Abstract
Water deficit is a significant impediment to enhancing rice yield. Genetic engineering tools have enabled agriculture researchers to develop drought-tolerant cultivars of rice. A common strategy to achieve this involves expressing drought-tolerant genes driven by constitutive promoters such as CaMV35S. However, the use of constitutive promoters is often limited by the adverse effects it has on the growth and development of the plant. Additionally, it has been observed that monocot-derived promoters are more successful in driving gene expression in monocots than in dicots. Substitution of constitutive promoters with stress-inducible promoters is the currently used strategy to overcome this limitation. In the present study, a 1514 bp AP2/ERF promoter that drives the expression of a transcription factor was cloned and characterized from drought-tolerant Indian rice genotype N22. The AP2/ERF promoter was fused to the GUS gene (uidA) and transformed in Arabidopsis and rice plants. Histochemical GUS staining of transgenic Arabidopsis plants showed AP2/ERF promoter activity in roots, stems, and leaves. Water deficit stress and ABA upregulate promoter activity in transformed Arabidopsis and rice. Quantitative PCR for uidA expression confirmed induced GUS activity in Arabidopsis and rice. This study showed that water deficit inducible Os-AP2/ERF-N22 promoter can be used to overcome the limitations of constitutive promoters. Transformants overexpressing Os-AP2/ERF-N22 showed higher relative water content, membrane stability index, total chlorophyll content, chlorophyll stability index, wax content, osmotic potential, stomatal conductance, transpiration rate, photosynthetic rate and radical scavenging activity. Drought tolerant (N22) showed higher expression of Os-AP2/ERF-N22 than the susceptible (MTU1010) cultivar.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-022-01246-9.
Keywords: Abiotic stress, Water deficit stress, Arabidopsis, Transgenic, Agrobacterium
Introduction
Rice is among the most widely cultivated and consumed cereals in the world. Around 92% of the rice grown the world over is produced in Asia, and 40% of the cultivated area is rainfed and experiences water stress which accounts for losses estimated at 200 million tons/year. Thus, rice cultivars that are tolerant to drought are the need in the present time when the world is witnessing climate change. A common strategy for the development of plant varieties that are tolerant to drought is to express the drought-responsive genes under the control of a strong constitutive promoter. (Rahman et al. 2016, Novak et al. 2013; Withanage et al. 2015; Du et al. 2016). The cauliflower mosaic virus (CaMV) 35S is the most commonly used promoter for this purpose (Odell et al. 1985). The 35S promoter and its derivatives have been used to regulate the transgene expression in monocots as well as dicots (Battraw and Hall, 1990; Benfey et al. 1990). However, its activity is less in monocots as compared to dicots (Gupta et al. 2001; McElroy et al. 1991; Cornejo et al. 1993). Therefore, it is necessary to develop monocot and dicot-specific promoters for better success of transgenic plants. The constitutive expression of transgenes regulated by the constitutive promoter may also adversely impact plant development and metabolism, which ultimately compromises plant growth and yield (Homrich et al. 2012). To overcome the undesirable effects of constitutive promoters, the genes responsible for drought tolerance are regulated by tissue-specific and/or stress-inducible promoters (Banerjee et al. 2013; Yan et al. 2015). Various stress-inducible promoters like AtRD29A (Yagamuchi-Shinozaki and Shinozaki 1994), GmMYB363P (Li et al. 2014), BBX24 (Imtiaz et al. 2015), ZmGAPP (Hou et al. 2016), PeNAC1 (Wang et al. 2016), and OsbZIP23 (Dey et al. 2016) have been reported. However, RD29 A (Yagamuchi-Shinozaki and Shinozaki 1994) promoter remains the preferred choice for regulating the expression of stress-specific genes in different plant species (Polizel et al. 2011; Siant Pierre et al. 2012; Engels et al. 2013; Bihmidine et al. 2013). There is a constant endeavour to look for new constitutive and inducible promoters from both monocots and dicots for scientific and commercial purposes.
Transcription factors (TFs) are the key regulatory elements that bind to specific cis-elements in the promoter region and thus regulate the expression of target genes responsible for controlling the expression of gene clusters. The transcriptional regulons regulating gene expression under abiotic stress have been identified in Arabidopsis and rice (Sahu et al. 2016). The AP2/ERF is the largest among the plant-specific TF families and has four major subfamilies i.e., AP2, ERF, DREB (dehydration-responsive element-binding protein), and RAV (RELATED TO ABI3/VP1). Transcription factors belonging to these subfamilies regulate gene expression in response to cold, dehydration, heat shock, ethylene, and the development of flowers, embryos, and seeds (Mizoi et al. 2012; Dietz et al. 2010; Kagaya and Hatori 2009). In our group's previous studies, a gene encoding drought and ABA-responsive transcription factor were identified from drought-tolerant rice genotype N22 (Mawlong et al. 2014, 2015, 2018; Kumar et al. 2018). The expression of this gene named Os-AP2/ERF-N22 was studied at transcript and protein levels after exposing rice plants to drought, drought + ABA, and ABA treatment. It was found that the expression of Os-AP2/ERF-N22 increased at both transcript and protein levels under the effect of drought and ABA (Kumar, 2018). Based on this finding, we hypothesized that the promoter of Os-AP2/ERF-N22 could be utilized for regulating the expression of drought-inducible genes. To test this hypothesis, the 1514 bp Os-AP2/ERF promoter region before the transcriptional start site of the AP2/ERF gene was isolated and characterized. The activity and drought inducibility of this promoter was analyzed in the leaves of transformed Arabidopsis and rice plants. There are no previous reports available on the isolation and characterization of drought and ABA-responsive promoter of the AP2/ERF family. Os-AP2/ERF-N22 was overexpressed in rice and physio-biochemical analysis of transformants was done. Relative expression of Os-AP2/ERF-N22 was also studied in contrasting cultivars for drought tolerance.
Materials and methods
Experimental site
The experiment was conducted at the National phytotron facility, IARI, New Delhi, India, which is located at 77° 09 E longitude, 28°38 N latitude, and 228 m above the mean sea level.
Biological materials and chemicals
Drought tolerant (N22) and drought susceptible (MTU 1010) cultivars of rice were used in this study, and plants were grown at 30 °C ± 2 °C temperature, 90% relative humidity at the National phytotron facility, Indian Agricultural Research Institute, New Delhi in earthen pots of 24 cm diameter that were filled with sterilized clay and farm yard manure (2:1 ratio). The plants were watered every morning. Drought was induced by withholding water for two days, four days, and six days respectively. Arabidopsis thaliana wild-type (ecotype Col-0) plants were used for the experiments. The plants were grown at a temperature of 22 ± 2 °C, 16-h light, and 8-h dark cycle at the National Phytotron Facility, New Delhi. pGEM-T Easy vector and the pORE R2 binary vector were procured from Promega, the USA, and NRCPB, New Delhi, respectively. New England Biolabs (England) supplied E. coli (DH5α), restriction enzymes, and Phusion DNA polymerase. T4 DNA Ligase, dNTPs, Gene Ruler 1 kb DNA ladder, and TRI reagent were purchased from Thermo Fisher Scientific (USA). X-gal, IPTG, kanamycin, and ampicillin were purchased from Sigma Aldrich (USA). LB, LA, agarose and rifampicin were procured from HiMedia (India).
Quantitative gene expression analysis of Os-AP2/ERF-N22
For qRT-PCR, the total RNA of rice seedlings was extracted using a TRIZOL reagent. To synthesize the first strand cDNA, Thermo Fisher Scientific (USA) RevertAid first strand cDNA synthesis kit (Cat. No. K1622) was used. cDNA of different samples was used as a template to amplify the Os-AP2/ERF-N22 transcript. The PCR reaction mixture comprised of 10 µl 2X KAPA SYBER FAST qPCR master Mix Universal, 1 µl diluted cDNA, 0.4 µl forward primer (10 µM), 0.4 µl reverse primer (10 µM), and 8.2 µl nuclease-free water. OsActin served as an internal reference. The specific primers for Os-AP2/ERF-N22 gene, Os-AP2/ERF-N22 promoter (Forward: F1 and Reverse: R1) and actin (Forward: F2 and Reverse: R2) were designed using primer 3 software (Table S2). The samples were taken in triplicate for quantitative analysis under the standard thermal cycling programme. The Ct value thus obtained was used to calculate the relative change in the expression of Os-AP2/ERF-N22 (2^-ddct). Results were presented as fold change in the transcript level of Os-AP2/ERF-N22 transcription factor normalized to the Actin gene.
5′ RACE for mapping of TSS (Transcriptional start site)
The TSS (Transcriptional start site) of Os-AP2/ERF-N22 transcript was identified by 5′ RACE (Rapid amplification of cDNA end). 5′ First Choice RLM-RACE kit by Ambion, USA was used. Total RNA from the leaf was used as a template for 5′ RACE PCR analysis. The 5′ RACE outer forward primer (F3), 5′ RACE Os-AP2/ERF-N22 specific outer reverse primer (R3), 5′ RACE inner forward primer (F4), and 5′ RACE Os-AP2/ERF-N22 specific inner reverse primer (R4) were used (Table S2).
Cis-element analysis
Approximately 1.5 kb DNA ahead of the start codon of Os-AP2/ERF-N22 was identified from the phytozome. The cis-elements in the promoter were identified with the help of PLACE (https://integbio.jp/dbcatalog/en/record/nbdc00168) and the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Cloning of Os-AP2/ERF promoter in pGEMT-Easy and pORE R2 binary vector
Genomic DNA was isolated from rice seedlings using the CTAB method (Doyle and Doyle, 1987). Approximately 1.5 kb nucleotide stretch upstream of the start codon of Os-AP2/ERF-N22 was identified from chromosome 6 of the Oryza sativa DNA sequence available at Phytozome. From this sequence, the Forward (F5) and Reverse (R5) primers were designed. The XhoI site in the forward primer (F5) and the BamHI site in the reverse primer (R5) was introduced (Table S2). The promoter sequence was amplified from the genomic DNA following the standard PCR program. Taq polymerase and Phusion polymerase were used to amplify the promoter. The amplified promoter was purified using a PCR purification kit from Qiagens and then cloned in pGEMT-Easy and also in the pORE R2 binary vector that has the GUS reporter gene (uid A). The recombinant plasmids were transformed into E. coli host (DH5α competent cell). Transformed plasmids were identified (Sambrook and Russel, 2001) and restricted with XhoI and BamHI. The recombinant plasmids were given for Sanger sequencing to GCC Biotech. Ltd. The in-silico analysis of cis-regulatory elements present in the promoter was done with the help of the PLANTCARE database.
Agrobacterium-mediated transformation of Arabidopsis
The Os-AP2/ERF promoter was cloned in a promoterless pORE R2 binary vector. Os-AP2/ERF promoter- pORE R2 construct was transformed in Agrobacterium tumefaciens strain EHA 105 by the freeze-thaw procedure. Kanamycin and rifampicin were used to select the transformed colonies of Agrobacterium tumefaciens. Screening of recombinant colonies was done with colony PCR using Os-AP2/ERF promoter-specific primers (F5 and R5 mentioned in Table S2). Arabidopsis plants at the four-week stage with sufficient inflorescence were transformed with recombinant Agrobacterium using the floral dip protocol (Zhang et al. 2006). The transformed Arabidopsis plants were stored in the dark for 12 h, after which they were grown under normal conditions. The seeds were collected after the siliques matured, and dried. After sterilization, they were subjected to kanamycin selection (30 μg/ml) on ½ MS media. The transformation efficiency of Arabidopsis plants with Os-AP2/ERF promoter-pORE R2 construct was found to be 0.67 (Table S1). Once the transformed Arabidopsis plants attained the four-leaf stage, those that survived the kanamycin selection developed roots. These plants having roots were transferred to pots containing soilrite media and were grown till their maturity to get T2 seeds. The sterilized T2 seeds were grown on kanamycin-containing media for further selection. A total of ten Arabidopsis plants having Os-AP2/ERF promoter-pORE R2 construct survived kanamycin-based selection in the T2 generation. Arabidopsis plants transformed with CaMV 35S promoter to be used as positive control showed kanamycin resistance. Next, genomic DNA was extracted from the leaves of the ten T2 plants (approximately four weeks old) having Os-AP2/ERF promoter-pORE R2 construct. The transformed plants were screened through PCR using this genomic DNA and primers specific for Os-AP2/ERF promoter (F5 and R5). The PCR reaction mixture comprised of genomic DNA of transformed Arabidopsis plants (100 ng), forward and reverse primers (0.4 μM each), dNTP (0.2 mM), Taq buffer (10X), Taq polymerase (1.25 U), and water (nuclease-free). The final volume was made up to 20 μl. The PCR program was the same as the one used for the amplification of rice Os-AP2/ERF promoter with Taq polymerase, as described earlier. The transformed Arabidopsis thaliana plants were also screened with GUS (uid A) specific primers F6 and R6 (Table S2). For this, the reaction mixture comprised of genomic DNA (100 ng), forward and reverse primers (0.4 μM each), dNTP (0.2 mM), Taq buffer (10X), Taq polymerase (1.25 U), and water (nuclease-free). Volume was made up to 20 μl. PCR program used was a. initial denaturation: at 94 °C for 5 min b. denaturation: at 94 °C for 1 min, annealing: at 54 °C for 45 s, extension: at 72 °C for 1 min, c. 35 cycles of steps b, d. final extension at 72 °C for 10 min. PCR screening showed that from the total of ten Arabidopsis plants that survived on kanamycin in the T2 generation, only two were positive for Os-AP2/ERF promoter as well as uid A. The histochemical GUS staining was performed on these two plants.
Histochemical GUS staining
Fresh tissue from Arabidopsis plants was harvested and kept in the GUS staining solution (Composition: sodium phosphate buffer (pH 7.2, 50 mM), potassium ferricyanide (2 mM), potassium ferrocyanide (2 mM), X-Gluc (2 mM), methanol (20%), and Triton X 100 (1%), (Jefferson et al. 1987; Vijyan et al. 2015). After staining, the chlorophyll was removed using 70% ethanol. These leaves were then examined under a compound light microscope, and the image was captured.
Quantitative expression of GUS (uid A) gene
For quantitative estimation of GUS expression, qRT PCR of the uidA gene was done according to the method discussed by Tewari et al. (2018). RNA from the leaves of GUS-positive plants was isolated and quantified. Its integrity was checked through the 260/280 absorbance ratio and also by agarose gel electrophoresis. The first strand cDNA was synthesized using the Thermo Fisher Scientific (USA) Revert Aid first strand cDNA synthesis kit. qRT PCR was done on a Real-Time PCR Detection System of Bio-Rad, USA. Arabidopsis thaliana and rice actin genes served as reference gene for qRT PCR analysis. Each sample was taken in triplicate for qRT PCR analysis. The 20 μl reaction mixture comprised of 100 ng cDNA, kappa SYBR Green fast qPCR kit master mix (2×), respective primer pairs for GUS (Forward: F7 and Reverse: R7), Actin of Arabidopsis (Forward: F8 and Reverse: R8), and rice (Forward: F2 and Reverse: R2) (Table S2). Reactions were done in triplicates, and the average Ct value was determined.
Southern blot analysis
Genomic DNA was isolated from transformed Arabidopsis leaves using the CTAB-based protocol (Doyle and Doyle 1987), and its concentration was estimated using nanodrop. Approximately 2.5 mg of isolated DNA was digested with EcoRI restriction enzyme and electrophoresed in a 1% agarose gel in TBE (1X) buffer. After depurination, denaturation, and neutralization treatments, the DNA was transferred to the nylon membrane (Sambrook et al. 2001). [α32]-dCTP using DecaLabelTM labelling kit from Fermentas, was used to label the probe DNA. Hybridization and washing steps by Ramanathan and Veluthambi (1995) were followed. The X-ray film was used to get an autoradiographic image, and the image was developed using Photon X-ray developer.
In-planta transformation of rice
Transformation of rice for Os-AP2/ERF-N22 promoter.
MTU1010 genotype of rice was transformed with Agrobacterium containing Os-AP2/ERF-N22 promoter-pORE R2 recombinant plasmid by the in-planta transformation method. Hundreds of rice seeds were surface sterilized with Bavistin (1% w/v) for 10 min, followed by HgCl2 (0.1%) for a few seconds. Seeds were soaked overnight in double distilled water and then germinated on sterile wet blotting paper in Petri plates at a temperature of 30 °C. The Agrobacterium tumefaciens (strain EHA 105) containing Os-AP2/ERF-N22-promter-pORE construct was inoculated in a 5 ml Luria broth (LB) medium having kanamycin (50 mg/ml) and rifampicin (10 mg/ml) and kept at 180 rpm in a shaker for 24 h to get primary culture. Three ml of primary culture was resuspended into 50 ml of AB media (pH 5.2) and kept at 180 rpm in a shaker for 18 h to get secondary culture. Tobacco leaves (8 g) were crushed in 20 ml of double distilled water to prepare the tobacco leaf extract. It was added to Agrobacterium secondary culture 5 h before infection. The apical meristem and inter-cotyledonary region of rice seedlings with plumule were pricked with a sterile needle and dipped in Agrobacterium secondary culture for 1 h. After that, the seedlings were washed with sterile water and germinated under the aseptic condition at National Phytotron Facility, IARI, New Delhi. Seventy-three transformed seedlings that germinated were then further grown on soil rite media for the next 15 days. The GUS activity test was done on ten randomly selected rice seedlings, two days after the in-planta transformation to check the transformation efficiency. Three of these seedlings developed blue colour, which showed that they were GUS-positive and transformants. The remaining sixty-three seedlings were transferred from soil rite media to soil and grown till maturity to get T1 seeds. The sterilized T1 seeds were grown on soil rite media, healthy seedlings were transferred to soil, and grown for further selection. Genomic DNA was isolated from ten healthy one-month-old rice plants. With this genomic DNA as a template, PCR with GUS-specific primers F6 and R6 (Table S2) was done using the GUS sequence present in the pORE R2 binary vector. PCR-positive plants were grown in phytotron till maturity to get T2 seeds. The sterilized T2 seeds were grown in a phytotron and PCR-based screening using GUS-specific primers F6 and R6 (Table S2) was done in one-month-old rice plants.
Transformation of rice for overexpression of Os-AP2/ERF-N22
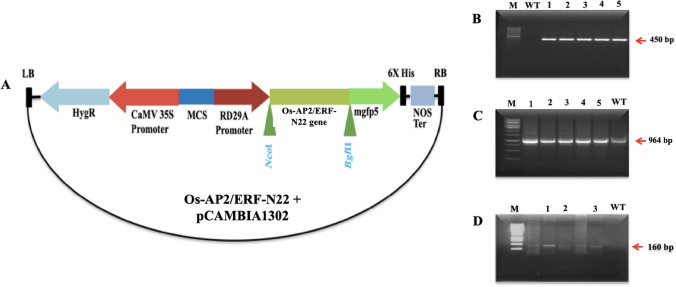
The PCR amplified Os-AP2/ERF-N22 and binary vector pCAMBIA1302 were restricted with restriction enzymes NcoI and BglII. The restricted Os-AP2/ERF-N22 was cloned in pCAMBIA1302. The recombinant construct was named pCAMBIA1302:RD29A-Os-AP2/ERF-N22 (Fig. 3A). The primers F9 and R9 were designed for the amplification of RD29A promoter using primer 3 software with an expected amplicon size of 450 bps (Table S2). MTU1010 genotype of rice was transformed with pCAMBIA1302:RD29A-Os-AP2/ERF-N22 by the in-planta transformation method. PCR analysis was done to confirm the integration of the AP2/ERF gene, RD29A promoter, and hpt marker, and only those giving the required amplicon size of 964, 450, and 160 bp, respectively were raised to maturity. Southern blot analysis for the integration of the promoter was done using RD29A as a probe.
Fig. 3.
(A) Schematic representation of recombinant pCAMBIA 1302 vector with Os-AP2/ERF-N22 gene. (B) PCR screening of transformed rice plants (T1) using RD 29A-specific primers. (C) PCR screening of transformed rice plants (T1) using Os-AP2/ERF-N22 specific primers. (D) PCR screening of transformed rice plants (T1) using hpt marker-specific primers
Relative water content (RWC)
The RWC of rice leaves was measured according to the method discussed by Barrs and Weatherly (1962).
Membrane stability index (MSI)
The MSI was estimated using the method reported by Sairam et al. (1997).
Total chlorophyll content
The total chlorophyll content was estimated according to the method of Hiscox and Israelstam (1979).
Chlorophyll stability index (CSI)
CSI was estimated using the method of Koleyoreas (1958).
Wax content
Wax content was measured using the colorimetric method of Ebercon et al. 1977.
Osmotic potential measurement
The leaf osmotic potential of the sample was measured according to the protocol given by Arndt et al. (2015).
Photosynthetic rate, stomatal conductance, and transpiration rate
Photosynthetic rate, stomatal conductance, and transpiration rate were estimated in completely expanded flag leaves of rice using IRGA (Infrared gas analyzer), LI-6400 Model. These parameters were determined between 10.00 am and 12.00 pm.
Radical scavenging activity (RSA)
RSA was estimated using the method of Mensor et al. (2001).
Seed yield
Seed yield was estimated by collecting the rice tillers from plants and presented in terms of gram/plant.
Results
An AP2/ERF transcription factor was identified from the Phytozome database. It had cis-acting elements ABRE and DRE within 500 bp upstream of its initiation codon. Intrigued by the presence of cis-elements pertaining to drought and ABA response in its promoter, we isolated and characterized the corresponding gene from the N22 cultivar of rice and named as Os-AP2/ERF-N22 (Mawlong et al. 2014, 2015). Relative expression of Os-AP2/ERF-N22 was studied in contrasting cultivars for drought tolerance. Os-AP2/ERF-N22 was overexpressed in drought-susceptible (MTU 1010) cultivar of rice and physio-biochemical analysis of transformants (T2) was done under imparted drought. The promoter of Os-AP2/ERF-N22 was cloned in promoter less pORE R2 binary vector and characterized in Arabidopsis thaliana and rice by the GUS reporter system under drought and ABA.
Identification of transcription start site (TSS) of Os-AP2/ERF-N22
The in-silico analysis of the genes adjacent to Os-AP2/ERF-N22 on chromosome no. 6 of rice revealed that the inter-gene distance between Os-AP2/ERF-N22 encoding gene (ID: LOC_OS06g40150) and next adjacent gene (ID: LOC_OS06g40140) is about 6.706 kb. To localize the cis-regulatory motifs upstream of Os-AP2/ERF-N22, the TSS was determined by 5′ RACE (Fig. 1). The PCR products of outer 5′ RLM RACE (200 bp) and inner 5′ RLM RACE (154 bp) were run on 1.2% agarose gel (Fig. 1). The purified PCR products were sequenced to determine 5′ ends of the products. The 43 nucleotides (GGGAGAATTCTTCAGGGACAGTATCCCTGCAGAGGTGAGATC) were confirmed as TSS of Os-AP2/ERF-N22 based on the sequencing result (Fig. 7). The ATG codon immediately after TSS is considered as the translational start site of Os-AP2/ERF-N22.
Fig. 1.
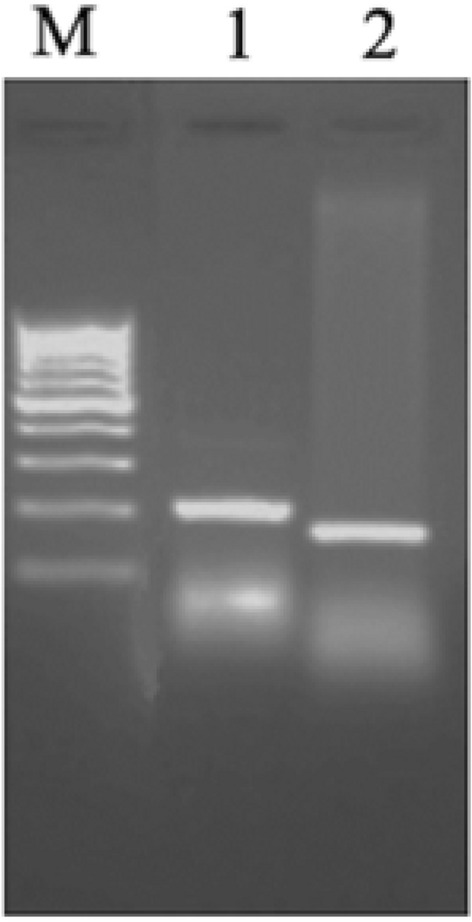
Mapping of the transcription start site of Os-AP2/ERF-N22. 5′ RACE (Rapid Amplification of cDNA End) PCR analysis; Lane M: 100 bp gene ruler, Lane 1: 200 bp amplicon obtained by 5′ RACE Os-AP2/ERF specific outer primer and 5′ RACE outer primer, and Lane 2: 154 bp amplicon obtained by 5′ RACE Os-AP2/ERF specific inner primer and 5′ RACE inner primer
Fig. 7.
Promoter sequence with different stress-responsive, TATA-box and CAAT-box cis-elements
Expression of Os-AP2/ERF-N22 in a tolerant and susceptible cultivar of rice in response to Water deficit stress (WDS)
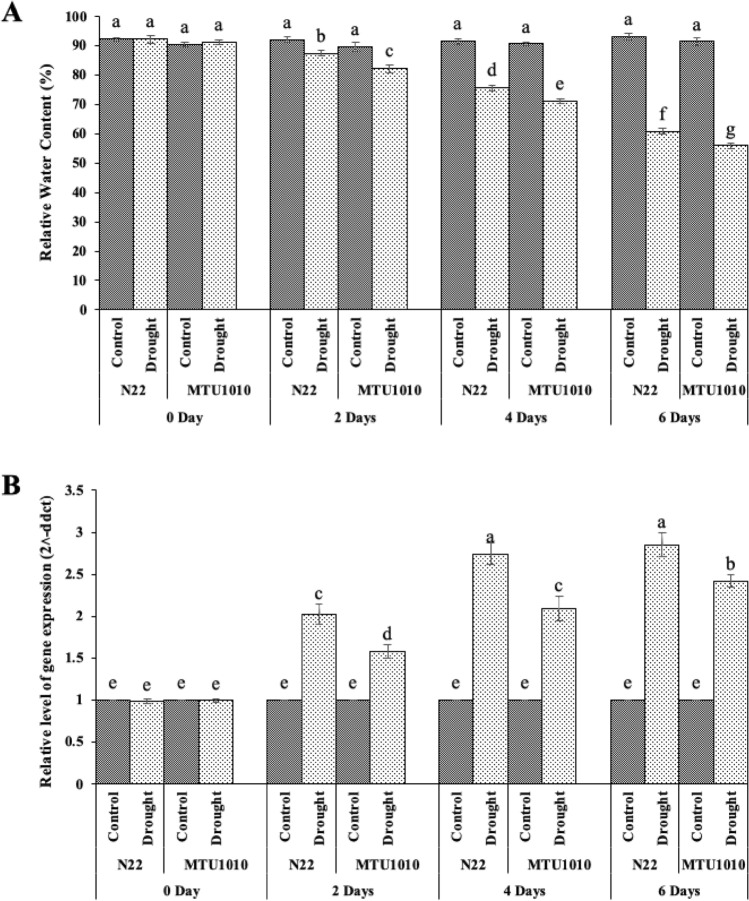
Differential expression of Os-AP2/ERF-N22 transcript and relative water content (RWC) in the leaves of drought tolerant (N22) and drought susceptible (MTU1010) cultivars of rice were assessed after subjected to drought for 2, 4 and 6 days respectively. RWC decreased from 82.14 to 55.89% in MTU1010 and 87.48 to 60.77% in N22 after withholding water for 2, 4, and 6 days respectively, in case of drought treatment (Fig. 2A). Among cultivars, N22 maintained a higher RWC than IR64 at different stress levels. Relative expression of Os-AP2/ERF-N22 increased as stress progressed, and higher expression was found in N22 (2.85-fold) as compared to MTU1010 (2.42-fold) after six days of withholding water (Fig. 2B). This difference in the relative fold expression can be explained by the draught inducible character of this transcription factor.
Fig. 2.
Relative water content (RWC) and expression of Os-AP2/ERF-N22 transcripts in a tolerant (N22) and susceptible (MTU1010) cultivar of rice in response to water deficit stress. (A) Relative water content (%), and (B) quantitative Real-Time PCR analysis of Os-AP2/ERF-N22 transcript after withholding water for 2, 4, and 6 days. Values represent mean ± SEs, n = 3. The different letters which mark the mean values indicate significant differences between treatments at P ≤ 0.05
Physio-biochemical analysis of rice transformants overexpressing Os-AP2/ERF-N22
Seven healthy transgenic rice plantlets were used for PCR-mediated selection to confirm the integration of the Os-AP2/ERF-N22, RD29A promoter, and hpt marker, and only those giving the required amplicon size of 964, 450, and 160 bp, respectively were raised to maturity. Out of these, three seedlings were found PCR positive for all three (Fig. 3B). To evaluate the drought tolerance capability of Os-AP2/ERF-N22 transformants, transformants and wild-type plants were exposed to drought treatment, and various physio-biochemical indices associated with WDS were analyzed.
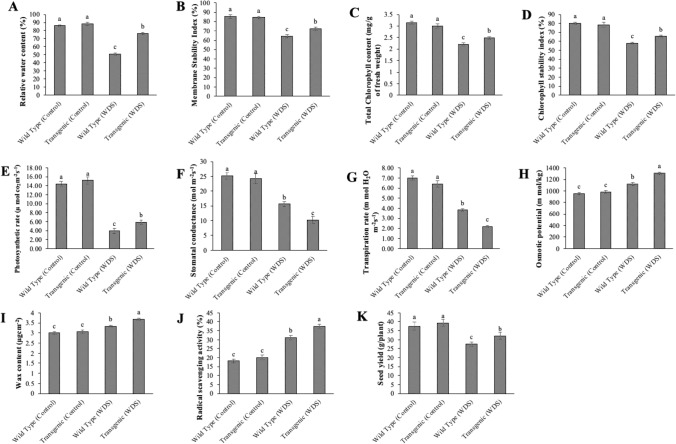
No significant difference was observed in the leaf RWC, MSI, total Chlorophyll content, CSI, Photosynthetic rate, and Seed yield of transgenics and wild type under control conditions. However, the transgenic line showed significantly higher leaf RWC, MSI, Total chlorophyll content, CSI, Photosynthetic rate, and Seed yield under water deficit stress (Fig. 4A–E and K). Under WDS conditions, the percentage decrease in these physio-biochemical parameters was higher in the wild type than in transgenics.
Fig. 4.
Physico-biochemical analysis of transgenic rice (T1) leaves: (A) Relative water content; (B) Membrane stability Index; (C) Total chlorophyll content; (D) Chlorophyll stability Index; (E) Photosynthetic rate; (F) Stomatal conductance; (G) Transpiration rate; (H) Osmotic potential; (I) Wax content; (J) Radical scavenging activity; and (K) Seed yield. Means ± SEs, n = 3. The means marked with different letters indicate significant differences between treatments at P ≤ 0.05 according to Duncan’s multiple range test
Stomatal conductance indicates the degree of stomatal opening and can be used as an indicator of plant water status. Transpiration means the loss of water through aerial parts in plants. Plants experience water deficit stress when the transpiration rate becomes very high. Under control conditions, there was no significant difference between the stomatal conductance and transpiration rate of wild-type and transgenics. However, under WDS, a decline in the stomatal conductance and transpiration rate of wild type and transgenics was observed. The declination was more in transgenics than wild type (Fig. 4F, G).
No significant difference was observed in the osmotic potential and wax content of wild-type and transgenics under control conditions. However, under water deficit stress, the osmotic potential and wax content was found to be more in transgenics than wild type (Fig. 4H, I). An increase in osmotic potential was due to increased hydrolysis of macromolecules into simpler ones.
The radical scavenging activity under control conditions was found to be 18.12% in the wild type and 20.16% in the transgenic line. Under WDS, the radical scavenging activity was found to be more in transgenics (37.47%) than wild type (31.17%) (Fig. 4J). Thus, the ability of transgenics to tolerate WDS by scavenging the reactive oxygen species was more than the wild type.
Isolation and cloning of Os-AP2/ERF promoter from rice
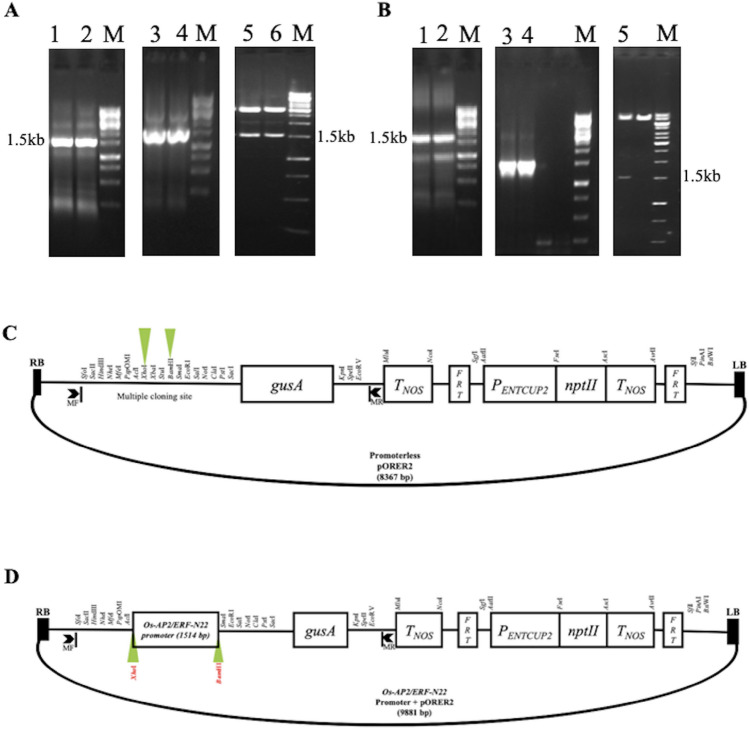
Approximately 1.5 kb promoter sequence upstream of the Os-AP2/ERF transcription start site was amplified from the N22 cultivar. The amplified sequence was first cloned and sequenced in the pGEM®-T Easy vector (Fig. 5A) and then into the promoter less pORE R2 binary vector (Fig. 5B). Based on the results from the sequencing of Os-AP2/ERF- pGEM®-T Easy and Os-AP2/ERF- pORE R2 clones, the Os-AP2/ERF promoter sequence was assembled and deposited in NCBI Gene Bank (accession no. KJ580618). Cloning of the Os-AP2/ERF-N22 promoter in the pORE R2 vector enabled the GUS gene (uidA) present in this vector to be regulated by the Os-AP2/ERF-N22 promoter (Fig. 5C, D).
Fig. 5.
Cloning of Os-AP2/ERF-N22 promoter. (A) Cloning in pGEMT vector; Lanes 1 and 2: 1.5 kb PCR amplified promoter region of Os-AP2/ERF-N22; Lanes 3 and 4: Colony PCR screening for recombinants; lane 5: Restriction analysis of Os-AP2/EFR-N22 promoter ligated in vector with BamHI and XhoI; lane M: 1 kb gene ruler. (B) Cloning in pORE R2 binary vector; Lanes 1 and 2: 1.5 kb PCR amplified promoter region of Os-AP2/ERF-N22; Lanes 3 and 4: Colony PCR screening for recombinants; lane 5: Restriction analysis of Os-AP2/EFR-N22 promoter ligated in vector with BamHI and XhoI; lane M: 1 kb gene ruler. (C) Schematic representation of promoterless pORE R2 binary vector. (D) Schematic representation of recombinant pORE R2 binary vector with Os-AP2/ERF-N22 promoter
In-silico analysis of cis-regulatory elements in water deficit stress responsive Os-AP2/ERF-N22 promoter
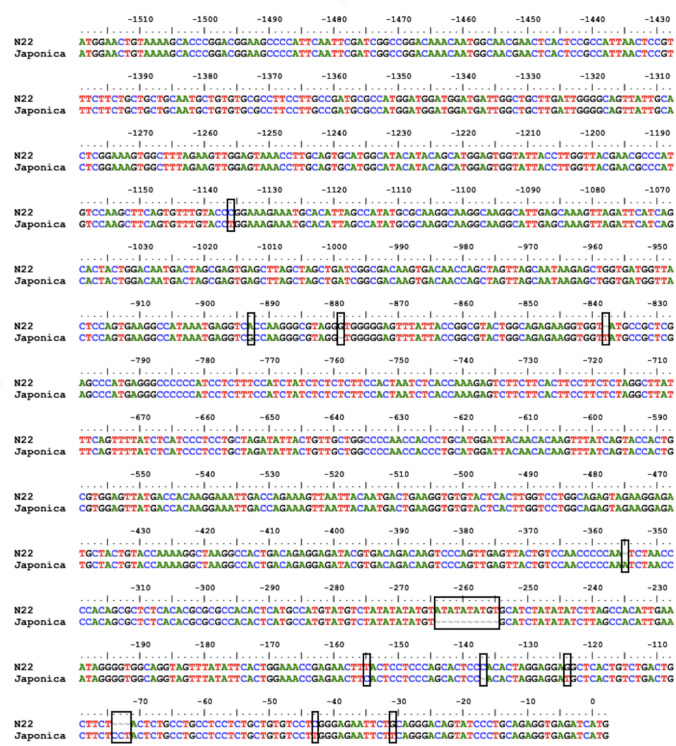
The Os-AP2/ERF-N22 promoter sequence of Oryza sativa sp. indica (N22) was compared with the Os-AP2/ERF promoter sequence of Oryza sativa sp. japonica (available in the Phytozome database) and differences were observed in their nucleotide sequence which included six single nucleotide polymorphisms and deletions at six positions (Fig. 6). Further, insilico analysis of important cis-regulatory elements of Os-AP2/ERF promoter from Oryza sativa sp. indica and Oryza sativa sp. japonica using PLACE and PlantCARE database revealed the presence of thirteen important cis-regulatory elements associated with drought and ABA responsiveness in both of these promoters (Alessandra et al. 2017). The identified cis-regulatory elements are MYBCORE, MYB2CONSENSUSAT, ABRELATERD1, ACGTATERD1, LTRECOREATCOR15, DRE2COREZMRAB17, DRECRTCOREAT, MYBATRD22, MYB1AT, MYB2AT, ABRE, MBS and WRKY71OS (Fig. 7 and Table 1). Eighteen TATA-box and 14 CAAT-box were also found upstream of the translational start site (Fig. 7). The predicted position, sequence, and function of TATA-box and CAAT-box are mentioned in Table S3. The Os-AP2/ERF promoter from indica and japonica species differ with respect to the copy number of MYB1AT and WRKY71OS cis-regulatory elements. In the Os-AP2/ERF-N22 promoter from indica species, there are two copies of MYB1AT cis-regulatory elements, whereas the Os-AP2/ERF promoter from japonica species has three copies of MYB1AT. Likewise, there are eleven copies of WRKY71OS in the Os-AP2/ERF-N22 promoter from indica species, whereas the Os-AP2/ERF promoter from japonica species has ten copies of WRKY71OS (Table 1).
Fig. 6.
Single nucleotide polymorphisms (SNPs) and deletions in between promoter region of Indica (N22 genotype) and Japonica (sequence available at phytozome database) group of rice
Table 1.
Comparison of drought-responsive Cis-elements in Os-AP2/ERF promoter of Oryza sativa sp. indica (N22) and Oryza sativa sp japonica
| Cis-regulatory elements | Core sequence | Number of cis-elements | Putative function | |
|---|---|---|---|---|
| Indica (N22 genotype) | Japonica | |||
| MYBCORE | CNGTTR | 6 | 6 | High salinity, cold, Heat, ABA and dehydration responsive |
| MYB2CONSENSUSAT | YAACKG | 4 | 4 | Dehydration responsive |
| ABRELATERD1 | ACGTG | 1 | 1 | Dehydration responsive |
| ACGTATERD1 | ACGT | 2 | 2 | Dehydration responsive |
| LTRECOREATCOR15 | CCGAC | 1 | 1 | Low temperature, Drought response |
| DRE2COREZMRAB17 | ACCGAC | 1 | 1 | Dehydration, high salinity and cold responsive |
| DRECRTCOREAT | RCCGAC | 1 | 1 | Dehydration, high salinity and cold responsive |
| MYBATRD22 | CTAACCA | 1 | 1 | ABA and Dehydration responsive |
| MYB1AT | WAACCA | 2 | 3 | ABA and Dehydration responsive |
| MYB2AT | TAACTG | 2 | 2 | Drought responsive |
| ABRE | TACGTG | 1 | 1 | ABA responsive |
| MBS | CAACTG | 2 | 1 | Drought responsive |
| WRKY71OS | TGAC | 11 | 10 | Drought responsive |
The symbol N represents A or C or G or T; R represents A or G; K represents T or A; Y represents C or T; and W represents A or T
Activity of rice Os-AP2/ERF-N22 promoter in transgenic Arabidopsis
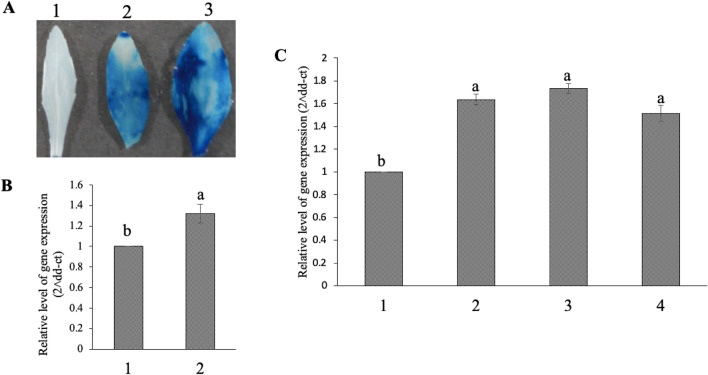
In order to analyze the activity of the promoter, the Os-AP2/ERF-N22 promoter-pORE R2 recombinant plasmid was first mobilized in Agrobacterium tumefaciens (EHA 105) and then in Arabidopsis thaliana by floral dip method (Zhang et al. 2006). Transformed Arabidopsis seeds were selected by kanamycin selection (Fig. 8), and at a later stage the transformed seedlings (T2) were subjected to PCR-mediated selection to confirm the presence of uid A gene (Fig. 9A) and Os-AP2/ERF-N22 promoter (Fig. 9B). Out of ten, two plants were found positive for both uid A gene as well as Os-AP2/ERF-N22 promoter in PCR mediated selection. The southern blot analysis also confirmed the presence of a single copy of Os-AP2/ERF-N22 promoter in the transgenic Arabidopsis (Fig. 9C). The GUS staining assay showed the activity of this promoter in the leaves, roots as well as stem of the transgenic Arabidopsis. In a comparison of GUS activity in transgenic Arabidopsis plants with Os-AP2/ERF-N22 promoter-driven GUS expression and those with CaMV35S constitutive promoter-driven GUS expression, it was observed that the intensity of blue colour developed in the GUS assay was higher in the plants having CaMV35S driven GUS expression (Fig. 10). Molecular analysis of transgenics was continued through T-1 and T-2 stage. GUS activity was further analyzed in the leaves of 30 days old T1 transgenic Arabidopsis seedlings having Os-AP2/ERF-N22 promoter-pORE R2 construct under osmotic stress conditions. The leaves from these plants were subjected to osmotic stress by PEG treatment (10 g/L). It was observed that these PEG-treated leaves developed more blue colour as compared to the leaves from control plants (transformed Arabidopsis) that were not subjected to PEG treatment (Fig. 11A). This observation was further validated by quantitative relative expression analysis of GUS (uid A) gene in the T1 transgenic Arabidopsis seedlings. The results showed a 1.32-fold higher expression of GUS (uid A) in the leaves subjected to 10 g/L PEG treatment compared to the leaves from control plants (transformed arabidopsis) that were not subjected to PEG treatment (Fig. 11B). Quantitative relative expression analysis of GUS (uid A) gene was also performed in the ABA treated (100 mM) leaves as well as PEG treated leaves (with two different PEG concentrations i.e. 5 and 10 g/L) of 30 days old T2 transgenic Arabidopsis seedlings having Os-AP2/ERF-N22 promoter-pORE R2. The results showed 1.51-fold higher expression of GUS (uid A) in the leaves subjected to ABA treatment as compared to the control (transformed Arabidopsis seedling without osmotic stress). In the leaves subjected to 10 g/L PEG treatment, 1.73-fold higher expression of GUS (uid A) was observed, whereas the leaves treated with 5 g/L PEG showed 1.63-fold higher expression of GUS (uid A) as compared to control (Fig. 11C). It was thus confirmed that Os-AP2/ERF-N22 promoter in T2 Arabidopsis responds to osmotic stress as well as ABA treatment. Higher GUS (uid A) expression was observed in stable T2 transformants under osmotic stress (PEG 10 g/L) as compared to T1.
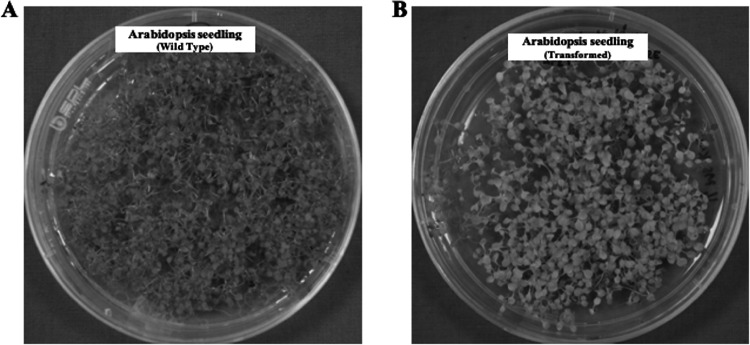
Fig. 8.
Selection of transformed Arabidopsis thaliana seedlings. (A) Arabidopsis thaliana (wild type) seeds on ½ MS media alone (control); (B) Arabidopsis thaliana seeds (from plants transformed with Os-AP2/ERF-N22 promoter-pORE R2 construct) grown on ½ MS media also containing kanamycin. Only the kanamycin-resistant transgenic seedlings retain the dark green colour, whereas the non-transformed seedlings are of pale light green colour
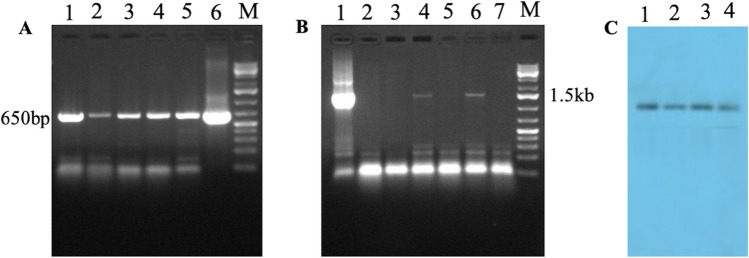
Fig. 9.
Screening of transformed Arabidopsis thaliana plants. (A) PCR mediated screening of Arabidopsis thaliana plants (with Os-AP2/ERF-N22 promoter-pORE R2 construct) using GUS (uid A) gene specific primers; Lanes 1–6: PCR amplified product of 650 bp. (B) PCR screening of Arabidopsis thaliana (with Os-AP2/ERF-N22 promoter-pORE R2 construct) using Os-AP2/ERF-N22 promoter-specific primers; Lane 1: Control, Lanes 4 and 6: PCR amplified product of ~ 1.5 kb. (C) Southern blot analysis of Arabidopsis thaliana plants for Os-AP2/ERF-N22 promoter using promoter-specific probe; Lanes 1 & 2: 1st Arabidopsis transformant, Lanes 3 & 4: 2nd Arabidopsis transformant
Fig. 10.
GUS staining of Arabidopsis thaliana plants. (A) The untransformed Arabidopsis thaliana plants (wild type) after GUS staining. (B) The transformed Arabidopsis thaliana plants (with CaMV35S constitutive promoter-driven GUS gene) after GUS staining (C) The transformed Arabidopsis thaliana plants (with Os-AP2/ERF-N22 promoter-driven GUS gene) after GUS staining
Fig. 11.
Drought and ABA responsiveness of transgenic Arabidopsis thaliana plants. (A) GUS staining Arabidopsis thaliana; 1: Leaf of wild type 2: Leaf without osmotic stress, 3: Leaf subjected to osmotic stress by treatment of PEG (10 g/L). (B) analysis of relative GUS expression in leaves of T1 Arabidopsis thaliana (transformed with Os-AP2/ERF-N22-pORE R2 construct) by qRT PCR; 1: Leaf without osmotic stress, 2: Leaf subjected to osmotic stress by treatment of PEG (10 g/L). (C) qRT PCR analysis of relative GUS expression in the leaves of T2 transgenic Arabidopsis thaliana having Os-AP2/ERF-N22 promoter-driven GUS (uid A) under different treatments 1: T2 Arabidopsis transformants without osmotic stress, 2: T2 Arabidopsis transformants subjected to osmotic stress (PEG 5 g/L), 3: T2 Arabidopsis transformants subjected to osmotic stress (PEG 10 g/L), and 4: T2 Arabidopsis transformants subjected to ABA (100 μM). Values represent mean ± SEs, n = 3. The different letters which mark the mean values indicate significant differences between treatments at P ≤ 0.05
Drought-responsiveness of Os-AP2/ERF-N22 promoter in rice transformants
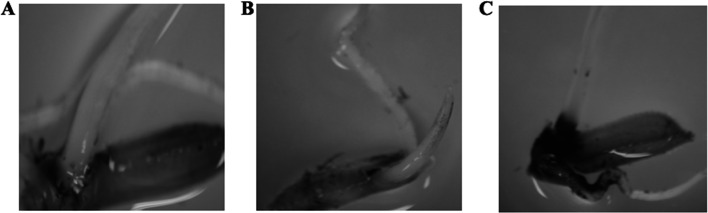
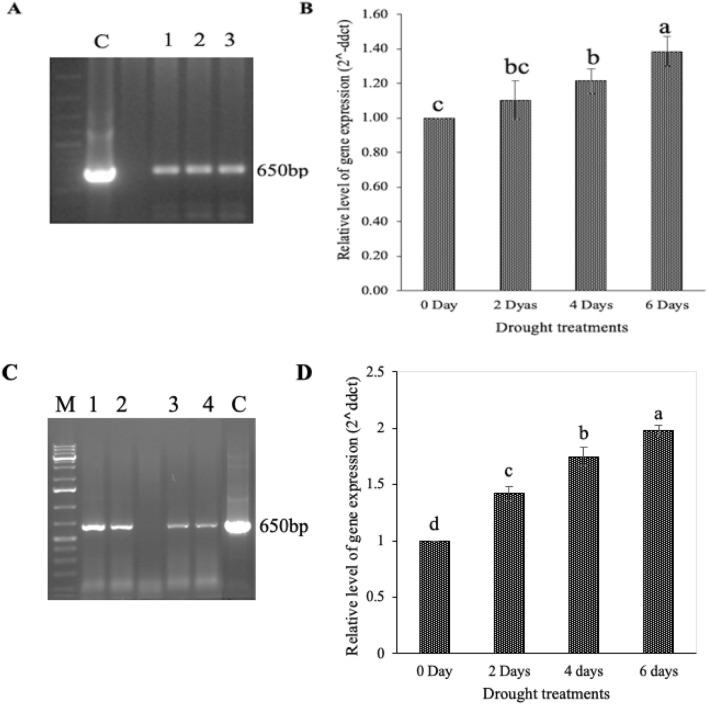
To investigate the drought responsiveness of Os-AP2/ERF-N22 promoter in rice plants, we transformed the MTU1010 genotype of rice with Os-AP2/ERF-N22 promoter-pORE R2 recombinant plasmid by the in-planta transformation method. In order to compare the GUS activity of Os-AP2/ERF-N22 promoter with CaMV35S constitutive promoter, we transformed the MTU1010 genotype with CaMV35S constitutive promoter-driven GUS gene. To check the transformation efficiency, the GUS activity test was done two days after transformation in randomly selected ten rice seedlings out of the seventy-three rice seedlings that were transformed. Three seedlings out of the ten that were tested developed blue colour, which shows that they were GUS positive and transformants (Fig. 12). It was also observed that the intensity of blue colour developed was higher in the plants having CaMV35S driven GUS expression as compared to those having Os-AP2/ERF-N22 promoter-driven GUS expression (Fig. 12). The remaining sixty-three seedlings (transformed with Agrobacterium containing Os-AP2/ERF-N22 promoter-pORE R2 recombinant plasmid) were grown in phytotron to get T1 seeds. Further, T1 seeds were grown in phytotron to be used later for PCR-mediated screening for the selection of seedlings having the uid A gene. Three of these seedlings were found PCR positive when screened for uid A (Fig. 13A). These PCR-positive plants were grown in a phytotron till maturity to get T2 seeds. Further, T2 transformants were subjected to PCR-mediated screening for uid A. Four of these seedlings were found PCR positive for uid A (Fig. 13C). To check the drought responsiveness of Os-AP2/ERF-N22 promoter in transformed rice plants (T1 and T2), the quantitative relative expression analysis of the GUS (uid A) was done in leaves from 30 days old transformed rice plants (T1 and T2), subjected to water deficit stress by withholding water for 2, 4 and 6 days consecutively. Our results showed that the expression of GUS in the transformed rice seedlings was enhanced by 1.11-fold, 1.21-fold, and 1.39-fold with respect to control in T-1 and 1.42-fold, 1.75-fold, and 1.98-fold with respect to control in T2 after withholding water for 2, 4, and 6 days respectively, in GUS positive plants, thus reflecting the increase in the activity of Os-AP2/ERF-N22 promoter upon water deficit stress (Fig. 13B, D).
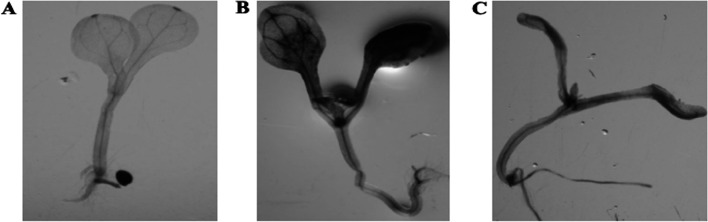
Fig. 12.
GUS staining of rice seedling (T0) after Agrobacterium-mediated in-planta transformation. (A) Wild type rice seedling (control), (B) GUS-stained rice seedling transformed with Os-AP2/ERF-N22 promoter-pORE R2 recombinants, and (C) GUS stained rice seedling transformed with the CaMV35S constitutive promoter-driven GUS (uid A) gene
Fig. 13.
Drought-responsiveness of Os-AP2/ERF-N22 promoter in rice transformants. (A) PCR screening of T1 transformed rice using GUS-specific primers; lane C: Positive PCR control; lanes 1, 2 and 3: 650 bp amplicon obtained from three different T1 transformed rice seedlings; M:1 kb gene ruler. (B) Analysis of relative GUS expression in the leaves of T1 rice transformants (Os-AP2/ERF-N22 promoter-driven GUS) in response to drought (created by withholding water for 2, 4, and 6 days) by qRT PCR. (C) PCR screening of T2 transformed rice using GUS-specific primers; lane C: Positive PCR control; lanes 1, 2, 3 and 4: 650 bp amplicon obtained from four different T2 transformed rice seedlings; M:1 kb plus gene ruler. (D) Analysis of relative GUS expression in the leaves of T2 rice transformants (Os-AP2/ERF-N22 promoter-driven GUS) in response to drought (created by withholding water for 2, 4, and 6 days) by qRT PCR. Values represent mean ± SEs, n = 3. The different letters which mark the mean values indicate significant differences between treatments at P ≤ 0.05
Discussion
Constitutive promoters are most often used in genetic engineering. The CaMV 35S promoter of the cauliflower mosaic virus drives gene expression in nearly all tissues and at all developmental stages (Fang et al. 1989). The constitutive expression of transgenes by constitutive promoters can conceal the function of transgenes related to signal transduction and energy transformation. Additionally, it may lead to metabolic burden or toxicity in transgenic plants. The repetitive use of any promoter is also a reason for transgenic silencing (Bhullar et al. 2003; Charrier et al. 2000; Xu et. al. 2010). Also, considering the low activity of dicot constitutive and inducible stress promoters such as CaMV35S and RD 29 A in monocots, it is imperative to have inducible promoters from monocots.
Till date, many abiotic stress-responsive promoters have been cloned and characterized mostly in Arabidopsis and some other plants. For example, Zhang et al. (2017) isolated an abiotic stress-responsive promoter of TaSnRK 2.8 (Sucrose non-fermenting 1-related protein kinase 2) from wheat and characterized it in Arabidopsis. Likewise, pGMRD26, a drought-responsive promoter from soybean, was characterized in Arabidopsis (Freitas et al. 2019). Alessandra et al. (2017) isolated the salt, osmotic stress and dehydration responsive promoter of soybean a-galactosidase gene (GlymaGal) and characterized it in both Arabidopsis and soybean. Rerksiri et al. (2013) isolated and characterized promoters of three heat-inducible genes (OsHsf B2cp, PM19p and HSP 90p) of rice. Characterization of OsbZIP23 promoter from drought tolerant O. rufipogon and drought-sensitive IR-20 cultivar of rice showed variation in the number of stress-responsive cis-elements and promoter activity (Dey et al. 2016). Similarly, Xue et al. (2018) isolated a promoter of OsGSE (Green tissue gene) from wild rice (Oryza rufipogon Griff) and functionally characterized it in Arabidopsis.
In the current study, the cloning and characterization of the promoter of Os-AP2/ERF-N22, a transcription factor encoding gene from the rice was carried out. In-silico analysis of the genes present on chromosome no. 6 of rice (Oryza sativa sp.) revealed that the inter-gene distance between the TF encoding gene (Os-AP2/ERF-N22, ID: LOC_OS06g40150) and the next adjacent gene on chromosome 6 (ID: LOC_OS06g40140) is about 6.706 Kb which indicated that the 1.5 kb DNA stretch before the TSS of Os-AP2/ERF-N22 does not contain the coding sequence of any other gene and can therefore be used to study the Os-AP2/ERF-N22 promoter. We, therefore, decided to clone this 1.5 kb region upstream of the Os-AP2/ERF-N22 coding sequence. This is the first report in which the promoter of drought and ABA-responsive AP2/ERF transcription factor encoding gene of rice has been cloned and characterized. Gene expression under stress is primarily regulated at the transcriptional level (Shinozaki and Yamaguchi-Shinozaki, 2007) via specific binding of TFs to the specific cis-elements present in the promoter (Passricha et al. 2017). The level of gene expression is also decided by the pattern and distribution of cis-elements in the promoter and intronic regions (Rombauts et al. 2003; Brown et al. 2007; Zou et al. 2011; Hernandez-Garcia and Finer, 2014).
The in-silico analysis of Os-AP2/ERF-N22 promoter had many cis-regulatory elements such as MYBCORE, MYB2CONSENSUSAT, ABRELATERD1, DRE2COREZMRAB17, MYBATRD22, MBS and WRKY71OS. These motifs present in the Os-AP2/ERF-N22 promoter are the binding site for major stress-inducible transcription factors like AREB, MYB, DREB, and WRKY. MYBCORE, a cis-element, binds to MYB transcription factors AtMYB1 and AtMYB2. The MYB transcription factors regulate the ABA-dependent stress signaling to upregulate many genes responsive to abiotic stress. AtMYB2 activates RD22 expression in response to dehydration and ABA (Urao et al. 1993; Abe et al. 2003). The Os-AP2/ERF-N22 promoter had the MYBCORE sequence, the binding site for the AtMYB2 transcription factor, which emphasizes the importance of this promoter under dehydration. Overexpression of AtMYB2 enhanced sensitivity to ABA and also improved the osmotic tolerance (Abe et al. 2003).
The 9 bp conserved sequence (5′-TACCGACAT-3′) of dehydration responsive element (DRE) was identified in RD29A, a drought-responsive gene promoter (Yamaguchi-Shinozaki and Shinozaki, 1994). The DRE2COREZMRAB17 cis-element (DRE2 core found in maize rab17 gene promoter) in the Os-AP2/ERF-N22 promoter binds to the DREB transcription factor to regulate gene expression under abiotic stress (Mizoi et al. 2012). The DREB1 (A-1) sub-group of DREB transcription factors comprises of six members, out of which DREB1D/CBF4 is responsive to drought as well as ABA (Mizoi et al. 2012). Under conditions of water deficit, the transgenic rice plants overexpressing DREB1 (A-1) regulated by a stress-inducible promoter showed higher spikelet fertility and yield as compared to wild-type plants (Xiao et al. 2009). The DREB2 (A-2) subgroup of DREB transcription factors comprises of eight members in Arabidopsis, out of which DREB2A is the best characterized one. The DREB2A gene is slightly upregulated by ABA but strongly induced in response to drought, salt, and temperature stress (Liu et al. 1998; Nakashima et al. 2000). Constitutive, over-expression of DREB2A, has been reported to improve tolerance against drought, high salinity, and heat shock but also retarded the growth of transgenic plants. The genes upregulated by DREB2A are also inducible by heat shock or drought (Sakuma et al. 2006).
ABRELATERD1 (ABRE-like sequence required for etiolation-induced expression of erd1) in Arabidopsis is also an important cis-element observed in the Os-AP2/ERF-N22 promoter. It binds to the ABA-responsive element (ABRE) binding proteins/factors (AREBs/ABFs). These transcription factors belong to the bZIP transcription factor family. Overexpression of AREB2/ABF4 transcription factor showed hypersensitivity to ABA, reduced transpiration, and enhanced drought tolerance in Arabidopsis (Kang et al. 2002; Fujita et al. 2005).
Our study involving transgenic Arabidopsis and rice plants containing the Os-AP2/ERF-N22 promoter-GUS construct showed GUS expression in the leaves, stem, and roots which implies that at the basal level, expression governed by this promoter is not localized to any particular plant part. Under water deficit stress induced by PEG treatment, the enhancement observed in the activity of this promoter confirmed the drought-inducible nature of this promoter. Treatment with different concentrations of PEG showed that increasing the intensity of stress (from 5 to 10 g/L) increases the activity of this promoter. Alessandra et al. (2017) observed that PEG-mediated osmotic stress enhanced the activity of the soybean α- galactosidase promoter in Arabidopsis. Tao et al. (2014) found that the activity of Zea mays RXO 1 (a nucleotide binding site leucine-rich repeat type of R gene in maize) promoter increased in transgenic Arabidopsis plants having pRXO1-GUS when subjected to PEG treatment.
We also observed that ABA treatment enhanced the expression of this promoter as reflected by GUS expression in transgenic Arabidopsis plants (containing the Os-AP2/ERF-N22 promoter-GUS construct), which was expected as the ABRELATERD1 cis-element in this promoter provides the binding site of ABA, and this explains the ABA inducibility of this promoter. The qRT PCR results also confirmed the PEG and ABA inducibility of this promoter. Further, the drought and ABA-inducible nature of this promoter were also established in transgenic rice plants by using the histochemical GUS assay. As in Arabidopsis, it was observed that increasing the intensity of stress also increased the activity of this promoter in rice.
Our results demonstrate that Os-AP2/ERF-N22 transcription factor is important for ABA-dependent response to drought. Physio-biochemical analysis of transgenics overexpressing Os-AP2/ERF-N22 transcription factor revealed higher relative water content, membrane stability index, osmotic potential, radical scavenging activity, chlorophyll stability index, photosynthetic rate, wax content, and almost similar chlorophyll content under water deficit stress and showed no phenotypic aberrations. AP2/ERF-N22 is the ortholog of Arabidopsis SHN proteins. SHN proteins, when overexpressed, display increased cuticular wax biosynthesis (Mawlong et. al. 2014). Our hypothesis is that AP2/ERF-N22 activated cuticular wax biosynthesis. Higher wax content also contributed to decreased stomatal conductance and transpiration rate and additionally influenced higher MSI, cell turgor, and osmotic potential in transgenics. Higher osmolytes in transgenics resulted in higher antioxidant potential and thus maintained higher MSI and cell turgor. A somewhat similar result was observed in Arabidopsis for the AtERF53 transcription factor (Cheng et al. 2012). Thus, enhanced expression of the Os-AP2/ERF-N22 gene under different levels of water deficit stress at vegetative, as well as anthesis stages, confirmed its crucial role in plant stress tolerance.
Future efforts toward identifying and characterizing multiple stress-responsive transcription factor genes will expand our understanding of stress tolerance in plants (Prasch and Sonnewald 2015). The simultaneous manipulation of many stress-responsive transcription factors using genetic and molecular techniques is a very promising approach for improving tolerance against drought rather than manipulating any one functional gene at a time. Further identification and characterization of novel drought-inducible promoters are equally important to overcome the drawbacks of using constitutively active promoters while manipulating the drought-responsive genes. Therefore, drought and ABA-responsive promoter characterized here will be useful in regulating the expression of stress-responsive genes for different crop engineering purposes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the support of Incharge, National Phytotron Facility, ICAR-IARI, New Delhi and Dr. Rohini Sreevathsa (Principal scientist, ICAR-NIPB, New Delhi) to carry out this work.
Author contributions
VK and AT conceptualized and designed the work. VK, AK, KT, NKG, SSC and KA conducted the experiments. VK, AK, KT and AT analysed data and wrote the manuscript.
Funding
The authors thankfully acknowledge the financial assistance of SERB, Department of Science and Technology, Government of India, New Delhi.
Declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any financial, commercial or other relationship that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vaibhav Kumar, Email: vaibhavchf@gmail.com.
Aruna Tyagi, Email: arunatyagi19@yahoo.com.
References
- Abe H, Urao T, Ito T, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15(1):63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandra JC, Guimaraes-Dias F, Nevas-Borges AC, Malato MB, Whipps DF, Ferreira MA. Isolation and characterization of a promoter responsive to salt, osmotic and dehydration stresses in soybean. Genet Mol Biol. 2017;40(1):226–237. doi: 10.1590/1678-4685-GMB-2016-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt SK. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol Plant. 2015;155:355–368. doi: 10.1111/ppl.12380. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Sahoo DK, Dey N, Houtz RL, Maiti IB. An intergenic region shared by At4g35985 and At4g35987 in Arabidopsis thaliana is a tissue specific and stress inducible bidirectional promoter analyzed in transgenic Arabidopsis and tobacco plants. PLoS ONE. 2013;8:e79622. doi: 10.1371/journal.pone.0079622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bars HD, Weatherly PE. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Battraw MJ, Hall TC. Histochemical analysis of CaMV 35S promoter-β- glucuronidase gene expression in transgenic rice plants. Plant Mol Biol. 1990;15:527–538. doi: 10.1007/BF00017828. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Ren L, Chua NH. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J. 1990;9:1677–1684. doi: 10.1002/j.1460-2075.1990.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar S, Chakravarthy S, Advani S, Datta S, Pental D, Burma PK. Strategies for development of functionally equivalent promoters with minimum sequence homology for transgene expression in plants: cis-elements in a novel DNA context versus domain swapping. Plant Physiol. 2003;132:988–998. doi: 10.1104/pp.103.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihmidine S, Lin J, Stone JM, Awada T, Specht JE, Clemente TE. Activity of the Arabidopsis RD29A and RD29B promoter elements in soybean under water stress. Planta. 2013;237:55–64. doi: 10.1007/s00425-012-1740-9. [DOI] [PubMed] [Google Scholar]
- Brown CD, Johnson DS, Sidow A. Functional architecture and evolution of transcriptional elements that drive gene. Science. 2007;317:1557–1560. doi: 10.1126/science.1145893. [DOI] [PubMed] [Google Scholar]
- Charrier B, Scollan C, Ross S, Zubko E, Meyer P. Co-silencing of homologous transgenes in tobacco. Mol Breed. 2000;6:407–419. doi: 10.1023/A:1009672714835. [DOI] [Google Scholar]
- Cheng MC, Hsieh EJ, Chen JH, Chen HY, Lin TP. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012;158:363–375. doi: 10.1104/pp.111.189738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE. Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol. 1993;23:567–581. doi: 10.1007/BF00019304. [DOI] [PubMed] [Google Scholar]
- Dey A, Samanta MK, Gayen S, Sen SK, Maiti MK. Enhanced gene expression rather than natural polymorphism in coding sequence of the OsbZIP23 determines drought tolerance and yield improvement in rice genotypes. PLoS ONE. 2016;11(3):e0150763. doi: 10.1371/journal.pone.0150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Vigel MO, Viehhauser A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma. 2010;245(1–4):3–14. doi: 10.1007/s00709-010-0142-8. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Du H, Shen X, Huang Y, Huang M, Zhang Z. Overexpression of Vitreoscilla hemoglobin increases waterlogging tolerance in Arabidopsis and maize. BMC Plant Biol. 2016;16:35. doi: 10.1186/s12870-016-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebercon A, Blum A, Jordan WR. A rapid colorimetric method for epicuticular wax content of sorghum leaves. Crop Sci. 1977;17:179–180. doi: 10.2135/cropsci1977.0011183X001700010047x. [DOI] [Google Scholar]
- Engels C, Fuganti-Pagliarini R, Marin SRR, Marcelino-Guimarães FC, Oliveira MCN, Kanamori N, Mizoi J, Nakashima K, Yamaguchi-Shinozaki K, Nepomuceno AL. Introduction of the rd29A: AtDREB2A CA gene into soybean (Glycine max L. Merril) and its molecular characterization in leaves and roots during dehydration. Genet Mol Biol. 2013;36:556–565. doi: 10.1590/S1415-47572013000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RX, Nagy F, Sivasuramaniam S, Chua NH. Multiple cis-regulatory elements for maximal expression of the Cauliflower Mosaic Virus 35S promoter in transgenic plants. Plant Cell. 1989;1:141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas EO, Melo BP, Lourenço-Tessutti IT. Identification and characterization of the GmRD26 soybean promoter in response to abiotic stresses: potential tool for biotechnological application. BMC Biotechnol. 2019;19:79. doi: 10.1186/s12896-019-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takaji M, Shinozaki K, Yamaguchi-Shinozaki K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17:3470–3488. doi: 10.1105/tpc.105.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Raghuvanshi S, Tyagi AK. Assessment of the efficiency of various gene promoters via biolistics in leaf and regenerating seed callus of millets, Eleusine coracan aand Echinochloa crusgalli. Plant Biotechnol. 2001;18:275–282. doi: 10.5511/plantbiotechnology.18.275. [DOI] [Google Scholar]
- Hernandez-Garcia CM, Finer JJ. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014;217(218):109–119. doi: 10.1016/j.plantsci.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57(12):1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Homrich MS, Wiebke-Strohm B, Weber RLM, Bodanese- Zanettini MH. Soybean genetic transformation: a valuable tool for the functional study of genes and the production of agronomically improved plants. Genet Mol Biol. 2012;35:998–1010. doi: 10.1590/S1415-47572012000600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Jiang P, Qi S, Zhang K, He Q, Xu C, Ding Z, Zhang K, Li K. Isolation and functional validation of salinity and osmotic stress inducible promoter from the maize type-II H+-pyrophosphatase gene by deletion analysis in transgenic tobacco plants. PLoS ONE. 2016;11:e0154041. doi: 10.1371/journal.pone.0154041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz M, Yang Y, Liu R, Xu Y, Khan MA, Wei Q, Gao J, Hong B. Identification and functional characterization of the BBX24 promoter and gene from Chrysanthemum in Arabidopsis. Plant Mol Biol. 2015;89:1–19. doi: 10.1007/s11103-015-0347-5. [DOI] [PubMed] [Google Scholar]
- Jagtap V, Bhargava S, Sterb P, Feierabend J. Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) J Exp Bot. 1998;49:1715–1721. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya Y, Hattori T. Arabidopsis transcription factors, RAV1 and RAV2, are regulated by touch-related stimulin in a dose dependent and biphasic manner. Genes Genet Syst. 2009;84:95–99. doi: 10.1266/ggs.84.95. [DOI] [PubMed] [Google Scholar]
- Kang JY, Choi HI, Im MY, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signalling. Plant Cell. 2002;14:343–357. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleyoreas SA. A new method for determining drought resistance. Plant Physiol. 1958;33:22. doi: 10.1104/pp.33.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Kumar A, Ali K, Tewari K, Garg NK, Changan SS, Tyagi A. Cloning and Heterologous expression of Os-AP2/ERF-N22 drought inducible rice transcription factor in E. Coli. Indian J Agric Sci. 2018;88(10):1515–1520. [Google Scholar]
- Kumar V (2018) Expression analysis of AP2/ERF family transcription factor from rice under water deficit stress and characterization of its promoter region. Ph.D Thesis ICAR-Indian Agricultural Research Institute Pusa New Delhi India
- Li H, Fa W, Zhang J, Wang N, Li Q, Huan C (2014) Soybean adverse situation induced gene promoter and application thereof. CN 103820451 A In Google Patents 2014 May 28
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlong I, Kurup D, Ali K, Yadav S, Tyagi A. Isolation and characterization of an AP2/ ERF-type drought stress inducible transcription factor encoding gene from rice. J Plant Biochem Biotechnol. 2014;23(1):42–51. doi: 10.1007/s13562-012-0185-3. [DOI] [Google Scholar]
- Mawlong I, Ali K, Srinivasan R, Rai RD, Tyagi A. Functional validation of a drought-responsive AP2/ERF family transcription factor-encoding gene from rice in Arabidopsis. Mol Breed. 2015;35:163–174. doi: 10.1007/s11032-015-0290-9. [DOI] [Google Scholar]
- Mawlong I, Ali K, Tyagi A. Functional validation of a water deficit stress responsive AP2/ERF family transcription factor encoding gene in Oryza sativa. Indian J Biochem Biophys. 2018;55:17–25. [Google Scholar]
- McElroy D, Blowers AD, Jenes B, Wu R. Construction of expression vectors based on the rice actin1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet. 1991;231:150–160. doi: 10.1007/BF00293832. [DOI] [PubMed] [Google Scholar]
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Shinwari ZK, Sakuma Y, Seki M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol Biol. 2000;42:657–665. doi: 10.1023/A:1006321900483. [DOI] [PubMed] [Google Scholar]
- Novák J, Pavlu J, Novák O, Nozková-Hlavácková V, Spundová M, Hlavinka J, Koukalová S, Skalák J, Cerny M, Brzobohaty B. High cytokinin levels induce a hypersensitive-like response in tobacco. Ann Bot. 2013;112:41–55. doi: 10.1093/aob/mct092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of cauliflower mosaic virus 35S promoter. Nature. 1985;313:810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Passricha N, Saifi S, Ansari MW, Tuteja N. Prediction and validation of cis-regulatory elements in 5′ upstream regulatory regions of lectin receptor-like kinase gene family in rice. Protoplasma. 2017;254(2):669–684. doi: 10.1007/s00709-016-0979-6. [DOI] [PubMed] [Google Scholar]
- Polizel AMM, Nakashima K, Yamanaka N, Farias JR, de Oliveira MC, Marin SR, Abdelnoor RV, Marcelino-Guimarães FC, Fuganti R, Rodrigues FA. Molecular, anatomical and physiological properties of a genetically modified soybean line transformed with rd29A: AtDREB1A for the improvement of drought tolerance. Genet Mol Res. 2011;4:3641–3656. doi: 10.4238/2011.October.21.4. [DOI] [PubMed] [Google Scholar]
- Prasch CM, Sonnewald U. Signaling events in plants: stress factors in combination change the picture. Environ Expt Bot. 2015;114:4–14. doi: 10.1016/j.envexpbot.2014.06.020. [DOI] [Google Scholar]
- Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Muthurajan R. Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol. 2016;16(suppl_1):7–20. doi: 10.1186/s12896-016-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan V, Veluthambi K. Transfer of non-T-DNA portions of the agrobacterium tumefaciens Ti plasmid pTiA6 from the left terminus of TL-DNA. Plant Mol Biol. 1995;28:1149–1154. doi: 10.1007/BF00032676. [DOI] [PubMed] [Google Scholar]
- Rerksiri W, Zhang X, Xiong H, Chen X (2013) Expression and Promoter Analysis of Six Heat Stress-Inducible Genes in Rice. Sci World J 397401 [DOI] [PMC free article] [PubMed]
- Rombauts S, Florquin K, Lescot M, Marchal K, Rouze R, Van de Peer Y. Computational approaches to identify promoters and cis- regulatory elements in plant genomes. Plant Physiol. 2003;132:1162–1176. doi: 10.1104/pp.102.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu H, Rao AR, Bansal KC, Muthusamy SK, Chinnusamy V (2016) Genome-wide analysis and identification of abiotic stress responsive transcription factor family genes and miRNAs in bread wheat (Triticum aestivum L.): genomic study of bread wheat. IEEE Digital Library 1–4
- Saint Pierre C, Crossa JL, Bonnett D, Yamaguchi-Shinozaki K, Reynolds MP. Phenotyping transgenic wheat for drought resistance. J Exp Bot. 2012;63:1799–1808. doi: 10.1093/jxb/err385. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–177. doi: 10.1111/j.1439-037X.1997.tb00486.x. [DOI] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular cloning: a laboratory manual. Cold Spring Harbour: Cold Spring Harbour Laboratory; 2001. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58(2):221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Tao Y, Wang F, Jia D, Li J, Zhang Y, Jia C, Wang D, Pan H. Cloning and functional analysis of the promoter of a Stress-inducible gene (ZmRXO1) in Maize. Plant Mol Biol Rep. 2014;33(2):200–208. doi: 10.1007/s11105-014-0741-1. [DOI] [Google Scholar]
- Tewari K, Kumar V, Kumar A, Bansal N, Vinutha T, Ali K, Sachdev A, Kumari S, Dahuja A. Molecular cloning and functional analysis of the promoter of γ-Tocopherol Methyl Transferase (γ-TMT) gene of soybean (Glycine max) 3 Biotech. 2018;8(8):325. doi: 10.1007/s13205-018-1347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K. An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell. 1993;5:1529–1539. doi: 10.1105/tpc.5.11.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan J, Devanna BN, Singh NK, Sharma TR. Cloning and functional validation of early inducible Magnaporthe oryzae responsive CYP76M7 promoter from rice. Front Plant Sci. 2015;6:371. doi: 10.3389/fpls.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Wang JP, Yang HF. Identification and functional characterization of the NAC gene promoter from Populus euphratica. Planta. 2016;244:417–427. doi: 10.1007/s00425-016-2511-9. [DOI] [PubMed] [Google Scholar]
- Withanage SP, Hossain MA, Kumar MS, Roslan HAB, Abdullah MP, Napis SB, Shukor NAA. Overexpression of Arabidopsis thaliana gibberellic acid 20 oxidase (AtGA20ox) gene enhance the vegetative growth and fiber quality in kenaf (Hibiscus cannabinus L.) plants. Breed Sci. 2015;65:177–191. doi: 10.1270/jsbbs.65.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BZ, Chen X, Xiang CB, Tang N, Zhang QF, Xiong LZ. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol Plant. 2009;2:73–83. doi: 10.1093/mp/ssn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ye R, Zheng Y, Wang Z, Zhou P, Lin Y, Li D. Isolation of the endosperm-specific LPAAT gene promoter from coconut (Cocos nucifera L.) and its functional analysis in transgenic rice plants. Plant Cell Rep. 2010;29:1061–1068. doi: 10.1007/s00299-010-0892-y. [DOI] [PubMed] [Google Scholar]
- Xue M, Long Y, Zhao Z. Isolation and Characterization of a Green-Tissue Promoter from Common Wild Rice (Oryza rufipogon Griff.) Int J Mol Sci. 2018;19(7):2009. doi: 10.3390/ijms19072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki KA. Novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6(2):251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Ma L, Wang Z, Lin Z, Su J, Lu BR. Multiple tissue-specific expression of rice seed-shattering gene SH4 regulated by its promoter pSH4. Rice. 2015;8:1–10. doi: 10.1186/s12284-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Henriques R, Lin S, Niu Q, Chua N. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):1–6. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jing R, Mao X. Functional characterization of TaSnRK2.8 promoter in response to abiotic stresses by deletion analysis in transgenic Arabidopsis. Front Plant Sci. 2017;8:1198. doi: 10.3389/fpls.2017.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Sun K, Mackaluso JD, Seddon AE, Jin R, Thomashow MF. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc Natl Acad Sci. 2011;108:14992–14997. doi: 10.1073/pnas.1103202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.