Abstract
Genetic syndromes which develop one or more nervous system (NS) tumors as one of the manifestations can be grouped under the umbrella term of NS tumor predisposition syndromes. Understanding the underlying pathological pathways at the molecular level has led us to many radical discoveries, in understanding the mechanisms of tumorigenesis, tumor progression, interactions with the tumor microenvironment, and development of targeted therapies. Currently, at least 7–10% of all pediatric cancers are now recognized to occur in the setting of genetic predisposition to cancer or cancer predisposition syndromes. Specifically, the cancer predisposition rate in pediatric patients with NS tumors has been reported to be as high as 15%, though it can approach 50% in certain tumor types (i.e., choroid plexus carcinoma associated with Li Fraumeni Syndrome). Cancer predisposition syndromes are caused by pathogenic variation in genes that primarily function as tumor suppressors and proto-oncogenes. These variants are found in the germline or constitutional DNA. Mosaicism, however, can affect only certain tissues, resulting in varied manifestations. Increased understanding of the genetic underpinnings of cancer predisposition syndromes and the ability of clinical laboratories to offer molecular genetic testing allows for improvement in the identification of these patients. The identification of a cancer predisposition syndrome in a CNS tumor patient allows for changes to medical management to be made, including the initiation of cancer surveillance protocols. Finally, the identification of at-risk biologic relatives becomes feasible through cascade (genetic) testing. These fundamental discoveries have also broadened the horizon of novel therapeutic possibilities and have helped to be better predictors of prognosis and survival. The treatment paradigm of specific NS tumors may also vary based on the patient’s cancer predisposition syndrome and may be used to guide therapy (i.e., immune checkpoint inhibitors in constitutional mismatch repair deficiency [CMMRD] predisposition syndrome) [8]. Early diagnosis of these cancer predisposition syndromes is therefore critical, in both unaffected and affected patients. Genetic counselors are uniquely trained master’s level healthcare providers with a focus on the identification of hereditary disorders, including hereditary cancer, or cancer predisposition syndromes. Genetic counseling, defined as “the process of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease” plays a vital role in the adaptation to a genetic diagnosis and the overall management of these diseases. Cancer predisposition syndromes that increase risks for NS tumor development in childhood include classic neurocutaneous disorders like neurofibromatosis type 1 and type 2 (NF1, NF2) and tuberous sclerosis complex (TSC) type 1 and 2 (TSC1, TSC2). Li Fraumeni Syndrome, Constitutional Mismatch Repair Deficiency, Gorlin syndrome (Nevoid Basal Cell Carcinoma), Rhabdoid Tumor Predisposition syndrome, and Von Hippel-Lindau disease. Ataxia Telangiectasia will also be discussed given the profound neurological manifestations of this syndrome. In addition, there are other cancer predisposition syndromes like Cowden/PTEN Hamartoma Tumor Syndrome, DICER1 syndrome, among many others which also increase the risk of NS neoplasia and are briefly described. Herein, we discuss the NS tumor spectrum seen in the abovementioned cancer predisposition syndromes as with their respective germline genetic abnormalities and recommended surveillance guidelines when applicable. We conclude with a discussion of the importance and rationale for genetic counseling in these patients and their families.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01277-w.
Keywords: Brain tumor, Cancer genetics, Cancer predisposition syndromes, Nervous system surveillance of genetic syndromes
Introduction
Genetic syndromes which develop one or more nervous system (NS) tumors as one of the manifestations can be grouped under the umbrella term of NS tumor predisposition syndromes. Understanding the underlying pathological pathways at the molecular level has led us to many radical discoveries, in understanding the mechanisms of tumorigenesis, tumor progression, interactions with the tumor microenvironment, and development of targeted therapies. Currently, at least 7-10% of all pediatric cancers are now recognized to occur in the setting of genetic predisposition to cancer, or cancer predisposition syndromes [1, 2]. Specifically, the cancer predisposition rate in pediatric patients with NS tumors has been reported to be as high as 15%, though it can approach 50% in certain tumor types (i.e., choroid plexus carcinoma associated with Li Fraumeni Syndrome).
Cancer predisposition syndromes are caused by pathogenic variation in genes that primarily function as tumor suppressors and proto-oncogenes [3, 4]. These variants are found in the germline, or constitutional DNA. Mosaicism is defined as the presence in an individual of at least two cell lines differing in genotype and arising from a single zygote [5]. Mosaicism however, can affect only certain tissues, resulting in varied manifestations [6]. Increased understanding of the genetic underpinnings of cancer predisposition syndromes and the ability of clinical laboratories to offer molecular genetic testing allows for improvement in the identification of these patients. The identification of a cancer predisposition syndrome in a CNS tumor patient allows for changes to medical management to be made, including the initiation of cancer surveillance protocols. Finally, the identification of at-risk biologic relatives becomes feasible through cascade (genetic) testing [7, 8].
These fundamental discoveries have also broadened the horizon of novel therapeutic possibilities and have helped to be better predictors of prognosis and survival. The treatment paradigm of specific NS tumors may also vary based on the patient’s cancer predisposition syndrome and may be used to guide therapy (example: immune checkpoint inhibitors in constitutional mismatch repair deficiency [CMMRD] predisposition syndrome and targeted therapies like MEK inhibitors, described later) [9]. Early diagnosis of these cancer predisposition syndromes along with early involvement of genetic counselors is therefore critical, in both unaffected and affected patients [10].
Cancer predisposition syndromes that increase risks for NS tumor development in childhood include classic neurocutaneous disorders like neurofibromatosis type 1 (NF1), schwannomatosis and its subtypes (e.g.,: NF2 , SMARCB1 and LZTR1) and tuberous sclerosis complex (TSC) type 1 and 2 (TSC1, TSC2). Li Fraumeni Syndrome, Constitutional Mismatch Repair Deficiency, Gorlin syndrome (Nevoid Basal Cell Carcinoma), Rhabdoid Tumor predisposition syndrome, Von Hippel-Lindau disease, and Ataxia Telangiectasia will also be discussed given the profound neurological manifestations of these syndromes. In addition, there are other cancer predisposition syndromes like Cowden/PTEN Hamartoma Tumor Syndrome, DICER1 syndrome, among many others which also increase the risk of NS neoplasia and are briefly described.
Herein, we discuss the NS tumor spectrum seen in the abovementioned cancer predisposition syndromes as with their respective germline genetic abnormalities and recommended surveillance guidelines when applicable. We conclude with a discussion of the importance and rationale for genetic counseling in these patients and their families.
Neurofibromatosis Type 1 (NF 1)
NF1 is among the most common genetic disorders. The incidence is about one in every 2500–4000 live births making it one of the most common cancer predisposition syndromes. The incidence does not vary across gender or ethnicity [11, 12]. It is inherited in an autosomal dominant fashion, though 50% of cases are thought to occur de novo (are not inherited from an affected parent), with near-complete penetrance, though the clinical manifestations and phenotypes can vary tremendously. Individuals with NF1 are at risk of developing tumors in multiple organ systems, particularly the central and peripheral nervous systems.
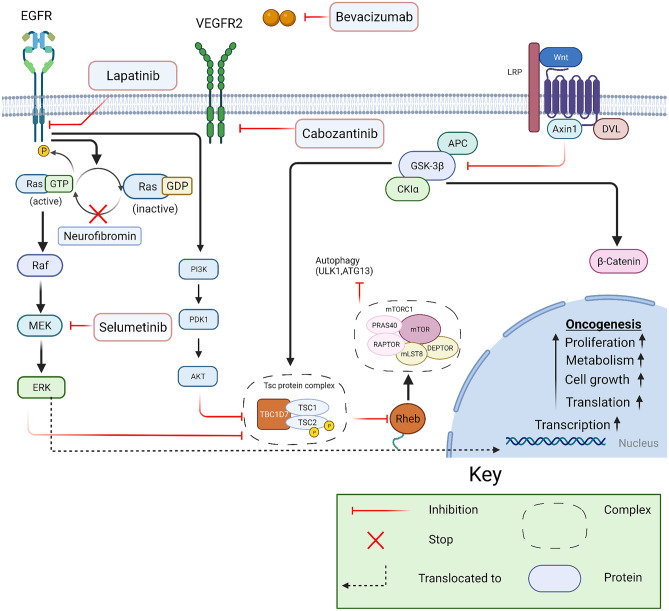
Neurofibromin is a negative regulator of the RAS-MAP kinase and mammalian target of rapamycin (mTOR) signaling pathways, and it is a well-known proto-oncogene. The role of the MAPK pathway is well-established in cell growth and proliferation (Fig. 1). The neurofibromin protein is coded by the NF1 gene located on the long arm of chromosome 17. Germline mutations of NF1 result in a multitude of manifestations that can be seen throughout the body [13]. Common nervous system tumors that develop in NF1 include low-grade gliomas (LGGs), cutaneous neurofibromas, plexiform neurofibromas (PNs), malignant peripheral nerve sheath tumors (MPNSTs), and high-grade gliomas (HGGs). Other non-nervous system tumors seen in the NF1 population are juvenile myelomonocytic leukemia (JMML), embryonal rhabdomyosarcoma, gastrointestinal stromal tumors, pheochromocytoma, breast cancers and endocrine tumors a monng others. The diagnostic criteria for NF1 are well-established, and recommended surveillance guidelines have been published (Table 1).
Fig. 1.
Molecular pathways associated with pathogenesis of NF and TSC with point of inhibition
Table 1.
Revised diagnostic criteria for neurofibromatosis type 1: an international consensus recommendation (NF1) [19]
| Revised diagnostic criteria for neurofibromatosis type 1: an international consensus recommendation |
|---|
| A: The diagnostic criteria for NF1 are met in an individual who does not have a parent diagnosed with NF1 if two or more of the following are present: |
| • Six or more café-au-lait macules over 5 mm in greatest diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals a |
| • Freckling in the axillary or inguinal region a |
| • Two or more neurofibromas of any type or one plexiform neurofibroma |
| • Optic pathway glioma |
| • Two or more iris Lisch nodules identified by slit lamp examination or two or more choroidal abnormalities (CAs)—defined as bright, patchy nodules imaged by optical coherence tomography (OCT)/near-infrared reflectance (NIR) imaging |
| • A distinctive osseous lesion such as sphenoid dysplasia, b anterolateral bowing of the tibia, or pseudarthrosis of a long bone |
| • A heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue such as white blood cells |
| B: A child of a parent who meets the diagnostic criteria specified in A merits a diagnosis of NF1 if one or more of the criteria in A are present |
aIf only café-au-lait macules and freckling are present, the diagnosis is most likely NF1 but exceptionally the person might have another diagnosis such as Legius syndrome. At least one of the two pigmentary findings (café-au-lait macules or freckling) should be bilateral
bSphenoid wing dysplasia is not a separate criterion in case of an ipsilateral orbital plexiform neurofibroma
Created with BioRender.com
Low-grade gliomas (LGG)
Optic pathway gliomas (OPGs) are the most common central nervous system tumor seen in NF1. OPGs are seen in up to 20% of the patients with NF1 and mainly present within the first 8 years of life, with a mean age of 4.5 years. On rare occasions, OPGs can present at an older age [14]. These lesions can often be indolent, but sometimes, they are progressive and may cause vision impairment. Once diagnosed with NF1, patients should undergo annual age-appropriate vision exams starting at the age of 1 year to 8 years; the recommendations for MRI screening after the age of 8 years are variable and considered on a case by case basis. This recommendation is based upon the relatively lower incidence of OPGs and vision dysfunction in this older age group [14, 15]. Tumors can arise anywhere from the retro-orbital optic nerve to optic radiations. In up to 50% of the patients, there may be no eye or vision symptoms associated with the tumors. Current recommendations are to treat only symptomatic tumors, particularly if there is a worsening in visual acuity. Due to the high degree of variability in symptoms and lack of reliable biomarkers, ophthalmologic exams and quantitative vision testing serve as the primary surveillance tools. Screening baseline MRI evaluations are not indicated for NF1-OPGs, as detecting these tumors rarely changes management in the absence of clinical symptoms or signs [16]. Asymptomatic tumors are hence monitored with annual to biannual ophthalmologic evaluations. Symptomatic tumors are often treated with chemotherapy and monitored with more frequent MRI surveillance and ophthalmologic exams [17, 18]. These practices may vary based on institution-specific protocols.
NF1-associated gliomas can occur in other parts of the central nervous system (CNS), including the brain, spinal cord, and cerebellum. Management options include maximum safe surgical resection symptomatic tumors. If surgery is not a safe option, chemotherapy is often utilized. Typically, it is the same chemotherapy used for LGG not associated with NF1, like the combination of carboplatin and vincristine, vinblastine monotherapy, and in the modern era, MAP kinase–targeted therapies, like Mek inhibitors as discussed in below. NF1-associated LGGs, including OPGs, have a more favorable prognosis when compared to the subgroup without NF1.
Surgery has a limited role in treating OPGs and is typically offered when vision is already significantly compromised and non-visual symptoms need management [16, 20]. Radiation is seldom used as a treatment modality in NF1-associated LGG due to the high risk of radiation-induced secondary high-grade malignancies, moyamoya disease and other radiation toxicities [21]. Chemotherapy is the main therapy option for OPGs and other NF-1-associated LGGs that require treatment. Packer et al. proposed chemotherapy involving vincristine and carboplatin in the early 1990s [22]. This was followed by the vinblastine regimen proposed by Buffet et al. [23] in the 2000s; both the regimens have similar effectiveness in stabilizing tumors and are often offered as the standard of care. Bevacizumab has been reported to improve visual outcomes in select cases; currently, studies are underway to evaluate an objective response [24]. In many clinical trials, targeted therapies and combinations are being studied with recent advancements in understanding molecular pathways. The MAP kinase pathway can be inhibited at many target points. One such trial studying MEK1/2 inhibitor selumetinib showed 40% sustained partial response and 96% of patients and progression-free survival and was tolerated well with minor side effects; completion of the studies might replace the current first-line chemotherapy protocols with MEK inhibitors especially in OPGs associated with NF1 [25] (Figure 1).
Plexiform Neurofibromas (PNs) and Malignant Peripheral Nerve Sheath Tumors (MPNSTs)
Plexiform neurofibromas and malignant peripheral nerve sheath tumors arise from schwann cells, grow along the nerve, and are seen in up to 50% of patients with NF1. These tumors are rarely encountered outside the diagnosis of NF 1. PNs can be present at birth and classically show maximum growth during childhood [26, 27]. While many PNs may be asymptomatic, some can present with significant complications and morbidities such as pain, cosmetic issues, and disfigurement which may compromise motor function. They can also cause pressure symptoms on the surrounding tissue, for example, around the carotid arteries or trachea which may lead to cardiovascular or respiratory compromise [27, 28–32]. Unlike LGGs, PNs are not sensitive to traditional cytotoxic chemotherapy regimens. Surgery is limited to debulking of symptomatic PNs. While it can be curative, complete resection is rarely safely achieved due to a high degree of involvement of the surrounding tissue and the underlying nerves. Various treatment options, including antihistamines (ketotifen), antifibrotic (pirfenidone), and immunotherapies (thalidomide and interferon), have been studied with limited success [27, 33–38]. However, one recent phase 2 trial using pegylated interferon (PI) showed more than doubling of the time to progression compared with placebo, further studies are needed to understand the role of immunotherapy in treating PNs [39]. Among the targeted therapies, MEK inhibitors have shown the most promising results. Recently completed phase 2 trial, most children with neurofibromatosis type 1 and inoperable PNs had durable tumor shrinkage and meaningful clinical benefit from selumetinib [40, 41]. Selumetinib is currently FDA approved for children with NF1 with inoperable and symptomatic PNs, after the age of 2 years. Another such therapy with limited evidence but early data suggestive of benefits is cabozantinib, a broad receptor tyrosine kinase (RTK) inhibitor. RTK inhibition has been shown to reduce the interaction between schwann cells and the surrounding tumor microenvironment. In a recently published phase 2 trial evaluating the response of PNs to cabozantinib, of the 19 participants assessed for response, 8 (42%) had a partial response (PR), and 11 had stable disease (SD). No patient had disease progression while on the study. PR was defined as ≥20% reduction in tumor volume from baseline on MRI [42]. Other trials targeting the mammalian target of rapamycin (mTOR) pathway, which has shown success in treating tumors related to neurofibromatosis 2 (NF2), have failed to show similar benefit in NF1 related PNs [43–45]. Future trials are underway to study the benefit of these medications in combination in addition to evaluating late effects. In approximately 8–13% of cases, PNs can progress to high-grade sarcoma-like tumors known as malignant peripheral nerve sheath tumors (MPNST) which is the leading cause of mortality in NF1 patients [46, 47]. Due to the risk of transformation of PNs to MPNSTs, radiation is not used to treat a classic PN [27, 48, 49] (Fig. 1).
Malignant Peripheral Nerve Sheath Tumors
Sequential inactivation of tumor suppressor genes is proposed as the underlying mechanism of MPNST. Other somatic alterations that contribute to high-grade cancerous behavior of these tumors in addition to biallelic inactivation of the NF1 gene include inactivation of tumor suppressors, namely CDKN2A/B, TP53, PTEN, and polycomb repressor complex 2 (PRC2). Additionally, the involvement of growth-promoting genes, such as PDGFR and EGFR, has also been implicated along with H3K27me3 alteration [27, 50, 51]. Risk factors for PNs transforming to MPNSTs include NF1 gene microdeletion, overall larger PN burden, atypical lesions, nodularity, and prior history of radiation [52]. Clinically, change in consistency or hardening of PNs, development of new neurological symptoms in the area involved, rapid growth, distinct nodular lesions (DNLs), and refractory pain are considered red flags for transformation and warrant further investigation with MRI sequences including diffusion-weighted imaging .18Fluorodeoxyglucose (FDG)-PET can also be considered, and FDG-PET scan can sometimes distinguish between benign growth of PNs versus malignant MPNST by demonstrating increased FDG uptake in the latter. Similarly, a combination of mean apparent diffusion coefficient (ADC) value and absence of split fat is excellent for discriminating malignant from benign PNSTs [12, 52–54]. Though tissue biopsy is still the gold standard for diagnosis, it might not always be safe to perform.
MPNSTs are aggressive and pose significant treatment challenges. Similar to PNs, there is a limited role of cytotoxic chemotherapy and radiation. Surgical options, including those using fluorescence techniques, are considered the first line to reduce tumor burden. Currently, many clinical trials are underway to study the role of targeted therapies alone and in combination. Many immune checkpoint inhibitors and vaccine therapies are also currently being tested, details of which are beyond the scope of the article.
High-Grade Gliomas (HGG)
NF1 patients are also at increased risk of developing HGG and most cases present in adulthood. The somatic molecular landscape of these HGGs is similar to that of MPNSTs, and similar additional secondary mutations are seen mainly in CDKN2A/B, TP53, ATRX, TERT, and PI3 kinase pathways along with the underlying germline NF1 mutation [15, 55]. It should be noted that alterations in IDH1 and histone proteins seen in adult HGGs are not typically seen in NF1-associated HGGs [56]. Overall, the prognosis of NF1-associated HGGs is better than in patients without NF1 [15, 57]. Due to its relative rarity, the treatment paradigm is yet to be universally standardized. Radiation along with temozolomide are often used, similarly to sporadic HGG, as they share similar tumor biology and harbor similar genetic mutations as mentioned above. Other novel therapies are still being tested in this population.
Neurofibromatosis Type 2 (NF 2)
NF2 was previously grouped under the umbrella term of neurofibromatosis and then considered a separate syndrome only after discovering the underlying genetic mutation in the NF2 gene on chromosome 22. NF2 is an autosomal dominant disease with distinct clinical manifestations and therapeutic challenges as compared to NF1.
The constellation of these manifestations along with the family history is used in the Manchester criteria, which remains the standard for diagnosis (Table 2). Due to significant mosaicism, genetic testing might be negative in up to 15% of the patients even if they meet the clinical criteria, in such cases testing for NF2 mutation in tumor tissue and germline might have a better yield. Genetic testing is often not required for the diagnosis. NF2 affects 1 in 25000 people worldwide [58, 59]. Common nervous system tumors in NF2 involve the cells covering the cranial nerves (schwannomas), brain (meningiomas), and ventricles and spinal canal (ependymomas). Most of the tumors arising in NF2 are low-grade, and malignant transformation is rare. However, patients may suffer from significant morbidity due to pressure symptoms on surrounding cranial nerves (CNs) and brainstem and spine, creating chronic progressive symptoms which are often challenging to treat and ultimately lead to death [60, 61].
Table 2.
Manchester criteria for diagnosis of NF2, diagnosis of Nf2 requires to meet primary and added features as mentioned below
| Primary finding | Added features needed for diagnosis |
|---|---|
| Bilateral Vestibular Schwannoma | None |
| First-degree relative with NF2 | Unilateral Vestibular Schwannoma, or Any two (2) other NF2-Associated lesions: Meningioma, Schwannoma, Ependymoma, or Juvenile Cataracts |
| Unilateral Vestibular Schwannoma | Any two (2) other NF2-Associated lesions: Meningioma, Schwannoma, Ependymoma, or Juvenile Cataract |
| Multiple Meningiomas | Unilateral Vestibular Schwannoma, or Any two (2) other NF2-Associated lesions:Schwannoma, Ependymoma, or Juvenile Cataracts |
The relationship between the severity of the symptoms and underlying germline NF2 variant is becoming more evident. It is now known that biallelic loss of NF2 gene is necessary for tumor development in schwannomas. Patients with truncated mutations present at an earlier age and have more severe symptoms when compared to missense mutations which have a milder course [62].
Schwannomas
As the name suggests, schwannomas arise from the schwann cells which are responsible for the production of myelin and play a similar role to the oligodendrocyte in the central nervous system [63]. NF2-associated schwannomas most commonly arise within the 8th cranial nerve (CN), and multiple foci along the same nerve can be involved. Symptoms are mainly due to the pressure effects on the surrounding cranial nerves and brainstem. For example, schwannoma of 8th CN often presents clinically with hearing loss due to direct effects upon the cochlear part of the CN 8. This can then progress to vestibular symptoms and facial weakness as the vestibular part of the 8th CN and facial nerve lie in close anatomic proximity. Current data suggests that the size of the tumor does not directly correlate to the severity of hearing loss [64, 65].
Since the schwannoma typically does not lead to rapid mortality, the management is mainly on preserving the function of the nerve and patient functional outcomes such as hearing whenever possible. This limits the role of surgery and radiation in managing the disease. Currently, bevacizumab a VEGF inhibitor (Figure 1) is considered the first choice in treating symptomatic CN8 schwannomas, as multiple studies have shown benefit in tumor size and hearing [66–68]. Bevacizumab has shown to cause tumor volume reduction in 40% of the tumors and 40% improvement in hearing loss. While bevacizumab may be used to control size for either unilateral (hearing ear) or bilateral tumors, when used for hearing loss alone, it is generally withheld until an only functioning/hearing ear begins to fail. Another phase 2 study using lapatinib, an EGFR and Erb2 inhibitor, has also demonstrated some improvements in both tumor volumes and hearing and may be considered in the management [69].
With recent advancements in the understanding of genetic and molecular underpinnings, several clinical trials are underway investigating different therapeutic options. Some of them include the COX2 inhibitor aspirin (NCT03079999), MEK inhibitor selumetinib (NCT03095248), tyrosine kinase inhibitor brigatinib (NCT04374305), and crizotinib, which inhibits focal adhesion kinase 1 (FAK1) (NCT04283669).
Meningiomas
Meningiomas are the second most common tumor typical of NF2. These can develop anywhere in the craniospinal axis through the length of meninges; however, they typically occur intracranially in the majority of the cases [70]. Upto 80% of patients with NF2 develop meningiomas and multifocal disease is seen in almost all of these patients. The presence of multiple meningiomas or early onset meningiomas should raise suspicion of NF2, as this might be the first presenting sign [71–73]. Treatment options for meningiomas are limited. Surgical resection is typically reserved for symptomatic patients. Radiation therapy is used on a case-by-case basis when complete surgical resection is not possible, radiation is used with caution in the setting of NF2 meningiomas. lapatinib, everolimus, and combination everolimus + octreotide and bevacizumab + everolimus have shown benefit in slowing the tumor growth, none have shown any benefit in tumor shrinkage [74–78].
Meningioangiomatosis
Meningioangiomatosis was initially considered a benign hamartomatous lesion with meningeal and vascular components; however, it is increasingly being recognized as a precancerous lesion. It can be unifocal or multifocal in nature and clinicians are advised to follow with serial MRIs [79–82].
Ependymomas
Ependymomas are seen in about 35–50% of the patients with NF2, but only 20% of the patients report symptoms. These most commonly arise in the spinal cord and cervical medullary junction [83, 84]. Ependymomas are more often seen in patients with truncating NF2 mutations, highlighting that those with truncating mutations have a more aggressive disease course [72]. Surgery is considered the standard of care with a goal of gross total resection when feasible and safe, especially in symptomatic patients. Radiation therapy (both newer proton beam and traditional photon therapy) is well established in the treatment of sporadic ependymomas and is considered with caution considering the underlying NF2 mutation [27, 85, 86].
Schwannomatosis
Schwannomas can also arise in other conditions which have overlapping clinical manifestations with NF2. These conditions are known as schwannomatosis and can be further classified as SMARCB1 (previously INI) and LZTR1-related schwannomatosis based on underlying germline mutation. Schwannomatosis is yet to be well understood. It is an autosomal dominant disorder with incomplete penetrance. About 5% of patients with germline pathogenic SMARCB1 variants might develop meningiomas. Germline pathogenic LZTR1 mutations can present with only unilateral vestibular schwannomas along with other intradermal tumors like NF2 but often lack other ocular manifestations like posterior subcapsular cataracts and retinal findings and skin findings [27, 87, 88]. It is important to make the distinction between these three overlapping conditions as the course of the tumors, management, and surveillance guidelines may change based on the diagnosis. Hence, the revised Manchester criteria differentiate LZTR1 as a separate condition from NF2 [87, 89] (Table 2).
Constitutional Mismatch Repair Deficiency (CMMRD)
CMMRD is one of the most aggressive cancer predisposition syndromes, with typical onset of malignancy in childhood. This rare autosomal recessive syndrome is caused by bi-allelic pathogenic variants in one of the mismatch repair genes: MLH1, MSH2, MSH6, and PMS2, and rarely EPCAM or MSH3. Single variants in these genes cause autosomal dominant Lynch Syndrome (LS), or Non-polyposis Hereditary Colorectal Cancer Syndrome. LS involves a predisposition to the development of gastrointestinal and genitourinary tract cancers with a mean age of first tumor onset of 45 years old [90–92]. While patients with Lynch syndrome can develop CNS tumors, it is rare. This syndrome is considered an adult-onset cancer predisposition syndrome. Therefore, current LS surveillance guidelines do not recommend screening for CNS tumors or any cancer screening in childhood [93, 94].
The process of DNA replication is a highly conserved process, which relies on DNA polymerases and the mismatch repair (MMR) system. Specifically, the exonuclease domains of DNA polymerases along with the mismatch repair complex are responsible for monitoring and repairing DNA replication errors. As a result, the hallmark of mismatch repair deficiency is an accumulation of point mutations and microsatellite instability [95].
Cancers in constitutional mismatch repair deficiency can arise as young as infancy and throughout childhood. The mean age for the first tumor presentation is reported to be 7.5 years old, though there is a wide range reported (0.4–39) [94]. Brain tumors are the most common tumors reported in patients with CMMRD and present at an average age of 10 years. Although a variety of brain tumors have been reported, including medulloblastomas, high-grade gliomas (HGG) are the most common type seen [93, 94, 96, 97]. Non-CNS malignancies reported in CMMRD include T cell lymphoblastic lymphoma, colorectal carcinoma, and other blood and gastrointestinal malignancies along with some of the neurocutaneous features described below.
Surveillance guidelines for patients with CMMRD have been proposed by both the “Care 4 CMMRD,” an International Biallelic Mismatch Repair Deficiency (BMMRD) Consortium and by the American Association of Cancer Research (AACR) Childhood Cancer Predisposition Workshop. They include brain MRI imaging at diagnosis and every 6 months thereafter. In addition, whole body MRI (WB-MRI) should begin by age 6 y/o (when the need for anesthesia is lessened) and be performed annually. Since body MRIs are not sensitive enough for brain tumors, brain MRIs are still recommended bi-annually [93, 94, 98]. Additional surveillance recommendations are included in Table 3.
Table 3.
Summary of cancer predisposition syndromes and recommended surveillance in childhood
|
Cancer predisposition syndrome (inheritance) |
Genes involved | CNS tumor risk/spectrum | Other neoplasia risk and features |
AACR Childhood Cancer Predisposition Workshop: Surveillance Guidelines (for those without active disease) |
|---|---|---|---|---|
| Neurofibromatosis type 1 (AD) | NF1 | Optic pathway gliomas, Gliomas (low and high grade) |
Malignant peripheral nerve sheath tumor Leukemia Lymphoma Embryonal rhabdomyosarcoma Breast cancer Pheochromocytoma Gastrointestinal stromal tumor |
Annual neurologic exam and physical exam Ophthalmic assessments from birth to 8 years, every 6 months to 12 months MRI surveillance is not currently recommended unless symptomatic or with an already diagnosed tumor Annual Screening for Breast cancer and Hypertension after the age of 30 years |
|
Neurofibromatosis type 2 (AD) |
NF2 | Schwannoma, meningioma, ependymoma |
Annual history and physical exam (including audiology with measurement of pure−tone thresholds and Word Recognition Scores) Annual (consider twice yearly in first year since diagnosis or signs of rapid growth) brain MRI starting at 10 years of age. If baseline imaging shows no characteristic sites of involvement, reduce frequency of screening to every 2 years Surveillance spinal MRI is recommended at 24− to 36−month intervals beginning at 10 years of age |
|
| Li Fraumeni Syndrome (AD) | TP53 | High-grade glioma, anaplastic astrocytoma, choroid plexus carcinoma medulloblastoma |
Sarcomas Breast Cancer Adrenocortical Carcinoma Leukemia and Lymphoma |
Complete physical examination and neurologic exam every 3–4 months US of abdomen and pelvis every 3–4 months Annual brain MRI beginning at diagnosis Annual Whole-Body MRI beginning at diagnosis |
| Von Hippel Lindau Syndrome (AD) | VHL | CNS Hemangioblastoma | Pheochromocytomas, retinal hemangioblastomas, ELST |
Biennial brain and spine MRI beginning at age 8 Annual ophthalmologic examination beginning at birth Annual physical exam Annual plasma free metanephrines beginning at age 2 Biennial audiogram beginning at age 5 Annual abdominal MRI beginning at age 10 |
|
Constitutional Mismatch Repair Deficiency ( CMMRD) (AR) |
MLH1, MSH2, MSH6 PMS2 EPCAM |
High-grade glioma Medulloblastoma |
Hematological malignancies Gastrointestinal malignancies |
Brain MRI at diagnosis and once every 6 months Whole-body MRI annually beginning at age 6 Abdominal ultrasound q6 months beginning at age 1 CBC q 6 months beginning at age 1 Upper gastrointestinal endoscopy; VCE, ileocolonoscopy annually beginning at age 4–6 |
|
Gorlin Syndrome / Nevoid Basal Cell Carcinoma Syndrome ( AD) |
PTCH1 SUFU |
Medulloblastoma, especially desmoplastic and SHH-type |
Basal cell carcinoma, cardiac and ovarian fibromas keratocystic odontogenic tumors |
Brain MRI can be considered in those with a SUFU variant every 4 months until age 3 and then every 6 months until age 5 Basic echocardiogram in infancy Dental exams with jaw X-ray every 12 to 18 months beginning at age 8 for PTCH1 mutation carriers Ovarian ultrasound by age 18 Basal cell carcinoma screening annually to begin by age 10 |
| Rhabdoid Tumor Predisposition Syndrome (AD) |
SMARCB1 SMARCA4 |
Atypical teratoid rhabdoid tumors Schwannomatosis Meningioma |
Rhabdoid tumors Non-small cell carcinoma of the ovary hypercalcemic type |
Brain MRI every 3 months till age 5 Consider whole-body MRI annually till age 5 Abdominal ultrasound every 3 months to age 5 (Recommendations are only made for SMARCB1 loss of function mutations) |
AD autosomal dominant, AR autosomal recessive, ELST endolymphatic sac tumor
Interestingly, there is an overlap between some neurocutaneous findings between CMMRD and NF1; patients with both diseases can develop café au-lait macules (CALM). While 99% of patients with NF1 develop CALMs by the age of 1 year, CALMs are known to develop in 62–71% of the patients with CMMRD [93, 96, 97]. However, many CMMRD patients do not have greater than 5 CALMs with the required measurements and age to meet the criteria for NF1 (Table 1). Furthermore, there appears to be a subtle difference between the morphologic appearance of CALMs. Patients with CMMRD develop a jagged “coast of Maine appearance” due to varying degrees of pigmentation and appear different from the more uniformly pigmented and smooth margined CALMs seen in NF 1 patients [93, 99–102]. Axillary freckling, Lisch nodules, cutaneous neurofibromas, and tibial pseudoarthrosis, all of which are features of NF1, are also reported in CMMRD with varying frequency. Hence, if the patient shows phenotypic manifestations of NF1 but tests negative for NF1 and SPRED1 gene alteration (causing legius syndrome), CMMRD should be considered in the differential diagnosis [93, 96, 97, 103–106].
Tuberous Sclerosis Complex (TSC)
TSC is another neurocutaneous syndrome that involves the development of a unique set of symptoms involving multiple organ systems. TSC is the result of suppression of one of the tumor suppressor genes namely TSC1 located on chromosome 9 and TSC 2 on chromosome 16. TSC1 and TSC2 encode for proteins hamartin and tuberin respectively, both of which are involved in the suppression of the mTOR pathway via RAS homologue enriched in the brain (Rheb). These are inherited in an autosomal dominant fashion with near-complete penetrance, but of cases are thought to be from de novo mutations [107–110]. The spectrum of manifestations includes typical skin findings, such as hypomelanotic macules, ungual fibromas, shagreen patch, and angiofibromas as well as angiofibromas lymphangiomyomatosis, and hamartomatous growths in multiple organ systems.
In the brain, hamartomatous growth, focal cortical dysplasia, and other developmental anomalies can present even before birth. Epilepsy develops in 70–80% of patients with TSC and is often refractory to traditional anticonvulsants. Frequently, patients develop epileptic encephalopathy in the form of infantile spasms. Current treatment guidelines recommend using ACTH and vigabatrin as the first-line therapy. Recently, EPISTOP demonstrated that when used prophylactically in patients with TSC (without epilepsy), vigabatrin reduces the risk and severity of epilepsy [111].
Another unique finding in TSC is the clustering of cells lining the ventricles named subependymal nodules (SEN) which occur in 80–90% of the patients. Approximately 15% of subependymal nodules transform into a more aggressive subependymal giant cell astrocytoma (SEGA) [112–114]. Other features with diagnostic criteria are listed in Table 4 (Washington DC 2012 TSC meeting report). A better understanding of the mTOR pathway and its role in TS led to clinical trials testing mTOR inhibitors, ultimately establishing everolimus as an FDA approved drug in the management of TSC-associated SEGAs as discussed below.
Table 4.
Tuberous sclerosis complex clinical features and diagnosis
| Diagnostic criteria for tuberous sclerosis complex 2012 | |||
|---|---|---|---|
| A. Genetic diagnostic criteria | The identification of either a TSC1 or a TSC2 pathogenic mutation in DNA from normal tissue is sufficient enough to make a definite diagnosis of Tuberous Sclerosis Complex (TSC) | ||
| B. Clinical diagnostic criteria | Major features |
1. Hypomelanotic macules (≥ 3, at least 5-mm diameter) 2. Angiofibromas (≥ 3) or fibrous cephalic plaque 3. Ungual fibromas (≥ 2) 4. Shagreen patch 5. Multiple retinal hamartomas 6. Cortical dysplasias a 7. Subependymal nodules 8. Subependymal giant cell astrocytoma 9. Cardiac rhabdomyoma 10. Lymphangioleiomyomatosis (LAM)b 11. Angiomyolipomas (≥ 2) b |
Definite diagnosis: Two major features or one major feature with ≥ 2 minor features Possible diagnosis: Either one major feature or ≥ 2 minor features |
| Minor features |
1. “Confetti” skin lesions 2. Dental enamel pits (> 3) 3. Intraoral fibromas (≥ 2) 4. Retinal achromic patch 5. Multiple renal cysts 6. Nonrenal hamartoma |
||
aIncludes tubers and cerebral white matter radial migration lines
bA combination of the two major clinical features (LAM and angiomyolipomas) without other features does not meet criteria for a definite diagnosis. A combination of these two major clinical features does not meet criteria for a definite diagnosis
SEGA
Subependymal nodules and low-grade hamartomas are mostly asymptomatic. However, depending on the location within the ventricles, these might cause blockage of CSF flow resulting in obstructive hydrocephalus. While SENs mostly develop in the first 2 decades of life, some congenital cases have been reported. When diagnosed congenitally, they are known to grow at a faster rate when compared to non-congenital SEGAs [115–123]. In one large multicenter study of 2200 patients with TSC, it was found that SEGAs developed in 25% of the patients. There is a higher rate in patients with a TSC2 (33%) compared to patients with a TSC1(13%). SEGAs were symptomatic in 42% of patients, and the most common presenting symptoms included the following: seizures or increased seizure frequency (15%), regression of milestones (10%) or behavioral change (12%) [124].
SEGAs are typically classified as low-grade gliomas and mostly have an indolent course. Currently, they are defined by imaging criteria set in 2013 by an international panel of experts [125]. Once a diagnosis of TSC is made, a baseline MRI is recommended to evaluate for underlying SENs and SEGAs [126]. SEGAs show contrast enhancement, and classically, continued tumor growth on serial surveillance MRIs. These characteristics help differentiate them from SENs. Like many SENs, SEGAs can also be asymptomatic. Current recommendations are to treat symptomatic SEGAs and to monitor asymptotic SEGAs and SNs with serial surveillance MRIs.
Everolimus is FDA approved for the treatment of SEGAs, and in the modern era, surgery is reserved for select patients, typically with acute symptoms such as hydrocephalus [110, 127–130]. Everolimus has shown to be effective in shrinking SEGAs by 30–50% and has also shown benefit in the control of underlying epilepsy which can often be refractory to classic anticonvulsants. Similar effects are also seen on renal angiomyolipomas [129, 131–133]. The role of mTOR in other manifestations of TSC including TSC-associated neuropsychiatric disorder (TAND) is yet to be well understood.
Von Hippel–Lindau Syndrome (VHL)
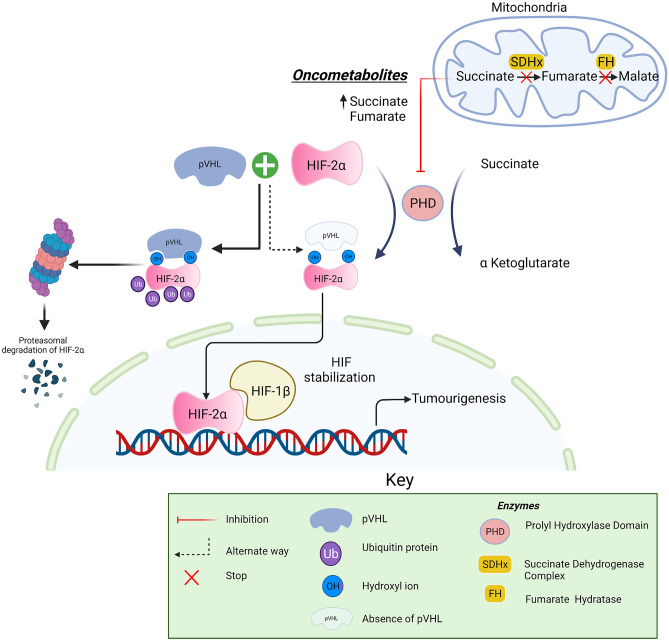
VHL is caused by pathogenic germline variants in the VHL gene, located on chromosome 3. This encodes for the pVHL protein, which regulates the response of a cell to hypoxia via hypoxia-inducible transcription factor (HIF) 1 and 2. The loss of a pVHL allele results in a skewed ratio of HIF 1 to HIF 2 resulting in an imbalance of the VHL/HIF axis and tumorigenesis [134]. VHL is inherited in an autosomal dominant fashion with an incidence of around 1 in 36000 live births, but up to 25% of cases occur due to a de novo mutation, but up to 25% of cases occur due to a de novo mutation [135–142].
VHL is not associated with malignant tumors of nervous system origin; however, hemangioblastomas are seen in 60–80% of patients with VHL and most often develop along the craniospinal axis, often causing neurologic symptoms due to direct pressure effects on adjacent structures or spontaneous hemorrhages [143, 144]. Patients with VHL are also known to develop endolymphatic sac tumors in the temporal bone. About 15% can also present with hearing loss, vertigo, tinnitus, and headaches [145, 146]. Hemangioblastomas can remain dormant for long periods of time before becoming symptomatic due to sudden growth or hemorrhage [147].
Other manifestations of VHL include neuroendocrine tumors of the pancreases, renal angiomas, renal cell carcinomas, serous cystadenomas, paragangliomas, and pheochromocytoma (PPGLS). VHL can be further classified as type 1 (truncating or missense mutation developing pheochromocytoma) and type 2 (missense mutation usually not developing pheochromocytoma). A better understanding of the VHL axis may lead to unique surveillance guidelines in the future based upon type (1 or 2) [136]. It should be noted that patients with VHL show variable degrees of mosaicism impacting clinical management and counseling [148].
Pheochromocytomas and paragangliomas (PPGLs) arising from adrenal chromaffin cells can also be seen in other mutations along the VHL/HIF axis, including PHD, VHL, HIF-2A (EPAS1), and SDHX (VHL Figure 2). About 40% of PPGLs result from germline mutations [149].
Fig. 2.
Pathogenesis of Von Hippel-Lindau syndrome in VHL/HIF axis
Created with BioRender.com
Current NIH and VHL Alliance recommendations for surveillance in individuals with VHL are to obtain MRI imaging of the brain and spinal cord with contrast every two years from the age of 11 years old onward [150]. If neurological symptoms are seen, then imaging should be considered sooner. Additionally, MRI without contrast is recommended around the 4th month of pregnancy to evaluate for the need for cesarean section in the presence of retinal, brain, or spinal lesions within the developing fetus [136, 140, 147, 151, 152]. This is accompanied by detailed neurological testing, ophthalmologic and hearing tests as mentioned in Table 3.
Recently, Belzutifan a hypoxia-inducible factor inhibitor was approved to treat adults with VHL-associated renal cell carcinoma, central nervous system hemangioblastomas, and pancreatic neuroendocrine tumors that do not require immediate surgery [153].
Genetic counseling should be a part of management and should be offered early. As 80% of the patients have an affected parent and 20% of patients have de novo mutations, all affected individuals have a 50% chance of passing down VHL to their biological children.
Ataxia Telangiectasia (AT)
As the name suggests, this syndrome is primarily characterized by ataxia secondary to progressive cerebellar atrophy and development of telangiectasias. AT is an autosomal recessive disorder with an incidence of 1 in 20,000 to 100,000 people [154]. Mutations in the “ataxia telangiectasia mutated” (ATM) gene located on chromosome 11 are known to cause carcinogenesis and can promote malignant transformation. Gene products of ATM genes are normally involved in repairing double-strand DNA breaks. Although reported, primary CNS tumors are not a cardinal manifestation. Other characteristic cancers of AT are lymphomas, leukemias, breast, and gastric cancers. Patients are particularly radiosensitive and ionizing radiation should be avoided [147, 155, 156].
Surveillance guidelines for individuals with AT have been recommended by the Pediatric Cancer Predisposition Workshop [157]. Routine MRI surveillance in patients with brain and spinal lesions should be considered. If neurological symptoms arise, surgery can be considered with caution. Although no targeted therapies have been successful in treating the underlying disorder or manifestations, betamethasone has shown some improvement in ataxia and can be tried if ambulation is affected [158]. Patients with AT can have poor ambulation and often develop early wheelchair dependency. Only 50% of the patients will survive into their 20s [159, 160].
Nevoid Basal Cell Carcinoma Syndrome (NBCCS or Gorlin Syndrome)
NBCCS or Gorlin syndrome has an incidence of 1:15,000 to19,000 births and is caused by an underlying germline mutation in the sonic hedgehog (SHH) pathway, specifically in patched1 (PATCH1) gene and suppressor of fused (SUFU) gene, which are inherited in an autosomal dominant fashion [161–164]. About 5% of patients develop medulloblastomas in the course of the disease, and most of them arise before the age of 3 years old [165–167]. Often medulloblastoma might be the first presenting sign, and therefore, a high level of suspicion is needed. Detailed family history and a detailed skin exam should be performed in those children less than 3 years old presenting with medulloblastoma, particularly if the subgrouping is SHH. Other features that help in the clinical diagnosis include jaw keratocyst, palmar and plantar pits, multiple basal cell carcinomas, and MRI findings of lamellar calcification of falx or clear evidence of calcification in a young child. The Jones criteria are often used to make the diagnosis and is described in Table 5 [168]. Patients who receive radiation for treatment of their medulloblastoma have the risk of developing a large number of basal cell carcinomas within the radiation entrance fields, further stressing the importance of early diagnosis [169, 170].
Table 5.
Jones criteria for diagnosis of Gorlin syndrome. A diagnosis can be made when 2 major or 1 major and 2 minor criteria are fulfilled
| Major criteria | Minor criteria |
|---|---|
| Lamellar (sheet-like) calcification of the falx or clear evidence of calcification in an individual younger than age of 20 years | Childhood medulloblastoma |
| Lympho-mesenteric or pleural cysts | |
| Jaw keratocyst | Macrocephaly (OFC > 97th centile) |
| 2 or more palmar/plantar pits | Cleft lip/palate |
| Multiple BCCs (more than five in a lifetime) or a BCC before the age of 30 years | Vertebral/rib anomalies such as bifid/splayed/extra ribs or bifid vertebrae |
| First degree relative with Gorlin Syndrome | Preaxial or postaxial polydactyly |
| Ovarian/cardiac fibromas | |
| Ocular anomalies (cataract, developmental defects, and pigmentary changes of the retinal epithelium) |
Rhabdoid Tumor Predisposition Syndrome (RTPS)
RTPS results from germline loss-of-function mutations in the SMARCB1 gene and differs phenotypically from schwannomatosis though both the syndromes share common genetic mutation. More recently, germline SMARCA4 mutations have also been associated with RTPS. These are both transmitted in an autosomal dominant fashion. Patients with germline mutations in SMARCB1 are classified as RTPS type 1 and patients with germline mutations in SMARCA4 are categorized as RTPS type 2 [171–174]. Patients with SMARCB1 mutations have shown more aggressive tumor types like atypical teratoid rhabdoid tumors (AT/RT) and intracranial meningiomas, schwannomas, and malignant peripheral nerve sheath tumors in the peripheral nervous system. Whereas, patients with SMARCA4 pathogenic variants harbor more risk of ovarian and thoracic carcinoma, in addition to the rhabdoid tumors. AT/RT can be the first manifestation, and hence, the current recommendation is to test for germline mutations in both the genes in all newly diagnosed cases of AT/RT [175–177]. Underlying germline mutation in SMARCB1 also indicates an overall poor prognosis.
Initial diagnosis of RTPS can be established after genetic confirmation of germline heterozygous mutation in either of the two genes in a patient with rhabdoid tumor anywhere in the body, and/ or a family history of rhabdoid tumors and/or other typical tumors as described above [178].
Surveillance recommendations have been published by the AACR Pediatric Cancer Predisposition Workshop and suggest MRI brain and ultrasound of the abdomen every three months from the day of diagnosis till the age 5 years and whole-body MRI at the age 5 years [171]. This recommendation was made only for truncating/loss of function mutations in the SMARCB1 gene. No recommendations were made on brain imaging of SMARCA4 genes and germline missense mutations of SMARCB1. The only recommendation for SMARCA4 was abdominal ultrasound every 6 months and consideration of oophorectomy if considered high risk after childhood [171].
Li-Fraumeni Syndrome (LFS)
LFS is an autosomal dominant disorder resulting from germline pathogenic variants in the TP53 gene. The TP53 tumor suppressor gene is located on chromosome 17. In addition to germline variants causing LFS, somatic (tumor) mutations in TP53 are some of the most common genetic alterations in human cancer [179].
Diagnosis of Li-Fraumeni can be made in a patient with a heterozygous germline mutation in TP53 and/or a patient meeting all the 3 diagnostic criteria, including the following: development of sarcoma before 45 years; one first-degree relative with any cancer diagnosed before 45 years; and one first or a second-degree relative with any cancer diagnosed before age of 45 years or a sarcoma at any age [180].
The risk of CNS tumors in LFS is well-established, and numerous brain tumors have been described such as medulloblastomas, astrocytomas, and choroid plexus carcinomas. The incidence of brain tumors in LFS can be estimated to be around 12% and appear to have a bimodal distribution with the first peak occurring around infancy to early childhood and a second, smaller peak at about the 3rd to 4th decade of life [181–183]. Females have a higher risk of tumors including brain tumors when compared to males [184, 185]. The implications of germline TP53 abnormalities on medical decision-making and prognosis remain poorly understood; however, all individuals with LFS are highly susceptible to the DNA-damaging effect of radiation, increasing the risk for secondary malignancies within radiation therapy fields [186].
Currently, the modified Toronto protocol of 2017 recommends annual brain MRIs; the first one should be performed with contrast and the subsequent ones without contrast if no abnormality is observed [187].
Surveillance guidelines for pediatric patients with LFS are available through the AACR Pediatric Cancer Predisposition Workshop and summarized in Table 3 [187].
Other Cancer Predispositions with CNS Tumors
Other rare cancer predisposition syndromes with a risk of developing CNS tumors are described below.
Multiple Endocrine Neoplasia Type 1 (MEN-1)
This autosomal dominant disorder of the tumor suppressor gene MEN1 can present with pituitary adenoma or tumors of the pancreas and parathyroid glands. The pituitary adenomas are likely to be prolactinomas and are more resistant to treatment when compared to the ones seen in the general population [188–190]. Neurologic symptoms mainly arise due to direct local pressure on the pituitary often causing vision loss and disturbances in the hypothalamic-pituitary axis.
Rubinstein Taybi Syndrome (RTS)
RTS is an autosomal dominant disorder linked to CREBBP gene products which plays a regulatory role in SHH and AMP regulated gene expression. An increased risk of medulloblastoma, oligodendroglioma, and meningiomas has been reported. Other morphological features that help raise suspicion of this diagnosis include broad thumbs, broad great toes, and distinctive facial features [191–194].
Cowden Syndrome (CS)
CS is an autosomal dominant syndrome with an underlying mutation in PTEN which has been linked to a higher risk of brain tumors, specifically cerebellar dysplastic gangliocytoma [195–197]. PTEN mutations have also been described in various other types of cancers.
Melanoma-Astrocytoma Syndrome
First described by Kaufman et al in 1993, melanoma-astrocytoma syndrome is now known to have mutations in CDKN2A. Many families have been described presenting with both melanomas and astrocytomas. Genetic testing should be considered if skin findings are concerning [198–204].
DICER1 Syndrome
DICER1 Syndrome, previously called Pleuropulmonary Blastoma (PPB) Syndrome, is caused by germline pathogenic variants in the DICER1 gene. This autosomal dominant syndrome increases the risk for a broad spectrum of both benign and malignant neoplasia, including pleuropulmonary blastoma, cystic nephroma, genitourinary embryonal rhabdomyosarcoma, ovarian Sertoli Leydig cell tumors, and thyroid carcinoma. Specifically, CNS manifestations can include metastatic PPB, pituitary blastoma, pineoblastoma, and ciliary body medulloepithelioma [205].
Genetic Counseling
All individuals suspected to have a hereditary cancer predisposition syndrome should be referred to a cancer genetic counselor or a pediatric genetics clinic. Genetic counselors are trained to provide pre-test counseling, obtain informed consent for genetic testing, and select the optimal genetic test for each patient. Once genetic testing results become available, genetic counselors are trained to facilitate a discussion of recommended cancer surveillance for children and their families. Genetic counselors are also trained to provide psychosocial support to patients undergoing an evaluation for a cancer predisposition syndrome, as well as to coping and adapting to living with the information once results become available).
Conclusion
Cancer predisposition syndromes might present to neurologists and oncologists alike in pediatric population or later as adults. Early genetic counseling is imperative and should be part of routine medical care. A detailed clinical history and examination will help raise suspicion, further confirmed with appropriate genetic testing. Patients and families can thus be appropriately subjected to screening measures, aiding not only in potential early diagnosis and management altering the course of the disease but also in family planning. As we continue to understand the complex interplay of genetic and molecular pathways, more diagnostic and therapeutic possibilities are being uncovered and surveillance guidelines continue to be updated in line with newer research.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Form
Disclosure form provided by the author are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong M, Mayoh C, Lau L, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–1753. doi: 10.1038/s41591-020-1072-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373:2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinzler K W, Vogelstein B. Lessons from hereditary colorectal cancer. Cell press.1996;87:159–70. [DOI] [PubMed]
- 4.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin KD, Hall JG. Nontraditional inheritance. Pediatr Clin North Am. 1992;39(2):335–348. doi: 10.1016/s0031-3955(16)38298-0. [DOI] [PubMed] [Google Scholar]
- 6.Milani D A Q, Chauhan P R. Genetics, Mosaicism. In: StatPearls [Internet]. vol. 2022. StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559193/. [PubMed]
- 7.Druker H, Zelley K, Mcgee RB, et al. Genetic counselor recommendations for cancer predisposition evaluation and surveillance in the pediatric oncology patient. Clin Cancer Res. 2017;23:91–97. doi: 10.1158/1078-0432.CCR-17-0834. [DOI] [PubMed] [Google Scholar]
- 8.NCI Dictionary of Genetic Terms [online]. Available from: https://www.cancer.gov/publications/dictionaries/genetics-dictionary/def/cascade-screening. Accessed 3 Aug 2022.
- 9.Bouffet E, Larouche V, Campbell BB, et al. immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34:2206–2217. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 10.Resta R, Biesecker BB, Bennett RL, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15:77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- 11.Walker JA, Upadhyaya M. Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin Ther Targets. 2018;22:419–437. doi: 10.1080/14728222.2018.1465931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirbe AC, Gutmann D. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843. doi: 10.1016/S1474-4422(14)70063-8. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann DH, Parada LF, Silva AJ, Ratner N. Neurofibromatosis type 1: modeling CNS dysfunction. J Neurosci. 2012;32:14087–14093. doi: 10.1523/JNEUROSCI.3242-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology. 2004;63:1944–1946. doi: 10.1212/01.WNL.0000144341.16830.01. [DOI] [PubMed] [Google Scholar]
- 15.Packer R J, Iavarone A, Jones DTW. Implications of new understandings of gliomas in children and adults with NF1:report of a consensus conference. 2009;22:773–784. [DOI] [PMC free article] [PubMed]
- 16.King A, Listernick R, Charrow J, Piersall L, Gutmann D. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 17.De BP, Fisher MJ, Liu GT. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37:23–32. doi: 10.1097/WNO.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Listernick R, Charrow J. Knowledge without truth: screening for complications of neurofibromatosis type 1 in childhood. Am J Med Genet A. 2004;127:221–223. doi: 10.1002/ajmg.a.20654. [DOI] [PubMed] [Google Scholar]
- 19.Legius E, Messiaen L, Wolkenstein P, International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC), Huson SM, Evans DG, Plotkin SR. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. 2021;23(8):1506–1513. 10.1038/s41436-021-01170-5. (Epub 2021 May 19. PMID: 34012067; PMCID: PMC8354850). [DOI] [PMC free article] [PubMed]
- 20.Listernick R, Ferner RE, Liu GT. Optic pathway gliomas in neurofibromatosis-1: Controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68:932–938. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 22.Packer RJ, Lange B, Ater J. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 23.Bouffet E, Jakacki R, Goldman S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 24.Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132:111–114. doi: 10.1001/jamaophthalmol.2013.5819. [DOI] [PubMed] [Google Scholar]
- 25.Fangusaro J, Onar-Thomas A, Poussaint TY, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019;20:1011–1022. [DOI] [PMC free article] [PubMed]
- 26.Tucker T, Friedman JM, Friedrich RE, Wenzel R, Fünsterer C, Mautner VF. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet. 2008;46:81–85. doi: 10.1136/jmg.2008.061051. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez L D, Bui A, Klesse L J. Targeted therapies for the neurofibromatoses cancers (Basel). 2021;13:6032–6032. [DOI] [PMC free article] [PubMed]
- 28.Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20:1643–1651. doi: 10.1093/neuonc/noy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10:593–598. doi: 10.1215/15228517-2008-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433–1443. doi: 10.1212/WNL.56.11.1433. [DOI] [PubMed] [Google Scholar]
- 31.Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89:31–37. doi: 10.1002/(SICI)1096-8628(19990326)89:1<31::AID-AJMG7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: Frequency and associated clinical deficits. J Pediatr. 2011;159:652–655. doi: 10.1016/j.jpeds.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol. 1993;129:577–581. doi: 10.1001/archderm.1993.01680260047004. [DOI] [PubMed] [Google Scholar]
- 34.Riccardi VM. Mast-cell stabilization to decrease neurofibroma growth. Preliminary experience with ketotifen Arch Dermatol. 1987;123:1011–1016. [PubMed] [Google Scholar]
- 35.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61:1598–1602. doi: 10.1002/pbc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289:211–218. [PubMed] [Google Scholar]
- 37.Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol. 1999;276:311–318. doi: 10.1152/ajplung.1999.276.2.L311. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Cohen BH, Ruggieri P, Packer RJ, Phillips PC. Phase I study of thalidomide for the treatment of plexiform neurofibroma in neurofibromatosis 1. Neurology. 2003;60:130–132. doi: 10.1212/01.WNL.0000042321.94839.78. [DOI] [PubMed] [Google Scholar]
- 39.Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017;19:289–297. doi: 10.1093/neuonc/now158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dombi E, Baldwin A, Marcus LJ. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–2560. doi: 10.1056/NEJMoa1605943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382:1290–1290. doi: 10.1056/NEJMoa1912735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher MJ, Shih CS, Rhodes SD, et al. Cabozantinib for neurofibromatosis type 1-related plexiform neurofibromas: a phase 2 trial. Neurofibromatosis Clinical Trials Consortium. 2021;27:165–173. doi: 10.1038/s41591-020-01193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr Blood Cancer. 2014;61:982–986. doi: 10.1002/pbc.24873. [DOI] [PubMed] [Google Scholar]
- 44.Zehou O, Ferkal S, Brugieres P, et al. Absence of efficacy of everolimus in neurofibromatosis 1-related plexiform neurofibromas: results from a phase 2a trial. J Invest Dermatol. 2018;139:718–720. doi: 10.1016/j.jid.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2012;381:116–116. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 46.Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15:290–301. doi: 10.1038/nrc3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans DG, Baser ME, Mcgaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22:443–462. doi: 10.1517/13543784.2013.772979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wentworth S, Pinn M, Bourland JD, et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys. 2008;73:208–213. doi: 10.1016/j.ijrobp.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 50.Pemov A, Li H, Presley W, Wallace MR, Miller DT. Genetics of human malignant peripheral nerve sheath tumors. Neurooncol Adv. 2019;2:50–61. doi: 10.1093/noajnl/vdz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spyk SL, Thomas N, Cooper DN, Upadhyaya M. Neurofibromatosis type 1-associated tumours: their somatic mutational spectrum and pathogenesis. Hum Genomics. 2011;5:623–690. [DOI] [PMC free article] [PubMed]
- 52.Peltonen S, Kallionpää RA, Rantanen M. Pediatric malignancies in neurofibromatosistype1: a population-based cohort study. Int J Cancer. 2019;145:2926–2932. [DOI] [PMC free article] [PubMed]
- 53.Evans DG, Salvador H, Chang VY, et al. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res. 2017;23:46–53. [DOI] [PubMed]
- 54.Yun JS, Lee MH, Lee SM, Lee JS, Kim HJ, Lee SJ, Chung HW, Lee SH, Shin MJ. Peripheral nerve sheath tumor: differentiation of malignant from benign tumors with conventional and diffusion-weighted MRI. Eur Radiol. 2021;31(3):1548–1557. doi: 10.1007/s00330-020-07234-5. [DOI] [PubMed] [Google Scholar]
- 55.D'Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med. 2019;25:176–187. [DOI] [PMC free article] [PubMed]
- 56.Lobbous M, Bernstock JD, Coffee E, et al. An update on neurofibromatosis type 1-associated gliomas. Cancers (Basel) 2020;12:114. doi: 10.3390/cancers12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theeler BJ, Ellezam B, Yust-Katz S, Slopis JM, Loghin ME, De GJF. Prolonged survival in adult neurofibromatosis type I patients with recurrent high-grade gliomas treated with bevacizumab. J Neurol. 2014;261:1559–1564. doi: 10.1007/s00415-014-7292-0. [DOI] [PubMed] [Google Scholar]
- 58.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: Higher incidence than previously thought. Otol Neurotol. 2005;26:93–97. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 59.Kluwe L, Mautner V, Heinrich B, et al. Molecular study of frequency of mosaicism in neurofibromatosis 2 patients with bilateral vestibular schwannomas. J Med Genet. 2003;40:109–114. doi: 10.1136/jmg.40.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maniakas A, Saliba I. Neurofibromatosis type 2 vestibular schwannoma treatment: a review of the literature, trends, and outcomes. Otol Neurotol. 2014;35:889–894. doi: 10.1097/MAO.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 61.King AT, Rutherford SA, Hammerbeck-Ward C, et al. Malignant peripheral nerve sheath tumors are not a feature of neurofibromatosis type 2 in the unirradiated patient. Neurosurgery. 2018;83:38–42. doi: 10.1093/neuros/nyx368. [DOI] [PubMed] [Google Scholar]
- 62.Halliday D, Emmanouil B, Pretorius P, et al. Genetic severity score predicts clinical phenotype in NF2. J Med Genet. 2017;54:657–664. [DOI] [PMC free article] [PubMed]
- 63.Graça DL, Bondan EF, Pereira LA, Fernandes CG, Maiorka PC. Behaviour of oligodendrocytes and Schwann cells in an experimental model of toxic demyelination of the central nervous system. Arq Neuropsiquiatr. 2001;59:358–361. doi: 10.1590/S0004-282X2001000300009. [DOI] [PubMed] [Google Scholar]
- 64.Badie B, Pyle GM, Nguyen PH, Hadar EJ. Elevation of internal auditory canal pressure by vestibular schwannomas. Otol Neurotol. 2001;22:696–700. [DOI] [PubMed]
- 65.Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012;33:473–480. [DOI] [PMC free article] [PubMed]
- 66.Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33:1046–1052. doi: 10.1097/MAO.0b013e31825e73f5. [DOI] [PubMed] [Google Scholar]
- 67.Lu VM, Ravindran K, Graffeo CS, et al. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J Neurooncol. 2019;144:239–248. doi: 10.1007/s11060-019-03234-8. [DOI] [PubMed] [Google Scholar]
- 68.Plotkin SR, Duda DG, Muzikansky A, et al. Prospective, phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019;37:3446–3454. doi: 10.1200/JCO.19.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karajannis MA, Legault G, Hagiwara M, et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14:1163–1170. doi: 10.1093/neuonc/nos146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mautner VF, Tatagiba M, Lindenau M, Fünsterer C, Pulst SM, Baser ME. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol. 1995;165:951–955. doi: 10.2214/ajr.165.4.7676998. [DOI] [PubMed] [Google Scholar]
- 71.Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60:994–1003. doi: 10.1093/jnen/60.10.994. [DOI] [PubMed] [Google Scholar]
- 72.Coy S, Rashid R, Stemmer-Rachamimov A, Santagata S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2019;139:643–665. doi: 10.1007/s00401-019-02029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith MJ, Higgs JE, Bowers NL, et al. Cranial meningiomas in 411 neurofibromatosis type 2 (NF2) patients with proven gene mutations: clear positional effect of mutations, but absence of female severity effect on age at onset. J Med Genet. 2011;48(4):261–5. [DOI] [PubMed]
- 74.Alanin MC, Klausen C, Caye-Thomasen P, et al. Effect of bevacizumab on intracranial meningiomas in patients with neurofibromatosis type 2-A retrospective case series. Int J Neurosci. 2016;126:1002–1006. doi: 10.3109/00207454.2015.1092443. [DOI] [PubMed] [Google Scholar]
- 75.Osorio DS, Hu J, Mitchell C, et al. Effect of lapatinib on meningioma growth in adults with neurofibromatosis type 2. J Neurooncol. 2018;139:749–755. doi: 10.1007/s11060-018-2922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nunes FP, Merker VL, Jennings D, et al. Bevacizumab treatment for meningiomas in NF2: A retrospective analysis of 15 patients. PLoS ONE. 2013;8:59941–59941. doi: 10.1371/journal.pone.0059941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graillon T, Sanson M, Campello C, et al. Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM. Trial Clin Cancer Res. 2020:552–557. [DOI] [PubMed]
- 78.Shih KC, Chowdhary S, Rosenblatt P, et al. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol. 2016;129:281–288. doi: 10.1007/s11060-016-2172-3. [DOI] [PubMed] [Google Scholar]
- 79.Tomkinson C, Lu JQ. Meningioangiomatosis: a review of the variable manifestations and complex pathophysiology. J Neurol Sci. 2018;15:130–136. doi: 10.1016/j.jns.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 80.Perry A, Kurtkaya-Yapicier O, Scheithauer BW, Robinson S, Prayson RA, Kleinschmidt-Demasters BK. Insights into meningioangiomatosis with and without meningioma: a clinicopathologic and genetic series of 24 cases with review of the literature. Brain Pathol. 2005;15:55–65. doi: 10.1111/j.1750-3639.2005.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiebe S, Munoz DG, Smith S, Lee DH. Meningioangiomatosis. A comprehensive analysis of clinical and laboratory features Brain. 1999;122:709–726. doi: 10.1093/brain/122.4.709. [DOI] [PubMed] [Google Scholar]
- 82.Rossi S, Brenca M, Zanatta L, Trincia E, Guerriero A, Pizzato C. A pediatric intra-axial malignant SMARCB1-deficient desmoplastic tumor arising in meningioangiomatosis. J Neuropathol Exp Neurol. 2018;77:883– 889. [DOI] [PubMed]
- 83.Plotkin SR, Donnell CC, Curry WT, Bove CM, Maccollin M, Nunes FP. Spinal ependymomas in neurofibromatosis Type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14:543–547. doi: 10.3171/2010.11.SPINE10350. [DOI] [PubMed] [Google Scholar]
- 84.Kalamarides M, Essayed W, Lejeune JP, et al. Spinal ependymomas in NF2: A surgical disease? J Neurooncol. 2018;136:605–611. doi: 10.1007/s11060-017-2690-7. [DOI] [PubMed] [Google Scholar]
- 85.Snyder MH, Ampie L, Didomenico JD, Asthagiri AR. Bevacizumab as a surgery-sparing agent for spinal ependymoma in patients with neurofibromatosis type II: Systematic review and case. J Clin Neurosci. 2021;86:79–84. doi: 10.1016/j.jocn.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Farschtschi S, Merker VL, Wolf D, et al. Bevacizumab treatment for symptomatic spinal ependymomas in neurofibromatosis type 2. Acta Neurol Scand. 2016;133:475–480. doi: 10.1111/ane.12490. [DOI] [PubMed] [Google Scholar]
- 87.Smith MJ, Bowers NL, Bulman M, et al. Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology. 2016;88:215–215. [DOI] [PMC free article] [PubMed]
- 88.Mansouri S, Suppiah S, Mamatjan Y, et al. Epigenomic, genomic, and transcriptomic landscape of schwannomatosis. Acta Neuropathol. 2021;141:101–116. doi: 10.1007/s00401-020-02230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schraepen C, Donkersloot P, Duyvendak W, et al. What to know about schwannomatosis: a literature review. Br J Neurosurg. 2020;2:1–4. doi: 10.1080/02688697.2020.1836323. [DOI] [PubMed] [Google Scholar]
- 90.Yurgelun MB, Hampel H. Recent advances in lynch syndrome: diagnosis, treatment, and cancer prevention. Am Soc Clin Oncol Educ Book. 2018;38:101–109. doi: 10.1200/EDBK_208341. [DOI] [PubMed] [Google Scholar]
- 91.Backes FJ, Cohn DE. Lynch syndrome. Clin Obstet Gynecol. 2011;54:199–214. doi: 10.1097/GRF.0b013e3182185a41. [DOI] [PubMed] [Google Scholar]
- 92.Tanakaya K. Current clinical topics of Lynch syndrome. Int J Clin Oncol. 2009;24:1013–1019. doi: 10.1007/s10147-018-1282-7. [DOI] [PubMed] [Google Scholar]
- 93.Wimmer K, Kratz CP, Vasen HF. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD) J Med Genet. 2014;51:355–365. doi: 10.1136/jmedgenet-2014-102284. [DOI] [PubMed] [Google Scholar]
- 94.Tabori U, Hansford JR, Achatz MI. Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood. Clin Cancer Res. 2017;23:32–37. doi: 10.1158/1078-0432.CCR-17-0574. [DOI] [PubMed] [Google Scholar]
- 95.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–381. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 96.Lavoine N, Colas C, Muleris M. Constitutional mismatch repair deficiency syndrome: clinical description in a French cohort. J Med Genet. 2015;52:770–778. doi: 10.1136/jmedgenet-2015-103299. [DOI] [PubMed] [Google Scholar]
- 97.Bakry D, Aronson M, Durno C. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: report from the constitutional mismatch repair deficiency consortium. Eur J Cancer. 2014;50:987–996. doi: 10.1016/j.ejca.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Durno C, Boland CR, Cohen S, et al. Recommendations on Surveillance and Management of Biallelic Mismatch Repair Deficiency (BMMRD) Syndrome: a Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1605–1614. doi: 10.1053/j.gastro.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 99.De VM, Hayward BE, Charlton R. PMS2 mutations in childhood cancer. J Natl Cancer Inst. 2006;98:358–361. doi: 10.1093/jnci/djj073. [DOI] [PubMed] [Google Scholar]
- 100.Scott RH, Homfray T, Huxter NL. Familial T-cell non-Hodgkin lymphoma caused by biallelic MSH2 mutations. J Med Genet. 2007;83–83. [DOI] [PMC free article] [PubMed]
- 101.Me LM, Lynch E. Biallelic PMS2 mutations and a distinctive childhood cancer syndrome. J Pediatr Hematol Oncol. 2008;30:254–257. doi: 10.1097/MPH.0b013e318161aa20. [DOI] [PubMed] [Google Scholar]
- 102.Kruger S, Kinzel M, Walldorf C. Homozygous PMS2 germline mutations in two families with early-onset haematological malignancy, brain tumours, HNPCC-associated tumours, and signs of neurofibromatosis type 1. Eur J Hum Genet. 2008;16:62–72. doi: 10.1038/sj.ejhg.5201923. [DOI] [PubMed] [Google Scholar]
- 103.Auclair J, Leroux D, Desseigne F. Novel biallelic mutations in MSH6 and PMS2 genes: gene conversion as a likely cause of PMS2 gene inactivation. Hum Mutat. 2007;28:1084–1090. doi: 10.1002/humu.20569. [DOI] [PubMed] [Google Scholar]
- 104.Wang Q, Lasset C, Desseigne F. Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res. 1999;59:294–297. [PubMed] [Google Scholar]
- 105.Baris HN, Barnes-Kedar I, Toledano H. Constitutional mismatch repair deficiency in Israel: high proportion of founder mutations in MMR genes and consanguinity. Pediatr Blood Cancer. 2016;63:418–427. doi: 10.1002/pbc.25818. [DOI] [PubMed] [Google Scholar]
- 106.Ricciardone MD, Ozcelik T, Cevher B. Human MLH1 deficiency predisposes to hematological malignancy and neurofibromatosis type 1. Cancer Res. 1999;59:290–293. [PubMed] [Google Scholar]
- 107.Dixon BP, Hulbert JC, Bissler JJ. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol. 2011;118:15–20. doi: 10.1159/000320891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slegtenhorst MV, Hoogt RD, Hermans C, Nellist M, Janssen B, Nellist M. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. [DOI] [PubMed]
- 109.European chromosome 16 tuberous sclerosis consortium, identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1320. [DOI] [PubMed]
- 110.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. [DOI] [PubMed]
- 111.Kotulska K, Kwiatkowski DJ, Curatolo P, et al. Prevention of epilepsy in infants with tuberous sclerosis complex in the EPISTOP trial. Ann Neurol. 2020;89:304–314. doi: 10.1002/ana.25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 113.Matyja E, Grajkowska W, Kotulska K, Jurkiewicz E. Brain lesions in tuberous sclerosis complex. Review Folia Neuropathologica. 2010;48:139–149. [PubMed] [Google Scholar]
- 114.Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2012;2013(49):243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raju GP, Urion DK, Sahin M. Neonatal subependymal giant cell astrocytoma: new case and review of literature. PediatrNeurol. 2007;36:128–131. doi: 10.1016/j.pediatrneurol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 116.Phi J. Congenital subependymal giant cell astrocytoma: clinical considerations and expression of radial glial cell markers in giant cells. Childs Nerv Syst. 2008;24:1449–1503. [DOI] [PubMed]
- 117.Cuccia V. Subependymal giant cell astrocytoma in children with tuberous sclerosis. Childs Nerv Syst. 2003;19:232–243. doi: 10.1007/s00381-002-0700-2. [DOI] [PubMed] [Google Scholar]
- 118.Mitra AG, Dickerson C. Central nervous system tumor with associated uni-lateral ventriculomegaly: unusual prenatal presentation of subsequently diagnosed tuberous sclerosis. J Ultrasound Med. 2000;19:651–654. doi: 10.7863/jum.2000.19.9.651. [DOI] [PubMed] [Google Scholar]
- 119.Park KJ. Gamma knife surgery for subependymal giant cell astrocytomas: clinical article. J Neurosurg. 2011;114:808–813. doi: 10.3171/2010.9.JNS10816. [DOI] [PubMed] [Google Scholar]
- 120.Isik U, Dincer A, Sav A, Ozek MM. Basal ganglia location of subependymal giant cell astrocytomas in two infants. Pediatr Neurol. 2010;42:157–159. doi: 10.1016/j.pediatrneurol.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Hussain N. Congenital subependymal giant cell astrocytoma diagnosed on fetal MRI. Arch Dis Child. 2006;91:520–520. doi: 10.1136/adc.2005.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goyer I, Dahdah N, Major P. Use of mTOR inhibitor everolimus in three neonates for treatment of tumors associated with tuberous sclerosis complex. Pediatr Neurol. 2014;52:450–453. doi: 10.1016/j.pediatrneurol.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 123.Kotulska K. Congenital subependymal giant cell astrocytomas in patients with tuberous sclerosis complex. Childs Nerv Syst. 2014;30:2037–2042. doi: 10.1007/s00381-014-2555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jansen AC, Belousova E, Benedik MP. Clinical characteristics of subependymal giant cell astrocytoma in tuberous sclerosis complex. Front Neurol. 2019;10:705–705. doi: 10.3389/fneur.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roth J, Roach ES, Bartels U, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr Neurol. 2013;49:439–44. [DOI] [PubMed]
- 126.Randle SC. Tuberous. Sclerosis Complex: A Review Pediatric Annals. 2017;46:166–171. doi: 10.3928/19382359-20170320-01. [DOI] [PubMed] [Google Scholar]
- 127.Ffranz DN. Everolimus in the treatment of subependymal giant cell astrocytomas, angiomyolipomas, and pulmonary and skin lesions associated with tuberous sclerosis complex. Biologics. 2013;7:211–221. doi: 10.2147/BTT.S25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Franz DN. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLoS ONE. 2016;11:0158476–0158476. doi: 10.1371/journal.pone.0158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.French JA, Lawson JA, Yapici Z, Ikeda H, Polster T. Adjunctive everolimus therapy for treatment resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:2153–2163. [DOI] [PubMed]
- 130.Chan DL, Kennedy SE, Sarkozy VE, et al. Congenital subependymal giant cell astrocytoma in children with tuberous sclerosis complex: growth patterns and neurological outcome. Pediatr Res. 2009;89:1447–1451. doi: 10.1038/s41390-020-1002-7. [DOI] [PubMed] [Google Scholar]
- 131.Zhou M, Chen Y, Yang C, et al. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: a meta-analysis. Orphanet J Rare Dis. 2019;14:39–39. doi: 10.1186/s13023-019-1012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun P, Kohrman M, Liu J, Guo A, Rogerio J, Krueger D. Outcomes of resecting subependymal giant cell astrocytoma (SEGA) among patients with SEGA-related tuberous sclerosis complex: a national claims database analysis. Curr Med Res Opin. 2012;28:657–663. doi: 10.1185/03007995.2012.658907. [DOI] [PubMed] [Google Scholar]
- 133.Bakhtiary H, Barzegar M, Shiva S, et al. The effect of everolimus on subependymal giant cell astrocytoma (SEGA) in children with tuberous sclerosis complex. Iran J Child Neurol. 2021;15:15–25. doi: 10.22037/ijcn.v15i4.30591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 135.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–142. doi: 10.1016/S0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 136.Maher ER, Neumann HP, Von Hippel Richard S. Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–640. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Varshney N, Kebede AA, Owusu-Dapaah H, Lather J, Kaushik M, Bhullar JS. A review of Von Hippel-Lindau Syndrome. J Kidney Cancer. 2017;4:20–29. doi: 10.15586/jkcvhl.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iliopoulos O, Ohh M, Kaelin WG. pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc Natl Acad Sci U S A. 1998;95:11661–11666. doi: 10.1073/pnas.95.20.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaelin WG Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. 2008;8:865–873. [DOI] [PubMed]
- 140.Richard S, Graff J, Lindau J. Resche F. von Hippel-Lindau disease. Lancet. 2004;363:1231–1235. doi: 10.1016/S0140-6736(04)15957-6. [DOI] [PubMed] [Google Scholar]
- 141.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 142.Van Leeuwaarde RS, Links AS, Links TP. Von Hippel-Lindau Syndrome. Seattle. WA; Washington, Seattle; 1993. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1463/?report=classic.
- 143.Chittiboina P, Lonser RR. Von Hippel-Lindau disease. Handb Clin Neurol. 2015;132:139–156. [DOI] [PMC free article] [PubMed]
- 144.Cassol C, Mete O. Endocrine manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med. 2015;139:263–268. doi: 10.5858/arpa.2013-0520-RS. [DOI] [PubMed] [Google Scholar]
- 145.Shanbhogue KP, Hoch M, Fatterpaker G, Chandarana H, Hippel Von. von Hippel-Lindau disease: review of genetics and imaging. Radiol Clin North Am. 2016;54:409–422. [DOI] [PubMed]
- 146.Choo D, Shotland L, Mastroianni M. Endolymphatic sac tumors in von Hippel-Lindau disease. J Neurosurg. 2004;100:480–487. doi: 10.3171/jns.2004.100.3.0480. [DOI] [PubMed] [Google Scholar]
- 147.Vijapura C. Aldin E Saad, Capizzano A A, Policeni B, Sato Y, Moritani T. Genetic syndromes associated with central nervous system tumors. 2016;37:258–280. doi: 10.1148/rg.2017160057. [DOI] [PubMed] [Google Scholar]
- 148.Sgambati MT, Stolle C, Choyke PL, et al. Mosaicism in von Hippel-Lindau disease: lessons from kindreds with germline mutations identified in offspring with mosaic parents. Am J Hum Genet. 2000;66(1):84–91. doi: 10.1086/302726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng S, Zhang J, Tan X, et al. The VHL/HIF axis in the development and treatment of pheochromocytoma/paraganglioma. Front Endocrinol (Lausanne). 2020;11:586857–586857. [DOI] [PMC free article] [PubMed]
- 150.vhl alliance active surveillance guidelines for vhl syndrome. 2020. https://www.vhlgr.org/content/91/vhla-suggested-active-surveillance-guide_lines-d-/.
- 151.Frantzen C, Klasson TD, Links TP, Giles RH. Von Hippel-Lindau syndrome. GeneReviews. 2015.
- 152.Wind JJ, Lonser RR. Management of von Hippel-Lindau disease-associated CNS lesions. Expert Rev Neurother. 2011;11:1433–1433. doi: 10.1586/ern.11.124. [DOI] [PubMed] [Google Scholar]
- 153.In: FDA approves belzutifan for cancers associated with von Hippel-Lindau disease [online]. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-cancers-associated-von-hippel-lindau-disease. Accessed 13 Aug 2021.
- 154.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316:1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 155.Malmer BS, Feychting M, Lönn S. Genetic variation in p53 and ATM haplotypes and risk of glioma and meningioma. J Neurooncol. 2007;82:229–237. doi: 10.1007/s11060-006-9275-1. [DOI] [PubMed] [Google Scholar]
- 156.Taylor AM, Harnden DG, Arlett CF, et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975;258:427–436. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- 157.Walsh MF, Chang VY, Kohlmann WK, et al. Recommendations for childhood cancer screening and surveillance in DNA repair disorders. Clin Cancer Res. 2017;23:23–31. doi: 10.1158/1078-0432.CCR-17-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zannolli R, Buoni S, Betti G, et al. A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia. Mov Disord. 2012;27:1312–1316. doi: 10.1002/mds.25126. [DOI] [PubMed] [Google Scholar]
- 159.Wallis LI, Griffiths PD, Ritchie SJ, Romanowski CA, Darwent G, Wilkinson ID. Proton spectroscopy and imaging at 3T in ataxia telangiectasia. AJNR Am J Neuroradiol. 2007;28:79–83. [PMC free article] [PubMed] [Google Scholar]
- 160.Nissenkorn A, Ben-Zeev B. Ataxia telangiectasia. Handb Clin Neurol. 2015;132:199–214. doi: 10.1016/B978-0-444-62702-5.00014-7. [DOI] [PubMed] [Google Scholar]
- 161.Farndon PA, Del Mastro RG, Evans DG, Kilpatrick MW. Location of gene for Gorlin syndrome. Lancet. 1992;339:581–582. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- 162.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/S0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 163.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 164.Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32:4155–4161. doi: 10.1200/JCO.2014.58.2569. [DOI] [PubMed] [Google Scholar]
- 165.Schofield D, West DC, Anthony DC, Marshal R, Sklar J. Correlation of loss of heterozygosity at chromosome 9q with histological subtype in medulloblastomas. Am J Pathol. 1995;146:472–480. [PMC free article] [PubMed] [Google Scholar]
- 166.Amlashi SF, Riffaud L, Brassier G, Morandi X. Nevoid basal cell carcinoma syndrome: Relation with desmoplastic medulloblastoma in infancy. A population-based study and review of the literature. Cancer. 2003;98:618–624. [DOI] [PubMed]
- 167.Evans DG, Farndon PA, Burnell LD, Gattamaneni HR, Birch JM. The incidence of Gorlin syndrome in 173 consecutive cases of medulloblastoma. Br J Cancer. 199;64:959–61. [DOI] [PMC free article] [PubMed]
- 168.Jones EA, Sajid MI, Shenton A, Evans DG. Basal cell carcinomas in gorlin syndrome: a review of 202 patients. J Skin Cancer. 2011;2011:217378. doi: 10.1155/2011/217378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Strong LC. Genetic and environmental interactions. Cancer. 1977;40:1861–1867. doi: 10.1002/1097-0142(197710)40:4+<1861::AID-CNCR2820400815>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 170.Evans DG, Birch JM, Orton CI. Brain tumours and the occurrence of severe invasive basal cell carcinoma in first degree relatives with Gorlin syndrome. Br J Neurosurg. 1991;5:643–646. doi: 10.3109/02688699109002890. [DOI] [PubMed] [Google Scholar]
- 171.Foulkes WD, Kamihara J, Evans DGR, et al. Cancer surveillance in Gorlin Syndrome and rhabdoid tumor predisposition syndrome. Clin Cancer Res. 2017;23:62–67. doi: 10.1158/1078-0432.CCR-17-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sredni ST, Tomita T. Rhabdoid tumor predisposition syndrome. Pediatr Dev Pathol. 2015;18:49–58. [DOI] [PubMed]
- 173.Bourdeaut F, Lequin D, Brugières L, Reynaud S, Dufour C, Doz F. Frequent hSNF5/INIl germline mutations in patients with rhabdoid tumor. Clin Cancer Res. 2011;17:31–38. doi: 10.1158/1078-0432.CCR-10-1795. [DOI] [PubMed] [Google Scholar]
- 174.Eaton KW, Tooke LS, Wainwright LM, Judkins AR, Biegel JA. Spectmm of SMARCB 1/INI 1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56:7–15. doi: 10.1002/pbc.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chun HJ, Lim EL, et al. Genome-wide profiles of extra-cranial malignant rhabdoid tumors reveal heterogeneity and dysregulated developmental pathways. Cancer Cell. 2016;29:394–406. [DOI] [PMC free article] [PubMed]
- 176.Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic sub-groups with distinct enhancer landscapes. Cancer Cell. 2016;29:379–393. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 177.Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1-deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med. 2021;145:90–98. doi: 10.5858/arpa.2019-0633-OA. [DOI] [PubMed] [Google Scholar]
- 178.Nemes K, Bens S, Bourdeaut F, et al. Rhabdoid tumor predisposition syndrome 2017 Dec 7. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. 2017;1993–2022.
- 179.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 180.Schneider K, Zelley K, Nichols KE, et al. Li-Fraumeni Syndrome. GeneReviews®[Internet]. 1993.
- 181.Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016;37:27328919–27328919. doi: 10.1002/humu.23035. [DOI] [PubMed] [Google Scholar]
- 182.Malkin D, Li FP, Strong LC, Fraumeni JF, Nelson CE, Kim DH. Germline p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 183.Kleihues P, Schauble B, Hausen Zur, Esteve A, Ohgaki JH. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150:1–13. [PMC free article] [PubMed]
- 184.Wu CC, Shete S, Amos CI, Strong LC. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res. 2006;66:8287–8292. doi: 10.1158/0008-5472.CAN-05-4247. [DOI] [PubMed] [Google Scholar]
- 185.Hwang SJ, Lozano G, Amos CI, Strong LC. Germline p53 mutations in a cohort with childhood sarcoma: sex differences in cancer risk. Am J Hum Genet. 2003;72:975–983. doi: 10.1086/374567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Le AN, Harton J, Desai H, et al. Frequency of radiation-induced malignancies post-adjuvant radiotherapy for breast cancer in patients with Li-Fraumeni syndrome. Breast Cancer Res Treat. 2020;181:181–188. doi: 10.1007/s10549-020-05612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kratz CP, Achatz MI, Brugières L, et al. Cancer screening recommendations for individuals with Li-Fraumeni Syndrome. Clin Cancer Res. 2017;23:38–45. doi: 10.1158/1078-0432.CCR-17-0408. [DOI] [PubMed] [Google Scholar]
- 188.Brandi ML, Gagel RF, Angeli A. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 189.Chandrasekharappa SC, Guru SC, Manickam P. Positional cloning of the gene for multiple endocrine neoplasia type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 190.Thakker RV, Newey PJ, Walls GV. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 191.Hou JW. Rubinstein-Taybi syndrome: clinical and molecular cytogenetic studies. Acta Paediatr Taiwan. 2005;46:143–148. [PubMed] [Google Scholar]
- 192.Petrij F, Giles RH, Dauwerse HG, et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 193.Hallam TM, Bourtchouladze R. Rubinstein-Taybi syndrome: molecular findings and therapeutic approaches to improve cognitive dysfunction. Cell Mol Life Sci. 2006;63:1725–1735. doi: 10.1007/s00018-005-5555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stevens CA, Adam MP, Ardinger HH, et al. Rubinstein-Taybi Syndrome. Seattle (WA; University of Washington, Seattle. (PMID;1993.20301699–20301699). [PubMed]
- 195.Tachibana I, Smith JS, Sato K, Hosek SM, Kimmel DW, Jenkins RB. Investigation of germline PTEN, p53, p16(INK4A)/p14(ARF), and CDK4 alterations in familial glioma. Am J Med Genet. 2000;92:136–141. [DOI] [PubMed]
- 196.Yakubov E, Ghoochani A, Buslei R, Buchfelder M, Eyüpoglu IY, Savaskan N. Hidden association of Cowden syndrome. PTEN mutation and meningioma frequency Oncoscience. 2016;3:149–155. doi: 10.18632/oncoscience.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607–1616. doi: 10.1093/jnci/djt277. [DOI] [PubMed] [Google Scholar]
- 198.Kaufman DK, Kimmel DW, Parisi JE, Michels VV. A familial syndrome with cutaneous malignant melanoma and cerebral astrocytoma. Neurology. 1993;43:1728–1731. [DOI] [PubMed]
- 199.Goldstein AM, Chan M, Harland M, Gillanders EM, et al. EA High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res. 2006;66:9818–9828. [DOI] [PubMed]
- 200.Baker MJ, Goldstein AM, et al. An interstitial deletion within 9p21.3 and extending beyond CDKN2A predisposes to melanoma, neural system tumours and possible haematological malignancies. J Med Genet. 2016;53:721– 727. [DOI] [PMC free article] [PubMed]
- 201.Petronzelli F, Sollima D, Coppola G, Martini‐Neri ME, Neri G, Genuardi M. CDKN2A germline splicing mutation affecting both p16(ink4) and p14(arf) RNA processing in a melanoma/neurofibroma kindred. Genes Chromosomes Cancer. 2001;31:398–401. [DOI] [PubMed]
- 202.Pasmant E, Laurendeau I, Héron D, Vidaud M, Vidaud D, Bièche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67:3963–3972. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 203.Frigerio S, Disciglio V, Manoukian S, Peissel B, Della Torre G, et al. 3 deletion in a girl affected by astrocytoma and multiple melanoma. BMC Med Genet. 2014;15:59– 59. [DOI] [PMC free article] [PubMed]
- 204.Chan AK, Han SJ, Choy W. Familial melanoma-astrocytoma syndrome: synchronous diffuse astrocytoma and pleomorphic xanthoastrocytoma in a patient with germline CDKN2A/B deletion and a significant family history. Clin Neuropathol. 2017;36:213–221. doi: 10.5414/NP301022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.de Kock L, Priest JR, Foulkes WD, Alexandrescu S. An update on the central nervous system manifestations of DICER1 syndrome. Acta Neuropathol. 2020;139:689–701. doi: 10.1007/s00401-019-01997-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.