Abstract
Vegetable crops are known as protective foods due to their potential role in a balanced human diet, especially for vegetarians as they are a rich source of vitamins and minerals along with dietary fibers. Many biotic and abiotic stresses threaten the crop growth, yield and quality of these crops. These crops are annual, biennial and perennial in breeding behavior. Traditional breeding strategies pose many challenges in improving economic crop traits. As in most of the cases the large number of backcrosses and stringent selection pressure is required for the introgression of the useful traits into the germplasm, which is time and labour-intensive process. Plant scientists have improved economic traits like yield, quality, biotic stress resistance, abiotic stress tolerance, and improved nutritional quality of crops more precisely and accurately through the use of the revolutionary breeding method known as clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein-9 (Cas9). The high mutation efficiency, less off-target consequences and simplicity of this technique has made it possible to attain novel germplasm resources through gene-directed mutation. It facilitates mutagenic response even in complicated genomes which are difficult to breed using traditional approaches. The revelation of functions of important genes with the advancement of whole-genome sequencing has facilitated the CRISPR-Cas9 editing to mutate the desired target genes. This technology speeds up the creation of new germplasm resources having better agro-economical traits. This review entails a detailed description of CRISPR-Cas9 gene editing technology along with its potential applications in olericulture, challenges faced and future prospects.
Keywords: genome-editing technology, CRISPR-cas application, vegetable crops, advanced, cutting-edge
Introduction
The world population is estimated to increase by 10 billion in the next three decades, thereby the demand for food crops is likely to increase by 25–70% (Hunter et al., 2017). Contemporary agriculture will eventually face enormous challenges to produce crops having high yield and better quality which require few inputs (Tilman et al., 2011). Vegetable crops act as protective foods that provide essential nutrients in the human diet due to their richness in vitamins, minerals, dietary fiber and phytochemicals (Dias, 2012). More than 400 g of fruits and vegetables should be consumed daily per person to reduce the risk of cardiovascular diseases. (Bazzano et al., 2002). or cancer (Aune et al., 2017). Like any other food crop, vegetable crops too are prone to many biotic and abiotic stresses (Jaganathan et al., 2018; Boscaiu and Fita 2020) thereby necessitating the development of next-generation architectured crops which are able to sustain adverse environmental stresses (Karkute et al., 2017).
Till now conventional breeding techniques have been extensively used to improve the yield and agronomic performance which is a complex, time-consuming and labor-intensive process (Zhang et al., 2018). It is a selection of improved individuals utilizing genetic variation in the population (Breseghello and Coelho, 2013). It also recombines the desired gene pools resulting in new genotypes or cultivars (Holme et al., 2019). However, when the desirable genetic variation is not available in the gene pool, then it can be generated and used for selection by inducing mutations using various mutagenic agents like non-ionizing radiation i.e. UV rays or ionizing radiation i.e. X and gamma rays, alpha and beta rays, fast and slow neutrons. Some chemicals can also be used as mutagenic agents like ethyl methane sulphonate (EMS), methyl methane sulphonate (MMS), hydrogen fluoride (HF), sodium azide, N-methyl-N-nitrosourea (MNU) and hydroxylamine) (Parry et al., 2009). Mutation breeding has not been extensively utilized in vegetable crop improvement except for a few exceptions being low in its efficiency. Over the last few decades, there have been a large number of significant developments in the molecular biology approaches to improve crop yield and quality. Recently, tremendous progress has been made in genome editing tools like site-directed nucleases (SDNs) which are able to edit the crops at high speed and possess great potential in shaping up the novel genetic makeup of vegetable crops (Tian et al., 2021). Genome editing tools can precisely engineer the genes by either deleting, replacing or inserting specific sequences at the specific targeted location in the target genome to generate novel traits (Corte et al., 2019). Zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated enzymes are the tools for genome editing used to modify plants (Miller et al., 2007). Site-specific double-stranded breaks (DSBs) are enabled by Crispr/Cas which further activate the cellular DNA repair systems (Zhu et al., 2020; Gaj et al., 2013). These DSBs can either be corrected by the non-homologous end joining (NHEJ) pathway or through the homology-directed repair (HDR) pathway (O’Driscoll and Jeggo, 2006; Wang T. et al., 2019a). The use of first-generation technologies like ZFNs and TALENs has been limited due to their adverse mutagenic outcome, low editing efficiency, time-consuming process and labor-intensive selection and screening process (Gaj et al., 2013; Jaganathan et al., 2018). The second-generation genome editing technology i. e. CRISPR/Cas9 is easier to design, and execute and more cost-effective. The use of CRISPR/Cas9 in vegetable crops has substantially expanded gene editing technology and made it possible to create novel genotypes with desired phenotypic features and altered genomic functions at the base pair level (Abdallah et al., 2015; Nunez de Caceres Gonzalez and De la Mora Franco, 2020). We will first go over CRISPR/Cas9 history and development before summarising how it is currently used to modify vegetable crops. Finally, we will talk about the real-world challenges in enhancing vegetable crops with the desired traits.
Clustered regularly interspaced short palindromic repeats-CRISPR-associated protein-9
CRISPR-Cas9 is an advanced genome editing technique that enables scientists to change, add, or remove specific DNA sequences to modify specific regions of the genome. In general, there are three main types (I-III) of CRISPR-Cas systems utilized for target interference (Rouillon et al., 2013). Type II uses its two distinctive nuclease domains, RuvC and HNH, to achieve interference with only a basic effector-module design (Gasiunas et al., 2012). Type II Cas9 from Streptococcus pyogenes (SpCas9) is the most popular CRISPR nuclease employed in CRISPR-Cas technology (Doudna and Charpentier, 2014). The protospacer adjacent motif (PAM) is recognized by the sgRNA-Cas complex, and Cas9 cleaves the target DNA to create a double-strand break (DSB), activating cellular DNA repair processes (Figure 1).
FIGURE 1.
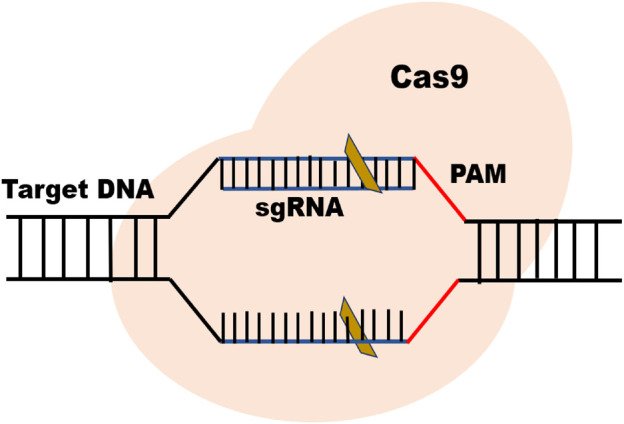
Basic structure of CRISPR/Cas9 system.
How does it work
The two essential parts of the CRISPR-Cas9 system that modify DNA are Cas 9 enzyme and guiding RNA (gRNA). The “genetic scissors” known as Cas9 (enzyme), cut the two DNA strands at an exact place in the genome to allow for the addition or deletion of DNA fragments and the guiding RNA (gRNA) is made up of two parts: Crispr RNA (crRNA), a 17–20 nucleotide sequence complementary to the target DNA, and a transactivating Crispr RNA (tracr RNA), which serves as a binding platform for the Cas9 nuclease (Mei et al., 2016) (Figure 1). Each crRNA hybridizes with tracr RNA, and these two RNAs jointly make a complex with the Cas9 nuclease (Deltcheva et al., 2011). The goal of the guide RNA is to find and bind to a target DNA sequence that is complementary to its RNA bases. Double strand breaks, or DSBs, are created when the Cas9 enzyme cuts across both DNA strands at the same location in the DNA sequence as the guide RNA (Jinek et al., 2012; Jiang et al., 2013). The DSBs inflicted by Cas-9 protein are repaired by two mechanisms i.e., non-homologous end-joining (NHEJ) and homology-directed repair (HDR) (Liu et al., 2019) (Figure 2). NHEJ needs enzymes in the repair mechanism in which different DNA segments are joined by excluding a homologous DNA template (Shuman and Glickman, 2007). It is an extraordinarily effective cell repair mechanism that is most often exploited but is prone to errors that can cause minor, spontaneous insertions or deletions. (Yang et al., 2020). The HDR however is quite precise in gene insertion or replacement of DNA segments at the predicted DSB site but requires a large amount of homologous DNA template (Liu et al., 2019; Yang et al., 2020).
FIGURE 2.
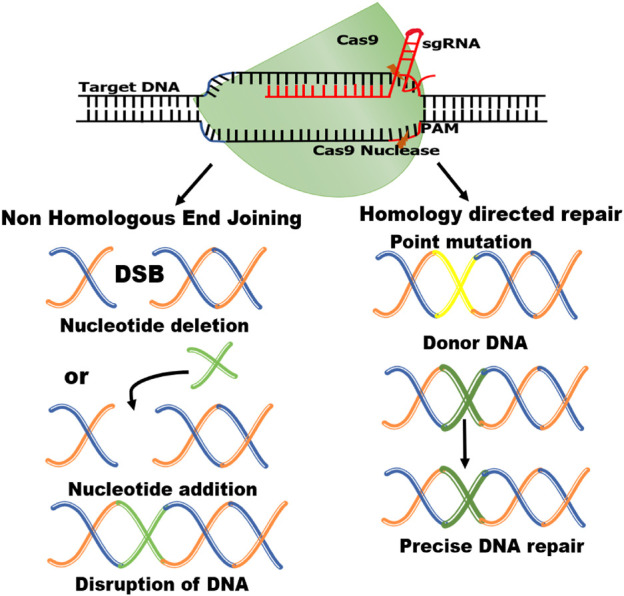
Schematic diagram of CRISPR/Cas9 mechanism. Double-strand breaks (DSBs) are induced when Cas9 enzyme and gRNA bind with targeted double-stranded DNA. These DSBs are repaired by Homology-directed repair (HDR) and Non-homologous end-joining (NHEJ) mechanisms.
Modifications in clustered regularly interspaced short palindromic repeats genome editing system
Several new CRISPR systems have been developed to overcome the limitations of CRISPR/Cas 9 and improve its specificity for more effective genome editing. These cutting-edge technologies are detailed below and could be crucial resources for molecular crop breeding.
Cas protein Cpf1 (also known as Cas12a) is with RNA-guided system being widely used in genome editing (Kim et al., 2017; Moon et al., 2018; Chen et al., 2019). It uses a T-rich PAM sequence to identify the target site, extending the editing sites beyond the G-rich PAM sequences preferred by Cas9. The target site of Cpf1 is located at the distal position and downward the PAM sequence. Cpf1’s guide RNA has 43 base pairs and is shorter than the sgRNA of Cas9 (about 100 bp) (Kim et al., 2017; Chen et al., 2019). Cpf1 generates staggered-ended double-strand breaks, which offers more advantages than Cas9 and improves the effectiveness of the NHEJ-based gene insertion. (Kim et al., 2017; Moon et al., 2018). Cpf1-based genome editing has been reported in rice and soybean (Kim et al., 2017; Xu et al., 2017). Additional research is required to analyze the specificity of Cpf1 in other crops and to enhance the current Cas12a-based applications Schindele and Puchta (2020).
Cas12b prefers T-rich PAM, produces staggered double-strand breaks, and needs both a crRNA and a trans-activating crRNA. Cas12b protein is smaller than Cas9 and Cas12a. Cas12b/C2c1 has been effectively used to carry out multiplex genome editing, induce mutations, and cause deletions at many loci in Arabidopsis (Wu et al., 2020). However, Cas12b shows its optimum activity at higher temperature (Teng et al., 2018), it needs to be altered for making it more useful in crop applications.
Cas13 is a recently identified CRISPR effector which targets specific RNAs in plant cells. This system has high RNA target specificity and efficiency. Cas13 protein belongs to class 2 type VI and contains unique higher eukaryotes and prokaryotes nucleotide-binding domains that are exclusively associated with RNase activity (Wolter and Puchta 2018). Till now, three different Cas13 protein classes, such as Cas13a, Cas13b, and Cas13d, have been used for RNA editing in plants (Schindele et al., 2019), mainly to target RNA for cleavage, for combating RNA viruses (Aman et al., 2018; Wolter and Puchta 2018). It has been demonstrated that CRISPR/LshCas13a system is used to create potyvirus resistance in plants, which suggests that this system can be employed for agricultural and biotechnological applications (Aman et al., 2018).
Cas14a is a highly compact protein, which can be used as an RNA-guided DNA nuclease for target-specific single-stranded DNA (ssDNA) cleavage (Harrington et al., 2018; Khan M. Z. et al., 2019b). Due to its sequence-independent and unrestricted cleavage, it has evolved into an excellent tool for building resistance to economically significant plant ssDNA viruses (Harrington et al., 2018; Khan M. S. S. et al., 2019). Cas14a is only one-third the size of Cas9 and is the smallest working CRISPR system to date. (Harrington et al., 2018). It has the potential to generate resistance against ssDNA viruses that belong to the Geminiviridae and Nanoviridae families (Khan M. S. S. et al., 2019a).
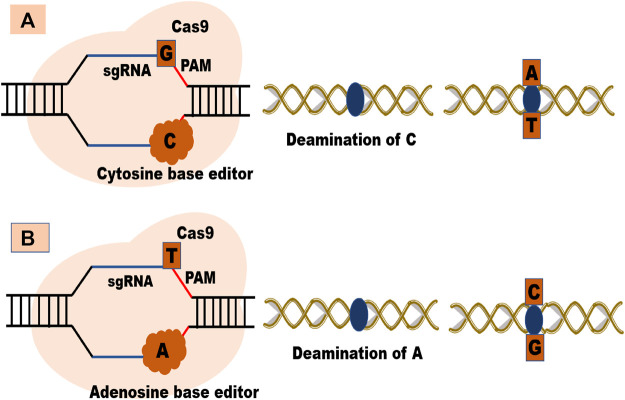
Besides these systems, base editing provides effective, concise, and well-recognized strategies for specific base replacement at the target site without the need for DSBs or donor DNA and independent of homology-directed repair (HDR) (Chen et al., 2019). They are helpful when there is a requirement for desired protein-coding genes to create variations with enhanced economic traits (Li H. et al., 2020c). At present, there are two types of base editors: Cytosine base editors (CBE) (which converts C-G pair to T-A pair) and adenine base editors (ABE) (converts A-T base pair to G-C base pairs (Komor et al., 2016; Gaudelli et al., 2017) Figures 3A,B. Both these editors largely depend upon the availability of PAM sequence as they use DNA binding proteins to induce point mutations at the targeted site (Jin et al., 2019). The major limitation of base editors is their inability to generate precise base edits in point mutations. Research is ongoing to improve the efficiency and role of base editing in random and targeted mutagenesis (Li H. et al., 2020c).
FIGURE 3.
(A) Cytosine base editor and (B) Adenosine base editor.
Prime editing, a recent genome-editing tool that induced all kinds of mutations and base substitutions (insertion/deletion) without donor DNA or double-strand breaks. Prime editing uses proteins fused to Cas 9 nickase and prime edited guide RNA with reverse transcriptase to induce mutations. The pegRNA contains not only complimentary sequence as the target sites that directs nCas9 to its target sequence, but also an additional sequence creating desired sequence changes. The Cas9 nicked the PAM containing DNA strand which act as a primer for reverse transcriptase to create extensions in the nicked strand using pegRNA as template and ultimately modifying the target site (Anzalone et al., 2020). PE has been successfully applied in rice and wheat to generate stable edited lines with price gene edits (Butt et al., 2020; Li H. et al., 2020c). It is advantageous over other genome editing tools in crop science with respect to reduced off-target mutations and less requirement of PAM sequences due to RNA template and high-efficiency rates but still experiments are need to be conducted regarding its specificity and potential. The various tools and Databases used for CRISPR/Cas9 mediated genome editing in plant systems are being presented in Tables 1, 2.
TABLE 1.
List of available tools used for CRISPR/Cas9 genome editing.
TABLE 2.
List of available databases for CRISPR/Cas9 for plants.
| Database name | Purpose | Access link | References | |
|---|---|---|---|---|
| 1) | CRISPR Plant v2 | For highly specific sgRNAs | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330547/ | Minkenberg et al. (2019) |
| 2) | PGED (Plant Genome Editing Database) | Stores information about mutants | http://plantcrispr.org/cgi-bin/crispr/index.cgi | Zheng et al. (2019) |
| 3) | CRISPRlnc | Manual database of sgRNAs | https://www.crisprlnc.org/ | Chen et al. (2019) |
| 4) | Cpf1- Database | Design tool for Cpf1 | http://www.rgenome.net/cpf1-database/ | Park and Bae (2017) |
| 5) | Cas-Database | Design tool for Cas9 nucleases | http://www.rgenome.net/cas-database/ | Park et al. (2016) |
| 6) | CrisprGE | CRISPR/Cas-Central repository | http://crdd.osdd.net/servers/crisprge/ | Kaur et al. (2015) |
Applications of clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9 in vegetable crops
As vegetables are susceptible to various abiotic and biotic stresses which reduce optimum production, which highlights the significance of developing resistant/tolerant cultivars. Additionally, in vegetable crops, various quality traits including flavor and nutritional profile, plant architecture, and shelf life can be improved. Various gene editing systems have good potential to improve the quality and yield of vegetables among which CRISPR/Cas is very popular. The major applications of CRISPR/Cas9 in vegetable crops is being discussed below under different sections and the list of various traits modified by CRISPR/Cas is being presented in Table 3.
TABLE 3.
List of traits modified by Crispr/Cas in vegetable crops.
| Crop | Target gene | Trait modification | References |
|---|---|---|---|
| Tomato | |||
| SlAMS | Affects pollen viability | Bao et al. (2022) | |
| SlHyPRP1 | Salt tolerance | Tran et al. (2021) | |
| SlPelo and SlMlo1 | Tomato yellow leaf curl virus (TYLCV) and Powdery mildew fungus | Pramanik et al. (2021) | |
| CCD8 | Host resistance to plants | Bari et al. (2021) | |
| K transporter HKT 1,2 | Salt tolerance | Vu et al. (2020) | |
| SlMAPK3 | Enhances heat stress tolerance | Yu et al. (2019) | |
| SlMAPK3 | Drought stress response | Wang et al. (2017) | |
| BZR 1 | Regulates heat stress tolerance | Yin et al. (2018) | |
| Coat protein, Replicase from Tomato yellow leaf curl virus | Induced resistance to tomato yellow leaf curl virus | Tashkandi et al. (2018) | |
| SlMlo1 | Resistance to powdery mildew | Nekrasov et al. (2017) | |
| Pectate lyase (PL), polygalacturonase 2a (PG2a and beta-galactanase (TBG4) | Development of cell wall and modification of fruit color and weight | Wang et al. (2018a) | |
| APETALA2a (AP2a), NON-RIPENING (NOR) and FRUITFULL (FUL1/TDR4 and FUL2/MBP7) | Development and ripening of fruit | Wang et al. (2018b) | |
| SlGAI | Gibberellin responsive dwarf mutant | Tomlison et al. (2019) | |
| SBPase | Induced Leaf senescence in SBPase mutants | Ding et al. (2018) | |
| Psy1 and CrtR-b2 | Change in Carotenoid biosynthesis | D’Ambrosio et al. (2018) | |
| lncRNA1459 | Alters fruit ripening, lycopene, ethylene and carotenoid biosynthesis | Li et al. (2018a) | |
| SGR1, Blc, LCY-E, LCY-B1, LCY-B2 | Increased lycopene content | Li et al. (2018b) | |
| SlCBF | The sharp decrease in chilling stress tolerance | Li et al. (2018c) | |
| PDS and GABA-TP1, GABA-TP2, GABA-TP3, CAT9 and SSADH | Increased γ-aminobutyric acid content | Li et al. (2018d) | |
| SlNPR1 | Reduced drought tolerance | Li. et al. (2019) | |
| SlMYB12 | Pink fruit color | Deng et al. (2018) | |
| RIN | Fruit ripening | Jung et al. (2018) | |
| SP, MUILT, FAS, CyCb, OVUTE and FW2.2 | Fruit size and lycopene accumulation | Zsogon et al. (2018) | |
| SP, SP5, CLV3 and WUS, GGP1 | Plant structure, Fruit ripening, Day-length response, Vitamin-C and fruit size | Li et al. (2018e) | |
| RIN and ethylene | Fruit ripening | Li et al. (2020a) | |
| RIN | Ethylene production and fruit ripening | Ito et al. (2017) | |
| SlORRM4 | Fruit ripening | Yang et al. (2017) | |
| Alc | Shelf life | Yu et al. (2017) | |
| CLAVATA-WUSICHEL | Altered locule number | Rodriguez-Leal et al. (2017) | |
| Solyc12g038510 | Jointless mutant, abscission | Roldan et al. (2017) | |
| phytoene synthase (PSY) | Fruit color | Filler et al. (2017) | |
| LEAFYCOTYLEDON1-LIKE4 | Fruit metabolism | Gago et al. (2017) | |
| SlIAA9 | Parthenocarpy | Ueta et al. (2017) | |
| SlAGL6 | Parthenocarpic fruits | Klap et al. (2017) | |
| ANT1 | Anthocyanin biosynthesis | Cermak et al. (2015) | |
| SlALS1 | Enhanced herbicide resistance | Danilo et al. (2019) | |
| ALS | Herbicide resistance | Veillet et al. (2019) | |
| Brinjal | |||
| SmelPPO4, SmelPPO5, and SmelPPO6 genes | Reduced levels of flesh browning | Maioli et al. (2020) | |
| Potato | |||
| StDND1, StCHL1, and DMG400000582(StDMR6-1) | Late blight resistance | Kieu et al. (2021) | |
| GBSS genes | Starch biosynthesis | Andersson et al. (2018) | |
| GBSS1 | Starch biosynthesis | Kusano et al. (2018) | |
| GBSS | Starch and tuber quality | Andersson et al. (2017) | |
| St16DOX | Glycoalkaloids metabolism | Nakayasu et al. (2018) | |
| Coilin gene | Biotic (PVY) and abiotic stress resistance | Makhotenko et al. (2019) | |
| Carrot | |||
| F3H | Change in the anthocyanin biosynthesis pathway | Klimek-Chodacka et al. (2018) | |
| DcMYB113-like | Anthocyanin biosynthesis | Xu et al. (2019b) | |
| Watermelon | |||
| ALS | Enhanced herbicide resistance | Tian et al. (2018) | |
| Pumpkin | |||
| GRF12, AHA1, and HAK5 | Salt sensitivity | Huang et al. (2019) | |
| Lettuce | |||
| LsNCED4 | Seed germination inhibition | Bertier et al. (2018) | |
| Chinese Cabbage | |||
| BraFLCs | The early-flowering phenotype that did not depend on vernalization | Jeong et al. (2019) | |
Albino phenotype
Some plants lack chlorophyll pigmentation as a result of phytoene desaturase (PDS) gene disruption, which affects the formation of carotenoids and chlorophyll, leading to albino plant phenotypes. There is little information on the albino plant phenotypes in vegetables except for a few publications where albinism has been used to standardize the gene-editing method utilizing CRISPR-Cas. Fully albino plants (pds mutants) were generated in various vegetable crops like tomato, watermelon, melon, cabbage and carrot via editing phytoene desaturase gene through Agrobacterium tumefaciens-mediated transformation (Pan et al., 2016; Tian et al., 2017; Xu J. et al., 2019; Hooghvorst et al., 2019; Ma et al., 2019).
Abiotic stress
Vegetable crops face many abiotic stresses caused due to temperature, drought, salinity and heat which adversely affect crop productivity. Although traditional breeding techniques are able to combat stresses to certain extent, new innovative technologies like CRISPR-Cas 9 offers the possibility to generate more resilient germplasm in dealing with these stresses (Haque et al., 2018). High temperature is a major stress factor that inhibits the growth and productivity of vegetable crops. It leads to the overproduction of reactive oxygen species (ROS) which causes oxidative damage, ultimately impairing the normal function of plant cells. Highly conserved protein kinases called mitogen-activated protein kinases (MAPKs) are involved in the response to heat stress of vegetable crops (Sharma et al., 2020). Knockout of BRASSINAZOLE RESISTANT 1 (BZR1) impaired the induction of RESPIRATORY BURST OXIDASE HOMOLOG1 (RBOH1) and induced production of H2O2 and heat tolerance in tomato. This exogenous H2O2 recovered the heat tolerance in bzr1 tomato mutant plants (Yin et al., 2018). In addition to this, mutations induced through CRISPR-Cas9 in slmapk3 imparts higher heat stress tolerance in tomato. Slmapk3 mutant also showed less wilting, mild membrane damage, low production of reactive oxygen species and improved antioxidant enzymatic activity under heat stress as reported by Yu et al. (2019). Similarly, in lettuce by using CRISPR/Cas9, LsNCED4 (9-cis-EPOXYCAROTENOIDDIOXYGENASE4) gene, resulting in thermo-inhibition of seed germination was knocked out, which significantly resulted in high-temperature germination in both cultivars (Salinas and Cobham Green), capable of germinating more than 70% at 37°C (Bertier et al., 2018).
One of the most harmful environment factors causing damage to vegetable crops is drought stress. Important signalling molecules that react to drought stress include mitogen-activated protein kinases (MAPKs). In 2017, Wang and his co-workers utilized CRISPR/Cas9) mediated mutagenesis to generate slmapk3 mutants in tomato. In comparison to wild type plants, the slmapk3 mutants had more severe wilting symptoms, greater hydrogen peroxide levels, low antioxidant enzyme activity, and experienced more membrane damage. They concluded that slmapk3 is involved in drought response in tomato by protecting from membrane damage and stimulating transcription of some stress related genes. Chilling stress is the primary obstacle that prevents the growth of some vegetable crops, like tomato, brinjal, and chilli as they are sensitive to severe chilling injury. The highly conserved C-repeat binding factors (CBFs) are involved in regulating cold tolerance. As in tomato, the slcbf mutants were generated using the CRISPR-Cas9 system, but these mutants exhibited severe chilling- injury symptoms as shown by the down-regulation of CBF-related genes as compared to wild-type (WT) plants (Li et al., 2018a). Additionally in both abovementioned studies, the mutants exhibited lower content of proline and protein and more amount of hydrogen peroxide contents and antioxidants than WT plants which further altered hormonal level of plants and reduced the expression of genes. So, there is a need to study the regulatory mechanism of CBF and mitogen-activated protein kinases (MAPKs) genes in order to understand their molecular mechanism.
In addition to this, the SlUVR8 gene was knocked out in tomato to increase tolerance to high UV-B stress using CRISPR-CAS9 gene editing approach by generating sluvr8 mutant lines which confirmed that SlUVR8 plays a significant function in tomato seedling growth and UV-B stress resistance Liu et al. (2020). Excessive concentration of salts within the plant tissues will reduce growth and productivity, as they can affect several pivotal processes, such as germination, photosynthesis, nutrient balance and redox balance, among others (Parihar et al., 2015; Petretto et al., 2019). Recently, the HKT1&2 allele was edited and inserted into tomato Hongkwang cultivar via the CRISPR/Cpf1-mediated homology-directed repair (HDR) mechanism which showed stable inheritance for salt tolerance (Vu et al., 2020). Furthermore, by precise deletion of one or more SlHyPRP1’s functional motifs using CRISPR/Cas9-based multiplexed editing, salt stress-tolerant events in cultivated tomatoes were produced (Tran et al., 2021).
Biotic stresses
Globally, major losses in the production of vegetable crops are caused by a diverse variety of diseases. A sustainable strategy for supplying the world’s expanding population with food is the development of disease-resistant cultivars. (Thomazella et al., 2016). Traditional plant breeding has been utilized for centuries to develop new varieties, but modern technologies, like genome editing, have the ability to produce improved varieties more quickly, by accurately introducing favourable alleles into locally adapted types (Nekrasov et al., 2017). In tomato, SlDMR6-1 orthologue Solyc03g080190.2 is up-regulated when infected due to Pseudomonas syringae pv. tomato and Phytophthora capsici. The tomato homologue genes were knocked out using CRISPR-Cas9 to cause mutations in DMR6, which resulted in broad-spectrum resistance to Pseudomonas, Phytophthora, and Xanthomonas spp. (Thomazella et al., 2016). Wild-type MILDEW RESISTANT LOCUS O (Mlo) alleles, encode a protein, which provides fungal sensitivity causing powdery mildew disease. In tomato, homozygous loss-of-function of SlMlo1 gene through CRISPR-mediated mutations resulted in resistance to powdery mildew (Nekrasov et al., 2017).
Pseudomonas syringae pv. tomato (Pto) DC3000, a causative agent of tomato bacterial speck disease releases coronatine (COR) which stimulates stomatal opening and encourages the bacterial colonization in the leaves. Ortigosa et al. (2019) developed a tomato genotype resistant to bacterial speck by editing the SlJAZ2 gene (a key co-receptor for coronatine in stomatal guard cells) via the CRISPR/Cas9 system to produce dominating Jasmonate-Zim Domain (JAZ2) repressors (SlJAZ2jas), which prevents coronatine from reopening stomata and provided resistance to PtoDC3000. Jeon et al. (2020) identified biosynthetic gene clusters (ACET1a, ACET1b and Solyc12g100270) in tomato plants required for the production of falcarindiol in response to biotic stress. Mutagenesis through CRISPR revealed the direct role of the cluster in synthesis of falcarindiol which imparts resistance against fungal and bacterial pathogens in tomato. In 2018, through the use of the CRISPR/Cas9 system, tomato plants were made resistant to the tomato yellow leaf curl virus by focusing Through the use of the CRISPR/Cas9 system, tomato plants were made resistant to the tomato yellow leaf curl virus by focusing on the coat protein and replicase sites (Tashkandi et al., 2018).
In order to speed up the breeding of potatoes for resistance to late blight (Phytophthora infestans) and potato virus Y (PVY), CRISPR/Cas has emerged as a substitute and effective method. Targeting P3, CI, Nib, and CP viral genes, Cas13a protein was used to give resistance to three PVY strains (RNA viruses) (Zhan et al., 2019). Similarly, the functional knockouts of StDND1, StCHL1, DMG400000582 (StDMR6-1) and caffeoyl-CoA-O-methyltransferase gene generated potato plants with increased late blight resistance (Hedge et al., 2021; Kieu et al., 2021). Knocking out of Clpsk1 gene, which encodes the PSK precursor, in watermelon to provide increased resistance to Fusarium oxysporum f.sp. niveum (Zhang et al., 2020), whereas, in tomatoes, Solyc08g075770-knockout via CRISPR-Cas9 resulted Fusarium wilt disease sensitivity in the plants (Prihatna et al., 2018). Virus resistance can be induced in cucumber plants by disrupting the function of the recessive eIF4E (eukaryotic translation initiation factor 4E) gene using Cas9/subgenomic RNA (sgRNA) technology (Chandrasekaran et al., 2016). Similarly in Brassica napus, BnWRKY11 and BnWRKY70 were edited with CRISPR/Cas9 vectors and mutations induced in BnWRKY70 generated mutants with enhanced resistance to Sclerotinia spp. (Sun et al., 2018).
Vegetable quality improvement
Fruit and vegetables (F&V) are highly perishable food products that need advanced post-harvest technologies to maintain their storage stability and extended shelf life (Gallagher and Mahajan., 2011). In tomato, the homology-directed repair (HDR) pathway was used to replace the allele of ALC with the alc gene, resulting in T1 homozygous plants with long shelf life. (Yu et al., 2017). Klap et al. (2017) used CRISPR/Cas9 technology to delete tomato SlAGL6 (SlAGAMOUS-LIKE6) which led to the development of parthenocarpy under high-temperature stress conditions without compromising the weight, fruit shape, or pollen vitality. Other vegetable crops, like the in-demand seedless watermelon or less-seeded fruits, can also use this approach to generate parthenocarpy.
Lycopene is an important plant nutrient with strong antioxidant properties that helps to protect the cells from damage. Lycopene accumulation in the fruit is facilitated by the knockdown of a few genes linked to the carotenoid metabolic pathway. The amount of lycopene was successfully increased to about 5.1 times in genome-edited tomato fruits. These results suggested that the CRISPR/Cas9 system has the potential to greatly increase the amount of lycopene in tomato fruit due to its high effectiveness, infrequent off-target mutations, and stable heredity Li et al. (2018a). The non-proteinogenic amino acid gamma-aminobutyric acid (GABA) has hypotensive properties. Nonaka et al. (2017) To boost GABA accumulation by 7–15 times in tomato fruits, researchers employed CRISPR/CRISPR-associated protein (Cas)9 technology to remove the autoinhibitory domain of SlGAD2 and SlGAD3 and insert a stop codon right before the autoinhibitory domain.
Potato starch quality is important in various food applications. Improved starch quality with full knockout of granule-bound starch synthase (GBSS), starch synthase gene (SS6) and starch-branching enzymes (SBEs) genes SBE1, and SBE2 was reported in potato using CRISPR mediated genome editing (Andersson et al., 2017; Andersson et al., 2018; Kusano et al., 2018; Johansen et al., 2019; Veillet et al., 2019; Sevestre et al., 2020; Zhao et al., 2021). The enzyme polyphenol oxidase (PPO) catalyzes the oxidation of phenolic compounds into highly reactive quinones that cause postharvest browning of cut or bruised fruit (Araji et al., 2014). In the tetraploid potato cultivar Desiree, Gonzalez et al. (2020) investigated the use of the CRISPR/Cas9 system to introduce mutations into the StPPO2 gene. Mutations induced in the four alleles of the StPPO2 gene led to lines with reduced PPO activity (69%) in tubers. CRISPR/Cas has also been utilized in potato for improving traits like carotenoid biosynthesis (Khromov et al., 2018; Banfalvi et al., 2020; Butler et al., 2020) and glycoalkaloids (Nakayasu et al., 2018). Reducing the amount of steroidal glycoalkaloids (SGAs), such as α-solanine and α -chaconine, in tubers is necessary for breeding excellent potatoes since their presence may give potatoes a bitter flavor and have other unfavorable effects on humans. Two SGA-free potato lines were generated by selectively inhibiting a steroid 16-hydroxylase (St16DOX) that is involved in the synthesis of steroidal glycoalkaloids (SGA) in potato (Nakayasu et al., 2018).
Similarly, in brinjal, the three-polyphenol oxidase (PPO) genes SmelPPO4, SmelPPO5, and SmelPPO6 in brinjal were linked to enzymatic browning. To stop the browning of fruit flesh, these three target PPO genes have been eliminated using CRISPR-Cas9-based mutagenesis. (Maioli et al., 2020). It paves the way for the creation of genotypes of eggplant with reduced levels of flesh browning and higher levels of berry polyphenols as this is the first time the CRISPR/Cas9 system has been applied to eggplant for biotechnological uses.
Yield
Cucumber gynoecious inbred lines are very important because of their better production yield and cheaper labour cost for crossing. Hu et al. (2017) used CRISPR-Cas9 technique to create Cswip1 mutants by targeting the WPP trp/pro/pro domain Interacting Protein1 (CsWIP1) gene, which encodes a zinc-finger transcription factor. Cswip1 T0 mutants had a gynoecious phenotype with exclusively female flowers and these gynoecious mutants will be beneficial in heterosis breeding to produce high-yielding hybrids.
Similarly, Zhang et al. (2019a) created artificial gynoecious watermelon lines by editing ClWIP1gene using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated system. Additionally, SP5G mutations in field tomatoes hasten the blooming process and alter the compact growth habit, resulting in a short flowering interval and an early harvest (Soyk et al., 2017).
Herbicide resistance
Weeds are a significant stress factor that affects the yield and quality of vegetables, and selective herbicides are frequently used to control the growth and development of weeds during cultivation. The herbicide target gene acetolactate synthase (ALS) has been edited by using CRISPR-Cas9 technology in vegetables like tomato, watermelon, soybean, and potato for developing herbicide resistance in plants (Danilo et al., 2019; Tian et al., 2018; Li et al., 2015; Veillet et al., 2019).
Phelipanche aegyptiaca, an obligatory weedy plant parasite, requires the presence of the plant hormone strigolactone (SL) to encourage seed germination. Carotenoid dioxygenase 8 (CCD8), a crucial enzyme in the carotenoid synthesis pathway that generates strigolactone in tomatoes, and More Axillary Growth1 (MAX1), which is involved in strigolactone synthesis, were modified using CRISPR-Cas9 to significantly lower SL content and produce tomato plants resistant to P. aegyptiaca (Bari et al., 2019; Bari et al., 2021).
Recently, Yang et al. (2022) designed and tested the efficiency of sgRNA to use with Crispr/Cas system to edit herbicide-related genes pds (phytoene desaturase), ALS (acetolactate synthase), and EPSPS (5-Enolpyruvylshikimate-3-phosphate synthase) in tomato. The outcomes of the sgRNA efficiency tests confirmed that the transformation process could alter the target locations. They verified that 19 different transgenic tomatoes had adequately been edited by ALS2 P or ALS1 W sgRNAs, and 2 of them carried three base mutations that are likely to change their herbicide resistance.
Regulation of genome-edited crops
Genome/gene editing refers to the precise change in either of DNA or RNA sequence of any target organism. This editing can lead to change in a single base pair to completely reorganization of the large genomic region. Sometimes, genes that are not present in the natural gene pool are also introduced into the target individual to generate novel traits. As this technique involve genetic manipulation either by altering genome sequence or by addition of foreign genomic sequence therefore it becomes mandatory to enforce the regulations of the Cartagena Protocol by any country. The Cartagena Protocol on Biosafety set the foundation for regulating the release and international trade of genetically modified organisms. However, there have been differences in the patterns of GM crop cultivation, utilization and legislation. While some nations restrict production and deny consumption, others actively cultivate and consume them (Garcia Ruiz et al., 2018). Some countries regulate the process while others are involved in the regulation of the product (Eckerstorfer et al., 2019; Van Vu et al., 2019).
For instance, in 2018, as per the guide lines of United States Department of Agriculture (USDA) genome editing through CRISPR-Cas 9 is like conventional breeding therefore does not need any regulation under American Regulatory Standards and are exempted from the regulatory frameworks (Waltz, 2016a). This gives advantage in minimizing the time and resources needed for the testing and legislation of the release of the CRISPR edited crops. Growing research output is authentic evidence that CRISPR-Cas edited crops holds significant promise in improving the yield and quality of crops for the consumers across the world.
In the year 2018, Canadian legislation stated that any gene editing technology which produces a novel product must be subjected to further regulatory supervision on toxicity, allergenicity and any effects on other organisms except the target (Smyth, 2017). For example, non-browning apples and non-dark spot potatoes were approved in Canada after a long examination process which ensured that the changes made in these two products were not harmful to the human being.
However, the European Court of Justice (ECJ) has approved many mutagenic crops developed through chemical and physical mutagens (Waltz, 2016b) but considered gene-edited crops under same strict rules as traditional genetically modified (GM) plants. Among the South American countries like Argentina has developed a regulation system as per the guidelines of Cartagena Protocol on Biosafety for the approval of genome-edited products and relies on the case-by-case evaluation with the exemption from the regulation in the absence of transgene (Whelan and Lema, 2015). Chile and Brazil also followed the same regulatory system regarding genome editing as Argentina. Chile has given regulations in 2017 while Brazil in January 2018 (Duensing et al., 2018).
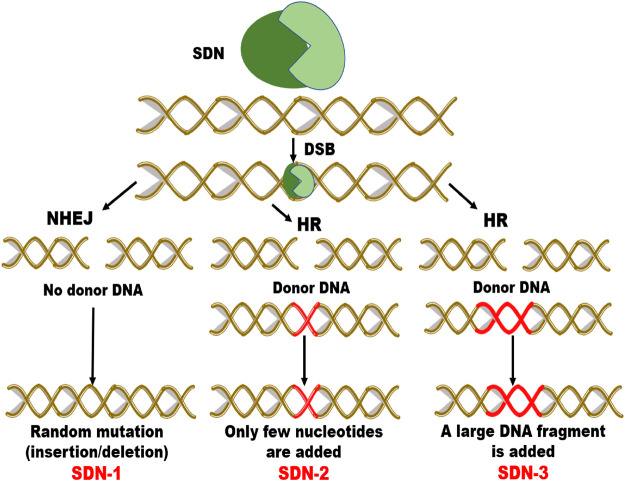
In Australia, the regulatory framework is set by the Gene Technology Act 2000 (GT Act) and GT Regulations 2001 (GT Regulations) with the purpose to protect people’s health, safety and the environment by recognizing threats posed as a consequence of genetic manipulation. The proposed amendments relevant to genome editing would exclude organisms developed with site-directed nucleases (SDN-1) from regulation and stated that organisms developed with SDN2 or SDN-3 are regulated as GMOs (Thysegen, 2019) (Please see Figure 4 depicting mechanism of SDN-1, 2 and 3 types). In the New Zealand, release of genetically modified plants is regulated by the Hazardous Substances and New Organisms (HSNO) Act 1996. The Act generally defines a GMO as any organism whose genome or genetic information has been altered by in vitro methods (Fritsche et al., 2018).
FIGURE 4.
Schematic diagram of modification by site directed nuclease (SDN- 1, SDN-2 and SDN-3) types. Double strand break (DSB) is repaired via non-homologous end joining (NHEJ) or homologous recombination (HR). SDN1 results in random insertion/deletion, SDN2 induces addition of few nucleotides and SDN3 inserts a DNA fragment.
In India, all the activities related to the development and use of genetically modified products are regulated as per the ‘‘Rules for the Manufacture/Use/Import/Export and Storage of Hazardous Microorganisms, Genetically Engineered Organisms or Cells’’, 1989 (Rules 1989) which covers new genome editing technologies including CRISPR/Cas9 and notified under the Environment (Protection) Act, 1986, by Genetic Engineering Appraisal Committee (GEAC). (Warrier and Pande 2016). This committee is responsible for granting permits to conduct experimental and large-scale open field trials and also granting approval for the commercial release of genetically altered crops.
Challenges and future prospects of clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9 genome editing
The CRISPR/Cas9 system is the latest cutting-edge technology that augments crop improvement by generating high-yielding, better quality, and resistant crop plants to biotic and abiotic stresses crops in a short span of time (Doudna and Charpentier, 2014; Langner et al., 2018). The NHEJ-mediated gene repair creates precise alterations to knock out or change the function of a particular target gene(s) which plays an important role in crop-trait-specific applications, but still there exist many challenges which must be overcome. Foremost is the selection of genes that are to be targeted for mutations and the types of mutation to avoid off-target gene editing. Moreover, it is difficult to carry out genome editing in the target organisms without genome sequencing. Editing a single gene does not result in desired phenotypic changes, because significant agronomic factors are quantitative. To add desired mutant alleles, effective CRISPR-Cas-mediated target site-specific insertion, deletion, and chromosomal recombination procedures can be applied (Zhu et al., 2020).
Once a gene has been identified, the second major challenge is to deliver CRISPR-Cas gene-editing agents into plant cells and the procedure to regenerate the putative edited plants (Chuang et al., 2021; Lino et al., 2018). Actually, it is quite challenging to create a universal and effective genetic transformation and regeneration system for vegetable crops (Niazian et al., 2017). In addition to this, for successful genetic transformation of vegetable crops editing efficiency is to be considered, which is further influenced by various factors, such as the number of sgRNA and GC amount; the expression levels of sgRNA and Cas9; and the secondary structure of the paired sgRNA and target sequence. (Kumlehn et al., 2018; Hu et al., 2019). Since genome editing in vegetable crops has such huge potential, we expect that strategies will be formulated to overcome these challenges in the near future.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdallah N. A., Channapatna S. P., McHughen A. G. (2015). GM Crops & Food, 6. Taylor & Francis Group, 183–205. 10.1080/21645698.2015.1129937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman R., Ali Z., Butt H., Mahas A., Aljedaani F., Khan M. Z., et al. (2018). RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 19, 1. 10.1186/s13059-017-1381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Turesson H., Nicolia A., Falt A. S., Samuelsson M., Hofvander P. (2017). Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 36, 117–128. 10.1007/s00299-016-2062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Turesson H., Olsson N., Falt A. S., Ohlsson P., Gonzalez M. N., et al. (2018). Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol. Plant. 164, 378–384. 10.1111/ppl.12731 [DOI] [PubMed] [Google Scholar]
- Anzalone A. Z., Koblan L. W., Liu D. R. (2020). Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844. 10.1038/s41587-020-0561-9 [DOI] [PubMed] [Google Scholar]
- Araji S., Grammer T. A., Gertzen R., Anderson S. D., Mikulic-Petkovsek M., Veberic R., et al. (2014). Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 164, 1191–1203. 10.1104/pp.113.228593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D., Giovannucci E., Boffetta P., Fadnes L. T., Keum N., Norat T., et al. (2017). Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose response meta-analysis of prospective studies. Int. J. Epidemiol. 46, 1029–1056. 10.1093/ije/dyw319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Park J., Kim J. S. (2014). Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475. 10.1093/bioinformatics/btu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfalvi Z., Csakvari E., Villanyi V., Kondrak M. (2020). Generation of transgene-free PDS mutants in potato by agrobacterium-mediated transformation. BMC Biotechnol. 20, 25. 10.1186/s12896-020-00621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Ding Y., Yang Fei., Zhang J., Xie J., Zhao C., et al. (2022). Gene silencing, knockout and over-expression of a transcription factor ABORTED MICROSPORES (SlAMS) strongly affects pollen viability in tomato (Solanum lycopersicum). BMC Genomics 23, 346. 10.1186/s12864-022-08549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari V. K., Nassar J. A., Kheredin S. M., Gal-On A., Ron M., Britt A., et al. (2019). CRISPR/Cas9-mediated mutagenesis of CAROTENOID CLEAVAGE DIOXYGENASE 8 in tomato provides resistance against the parasitic weed Phelipanche aegyptiaca . Sci. Rep. 9, 11438. 10.1038/s41598-019-47893-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari V. K., Nassar J. A., Aly R. (2021). CRISPR/Cas9 mediated mutagenesis of MORE AXILLARY GROWTH 1 in tomato confers resistance to root parasitic weed Phelipanche aegyptiaca . Sci. Rep. 11, 3905. 10.1038/s41598-021-82897-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano L. A., He J., Ogden L. G., Loria C. M., Vupputuri S., Myers L., et al. (2002). Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first national health and nutrition examination survey epidemiologic follow-up study. Am. J. Clin. Nutr. 76, 93–99. 10.1093/ajcn/76.1.93 [DOI] [PubMed] [Google Scholar]
- Beeber D., Chain F. J. J. (2020). crispRdesignR: A versatile guide RNA design package in R for CRISPR/cas9 applications. J. Genomics 8, 62–70. 10.7150/jgen.41196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertier L. D., Ron M., Huo H., Bradford K. J., Britt A. B., Michelmore R. W. (2018). High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-Induced modifications of NCED4 in lettuce (Lactuca sativa). G3 (Bethesda) 8, 1513–1521. 10.1534/g3.117.300396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscaiu M., Fita A. (2020). Physiological and molecular characterization of crop resistance to abiotic stresses. Agronomy 10, 1308. 10.3390/agronomy10091308 [DOI] [Google Scholar]
- Breseghello F., Coelho A. S. (2013). Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.) J. Agric. Food Chem. 61, 8277–8286. 10.1021/jf305531j [DOI] [PubMed] [Google Scholar]
- Butler N. M., Jansky S. H., Jiang J. (2020). First-generation genome editing in potato using hairy root transformation. Plant Biotechnol. J. 18, 2201–2209. 10.1111/pbi.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H., Rao G. S., Sedeek K., Aman R., Mahfouz M. (2020). Engineering herbicide resistance via prime editing in rice. Plant Biotechnol. J. 18, 2370–2372. 10.1111/pbi.13399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T., Baltes N. J., Čegan R., Zhang Y., Voytas D. F. (2015). High frequency, precise modification of the tomato genome. Genome Biol. 16, 232. 10.1186/s13059-015-0796-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran J., Brumin M., Wolf D., Leibman D., Klap C., Pearlsman M., et al. (2016). Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 17, 1140–1153. 10.1111/mpp.12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Wang Y., Zhang R., Zhang H., Gao C. (2019). CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. 10.1146/annurev-arplant-050718-100049 [DOI] [PubMed] [Google Scholar]
- Corte E.-D., Mahmoud L. M., Moraes T. S., Mou Z., Grosser J. W., Dutt M. (2019). Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants 8, 601. 10.3390/plants8120601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang F. Y., Phipps J. A., Lin L. F., Hecht V., Hewitt W. A., Wang Y. P. (2021). Approach for in vivo delivery of CRSIPR/Cas system: a recent update and furture prospect. Cell. Mol. Life Sci. 78, 2683–2708. 10.1007/s00018-020-03725-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio C., Giorio G., Stigliani A. L. (2018). Knockout of NPTII marker gene in transgenic tomato plants using the CRISPR/Cas9 system. Transgenic Res. 54, 367–378. 10.1007/s11248-018-0079-9 [DOI] [Google Scholar]
- Danilo B., Perrot L., Mara K., Botton E., Nogue F., Mazier M. (2019). Efficient and transgene-free gene targeting using Agrobacterium-mediated delivery of the CRISPR/Cas9 system in tomato. Plant Cell Rep. 38, 459–462. 10.1007/s00299-019-02373-6 [DOI] [PubMed] [Google Scholar]
- Deltcheva E., Chylinski K., Sharma C. M., Gonzales K., Chao Y., Pirzada Z. A., et al. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607. 10.1038/nature09886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Wang H., Sun C., Li Q., Jiang H., Du M., et al. (2018). Efficient generation of pink-fruited tomatoes using CRISPR/Cas9 system. J. Genet. Genomics 45, 51–54. 10.1016/j.jgg.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Dias J. (2012). Nutritional quality and health benefits of vegetables: A review. Food Nutr. Sci.Food Nutr. Sci. 3, 1354–1374. 10.4236/fns.2012.310179 [DOI] [Google Scholar]
- Ding F., Wang M., Zhang S. (2018). Sedoheptulose-1, 7-bisphosphatase is involved in methyl jasmonate- and dark-induced leaf senescence in tomato plants. Int. J. Mol. Sci. 19, 3673. 10.3390/ijms19113673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J. G., Hartenian E., Graham D. B., Tothova Z., Hegde M., Smith I., et al. (2014). Rational design of highly active sgRNAs for CRISPR/Cas9–mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267. 10.1038/nbt.3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna J. A., Charpentier E. (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- Duensing N., Sprink T., Parrott W. A., Fedorova M., Lema M. A., Wolt J. D., et al. (2018). Novel features and considerations for ERA and regulation of crops produced by Genome editing. Front. Bioeng. Biotechnol. 6, 79. 10.3389/fbioe.2018.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerstorfer M. F., Engelhard M., Heissenberger A., Simon S., Teichmann H. (2019). Plants developed by new genetic modification techniques—Comparison of existing regulatory frameworks in the EU and non-EU countries. Front. Bioeng. Biotechnol. 7, 26. 10.3389/fbioe.2019.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler H. S., Melamed B. C., Levy A. A. (2017). Targeted recombination between homologous chromosomes for precise breeding in tomato. Nat. Commun. 8, 15605. 10.1038/ncomms15605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche S., Poovaiah C., MacRae E., Thorlby G. (2018). A New Zealand perspective on the application and regulation of gene editing. Front. Plant Sci. 9, 1323. 10.3389/fpls.2018.01323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago C., Drosou V., Paschalidis K., Guerreiro A., Miguel G., Antunes D., et al. (2017). Targeted gene disruption coupled with metabolic screen approach to uncover the LEAFY COTYLEDON1-LIKE4 (L1L4) function in tomato fruit metabolism. Plant Cell Rep. 36, 1065–1082. 10.1007/s00299-017-2137-9 [DOI] [PubMed] [Google Scholar]
- Gaj T., Gersbach C. A., Carlos F. (2013). ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405. 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M. J., Mahajan P. V. (2011). “Stability and shelf life of fruit and vegetables,” in Food and beverage stability and shelf-life. Editors Kilcast, Subramaniam (Cambridge, UK: Woodhead Publishing Ltd; ), 641–656. Chapter 22. [Google Scholar]
- Garcia Ruiz M. T., Knapp A. N., Garcia-Ruiz H. (2018). Profile of genetically modified plants authorized in Mexico. Gm. Crops Food 9, 152–168. 10.1080/21645698.2018.1507601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiunas G., Barrangou R., Horvath P., Siksnys V. (2012). Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. U. S. A. 109, E2579–E2586. 10.1073/pnas.1208507109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli N. M., Komor A. C., Rees H. A., Packer M. S., Badran A. H., Bryson D. I., et al. (2017). Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471. 10.1038/nature24644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. N., Massa A. G., Andersson M., Turesson H., Olsson N., Falt A. S., et al. (2020). Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front. Plant Sci. 9, 1649. 10.3389/fpls.2019.01649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussler M., Schonig K., Eckert H., Eschstruth A., Mianne J., Renaud J. B., et al. (2016). Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148. 10.1186/s13059-016-1012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque E., Taniguchi H., Hassan M., Bhowmik P., Karim M. R., Smiech M., et al. (2018). Application of CRISPR/Cas9 genome editing technology for the improvement of crops cultivated in tropical climates: Recent progress, prospects, and challenges. Front. Plt. Sci. 9, 617. 10.3389/fpls.2018.00617d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. B., Burstein D., Chen J. S., Paez-Espino D., Ma E., White I. P., et al. (2018). Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842. 10.1126/science.aav4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge N., Joshi S., Soni N., Kushalappa A. C. (2021). The caffeoyl-CoA O-methyltransferase gene SNP replacement in Russet Burbank potato variety enhances late blight resistance through cell wall reinforcement. Plant Cell Rep. 40, 237–254. 10.1007/s00299-020-02629-6 [DOI] [PubMed] [Google Scholar]
- Heigwer F., Kerr G., Boutros M. (2014). E-CRISP: Fast CRISPR target site identification. Nat. Methods 11, 122–123. 10.1038/nmeth.2812 [DOI] [PubMed] [Google Scholar]
- Holme I. B., Gregersen P. L., Brinch-Pedersen H. (2019). Induced genetic variation in crop plants by random or targeted mutagenesis: Convergence and differences. Front. Plant Sci. 14, 1468. 10.3389/fpls.2019.01468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghvorst I., Lopez-Cristoffanini C., Nogues S. (2019). Efficient knockout of phytoene desaturase gene using CRISPR/Cas9 in melon. Sci. Rep. 19, 17077. 10.1038/s41598-019-53710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Li D., Liu X., Qi J., Gao D., Zhao S., et al. (2017). Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol. Plant 10, 1575–1578. 10.1016/j.molp.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Hu N., Xian Z., Li N., Liu Y., Huang W., Yan F., et al. (2019). Rapid and user-friendly open-source CRISPR/Cas9 system for single- or multi-site editing of tomato genome. Hortic. Res. 6, 7. 10.1038/s41438-018-0082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Cao H., Yang L., Chen C., Shabala L., Xiong M., et al. (2019). Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 70, 5879–5893. 10.1093/jxb/erz328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. C., Smith R. G., Schipanski M. E., Atwood L. W., Mortensen D. A. (2017). Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 67, 386–391. 10.1093/biosci/bix010 [DOI] [Google Scholar]
- Hwang G. H., Bae S. (2021). Web-Based Base Editing Toolkits: BE-Designer and BE-Analyzer. Methods Mol. Biol. 2189, 81–88. 10.1007/978-1-0716-0822-7_7 [DOI] [PubMed] [Google Scholar]
- Hwang G. H., Park J., Lim K., Kim S., Yu J., Yu E., et al. (2018). Web-based design and analysis tools for CRISPR base editing. BMC Bioinforma. 19, 542. 10.1186/s12859-018-2585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Nishizawa-Yokoi A., Endo M., Mikami M., Shima Y., Nakamura N., et al. (2017). Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat. Plants 3, 866–874. 10.1038/s41477-017-0041-5 [DOI] [PubMed] [Google Scholar]
- Jaganathan D., Ramasamy K., Sellamuthu G., Jayabalan S., Venkataraman G. (2018). CRISPR for crop improvement: An update review. Front. Plant Sci. 9, 985. 10.3389/fpls.2018.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J. E., Kim J. G., Fischer C. R., Mehta N., Dufour-Schroif C., Wemmer K., et al. (2020). A pathogen-responsive gene cluster for highly modified fatty acids in tomato. Cell 180, 176–187. 10.1016/j.cell.2019.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. Y., Ahn H., Ryu J., Oh Y., Sivanandhan G., Won K. H., et al. (2019). Generation of early-flowering Chinese cabbage (Brassica rapa spp. pekinensis) through CRISPR/Cas9-mediated genome editing. Plant Biotechnol. Rep. 13, 491–499. 10.1007/s11816-019-00566-9 [DOI] [Google Scholar]
- Jiang W., Bikard D., Cox D., Zhang F., Marraffini L. A. (2013). RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239. 10.1038/nbt.2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Zong Y., Gao Q., Zhu Z., Wang Y., Qin P., et al. (2019). Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295. 10.1126/science.aaw7166 [DOI] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen I. E., Liu Y., Jørgensen B., Bennett E. P., Andreasson E., Nielsen K. L., et al. (2019). High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Sci. Rep. 9, 17715. 10.1038/s41598-019-54126-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. J., Lee G. J., Bae S., Kang K. K. (2018). Reduced ethylene production in tomato fruits upon CRSPR/Cas9-mediated LeMADS-RIN mutagenesis. Hortic. Sci. 36, 396–405. 10.12972/kjhst.20180039 [DOI] [Google Scholar]
- Karkute S. G., Singh A. K., Gupta O. P., Singh P. M., Singh B. (2017). CRISPR/Cas9 Mediated Genome Engineering for Improvement of Horticultural Crops. Front Plant Sci. 22, 1635. 10.3389/fpls.2017.01635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Tandon H., Gupta A. K., Kumar M. (2015). CrisprGE: A central hub of CRISPR/Cas-based genome editing. Database (Oxford) 27, bav055. 10.1093/database/bav055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S. S., Basnet R., Islam S. A., Shu Q. (2019a). Mutational analysis of OsPLDα1 reveals its involvement in phytic acid biosynthesis in rice grains. J. Agric. Food Chem. 41, 11436–11443. 10.1021/acs.jafc.9b05052 [DOI] [PubMed] [Google Scholar]
- Khan M. Z., Haider S., Mansoor S., Amin I. (2019b). Targeting plant ssDNA viruses with engineered miniature CRISPR-Cas14a. Trends Biotechnol. 37, 800–804. 10.1016/j.tibtech.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Khromov A. V., Gushchin V. A., Timberbaev V. I., Kalinina N. O., Taliansky M. E., Makarov V. V. (2018). Guide RNA design for CRISPR/Cas9-mediated potato genome editing. Dokl. Biochem. Biophys. 479, 90–94. 10.1134/s1607672918020084 [DOI] [PubMed] [Google Scholar]
- Kieu N. P., Lenman M., Wang E. S., Petersen L. B., Andreasson E. (2021). Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 11, 4487. 10.1038/s41598-021-83972-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim S. T., Ryu J., Kang B. C., Kim J. S., Kim S. G. (2017). CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 8, 14406. 10.1038/ncomms14406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klap C., Yeshayahou E., Bolger A. M., Arazi T., Gupta S. K., Shabtai S., et al. (2017). Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 15, 634–647. 10.1111/pbi.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek-Chodacka M., Oleszkiewicz T., Lowder L. G., Qi Y., Baransk R. (2018). Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant Cell Rep. 37, 575–586. 10.1007/s00299-018-2252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor A. C., Kim Y. B., Packer M. S., Zuris J. A., Liu D. R. (2016). Programmable editing of a target base in genomic DNA without double stranded DNA cleavage. Nature 533, 420–424. 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlehn J., Pietralla J., Hensel G., Pacher M., Puchta H. (2018). The CRISPR/Cas revolution continues: From efficient gene editing for crop breeding to plant synthetic biology. J. Integr. Plant Biol. 60, 1127–1153. 10.1111/jipb.12734 [DOI] [PubMed] [Google Scholar]
- Kusano H., Ohnuma M., Mutsuro-Aoki H., Asahi T., Ichinosawa D., Onodera H., et al. (2018). Establishment of a modified CRISPR/Cas9 system with increased mutagenesis frequency using the translational enhancer dMac3 and multiple guide RNAs in potato. Sci. Rep. 8, 13753. 10.1038/s41598-018-32049-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K., Montague T. G., Gagnon J. A., Thyme S. B., Valen E. (2016). CHOPCHOP v2: A web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44, W272–W276. 10.1093/nar/gkw398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner T., Kamoun S., Belhaj K. (2018). CRISPR Crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 56, 479–512. 10.1146/annurev-phyto-080417-050158 [DOI] [PubMed] [Google Scholar]
- Li Z., Liu Z. B., Xing A., Moon B. P., Koellhoffer J. P., Huang L., et al. (2015). Cas9-Guide RNA directed genome editing in soybean. Plant Physiol. 169, 960–970. 10.1104/pp.15.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhang L., Wang L., Chen L., Zhao R., Sheng J., et al. (2018a). Reduction of tomato-plant chilling tolerance by CRISPR-Cas9- mediated SlCBF1 mutagenesis. J. Agric. Food Chem. 66, 9042–9051. 10.1021/acs.jafc.8b02177 [DOI] [PubMed] [Google Scholar]
- Li R., Fu D., Zhu B., Luo Y., Zhu H. (2018b). CRISPR/Cas9-mediated mutagenesis of lncRNA1459 alters tomato fruit ripening. Plant J. 94, 513–524. 10.1111/tpj.13872 [DOI] [PubMed] [Google Scholar]
- Li X., Wang Y., Chen S., Tian H., Fu D., Zhu B., et al. (2018c). Lycopene is enriched in tomato fruit by CRISPR/Cas9 mediated multiplex genome editing. Front. Plant Sci. 1-12, 559. 10.3389/fpls.2018.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Li R., Li X., Fu D., Zhu B., Tian H., et al. (2018d). Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum . Plant Biotechnol. J. 16, 415–427. 10.1111/pbi.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Yang X., Yu Y., Si X., Zhai X., Zhang H., et al. (2018e). Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36, 1160–1163. 10.1038/nbt.4273 [DOI] [PubMed] [Google Scholar]
- Li R., Liu C., Zhao R., Wang L., Chen L., Sheng J., et al. (2019). CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 19, 38. 10.1186/s12870-018-1627-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhu B., Pirello J., Xu C., Zhang B., Bouzayen M., et al. (2020a). Roles of RIN and ethylene in tomato fruit ripening and ripening‐associated traits. New Phytol. 226, 460–475. 10.1111/nph.16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li X., Xu Y., Liu H., He M., Tian X., et al. (2020c). High-efficie.ncy reduction of rice amylose content via CRISPR/Cas9-mediated base editing. Rice Sci. 27, 445–448. 10.1016/j.rsci.2020.09.001 [DOI] [Google Scholar]
- Lino C. A., Harper J. C., Carney J. P., Timlin J. A. (2018). Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 25, 1234–1257. 10.1080/10717544.2018.1474964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Ding Y., Zhou Y., Jin W., Xie K., Chen L. L. (2017). CRISPR-P 2.0: An improved CRISPR/Cas9 tool for genome editing in plants. Mol. Plant 10, 530–532. 10.1016/j.molp.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Liu M., Rehman S., Tang X., Gu K., Fan Q., Chen D., et al. (2019). Methodologies for improving HDR efficiency. Front. Genet. 9, 691–699. 10.3389/fgene.2018.00691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Q., Yang G., Zhang C., Dong H., Liu Y., et al. (2020). Pivotal roles of Tomato photoreceptor SlUVR8 in seedling development and UV-B stress tolerance. Biochem. Biophys. Res. Commun. 522, 177–183. 10.1016/j.bbrc.2019.11.073 [DOI] [PubMed] [Google Scholar]
- Ma C., Liu M., Li Q., Si J., Ren X., Song H. (2019). Efficient BoPDS gene editing in cabbage by the CRISPR/Cas9 system. Hortic. Plant J. 5, 164–169. 10.1016/j.hpj.2019.04.001 [DOI] [Google Scholar]
- Maioli A., Gianoglio S., Moglia A., Acquadro A., Valentino D., Milani A. M., et al. (2020). Simultaneous CRISPR/Cas9 editing of three PPO genes reduces fruit flesh browning in Solanum melongena L. Front. Plant Sci. 11, 607161. 10.3389/fpls.2020.607161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhotenko A. V., Khromov A. V., Snigir E. A., Makarova S. S., Makarov V. V., Suprunova T. P., et al. (2019). Functional analysis of coilin in virus resistance and stress tolerance of potato Solanum tuberosum using CRISPR-cas9 editing. Dokl. Biochem. Biophys. 484, 88–91. 10.1134/S1607672919010241 [DOI] [PubMed] [Google Scholar]
- Mei Y., Wang Y., Chen H., Sun Z. S., Da J. X. (2016). Recent progress in CRISPR/Cas9 technology. J. Genet. Genomics. 43, 63–75. 10.1016/j.jgg.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Miller J. C., tis M. C., Wang J. B., Guschin D. Y., Lee Y. L., Rupniewski I., et al. (2007). An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 25, 778–785. 10.1038/nbt1319 [DOI] [PubMed] [Google Scholar]
- Minkenberg B., Zhang J., Xie K., Yang Y. (2019). CRISPR-PLANT v2: An online resource for highly specific guide RNA spacers based on improved off-target analysis. Plant Biotechnol. J. 17, 5–8. 10.1111/pbi.13025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague T. G., Cruz J. M., Gagnon J. A., Church G. M., Valen E. (2014). CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, 401–407. 10.1093/nar/gku410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S. B., Lee J. M., Kang J. G., Lee N. E., Ha D. I., Kim D. Y., et al. (2018). Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3’-overhang. Nat. Commun. 9, 3651. 10.1038/s41467-018-06129-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mateos M. A., Vejnar C. E., Beaudoin J. D., Fernandez J. P., Mis E. K., Khokha M. K., et al. (2015). CRISPRscan: Designing highly efficient sgRNAs for CRISPR/Cas9 targeting in vivo . Nat. Methods 12, 982–988. 10.1038/nmeth.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Hino K., Bono H., Ui-Tei K. (2014). CRISPRdirect: Software for designing CRISPR/cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123. 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu M., Akiyama R., Lee J. H., Osakabe K., Osakabe Y., Watanabe B., et al. (2018). Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol. biochem. 131, 70–77. 10.1016/j.plaphy.2018.04.026 [DOI] [PubMed] [Google Scholar]
- Nature Plants Editorial (2018). A CRISPR definition of genetic modification. Nat. Plants 4, 233. 10.1038/s41477-018-0158-1 [DOI] [PubMed] [Google Scholar]
- Nekrasov V., Wang C., Win J., Lanz C., Weigel D., Kamoun S. (2017). Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 7, 482. 10.1038/s41598-017-00578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazian M., Sadatnoori S. A., Galuszka P., Mortazavian S. M. M. (2017). Tissue culture-based Agrobacterium-mediated and in Planta transformation methods. Czech J. Genet. Plant Breed. 53, 133–143. 10.17221/177/2016-CJGPB [DOI] [Google Scholar]
- Nonaka M., Arai M., akayama M., Matsukura M., Ezura M. (2017). Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 7, 7057. 10.1038/s41598-017-06400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez de Caceres Gonzalez F. F., De la Mora Franco D. (2020). “Vegetable crop improvement using CRISPR/Cas9,” in CRISPR/Cas genome editing: Strategies and Potential for crop improvement. Editors Bhattacharya A., Parkhi V., Char B. (Cham: Springer International Publishing; ), 119–129. [Google Scholar]
- O’Driscoll M., Jeggo P. A. (2006). The role of double-strand break repair—Insights from human genetics. Nat. Rev. Genet. 7, 45–54. 10.1038/nrg1746 [DOI] [PubMed] [Google Scholar]
- Oliveros J. C., Franch M., Tabas-Madrid D., San-Leon D., Montoliu L., Cubas P., et al. (2016). Breaking-Cas—Interactive design of guide RNAs for CRISPR-cas experiments for ENSEMBL genomes. Nucleic Acids Res. 44, W267–W271. 10.1093/nar/gkw407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigosa A., Gimenez-Ibanez S., Leonhardt N., Solano R. (2019). Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol. J. 17, 665–673. 10.1111/pbi.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C., Ye L., Qin L., Liu X., He Y., Wang J., et al. (2016). CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci. Rep. 6, 24765. 10.1038/srep24765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar P., Singh S., Singh R., Singh V. P., Prasad S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 22, 4056–4075. 10.1007/s11356-014-3739-1 [DOI] [PubMed] [Google Scholar]
- Park J., Bae S. (2017). Cpf1-database: Web-based genome-wide guide RNA library design for gene knockout screens using CRISPR-cpf1. Bioinformatics 34, 1077–1079. 10.1093/bioinformatics/btx695 [DOI] [PubMed] [Google Scholar]
- Park J., Kim J. S., Bae S. (2016). Cas-database: Web-based genome-wide guide RNA library design for gene knockout screens using CRISPR/Cas9. Bioinformatics 32, 2017–2023. 10.1093/bioinformatics/btw103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. A. J., Madgwick P. J., Bayon C., Tearall K., Hernandez-Lopez A., Baudo M., et al. (2009). Mutation discovery for crop improvement. J. Exp. Bot. 60, 2817–2825. 10.1093/jxb/erp189 [DOI] [PubMed] [Google Scholar]
- Paul H. M., Istanto D. D., Heldenbrand J., Hudson M. E. (2022). Cropsr: An automated platform for complex genome-wide CRISPR gRNA design and validation. BMC Bioinforma. 23, 74. 10.1186/s12859-022-04593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A. R., Pritykin Y., Vidigal J. A., Chhangawala S., Zamparo L., Leslie C. S., et al. (2017). GuideScan software for improved single and paired CRISPR guide RNA design. Nat. Biotechnol. 35, 347–349. 10.1038/nbt.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petretto G. L., Urgeghe P. P., Massa D., Melito S. (2019). Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. biochem. 141, 30–39. 10.1016/j.plaphy.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Pliatsika V., Rigoutsos I. (2015). Off-spotter”: Very fast and exhaustive enumeration of genomic lookalikes for designing CRISPR/cas guide RNAs. Biol. Direct 10, d4. 10.1186/s13062-015-0035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel R., Rodriguez L. T., Reisch C. R., Rivers A. R. (2022). GuideMaker: Software to design CRISPR-Cas guide RNA pools in non-model genomes. Gigascience 11, giac007–8. 10.1093/gigascience/giac007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik D., Shelake R. M., Park J., Kim M. J., Hwang I., Park Y., et al. (2021). CRISPR/Cas9-Mediated generation of pathogen-resistant tomato against tomato yellow leaf curl virus and powdery mildew. Int. J. Mol. Sci. 22, 1878. 10.3390/ijms22041878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihatna C., Barbetti M. J., Barker S. J. (2018). A novel tomato Fusarium wilt tolerance gene. Front. Microbiol. 9, 1226. 10.3389/fmicb.2018.01226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prykhozhij S. V., Rajan V., Gaston D., Berman J. N. (2015). CRISPR multitargeter: A web tool to find common and unique CRISPR single guide RNA targets in a set of similar sequences. PLoS One 10, e0119372. 10.1371/journal.pone.0119372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Leal D., Lemmon Z. H., Man J., Bartlett M. E., Lippman Z. B. (2017). Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480. 10.1016/j.cell.2017.08.030 [DOI] [PubMed] [Google Scholar]
- Roldan M. V. G., Perilleux C., Morin H., Huerga-Fernandez S., Latrasse D., Benhamed M., et al. (2017). Natural and induced loss of function mutations in SlMBP21 MADS-box gene led to jointless-2 phenotype in tomato. Sci. Rep. 7, 4402. 10.1038/s41598-017-04556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon C., Zhou M., Zhang J., Politis A., Beilsten-Edmands V., Cannone G., et al. (2013). Structure of the CRISPR interference complex CSM reveals key similarities with cascade. Mol. Cell 52, 124–134. 10.1016/j.molcel.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindele P., Puchta H. (2020). Engineering CRISPR/LbCas12a for highly efficient, temperature-tolerant plant gene editing. Plant Biotechnol. J. 8, 1118–1120. 10.1111/pbi.13275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevestre F., Facon M., Fabrice Wattebled F., Nicolas Szydlowski N. (2020). Facilitating gene editing in potato: A single-nucleotide polymorphism (SNP) map of the Solanum tuberosum L. Cv. Desiree genome. Sci. Rep. 10, 2045. 10.1038/s41598-020-58985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M., Xu T., Chen C. (2016). The big bang of genome editing technology: Development and application of the CRISPR/CAS9 system in disease animal models. Sci. Press Zool. Res. 37, 191–204. 10.13918/j.issn.2095-8137.2016.4.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Verma N., Pandey C., Verma D., Bhagat P., Noryang S., et al. (2020). “MAP kinase as regulators for stress responses in plants: An overview,” in book: Protein kinases and stress signaling in plants, 369–392. 10.1002/9781119541578.ch15 [DOI] [Google Scholar]
- Shuman S., Glickman M. S. (2007). Bacterial DNA repair by non-homologous end joining. Nat. Rev. Microbiol. 5, 852–861. 10.1038/nrmicro1768 [DOI] [PubMed] [Google Scholar]
- Siegner S. M., Karasu M. E., Schroder M. S., Kontarakis Z., Corn J. E. (2021). PnB designer: A web application to design prime and base editor guide RNAs for animals and plants. BMC Bioinforma. 22, 101. 10.1186/s12859-021-04034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth S. J. (2017). Canadian regulatory perspectives on genome engineered crops. Gm. Crops Food 8, 35–43. 10.1080/21645698.2016.1257468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyk S., Muller N., Ju Park S., Schmalenbach I., Jiang K., Hayama R., et al. (2017). Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 49, 162–168. 10.1038/ng.3733 [DOI] [PubMed] [Google Scholar]
- Sun Q., Lin L., Liu D., Wu D., Fang Y., Wu J., et al. (2018). CRISPR/Cas9-Mediated multiplex genome editing of the BnWRKY11 and BnWRKY70 genes in Brassica napus L. Int. J. Mol. Sci. 19, 2716. 10.3390/ijms19092716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Liu H., Liu J., Cheng S., Peng Y., Zhang Q., et al. (2019). CRISPR-local: A local single-guide RNA (sgRNA) design tool for non-reference plant genomes. Bioinformatics 35 (14), 2501–2503. 10.1093/bioinformatics/bty970 [DOI] [PubMed] [Google Scholar]
- Tashkandi M., Ali Z., Aljedaani F., Shami A., Mahfouz M. M. (2018). Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant Signal. Behav. 13, e1525996. 10.1080/15592324.2018.1525996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F., Cui T., Feng G., Guo L., Xu K., Gao Q., et al. (2018). Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 4, 63. 10.1038/s41421-018-0069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazella P. d. T., Brail Q., Dahlbeck D., Staskawicz B. (2016). “CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance,” in Proceedings of the national academy of sciences (Berkeley, CA: bioRxiv; ), 1–23. 10.1101/064824:064824 [DOI] [Google Scholar]
- Thysegen P. (2019). Clarifying the regulation of genome editing in Australia: Situation for genetically modified organisms. Transgenic Res. 28, 151–159. 10.1007/s11248-019-00151-4 [DOI] [PubMed] [Google Scholar]
- Tian S., Jiang L., Gao Q., Zhang J., Zong M., Zhang H., et al. (2017). Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 36, 399–406. 10.1007/s00299-016-2089-5 [DOI] [PubMed] [Google Scholar]
- Tian S., Jiang L., Cui X., Zhang J., Guo S., Li M., et al. (2018). Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 37, 1353–1356. 10.1007/s00299-018-2299-0 [DOI] [PubMed] [Google Scholar]
- Tian S. W., Xing S. N., Yong X. (2021). Advances in CRISPR/Cas9-mediated genome editing on vegetable crops. Vitro Cell. Dev. Biol. -Plant. 4, 672–682. 10.1007/s11627-021-10187-z [DOI] [Google Scholar]
- Tilman D., Balzer C., Hill J., Befort B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U. S. A. 108, 20260–20264. 10.1073/pnas.1116437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilson L., Yang Y., Emenecker R., Smoker M., Taylor J., Parkins S., et al. (2019). Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol. J. 17, 132–140. 10.1111/pbi.12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M. T., Doan D. T. H., Kim J., Song Y. J., Sung Y. W., Das S., et al. (2021). CRISPR/Cas9-based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep. 40, 999–1011. 10.1007/s00299-020-02622-z [DOI] [PubMed] [Google Scholar]
- Ueta R., Takahito Watanabe T., Sugano S. S., Ishihara R., Ezura H., Osakabe Y., et al. (2017). Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci. Rep. 7, 507. 10.1038/s41598-017-00501-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vu T., Sung Y. W., Kim J., Doan D. T. H., Tran M. T., Kim J.-Y. (2019). Challenges and perspectives in homology-directed gene targeting in monocot plants. Rice 12, 95. 10.1186/s12284-019-0355-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillet F., Perrot L., Chauvin L., Kermarrec M. P., Guyon-Debast A., Chauvin J. E., et al. (2019). Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 20, 402. 10.3390/ijms20020402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. V., Sivankalyani V., Kim E. J., Doan D. T. H., Tran M. T., Kim J., et al. (2020). Highly efficient homology‐directed repair using CRISPR/Cpf1‐gemini viral replicon in tomato. Plant Biotechnol. J. 18, 2133–2143. 10.1111/pbi.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz E. (2016a). CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 34, 582. 10.1038/nbt0616-582 [DOI] [PubMed] [Google Scholar]
- Waltz E. (2016b). Gene-edited CRISPR mushroom escapes US regulation. Nature 532, 293. 10.1038/nature.2016.19754 [DOI] [PubMed] [Google Scholar]
- Wang L., Chen L., Li R., Zhao R., Yang M., Sheng J., et al. (2017). Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 65, 8674–8682. 10.1021/acs.jafc.7b02745 [DOI] [PubMed] [Google Scholar]
- Wang X. L. Y., Chen S., Tian H., Fu D., Zhu B., Luo Z. Y., et al. (2018a). Lycopene is enriched in tomato fruit by CRISPR/Cas9-Mediated multiplex genome editing. Front. Plant Sci. 26, 559. 10.3389/fpls.2018.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Samsul Rizal N., Yan C., Allcock N., Craigon J., Blanco-Ulate B., et al. (2018b). Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 179, 544–557. 10.1104/pp.18.01187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zhang H., Zhu H. (2019a). CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 6, 77. 10.1038/s41438-019-0159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Tavano E. C. D., Lammers M., Martinelli A. P., Angenent G. C., Maagd R. A. (2018b). Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Sci. Rep. 9, 1696. 10.1038/s41598-018-38170-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier R., Pande H. (2016). Genetically engineered plants in the product development pipeline in India. Gm. Crops Food 7, 12–19. 10.1080/21645698.2016.1156826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan A. I., Lema M. A. (2015). Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. Gm. Crops Food 6, 253–265. 10.1080/21645698.2015.1114698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F., Puchta H. (2018). The CRISPR/Cas revolution reaches the RNA world: Cas13, a new Swiss Army knife for plant biologists. Plant J. 94, 767–775. 10.1111/tpj.13899 [DOI] [PubMed] [Google Scholar]
- Wong N., Liu W., Wang X. (2015). Wu-CRISPR: Characteristics of functional guide RNAs for the CRISPR/Cas9 system. Genome Biol. 16, 218. 10.1186/s13059-015-0784-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Qiao X., Zhao Y., Zhang Z., Gao Y., Shi L., et al. (2020). Targeted mutagenesis in Arabidopsis thaliana using CRISPR-Cas12b/C2c1. J. Integr. Plant Biol. 11, 1653–1658. 10.1111/jipb.12944 [DOI] [PubMed] [Google Scholar]
- Xie X., Li F., Tan X., Zeng D., Liu W., Zeng W., et al. (2022). BEtarget: A versatile web-based tool to design guide RNAs for base editing in plants. Comput. Struct. Biotechnol. J. 20, 4009–4014. 10.1016/j.csbj.2022.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Qin R., Li H., Li D., Li L., Wei P., et al. (2017). Generation of targeted mutant rice using a CRISPR-Cpf1 system. Plant Biotechnol. J. 15, 713–717. 10.1111/pbi.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Kang B. C., Naing A. H., Bae S. J., Kim J. S., Kim H., et al. (2019a). CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 18, 287–297. 10.1111/pbi.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. S., Feng K., Xiong A. S. (2019b). CRISPR/Cas9-mediated multiply targeted mutagenesis in orange and purple carrot plants. Mol. Biotechnol. 61, 191–199. 10.1007/s12033-018-00150-6 [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhu G., Li R., Yan S., Fu D., Zhu B., et al. (2017). The RNA editing factor SlORRM4 is required for normal fruit ripening in tomato. Plant Physiol. 175, 1690–1702. 10.1104/pp.17.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Ren S., Yu S., Pan H., Li T., Ge S., et al. (2020). Methods favoring homology-directed repair choice in response to CRISPR/cas9 induced-double strand breaks. Int. J. Mol. Sci. 21, 6461. 10.3390/ijms21186461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. H., Kim E., Park H., Koo Y. (2022). Selection of the high efficient sgRNA for CRISPR-Cas9 to edit herbicide related genes, PDS, ALS, and EPSPS in tomato. Appl. Biol. Chem. 65, 13. 10.1186/s13765-022-00679-w [DOI] [Google Scholar]
- Yin Y., Qin K., Song X., Zhang Q., Zhou Y., Xia X., et al. (2018). BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 59, 2239–2254. 10.1093/pcp/pcy146 [DOI] [PubMed] [Google Scholar]
- Yu Q. H., Wang B., Li N., Tang Y., Yang S., Yang T., et al. (2017). CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long shelf-life tomato lines. Sci. Rep. 7, 11874. 10.1038/s41598-017-12262-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Wang L., Zhao R., Shen G. J., Zhang S., Li R., et al. (2019). Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol. 19, 354. 10.1186/s12870-019-1939-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Zhang F., Zhong Z., Chen R., Wang Y., Chang L., et al. (2019). Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol. J. 17, 1814–1822. 10.1111/pbi.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Karen M., Ian D. G., Caixia G. (2018). Applications and potential of genome editing in crop improvement. Genome Biol. 19, 210. 10.1186/s13059-018-1586-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Guo S., Ji G., Zhao H., Sun H., Ren Y., et al. (2019a). A unique chromosome translocation disrupting ClWIP1 leads to gynoecy in watermelon. Plant J. 101, 265–277. 10.1111/tpj.14537 [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Q., Yang X., Xu J., Liu G., Yao X., et al. (2020). CRISPR/Cas9-mediated editing of ClPSK1 enhanced watermelon resistance to Fusarium oxysporum . Plant Cell Rep. 39, 589–595. 10.1007/s00299-020-02516-0 [DOI] [PubMed] [Google Scholar]
- Zhao X., Jayarathna S., Turesson H., Falt A-S., Nestor G., Gonzalez M. N., et al. (2021). Amylose starch with no detectable branching developed through DNA-free CRISPR-Cas9 mediated mutagenesis of two starch branching enzymes in potato. Sci. Rep. 11, 4311. 10.1038/s41598-021-83462-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N. Z., Martin G. B., Fei Z. (2019). Plant genome editing database (pged): A call for submission of information about genome-edited plant mutants. Mol. Plant 12, 127–129. 10.1016/j.molp.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Zhu L. J., Holmes B. R., Aronin N., Brodsky M. H. (2014). CRISPRseek: A bioconductor package to identify target-specific guide RNAs for CRISPR/Cas9 genome-editing systems. PLoS One 9, e108424. 10.1371/journal.pone.0108424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Li C., Gao C. (2020). Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677. 10.1038/s41580-020-00288-9 [DOI] [PubMed] [Google Scholar]
- Zsogon A., Cermak T., Naves E. R., Notini M. M., Edel K. H., Weinl S., et al. (2018). De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36, 1211–1216. 10.1038/nbt.4272 [DOI] [PubMed] [Google Scholar]