A 55-year-old man with non-insulin-dependent diabetes, hypertension, and obesity (BMI, 35) presented with 5 days of progressive shortness of breath with exertion and bilateral lower leg pain. He had received a single-shot COVID-19 vaccine 1 week before presentation.
On arrival, his vital signs were temperature, 36.1 °C; pulse, 80 beats/min; BP, 160/101 mm Hg; respiratory rate, 28 breaths/min; oxygen saturation, 82% on room air. He was placed on 4 L nasal cannula. Relevant laboratory analysis included hemoglobin, 15.0 g/dL; creatinine, 1.1 mg/dL; troponin, 0.91 ng/mL; platelets, 176 (× 103/mL).
On examination, the patient appeared ill. He was tachypneic. He had clear lungs bilaterally and mild bilateral lower extremity edema. Point-of-care ultrasound (POCUS) examination of the heart was performed (Video 1).
Question: Based on the findings of the POCUS examination, what is the most likely diagnosis?
Answer: A mobile saddle pulmonary embolus (PE) is seen on the parasternal short axis (PSAX) view at the bifurcation of the main pulmonary artery (PA). A D-shaped interventricular septum along with RV (right ventricle) enlargement and dysfunction in PSAX and four-chamber views is seen. Tricuspid annular plane systolic excursion (TAPSE) is reduced.
The patient’s saddle PE was subsequently confirmed on CT angiography imaging with extension into lobar and segmental branches of all pulmonary lobes and evidence of RV strain (Fig 1 ). Further POCUS 2-point deep venous thrombosis examination showed a right popliteal deep venous thrombosis.
Figure 1.
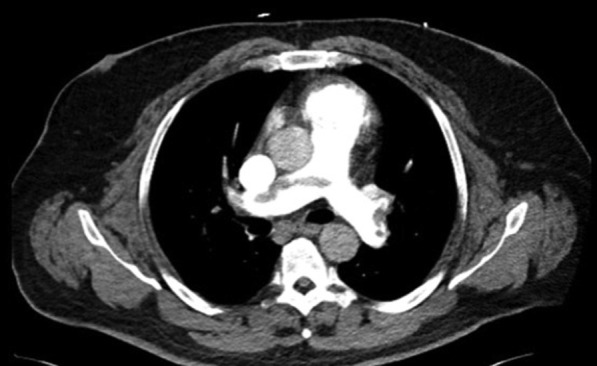
CT angiography of the chest, showing patient’s saddle pulmonary embolus with extension into right and left pulmonary arteries.
Given the patient’s low normal platelet count and recent COVID-19 vaccine, initial suspicion for vaccine-induced immune thrombotic thrombocytopenia was considered. He was started on argatroban infusion, and heparin products were avoided. Given his relatively low oxygen requirement and hemodynamic stability, a conservative approach was undertaken, and thrombolytics or catheter-directed approaches were not pursued.
The patient improved clinically over the next 72 h and was weaned off oxygen. Repeat POCUS at 72 h showed resolution of the saddle embolism at the bifurcation of the main PA, along with some improvement in RV function (TAPSE, 1.21 to > 1.41) (Video 2). Platelet count remained stable throughout hospitalization, and associated vaccine-induced immune thrombotic thrombocytopenia testing was negative. The patient was transitioned to apixaban and discharged on hospital day 3.
Discussion
Saddle PE visualization on transthoracic ultrasound is an infrequent finding, and only a small number of cases have been described.1, 2, 3, 4 A saddle PE is defined as a PE occurring at the bifurcation of the main pulmonary artery. Approximately 5% of patients with PE present with a saddle PE.5 Although most patients with saddle PE have RV dysfunction, overall mortality appears to be low and maybe not as deadly as traditionally thought.6 In a retrospective analysis, there was no significant difference in mortality or hospital length of stay between saddle PE and non-saddle PE groups; however, patients with saddle PE did have increased use of systemic thrombolysis for late (> 6 h after admission) decompensation.7 Patients with saddle PE can have a large clot burden, and thus early detection and treatment along with close clinical monitoring for decompensation is essential.
The PSAX view of the pulmonic valve and main pulmonary artery can sometimes be difficult to obtain, particularly in obese and tachypneic patients in a point-of-care setting. However, with practice and optimal patient positioning, the pulmonic valve and main PA bifurcation can be visualized in most patients with a POCUS examination. In patients with suboptimal parasternal views because of lung hyperinflation or body habitus, the main PA can be visualized from a subcostal short axis view as well. Although finding a saddle PE on POCUS is rare, we believe that rigorous evaluation with 2D (two-dimensional) and Doppler imaging of the right ventricular outflow tract (RVOT), pulmonic valve, and main PA up to and including the bifurcation should be attempted in all suspected patients with PE in addition to classic RV dysfunction parameters.
Video 1, along with showing the large saddle PE, illustrates several of these classic RV dysfunction parameters seen in PE. A D-shaped interventricular septum predominantly in systole, reflecting RV pressure overload, is visualized in PSAX. An increased RV:LV ratio (seen here increased approximately to 1:1), occurs because of a thin-walled RV that is unable to cope with the sudden increase in RV afterload caused by a PE. In addition, the RV longitudinal contraction is reduced, characterized by a decreased TAPSE, measured in M-mode from the apical four-chamber view.
Other indirect echocardiographic signs of PE using 2D and Doppler imaging such as McConnell’s sign and 60/60 sign are often relied on during POCUS to help diagnose PE, but no one sign has shown adequate diagnostic accuracy for a definitive diagnosis. Renewed interest has been seen in notching patterns of the RVOT pulse wave (PW) profile as another potential clue for PE. “Notching” of the PW signal is often found in pulmonary hypertension and can give insight into the hemodynamics of pulmonary vascular disease.8 Early systolic notching is an RVOT PW Doppler pattern that has been shown to be helpful in identifying massive and submassive PE.9 The early systolic notching “spike and dome” appearance may reflect the early arrival of pressure wave reflection caused by submassive and massive PE in the pulmonary vasculature.9 , 10 The patient exhibited this disturbed RVOT ejection pattern (Fig 2 ), and this was helpful to confirm our 2D findings. Qualitative RVOT PW Doppler waveforms can be interrogated in patients suspected of having submassive or massive PE.
Figure 2.
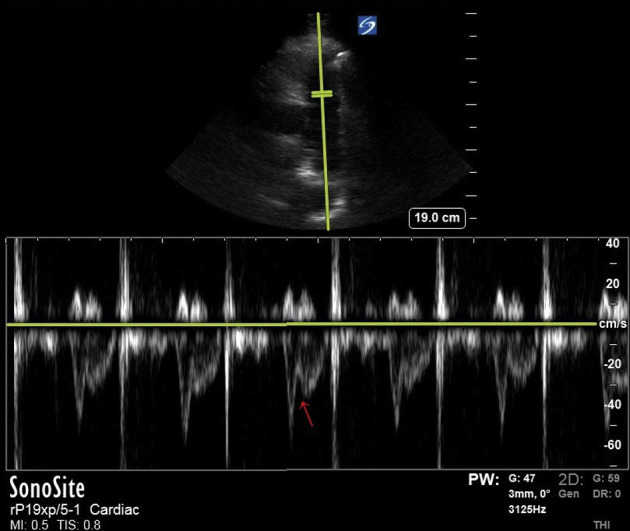
Early systolic notching (red arrow) pulse wave Doppler pattern seen in right ventricular outflow tract with “spike and dome” appearance.
We repeated the POCUS examination 72 h after initial presentation on the patient, which showed resolution of the saddle embolus. D-shaped septum and RV enlargement had not yet resolved, but TAPSE was improving (Video 2). Such follow-up is not routine or necessary with CT imaging, given risks associated with recurrent radiation and contrast exposure. The noninvasive nature of POCUS allows repeat examinations and, in this case, illustrates the effectiveness of anticoagulation for the treatment of PE. Repeating POCUS examinations also can be helpful in PE cases that are initially stable on admission, but then become acutely unstable later in the hospitalization. Rapid detection of worsening RV failure or other new causes of hemodynamic instability can be detected and treated early. See Narration Video for a detailed explanation of Videos 1 and 2.
Reverberations
-
1.
Saddle PE can occasionally be directly visualized with POCUS .
-
2.
Saddle PE is not always a massive PE or life-threatening .
-
3.
RVOT Doppler waveforms can be evaluated during POCUS for PE .
-
4.
Follow-up POCUS examinations in patients with PE may be helpful to monitor response to treatment or to detect new clinical changes .
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Additional information: Videos for this case are available under "Supplementary Data".
Supplementary Data
References
- 1.Secko M., Legome E., Rinnert S. Saddle embolism diagnosed by point-of-care transthoracic echocardiography before computed tomography angiogram of the chest. Am J Emerg Med. 2016;34(12):2467. doi: 10.1016/j.ajem.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Kanjanauthai S., Couture L.A., Fissha M., Gentry M., Sharma G.K. Saddle pulmonary embolism visualized by transthoracic echocardiography. J Am Coll Cardiol. 2010;56(11):e21. doi: 10.1016/j.jacc.2009.10.097. [DOI] [PubMed] [Google Scholar]
- 3.Baran J., Kabłak-Ziembicka A., Sobczyk D., Przewłocki T., Gackowski A. Saddle pulmonary embolism diagnosed by bedside transthoracic echocardiography. Pol Arch Intern Med. 2019;129(5):346–347. doi: 10.20452/pamw.4436. [DOI] [PubMed] [Google Scholar]
- 4.Letourneau M.M., Wilczynski S., Rao S., Osman A. Two-for-one saddles: a case of mobile double pulmonary embolism. CASE (Phila) 2018;2(2):66–68. doi: 10.1016/j.case.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardi A., Gluskin J., Guttentag A., Kotler M.N., Braitman L.E., Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011;39(11):2413–2418. doi: 10.1097/CCM.0b013e31822571b2. [DOI] [PubMed] [Google Scholar]
- 6.Ryu J.H., Pellikka P.A., Froehling D.A., Peters S.G., Aughenbaugh G.L. Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features and outcome. Respir Med. 2007;101(7):1537–1542. doi: 10.1016/j.rmed.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Alkinj B., Pannu B.S., Apala D.R., Kotecha A., Kashyap R., Iyer V.N. Saddle vs nonsaddle pulmonary embolism: clinical presentation, hemodynamics, management, and outcomes. Mayo Clin Proc. 2017;92(10):1511–1518. doi: 10.1016/j.mayocp.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Arkles J.S., Opotowsky A.R., Ojeda J., et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(2):268–276. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 9.Afonso L., Sood A., Akintoye E., et al. A Doppler echocardiographic pulmonary flow marker of massive or submassive acute pulmonary embolus. J Am Soc Echocardiogr. 2019;32(7):799–806. doi: 10.1016/j.echo.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Bernard S., Namasivayam M., Dudzinski D.M. Reflections on echocardiography in pulmonary embolism—literally and figuratively. J Am Soc Echocardiogr. 2019;32(7):807–810. doi: 10.1016/j.echo.2019.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


