Abstract
INTRODUCTION:
We investigated the association of the area deprivation index (ADI) with cognitive decline, mild cognitive impairment (MCI), and dementia in older adults (≥50 years old). ADI is a neighborhood socioeconomic disadvantage measure assessed at the census block group-level.
METHODS:
The study included 4,699 participants, initially without dementia, with available ADI values for 2015 and at least one study visit in 2008 – 2018. Using logistic regression and Cox proportional hazards models with age as the time scale, we assessed the odds for MCI and the risk for dementia, respectively.
RESULTS:
In cognitively unimpaired (CU) at baseline, the risk for progression to dementia increased for every decile increase in the ADI State ranking (HR =1.06, 95%CI (1.01–1.11), p=0.01). Higher ADI values were associated with subtly faster cognitive decline.
DISCUSSION:
In older CU adults, higher baseline neighborhood socioeconomic deprivation levels were associated with progression to dementia and slightly faster cognitive decline.
Keywords: Area deprivation index, neighborhood socioeconomic disadvantage, dementia, mild cognitive impairment
1 |. INTRODUCTION
The conditions of the environment people spend their time in (e.g., work, play, worship, age) may affect their health, everyday functioning, quality of life and comprise the social determinants of health. [1] Where people live, and their risk of morbidity, disability, and death are related, even after controlling for individual-level socioeconomic status (SES). [2] In addition, as people age and their health declines, the need for services also increases, resulting in older adults potentially being more dependent on local environments and resources. [2]
Alzheimer’s disease (AD) and related dementias disproportionately affect minority and socially disadvantaged populations. [1] Living in a socioeconomically disadvantaged neighborhood has been shown to affect health negatively (e.g., higher rates of cardiovascular diseases, diabetes, premature mortality), health behaviors, stress levels, access to food, safety, and education. [3–5] Many of these conditions could affect brain health and are associated with mild cognitive impairment (MCI) and dementia risk, suggesting that area-level socioeconomic conditions could contribute to late-life cognitive impairment. [6–8]
Neighborhoods are complex constructs, and their characteristics could influence health and contribute to health disparities due to having variable environmental exposures, health care access, educational and economic opportunities, or social interactions. [9, 10] Although benefits in cognitive changes associated with neighborhood characteristics (e.g., available neighborhood resources like community centers or proximity to public transit) seem to be much smaller than benefits in changes associated with individual health behaviors, changes in neighborhoods might be easier to implement than trying to change individuals’ health behaviors in an environment deprived of resources.[11] On the other hand, chronic illnesses can have variable consequences such as on employment, income, and educational attainment [12, 13], characteristics included by the ADI measure. In addition, it is possible that an underlying common factor (known or unknown) could influence both the place of residence and health. Thus, the relation of ADI to cognitive health is likely to be complex and bidirectional.
Area level deprivation measures, like the area deprivation index (ADI), [3] comprise geographic area-based estimates of the socioeconomic deprivation of neighborhoods that integrate social determinants of health [1], such as indicators of poverty, education, housing, and employment. [3, 5] The ADI data is freely available for every neighborhood of the United States through the National Institutes of Health (NIH)–funded Neighborhood Atlas®, [3] and its use in aging research is strongly supported by the National Institute on Aging (NIA) [14]. ADI provides an opportunity to assess health disparities in socioeconomically disadvantaged populations that are included in the priority populations for health disparities research in aging, as suggested by the NIA Health Disparities Research Framework. [15, 16]
Our study aimed to examine the association of neighborhood socioeconomic disadvantage, as assessed by the ADI, with cognitive impairment (i.e., MCI and dementia) and cognitive decline in community-dwelling adults, ≥50 years old, free of dementia at study baseline, in the population-based Mayo Clinic Study of Aging (MCSA).
2 |. METHODS
2.1 |. Area Deprivation Index
ADI, an area-level measure of neighborhood socioeconomic disadvantage, is a composite measure that uses 17 census measures capturing education, employment, income, poverty, and housing characteristics.[17, 18] We downloaded the 2015 ADI data from the Neighborhood Atlas® website (https://www.neighborhoodatlas.medicine.wisc.edu/) by the University of Wisconsin, School of Medicine and Public Health.[3, 18] Complete details of the ADI construction can be found at the Neighborhood Atlas® website. The Census Block Group (i.e., the closest approximation to a “neighborhood”) was the geographic unit of construction. The Neighborhood Atlas® does not provide the raw ADI score a “neighborhood” receives, as the ADI needs to be used in a rank-type format. Thus, the ADI values were provided in national percentile rankings at the block group level from 1 to 100, so that a block group with a ranking of 1 indicates the lowest level of “disadvantage” within the nation and an ADI with a ranking of 100 indicates the highest level of “disadvantage”. The ADIs are also provided in deciles that are created by ranking the ADI from low to high within each state. In a similar manner, a block group with a ranking of 1 indicated the lowest level of “disadvantage” within the state (i.e., Minnesota in the present study), and 10 indicated the highest ADI (most disadvantaged) within Minnesota. The 2015 ADI uses the American Community Survey (ACS) data for 2015, a 5-year average of ACS data obtained from 2011–2015. Thus, we linked the participants’ geocoded addresses at study baseline (first study visit in 2008–2018, as explained in the “Study sample” section) with the ADI data to obtain both sets of rankings: one relative to the state of Minnesota and one relative to the nation.
2.2 |. Study sample
The MCSA is a population-based cohort study initially established in 2004 in Olmsted County, MN, to identify MCI and dementia risk factors. Using the Rochester Epidemiology Project (REP) resources, an age- and sex-stratified random sample of Olmsted County residents was invited to participate in the MCSA. The MCSA initially recruited 70–89 years old residents; in 2012, recruitment expanded to 50–69 year olds. Participants are also invited for follow-up visits every 15 months, following the same evaluation protocol.
At each MCSA visit, a study coordinator collected sociodemographic factors, asked questions on memory, neuropsychiatric symptoms, and activities of daily living (to an informant). [19, 20] In addition, a physician reviewed the participant’s medical history, administered the Short Test of Mental Status [21], and performed a neurological examination. Nine neuropsychological tests, administered by a psychometrist, were used to assess cognitive performance in four domains: memory, attention/executive function, language, and visuospatial skills. [19, 20] At each MCSA visit, the final diagnosis (cognitively unimpaired (CU), MCI, dementia) was based on a consensus agreement between the study coordinator, the physician, and a neuropsychologist after reviewing all the information for each participant. MCI was diagnosed [22] by a combination of (1) cognitive concerns by the individual, study partner, and/or physician, (2) impairment in one or more cognitive domains, (3) essentially normal functional activities, and (4) does not meet criteria for dementia. Participants were diagnosed with dementia if they met the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for dementia [23]. Individuals who performed in the normal range and did not meet the MCI or dementia criteria were classified as CU. Apolipoprotein E (APOE) ε4 status was determined from blood draw at MCSA baseline assessment. An extensive medical record review occurs every five years for participants lost to follow-up to ascertain incident dementia cases when they reach 70 years of age.
The current study included all MCSA participants residing in Olmsted County, ≥50 years old, with available 2015 ADI values, and at least one visit free of dementia between 2008 and 2018. The time interval of 2008–2018 was chosen blindly (without looking at the data) as the 2015 ADI is based on 2011–2015 ACS data, and we allowed three additional years of observation before and after 2011–2015. There were 4,796 eligible participants for the study. Each participant’s geolocation was calculated by linking addresses to the TIGER/Line address range shapefile provided by the US Census. [24] We were not able to geocode the addresses of 97 participants (18 participants had a non-Olmsted County address, 38 had PO boxes, and 41 had incomplete address details to be safely used). Thus, a total of 4,699 MCSA participants were included in the present study (581 with MCI and 4,118 CU at study baseline).
2.3 |. Standard protocol approvals, registrations, and patient consents
Study approval was obtained from the IRBs of the Mayo Clinic and Olmsted Medical Center in Rochester, Minnesota. Written informed consent was obtained from all participants or, in the case of persons with cognitive impairment sufficient to interfere with capacity, from a legally authorized representative.
2.4 |. Statistical analysis
The study exposure variables were the ADI State and National ranking values. For analytical purposes, the raw scores for the neuropsychological tests in each cognitive domain were z-scored and averaged to create domain-specific cognitive z-scores (i.e., memory, attention/executive, language, and visuospatial skills); a global z-score for overall cognitive performance was created by averaging the four domain z-scores. The outcomes examined in analyses were MCI, dementia, and change in global cognitive z-score. Participants’ characteristics were summarized by ADI quintile using “count (%)” or mean (SD) and compared using χ2 or ANOVA tests as appropriate.
The primary analysis focused on the numeric State and National ADI ranking values. For uniformity, we used deciles in both ADI rankings. Based on the primary analysis findings, sensitivity analyses were employed using the State and National ADI ranking values categorized in quintiles or dichotomous (quintile 5 (most deprived) vs. quintiles 1–4), as was also presented by previous ADI studies, [4, 10] noting that the analyses may not be adequately powered based on the low sample size in quintile 5 for the National ADI ranking values (n=97) specifically.
We considered unadjusted models first and then adjusted for important potential risk factors for cognitive impairment (i.e., age, education, sex, and APOE ε4 allele status (any ε4 vs. none).[25] In cross-sectional analysis, we used logistic regression models and estimates are presented as odds ratios (OR) and 95% confidence intervals (95% CI). Hazard ratios (HR) (95% CI) for progression to dementia were estimated using Cox proportional hazards models using age as the time scale. There were 207 CU participants who did not have study follow-up nor medical record review for inclusion in the Cox models. We also examined the association of baseline ADI (exposure) and change in global cognitive z-score, using mixed-effects models, also adjusted for whether the administration of the cognitive tests was the first time ever (i.e., practice effect), allowing for random subject-specific intercepts and slopes. Estimates are presented as β (95% CI).
Longitudinal analyses (i.e., using Cox proportional hazards models and mixed effects models) in participants with MCI at baseline did not reveal statistically significant associations, possibly due to small numbers and thus lower statistical power; results are not included in the tables.
In addition, we calculated the chronic disease burden at the study baseline from a modified Charlson comorbidity index (Charlson Index) [26] score based on electronic diagnosis codes; dementia codes were not included in the index, as MCSA assesses cognitive status. Adding the Charlson Index in the models did not change estimates appreciably; thus, models are not included in the tables.
Potential effect modification by sex in the association between ADI and dementia was examined using an interaction term between ADI (State and National ranking in deciles) and sex in the Cox models adjusting for sex and education (years) using age as the time scale; no statistically significant interactions were detected. All analyses were considered statistically significant at a p-value <0.05 and were performed using the SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina).
3 |. RESULTS
3.1 |. Participants’ characteristics
The 4,699 participants had a mean age (standard deviation (SD)) of 72.9 (10.5) years, 2,366 (50.4%) were males, 4,598 (97.9%) were white, and 4,656 (99.1%) were not Hispanic or Latino. The characteristics of the participants are presented in Tables 1 and 2 by State and National ADI ranking quintiles for participants without dementia at baseline (Supplementary Tables 1 and 2 present the characteristics of CU participants at baseline, n=4,118). The ADI distribution was skewed, with most participants being in the Q1-Q4 quintiles. Participants in the highest (Q5) ADI National ranking quintile (highest deprivation; Tables 2) were on average older, more likely to be women and have MCI (vs. CU) at study baseline compared to participants in Q1. We were able to geocode 98% of the eligible participants (n=4699). We could not geocode the baseline addresses of 97 (2%) participants with visits in 2008–2018; thus, they were not linked to the ADI values. Participants without geocoding (n=97) were older [77.5 (10.9) vs. 72.9 (10.5) years old], had lower mean years of education [13.5 (2.9) vs. 14.5 (2.7)], and were more likely to have prevalent MCI (22.7% vs. 12.4%), less follow-up time (3.7 (3.2) vs, 4.5 (3.4) years), fewer evaluation visits (3.7 (3.2) vs. 4.5 (3.4)) and shorter follow-up for incident dementia diagnosis (4.8 (2.9) vs. 5.6 (3.2) years) (than participants with successful geocoding).
Table 1.
Characteristics by MN State area deprivation index ranking quantile in participants without dementia.
| State Area Deprivation Index Ranking Quintiles |
||||||
|---|---|---|---|---|---|---|
| Characteristics‡ | 1st (N=1094) | 2nd (N=1057) | 3rd (N=962) | 4th (N=1158) | 5th (N=428) | p value† |
|
| ||||||
|
Study baseline (first visit between 2008–2018) |
||||||
| Age (years) | 71.3 (10.1) | 71.0 (11.0) | 72.8 (10.2) | 75.7 (9.9) | 74.4 (11.2) | <0.001 |
| Sex (male), N (%) | 617 (56.4) | 545 (51.6) | 466 (48.4) | 544 (47.0) | 194 (45.3) | <0.001 |
| APOE ε4, any ε4, N (%) (n=4491) | 274 (26.5) | 280 (28.1) | 260 (28.1) | 318 (28.4) | 113 (27.4) | 0.88 |
| Education (years), (n=4693) | 15.0 (2.8) | 14.6 (2.6) | 14.4 (2.7) | 14.2 (2.7) | 13.7 (2.6) | <0.001 |
| Global cognitive z- score§ (n=4364) | -0.1 (1.1) | -0.1 (1.2) | -0.2 (1.1) | -0.5 (1.1) | -0.5 (1.2) | <0.001 |
| MCI (yes), N (%) | 104 (9.5) | 124 (11.7) | 111 (11.5) | 167 (14.4) | 75 (17.5) | <0.001 |
| Charlson Index ± | 2.9 (3.1) | 2.9 (3.1) | 2.8 (2.8) | 3.6 (3.3) | 3.6 (3.1) | <0.001 |
|
Follow-up
|
||||||
| Number of study visits | 4.4 (2.5) | 4.3 (2.4) | 4.5 (2.6) | 4.4 (2.6) | 4.1 (2.6) | 0.070 |
| Study follow-up (years) ^ | 4.5 (3.4) | 4.4 (3.2) | 4.7 (3.4) | 4.6 (3.5) | 4.2 (3.4) | 0.078 |
| Follow-up for incident dementia (years) ~ | 5.5 (3.2) | 5.4 (3.1) | 5.8 (3.2) | 5.9 (3.3) | 5.5 (3.3) | 0.003 |
| Incident Dementia,≠ N (%) (n=4678) | 86 (7.9) | 84 (8.0) | 92 (9.6) | 154 (13.4) | 76 (17.8) | <0.001 |
Abbreviations: APOE = apolipoprotein E; MCI= Mild cognitive impairment.
Mean (standard deviation) unless otherwise specified; the column also includes the number of participants with available data for variables that had missing data, otherwise n=4699.
ANOVA (continuous variables) or Chi-Square test (categorical variables).
Global cognitive z scores computed after scaling raw cognitive test scores (mean = 0, SD = 1) using data for cognitively unimpaired participants at baseline. Domain specific z scores are summed and scaled to obtain global z scores.
Excluding dementia.
From baseline to last in-person follow-up.
Either until last active follow-up visit or last medical record review date for participants who dropped from active follow-up.
Dementia cases ascertained by medical record review were also included.
Table 2.
Characteristics by National area deprivation index ranking quantiles in participants without dementia.
| National Area Deprivation Index Ranking Quintiles |
||||||
|---|---|---|---|---|---|---|
| Characteristics‡ | 1st (N=551) | 2nd (N=1600) | 3rd (N=1578) | 4th (N=877) | 5th (N=93) | p value† |
|
| ||||||
|
Study baseline (first visit between 2008–2018) |
||||||
| Age (years) | 71.1 (9.9) | 71.1 (10.8) | 73.8 (10.0) | 75.2 (10.6) | 77.0 (10.7) | <0.001 |
| Sex (male), N (%) | 318 (57.7) | 844 (52.8) | 748 (47.4) | 420 (47.9) | 36 (38.7) | <0.001 |
| APOE ε4, any ε4, N (%) (n=4491) | 135 (26.3) | 419 (27.6) | 423 (27.9) | 244 (28.7) | 24 (26.4) | 0.90 |
| Education (years), (n=4693) | 15.4 (2.6) | 14.6 (2.7) | 14.2 (2.6) | 14.3 (2.8) | 13.4 (2.4) | <0.001 |
| Global cognitive z- score,§(n=4364) | 0.0 (1.1) | −0.1 (1.2) | −0.3 (1.1) | −0.5 (1.2) | −0.8 (1.3) | <0.001 |
| MCI (yes),# N (%) | 45 (8.2) | 183 (11.4) | 193 (12.2) | 139 (15.8) | 21 (22.6) | <0.001 |
| Charlson Index ± | 2.9 (3.0) | 2.9 (3.1) | 3.1 (3.0) | 3.6 (3.2) | 4.1 (3.2) | <0.001 |
|
Follow-up
|
||||||
| Number of study visits | 4.3 (2.6) | 4.3 (2.4) | 4.4 (2.6) | 4.5 (2.6) | 3.6 (2.3) | 0.020 |
| Study follow-up (years) ^ | 4.5 (3.4) | 4.4 (3.2) | 4.6 (3.4) | 4.6 (3.4) | 3.7 (3.2) | 0.084 |
| Follow-up for incident dementia (years) ~ | 5.4 (3.2) | 5.5 (3.1) | 5.8 (3.2) | 5.8 (3.3) | 5.4 (3.4) | 0.014 |
| Incident Dementia,≠ N (%) (n=4678) | 44 (8.0) | 126 (7.9) | 172 (11.0) | 126 (14.4) | 24 (25.8) | <0.001 |
Abbreviations: APOE = apolipoprotein E; MCI= Mild cognitive impairment.
Mean (standard deviation) unless otherwise specified; the column also includes the number of participants with available data for variables that had missing data, otherwise n=4699.
ANOVA (continuous variables) or Chi-Square test (categorical variables).
Global cognitive z scores computed after scaling raw cognitive test scores (mean = 0, SD = 1) using data for cognitively unimpaired participants at baseline. Domain specific z scores are summed and scaled to obtain global z scores.
Excluding dementia.
From baseline to last in-person follow-up.
Either until last active follow-up visit or last medical record review date for participants who dropped from active follow-up.
Dementia cases ascertained by medical record review were also included.
3.2 |. MCI, dementia, and cognitive decline
Higher baseline ADI National or State decile rankings at the block group level were associated with higher odds of MCI at study baseline [(OR =1.08, 95%CI (1.05, 1.12), p<0.001; per decile increase in State ADI ranking) and (OR =1.13, 95%CI (1.08, 1.19), p<0.001 per decile increase in National ADI ranking); (Table 3)]. Adjusting for years of education or age attenuated the associations (Table 3); additionally adjusting for sex and APOE ε4 status minimally changed estimates and statistical significance persisted in most models (Supplementary Table 3).
Table 3.
Cross-sectional associations between the area deprivation index and mild cognitive impairment at study baseline (first visit between 2008–2018).
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| ADI ranking, per decile | OR# | 95% CI | p-value | OR# | 95% CI | p-value | OR# | 95% CI | p-value |
|
| |||||||||
| State ADI ranking | 1.08 | (1.05, 1.12) | <0.001 | 1.04 | (1.00, 1.07) | 0.048 | 1.06 | (1.03, 1.10) | <0.001 |
| National ADI ranking | 1.13 | (1.08, 1.19) | <0.001 | 1.06 | (1.01, 1.12) | 0.012 | 1.10 | (1.05, 1.15) | <0.001 |
Abbreviations: ADI, area deprivation index; OR, odds ratio; CI, confidence interval.
OR (95% CI) retained from logistic regression models; model 1 includes ADI as the independent variable and was unadjusted for any other covariates (MCI/CU: 581/4118); model 2 was adjusted for age (MCI/CU:581/4118); model 3 was adjusted for years of education (MCI/CU:580/4113).
In CU participants at baseline, the risk for progression to dementia increased for every decile increase in the ADI State or National ranking [(HR =1.06, 95%CI 1.01,1.11, p=0.010; per decile increase in State ADI ranking) and (HR =1.09, 95%CI (1.02, 1.16), p=0.008), per decile increase in National ADI ranking; Table 4]. Findings did not change appreciably when analysis was adjusted for participant’s education, or education, sex and APOE ε4 status (Table 4).
Table 4.
Association between area deprivation index and incident dementia in cognitively unimpaired at baseline participants.
| Incident Dementia* (outcome) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ADI ranking, per decile | Model 1 | Model 2 | Model 3 | ||||||
|
|
|||||||||
| HR‡ | 95% CI | p-value | HR‡ | 95% CI | p-value | HR‡ | 95% CI | p-value | |
|
| |||||||||
| State ADI ranking | 1.06 | (1.01, 1.11) | 0.010 | 1.06 | (1.01, 1.11) | 0.012 | 1.06 | (1.01, 1.11) | 0.015 |
| National ADI ranking | 1.09 | (1.02, 1.16) | 0.008 | 1.08 | (1.02, 1.15) | 0.010 | 1.08 | (1.02, 1.15) | 0.013 |
Abbreviations: HR, hazards ratio; CI, confidence interval.
All dementia cases, including those ascertained by medical record review.
HR (95% CI) retained from Cox proportional hazards models with age as the time scale; model 1 is unadjusted (events/total: 293/3911; mean(SD) follow-up for events/non-events: 6.0(2.6)/5.9(3.2)); model 2 is adjusted for years of education (events/total: 293/3910; mean(SD) follow-up for events/non-events: 6.0(2.6)/5.9(3.2)); model 3 is additionally adjusted for sex, and Apolipoprotein E ε4 allele status (events total: 292/3786; mean(SD) follow-up for events/non-events: 6.0(2.6)/6.0(3.1)).
In CU participants at baseline, a higher ADI value (i.e., greater deprivation relative to the State or the Nation) was associated with subtly faster cognitive decline (Table 5). Differences in annualized rate of change in global cognitive z-score per State or National ADI ranking decile increase did not change appreciably when analysis was adjusted for age or years of education (Table 5), age sex, APOE ε4 status and years of education (Supplementary Table 4).
Table 5.
The associations between state and national area deprivation index ranking and change in global cognitive z-score in cognitively unimpaired at baseline.
| Change in global cognitive z-score (Outcome) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ADI ranking, per decile | Model 1 | Model 2 | Model 3 | ||||||
|
|
|||||||||
| ß* | (95% CI) | p-value | ß* | (95% CI) | p-value | ß* | (95% CI) | p-value | |
|
| |||||||||
| State ADI ranking | −0.050 | (−0.061, −0.038) | <0.001 | −0.024 | (−0.034, −0.014) | <0.001 | −0.035 | (−0.045, −0.024) | <0.001 |
| Time | −0.012 | (−0.022, −0.002) | 0.016 | −0.008 | (−0.017, 0.002) | 0.13 | −0.011 | (−0.021, −0.002) | 0.022 |
| State ADI ranking*time | −0.007 | (−0.009, −0.005) | <0.001 | −0.007 | (−0.008, −0.005) | <0.001 | −0.007 | (−0.009, −0.005) | <0.001 |
| National ADI ranking | −0.071 | (−0.087, −0.054) | <0.001 | −0.037 | (−0.051, −0.023) | <0.001 | −0.048 | (−0.063, −0.032) | <0.001 |
| Time | −0.007 | (−0.018, 0.005) | 0.26 | −0.002 | (−0.014, 0.009) | 0.71 | −0.006 | (−0.017, 0.006) | 0.31 |
| National ADI ranking*time | −0.009 | (−0.012, −0.007) | <0.001 | −0.009 | (−0.012, −0.007) | <0.001 | −0.009 | (−0.012, −0.007) | <0.001 |
Abbreviations: ADI, area deprivation index; CI, confidence interval.
Estimates of fixed effects from a linear mixed effects model allowing for random individual intercepts and slopes; slope estimates represent an annualized change from baseline.
All models include ADI, time, and the ADI*time interaction. Model 1 controls for whether or not the administration of the cognitive tests was the first time ever (i.e., practice effects); model 2 controls for age (centered for mean age in CU 72.0 years) and practice effects; model 3 controls for years of education (centered for mean years of education in CU 14.6 years) and practice effects. Mean (SD) study follow-up was 4.7 (3.4)) years for N=4,118 participants.
3.3 |. Sensitivity analyses
Participants in the highest State or National ADI deprivation quintile (Q5) had significantly higher odds of MCI (vs. Q1; Supplementary Table 5). Adjusting for education or age attenuated the estimates, while statistical significance was kept.
CU participants in the most disadvantaged neighborhood quintile (Q5), relative to the State ADI ranking, had a significantly higher risk for progression to dementia than the least deprived quintile (Q1) (Supplementary Table 6). Estimates did not change appreciably when models were adjusted for covariates. Similar associations were observed between National ADI quintiles and dementia risk; however, the estimates did not reach statistical significance and the low number of participants in Q5 (n=72) was a limitation, implying inadequate statistical power. Living in the most deprived State quintile vs. not (Q5 vs. Q1–4) was associated with higher risk for progression to dementia (Supplementary Table 6 and Figure 1).
Figure 1.
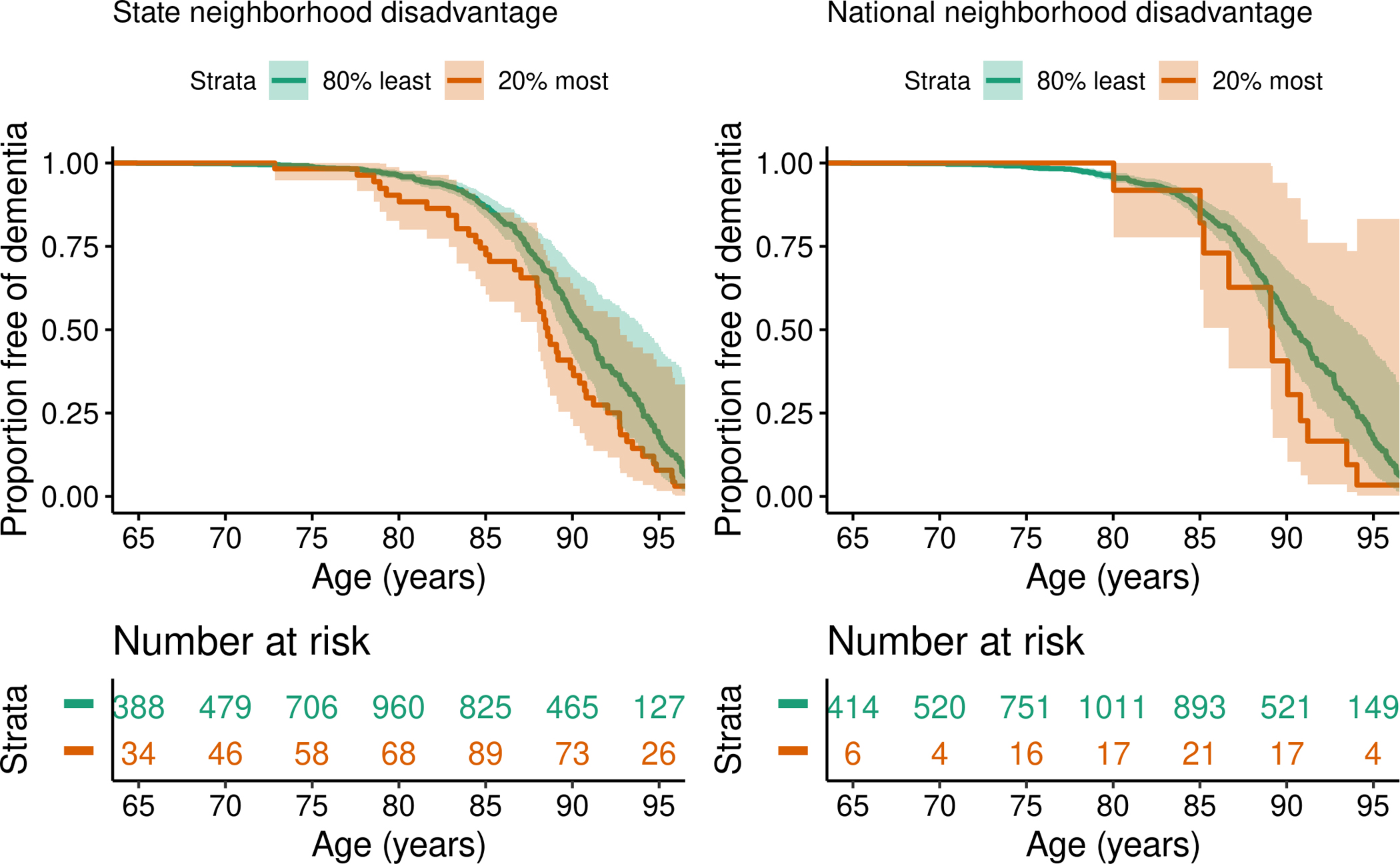
Risk of dementia for cognitively unimpaired participants in the 5th quintile (most deprived) compared to all others by (A) State neighborhood disadvantage (as assessed by the State ADI ranking), and (B) National neighborhood disadvantage (as assessed by the National ADI ranking), conditional on being cognitive unimpaired at age 65 years. Unadjusted analyses using age as the timescale.
Finally, participants in the higher quintiles (Q4 and Q5) for State or National ADI ranking had the greatest annualized decline in the global cognitive z-score from baseline (Supplementary Tables 7 and 8). Estimates did not change appreciably when models were adjusted for covariates.
4 |. DISCUSSION
In community-dwelling older adults (≥50 years old) without dementia at baseline, higher levels of neighborhood socioeconomic deprivation at study baseline, as assessed by ADI on the block group level, were associated with greater odds of MCI. Higher levels of neighborhood socioeconomic deprivation at study baseline were also associated with a higher risk for progression to dementia in CU participants. In sensitivity analysis, we observed that residing in the most socioeconomically deprived neighborhoods relative to the State increased risk for progression to dementia, compared with participants residing in the least deprived neighborhoods. Although differences in cognitive decline were very subtle, CU at baseline declined faster in the most deprived State or National ADI ranking quintiles (vs. the least deprived quintile).
Even within a well-resourced region such as Olmsted County, Minnesota, census block group-level neighborhood disadvantage was associated with an increased risk for progression to dementia and faster cognitive decline, consistent with prior studies, even after accounting for education (i.e., individual-level SES factor). [5, 10, 27] Previous research findings have been variable, with studies [10, 28] also not detecting a significant association between area-based SES measures and dementia risk, especially when individual-level SES factors were taken into consideration. It is plausible that individual-level SES variables partially explain the neighborhood-level SES effect, [28] keeping in mind also that neighborhood-level SES during mid- or late adulthood might not represent early life SES exposures as measures incorporating neighborhood life-course SES would. [29]
Neighborhoods, through their physical and social characteristics, could influence a person’s health behaviors and stress, while neighborhood-level environmental factors related to SES (e.g., air pollution or lead exposure), access to healthy food, or recreation opportunities could contribute to causal pathways between neighborhood-level SES and cognitive health. [9, 10, 29] Socioeconomically disadvantaged neighborhoods might have more stressors and less physical and social resources, hindering social, physical, and cognitively beneficial activities. [30, 31] For example, that poor individuals who live in wealthier neighborhoods could have better health outcomes than poor people living in the most deprived areas. [3] Thus, more emphasis and attention need to be given to how the neighborhood context can provide opportunities that facilitate healthy behaviors which support cognitive health.
The number of studies examining the association of neighborhood socioeconomic deprivation with cognitive decline and impairment is still limited, especially for population-based studies and for earlier cognitive impairment stages, including mild cognitive impairment (MCI), a precursor of dementia. [3, 5, 32, 33] Prior research suggests variable associations of neighborhood socioeconomic disadvantage and cognitive outcomes, also suggesting variable associations by individual-level socioeconomic characteristics, race, ethnicity. [34–36] Detailed cognitive evaluations are not included in all studies or prospective follow-up. MCSA, a population-based cohort study with detailed serial cognitive evaluations, adds significant findings to mounting evidence of an association between area-level socioeconomic deprivation and cognitive impairment and decline. MCSA provides multiple cognitive outcomes, with prospective follow-up, also facilitating comparisons with past and future studies of MCI, progression to dementia, and change in cognitive function. In addition, MCSA is among the first studies with cognitive outcomes to use the publicly available ADI data. Including both the State and National ADI ranking data would allow further comparisons with future studies both within the state and nationwide.
It is becoming more evident that together with the necessary downstream interventions (at the individual level), upstream (e.g., public policy) and midstream (e.g., community) interventions can be employed to improve cognitive health and reduce disparities. [37] Area-level deprivation could drive multiple disease-risk pathways causing health disparities and needs to be also considered when disease-modifying interventions are implemented. [38] Disease interventions that do not account for neighborhood deprivation could provide limited help or fail. [3]
MCSA invites participants into the study using an age- and sex-stratified random sample of Olmsted County residents. This is achieved using the REP resources; the REP Census counts are comparable to decennial US censuses (e.g., REP included 102.7% of 2000 census counts).[39] MCSA, going forward, will continue to use the publicly available ADI data to understand better how neighborhood context affects brain health and incorporate information as needed going forward. Study findings underline the importance and critical information that neighborhood context could provide for research, outreach and community-based care management, and policy.[3, 40] Area-level socioeconomic measures like ADI could help identify individuals at higher risk for developing cognitive impairment and inform clinical vigilance, as well as, research design, recruitment, and retention strategies that support participation in longitudinal studies and prevent underrepresentation of socioeconomically disadvantaged participants. To this end, soliciting stakeholder engagement from socioeconomically deprived communities could also improve recruitment and retention practices, facilitate dissemination of findings, and help design therapeutic and preventive interventions that reach individuals accounting also for the neighborhood socioeconomic status.[3, 10, 41]
The study should be considered in light of its limitations. We had only one measurement of ADI (2015) on the census block group level of the place of residence, and we cannot account for years of exposure at this ADI level and thus may not capture neighborhood deprivation exposure accurately across the life span. Study participants were 97.9% white, and 99.1% were not Hispanic or Latino. Thus, investigating these associations in areas with more racial and ethnic diversity would be desirable. The low number of participants in the most deprived National ADI ranking quintile should be considered in interpreting findings, probably resulting in inadequate statistical power in these analyses. Our study could not account for homelessness. We cannot be certain that unmeasured factors did not influence the observed associations. In addition, alternative interpretations of the findings could be considered, e.g., relating to selection bias, i.e., if persons living in disadvantaged neighborhoods would be more likely to participate in MCSA when worrisome symptoms are present compared to persons living in less disadvantaged areas. The underlying mechanisms of the area’s effects on health and dementia are expected to be intricate [2] and possibly bidirectional (e.g., chronic conditions could influence elements included in ADI measures such as income, employment, or education),[12, 13] emphasizing the need for further research, beyond the current study’s scope.
The study has important strengths. The MCSA is a well-characterized cohort with a comprehensive cognitive evaluation. The evaluation and diagnosis were made without ADI knowledge, blind to any previous clinical diagnosis, and followed the same protocol in all visits. The study uses ADI [38] a novel, publicly available, validated composite measure of neighborhood disadvantage.
In conclusion, higher baseline neighborhood socioeconomic deprivation levels, as assessed by ADI on the block group level, were associated with higher odds of MCI at baseline and higher risk for progression to dementia and faster cognitive decline in CU at baseline older adults. Continued research is needed on the association of neighborhood socioeconomic disadvantage and cognitive impairment to understand the potential pathways involved and provide further valuable insight for public policies, community interventions, social and health care to prevent and treat cognitive impairment.
Supplementary Material
Highlights.
The study used ADI, a composite freely available neighborhood deprivation measure.
Higher levels of neighborhood deprivation were associated with greater MCI odds.
Higher neighborhood deprivation levels were associated with higher dementia risk.
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the literature using PubMed and combinations of keywords (i.e., “area deprivation”, “area socioeconomic”, “socioeconomic deprivation”, “socioeconomic disadvantage”, “neighborhood deprivation” “neighborhood disadvantage”, “cognitive ”, “cognition”, “dementia”, “Alzheimer”). There is a limited number of studies examining the association of neighborhood socioeconomic deprivation and cognitive impairment and the relevant citations are appropriately cited.
Interpretation: Study findings suggest that higher levels of neighborhood deprivation were associated with greater odds of MCI at study baseline and higher risk for progression to dementia in cognitively unimpaired persons at study baseline.
Future directions: We need to continue examining the association of neighborhood socioeconomic deprivation and cognitive impairment to understand potential pathways involved, which are expected to be complex, and provide insight for public policy, facilitate community interventions, and support social and health care to prevent, postpone and treat cognitive impairment.
ACKNOWLEDGEMENTS
The study was supported by the National Institutes of Health (U01 AG006786, P30 AG062677, R01AG057708, R37 AG011378, R01 AG041851, R01 NS097495), the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation, the Schuler Foundation and was made possible by the Rochester Epidemiology Project (R01 AG034676). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Author Disclosures
Maria Vassilaki –Has received research funding from F. Hoffmann-La Roche Ltd and Biogen and consulted for F. Hoffmann-La Roche Ltd; she receives research funding from NIH and St. Anne’s University Hospital Brno, International Clinical Research Center, Czech Republic/EU and has equity ownership in Abbott Laboratories, Johnson and Johnson, AbbVie, Medtronic, and Amgen.
Jeremiah A. Aakre – Reports no disclosures.
Anna Castillo - Reports no disclosures.
Alanna M. Chamberlain - Receives research funding from NIH, PCORI, and EpidStrategies for research in collaboration with Amgen, Inc.
Patrick M. Wilson - Reports no disclosures.
Walter K. Kremers – Receives research funding from Department of Defense, NIH, Astra Zeneca, Biogen and Roche.
Michelle M. Mielke – Has consulted for Brain Protection Company and Biogen, and receives research funding from the NIH/NIA.
Yonas E. Geda – Receives funding from NIH and Roche, and previously served on Lundbeck Advisory Board.
Mary M. Machulda – Receives NIH funding.
Rabe E. Alhurani - Reports no disclosures.
David S. Knopman – Serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by Lilly Pharmaceuticals, Biogen and the Alzheimer’s Treatment and Research Institute at USC; and receives research support from the NIH.
Jonathan Graff-Radford - receives support from the NIH
Prashanthi Vemuri – Receives NIH funding
Val J. Lowe – Serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, Merck Research, AVID Radiolpharmaceuticals and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI)
Clifford R. Jack Jr - Serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH, the GHR Foundation and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic.
Ronald C. Petersen – Consultant for Roche, Inc., Biogen, Inc., Merck, Inc., Eli Lilly and Company, and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institute of Health.
REFERENCES
- [1].Powell WR, Buckingham WR, Larson JL, Vilen L, Yu M, Salamat MS, et al. Association of Neighborhood-Level Disadvantage With Alzheimer Disease Neuropathology. JAMA Netw Open. 2020;3:e207559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Basta NE, Matthews FE, Chatfield MD, Brayne C, Mrc C. Community-level socio-economic status and cognitive and functional impairment in the older population. European journal of public health. 2008;18:48–54. [DOI] [PubMed] [Google Scholar]
- [3].Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. 2018;378:2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chamberlain AM, Rutten LJF, Wilson PM, Fan C, Boyd CM, Jacobson DJ, et al. Neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. Bmc Public Health. 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCann A, McNulty H, Rigby J, Hughes CF, Hoey L, Molloy AM, et al. Effect of Area-Level Socioeconomic Deprivation on Risk of Cognitive Dysfunction in Older Adults. J Am Geriatr Soc. 2018;66:1269–75. [DOI] [PubMed] [Google Scholar]
- [6].Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roberts RO, Cha RH, Mielke MM, Geda YE, Boeve BF, Machulda MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84:1854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Resende EDF, Guerra JJL, Miller BL. Health and Socioeconomic Inequities as Contributors to Brain Health. JAMA Neurol. 2019;76:633–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–45. [DOI] [PubMed] [Google Scholar]
- [10].Hunt JFV, Vogt NM, Jonaitis EM, Buckingham WR, Koscik RL, Zuelsdorff M, et al. Association of Neighborhood Context, Cognitive Decline, and Cortical Change in an Unimpaired Cohort. Neurology. 2021;96:e2500–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clarke PJ, Weuve J, Barnes L, Evans DA, Mendes de Leon CF. Cognitive decline and the neighborhood environment. Ann Epidemiol. 2015;25:849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris JR, Wallace RB. The Institute of Medicine’s new report on living well with chronic illness. Prev Chronic Dis. 2012;9:E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mitchell R, Cameron CM, Lystad RP, Nielssen O, McMaugh A, Herkes G, et al. Impact of chronic health conditions and injury on school performance and health outcomes in New South Wales, Australia: a retrospective record linkage study protocol. BMJ Paediatr Open. 2019;3:e000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barr R The Neighborhood Atlas—Free social determinants of health data for all! 2019. https://www.nia.nih.gov/research/blog/2019/11/neighborhood-atlas-free-socialdeterminants-health-data-all. Accessed December 8, 2021
- [15].Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis. 2015;25:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].National Institute on Aging. Health Disparities Framework.
- [17].Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. [DOI] [PubMed] [Google Scholar]
- [22].Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- [23].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. ed 4. ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- [24].Bureau. USC. 2016. TIGER/Line shapefiles technical documentation. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html. Accessed June 15, 2021
- [25].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [26].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [27].Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2008;56:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A. Individual and Area-Based Socioeconomic Factors Associated With Dementia Incidence in England: Evidence From a 12-Year Follow-up in the English Longitudinal Study of Ageing. JAMA Psychiatry. 2018;75:723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].George KM, Lutsey PL, Kucharska-Newton A, Palta P, Heiss G, Osypuk T, et al. Life-Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2020;189:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Krell-Roesch J, Vemuri P, Pink A, Roberts RO, Stokin GB, Mielke MM, et al. Association Between Mentally Stimulating Activities in Late Life and the Outcome of Incident Mild Cognitive Impairment, With an Analysis of the APOE epsilon4 Genotype. JAMA Neurol. 2017;74:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krell-Roesch J, Syrjanen JA, Vassilaki M, Machulda MM, Mielke MM, Knopman DS, et al. Quantity and quality of mental activities and the risk of incident mild cognitive impairment. Neurology. 2019;93:e548–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Bmj. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marengoni A, Fratiglioni L, Bandinelli S, Ferrucci L. Socioeconomic status during lifetime and cognitive impairment no-dementia in late life: the population-based aging in the Chianti Area (InCHIANTI) Study. J Alzheimers Dis. 2011;24:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aneshensel CS, Ko MJ, Chodosh J, Wight RG. The urban neighborhood and cognitive functioning in late middle age. J Health Soc Behav. 2011;52:163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rosso AL, Flatt JD, Carlson MC, Lovasi GS, Rosano C, Brown AF, et al. Neighborhood Socioeconomic Status and Cognitive Function in Late Life. Am J Epidemiol. 2016;183:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sheffield KM, Peek MK. Neighborhood context and cognitive decline in older Mexican Americans: results from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Am J Epidemiol. 2009;169:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dopp AR, Lantz PM. Moving Upstream to Improve Children’s Mental Health Through Community and Policy Change. Administration and Policy in Mental Health and Mental Health Services Research. 2020;47:779–87. [DOI] [PubMed] [Google Scholar]
- [38].Zuelsdorff M, Larson JL, Hunt JFV, Kim AJ, Koscik RL, Buckingham WR, et al. The Area Deprivation Index: A novel tool for harmonizable risk assessment in Alzheimer’s disease research. Alzheimers Dement (N Y). 2020;6:e12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an Area Deprivation Index Measuring Patient Socioeconomic Status in an Integrated Health System: Implications for Population Health. EGEMS (Wash DC). 2016;4:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chamberlain AM, St Sauver JL, Finney Rutten LJ, Fan C, Jacobson DJ, Wilson PM, et al. Associations of Neighborhood Socioeconomic Disadvantage With Chronic Conditions by Age, Sex, Race, and Ethnicity in a Population-Based Cohort. Mayo Clin Proc. 2022;97:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


