Abstract
Background
This study aimed to evaluate the influence of maternal diabetes in the risk of neurodevelopmental disorders in offspring in the prenatal and postnatal periods.
Methods
This cohort study included singleton gestational diabetes mellitus (GDM) pregnancies >22 weeks’ gestation with live newborns between 1991 and 2008. The control group was randomly selected and matched (1:2) for maternal age, weeks of gestation and birth year. Cox regression models estimated the effect of GDM on the risk of attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and maternal type 2 diabetes mellitus (T2DM). Moreover, interaction between maternal T2DM and GDM-ADHD relationship was evaluated.
Results
Children (n=3,123) were included (1,073 GDM; 2,050 control group). The median follow-up was 18.2 years (interquartile range, 14.2 to 22.3) (n=323 with ADHD, n=36 with ASD, and n=275 from women who developed T2DM). GDM exposure was associated with ADHD (hazard ratio [HR]crude, 1.67; 95% confidence interval [CI], 1.33 to 2.07) (HRadjusted, 1.64; 95% CI, 1.31 to 2.05). This association remained significant regardless of the treatment (diet or insulin) and diagnosis after 26 weeks of gestation. Children of mothers who developed T2DM presented higher rates of ADHD (14.2 vs. 10%, P=0.029). However, no interaction was found when T2DM was included in the GDM and ADHD models (P>0.05). GDM was not associated with an increased risk of ASD (HRadjusted, 1.46; 95% CI, 0.74 to 2.84).
Conclusion
Prenatal exposure to GDM increases the risk of ADHD in offspring, regardless of GDM treatment complexity. However, postnatal exposure to maternal T2DM was not related to the development of ADHD.
Keywords: Diabetes, gestational; Diabetes mellitus, type 2; Neurodevelopmental disorders
INTRODUCTION
Gestational diabetes mellitus (GDM) is one of most common complications during pregnancy with a global prevalence of around 6% to 10% [1,2]. Its diagnosis has been associated with a higher risk of adverse pregnancy outcomes such as preeclampsia, cesarean section, prematurity, macrosomia, and neonatal hypoglycemia [3]. Moreover, exposure to maternal hyperglycemia during this period has been associated with an increased risk of psychiatric disorders in offspring such as attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) [4-8].
Hyperglycemia may predispose fetuses to stress, chronic inflammation, hypoxia and fetal hyperinsulinemia, which, in turn, may interfere with fetal brain development during critical prenatal windows and lead to neurobehavioral disorders later in life [9,10]. This hypothesis has been supported by previous cohort studies showing an association between a higher incidence of psychiatric disorders and greater severity of maternal diabetes especially pregestational diabetes (ASD: hazard ratio [HR], 1.98; 95% confidence interval [CI], 1.19 to 1,55; ADHD: HR, 1.36; 95% CI, 1.19 to 1.55) [4]. However, results related to GDM involving exposure to a lower degree of maternal hyperglycemia are inconsistent. Data from large cohorts showed an association between GDM and both ASD and ADHD, but these results were discordant regarding the characteristics of GDM. For example, early GDM (diagnosed before 26 weeks’ gestation) was associated with ASD [5], while the only risk factor for ADHD was GDM requiring medication [6]. Furthermore, a recent meta-analysis failed to demonstrate any relationship with ADHD [7], and a large nationwide Finnish cohort study including 649,043 newborns did not find any clear effect of GDM on ASD risk in normal-weight mothers [8]. Thus, knowledge about the impact of GDM on fetal brain development remains uncertain.
Genetic and well-known prenatal risk factors cannot completely explain the incidence of neurodevelopmental disorders. Therefore, postnatal factors could play a role with gene–environment interactions, such as exposure to artificial food colorings and flavorings, increasing the severity of ADHD [11]. In this regard, although women with a history of GDM are more likely to develop type 2 diabetes mellitus (T2DM) later in life [12], other factors beyond weight-related variables (environmental factors and behaviors) have been independently associated with T2DM. For instance, data from the primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-olive oil or nuts (PREDIMED) study, a randomized clinical trial, showed that the incidence of diabetes was reduced by 52% with the Mediterranean diet without calorie restriction [13]. We hypothesize that women who develop T2DM could potentially have been exposed to environmental factors inducing a negative influence on the neurodevelopment of their offspring.
With this background, the aim of this study was to evaluate the association of exposure to maternal hyperglycemia during pregnancy (GDM) and the risk of psychiatric disorders in offspring, as well as the role of maternal T2DM diagnosed later in life in this relationship. We also performed an in-depth analysis of different patterns of GDM related to the risk of T2DM.
METHODS
Study population
This cohort study was composed of singleton pregnancies >22 weeks of gestation with live newborns between January 1, 1991, and December 31, 2008 in a university hospital (University Hospital Mutua de Terrassa, Terrassa, Spain). The study protocol was conducted according to the principles of the Declaration of Helsinki and approved by the hospital Research Ethics Committee (EOINT2026). Informed consent was not required for this type of study, because no participants were contacted. The study and data analysis were conducted from June 4, 2020, to December 30, 2020.
All pregnancies with a diagnosis of GDM during the study period were included. For each GDM case, we randomly selected two pregnancies matched for maternal age, weeks of gestation and birth year for comparison. Data on pregnancy outcomes were collected at discharge (well-structured summary) and included: weeks of gestation, induction of labor, cesarean section, newborn sex, birth weight, and Apgar score at 1 and 5 minutes after birth.
Main exposure
According to national practice guidelines, the two-step approach recommended by the National Diabetes Data Group (NDDG) was used to diagnose GDM along the study period [1,14,15]. Women were screened for GDM with the 50-g 1-hour glucose challenge test (GCT). A positive GCT result was defined as a serum glucose level of ≥140 mg/dL (7.8 mmol/L). Women with a positive GCT underwent a 3-hour, 100-g diagnostic oral glucose tolerance test (OGTT). GDM was diagnosed if two or more of four glucose thresholds were met: fasting plasma glucose (FPG) ≥105 mg/dL (5.8 mmol/L), 1 hour ≥190 mg/dL (10.6 mmol/L), 2 hours ≥165 mg/dL (9.2 mmol/L), and 3 hours ≥145 mg/dL (8.1 mmol/L). Gestational age at GDM diagnosis was calculated using the date of the OGTT that met the GDM diagnosis criteria. Women diagnosed with pregestational diabetes during pregnancy were excluded.
Treatment for GDM was based on national practice guidelines, using insulin as antidiabetes medication (oral drugs such as metformin or sulfonylureas were not used) [15]. All women were referred to a nurse for diet counseling, instruction for self-blood glucose monitoring and insulin administration (if needed), weekly review of blood sugar logs and insulin doses, and modifications of treatment regimens established by an endocrinologist. Capillary glucose treatment goals to determine when insulin initiation or titration is needed were: fasting glucose <95 mg/dL (5.3 mmol/L); 1 hour post-prandial glucose <140 mg/dL (7.8 mmol/L).
Main outcomes
The diagnosis of T2DM in the mother was based on American Diabetes Association criteria: FPG ≥126 mg/dL (7.0 mmol/L) or 2-hour plasma glucose ≥200 mg/dL (11.1 mmol/L) during 75-g OGTT or glycosylated hemoglobin (HbA1c) ≥6.5% (48 mmol/mol) or a random plasma glucose value ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis. Information about plasma glucose levels was obtained from medical records.
Neurodevelopmental disorders in the offspring were identified from medical records in accordance with International Classification of Diseases (ICD)-10 codes: F84 for ASD and F90-91 for ADHD. These codes included children with and without medical treatment.
Statistical analysis
Data are presented as median (interquartile range [IQR]) or number (percentage) unless otherwise indicated. The Mann‐Whitney U test, Pearson’s chi‐squared test and analysis of variance (ANOVA) were performed, as appropriate, for comparisons between GDM and the control group.
For T2DM risk analysis, if there was more than one pregnancy from the same mother, the pregnancy with a GDM diagnosis was selected for the analysis. If all pregnancies were in the same category (GDM or control group), random selection was performed (243 pregnancies were excluded). For the analysis of psychiatric disorders of the offspring, children with no registered medical visits were excluded. Moreover, for both analyses, women with no plasma glucose determination in the last 5 years (for migration outside the hospital catchment area or no medical visits) and those diagnosed with type 1 diabetes mellitus (T1DM) were also excluded. The baseline characteristics in both analyses did not differ in the whole cohort (Table 1).
Table 1.
Baseline characteristics according to diabetes status and subgroup analysis
| Characteristic | Whole cohort |
T2DM risk analysis |
Offspring psychiatric disorders analysis |
|||
|---|---|---|---|---|---|---|
| Control group | GDM group | Control group | GDM group | Control group | GDM group | |
| Number | 2,298 | 1,149 | 1,934 | 1,012 | 2,050 | 1,073 |
| Maternal age, yr | 32.6 (29.7–35.8) | 32.6 (30.0–36.0) | 32.6 (29.7–35.8) | 32.6 (30.0–35.9) | 32.6 (29.7–35.8) | 32.8 (30.0–36.0) |
| Gestational age at delivery, wk | 39.7 (38.7–40.4) | 39.7 (38.6–40.6) | 39.7 (38.9–40.4) | 39.7 (38.7–40.6) | 39.7 (38.7–40.4) | 39.7 (38.7–40.6) |
| Labor induction | 494 (21.6) | 264 (23.0) | 417 (21.6) | 197 (19.5) | 447 (21.8) | 249 (23.2) |
| Cesarean section | 495 (21.5) | 290 (25.2)a | 414 (21.4) | 256 (25.3)a | 432 (21.7) | 275 (25.6)a |
| Female sex | 1,076 (46.8) | 539 (46.9) | 921 (47.6) | 456 (46.6) | 975 (47.6) | 500 (46.6) |
| Birthweight, g | 3,260 (2,980–3,550) | 3,230 (2,920–3,560) | 3,260 (2,980–35,650) | 3,230 (2,910–3,560) | 3,260 (2,990–3,555) | 3,230 (2,920–3,570) |
| Macrosomia (≥4,000 g) | 113 (4.9) | 69 (6.0) | 103 (5.3) | 61 (6.0) | 107 (5.2) | 63 (5.9) |
| Large for gestational age (>90th centile) | 282 (12.3) | 157 (13.7) | 244 (12.6) | 142 (14.0) | 282 (12.3) | 157 (13.7) |
| Preterm delivery (<37 wk) | 154 (6.7) | 86 (7.5) | 124 (6.4) | 81 (8.0) | 125 (6.1) | 82 (7.6) |
| Early preterm delivery (<34 wk) | 31 (1.3) | 15 (1.3) | 25 (1.3) | 11 (1.1) | 25 (1.2) | 14 (1.3) |
| Apgar (≤6) | 7 (0.3) | 4 (0.4) | 5 (0.3) | 3 (0.3) | 7 (0.3) | 4 (0.4) |
| Gestational age at GDM diagnosis, wk | NA | 28.5 (26.5–31.5) | NA | 28.5 (26.5–31.5) | NA | 28.5 (26.5–31.5) |
| GDM diagnosed (<26 wk) | NA | 213 (18.5) | NA | 175 (17.3) | NA | 195 (18.2) |
| Insulin therapy | NA | 457 (38.8) | NA | 388 (38.4) | NA | 418 (39.0) |
| Time of insulin therapy initiation, wk | NA | 33.5 (29.5–35.5) | NA | 34.5 (31.5–35.5) | NA | 33.5 (29.5–35.5) |
Values are presented as median (interquartile range) or number (%).
T2DM, type 2 diabetes mellitus; GDM, gestational diabetes mellitus; NA, not applicable.
P<0.05 control vs. GDM group calculated from chi-square test.
Cox proportional hazards modeling was used to estimate the association of exposure to maternal GDM with: (1) diagnosis of T2DM in the mother later in life, (2) offspring psychiatric disorders. Maternal gestational diabetes was included in the aforementioned models as an independent bivariate variable (yes/no) or categorical variable according to time of GDM diagnosis (no GDM, GDM< and ≥26 weeks’ gestation), type of treatment during pregnancy (no GDM, yes/no insulin therapy) or diagnosis of T2DM later in life (GDM absence/presence, with/without T2DM). Model 1 was adjusted for maternal age. Model 2 was adjusted for maternal age, weeks of gestation, cesarean section, Apgar ≤6 at 5 minutes after birth, and birth weight. In addition, an interaction analysis was performed in model 2 in order to evaluate the influence of maternal T2DM diagnosis in the relationship of GDM (as bivariate variable) and ADHD in offspring. HR with 95% CI were reported as measures of effect size. P values <0.05 were considered statistically significant. All statistical calculations were performed with the STATA version 14.0 (StataCorp., College Station, TX, USA) statistical package.
RESULTS
Participant characteristics
A total of 3,123 singleton pregnancies were included (Fig. 1). The GDM group presented significantly higher rates of cesarean section (25.6% vs. 21.7%, P=0.010) compared to the control group, and 39% used insulin therapy during pregnancy and 18.2% were classified as early GDM (diagnosed before 26 weeks of gestation). The range of gestational period at the time of diagnosis was 9.5 to 25.5 weeks of gestation in the early GDM group and 26.5 to 39 in the late GDM group. No other between-group differences were observed in pregnancy outcomes (Table 1, Supplementary Table 1).
Fig. 1.
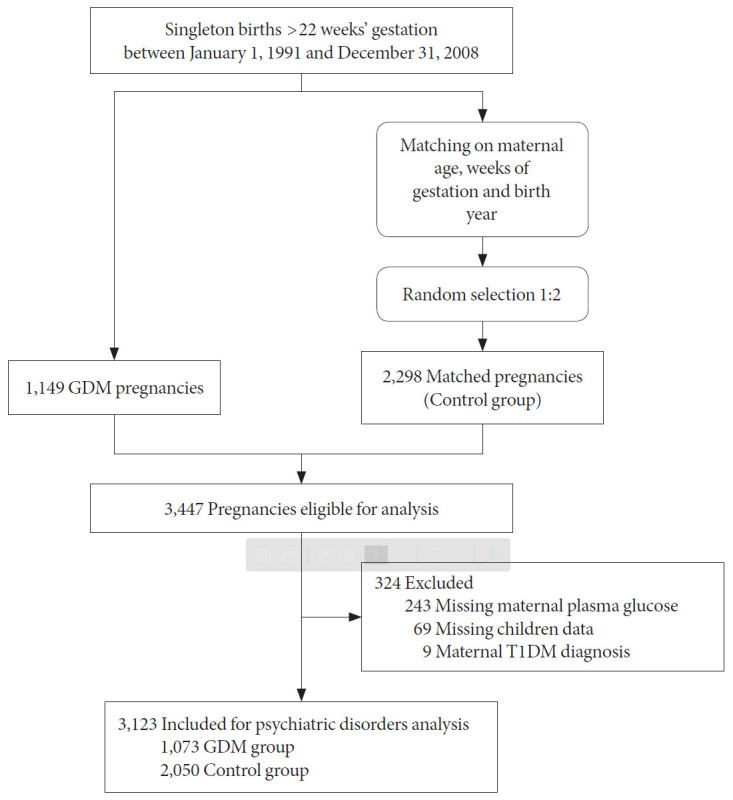
Flowchart of the cohort of gestational diabetes mellitus (GDM) pregnancies and matched pregnancies including detailed information on the pregnancies excluded. T1DM, type 1 diabetes mellitus.
Risk of development of T2DM
Among 2,946 women included in this analysis (n=1,012 in the GDM group and n=1,934 in the control group), 244 incident cases of T2DM were observed with a median follow-up of 18.6 years (IQR, 15.5 to 22.7) and a median time to the development of T2DM of 13.7 years (IQR, 9.1 to 18.7). A history of GDM was associated with a higher risk of later T2DM compared with non-diabetic pregnancies, both in the crude and in ageadjusted model (HRcrude, 9.06; 95% CI, 6.68 to 12.29) (HRadjusted, 8.95; 95% CI, 6.60 to 12.15). When the time of GDM diagnosis was evaluated, women with an early GDM diagnosis (before 26 weeks of gestation) showed a two-fold greater risk of T2DM than those with a late diagnosis (for early GDM [HRcrude, 19.41; 95% CI, 13.29 to 28.37], [HRadjusted, 18.81; 95% CI, 12.86 to 27.5]) (for late GDM [HRcrude, 7.41; 95% CI, 5.39 to 10.20], [HRadjusted, 7.34; 95% CI, 5.33 to 10.11]). In addition, the risk of T2DM was increased according to the complexity of GDM treatment (for only diet [HRcrude, 4.68; 95% CI, 3.24 to 6.77], [HRadjusted, 4.77; 95% CI, 3.30 to 6.90]) (for insulin use [HRcrude, 15.99; 95% CI, 11.60 to 22.03], [HRadjusted, 15.29; 95% CI, 11.07 to 21.11]).
Risk of psychiatric disorders in offspring
The median follow-up of the children was 18.2 years (IQR, 14.2 to 22.3), with 323 (10.3%) incident cases of ADHD, 36 (1.15%) cases of ASD and 275 (8.8%) children from mothers who developed T2DM later in life (with or without psychiatric disorder). The incidence rate of ADHD was significantly higher in pregnancies complicated by GDM compared to the control group (7.81 vs. 4.66 cases per 1,000 person-years, P<0.05). After adjustment for well-known risk factors such as maternal age, weeks of gestation, cesarean section, Apgar score and birth weight, offspring from GDM pregnancies still showed a higher risk of ADHD (HRadjusted, 1.64; 95% CI, 1.31 to 2.05) (Table 2). When GDM was divided according to the type of treatment during pregnancy or time of diagnosis, the association with ADHD remained statistically significant regardless of the treatment required. However, this association was blunted in the subgroup of early GDM, both in the crude and adjusted models (Table 2). Lastly, the impact of developing T2DM later in life in these mothers was studied. Children from mothers with T2DM presented higher rates of ADHD compared to those from non-diabetic mothers (14.2% vs. 10%, P=0.029). Furthermore, when children were grouped according to the history of GDM and the diagnosis of T2DM in their mothers, higher rates of ADHD were observed in the GDM groups (GDM–/T2DM– 173/1,989 [8.7%], GDM–/T2DM+ 6/61 [9.8%], GDM+/T2DM– 111/859 [12.9%], GDM+/T2DM+ 33/214 [15.4%]; P among groups <0.001). Fig. 2 depicts the crude cumulative incidences of ADHD by history of GDM and diagnosis of T2DM. The association of a history of GDM and ADHD in offspring was not different with or without the presence of T2DM in the mothers in either the crude model or after adjustment for well-known confounders (Table 3). Thus, no interaction was found when T2DM was included as a bivariate variable in the Cox proportional hazards models (P>0.05). Lastly, the length of exposure to maternal T2DM was evaluated. Although, as expected, the GDM group had longer exposure to maternal T2DM (6.1 years [1.3 to 12.3] vs. 3.2 years [1.3 to 6.5], P=0.007), no differences were observed in the rates of diagnosis of ADHD after the onset of maternal diabetes (50% vs. 51.2% in control and GDM groups, respectively).
Table 2.
Risk of ADHD in offspring in pregnancies complicated by GDM according to type of treatment during pregnancy or time of diagnosis
| Variable | No. with ADHD/total | Crude model |
Adjusted modela |
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| GDM considered as one group | |||||
| No GDM | 179/2,050 | 1 (reference) | 1 (reference) | ||
| GDM | 144/1,073 | 1.67 (1.33–2.07) | <0.001 | 1.64 (1.32–2.05) | <0.001 |
| GDM by time of diagnosisb | |||||
| No GDM | 179/2,050 | 1 (reference) | 1 (reference) | ||
| Early GDM | 23/195 | 1.48 (0.96–2.29) | 0.070 | 1.45 (0.94–2.25) | 0.094 |
| Late GDM | 119/878 | 1.69 (1.34–2.13) | <0.001 | 1.67 (1.32–2.11) | <0.001 |
| GDM by treatment | |||||
| No GDM | 179/2,050 | 1 (reference) | 1 (reference) | ||
| GDM only with diet | 87/655 | 1.68 (1.30–2.17) | <0.001 | 1.68 (1.30–2.18) | <0.001 |
| GDM with insulin use | 57/418 | 1.64 (1.22–2.22) | 0.001 | 1.59 (1.18–2.15) | 0.002 |
Cox regression models expressed as HR (95% CI).
ADHD, attention-deficit/hyperactivity disorder; GDM, gestational diabetes mellitus; HR, hazard ratio; CI, confidential interval.
Model adjusted for maternal age, weeks of gestation, cesarean section, Apgar <6 at 5 minutes after birth and birth weight,
Late/early GDM: diagnosis of GDM after or before 26 weeks’ gestation, respectively.
Fig. 2.
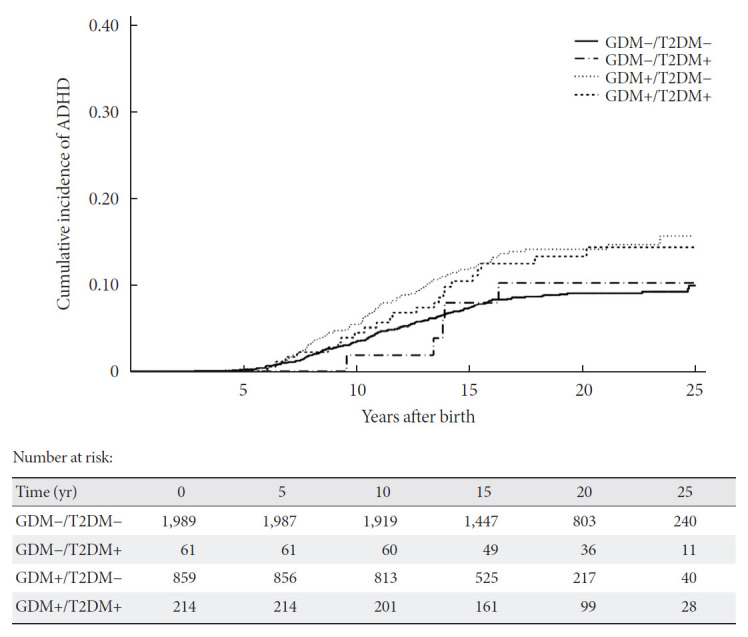
Crude cumulative incidence of attention-deficit/hyperactivity disorder (ADHD) by diabetes exposure in utero and diagnosis of type 2 diabetes mellitus (T2DM) later in life in their mothers. +/− indicates presence or absence of gestational diabetes mellitus (GDM) or/and T2DM.
Table 3.
Association between maternal diabetes during pregnancy and risk of attention-deficit/hyperactivity disorder in offspring by T2DM diagnosis in their mothers
| Crude model |
Adjusted modela |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| GDM–/T2DM– | 1 (reference) | 1 (reference) | ||
| GDM–/T2DM+ | 1.03 (0.45–2.32) | 0.949 | 1.01 (0.44–2.28) | 0.990 |
| GDM+/T2DM– | 1.64 (1.29–2.08) | <0.001 | 1.64 (1.29–2.08) | <0.001 |
| GDM+/T2DM+ | 1.78 (1.23–2.58) | 0.002 | 1.69 (1.16–2.47) | 0.007 |
Cox regression models expressed as HR (95% CI). +/– indicates presence or absence of GDM or/and T2DM.
T2DM, type 2 diabetes mellitus; HR, hazard ratio; CI, confidential interval; GDM, gestational diabetes mellitus.
Model adjusted for maternal age, weeks of gestation, cesarean section, Apgar <6 at 5 minutes after birth and birth weight.
Regarding the incidence of ASD in offspring, diabetes status during pregnancy was not associated with an increased risk compared to uncomplicated pregnancies in either the crude or adjusted models (0.77 vs. 0.52 cases per 1,000 person-years, GDM vs. control, P=0.16; HRcrude, 1.47; 95% CI, 0.75 to 2.86; HRadjusted, 1.46; 95% CI, 0.74 to 2.84). The low number of ASD cases precluded a sub-analysis according to the time of diagnosis and GDM treatment and an interaction analysis for the development of T2DM.
DISCUSSION
This study evaluated the long-term repercussion of GDM in both mothers and children, and the results confirm the negative impact of maternal hyperglycemia on fetal neurodevelopment as well as the development of T2DM in the mother later in life. Nonetheless, postnatal exposure to an environment related to a higher incidence of maternal T2DM did not have an impact on the incidence of psychiatric disorders. To the best of our knowledge, no previous study has related maternal conditions such as GDM and the development of T2DM after pregnancies to neurodevelopment disorders in the same cohort.
A previous diagnosis of GDM is an established risk factor for developing T2DM in later life. Thus, a recent meta-analysis, assessing a total of 1,332,373 individuals, showed that women with pregnancies complicated by GDM were 10-fold more likely to develop T2DM (relative risk, 9.51; 95% CI, 7.14 to 12.67) [12]. Our results are in accordance with a HR for T2DM of 8.95 (95% CI, 6.60 to 12.15). Furthermore, our data represent the current incidence of T2DM with the longest follow-up published to date (almost 20 years after pregnancy) using the most recent diabetes criteria. Finally, our findings are also in accordance with previous studies identifying risk factors of progression to T2DM, such as early GDM diagnosis and the use of insulin during pregnancy [16,17]. Altogether this highlights the importance of postpartum screening to identify women at higher risk of progression and the introduction of strategies for diabetes prevention.
The incidence of neurodevelopmental disorders has increased in the last years, suggesting that maternal factors could play a role. In this regard, exposure to maternal hyperglycemia in the prenatal period has been widely studied, especially pregestational diabetes [4,6,18-20]. However, results regarding milder hyperglycemia such as GDM have been inconsistent. We observed a higher risk of ADHD in offspring exposed compared to those unexposed to GDM. The effect observed (HRadjusted, 1.64; 95% CI, 1.31 to 2.05) was slightly higher to what has previously been published [8,21]. A large nationwide Finnish cohort study including 649,043 newborns found HRs for ADHD in children of 1.15 (95% CI, 1.01 to 1.30) among normal-weight mothers [8]. However, the birth year ranged from 2004 to 2014 with a short follow-up in the last period, reducing the chance of debut of psychiatric disorders. Furthermore, a retrospective cohort study with a longer follow-up (median 9.9 years) failed to demonstrate any relationship between GDM and ADHD (HR, 1.02; 95% CI, 0.96 to 1.09) [6]. However, when GDM was divided according to medication use during pregnancy, pregnancies requiring medication (insulin, metformin, or glyburide) were actually associated with a higher risk of ADHD (HR, 1.26; 95% CI, 1.14 to 1.41). Our results showed a higher risk independently of medication use during pregnancy. Unlike the aforementioned study, insulin was the only antidiabetic medication used in our cohort. In contrast to insulin [22], other antidiabetic medications such as metformin or glyburide are known to cross the placenta and have been associated with adverse neonatal outcomes such as preterm birth and neonatal hypoglycemia, respectively [23-25]. Thus, the effect of antidiabetic medication on neurodevelopment, indirectly as a consequence of adverse neonatal outcomes or through direct interference with fetal brain development, could not be ruled out in the previous studies. Lastly, our results reinforce recent data [21], reporting that exposure to hyperglycemia after 26 weeks of gestation is associated with ADHD in children. By contrast, no association was found with early GDM. Fetal brain development is continuous throughout gestation [26], and therefore, the degree of hyperglycemia could have a negative effect on the fetus regardless of gestational age. Nonetheless, evaluation of glycemic control in the GDM setting is challenging. Data from self-blood glucose monitoring is usually not available, and its evaluation by HbA1c is controversial [6,8,27]. Thus, further studies with glycemic data from continuous glucose monitoring could contribute to better understanding.
Brain function itself continues to develop after birth. Indeed, early interventions in children with ASD have shown to minimize core deficits and maximize functional independence [28]. In this context, we explored what happens to children of mothers who develop T2DM after pregnancy. Poor maternal behaviors lead to the development of similar behavioral phenotypes in their own children, as observed by the influence of parents’ dietary behaviors on children’s eating habits [29]. Our results showed a higher rate of ADHD in children of mothers who developed T2DM later in life, but when this was evaluated according to previous GDM, the possible maternal T2DM effect was blunted. No previous study has evaluated GDM, maternal T2DM, and ADHD in children in the same cohort. Current studies focus on the higher prevalence of cardiovascular risk factors such as obesity and diabetes in children with ADHD [30,31], and more recently, on the role of metabolic control in children with T1DM in the development of ADHD [32]. However, no study has evaluated the diagnosis of diabetes in mothers. Several factors underlie the diagnosis of T2DM, some of which are closely related to weight as well as environmental and behavior-related factors such as physical activity [33], diet quality [13], or socio-economic status [34], which, in turn, are related to ADHD [11,35]. We hypothesized that children from mothers who develop T2DM would be exposed to poor behaviors, and consequently, would be at higher risk of developing ADHD. Nevertheless, our data suggest that the intrauterine environment could be a stronger predictor of the development of psychiatric disorders than the postnatal period.
This study has several strengths. To our knowledge, this study reports the longest follow-up (almost 20 years) evaluating the effects of GDM, and, in addition, it is the first study to evaluate the repercussion of maternal diabetes in both the prenatal and postnatal periods in the same cohort. Second, the validity of our data regarding ADHD is supported by recently published data of a sample of 6,834 students aged 5 to 17 years in Spain reporting an overall prevalence of ADHD comparable to our results (10.3%) [36]. Third, in order to eliminate a possible selection bias, a control group matched for birth year was recruited. Protocols of obstetric and neonatal management, such as corticoid use in threatened preterm labor or the approach to neonatal hypoglycemia, both related to long-term repercussions in adulthood, have changed over time [37,38]. However, these changes did not interfere with our results. Fourth, despite being an observational study with no direct intervention, the diagnosis of T2DM was directly confirmed through plasma glucose determination in order to assure correct diagnosis and eliminate miscoding and misdiagnosis described in primary care [39]. Finally, regarding psychiatric disorders, ICD-10 codes were selected because of the complexity of the diagnosis of these disorders. Nonetheless, only children undertaking regular visits with a pediatrician/physician were included in the analyses to minimize ascertainment bias. These strengths reinforce the results observed, especially in the incidence of ADHD as well as T2DM.
We also acknowledge some limitations. First, pregestational maternal weight was not assessed because data were not readily available. Compared to normal-weight, maternal obesity increases the risk of ADHD by up to 2-fold. However, even in the normal-weight setting, a higher risk of ADHD has been described in offspring of women with GDM compared to non-diabetic counterparts [8,40]. Second, potential confounding owing to paternal risk factors, socio-economic status, causes of cesarean section, ethnicity, or drug consumption during pregnancy such as smoking substance consumption such as smoking during pregnancy could not be evaluated due to a lack of data. However, maternal and delivery risk factors (some of which are closely related to the former) such as maternal age, weeks of gestation, type of delivery, low Apgar score, and birth weight were taken into account and included in the multivariate models. In fact, prematurity and low birthweight have consistently been associated with ADHD, with family studies suggesting that these effects cannot be explained by genetic confounding [35]. Third, the low number of cases of ASD in the whole cohort and ADHD cases in the early GDM group limited the statistical analysis, and therefore caution should be taken in interpreting the findings. Fourth, in the present study, the birth year ranged from 1991 to 2008 using NDDG diagnostic criteria for GDM. But nowadays, 2013 World Health Organization criteria (with lower plasma glucose cutoffs) have been adopted by 67.9% of the European countries [41]; thus, the impact of GDM diagnosed by these new criteria on fetal brain development remains uncertain. Lastly, this was an observational study, and therefore causal inferences cannot be drawn.
In conclusion, the results of this study suggest that despite the higher rates of ADHD observed in children from mothers who develop T2DM later in life, this relationship is mediated by previous exposure to hyperglycemia during gestation. These findings highlight the role of mild hyperglycemia, such as GDM, in the prenatal period leading to adverse long-term consequences in mental health for offspring. Nonetheless, further large studies are needed to confirm these results and assess the impact of maternal glycemic control on the risk of psychiatric disorders in offspring in both the pre- and postnatal periods.
Acknowledgments
We are grateful to Donna Pringle for helping in the writing and editing of the manuscript.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: V.P.
Acquisition, analysis, or interpretation of data: V.P., X.U., M.V., M.M., A.C., E.E., G.E., N.P., O.G., T.G., A.S.S., A.D., N.A.C., C.Q., A.J.A., E.L., M.J.B.
Drafting the work or revising: V.P., A.J.A., E.L., M.J.B.
Final approval of the manuscript: V.P., X.U., M.V., M.M., A.C., E.E., G.E., N.P., O.G., T.G., A.S.S., A.D., N.A.C., C.Q., A.J.A., E.L., M.J.B.
FUNDING
None
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0340.
Baseline characteristics according to time of GDM diagnosis and subgroup analysis
REFERENCES
- 1.Ricart W, Lopez J, Mozas J, Pericot A, Sancho MA, Gonzalez N, et al. Potential impact of American Diabetes Association (2000) criteria for diagnosis of gestational diabetes mellitus in Spain. Diabetologia. 2005;48:1135–41. doi: 10.1007/s00125-005-1756-9. [DOI] [PubMed] [Google Scholar]
- 2.Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening: a review. Diabet Med. 2012;29:844–54. doi: 10.1111/j.1464-5491.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- 3.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto JM, Benham JL, Dewey D, Sanchez JJ, Murphy HR, Feig DS, et al. Neurocognitive and behavioural outcomes in offspring exposed to maternal pre-existing diabetes: a systematic review and meta-analysis. Diabetologia. 2019;62:1561–74. doi: 10.1007/s00125-019-4923-0. [DOI] [PubMed] [Google Scholar]
- 5.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–34. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 6.Xiang AH, Wang X, Martinez MP, Getahun D, Page KA, Buchanan TA, et al. Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care. 2018;41:2502–8. doi: 10.2337/dc18-0733. [DOI] [PubMed] [Google Scholar]
- 7.Guo D, Ju R, Zhou Q, Mao J, Tao H, Jing H, et al. Association of maternal diabetes with attention deficit/hyperactivity disorder (ADHD) in offspring: a meta-analysis and review. Diabetes Res Clin Pract. 2020;165:108269. doi: 10.1016/j.diabres.2020.108269. [DOI] [PubMed] [Google Scholar]
- 8.Kong L, Norstedt G, Schalling M, Gissler M, Lavebratt C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 2018;142:e20180776. doi: 10.1542/peds.2018-0776. [DOI] [PubMed] [Google Scholar]
- 9.Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, Fassett MJ, et al. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131:e53–61. doi: 10.1542/peds.2012-1298. [DOI] [PubMed] [Google Scholar]
- 10.Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–32. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, Grimshaw K, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370:1560–7. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- 12.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and metaanalysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salas-Salvado J, Bullo M, Babio N, Martinez-Gonzalez MA, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–9. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991;40 Suppl 2:197–201. doi: 10.2337/diab.40.2.s197. [DOI] [PubMed] [Google Scholar]
- 15.Acosta D, Balsells M, Ballesteros M, Bandres MO, Bartha JL, Bellart J, et al. Care of pregnancies complicated by diabetes. Clinical practice guidelines: 2014 update. Av Diabetol. 2015;31:45–59. [Google Scholar]
- 16.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59:1403–11. doi: 10.1007/s00125-016-3927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albareda M, Caballero A, Badell G, Piquer S, Ortiz A, de Leiva A, et al. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26:1199–205. doi: 10.2337/diacare.26.4.1199. [DOI] [PubMed] [Google Scholar]
- 18.Knorr S, Clausen TD, Vlachova Z, Bytoft B, Damm P, BeckNielsen H, et al. Academic achievement in primary school in offspring born to mothers with type 1 diabetes (the EPICOM Study): a register-based prospective cohort study. Diabetes Care. 2015;38:1238–44. doi: 10.2337/dc15-0223. [DOI] [PubMed] [Google Scholar]
- 19.Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK. Maternal type 1 diabetes and risk of autism in offspring. JAMA. 2018;320:89–91. doi: 10.1001/jama.2018.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TC, Nassar N, Shand AW, Jones H, Guastella AJ, Dale RC, et al. Association of maternal autoimmune disease with attention-deficit/hyperactivity disorder in children. JAMA Pediatr. 2021;175:e205487. doi: 10.1001/jamapediatrics.2020.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Zhao S, Dalman C, Karlsson H, Gardner R. Association of maternal diabetes with neurodevelopmental disorders: autism spectrum disorders, attention-deficit/hyperactivity disorder and intellectual disability. Int J Epidemiol. 2021;50:459–74. doi: 10.1093/ije/dyaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon RK, Cohen RM, Sperling MA, Cutfield WS, Mimouni F, Khoury JC. Transplacental passage of insulin in pregnant women with insulin-dependent diabetes mellitus: its role in fetal macrosomia. N Engl J Med. 1990;323:309–15. doi: 10.1056/NEJM199008023230505. [DOI] [PubMed] [Google Scholar]
- 23.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 24.Picon-Cesar MJ, Molina-Vega M, Suarez-Arana M, GonzalezMesa E, Sola-Moyano AP, Roldan-Lopez R, et al. Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: randomized prospective trial. Am J Obstet Gynecol. 2021;225:517. doi: 10.1016/j.ajog.2021.04.229. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu W, Chen H, Chen Q. Comparison of insulin, metformin, and glyburide on perinatal complications of gestational diabetes mellitus: a systematic review and meta-analysis. Gynecol Obstet Invest. 2021;86:218–30. doi: 10.1159/000515893. [DOI] [PubMed] [Google Scholar]
- 26.Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsakiridis I, Giouleka S, Mamopoulos A, Kourtis A, Athanasiadis A, Filopoulou D, et al. Diagnosis and management of gestational diabetes mellitus: an overview of national and international guidelines. Obstet Gynecol Surv. 2021;76:367–81. doi: 10.1097/OGX.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 28.Hyman SL, Levy SE, Myers SM, Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447. doi: 10.1542/peds.2019-3447. [DOI] [PubMed] [Google Scholar]
- 29.Mahmood L, Flores-Barrantes P, Moreno LA, Manios Y, Gonzalez-Gil EM. The influence of parental dietary behaviors and practices on children’s eating habits. Nutrients. 2021;13:1138. doi: 10.3390/nu13041138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kase BE, Rommelse N, Chen Q, Li L, Andersson A, Du Rietz E, et al. Longitudinal associations between symptoms of ADHD and BMI from late childhood to early adulthood. Pediatrics. 2021;147:e2020036657. doi: 10.1542/peds.2020-036657. [DOI] [PubMed] [Google Scholar]
- 31.Lindekilde N, Rutters F, Erik Henriksen J, Lasgaard M, Schram MT, Rubin KH, et al. Psychiatric disorders as risk factors for type 2 diabetes: an umbrella review of systematic reviews with and without meta-analyses. Diabetes Res Clin Pract. 2021;176:108855. doi: 10.1016/j.diabres.2021.108855. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Kuja-Halkola R, Larsson H, Lichtenstein P, Ludvigsson JF, Svensson AM, et al. Poor glycaemic control is associated with increased risk of neurodevelopmental disorders in childhood-onset type 1 diabetes: a population-based cohort study. Diabetologia. 2021;64:767–77. doi: 10.1007/s00125-020-05372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnard-Kelly KD, Chernavvsky D. Social inequality and diabetes: a commentary. Diabetes Ther. 2020;11:803–11. doi: 10.1007/s13300-020-00791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395:450–62. doi: 10.1016/S0140-6736(19)33004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosch R, Pagerols M, Rivas C, Sixto L, Bricolle L, EspanolMartin G, et al. Neurodevelopmental disorders among Spanish school-age children: prevalence and sociodemographic correlates. Psychol Med. 2021 Jan 13; doi: 10.1017/S0033291720005115. [Epub]. [DOI] [PubMed] [Google Scholar]
- 37.Dani C, Corsini I. Guidelines for management of neonatal hypoglycemia: are they actually applicable? JAMA Pediatr. 2020;174:638–9. doi: 10.1001/jamapediatrics.2020.0632. [DOI] [PubMed] [Google Scholar]
- 38.Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. Am J Obstet Gynecol. 2018;219:62–74. doi: 10.1016/j.ajog.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 39.de Lusignan S, Sadek N, Mulnier H, Tahir A, Russell-Jones D, Khunti K. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med. 2012;29:181–9. doi: 10.1111/j.1464-5491.2011.03419.x. [DOI] [PubMed] [Google Scholar]
- 40.Kong L, Nilsson IA, Brismar K, Gissler M, Lavebratt C. Associations of different types of maternal diabetes and body mass index with offspring psychiatric disorders. JAMA Netw Open. 2020;3:e1920787. doi: 10.1001/jamanetworkopen.2019.20787. [DOI] [PubMed] [Google Scholar]
- 41.Benhalima K, Mathieu C, Van Assche A, Damm P, Devlieger R, Mahmood T, et al. Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol. 2016;201:197–202. doi: 10.1016/j.ejogrb.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics according to time of GDM diagnosis and subgroup analysis


