Abstract
Purpose
Zona pellucida-free (ZP-free) embryos often fail to achieve good developmental outcomes and are routinely discarded in assisted reproductive laboratories. Existing attempts to rescue ZP-free embryos are not widely used due to operational complexity and high technical requirements. To handle cases with missing ZP, we applied modified sodium hyaluronate gel (MSHG) to embryo culture to determine if it can function as a substitute for human zona pellucida.
Methods
The developmental process and the blastocyst formation rate of embryos were analyzed in both mouse and human. The first clinical application of MSHG was reported, and the pregnancy outcome was continuously followed up.
Results
Human and mouse ZP-free embryos cultured with MSHG showed a blastocyst formation rate similar to ZP-intact embryos. MSHG improves blastocysts formation rate by maintaining blastomere spatial arrangement at early stages. Compared to ZP-free embryos, the proportion of tetrahedrally arranged blastomeres at the 4-cell stage increased significantly in embryos cultured with MSHG in humans. A ZP-free blastocyst cultured in MSHG with the highest score was successfully implanted after day 5 transplantation and developed normally.
Conclusion
These data demonstrate that MSHG can substitute the function of zona pellucida and rescue human ZP-free embryos during assisted reproductive technology.
Keywords: Modified sodium hyaluronate gel (MSHG), Zona pellucida, Assisted reproductive technology, Blastocyst
Introduction
As a hard shell encircling oocytes, zona pellucida plays an essential protective role during fertilization and early embryonic development, including recognition of and binding to the spermatozoa, induction of the acrosome reaction, blocking of polyspermy [1–4]. Also, zona pellucida helps to keep the adhesion among blastomeres, as blastomeres are loosely attached to each other and can easily be dissociated before compaction occurs [5]. In assisted reproduction laboratories, the existence of zona pellucida is good for performing fine micromanipulation on oocytes. However, during micromanipulation, such as oocyte retrieval or cumulus cell removal, zona pellucida are sometimes damaged accidentally, resulting in zona pellucida-free (ZP-free) oocytes. Such ZP-free oocytes are routinely discarded in patients with many oocytes retrieved. However, in low ovarian response patients or patients with only ZP-free oocytes, these oocytes need to be kept for transplantation.
In fact, some ZP-free human oocytes are still able to be normally fertilized and further develop to the blastocyst stage without adversely affecting subsequent pregnancy rates [6–9]. Also, one blastocyst from a ZP-free egg was reported to be able to undergo biopsy and have a normal chromosome complement [10]. Furthermore, the first live births of singleton and twins from ZP-free oocytes were reported in several case reports [11, 12]. However, the problem of loosely attached blastomeres and the risk of blastomere separation before compaction still exists in ZP-free embryos. Time-lapse imaging clearly showed this development process [13]. It has also been shown in mice that the developmental potential of ZF ZP-free embryos directly correlates with the number of cell contacts observed in a 4-cell-stage embryo[14].
To solve this problem, many attempts have been made to protect ZP-free embryos, such as alginate encapsulation [15], Well of the Well (WOW) system [16], “GO” culture system[17], and agarose capsules [18]. These attempts have achieved improvement, but they all have the characteristics of either operational complexity or high technical requirements. There are also concerns about insufficient exchange of nutrients and toxicity of materials, as embryos constantly consume nutrients from their surroundings and secrete factors that affect development [19, 20]. Therefore, there is a lack of methods for human ZP-free embryo culture and manipulation that can be widely used in the clinic.
Double-phase modified sodium hyaluronate gel (MSHG) is a medical product used for human facial dermal tissue injection. It is composed of crosslinked and free hyaluronic acid (HA). Hyaluronic acid is present in almost all biological fluids and tissues. It is particularly abundant in embryonic tissues and in the extracellular matrix (ECM) of adult soft connective tissues [21]. In physiological conditions, HA takes the form of a sodium salt that is negatively charged and highly hydrophilic [22, 23]. HA possesses exceptional physicochemical properties such as biocompatibility and biodegradability, non-inflammatory, non-toxicity, and non-immunogenic behavior [24]. It is widely used in osteoarthritis [25, 26], ophthalmological surgeries [27, 28], cosmetic regeneration, and reconstruction of soft tissue [29, 30]. Considering its non-toxicity and gel state, we use MSHG to assist the culture of ZP-free embryos and substitute the function of zona pellucida.
In this study, we first tested MSHG in a mouse embryo culture medium. The blastocyst formation rate of ZP-free embryos with MSHG was analyzed. We then applied MSHG in human embryos, and the blastocyst formation rate and the 4-cell-stage blastomere spatial arrangement were analyzed. Lastly, we reported the first case of pregnancy outcome from a patient who sought assisted reproduction, whose embryo was ZP-free and treated with MSHG.
Materials and methods
Human oocytes and embryos
Human oocytes were derived from clinically useless immature MI oocytes donated by patients after signing informed consent from May 2021 to November 2021. The MI oocytes were cultured in vitro maturation (IVM) medium at 37 ℃ in an atmosphere with 6% CO2 and 5% O2 for 18–24 h [31]. Intracytoplasmic sperm injection (ICSI) was performed on mature MII oocytes with donated sperm. A total of 156 zygotes with two pronuclei were included in this study, of which 116 ZP-free zygotes were produced with a micromanipulation system (NARISHIGE, Japan), following the steps below. After the zygotes were fixed by a holding pipette (Sunlight Medical, USA), two openings of different sizes were cut on zona pellucida with a PZD pipette (Sunlight Medical, USA). The small opening was in the 2 o’clock direction, and the size was about 20 μm; the large opening was located in the 6 o’clock direction, in the shape of a cross, with a size of about 100 μm. Then, the pressure in the blastomere biopsy pipette (35 μm, beveled tip, Sunlight Medical, USA) was released from the small opening to make the zygote escape from the large opening. At last, all the zygotes were cultured under 6% CO2 and 5% O2 in 16-well dishes (HUCHUANG Life Sciences, China) to the blastocyst stage. K-SICM and K-SIBM culture media (Cook, Australia) were used sequentially for embryo culture. The scoring of blastocysts was based on the expansion state of blastocysts and the consistency of inner cell mass and trophoblast cells. Only embryos with a score of 4BC, 5BC, 6BC, or above (AA, AB, BA, BB) are high-quality blastocysts [32]. Blastocyst formation rate was defined as the number of high-quality blastocysts/the number of 2PN zygotes.
Mouse oocytes and embryos
ICR mice purchased from Charles River were used in this experiment. All mice were housed under standard 12-h light–dark cycle conditions in a specialized and certified animal facility. Additional information about handling and injecting mice, as well as embryo recovery techniques, can be found in other resources [33]. To induce superovulation, 6- to 8-week-old female mice were injected intraperitoneally with 10 IU pregnant mare serum gonadotropin (PMSG) (#110,914,564, Ningbo Sansheng Biological Technology Co., LTD), followed by 10 IU human chorionic gonadotropin (hCG) (#110,911,282, Ningbo Sansheng Biological Technology Co., LTD) 48 h later. Oocytes were collected at 15 h after hCG injection. First, the collected oocytes were fertilized in G-IVF (#10,136, Vitrolife) with epididymal sperms from adult males capacitated in G-IVF for 1 h. A total of 720 zygotes with two pronuclei were included in the study, of which 480 ZP-free zygotes were produced in a way similar to humans. At last, all the zygotes were cultured in G1 medium (Vitrolife, Sweden) to the blastocyst stage under 6% CO2, 5% O2 at 37 ℃.
Preparation of culture medium with MSHG
For humans, the culture media K-SICM (Cook, Australia), paraffin oil (Vitrolife, Sweden), and double-phase modified sodium hyaluronate gel (MSHG, Bloomage Biotechnology Corporation Limited, China) were taken out from a 4 ℃ refrigerator and restored 30 min to room temperature. Double-phase MSHG is composed of modified sodium hyaluronate, sodium chloride, phosphate buffer, and water for injection. It has been approved by China National Medical Products Administration for facial dermal tissue injection. In the meanwhile, the mouth of a Pasteur pipette (ORIGIO, USA) was pinched into a flat shape with tweezers and a high-temperature flame. Then about 10 mg MSHG was added to each well of the 16-well dish and tiled gently at the bottom of the dish with the flat Pasteur pipette until the gel blocks are filled with the bottom of the culture dish. At last, 25 μl K-SICM was added to each well, covered with 3 ml paraffin oil. The culture medium was pre-equilibrated in an incubator under 6% CO2, 5% O2 at 37 ℃ for at least 5 h. Bubbles were removed from the culture medium with a new Pasteur pipette before use. When using, zygotes were transferred to the space between the gel blocks, one zygote per well. The culture medium was changed on D4 when the morula was formed. Approximately 80% of the existing medium was removed, then pre-equilibrated K-SIBM medium was added without disturbing the embryos.
For the mouse, 30 embryos were cultured together in each well of 4-well dishes (Thermo Scientific), with about 200 mg MSHG and 600 ul G1 in each well. The preparation procedure of the culture medium was similar to that of the human.
Statistical analysis
Proportional data were analyzed using the Fisher exact test or GraphPad. Differences were considered significant when p-values were less than 0.05.
Results
MSHG substitutes the function of zona pellucida in mouse embryos
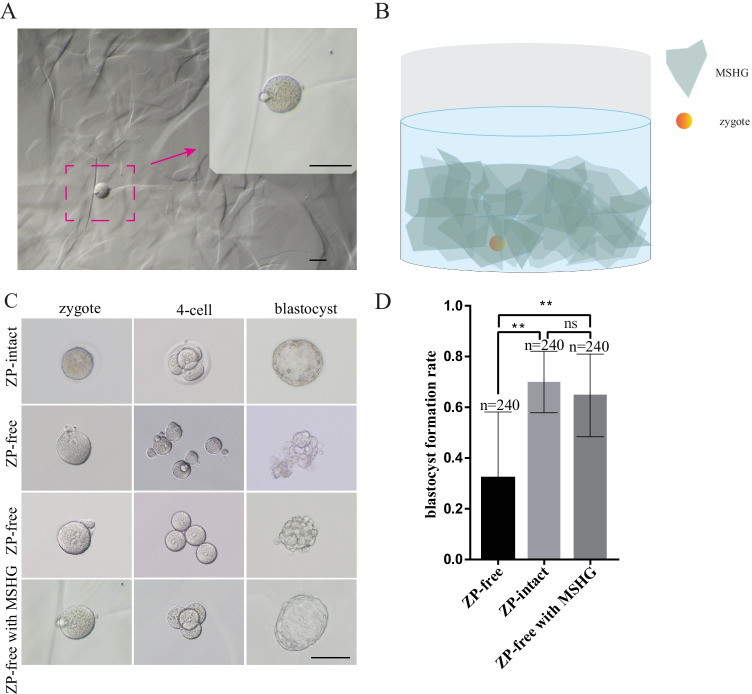
The gel blocks vary in size and resemble angular stones in shape. In the culture medium, 2–3 layers of gel blocks were uniformly deposited at the bottom of the culture dish. The embryo was placed in a proper position to ensure that the gel blocks can be well stacked around the embryo. When there was no external force, the relative position among the gel blocks was fixed, and a stable supporting structure was formed (Fig. 1B).
Fig. 1.
The validity of MSHG in mouse embryo culture. A Bright-field microscope shows an overview of MSHG in a culture medium under different microscope magnifications. B A schematic diagram of a mouse oocyte and gel blocks in the culture medium. C Bright-field microscope shows the developmental process of embryos from ZP-free, ZP-intact, and ZP-free with MSHG. D Comparison of blastocyst formation rate of ZP-free, ZP-intact, and ZP-free with MSHG. **p < 0.01; ns, not significant. Scale bar = 100 um
To address if MSHG could substitute the function of zona pellucida, we first tested the developmental potential of mouse embryos. The shape and size of mouse zygotes, as well as the pronuclear morphology, were not changed when placed in the culture medium with MSHG (Fig. 1A), meaning the hydrostatic pressure and osmotic pressure were kept balanced. When a zygote began to undergo divisions, MSHG performed its main function by surrounding the embryos and forming a barrier that prevented the dispersion of embryos (Fig. 1C). To test if nutrients can be efficiently exchanged among culture medium and MSHG gel blocks, blastocyst formation rate, which could reflect the developmental potential of embryos, was analyzed. The blastocyst formation rate of embryos with MSHG was slightly lower than in ZP-intact embryos, but still significantly higher than that in ZP-free embryos (Fig. 1D).
In conclusion, as a substitution for zona pellucida, MSHG is safe and functional for the culture of ZP-free embryos in the mouse.
Application of MSHG in human embryo culture
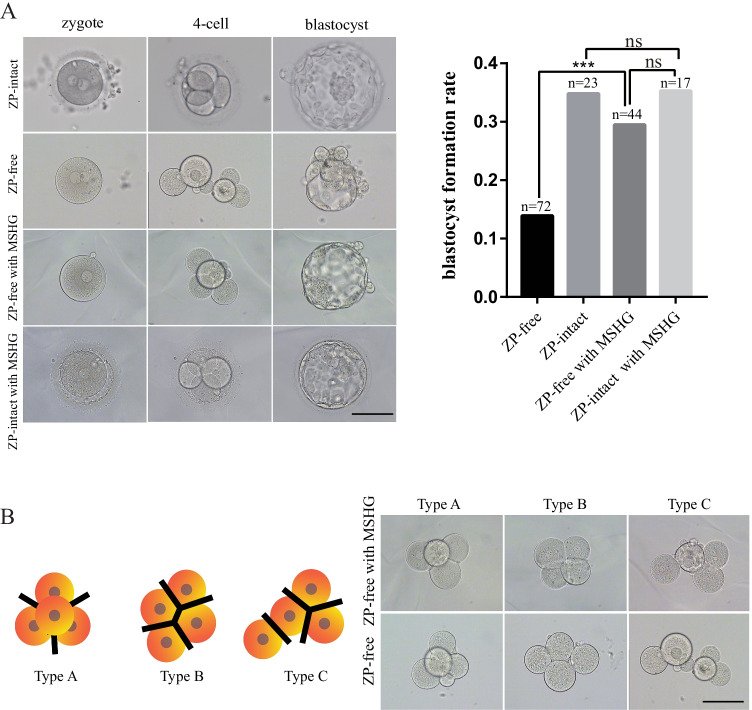
Having validated the usage of MSHG in mouse embryo culture medium, we employed MSHG in donated human embryos to test whether it can be used for clinical application. We first cultured ZP-intact embryos in a culture medium with MSHG, and those embryos had similar morphological status and high-quality blastocyst formation rate compared with ZP-intact embryos (Fig. 2A). Similar to mouse embryos, the high-quality blastocyst formation rate was low in ZP-free embryos and was largely rescued in embryos with MSHG. All embryos of the four groups showed normal developmental speed (4-cell stage, 44 ± 1 h; 8-cell stage, 68 ± 1 h; morula, 92 ± 2 h; blastocyst, 116 ± 2 h) [34].
Fig. 2.
Application of MSHG in human embryo culture. A Bright-field microscope shows the development process of embryos from ZP-free, ZP-intact, ZP-free with MSHG, and ZP-intact with MSHG. And comparison of blastocyst formation rate among the four groups. ***p < 0.01; ns, not significant. B Classification criteria of blastomere spatial arrangement. Type A, tetrahedrally arranged blastomeres, with 6 points of contact. Type B, planarly arranged blastomeres, with 5 points of contact. Type C, linearly arranged blastomeres, with 4 points of contact. Scale bar = 100 um
Blastomere arrangement was found to be associated with blastocyst formation rate in ZP-intact human embryos [35, 36]. In fact, the blastomeres of ZP-free embryos tended to move at the 4-cell stage, resulting in a variety of arrangements [6, 14, 37]. We hypothesized that, in addition to its main role in preventing the dispersion of embryos, MSHG plays a critical role in maintaining the spatial arrangement of blastomere during early embryo development, especially from 4-cell to the blastocyst stage. Considering that some embryos degenerated at early stages, we only analyzed blastomere spatial arrangement of the 4-cell stage in 23 high-quality blastocysts from ZP-free and ZP-free with MSHG groups. The blastomere spatial arrangement was classified into 3 types according to their blastomere contact (Fig. 2B). In the ZP-free with MSHG group, the proportion of type A was significantly higher than in the ZP-free group, while the proportion of type B was significantly lower than the ZP-free group. Both groups had a low proportion of type C, which was hardly seen in ZP-intact embryos (Table 1).
Table 1.
Comparison of the proportion of type A, type B, and type C in ZP-free and ZP-free with MSHG
| ZP-free | ZP-free with MSHG | P value | |
|---|---|---|---|
| Type A | 20 (2/10) | 69.2 (9/13) | 0.036 |
| Type B | 70 (7/10) | 23.1 (3/13) | 0.040 |
| Type C | 10(1/10) | 7.7 (1/13) | NS |
In conclusion, MSHG does not affect the morphological status and development speed of embryos, and it improves the high-quality blastocysts formation rate by maintaining blastomere spatial arrangement at early stages.
Clinical treatment by MSHG during assisted reproductive technology
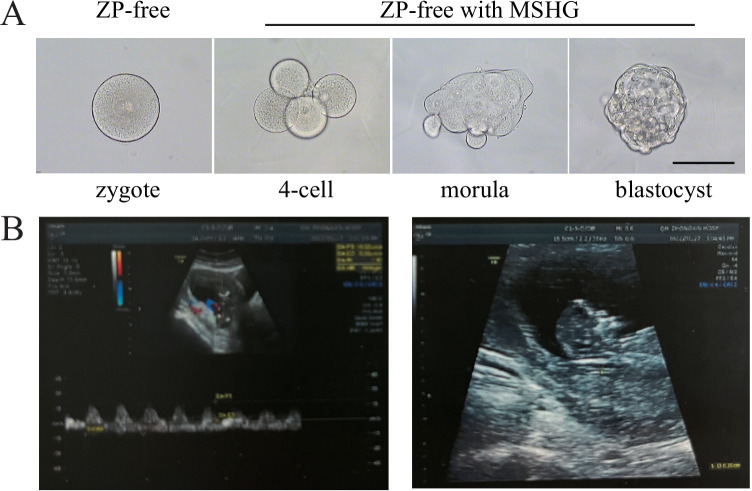
A 25-year-old secondary infertility female patient sought assisted reproductive treatment in our center due to fallopian tube obstruction. Her husband was 26 years old with relatively normal semen analysis (sperm concentration, 141 × 106/ml; motility, 42.2%; normal morphology, 2.87%). Controlled ovarian hyperstimulation was performed using a long GnRH-agonist protocol. During the egg retrieval, a total of 5 oocytes with cumulus complexes were obtained, and the common in vitro fertilization (IVF) was carried out later. When removing granulosa cells 5 h after insemination, one oocyte escaped from the ruptured zona pellucida and became ZP-free. The next morning, only 3 oocytes showed two pronuclei, including the ZP-free one. Considering the scarcity of embryos in this patient, the ZP-free zygote was transferred to a new culture medium with MSHG after signing informed consent by the patient. On day 5, the ZP-free embryo developed into a blastocyst with the highest score (4AB) (Fig. 3A), and the remaining two embryos developed both into low-grade blastocysts (4BC). After communication with the clinical doctor and the patients, the ZP-free blastocyst was chosen for transplantation. During the embryo transfer, the ZP-free blastocyst was picked from the culture medium with MSHG and cleaned several times with K-SIBM to ensure no gel blocks remained on the surface.
Fig. 3.
The ZP-free embryo that transferred during assisted reproductive technology. A Bright-field microscope shows the development process of the transplanted embryo. B Ultrasound image of fetus at 12 weeks. Scale bar = 100 um
Embryo transfer was carried out on November 23, 2021, and 12 days after embryo transfer, pregnancy was confirmed with a β-HCG value of 2092 IU/L. On January 11, 2022, a gestational sac was detected in the uterine cavity by ultrasound, with a size of about 6.2 × 5.1 cm. A fetal bud echo was detected in the gestational sac, with a length of 3.0 cm. Cardiac pulsation was clearly visible. On January 27, 2022, a fetal echo was detected in the uterine cavity. The head hip length was about 8.9 cm, and the fetal heartbeat was 149 times/min (Fig. 3B).
In our study, for the first time, MSHG was applied in the clinic.
Discussion
In this study, we examined if MSHG can functionally substitute zona pellucida in human embryos. Double-phase MSHG was composed of crosslinked sodium hyaluronate gel and free sodium hyaluronate. The free sodium hyaluronate is water-soluble, allowing nutrients exchange, and the crosslinked sodium hyaluronate gel can form numerous independent micro-spaces to provide the supporting structure for the embryo. Besides, it has been widely used in medical products [38]. Thus, MSHG has the property of biocompatibility and non-toxicity and is suitable for in vitro culture of ZP-free embryos.
After embryo division, the most important role of zona pellucida is to prevent the dispersion of embryos and further to maintain the spatial arrangement of blastomeres, especially at the 4-cell stage. In ZP-intact embryos, most of the 4-cell embryos were tetrahedrally arranged, and only a small number of embryos were planarly arranged [36]. Tetrahedrally arranged embryos have more cell–cell contacts than the other two spatial arrangements. Critical developmental benchmarks, such as the 8-cell stage, compaction, morula formation, and developing to an extended blastocyst, are aided by closer proximity of blastomeres and increased cell–cell contacts [39–43]. Embryos with planarly arrangement were associated with either a poor prognosis for blastocyst formation or a low implantation rate after the transfer [35, 44, 45]. When using MSHG, 2–3 layers of gel blocks are stacked around the embryos, which form a three-dimensional space like zona pellucida to keep the adhesion among blastomeres and further to maintain the spatial arrangement of blastomeres. In this study, we show that blastomeres without zona pellucida form closer contact with the help of MSHG, and thus, the proportion of tetrahedral arrangement is improved, increasing the blastocyst formation rate of ZP-free embryos.
Meanwhile, the application of MSHG is feasible in the clinic for human ZP-free embryo culture since it does not require any additional manipulation of the embryo and the preparation of the culture dish is conventional. The embryologist only needs to lay the gel on the bottom of the dish before adding the culture medium and confirm that the gel blocks are completely filled with the culture dish and that the position of the gel blocks is fixed. The numerous spaces formed between the gel blocks allow multiple ZP-free embryos to be cultured in one culture dish. When changing the culture medium, the gel blocks sometimes move as the culture medium decreases or increases. Therefore, the culture medium was changed only when the morula was formed.
Remarkably, we reported the first live fetus from a ZP-free embryo cultured with MSHG. This embryo developed into a higher-quality blastocyst compared with the other two ZP-intact embryos. The successful implantation of the blastocyst proves that MSHG can be applied in clinical conditions.
To conclude, our study proposed a novel method for the culture of ZP-free embryos. MSHG maintained the tight contact of blastomeres at the early embryo stage and significantly improved the development potential of ZP-free embryos. This gives embryologists another option when dealing with ZP-free embryos.
Acknowledgements
We would like to thank Xueping Guo, Ph.D. (Bloomage Biotechnology Corporation Limited), for providing double-phase modified sodium hyaluronate gel (MSHG).
Author contribution
Study conception and design were performed by Keliang Wu. Material preparation, data collection, and analysis were performed by Jinzhu Song, Jingye Zhang, Xinyi Yuan, Boyang Liu, Wenrong Tao, and Chuanxin Zhang. The first draft of the manuscript was written by Jinzhu Song and Jingye Zhang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by The National Key Research and Development Program of China (2021YFC2700300) and the National Natural Science Foundation of China (32170817, 32100650).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Reproductive Study Ethics Committee in the Center for Reproductive Medicine, Shandong University, China (2021/Ethical Review #01 and Ethical Review #56).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for the publication of the images in Fig. 3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinzhu Song and Jingye Zhang contributed equally to this work.
References
- 1.Wassarman P, Chen J, Cohen N, Litscher E, Liu C, Qi H, et al. Structure and function of the mammalian egg zona pellucida. J Exp Zool. 1999;285(3):251–258. doi: 10.1002/(SICI)1097-010X(19991015)285:3<251::AID-JEZ8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Bleil JD, Wassarman PM. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- 3.Fahrenkamp E, Algarra B. Mammalian egg coat modifications and the block to polyspermy 2020;87(3):326-40. 10.1002/mrd.23320 [DOI] [PMC free article] [PubMed]
- 4.Bhakta HH, Refai FH. The molecular mechanisms mediating mammalian fertilization. 2019;146(15). 10.1242/dev.176966. [DOI] [PubMed]
- 5.Vajta G, Rienzi L, Bavister BD. Zona-free embryo culture: is it a viable option to improve pregnancy rates? Reprod Biomed Online. 2010;21(1):17–25. doi: 10.1016/j.rbmo.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Rana N, Dmowski WP. Intracytoplasmic sperm injection into zona-free human oocytes results in normal fertilization and blastocyst development. Hum Reprod. 1999;14(2):476–478. doi: 10.1093/humrep/14.2.476. [DOI] [PubMed] [Google Scholar]
- 7.Ueno S, Bodri D, Uchiyama K, Okimura T, Okuno T, Kobayashi T, et al. Developmental potential of zona pellucida-free oocytes obtained following mild in vitro fertilization. Fertil Steril. 2014;102(6):1602–1607. doi: 10.1016/j.fertnstert.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Metwalley A, Brasha N, Esteves SC, Fawzy M, Brasha H, Hellani A, et al. Role of diagnostic intracytoplasmic sperm injection (ICSI) in the management of genetically determined zona pellucida-free oocytes during in vitro fertilization: a case report. Zygote. 2020;28(6):519–523. doi: 10.1017/s0967199420000441. [DOI] [PubMed] [Google Scholar]
- 9.Stanger JD, Stevenson K, Lakmaker A, Woolcott R. Pregnancy following fertilization of zona-free, coronal cell intact human ova: case report. Hum Reprod. 2001;16(1):164–167. doi: 10.1093/humrep/16.1.164. [DOI] [PubMed] [Google Scholar]
- 10.OA-5 - Growing zona pellucida-free (ZPF) oocytes from ICSI to biopsy: it works. Reproductive biomedicine online. 2016.
- 11.Shu Y, Peng W, Zhang J. Pregnancy and live birth following the transfer of vitrified-warmed blastocysts derived from zona- and corona-cell-free oocytes. Reprod Biomed Online. 2010;21(4):527–532. doi: 10.1016/j.rbmo.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Trolice MP. Live birth of twins derived from zona-free oocytes. Fertil Steril. 2016;105(5):1232–1235. doi: 10.1016/j.fertnstert.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Bodri D, Kato R, Kondo M, Hosomi N, Katsumata Y, Kawachiya S, et al. Time-lapse monitoring of zona pellucida-free embryos obtained through in vitro fertilization: a retrospective case series. Fertil Steril. 2015;103(5):e35. doi: 10.1016/j.fertnstert.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Togashi M, Adachi J, Toyoda Y. Developmental ability of zona-free mouse embryos is influenced by cell association at the 4-cell stage. Biol Reprod. 1995;53(1):78–83. doi: 10.1095/biolreprod53.1.78. [DOI] [PubMed] [Google Scholar]
- 15.Yániz JL, Santolaria P, López-Gatius F. In vitro development of bovine embryos encapsulated in sodium alginate. J Vet Med A Physiol Pathol Clin Med. 2002;49(8):393–395. doi: 10.1046/j.1439-0442.2002.00463.x. [DOI] [PubMed] [Google Scholar]
- 16.Vajta G, Peura TT, Holm P, Páldi A, Greve T, Trounson AO, et al. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev. 2000;55(3):256–264. doi: 10.1002/(sici)1098-2795(200003)55:3<256::aid-mrd3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Thouas GA, Jones GM, Trounson AO. The ‘GO’ system – a novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction. 2003;126(2):161–169. doi: 10.1530/rep.0.1260161. [DOI] [PubMed] [Google Scholar]
- 18.Nagatomo H, Yao T, Araki Y, Mizutani E, Wakayama T. Agarose capsules as new tools for protecting denuded mouse oocytes/embryos during handling and freezing-thawing and supporting embryonic development in vivo. Sci Rep. 2017;7(1):17960. doi: 10.1038/s41598-017-18365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortezzi SS, Garcia JS, Ferreira CR, Braga DP, Figueira RC, Iaconelli A, Jr, et al. Secretome of the preimplantation human embryo by bottom-up label-free proteomics. Anal Bioanal Chem. 2011;401(4):1331–1339. doi: 10.1007/s00216-011-5202-1. [DOI] [PubMed] [Google Scholar]
- 20.Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;86(3):678–685. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 22.Rice KG. The chemistry, biology, and medical applications of hyaluronan and its derivatives edited by T. C. Laurent. Portland Press, London, U.K. 1998. xvi + 341 pp. 17 × 25 cm. ISBN 1–85578–119–0. $127.50. J Med Chem. 1998;41(26):5336-. 10.1021/jm980609z.
- 23.Abatangelo G, Vindigni V, Avruscio G, Pandis L, Brun P. Hyaluronic acid: redefining its role. 2020;9(7). 10.3390/cells9071743. [DOI] [PMC free article] [PubMed]
- 24.Ahmadian E, Dizaj SM, Eftekhari A, Dalir E, Vahedi P, Hasanzadeh A, et al. The potential applications of hyaluronic acid hydrogels in biomedicine. Drug Res. 2020;70(1):6–11. doi: 10.1055/a-0991-7585. [DOI] [PubMed] [Google Scholar]
- 25.Bronstone A, Neary JT, Lambert TH, Dasa V. Supartz (Sodium hyaluronate) for the treatment of knee osteoarthritis: a review of efficacy and safety. Clin Med Insights Arthritis Musculoskelet Disord. 2019;12:1179544119835221. doi: 10.1177/1179544119835221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leighton R, Fitzpatrick J, Smith H, Crandall D, Flannery CR, Conrozier T. Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Access Rheumatol. 2018;10:43–54. doi: 10.2147/oarrr.s162127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo G, Villa-Collar C, Martin-Gil A, Serramito M, Santamaría L. Comparison between viscous teardrops and saline solution to fill orthokeratology contact lenses before overnight wear. Eye Contact Lens. 2018;44(Suppl 1):S307–S311. doi: 10.1097/icl.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 28.Simmons PA, Liu H, Carlisle-Wilcox C, Vehige JG. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol (Auckland NZ) 2015;9:665–675. doi: 10.2147/opth.s78184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick S, Rother S, Zimmermann H, Lee PS, Moeller S, Schnabelrauch M, et al. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application – the role of chondroitin sulfate and sulfated hyaluronan. Mater Sci Eng C Mater Biol Appl. 2017;79:15–22. doi: 10.1016/j.msec.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein-Oppenheimer CR, Brown DI, Coloma R, Morales P, Reyna-Jeldes M, Díaz MJ, et al. Design of a hybrid biomaterial for tissue engineering: biopolymer-scaffold integrated with an autologous hydrogel carrying mesenchymal stem-cells. Mater Sci Eng C Mater Biol Appl. 2017;79:821–830. doi: 10.1016/j.msec.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 31.Shirasawa H, Kumagai J, Sato W, Kumazawa Y, Sato N, Terada Y. Retrieval and in vitro maturation of human oocytes from ovaries removed during surgery for endometrial carcinoma: a novel strategy for human oocyte research. J Assist Reprod Genet. 2013;30(9):1227–1230. doi: 10.1007/s10815-013-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. Towards Reproductive Certainty Infertility & Genetics Beyond. 1999.
- 33.Hogan BLM, Costantini F, Lacy EE. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press. 1994.
- 34.The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human Reprod. 2011;26(6):1270-83. 10.1093/humrep/der037 [DOI] [PubMed]
- 35.Ebner T, Maurer M, Shebl O, Moser M, Mayer RB, Duba HC, et al. Planar embryos have poor prognosis in terms of blastocyst formation and implantation. Reprod Biomed Online. 2012;25(3):267–272. doi: 10.1016/j.rbmo.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Cauffman G, Verheyen G, Tournaye H, Van de Velde H. Developmental capacity and pregnancy rate of tetrahedral- versus non-tetrahedral-shaped 4-cell stage human embryos. J Assist Reprod Genet. 2014;31(4):427–434. doi: 10.1007/s10815-014-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham CF, Lehtonen E. Formation and consequences of cell patterns in preimplantation mouse development. J Embryol Exp Morphol. 1979;49:277–294. [PubMed] [Google Scholar]
- 38.Dai X, Li L, Peterson W, Baumgartner RR, Huang J, Baer-Zwick A, et al. Safety and effectiveness of hyaluronic acid dermal filler in correction of moderate-to-severe nasolabial folds in Chinese subjects. Clin Cosmet Investig Dermatol. 2019;12:57–62. doi: 10.2147/ccid.s187079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sozen B, Can A, Demir N. Cell fate regulation during preimplantation development: a view of adhesion-linked molecular interactions. Dev Biol. 2014;395(1):73–83. doi: 10.1016/j.ydbio.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Ziomek CA, Johnson MH. Cell surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell. 1980;21(3):935–942. doi: 10.1016/0092-8674(80)90457-2. [DOI] [PubMed] [Google Scholar]
- 41.Paternot G, Debrock S, De Neubourg D, D'Hooghe TM, Spiessens C. The spatial arrangement of blastomeres at the 4-cell stage and IVF outcome. Reprod Biomed Online. 2014;28(2):198–203. doi: 10.1016/j.rbmo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Desai N, Gill P. Blastomere cleavage plane orientation and the tetrahedral formation are associated with increased probability of a good-quality blastocyst for cryopreservation or transfer: a time-lapse study. Fertil Steril. 2019;111(6):1159–68.e1. doi: 10.1016/j.fertnstert.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Hentschke MR, Azambuja R, Dornelles VC, Cunegatto B, Hickman C, Hariharan R, et al. Is there a better evolutionary outcome in a 4-cell tetrahedron embryo? JBRA Assist Reprod. 2021;25(4):640–643. doi: 10.5935/1518-0557.20210034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebner T, Höggerl A, Oppelt P, Radler E, Enzelsberger SH, Mayer RB, et al. Time-lapse imaging provides further evidence that planar arrangement of blastomeres is highly abnormal. Arch Gynecol Obstet. 2017;296(6):1199–1205. doi: 10.1007/s00404-017-4531-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Clinical significance of intercellular contact at the four-cell stage of human embryos, and the use of abnormal cleavage patterns to identify embryos with low implantation potential: a time-lapse study. Fertil Steril. 2015;103(6):1485–91.e1. doi: 10.1016/j.fertnstert.2015.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.