabetes, Overweight, WC, IR, BP, Inflammation | 0.76 (0.74, 0.78) | 0.18 | 0.58 (0.49, 0.68) | 0.4 (0.31, 0.49) | 0.18 (0.15, 0.21) |
| Non-Blood Markers | Diabetes, Overweight, WC, BP | 0.74 (0.72, 0.75) | 0.15 | 0.38 (0.29, 0.48) | 0.34 (0.25, 0.43) | 0.04 (0.01, 0.07) |
All models were adjusted for sex, age, and ethnicity
Nagelkerke R2
Grant Support
This effort was funded by NIH National Institutes of Allergy and Infectious Disease (NIAID) R01AI127250 and National Institutes of Environmental Health Science (NIEHS) R01ES032470. This effort was also supported in part by Optum Health, Inc. Dr. Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, the Doris Duke Charitable Foundation Grant #2019085, Gilead Sciences Research Scholars Award, the Boston University School of Medicine Department of Medicine Career Investment Award and the Boston University Clinical Translational Science Institute UL1 TR001430. The funding sources had no role in writing of the manuscript. The corresponding author had full access to all of the data in the study and had responsibility for submission for publication.
Footnotes
Declaration of Interests:
The authors declare that they have no competing interests.
Data Transparency Statement
All data used in this study are publicly available.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020;158:1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 2.Wai-Sun Wong V, Kanwal F. On the Proposed Definition of Metabolic-Associated Fatty Liver Disease. Clin Gastroenterol Hepatol January 2021. [DOI] [PubMed] [Google Scholar]
- 3.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1717–1730. [DOI] [PubMed] [Google Scholar]
- 4.Pang Q, Zhang J-Y, Song S-D, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol 2015;21:1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaka T, Shimano H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J Diabetes Investig 2011;2:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruben N, Shiri-Sverdlov R, Koonen DPY, et al. Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2014;1842:2329–2343. [DOI] [PubMed] [Google Scholar]
- 7.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao E, Hercun J, Heller T, et al. Undiagnosed liver diseases. Transl Gastroenterol Hepatol 2021;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 10.Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.