Highlights
-
•
Brain-behavior relations and neuroplasticity in aphasia in first months post stroke.
-
•
Focus on damaged left Arcuate Fasciculus, but also ventral and intact right tracts.
-
•
Confirmation of dual-stream model: dorsal-phonological and ventral-semantic relations.
-
•
Neuroplasticity: early decrease in connection density (FBC) of Arcuate Fasciculus.
-
•
Acute FBC did not predict later language when initial language was accounted for.
Keywords: Post stroke aphasia, Early neuroplasticity, Predicting language recovery, Spherical deconvolution tractography, Arcuate fasciculus, Inferior fronto-occipital fasciculus
Abbreviations: AF, Arcuate Fasciculus; AR, Autoregressive; CSD, Constrained Spherical Deconvolution; DTI, Diffusion Tensor Imaging; FA, Fractional Anisotropy; FBC, Fiber Bundle Capacity; FLAIR, Fluid-Attenuated Inversion Recovery; HARDI, High Angular Resolution Diffusion Imaging; IFOF, Inferior Fronto-Occipital Fasciculus; ILF, Inferior Longitudinal Fasciculus; NIHSS, National Institutes of Health Stroke Scale; PWA, Patient(s) With Aphasia; SLF, Superior Longitudinal Fasciculus; UF, Uncinate Fasciculus
Abstract
A disruption of white matter connectivity is negatively associated with language (recovery) in patients with aphasia after stroke, and behavioral gains have been shown to coincide with white matter neuroplasticity. However, most brain-behavior studies have been carried out in the chronic phase after stroke, with limited generalizability to earlier phases. Furthermore, few studies have investigated neuroplasticity patterns during spontaneous recovery (i.e., not related to a specific treatment) in the first months after stroke, hindering the investigation of potential early compensatory mechanisms. Finally, the majority of previous research has focused on damaged left hemisphere pathways, while neglecting the potential protective value of their right hemisphere counterparts for language recovery. To address these outstanding issues, we present a longitudinal study of thirty-two patients with aphasia (21 males and 11 females, M = 69.47 years, SD = 10.60 years) who were followed up for a period of 1 year with test moments in the acute (1–2 weeks), subacute (3–6 months) and chronic phase (9–12 months) after stroke. Constrained Spherical Deconvolution-based tractography was performed in the acute and subacute phase to measure Fiber Bundle Capacity (FBC), a quantitative connectivity measure that is valid in crossing fiber regions, in the bilateral dorsal arcuate fasciculus (AF) and the bilateral ventral inferior fronto-occipital fasciculus (IFOF). First, concurrent analyses revealed positive associations between the left AF and phonology, and between the bilateral IFOF and semantics in the acute – but not subacute - phase, supporting the dual-stream language model. Second, neuroplasticity analyses revealed a decrease in connection density of the bilateral AF – but not the IFOF – from the acute to the subacute phase, possibly reflecting post stroke white matter degeneration in areas adjacent to the lesion. Third, predictive analyses revealed no contribution of acute FBC measures to the prediction of later language outcomes over and above the initial language scores, suggesting no added value of the diffusion measures for language prediction. Our study provides new insights on (changes in) connectivity of damaged and undamaged language pathways in patients with aphasia in the first months after stroke, as well as if/how such measures are related to language outcomes at different stages of recovery. Individual results are discussed in the light of current frameworks of language processing and aphasia recovery.
1. Introduction
In patients with aphasia after stroke, functional brain reorganization coincides with behavioral improvement (Hartwigsen and Saur, 2019, Kiran et al., 2019). However, little is known on structural reorganization of white matter tracts supporting language, especially not in the first months after stroke. Therefore, this study aims to fill this gap by examining white matter plasticity during the first months post stroke, and assessing its predictive value for later language outcomes.
Language is supported by large-scale networks made up of interactions between several brain regions along two processing streams (Fridriksson et al., 2016, Friederici, 2011, Hickok and Poeppel, 2004, Hickok and Poeppel, 2007). More specifically, the dual-stream model states that in neurotypical adults, phonological processing is driven by a left-lateralized dorsal stream, whereas semantic processing is driven by a bilateral ventral stream (Hickok and Poeppel, 2004, Hickok and Poeppel, 2007). At the structural level, we can use diffusion-weighted magnetic resonance imaging to non-invasively assess the main white matter tracts in the brain (Tournier et al., 2011). Relying on this technique, it has been indicated that for the dorsal stream the Arcuate Fasciculus (AF) is very important, especially given that its long segment (which directly connects posterior temporal and inferior frontal regions) seems to underlie phonological processing (Duffau, 2008, Glasser and Rilling, 2008, Vandermosten et al., 2012). For the connectivity within the ventral stream, the Inferior Fronto-Occipital Fasciculus (IFOF) plays a key role and has typically been linked with orthographic (Epelbaum et al., 2008, Vandermosten et al., 2012) and multimodal semantic processing (Martino et al., 2013, Turken and Dronkers, 2011). The white matter network for language is more complex than these two tracts (Dick and Tremblay, 2012, Tremblay and Dick, 2016), given that the dorsal stream is actually composed of several segments of the AF (and/or Superior Longitudinal Fasciculus [SLF]) and the ventral stream consists of other tracts in addition to the IFOF, such as the Inferior Longitudinal Fasciculus (ILF) and the Uncinate Fasciculus (UF) (Dick and Tremblay, 2012, Nakajima et al., 2020, Tremblay and Dick, 2016). However, the long AF segment and the IFOF are crucial as they directly connect inferior frontal (Broca’s) and posterior temporal (Wernicke’s) regions.
In persons with aphasia, several diffusion-derived measures of both white matter pathways have been related to concurrently measured language impairments after stroke, mostly in the chronic phase (for exceptions, see Kümmerer et al., 2013, Zhang et al., 2021a). A recent systematic review and meta-analysis of such studies (Zhang et al., 2021b) showed that, across studies, properties of the left ventral tracts – but not the dorsal tracts – significantly correlated with general aphasia severity, language comprehension, naming and reading ability, with the strongest correlations for the left IFOF. In contrast, the properties of the left dorsal AF significantly correlated with repetition – mainly involving phonological processing – and syntactic processing, while the IFOF did not. The latter converges with the dual-stream model presented above (Hickok and Poeppel, 2004, Hickok and Poeppel, 2007). However, the observed concurrent correlations between the dual-stream pathways and language behavior were almost all obtained in the chronic phase, hence they could also be the result of neural reorganization during language recovery.
Language intervention studies have indeed demonstrated that the brain is plastic after stroke, with especially evidence for the dorsal AF (Breier et al., 2011, van Hees et al., 2014, Schlaug et al., 2009, Wan et al., 2014, Zipse et al., 2012). However, these neuroplasticity studies have again mainly been carried out in the chronic phase and have all studied neuroplasticity in an intervention context, while information on white matter plasticity during “spontaneous” language recovery, i.e., not deliberately induced by a specific treatment in a research context, is missing (Cocquyt et al., 2017). Neuroplasticity patterns might be qualitatively different depending on the time post stroke and depending on experience, learning, and training (Gerstenecker and Lazar, 2019, Kiran and Thompson, 2019). In the acute and subacute phase, neural repair mechanisms, including resolution of diaschisis, are assumed to operate along neuroplastic processes adaptable to environmental input, such as highly targeted language interventions (Kleim, 2011). In the chronic phase, repair processes have more or less reached a stable state, whereas neuroplastic processes underlying the dynamic reorganization of the language network can continue for the duration of the patient’s life (Gerstenecker and Lazar, 2019, Kiran and Thompson, 2019). Thus, also in the field of white matter plasticity, studies in the acute and subacute phase after stroke are needed to complement the chronic results, in addition to studies on spontaneous language recovery.
Connectivity information has also been studied in a predictive context, in which diffusion-derived measures of bilateral dual-stream pathways are used to predict later language outcomes (Hope et al., 2013, Hope et al., 2018). In the left hemisphere, disruption (preservation) of the dorsal AF has been shown to be negatively (positively) associated with naming recovery (Bonilha et al., 2016, Hillis et al., 2018). This corresponds with previous findings that the degree to which the left AF can be reconstructed shortly after stroke (reflecting AF-specific damage) is related to language outcomes 6 months later (Kim and Jang, 2013) and that the loss of leftward asymmetry of the AF in acute stroke patients with left middle cerebral artery infarcts is related to the presence of aphasia at hospital discharge (Hosomi et al., 2009). In another study, diffusion-derived measures of the left ventral IFOF have been related to naming treatment success, over and above lesion volume (Meier et al., 2019).
Although most predictive studies have focused on left hemisphere predictors related to the brain damage, diffusion-derived measures of intact right-hemispheric tracts might carry information on the compensatory potential of a person (Osa García et al., 2020). Forkel and colleagues (Forkel et al., 2014, Forkel and Catani, 2018) showed that the acute volume of the long segment of the right AF was an important positive predictor for language outcomes 6 months after stroke. The role of both hemispheres in aphasia recovery remains up for debate (Cocquyt et al., 2017, Saur et al., 2006), with the exact interactions between both hemispheres presumably depending on factors such as the time post stroke, premorbid language lateralization, the nature of the language task, aphasia severity, lesion size and lesion location (Cocquyt et al., 2017, Hartwigsen and Saur, 2019, Kiran and Thompson, 2019). Clearly, more research is needed to define the – possible protective – role of intact right-hemispheric tracts in aphasia recovery.
An important methodological limitation of the existing body of work is the use of the diffusion tensor model (DTI) (Basser et al., 1994) to investigate white matter tracts. This can lead to erroneous conclusions in regions of crossing fibers because only one fiber direction per voxel can be estimated (Jones, 2009). This is particularly so for research on the AF and the IFOF, two long association fibers which cross several other pathways along their length. In addition, although fractional anisotropy (FA) is the most commonly derived tensor-based metric, it cannot disentangle microstructural (e.g., axon density, myelination) and macrostructural (e.g., axon architecture) tissue properties (Jones et al., 2013). By combining advanced non-tensor methods, such as multi-shell multi-tissue Constrained Spherical Deconvolution (CSD), with anatomically and microstructurally informed tractography algorithms, multiple fiber directions can be successfully disentangled within one voxel (Jeurissen et al., 2014, Tournier et al., 2004) and reliable and valid metrices can be derived to quantify connection density of a given pathway (Smith et al., 2020, Smith et al., 2012).
In summary, there remain quite a few knowledge gaps. Concerning aphasia-related neuroplasticity, most structural connectivity studies have been carried out in patients with chronic aphasia and have investigated intervention-related plasticity. Hence, we do not know what happens in earlier phases post stroke and during “spontaneous” (vs. treatment-related) aphasia recovery. Moreover, while the available studies have focused on the left AF, connectivity and neuroplasticity in the ventral IFOF and the right-hemispheric counterparts during language recovery are understudied. In this study, we aimed to address these shortcomings. By using a longitudinal design, we first investigated concurrent brain-behavior associations in the acute and subacute phase post stroke. We hypothesized that connectivity of the left dorsal AF is related to the phonological deficit, while connectivity of the bilateral IFOF is related to the semantic deficit. Second, we assessed early “spontaneous” neuroplasticity patterns in the dorsal AF and ventral IFOF and if/how these changes are related to language outcomes. Because to our knowledge, this has not been investigated yet, we did not formulate specific hypotheses. Third, we investigated the role of acute connectivity of the bilateral AF and IFOF in the prediction of later language outcomes. We expected a negative role of the damaged left AF and a positive role of the intact right AF in the prediction of later language outcomes, but no specific hypotheses were formulated regarding the IFOF. To address these outstanding issues, we used multi-shell multi-tissue CSD, which is optimized to quantify connectivity density in regions with crossing white matter fibers, as is the case in the language regions under investigation.
2. Materials and methods
2.1. Participants
This project was approved by the Medical Ethical Committee of the University Hospitals and University of Leuven (registration number B322201731747). Informed consent was obtained from all patients and/or their relatives. Details on patient recruitment are provided in Supplementary Information. A flowchart of patient recruitment is shown in Supplementary Fig. 1. Patients with a stroke lesion in the left hemisphere and a confirmed language deficit were followed from the acute phase post stroke (1–2 weeks post stroke, n = 65) to the chronic phase (9–12 months post stroke, n = 43), with an extra measurement in the subacute phase (3–6 months post stroke, n = 42). In the present longitudinal study, a subset of 32 patients with acute diffusion MRI and at least one behavioral follow-up moment was included. 27 of these patients also had a follow-up diffusion MRI scan. Table 1 shows the participant characteristics for this group.
Table 1.
Characteristics of the group of patients with aphasia under study.
| Variable | N = 32a | Median (Range) | NAb |
|---|---|---|---|
| Age (years) | 69.5 (10.6) | 70.5 (41.0–86.0) | |
| Sex (female/male) | 11/21 | ||
| Handedness (right-handed/other) | 28/4 | ||
| Education (years) | 13.8 (3.1) | 14.0 (8.0–22.0) | 1 |
| Stroke type (ischemia/hemorrhage) | 29/3 | ||
| Stroke laterality (left/bilateral) | 27/5 | ||
| History of stroke (no/yes) | 29/3 | ||
| Affected circulation area | |||
| ACM/ACP/Avert/Abas/multifocal | 22/5/1/1/3 | ||
| Acute lesion volume (cm³) | 44.49 (41.37) | 28.84 (1.12–149.51) | |
| Old lesion load (cm³) | 20.28 (16.84) | 14.91 (1.46–55.67) | |
| Acute NIHSS total score | 7 (6) | 4 (0–30) | |
| Days post stroke (acute)c | 5 (6) | 3 (0–29) | |
| Days post stroke (subacute)c | 117 (28) | 108 (85–185) | |
| Days post stroke (chronic)c | 287 (10) | 286 (272–315) |
aN is reported for categorical variables; M (SD) is reported for continuous variables.
bN indicates the number of participants for which the corresponding data are missing.
cFor behavioral language testing.
Note. ACM = arteria cerebri media, ACP = arteria cerebri posterior, Avert = arteria vertebralis, Abas = arteria basilaris, NIHSS = National Institutes of Health Stroke Scale (a higher score corresponds to a more severe stroke).
2.2. Procedure
In the acute phase, the ScreeLing (Visch-Brink et al., 2010) was administered to measure patients’ language impairment, and MRI data was acquired. The ScreeLing has been validated in an acute stroke population (Doesborgh et al., 2003, El Hachioui et al., 2012, El Hachioui et al., 2017) and is specifically designed to assess language functioning on three linguistic domains: semantics, phonology and syntax. Each domain is assessed with four tasks that each contain six test items. To assess dual-stream processing, only the subscores assessing phonological and semantic processing were considered. In the subacute phase, the ScreeLing (Visch-Brink et al., 2010) was readministered and follow-up MRI data were collected. Patients further completed a custom-made demographic questionnaire. Other measures collected, such as statistical learning measures, are outside the scope of this study and are reported elsewhere (Schevenels et al., 2022). In the chronic phase, the ScreeLing (Visch-Brink et al., 2010) was again readministered. Due to COVID-19, data collection in the acute phase was stopped prematurely, and follow-up moments were spread over a period of three months (subacute: 3–6 months post stroke, chronic: 9–12 months post stroke) instead of the foreseen 1 month (subacute: 3–4 months post stroke, chronic: 9–10 months post stroke).
2.3. Neuroimaging
2.3.1. Data acquisition
Image data were acquired on a 3-Tesla MRI system (Achieva dStream, Philips Medical Systems, Best, The Netherlands) using a 32-channel head coil. A multi-shell diffusion-weighted image series was acquired using a single-shot spin echo echo-planar imaging (EPI) sequence consisting of 20, 32 and 60 diffusion-sensitisation directions at b = 700, b = 1000 and b = 2000 s/mm2, respectively, with 7 b = 0 volumes. Other scan parameters were: 62 transverse slices, acquisition voxel size = 2.2 mm isotropic, reconstruction voxel size = 2.14 × 2.14 × 2.2 mm, TR/TE = 3593/88 ms, flip angle = 90°, multi-band factor = 2, EPI factor = 43, acquisition time = 7:38 min. In all but 2 patients, an additional pair of b = 0 images with reversed phase encoding was acquired immediately following the previous scan for estimation of the inhomogeneity field (Andersson et al., 2003). A T1-weighted anatomical contrast image for the purpose of tissue segmentation was acquired using a CS-SENSE TFE sequence with the following parameters: 240 sagittal slices, acquisition voxel size = 0.9 mm isotropic, reconstruction voxel size = 0.67 mm isotropic, TR/TE = 9.1/4.2 ms, acquisition time = 3:30 min. Finally, for the purpose of lesion segmentation, an additional diffusion-weighted sequence (1 b0 volume and 3 b1000 volumes) was acquired, as well as a Fluid-Attenuated Inversion Recovery (FLAIR) sequence. The FLAIR image was only acquired during the research scan if it had not been acquired clinically. For one patient, images were acquired on a different scanner (3-Tesla Philips Ingenia). A resting-state fMRI scan was additionally acquired but is outside the scope of this study and is not reported here.
2.3.2. Lesion segmentation
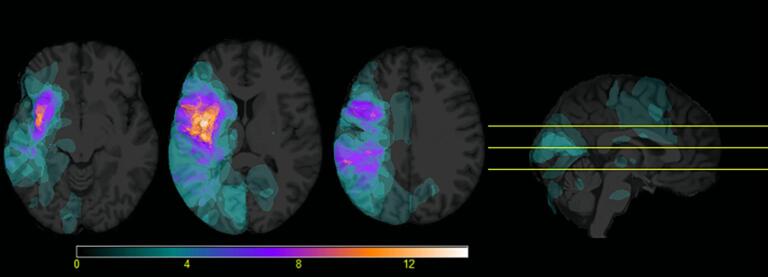
We created two lesion masks for every patient: an acute lesion mask and a full lesion mask. First, acute stroke lesions were manually delineated (by KS) in MRIcron (v. 02092019, available via https://www.nitrc.org/projects/mricron) and visually checked twice by a resident in neurology. Manual delineations were drawn on the FLAIR image (axial slices), guided by the diffusion-weighted image (b1000 and ADC [apparent diffusion coefficient]) for ischemic lesions. The acute lesion mask was used to determine acute lesion volume (in cm3) and to exclude recently damaged tissue from the tracts of interest (see further). Fig. 1 presents a lesion overlay image for the acute lesion masks.
Fig. 1.
Acute lesion overlay image (maximum overlap = 14) for the included participants (n = 32). Axial slices are shown in neurological orientation.
Second, we created a full lesion mask covering all burden of cerebrovascular disease. To do so, all FLAIR hyperintense lesions (acute stroke lesions, old stroke lesions as well as leukoaraiosis) were segmented using the Lesion Prediction Algorithm (Schmidt, 2017) as implemented in the Lesion Segmentation Toolbox version 3.0.0 (available via https://www.statistical-modelling.de/lst.html) for Statistical Parametric Mapping (SPM). The resulting map was then merged with the (manually drawn) acute lesion mask from the previous step, and manual delineations of old (FLAIR) hypointense stroke lesions where necessary. By subtracting the acute lesion mask from the full lesion mask, old lesion load (in cm3) was obtained.
2.3.3. Processing of anatomical images
T1-weighted images were used to anatomically inform the tractography algorithm. To achieve this, Virtual Brain Grafting (available via https://github.com/KUL-Radneuron/KUL_VBG) was run to generate lesion free T1-weighted images, which were subsequently fed to FreeSurfer’s recon-all (v. 6.0.0, available via https://surfer.nmr.mgh.harvard.edu/) (Fischl, 2012). We used the fully automated workflow provided by Virtual Brain Grafting (Radwan et al., 2021) to prevent FreeSurfer failure (Reid et al., 2016, Zhang et al., 2017) and to ensure reliable tissue parcellations in the presence of heterogeneous stroke lesions and leukoaraiosis (Dadar et al., 2021).
2.3.4. Processing of diffusion images
Multi-shell HARDI data were acquired to delineate the fibers of the bilateral dual-stream pathways under study. Diffusion images were first denoised (Cordero-Grande et al., 2019, Veraart et al., 2016a, Veraart et al., 2016b) and unringed (Kellner et al., 2016) in MRtrix3 (Tournier et al., 2019). Subsequently, images were corrected for (b0-paired) EPI distortions, B0-field inhomogeneities, eddy currents and inter-volume motion using topup and eddy tools in FMRIB Software Library (FSL, v. 6.0.1) (Jenkinson et al., 2012, Smith et al., 2004), called by MRtrix3′s preprocessing tool (Andersson et al., 2003, Andersson and Sotiropoulos, 2015, Bastiani et al., 2019, Holland et al., 2010, Smith et al., 2004). Finally, the images were corrected for bias fields (Tustison et al., 2010) and a brain mask was derived. The mean relative RMS value of the translational and rotational movement parameters was calculated by eddy (S)QUAD (Bastiani et al., 2019). This measure did not exceed 0.55 mm for any patient, for which reason no diffusion data was excluded from further analysis (Satterthwaite et al., 2012) (acute phase: M = 0.26 mm, SD = 0.07 mm; subacute phase: M = 0.24 mm, SD = 0.06 mm).
Following these preprocessing steps, fiber orientation distributions were computed using multi-shell multi-tissue CSD (default parameters) (Jeurissen et al., 2014, Tournier et al., 2004), with group averaged response functions for white matter, gray matter, and cerebrospinal fluid (Dhollander et al., 2016, Dhollander et al., 2019). To correct for global intensity differences between scans, intensity normalisation was performed (Dhollander et al., 2021, Raffelt et al., 2017b). Finally, to create the whole-brain tractogram, 10 million streamlines were generated using the probabilistic Second-order Integration over Fiber Orientation Distributions (iFOD2) algorithm (Tournier et al., 2010) with dynamic seeding and backtracking. We incorporated the anatomically-constrained tractography framework (Smith et al., 2012) (details in Supplementary Information) to improve biological plausibility of streamline generation. In addition, to ensure that streamline counts reliably reflect the underlying anatomical fiber density information, we performed Spherical deconvolution Informed Filtering of Tracts (SIFT2), resulting in a weight for every streamline (Smith et al., 2015).
2.3.5. Manual delineation of tracts of interest
To quantify connection density of the bilateral dorsal AF and the ventral IFOF, all four tracts were manually delineated in Trackvis (v 0.6.1.) according to Wakana’s protocol (Wakana et al., 2007). For each tract, two inclusion regions were drawn on the acute FA image (for details see tract #6 and tract #8 in Wakana et al., 2007). Because tractography in our study was based on a probabilistic algorithm using the underlying fiber orientation distributions, instead of a deterministic algorithm using the simpler diffusion tensor model (as in Wakana’s protocol), a maximum length threshold was specified for the AF (130 mm) and the IFOF (200 mm) to exclude spurious streamlines. To further exclude implausible streamlines, extra NOT regions were added. An overview of all inclusion and exclusion regions used for manual delineation of the tracts is provided in Supplementary Fig. 2. Importantly, the acute lesion mask was additionally used as an exclusion region (at both time points) for all tracts of interest (Gleichgerrcht et al., 2017). This was done because at the time of scanning, the patients varied in time post stroke from 1 day to 31 days, implying that there was some variability in whether damaged fibers already had degenerated. In the subacute phase, the acute lesion mask was again excluded to keep results between time points comparable, except for three patients (including two patients with hemorrhagic stroke), for whom a (newly delineated) subacute lesion mask was excluded because of considerable changes in their lesion.
To quantify connectivity, the streamline weights of the tracts of interest were summed and scaled with a patient-specific coefficient, which centered the distribution of weights around unity and consequently enabled comparison across patients. We will refer to this derived measure of connectivity throughout the paper as Fiber Bundle Capacity (FBC) (Smith, 2022). In short, FBC specifically addresses the limitations of raw streamline count as a metric of connectivity in the context of quantitative tractography. Ideally, FBC represents the total fiber intra-axonal cross-sectional area of a pathway, which should be a reasonable proxy of its information transfer capacity (Smith, 2022). In our dataset, FBC was highly correlated with the number of streamlines (r =0.96) and tract volume (r =0.97), but less with fractional anisotropy (r = − .33). Eventually, we ended up with four FBC measures per patient per time point (2 tracts × 2 hemispheres).
2.4. Statistical analysis
All statistical analyses were performed in R (v. 4.1.1) (R Core Team, 2021). A matrix representing pairwise correlations between all (dependent and independent) variables under study is provided in Supplementary Fig. 3. Prior to the statistical analyses, missing behavioral data were imputed for three variables: acute phonology score (1 data point missing), chronic phonology score (1 data point missing) and chronic semantics score (1 data point missing). Data were considered to be Missing At Random (details in Supplementary Information) and imputed using multivariate imputation by chained equations (MICE) as implemented in the mice package (Azur et al., 2011, van Buuren and Groothuis-Oudshoorn, 2011).
To characterize the patients’ behavioral recovery patterns, improvements in phonology and semantics over time were first investigated. Then, the main aims of this study were addressed. The first aim, ‘concurrent analyses’, was to assess concurrent associations between FBC of the dual-stream pathways under study and behavioral measures targeting phonological and semantic processing in the acute and subacute phase. To address this aim, we assessed to what extent acute (resp. subacute) language subscores could be explained by the acute (resp. subacute) FBC. Functional selectivity of the observed brain-behavior associations was further investigated with linear mixed effect models with random intercepts for patients and fixed effects for FBC, language component (semantics or phonology) and the interaction between both. The second aim, ‘neuroplasticity analyses’, was to assess early spontaneous neuroplasticity in the bilateral dorsal AF and ventral IFOF and its relation to language outcomes. To address this aim, we compared FBC of each tract between the acute and subacute phase, and assessed how the change in FBC was related to subacute and chronic language outcomes, controlling for acute language outcomes. By taking into account the initial language scores, we controlled for the autoregressive effect, i.e., the effect of an initial measurement on itself measured at a later time point. In this way, we could rule out that a possible predictive effect of change in FBC on later language outcomes was simply due to correlations between change in FBC and acute language subscores (Selig and Little, 2012). The third aim, ‘early prediction analyses’, was to assess the role of early connectivity of the bilateral dual-stream pathways under study in the prediction of later language outcomes. To address this aim, we assessed whether subacute or chronic language outcomes could be predicted by acute FBC. As in aim two, we again controlled for acute language outcomes to take into account the autoregressive effect.
All significant results were checked for their robustness when controlling for age, acute lesion volume, old lesion load and days post stroke at which the dependent (behavioral) variable was collected. To avoid overfitting, all four covariates were separately added to the corresponding model. The contribution of the variable of interest was then assessed over and above all significant covariates.
2.5. Data availability
The pseudonymized study data and code to reproduce the figures and findings of this study are publicly available at https://github.com/kschevenels/pwawm. Please note that the MRI data cannot be shared under any circumstance, as lesioned MRI data are person-specific and therefore cannot be considered anonymous.
3. Results
3.1. Language recovery over time
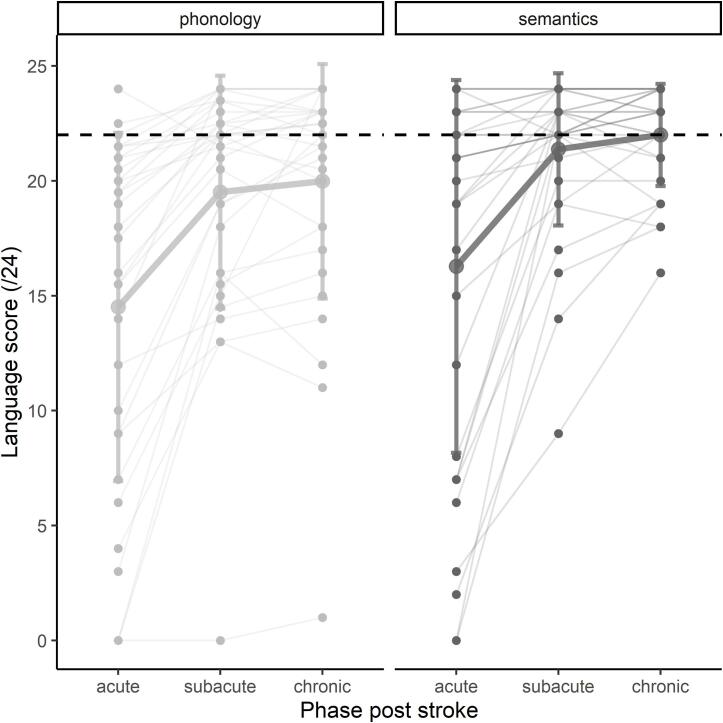
Fig. 2 provides the data on the phonology and semantics measures in the acute, subacute and chronic phase. Recovery of phonology and semantics over time was investigated with a linear mixed effect model with random intercepts for patients, two categorical main effects (language component and time point) as well as the interaction between both. There was a main effect of time point (F(2, 155) = 42.72, p < .001) and language component (F(1, 155) = 11.44, p = .001), but the interaction between both was not significant (F(2, 155) = 0.06, p = .947). For both language components, patients improved significantly from the acute to the subacute phase (t1-t2 = -4.95, 95% CI [-6.50, −3.40], t(1 5 5) = -7.55, p < .001), but not from the subacute to the chronic phase (t2-t3 = -0.56, 95% CI [-2.12, 1.00], t(1 5 5) = -0.85, p = .674). The semantic score was significantly higher than the phonology score across all time points (mean difference = -1.81, 95% CI [-2.87, −0.75], t(1 5 5) = -3.38, p = .001).
Fig. 2.
Language recovery over time. Individual data points and trajectories are shown, thick lines represent corresponding means with error bars at ± 1 SD. Dashed black lines represent the cut-off values for the tasks: a score lower than 22 can be interpreted as a phonological (left) or semantic (right) deficit.
3.2. Aim 1: Concurrent analyses
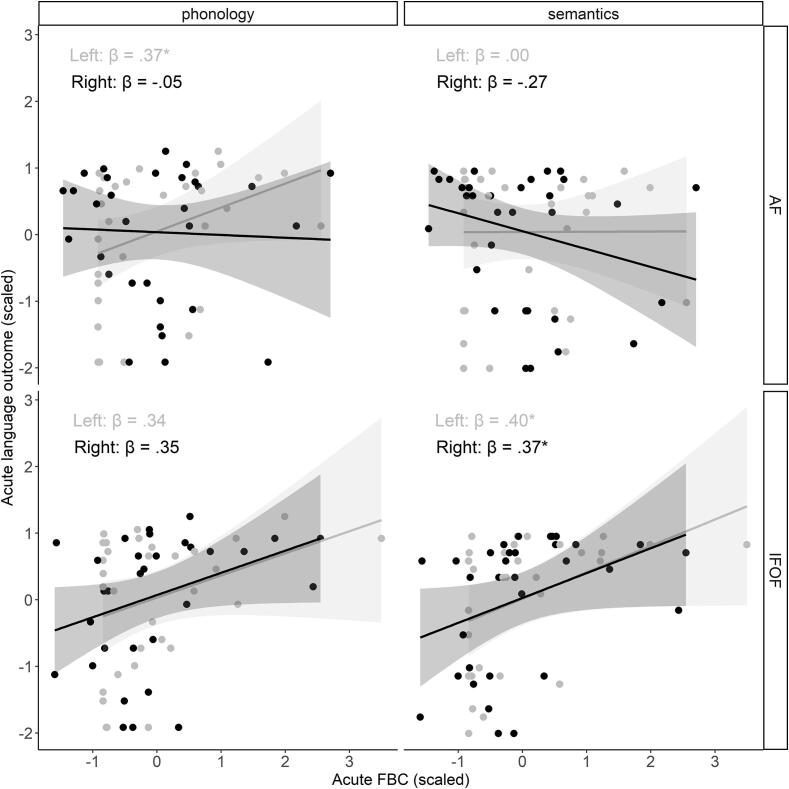
Concurrent analyses were performed with robust iterated re-weighted least squares regression to diminish the influence of extreme values in our modest sample, using the rlm function from the MASS package in R (Venables and Ripley, 2002). Concurrent regressions in the acute phase (Table 2, Fig. 3) indicated that the FBC of the left dorsal AF significantly contributed to the phonological model, but not the semantic model. The FBC of the right dorsal AF did neither significantly contribute to the phonological model, nor to the semantic model. On the other hand, the FBC of the left and right ventral IFOF significantly contributed to the semantic model, but not to the phonological model. The found concurrent effects in the left hemisphere disappeared when acute lesion volume – the only significant covariate – was added to the models (left AF – phonology: = 0.23, SE = 0.18, p = .210; left IFOF – semantics: = 0.22, SE = 0.19, p = .262), while the effect in the right hemisphere trended (right IFOF – semantics: = 0.33, SE = 0.16, p = .056).
Table 2.
Robust regression results of the concurrent analyses in the acute phase (without covariates). Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | .37 | 0.18 | .049 | .00 | 0.19 | .986 |
| Right | -.05 | 0.19 | .779 | -.27 | 0.18 | .149 | |
| IFOF | Left | .34 | 0.18 | .066 | .40 | 0.17 | .029 |
| Right | .35 | 0.18 | .063 | .37 | 0.17 | .037 | |
Fig. 3.
Concurrent relations in the acute phase in both hemispheres (gray = left hemisphere, black = right hemisphere). Robust regression lines with corresponding 95% confidence intervals are shown on the plot.
The functional selectivity of the observed association in the acute phase between left AF and phonology, and between bilateral IFOF and semantics was further investigated with three linear mixed effect models. In the model for the left AF, a significant interaction effect between FBC and language component (F(1, 30) = 10.30, p = .003) indicated that the relationship between the left AF and phonology was significantly stronger than the relationship between the left AF and semantics (fon-sem = 4.87, 95% CI [1.77, 7.96], t(30) = 3.21, p = .003). In contrast, in the models for the IFOF, the interaction terms were not significant (left IFOF: F(1, 30) = 0.33, p = .569; right IFOF: F(1, 30) = 0.18, p = .678), indicating that the relationship between the IFOF and semantics was not significantly stronger than the relationship between the IFOF and phonology (left IFOF: fon-sem = -7.73, 95% CI [-35.10, 19.65], t(30) = -0.58, p = .569; right IFOF: fon-sem = -5.56, 95% CI [–32.66, 21.55], t(30) = -0.42, p = .678).
In the subacute phase, we found no significant concurrent contributions of FBC of the dual-stream pathways under study to the semantic or phonological models (Table 3).
Table 3.
Robust regression results of the concurrent analyses in the subacute phase (without covariates). Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | -.01 | 0.15 | .955 | -.11 | 0.10 | .282 |
| Right | -.10 | 0.16 | .531 | -.17 | 0.10 | .111 | |
| IFOF | Left | .16 | 0.16 | .323 | .20 | 0.10 | .066 |
| Right | .11 | 0.15 | .481 | .09 | 0.10 | .358 | |
3.3. Aim 2: Neuroplasticity analyses
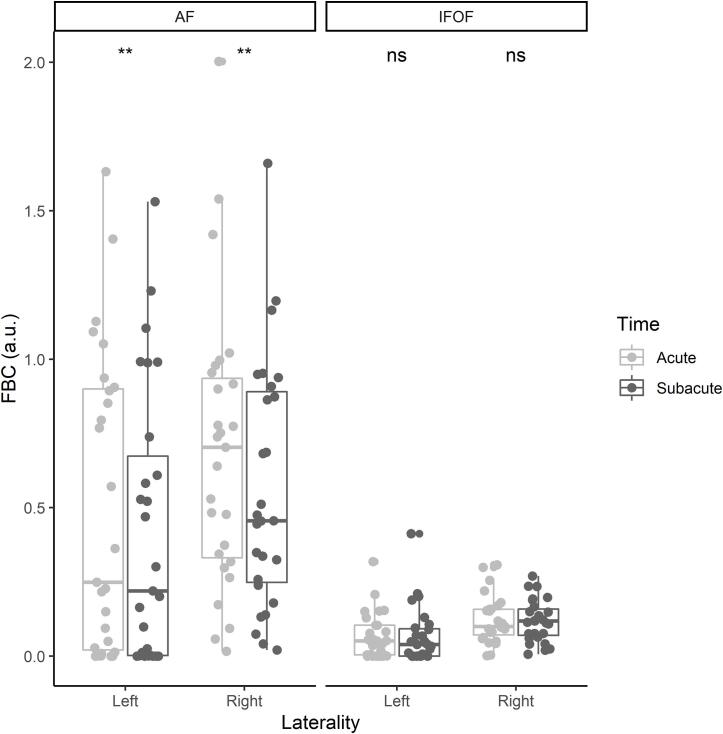
Fig. 4 shows the FBC of all four dual-stream pathways under study in the acute and subacute phase after stroke. Paired Wilcoxon signed-rank tests indicated that FBC of the left (W = 278, p = .002, Wilcoxon effect size r = 0.61) and right dorsal AF (W = 303, p = .005, Wilcoxon effect size r = 0.53) significantly decreased over time, while FBC of the bilateral ventral IFOF did not significantly change over time (left IFOF: W = 178, p = .230, Wilcoxon effect size r = 0.29; right IFOF: W = 208, p = .662, Wilcoxon effect size r = 0.09). For this reason, we only assessed how the change in FBC was related to subacute and chronic language outcomes for the bilateral dorsal AF.
Fig. 4.
Connection density in the bilateral dorsal AF and ventral IFOF throughout the acute and subacute phase after stroke (a.u. = arbitrary units).
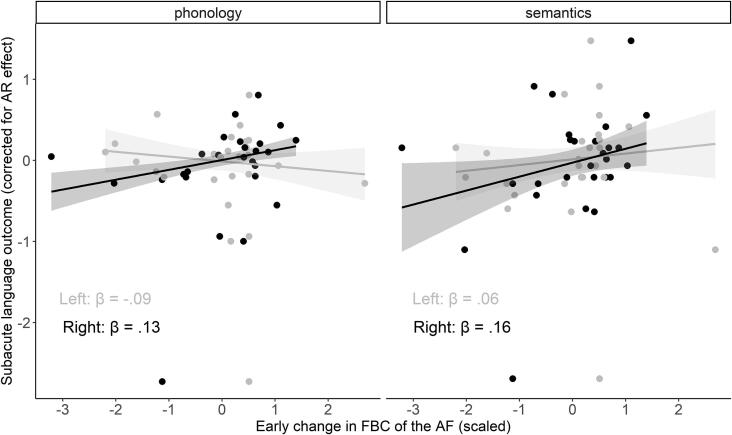
With robust regression analyses (such as for the concurrent analyses presented above), we did not find a significant contribution of an early (i.e., from acute to subacute) change in FBC of the left or right dorsal AF to subacute phonology or semantics scores, over and above the autoregressive effect. However, the effect of the FBC change in the right AF on subacute phonology scores trended (p = .054) (Table 4, Fig. 5). There were no significant covariates for the latter model. Similarly, in the chronic phase, the early change in FBC of the AF did not significantly predict chronic phonology or semantics scores, over and above the autoregressive effect (Table 5).
Table 4.
Robust regression results of the predictive (change in connectivity to subacute language) analyses (without covariates), corrected for the autoregressive effect. Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | -.09 | 0.08 | .246 | .06 | 0.10 | .561 |
| Right | .13 | 0.06 | .054 | .16 | 0.10 | .147 | |
Fig. 5.
Predictive relations between the connectivity change in the bilateral AF and subacute language outcomes corrected for the autoregressive (AR) effect (gray = left hemisphere, black = right hemisphere). Robust regression lines with corresponding 95% confidence intervals are shown on the plot.
Table 5.
Robust regression results of the predictive (change in connectivity to chronic language) analyses (without covariates), corrected for the autoregressive effect. Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | .06 | 0.12 | .614 | .03 | 0.13 | .809 |
| Right | .09 | 0.13 | .498 | .11 | 0.12 | .343 | |
3.4. Aim 3: Early prediction analyses
The results of the robust regression analyses indicated that the acute FBC of the dual-stream pathways under study could not significantly predict neither subacute (Table 6), nor chronic (Table 7) phonology or semantic scores, over and above the autoregressive effect of acute language scores.
Table 6.
Robust regression results of the predictive (acute connectivity to subacute language) analyses (without covariates), corrected for the autoregressive effect. Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | -.02 | 0.07 | .805 | -.07 | 0.09 | .465 |
| Right | -.07 | 0.07 | .329 | -.12 | 0.10 | .218 | |
| IFOF | Left | .01 | 0.07 | .928 | .09 | 0.09 | .335 |
| Right | .03 | 0.07 | .677 | -.08 | 0.10 | .460 | |
Table 7.
Robust regression results of the predictive (acute connectivity to chronic language) analyses (without covariates), corrected for the autoregressive effect. Standardized beta coefficients, corresponding standard errors and (uncorrected) p-values are reported.
|
Phonology |
Semantics |
||||||
|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | ||
| AF | Left | -.06 | 0.12 | .621 | .04 | 0.12 | .707 |
| Right | -.13 | 0.11 | .244 | -.10 | 0.11 | .411 | |
| IFOF | Left | .05 | 0.12 | .683 | .01 | 0.12 | .951 |
| Right | .08 | 0.12 | .504 | -.20 | 0.11 | .069 | |
4. Discussion
We present a longitudinal study on concurrent and predictive relations as well as neuroplasticity of the bilateral dorsal AF and ventral IFOF in post stroke aphasia recovery. A main step forward is that we investigated longitudinal brain-behavior associations within the first months after stroke, and not in the chronic phase like most previous studies. Patients with aphasia were followed up from the acute phase (1–2 weeks) to the subacute (3–6 months) and chronic phase (9–12 months) after stroke, with MRI acquisition in the acute and subacute phase. In our group of patients, we found positive concurrent associations in the acute phase – but not the subacute phase – between connection density of the left dorsal AF and phonological performance, and between the bilateral ventral IFOF and semantic performance. Whereas connection density of the bilateral IFOF did not significantly change in the first months post stroke, a decrease was observed in bilateral AF, of which larger decreases in the right hemisphere tended to cohere with worse subacute phonological scores when controlling for the autoregressive effect. Acute connectivity of none of the dual-stream pathways predicted later language performance when controlling for the autoregressive effect.
As to the concurrent analyses, at first sight our results in the acute phase are in line with previous literature investigating brain-behavior associations (Zhang et al., 2021b) in the context of the dual-stream framework of language processing (Hickok and Poeppel, 2004, Hickok and Poeppel, 2007), i.e., connectivity of the left dorsal AF was positively associated with phonology performance, while connectivity of the bilateral ventral IFOF was positively associated with semantic performance. In the subacute phase, we found no significant concurrent brain-behavior associations, probably caused by the decrease in variability (i.e., ceiling effects) of the language scores over time as patients recover (part of) their language abilities. To test the functional specificity of the concurrent correlations in the acute phase, we conducted extra analyses that showed that the relationship between the left AF and phonology was significantly stronger than the relationship between the left AF and semantics, confirming the specialization of left AF for phonological processing. However, the relationship between the bilateral IFOF and semantics was not significantly stronger than the relationship between the bilateral IFOF and phonology, hence we can not conclude that the association between the ventral IFOF and semantics was very specific. Other studies in acute stroke patients within 1 month post stroke (Boukrina et al., 2015, Zhang et al., 2018) indeed suggest a broader role of the IFOF in language processing, including semantic processing but possibly also extending to phonological processing. Given that in our study the associations between IFOF and phonology did not reach significance, the (lack of) functional specialization of the IFOF remains to be further investigated in larger samples. As to the right dorsal AF, we did not find a clear role of this tract in language processing, similar to several reports on residual language processing in the chronic phase (Breier et al., 2008, Geva et al., 2015, Ivanova et al., 2016, Ivanova et al., 2021, Meier et al., 2019; but see Kourtidou et al., 2021 for different results) and two studies in the acute phase (Forkel et al., 2014, Osa García et al., 2020). Overall, our concurrent findings are in line with the dual-stream model showing left dorsal-phonological and bilateral ventral-semantic associations, although the specificity of this relation could only be confirmed for the left dorsal AF. Different from previous studies that used classic DTI, we used multi-shell multi-tissue CSD and a state-of-the-art workflow optimized for lesioned images to obtain reliable and biologically-relevant measures of connectivity strength, such as FBC. Although FBC is a relatively novel measure, the confirmation of the dual route correlations in our study validates its use.
Note that in the left hemisphere, the observed concurrent brain-behavior associations in the acute phase disappeared when lesion size was taken into account (Meier et al., 2019). This is not unexpected because the acute lesion mask was used as an exclusion region during tract segmentation. As FBC is proportional to the number of streamlines in the segmentation, lesioned tracts have a lower connection density. Previous studies have similarly demonstrated associations between structural damage to specific left-hemispheric tracts and language deficits after stroke (Geller et al., 2019, Meier et al., 2019, Yang et al., 2017). In the right hemisphere, there was still a marginally significant positive association between connectivity of the right IFOF and semantic performance after controlling for lesion size. To the best of our knowledge, this is the first study to suggest a possible immediate protective role for the right IFOF in acute aphasia after stroke. With our study, we have extended the traditional focus in aphasia research from the left arcuate fasciculus only to the inclusion of ventral and right-hemispheric tracts. This not only allowed us to examine associations and neural (re)organization in damaged tracts, but additionally in intact tracts that can assist in language function and recovery.
As to the neuroplasticity analyses, we found that connectivity of the bilateral AF decreased in the first months after stroke. It is known that tract volume tends to decrease with age later in life (Lebel et al., 2012). However, it seems unlikely that the connectivity decrease was caused by increasing age as it was only present for AF (not IFOF), and we observed no negative correlation between age and FBC of the AF (see Supplementary Fig. 3). The maximum lesion overlap in our patient group was localized in and around the insula, i.e., adjacent to the AF. Therefore, the FBC decrease could be related to post stroke white matter degeneration in areas adjacent to or (structurally or functionally) connected to the lesion (i.e., diaschisis) (Carrera and Tononi, 2014, Egorova et al., 2020). A recent article even demonstrated remote white matter atrophy in close proximity to the AF specific to patients with aphasia (Egorova-Brumley et al., 2022). In the future, it might be interesting to further explore this connectivity decrease with fixel-based analyses, which can disentangle microscopic intra-axonal changes from macroscopic cross-sectional changes (Raffelt et al., 2017a). A smaller connectivity decrease of the right AF was marginally related to better subacute phonology scores over and above the autoregressive effect, however, this effect remains to be validated in larger patient samples. We are aware of only two recent (DTI) studies that investigated early structural neuroplasticity in bilateral white matter tracts during language recovery (Bae et al., 2022, Blom-Smink et al., 2020). Bae et al. (2022) demonstrated a decrease in FA of the bilateral AF in a group of 35 patients with aphasia from 1 month to 6 months after stroke, with a decrease in the left AF being associated with worse language outcomes. Blom-Smink et al. (2020) investigated treatment-related FA changes within a 1-month interval across dorsal and ventral tracts and found no significant change in FA between scanning sessions, but decreased FA in the right ILF was associated with limited naming improvement or a slight decline (Blom-Smink et al., 2020). The results of these studies converge with ours in that in the subacute phase, in the majority of patients, there is a reduction in tract integrity, possibly reflecting (remote) white matter degeneration, and this tends to be associated with less behavioral improvement individually. Importantly, the direction of change is vastly different from intervention studies conducted in the chronic phase (Breier et al., 2011, van Hees et al., 2014, Schlaug et al., 2009, Wan et al., 2014, Zipse et al., 2012), which underlines the importance of also including the early stages of recovery and of investigating plasticity patterns which are representative of day-to-day clinical practice (i.e., independently from highly targeted interventions with high intensity and frequency).
As to the early prediction analyses, we found no specific contribution of acute connectivity of the dual-stream pathways under study to the prediction of later language outcomes, over and above information on the initial language deficit. This was against our expectations, because a few previous studies have demonstrated an early role for the left (Hosomi et al., 2009, Keser et al., 2020, Kim and Jang, 2013) and right AF (Forkel et al., 2014, Forkel and Catani, 2018) in the prediction of language outcomes 6 months after stroke. However, these studies did not control for the autoregressive effect of the initial language deficit, hence they did not predict the degree of change but rather the absolute language outcomes which are very dependent on the initial language deficit. For comparison purposes, we ran the same models without correcting for initial language scores (reported in Supplementary Information). These exploratory analyses showed a significant contribution of the left ventral IFOF to subacute semantics, i.e., the stronger the acute connectivity of the left IFOF, the higher the subacute semantic scores. This probably reflects a lagged effect of tract-specific lesion load, which was already demonstrated in our concurrent findings of the acute phase. In addition, we found a significant contribution of the right dorsal AF to subacute semantics, i.e., the stronger the acute connectivity in the right AF, the lower the subacute semantic scores. In our concurrent analyses, there was no significant association between the right AF and semantics in the acute phase, but the standardized regression coefficient ( = -.27, medium effect size) indicates that both measures shared at least some variance. The fact that the found predictive effect of the right AF for subacute semantic scores disappeared when we controlled for initial semantic scores, suggests that it was confounded by the shared variance of both measures in the acute phase. Hence, this demonstrates the importance of controlling for the autoregressive effect even when concurrent correlations are not significant. In sum, our results suggest that, similar to the findings in the study by Osa García et al. (2020), connectivity information does not improve the prediction of later language outcomes when acute language scores have been accounted for (Osa García et al., 2020).
Some limitations of this study should be recognized. Despite the large-scale screening of stroke patients admitted to the stroke unit of our university hospital during a 1.5 year period (n = 400), the number of included patients with aphasia who underwent an MRI scan twice (i.e., in the acute and subacute phase) was relatively modest (n = 27). Despite our best efforts, we mainly recruited patients with minor and moderate stroke and aphasia severity (as measured by the NIHSS), and only a few patients with severe stroke and aphasia. In order to not discard these difficult to collect and interesting data points, robust regression methods were applied to decrease the weight assigned to these relatively extreme data points in the analyses. Furthermore, longitudinal clinical data frequently suffer from ceiling effects, because the measurements have to be administered to patients with a wide range of language abilities who are recovering over time to different extents (Bowman et al., 2021). As previously mentioned, this has caused a decrease in variability especially at later follow-up moments, possibly inflating model output. We acknowledge this issue, which could be addressed in the future by creating behavioral scales with more sensitive upper ends or unbounded upper limits (Bowman et al., 2021).
In addition, due to the onset of the COVID-19 pandemic during data collection, follow-up moments were spread out over a period of three months instead of the planned one month. To consider this extra variability, the exact timing of those moments was also entered (but ultimately not retained, due to non-significance) in the analyses. In the same vein, compared to other studies, our inclusion criteria were less strict, including patients with ischemic as well as hemorrhagic lesions, left-handed and right-handed individuals, and patients with first-ever stroke as well as history of stroke. We made this choice to ensure that the longitudinal research carried out was clinically valid and the population under study was representative for patients seen in the daily clinical environment. Note that, although the heterogeneity of the lesions in our sample can make the interpretation of the data more complex, we have performed the latest MRI processing tools to carefully handle the presence of brain lesions in the brain images (Radwan et al., 2021, Smith et al., 2012), which is often lacking in previous aphasia studies. Due to the relatively small sample size, we were not able to study different patient subgroups separately. Although the covariate “old lesion load” (including old stroke lesion load) did not significantly contribute to any of the regression models, future (larger-scale) independent studies are crucial to validate our findings (Poldrack et al., 2020) and could check whether the found effects hold or differ in various subgroups of patients (Wilson et al., 2022), such as patients with and without stroke history, patients with different stroke types or language lateralization patterns.
Finally, a variety of other associative fronto-temporal, parieto-temporal, occipito-temporal and fronto-frontal white matter connections are somehow involved in language processing, including (ventrally) the ILF and UF and (dorsally) the different segments of the AF/SLF and the Frontal Aslant Tract. One could also argue that, next to these cortical-cortical connections, the cortical-subcortical circuitry should also be considered as part of the language network (Cahana-Amitay and Albert, 2014, Dick and Tremblay, 2012). Nevertheless, it was beyond the scope of this study to provide a complete mapping of the language network. Instead, we decided to focus on two specific white matter pathways, i.e., the AF and the IFOF, that connect Broca’s and Wernicke’s area dorsally and ventrally, and that have been most consistently linked with the dual-system account of language processing (Dick and Tremblay, 2012).
5. Conclusion
Our longitudinal study provides new insights on early (neuroplasticity in) connectivity of dual-stream pathways in patients with post stroke aphasia, as well as if/how such measures are related to language outcomes at different stages of recovery. The concurrent analyses showed brain-behavior correlations in accordance with dual-stream models of language processing, but driven by lesion size in the left hemisphere. Our neuroplasticity analyses revealed connectivity decreases in the bilateral AF, possibly related to post stroke white matter neurodegeneration. When controlling for the autoregressive effect, the early prediction analyses showed no specific contribution of acute connectivity of the AF or IFOF to the prediction of later language outcomes. With a state-of-the-art methodology that addresses important limitations of classic DTI (i.e., interpretability and validity in crossing-fiber regions), we have paved the way towards a better understanding of early brain-behavior associations after stroke. Eventually, such knowledge is necessary to inform neuroimaging-based prediction of long-term language outcomes in patients with aphasia.
CRediT authorship contribution statement
Klara Schevenels: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing – original draft, Project administration, Funding acquisition. Robin Gerrits: Methodology, Formal analysis, Writing – review & editing. Robin Lemmens: Resources, Writing – review & editing. Bert De Smedt: Supervision, Methodology, Writing – review & editing. Inge Zink: Supervision, Writing – review & editing. Maaike Vandermosten: Supervision, Conceptualization, Methodology, Project administration, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are most grateful to all patients admitted to the Stroke Unit between September 2018 and March 2020 and their families for their participation in our study in such a troubled time. In addition, we are thankful to the nursing and (para)medical staff at the Stroke Unit to make this collaboration possible, with a special thanks to the study MR nurses Stefan, Guido and Kris. We also truly appreciate the help during behavioral data collection of Pauline D’Hondt, Floor Vandecruys, Mara Barberis, Anneleen Heyns, Mouna Vanlommel, Louise Depourcq, Pauline Spoormans, Aline Smets, Jill Kries, Lissa Billast, Nathan Schaltin, Reinhard Arnou, Emilie Bartsoen, Hanna Van Vaerenbergh, Charlien Janssen and Laura Schillebeeckx. Finally, we would like to thank Laura Michiels, Mara Barberis and Merel Dillen for their help during the lesion delineation process; and Ahmed Radwan, Ramtin Mehraram, Thanh Vân Phan and Maria Economou for their helpful advice on processing of the MRI data.
Funding
This work was supported by the Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek), Award ID 1S81620N to Klara Schevenels.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103271.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2015;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azur M.J., Stuart E.A., Frangakis C., Leaf P.J. Multiple imputation by chained equations: what is it and how does it work? Int. J. Methods Psychiatr. Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C.R., Na Y., Cho M., Hwang Y.M., Tae W.S., Pyun S.B. Structural changes in the arcuate fasciculus and recovery of post-stroke aphasia: a 6-month follow-up study using diffusion tensor imaging. Neurorehabil. Neural Repair. 2022;36(9):633–644. doi: 10.1177/15459683221121752. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., Lebihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bastiani M., Cottaar M., Fitzgibbon S.P., Suri S., Alfaro-Almagro F., Sotiropoulos S.N., Jbabdi S., Andersson J.L.R. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184(May 2018):801–812. doi: 10.1016/j.neuroimage.2018.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom-Smink M., Verly M., Spielmann K., Smits M., Ribbers G.M., van de Sandt-Koenderman M.W.M.E. Change in right inferior longitudinal fasciculus integrity is associated with naming recovery in subacute poststroke aphasia. Neurorehabil. Neural Repair. 2020;34(9):784–794. doi: 10.1177/1545968320940982. [DOI] [PubMed] [Google Scholar]
- Bonilha L., Gleichgerrcht E., Nesland T., Rorden C., Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil. Neural Repair. 2016;30(3):266–279. doi: 10.1177/1545968315593808.Success. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukrina O., Barrett A.M., Alexander E.J., Yao B., Graves W.W. Neurally dissociable cognitive components of reading deficits in subacute stroke. Front. Hum. Neurosci. 2015;9(May):1–14. doi: 10.3389/fnhum.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman H., Bonkhoff A., Hope T., Grefkes C., Price C. Inflated estimates of proportional recovery from stroke: the dangers of mathematical coupling and compression to ceiling. Stroke. 2021;52(5):1915–1920. doi: 10.1161/STROKEAHA.120.033031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier J.I., Hasan K.M., Zhang W., Men D., Papanicolaou A.C. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion. Am. J. Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier J.I., Juranek J., Papanicolaou A.C. Changes in maps of language function and the integrity of the arcuate fasciculus after therapy for chronic aphasia. Neurocase. 2011;17(6):506–517. doi: 10.1080/13554794.2010.547505.Changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahana-Amitay D., Albert M.L. Brain and language: Evidence for neural multifunctionality. Behav. Neurol. 2014;2014:1–16. doi: 10.1155/2014/260381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E., Tononi G. Diaschisis: past, present, future. Brain. 2014;137(9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Cocquyt E., Ley L.D., Santens P., Borsel J.V., Letter M.D. The role of the right hemisphere in the recovery of stroke-related aphasia: a systematic review. J. Neurolinguistics. 2017;44:68–90. doi: 10.1016/j.jneuroling.2017.03.004. [DOI] [Google Scholar]
- Cordero-Grande L., Christiaens D., Hutter J., Price A.N., Hajnal J.V. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage. 2019;200(March):391–404. doi: 10.1016/j.neuroimage.2019.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Potvin O., Camicioli R., Duchesne S. Beware of white matter hyperintensities causing systematic errors in FreeSurfer gray matter segmentations! Hum. Brain Mapp. 2021;42(9):2734–2745. doi: 10.1002/hbm.25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhollander T., Clemente A., Singh M., Boonstra F., Civier O., Duque J.D., Egorova N., Enticott P., Fuelscher I., Gajamange S., Genc S., Gottlieb E., Hyde C., Imms P., Kelly C., Kirkovski M., Kolbe S., Liang X., Malhotra A., Mito R., Poudel G., Silk T.J., Vaughan D.N., Zanin J., Raffelt D., Caeyenberghs K. Fixel-based analysis of diffusion MRI: methods, applications, challenges and opportunities. NeuroImage. 2021;241:118417. doi: 10.1016/j.neuroimage.2021.118417. [DOI] [PubMed] [Google Scholar]
- Dhollander, T., Mito, R., Raffelt, D., Connelly, A. (2019). Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. Proc. Intl. Soc. Mag. Reson. Med, May 11-16, 555.
- Dhollander, T., Raffelt, D., Connelly, A., 2016. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI, 35(September), 1–2. https://www.researchgate.net/publication/307863133.
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Doesborgh S.J.C., van de Sandt-Koenderman W.M.E., Dippel D.W.J., van Harskamp F., Koudstaal P.J., Visch-Brink E.G. Linguistic deficits in the acute phase of stroke. J. Neurol. 2003;250(8):977–982. doi: 10.1007/s00415-003-1134-9. [DOI] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46(4):927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Egorova N., Dhollander T., Khlif M.S., Khan W., Werden E., Brodtmann A. Pervasive white matter fiber degeneration in ischemic stroke. Stroke. 2020;51(5):1507–1513. doi: 10.1161/STROKEAHA.119.028143. [DOI] [PubMed] [Google Scholar]
- Egorova-Brumley N., Khlif M.S., Werden E., Brodtmann A., Bird L.J. Grey and white matter atrophy 1 year after stroke aphasia. Brain Communications. 2022;1–12 doi: 10.1093/braincomms/fcac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hachioui H., van de Sandt-Koenderman M.W.M.E., Dippel D.W.J., Koudstaal P.J., Visch-Brink E.G. The screeling: occurence of linguistic deficits in acute aphasia post-stroke. J. Rehabil. Med. 2012;44(5):429–435. doi: 10.2340/16501977-0955. [DOI] [PubMed] [Google Scholar]
- El Hachioui H., Visch-Brink E.G., de Lau L.M.L., van de Sandt-Koenderman M.W.M.E., Nouwens F., Koudstaal P.J., Dippel D.W.J. Screening tests for aphasia in patients with stroke: a systematic review. J. Neurol. 2017;264(2):211–220. doi: 10.1007/s00415-016-8170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S., Pinel P., Gaillard R., Delmaire C., Perrin M., Dupont S., Dehaene S., Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel S.J., Catani M. Lesion mapping in acute stroke aphasia and its implications for recovery. Neuropsychologia. 2018;115(March):88–100. doi: 10.1016/j.neuropsychologia.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel S.J., De Schotten M.T., Dell’Acqua F., Kalra L., Murphy D.G.M., Williams S.C.R., Catani M. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain. 2014;137(7):2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Yourganov G., Bonilha L., Basilakos A., Den Ouden D.-B., Rorden C. Revealing the dual streams of speech processing. Proc. Natl. Acad. Sci. U.S.A. 2016;113(52):15108–15113. doi: 10.1073/pnas.1614038114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Geller J., Thye M., Mirman D. Estimating effects of graded white matter damage and binary tract disconnection on post-stroke language impairment. Neuroimage. 2019;189:248–257. doi: 10.1016/j.neuroimage.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Gerstenecker A., Lazar R.M. Language recovery following stroke. Clin. Neuropsychol. 2019:1–20. doi: 10.1080/13854046.2018.1562093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva S., Correia M.M., Warburton E.A. Contributions of bilateral white matter to chronic aphasia symptoms as assessed by diffusion tensor MRI. Brain Lang. 2015;150:117–128. doi: 10.1016/j.bandl.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain’s language pathways. Cereb. Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E., Fridriksson J., Rorden C., Bonilha L. Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage: Clinical. 2017;16(April):461–467. doi: 10.1016/j.nicl.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., Saur D. Neuroimaging of stroke recovery from aphasia – Insights into plasticity of the human language network. Neuroimage. 2019;190(August 2017):14–31. doi: 10.1016/j.neuroimage.2017.11.056. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurol. 2007;8(May):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Beh Y.Y., Sebastian R., Breining B., Tippett D.C., Wright A., Saxena S., Rorden C., Bonilha L., Basilakos A., Yourganov G., Fridriksson J. Predicting recovery in acute poststroke aphasia. Ann. Neurol. 2018;83(3):612–622. doi: 10.1002/ana.25184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Kuperman J.M., Dale A.M. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T.M.H., Seghier M.L., Leff A.P., Price C.J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. YNICL. 2013;2:424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T.M.H., Leff A.P., Price C.J. Predicting language outcomes after stroke: Is structural disconnection a useful predictor? NeuroImage: Clinical. 2018;19(March):22–29. doi: 10.1016/j.nicl.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi A., Nagakane Y., Yamada K., Kuriyama N., Mizuno T., Nishimura T., Nakagawa M. Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Diagnostic Neuroradiol. 2009;51(9):549–555. doi: 10.1007/s00234-009-0534-7. [DOI] [PubMed] [Google Scholar]
- Ivanova M.V., Isaev D.Y., Dragoy O.V., Akinina Y.S., Petrushevskiy A.G., Fedina O.N., Shklovsky V.M., Dronkers N.F. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex. 2016;85:165–181. doi: 10.1016/j.cortex.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Ivanova M.V., Zhong A., Turken A., Baldo J.V., Dronkers N.F. Functional Contributions of the arcuate fasciculus to language processing. Front. Hum. Neurosci. 2021;15(June):1–15. doi: 10.3389/fnhum.2021.672665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Tournier J.D., Dhollander T., Connelly A., Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Jones, D.K., 2009. Chapter 3 – Gaussian Modeling of the Diffusion Signal. In H. Johansen-Berg & T. E. J. B. T.-. D. M. R. I. Behrens (Eds.), Diffusion MRI: From quantitative measurement to in vivo neuroanatomy (pp. 37–54). Academic Press. 10.1016/B978-0-12-374709-9.00003-1.
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kellner E., Dhital B., Kiselev V.G., Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016;76(5):1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Keser Z., Meier E.L., Stockbridge M.D., Hillis A.E. The role of microstructural integrity of major language pathways in narrative speech in the first year after stroke. J. Stroke Cerebrovasc. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Jang S.H. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. Am. J. Neuroradiol. 2013;34(4):785–790. doi: 10.3174/ajnr.A3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S., Meier E.L., Johnson J.P. Neuroplasticity in aphasia: A proposed framework of language recovery. J. Speech Lang. Hear. Res. 2019;62(11):3973–3985. doi: 10.1044/2019_JSLHR-L-RSNP-19-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S., Thompson C.K. Neuroplasticity of language networks in aphasia: advances, updates and future challenges. Front. Neurol. 2019;10(April):295. doi: 10.3389/FNEUR.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J.A. Neural plasticity and neurorehabilitation: Teaching the new brain old tricks. J. Commun. Disord. 2011;44(5):521–528. doi: 10.1016/j.jcomdis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kourtidou E., Kasselimis D., Angelopoulou G., Karavasilis E., Velonakis G., Kelekis N., Zalonis I., Evdokimidis I., Potagas C., Petrides M. The role of the right hemisphere white matter tracts in chronic aphasic patients after damage of the language tracts in the left hemisphere. Front. Hum. Neurosci. 2021;15(June):1–20. doi: 10.3389/fnhum.2021.635750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer D., Hartwigsen G., Kellmeyer P., Glauche V., Mader I., Klöppel S., Suchan J., Karnath H.O., Weiller C., Saur D. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136(2):619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Martino J., Da Silva-Freitas R., Caballero H., Marco De Lucas E., García-Porrero J.A., Vázquez-Barquero A. Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery. 2013;72(March):87–98. doi: 10.1227/NEU.0b013e318274294b. [DOI] [PubMed] [Google Scholar]
- Meier E., Johnson J., Pan Y., Kiran S. The utility of lesion classification models in predicting language abilities and treatment outcomes in persons with aphasia. Front. Hum. Neurosci. 2019;12 doi: 10.3389/conf.fnhum.2018.228.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Kinoshita M., Shinohara H., Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav. 2020;14(6):2817–2830. doi: 10.1007/s11682-019-00187-4. [DOI] [PubMed] [Google Scholar]
- Osa García A., Brambati S.M., Brisebois A., Désilets-Barnabé M., Bedetti C., Rochon E., Leonard C., Desautels A., Marcotte K. Predicting early post-stroke aphasia outcome from initial aphasia severity. Front. Neurol. 2020;11(February):120. doi: 10.3389/FNEUR.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Huckins G., Varoquaux G. Establishment of best practices for evidence for prediction – a review. JAMA Psychiat. 2020;77(5):534–540. doi: 10.1001/jamapsychiatry.2019.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A language and environment for statistical computing. https://www.r-project.org/.
- Radwan A.M., Emsell L., Blommaert J., Zhylka A., Kovacs S., Theys T., Sollmann N., Dupont P., Sunaert S. Virtual brain grafting: Enabling whole brain parcellation in the presence of large lesions. Neuroimage. 2021;229(January) doi: 10.1016/j.neuroimage.2021.117731. [DOI] [PubMed] [Google Scholar]
- Raffelt D., Dhollander T., Tournier J.D., Tabbara R., Smith R.E., Pierre E., Connelly A. Bias field correction and intensity normalisation for quantitative analysis of apparent fiber density. Proc. ISMRM. 2017;25:3541. https://www.researchgate.net/publication/315836355 [Google Scholar]
- Raffelt D.A., Tournier J.D., Smith R.E., Vaughan D.N., Jackson G., Ridgway G.R., Connelly A. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L.B., Cunnington R., Boyd R.N., Rose S.E. Surface-based fMRI-driven diffusion tractography in the presence of significant brain pathology: A study linking structure and function in cerebral palsy. PLoS One. 2016;11(8):1–25. doi: 10.1371/journal.pone.0159540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Lange R., Baumgaertner A., Schraknepper V., Willmes K., Rijntjes M., Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schevenels K., Michiels L., Lemmens R., De Smedt B., Zink I., Vandermosten M. The role of the hippocampus in statistical learning and language recovery in persons with post stroke aphasia. NeuroImage: Clinical. 2022;36(October) doi: 10.1016/j.nicl.2022.103243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G., Marchina S., Norton A. Evidence for plasticity in white-matter tracts of patients with chronic broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, P. (2017). Supervised segmentation of MS lesions. In Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. https://edoc.ub.uni-muenchen.de/20373/.
- Selig, J.P., Little, T.D., 2012. Autoregressive and Cross-Lagged Panel Analysis for Longitudinal Data. Handbook of Developmental Research Methods, Chapter 12, 265–278.
- Smith, R., 2022. Quantitative streamlines tractography: methods and inter-subject normalisation. Aperture Neuro, 2, 1–23. 10.52294/apertureneuro.2022.2.neod9565.
- Smith, R., Raffelt, D., Tournier, J.-D., Connelly, A., 2020. Quantitative streamlines tractography: methods and inter-subject normalisation. OSF Preprint, c, 1–27. 10.31219/osf.io/c67kn.
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Tournier J.D., Calamante F., Connelly A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62(3):1924–1938. doi: 10.1016/j.neuroimage.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Tournier J.D., Calamante F., Connelly A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. 2015;119:338–351. doi: 10.1016/j.neuroimage.2015.06.092. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Gadian D.G., Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23(3):1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Tournier J.-D., Calamante F., Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Ismrm. 2010;88(2003):2010. [Google Scholar]
- Tournier J.-D., Mori S., Leemans A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.D., Smith R., Raffelt D., Tabbara R., Dhollander T., Pietsch M., Christiaens D., Jeurissen B., Yeh C.H., Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202(August) doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- Tremblay P., Dick A.S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016;162:60–71. doi: 10.1016/j.bandl.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Turken, A. U., Dronkers, N. F., 2011. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5(FEBRUARY 2011), 1–20. 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S., Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45(3):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- van Hees S., Mcmahon K., Angwin A., Zubicaray G.D., Read S., Copland D.A. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil. Neural Repair. 2014;28(4):325–334. doi: 10.1177/1545968313508654. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. Springer; 2002. Modern applied statistics with S. 10.1198/tech.2003.s33. [Google Scholar]
- Veraart J., Fieremans E., Novikov D.S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 2016;76(5):1582–1593. doi: 10.1002/mrm.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J., Novikov D.S., Christiaens D., Ades-Aron B., Sijbers J., Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visch-Brink E.G., van de Sandt-Koenderman W.M.E., El Hachioui H. Bohn Stafleu van Loghum; 2010. ScreeLing. [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C.Y., Zheng X., Marchina S., Norton A., Schlaug G., Laboratories S.R., Deaconess B.I. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang. 2014;136:1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Entrup J.L., Schneck S.M., Onuscheck C.F., Levy D.F., Rahman M., Willey E., Casilio M., Yen M., Brito A., Kam W., Davis L.T., de Riesthal M., Kirshner H.S. Recovery from aphasia in the first year after stroke. Brain. 2022 doi: 10.1093/brain/awac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Li Y., Li J., Yao D., Liao W., Chen H. Beyond the arcuate fasciculus: damage to ventral and dorsal language pathways in aphasia. Brain Topogr. 2017;30(2):249–256. doi: 10.1007/s10548-016-0503-5. [DOI] [PubMed] [Google Scholar]
- Zhang, F., Kahali, P., Suter, Y., Norton, I., Rigolo, L., Savadjiev, P., Song, Y., Rathi, Y., Cai, W., Wells, W. M., Golby, A. J., & O’Donnell, L. J. (2017). Automated connectivity-based groupwise cortical atlas generation: Application to data of neurosurgical patients with brain tumors for cortical parcellation prediction. Proceedings – International Symposium on Biomedical Imaging, 22591, 774–777. 10.1109/ISBI.2017.7950633.
- Zhang J., Wei X., Xie S., Zhou Z., Shang D., Ji R., Yu Y., He F., Du Y., Ye X., Luo B. Multifunctional roles of the ventral stream in language models: advanced segmental quantification in post-stroke aphasic patients. Front. Neurol. 2018;9:89. doi: 10.3389/fneur.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zheng W., Shang D., Chen Y., Zhong S., Ye J., Li L., Yu Y., Zhang L.i., Cheng R., He F., Wu D., Ye X., Luo B. Fixel-based evidence of microstructural damage in crossing pathways improves language mapping in Post-stroke aphasia. NeuroImage: Clinical. 2021;31:102774. doi: 10.1016/j.nicl.2021.102774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhong S., Zhou L., Yu Y., Tan X., Wu M., Sun P., Zhang W., Li J., Cheng R., Wu Y., Yu Y., Ye X., Luo B. Correlations between dual-pathway white matter alterations and language impairment in patients with aphasia: a systematic review and meta-analysis. Neuropsychol. Rev. 2021;31(3):402–418. doi: 10.1007/s11065-021-09482-8. [DOI] [PubMed] [Google Scholar]
- Zipse, L., Norton, A., Marchina, S., Schlaug, G., 2012. When right is all that is left: plasticity of right-hemisphere tracts in a young aphasic patient. Ann N Y Acad Sci., April(1252), 237–245. 10.1111/j.1749-6632.2012.06454.x.When. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pseudonymized study data and code to reproduce the figures and findings of this study are publicly available at https://github.com/kschevenels/pwawm. Please note that the MRI data cannot be shared under any circumstance, as lesioned MRI data are person-specific and therefore cannot be considered anonymous.