Abstract
Candida albicans β-1,2-oligomannosides stimulate macrophage tumor necrosis factor alpha (TNF-α) but not NO release. This stimulation desensitized macrophages by altering β-1,2-oligomannoside-dependent TNF-α production and lipopolysaccharide-dependent TNF-α and NO secretion. Desensitization was not related to tyrosine phosphorylation signal transduction but was transferred by culture supernatants in which arachidonic acid derivatives were evidenced.
During the course of infection, Candida albicans disregulates the immune system (8), namely by altering macrophage functions (7, 20). It suppresses nitric oxide (NO) production by murine peritoneal macrophages stimulated by gamma interferon or bacterial lipopolysaccharide (LPS) (5). Although these results have pathophysiological relevance (26), the nature of C. albicans molecules responsible for either stimulation or suppression of macrophage activities has not been investigated yet.
Desensitization leading to suppression of macrophage functions in response to a second stimulation is generally a property of microbial molecules which act as macrophage major stimulants (6, 31). The prototypic example of microbial stimulants presenting these activities is the bacterial LPS. Among fungi, the cryptococcal capsular polysaccharide has been shown to display down-regulating activities concerning tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) secretion (14, 28).
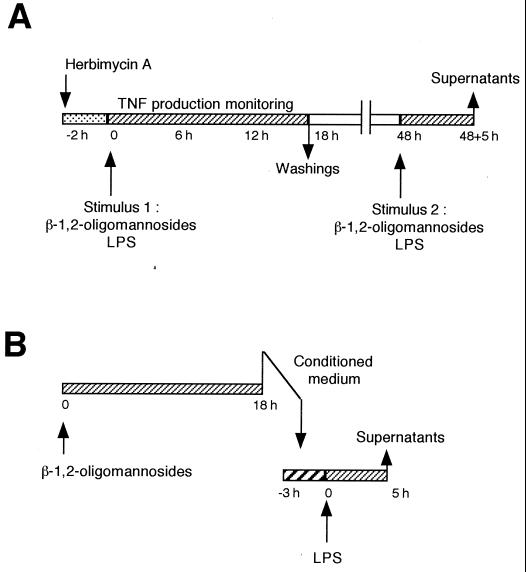
C. albicans yeast cells stimulate TNF-α production (12, 16), and different C. albicans-derived molecules have been shown to maintain these capacities (9, 11, 27). All these molecules display a polysaccharidic moiety presenting β-1,2-oligomannosides (25), a special type of sugars first described in the acid-labile fraction of the C. albicans cell wall phosphopeptidomannan (22). The β-1,2-oligomannosides are able per se to stimulate TNF-α production (13). This stimulation depended on the oligomer size, and the mannotetraose was the minimal C. albicans-derived molecular entity displaying TNF-α-inducing properties. With the aim of exploring the molecular mechanisms responsible for down-regulation of macrophage functions by C. albicans, we used this C. albicans-derived β-1,2 mannotetraose in a series of experiments (whose principle is shown in Fig. 1) with the J774 macrophage cell line.
FIG. 1.
Experimental design used in the study.
Cells were incubated with 50 μM β-1,2-mannotetraose purified from the cell wall of the VW32 strain of C. albicans (serotype A) as previously described (13). The effect on cell stimulation was first compared to that obtained with 1 μg of LPS per ml from Escherichia coli (0111B4). The cell response was examined through the measurement in the cell-free supernatants of TNF-α by using the L929 lytic bioassay (13). Comparable amounts and kinetics of TNF-α production were obtained with both stimuli: cytokine production peaked after 4 to 5 h of stimulation with values of 6.6 ± 3.0 and 6.7 ± 3.0 ng/ml upon β-1,2-oligomannoside and LPS stimulation, respectively, and decreased to an undetectable amount after 12 to 15 h. LPS-dependent cytokine induction involved signal transduction pathway based upon tyrosine phosphorylation (19). Treatment 2 h before addition of β-1,2-oligomannosides with the protein tyrosine kinase (PTK) inhibitor herbimycin A resulted in a dose-dependent inhibition of the TNF-α release in cell supernatants, 100% inhibition being obtained with 1 μg of herbimycin per ml. Nonetheless, stimulation with β-1,2-oligomannosides differed from the LPS-dependent stimulation. Although addition of 1 μg of LPS per ml to the cells led to a nitrite release detectable after a 12-h incubation and reached a maximum production after a 24-h incubation, it was not possible to detect NO production by the cells stimulated with β-1,2-oligomannosides, even after 48 h of incubation.
Whether incubation with β-1,2-oligomannosides led to a desensitization of the cells was therefore investigated (Fig. 1A). After a first stimulation similar to that applied as above, cells were washed to eliminate residual cytokine (and oligomannosides) and cultured in fresh medium. A second stimulation with either β-1,2-oligomannosides or LPS was then attempted, and after a further 5-h incubation corresponding to the time necessary to gain cytokine production, the amount of TNF-α released into the supernatants was determined. Compared to the control cells incubated in the same conditions but with medium alone, preincubation of cells with β-1,2-oligomannosides resulted in a strong inhibition of TNF-α release upon a second stimulation. This effect was evidenced both in the case of a second stimulation with LPS (74%; P < 0.05; n = 3) and with β-1,2-oligomannosides (81%; P < 0.05; n = 3). β-1,2-Oligomannoside-dependent desensitization also altered the NO production obtained after stimulation with LPS. The cells pretreated with 50 μM β-1,2-oligomannosides produced amounts of NO in response to LPS that were lower than those produced by cells preincubated with medium alone (58 ± 5 μM versus 34 ± 4 μM, respectively; P < 0.05 by the Student's t test). Thus, β-1,2-oligomannosides exert an inhibitory effect on the level of at least two cell activities, viz., cytokine production and NO release.
To investigate the mechanism of the β-1,2-oligomannoside-induced reprogramming we observed, we first studied whether the secondary desensitization could be altered if the signals involved in the first stimulation had been inhibited (Fig. 1A). Since β-1,2-oligomannoside-dependent stimulation involved PTK, we treated cells with 1 μg of herbimycin A per ml prior to the addition of the β-1,2-oligomannosides (50 μM). After 12 h, the cells were washed and cultured in fresh medium for 36 h as above. A second stimulation with the β-1,2-oligomannosides (50 μM) was then made, and the resulting TNF-α production was examined after 5 h. As a control, the capability of cells pretreated with herbimycin A to produce TNF-α without a first stimulation was examined. In this case, the cells were able to respond to the late stimulation, showing that herbimycin A treatment was inefficient for altering the cell response to the second stimulation. Although this PTK inhibitor significantly inhibits β-1,2-oligomannoside-dependent stimulation leading to TNF-α production, this treatment did not prevent down-regulation following multiple stimulations of the cells. Similar results have been obtained by West et al. (29), who have shown by using comparable approaches that the LPS-induced reprogramming was not related to a tyrosine phosphorylation-dependent pathway.
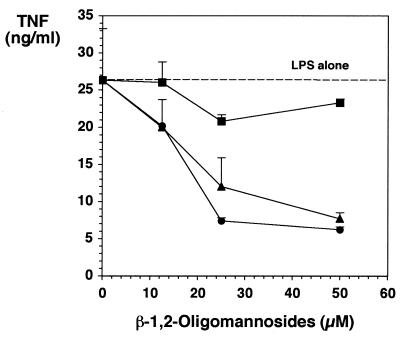
We then investigated whether the inhibitory effect we obtained after culturing the cells in the presence of β-1,2-oligomannosides could be related to the secretion of products in the cell-free supernatants of the β-1,2-oligomannoside-conditioned cells. Cells were thus incubated with different concentrations of β-1,2-oligomannosides. After 18 h, culture supernatants were collected (Fig. 1B). These conditioned media did not contain β-1,2-oligomannosides, since they did not lead to the production of TNF-α by fresh cells. Moreover, these media did not contain TNF-α, since they did not display any L929 lytic activities. Fresh cells were therefore incubated with these conditioned media for different periods of time before addition of LPS. Conditioned media from β-1,2-oligomannoside-treated cells gave rise to a strong inhibition of LPS-dependent TNF-α production by fresh cells (Fig. 2). This effect depended on the amount of β-1,2-oligomannosides used for producing the media, with a maximal effect observed for media from cells incubated with 50 μM β-1,2-oligomannosides. This inhibition also depended on the time of incubation of the cells with conditioned media before addition of the LPS, with a maximal effect (72% inhibition compared to cells incubated with LPS but in the absence of β-1,2-oligomannosides; P < 0.05; n = 3) obtained when conditioned media were added to the cells together with the LPS.
FIG. 2.
Effect of conditioned medium from β-1,2-oligomannoside-stimulated J774 cells on LPS-induced TNF-α production. Supernatants from J774 cells incubated for 18 h in the presence of different concentrations of β-1,2-oligomannosides were transferred onto fresh J774 cells for 3 h (■), 1 h (▴), or 0 h (●) before addition of LPS. After 5 h of further incubation, TNF-α production in cell-free supernatants was measured.
Several monocyte-derived immunomodulators that inhibit macrophage functions have been described (1, 15). Levitz et al. (15) have demonstrated that addition of either exogenous IL-10 or transforming growth factor β to human peripheral blood mononuclear cells inhibited C. albicans-induced TNF-α production by these cells. However, although not determined, the mechanism of C. albicans-induced inhibition of nitric oxide production by LPS-stimulated murine macrophages did not involve these immunosuppressive cytokines (5). Moreover, even though C. albicans cell wall mannoproteins with β-1,2-oligomannosides in their structure have been described as potent TNF-α inducers (25), they appear unable to stimulate cells to produce cytokines such as IL-10 (2).
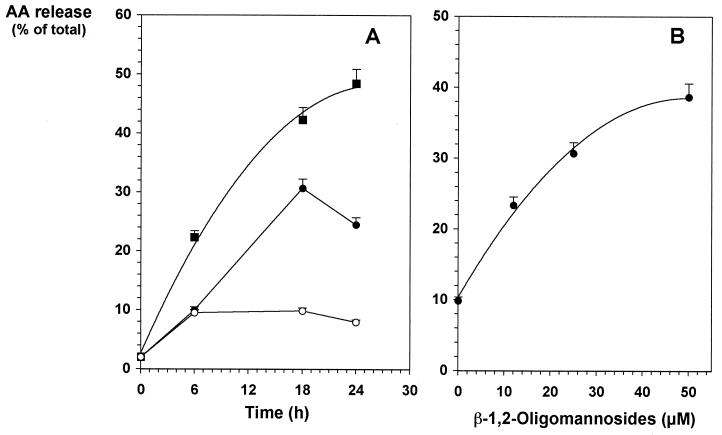
Arachidonic acid (AA) derivatives represent another important immunoregulator family whose release is induced by several microorganisms, including C. albicans. AA derivatives exert their immunosuppressive effects on different target cells, including B, T, and myeloid cells (17). Abnormal amounts of these immunoregulators have been shown to be produced in response to C. albicans infection (18), and their involvement in a disregulation of the immune response during the course of the infection has been demonstrated (30). It has already been evidenced that C. albicans cell wall phosphopeptidomannan stimulates the release of such lipid mediators by macrophages, and a participation in this process of the acid-labile fraction of the mannan has been suggested but not demonstrated (4). AA derivative synthesis has been reported to be independent of tyrosine phosphorylation but involves phosphorylation of serine proteins (21). Altogether, these observations led us to hypothesize that β-1,2-oligomannoside-induced desensitization of macrophages could be related to the secretion of such mediators by macrophages. [3H]AA-loaded J774 cells were incubated with medium alone or with different doses of either β-1,2-oligomannosides or LPS, which was used as a positive control for stimulation. After various incubation times over a 24-h period, culture supernatants were collected and the corresponding cells were extracted. As expected (24), when cells were stimulated with 0.1 μg of LPS per ml, significant amounts of [3H]AA were detected as soon as 6 h after incubation and reached a plateau corresponding to 50% of the total radioactivity after 24 h of incubation (Fig. 3A). Spontaneous release of [3H]AA did not exceed 10% of the total radioactivity. In contrast, 18 h of incubation with β-1,2-oligomannosides were needed to achieve optimal [3H]AA release (Fig. 3A). At this 18-h time point, [3H]AA release depended on the concentration of oligomannosides added to the cells and reached a plateau corresponding to 40% of the total radioactivity [(78 ± 5) × 103 cpm; n = 3] when the cells were incubated with the highest dose used for stimulation (50 μM) (Fig. 3B). Thus, macrophages liberate AA-derived products in a concentration- and time-dependent manner in response to stimulation by β-1,2-oligomannosides.
FIG. 3.
AA release by J774 cells after incubation with β-1,2-oligomannosides. (A) After incorporation of [3H]AA for 2.5 h, radiolabeled cells (5 × 105 cells) were incubated without stimulus (○) or with 0.1 μg of LPS (■) per ml or 25 μM β-1,2-oligomannosides (●). At different time points, [3H]AA release in the cell-free supernatants was measured. (B) Radiolabeled cells were incubated for 18 h with different concentrations of β-1,2-oligomannosides. [3H]AA release in the cell-free supernatants was then determined.
Altogether, these results suggest that the down-regulation of cell activity observed after the cells had been stimulated with β-1,2-oligomannosides is associated AA release. Further experiments are nevertheless needed to understand the exact mechanisms involved. However, these data bring to light new properties for β-1,2-oligomannosides in relation with the growing body of evidence, demonstrating their important role in the host-C. albicans interplay (3, 10, 12, 23).
Acknowledgments
We thank Jean-Claude Ameisen and Daniel Camus for their suggestions for experimental approaches and helpful discussions and Annie Robinet, John Boothroyd, and Donald W. R. Mackenzie for their help in the preparation of the manuscript.
REFERENCES
- 1.Alleva D, Burger C, Elgert K. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-α production. Role of tumor-derived IL-10, TGF-β, and prostaglandin E2. J Immunol. 1994;153:1674–1686. [PubMed] [Google Scholar]
- 2.Ausiello C M, Spagnoli G C, Boccanera M, Casalinuovo I, Malavasi F, Casciani C U, Cassone A. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol. 1986;22:195–202. doi: 10.1099/00222615-22-3-195. [DOI] [PubMed] [Google Scholar]
- 3.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–2624. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro M, Ralston N V, Morgenthaler T I, Rohrbach M S, Limper A H. Candida albicans stimulates arachidonic acid liberation from alveolar macrophages through alpha-mannan and beta-glucan cell wall components. Infect Immun. 1994;62:3138–3145. doi: 10.1128/iai.62.8.3138-3145.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinen T, Qureshi M H, Koguchi Y, Kawakami K. Candida albicans suppresses nitric oxide (NO) production by interferon-gamma (IFN-gamma) and lipopolysaccharide (LPS)-stimulated murine peritoneal macrophages. Clin Exp Immunol. 1999;115:491–497. doi: 10.1046/j.1365-2249.1999.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffee K, Halushka P, Ashton S, Tempel G, Wise W, Cook J. Endotoxin tolerance is associated with altered GTP-binding protein function. J Appl Physiol. 1992;73:1008–1013. doi: 10.1152/jappl.1992.73.3.1008. [DOI] [PubMed] [Google Scholar]
- 7.Danley D L, Polakoff J. Rapid killing of monocytes in vitro by Candida albicans yeast cells. Infect Immun. 1986;51:307–313. doi: 10.1128/iai.51.1.307-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel P, Lynch M, Sobel J. Candida-specific TH1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garner R E, Rubanowice K, Sawyer R T, Hudson J A. Secretion of TNF-α by alveolar macrophages in response to Candida albicans mannan. J Leukoc Biol. 1994;55:161–168. doi: 10.1002/jlb.55.2.161. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Kanbe T, Cherniak R, Cutler J. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouault T, Bernigaud A, Lepage G, Trinel P A, Poulain D. The Candida albicans phospholipomannan induces in vitro production of tumour necrosis factor-alpha from human and murine macrophages. Immunology. 1994;83:268–273. [PMC free article] [PubMed] [Google Scholar]
- 12.Jouault T, Fradin C, Trinel P A, Bernigaud A, Poulain D. Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid. J Infect Dis. 1998;178:792–802. doi: 10.1086/515361. [DOI] [PubMed] [Google Scholar]
- 13.Jouault T, Lepage G, Bernigaud A, Trinel P A, Fradin C, Wieruszeski J M, Strecker G, Poulain D. Beta-1,2-linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect Immun. 1995;63:2378–2381. doi: 10.1128/iai.63.6.2378-2381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami K, Zhang T, Qureshi M, Saito A. Cryptococcus neoformans inhibit nitric oxide production by murine peritoneal macrophages stimulated with interferon-γ and lipopolysaccharide. Cell Immunol. 1997;180:47–54. doi: 10.1006/cimm.1997.1166. [DOI] [PubMed] [Google Scholar]
- 15.Levitz S, Tabuni A, Nong S-H, Golenbock D. Effects of interleukin-10 on human peripheral blood mononuclear cell responses to Cryptococcus neoformans, Candida albicans, and lipopolysaccharide. Infect Immun. 1996;64:945–951. doi: 10.1128/iai.64.3.945-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meerpohl H, Bauknecht T. Role of prostaglandins on the regulation of macrophage proliferation and cytotoxic functions. Prostaglandins. 1987;31:961–966. doi: 10.1016/0090-6980(86)90026-2. [DOI] [PubMed] [Google Scholar]
- 18.Nelson R. Effects of Candida albicans mannans and mannan catabolites on human cell-mediated immune function in vitro. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanism. Tokyo, Japan: Saikon Publishing Co.; 1997. pp. 169–173. [Google Scholar]
- 19.Orlicek S L, Meals E, English B K. Differential effects of tyrosine kinase inhibitors on tumor necrosis factor and nitric oxide production by murine macrophages. J Infect Dis. 1996;174:638–642. doi: 10.1093/infdis/174.3.638. [DOI] [PubMed] [Google Scholar]
- 20.Redmond H P, Shou J, Gallagher H J, Kelly C J, Daly J M. Macrophage-dependent candidacidal mechanisms in the murine system. Comparison of murine Kupffer cell and peritoneal macrophage candidacidal mechanisms. J Immunol. 1993;150:3427–3433. [PubMed] [Google Scholar]
- 21.Serhan C, Haeggstrom J, Leslie C. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 22.Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as β-1,2-linked oligomannosyl residues. Infect Immun. 1992;60:4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki S. Immunochemical study on mannans of genus Candida structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr Top Med Mycol. 1997;8:57–70. [PubMed] [Google Scholar]
- 24.Tanamoto K-I. Induction of prostaglandin release from macrophages by bacterial endotoxin. Methods Enzymol. 1994;236:31–41. doi: 10.1016/0076-6879(94)36006-5. [DOI] [PubMed] [Google Scholar]
- 25.Trinel P, Faille C, Jacquinot P, Cailliez J, Poulain D. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect Immun. 1992;60:3845–3851. doi: 10.1128/iai.60.9.3845-3851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez T A, Balish E. Macrophages in resistance to candidiasis. Microbiol Mol Biol Rev. 1997;61:170–192. doi: 10.1128/mmbr.61.2.170-192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecchiarelli A, Puliti M, Torosantucci A, Cassone A, Bistoni F. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell Immunol. 1991;134:65–76. doi: 10.1016/0008-8749(91)90331-5. [DOI] [PubMed] [Google Scholar]
- 28.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West M, Bennet T, Seatter S, Clair L, Bellingham J. LPS pretreatment reprograms macrophage LPS-stimulated TNF and IL-1 release without protein tyrosine kinase activation. J Leukoc Biol. 1997;61:88–95. doi: 10.1002/jlb.61.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Witkin S, Hirsch J, Ledger W. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986;155:790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler-Heitbrock H, Blumenstein M, Kafferlein E, Kieper D, Petersmann I, Endres S, Flegel W, Northoff H, Riethmuller G, Haas J. In vitro desensitization to lipopolysaccharide suppresses tumor necrosis factor, interleukin-1 and interleukin-6 gene expression in a similar fashion. Immunology. 1992;75:264–268. [PMC free article] [PubMed] [Google Scholar]