Abstract
The germfree mouse model of Vibrio cholerae infection can be used to judge immune responses to V. cholerae vaccine and vector strains. In the original model, a single oral inoculation was administered on day 0, a booster oral inoculation was administered on day 14, and immune responses were analyzed with samples collected on day 28. Unfortunately, immune responses in this model frequently were low level, and interanimal variability occurred. In order to improve this model, we evaluated various primary and booster V. cholerae inoculation schedules. The most prominent systemic and mucosal antibody responses were measured in mice that received a multiple primary inoculation series on days 0, 2, 4, and 6 and booster inoculations on days 28 and 42. These modifications result in improved preliminary evaluation of V. cholerae vaccine and vector strains in mice.
Vibrio cholerae has a number of attributes that make it an attractive candidate for development as a vector for inducing mucosal immunity. Immune responses induced by V. cholerae are long lasting and involve both mucosal and systemic immune systems (9, 13). Attenuated strains of V. cholerae that have been shown to be both safe and immunogenic in humans have already been developed (1, 10–12, 19, 20). We have recently utilized the hemolysin operon of Escherichia coli to obtain secretion of large heterologous antigens in attenuated vaccine strains of V. cholerae and have shown that immunization with these vaccine vectors results in immune responses that are protective against subsequent challenge (15). We have also recently shown that attenuated vaccine and vector strains of V. cholerae can secrete immunoadjuvants, such as the nontoxic mutant of E. coli heat-labile enterotoxin LTR192G, in vivo, resulting in boosting of immune responses against coexpressed V. cholerae antigens (17). Additionally, we recently reported the development of a balanced lethal plasmid system, based on a complemented mutation of the glutamine synthetase gene of V. cholerae, glnA; this system permits high-level expression of heterologous antigens by attenuated vaccine and vector strains of V. cholerae (18).
The development of V. cholerae organisms as vectors for inducing mucosal immune responses against heterologous antigens has been limited by the paucity of animal models for preliminary in vivo evaluation of V. cholerae-based vaccines. V. cholerae is a human pathogen that is unable to colonize the intestinal tracts of most animal species (14). Rabbits have historically been used to evaluate V. cholerae immune responses; however, using the rabbit model of V. cholerae infection is time-consuming and labor-intensive (2, 4, 15). Additionally, a neonatal mouse lethality model has been used to judge pathogenicity of V. cholerae strains (6, 14); however, this model cannot be used to judge immune responses because of the rapidity of death and the immaturity of the immune system of the inoculated mice. We previously reported the development of an adult germfree mouse model for evaluating vector and vaccine strains of V. cholerae (3, 16–18). This model involves the use of 3- to 4-week-old germfree mice that are removed from their shipper and immediately inoculated with V. cholerae strains of interest. The mice are then housed under nongermfree conditions. This animal model is simple, the mice are housed and cared for under routine conditions, and the model permits the simultaneous evaluation of multiple V. cholerae vaccine strains. In our original germfree mouse model, a single oral inoculation of 250 μl was administered via rigid gavage tubing on day 0, an identical booster oral inoculation was administered on day 14, and immune responses were analyzed on samples collected on day 28 (16). Immune responses induced by this model, however, were low level, interanimal variability would occur, and inoculation-related mortality was problematic (16–18). Improvement of this animal model would facilitate more rapid preliminary in vivo evaluation of V. cholerae vaccine and vector strains.
Colonization studies with the germfree mouse model have shown that V. cholerae organisms are present in the stools of mice for 7 to 14 days after a single oral inoculation on day 0 (3, 16, 18), although the number of V. cholerae organisms isolated per stool pellet falls rapidly within the first 48 to 72 h after day 0 inoculation (data not shown). We therefore evaluated whether a primary vaccination series consisting of repetitive inoculations administered every 48 h for a week could result in improved immunological responses in mice that received attenuated strains of V. cholerae. Such increased immunogenicity occurs in humans who receive repetitive oral administration of the U. S. Food and Drug Administration-approved typhoid vaccine based on the attenuated Salmonella enterica serovar Typhi strain Ty21a (Vivotef Berna vaccine; Swiss Serum and Vaccine Institute, Bern, Switzerland) (5, 8); this vaccine is widely used in travelers, and standard administration is every 48 h for four oral doses.
We have also previously found that V. cholerae strains are recoverable from the stools of mice for only 1 to 2 days after day 14 or later booster inoculation, presumably due to increased competition from intestinal flora newly constituted after removal of mice from germfree conditions (16, 18). Despite such transient intestinal presence, we have previously found that reinoculation on day 14 results in boosting of immune responses in samples collected on day 28 (16). In order to evaluate the effect of additional booster inoculations on immune responses, and in order to judge optimal timing for immunological sampling, we compared two booster vaccination schedules and immune responses in samples collected 28, 42, and 56 days after day 0 inoculation.
For these experiments, we used a previously described balanced lethal plasmid system for high level expression of antigen (17). We used an attenuated vaccine strain of V. cholerae that is auxotrophic for glutamine, Peru2ΔglnA; this strain is a nontoxic derivative of V. cholerae O1 E1 Tor C6709 (ΔattRS1 ΔctxAB) and contains an internal in-frame 354-bp deletion in the chromosomal glnA gene (corresponding to amino acids phenylalanine-134 to glycine-251) (18). This strain expresses neither cholera holotoxin nor the nontoxic B subunit of cholera toxin (CtxB). Peru2ΔglnA is unable to grow in M9 minimal media lacking glutamine; this nutritional deficiency is complemented by pKEK71-NotI, a plasmid containing the S. enterica serovar Typhimurium glnA gene under the control of a high-level sigma 54-independent promoter (7). The auxotrophy is also complemented by plasmid pTIC5, a pKEK71-NotI derivative containing a 1.8-kbp fragment that directs expression of the nontoxic B subunit (CtxB) of cholera toxin with a 12-amino-acid epitope of the serine-rich Entamoeba histolytica protein fused to the amino terminus (SREHP-12–CtxB) (16, 18). We have previously shown that mice that receive Peru2ΔglnA(pTIC5) develop mucosal and systemic anti-CtxB immune responses that are more prominent than those induced by a vaccine strain of V. cholerae expressing SREHP-12–CtxB from the chromosome (18). We inoculated mice with either Peru2ΔglnA(pTIC5) or Peru2ΔglnA(pKEK71-NotI) using various inoculation schedules and measured systemic and mucosal anti-CtxB responses at a number of time points.
Immediately upon removal of mice from the shipping container, six groups of 5 to 25 germfree female Swiss mice, 3 to 4 weeks old (Taconic Farms, Inc., Germantown, N.Y.), were orally inoculated via gastric intubation with 250-μl inocula containing approximately 108 organisms of V. cholerae strains resuspended in 0.5 M NaHCO3 (pH 8.0) (4). In a change from our previous model, oral inocula were administered through soft polyethylene tubing (catalogue no. 427416: internal diameter, 0.76 mm; external diameter, 1.22 mm; Intramedic Clay Adams brand; Becton Dickinson & Co., Sparks, Md.) rather than through rigid gavage tips. Prior to inoculation, Peru2ΔglnA(pKEK71-NotI) and Peru2ΔglnA(pTIC5) were grown in M9 minimal media supplemented with 0.05 mM thiamine (Sigma Chemical Co., St. Louis, Mo.) and 0.3 mM cysteine (Sigma) but containing neither glutamine nor antibiotics. Mice were subsequently housed in nongermfree conditions. Neither antibiotic selection pressure nor specific nutritional supplementation was implemented in vivo. Mice were divided into three groups: one cohort was orally inoculated with Peru2ΔglnA(pTIC5) on day 0, with oral booster inoculations on days 14, 28, and 42. This group is referred to as the single primary inoculation group (number of inoculated mice, 12). The second cohort of mice received primary oral inoculations on days 0, 2, 4, and 6, with oral booster inoculations on days 28 and 42. This group is referred to as multiple primary inoculation group I (number of inoculated mice, 25). The third cohort of mice received primary oral inoculations on days 0, 2, 4, and 6, with oral booster inoculations on days 14, 28, and 42. This group is referred to as multiple primary inoculation group II (number of inoculated mice, 9). Groups of control mice for each group were similarly inoculated; these animals received V. cholerae control strain Peru2ΔglnA(pKEK71-NotI), which does not express CtxB. In the single primary inoculation control group, 10 mice were inoculated with Peru2ΔglnA(pKEK71-NotI); 6 control mice were inoculated in multiple primary inoculation group I, and 9 control mice were inoculated in multiple primary inoculation group II.
Blood was collected via tail bleeds on days 28 and 42 (16). Mice were sacrificed on day 56, at which point blood was collected via cardiac puncture; bile was also collected by hepatic dissection and aspiration of gall bladder contents (16, 17). Blood and bile samples were processed as previously described, divided into aliquots, and stored at −70°C for subsequent analysis (17).
Serum vibriocidal-antibody titers were measured by a microassay as previously described, with the modification that Luria-Bertani broth was used in place of brain heart infusion media (16, 17). V. cholerae O1 Peru2 was used as the vibriocidal target strain. Specific anti-CtxB immunoglobulin G (IgG) and IgA antibodies in sera were detected by using microtiter plates previously coated with ganglioside and CtxB and developed for peroxidase activity in an enzyme-linked immunosorbent assay (ELISA) as previously described (16–18). Optical density at 405 nm was detected kinetically with a Vmax microplate reader (Molecular Devices Corp., Sunnyvale, Calif.), and plates were read for 5 min at 19-s intervals; the maximum slope for an optical density change of 0.2 U was reported as milli-optical density units per minute (15, 17, 18).
To detect specific IgA antibody responses in bile, measurements of total bile IgA were first taken as previously described; comparisons to a mouse IgA standard were made (Kappa TEPC 15; Sigma) (17, 18). To detect specific anti-CtxB IgA antibody in bile, duplicate 200-μl samples of bile containing 200 ng of total IgA in phosphate-buffered saline (PBS)–0.05% Tween 20 (PBS-T; Sigma) were added to wells previously coated with ganglioside-CtxB (17, 18). After incubation of plates, a 1:2,000 dilution of goat anti-mouse IgA–biotin conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) in PBS-T was added. The plates were tested for horseradish peroxidase activity and the optical density at 405 nm was determined kinetically as described above.
Statistical analysis for comparison of geometric means was performed for normally distributed data with the independent-sample Student t test or with the Mann-Whitney U test for nonparametric data by use of SPSS for Windows 8.0 (17, 18). Data were plotted with Microsoft Excel 7.0a and GraphPad Prism 3.0.
Mice tolerated the modified inoculation procedures and schedules well. Procedure-related mortality was lower in mice that received oral inocula through soft polyethylene tubing than in those inoculated by the use of rigid gavage tubing (data not shown). With this modification, and with a subsequent lowering of the volume of oral inocula from 250 to 125 μl (6a), we have effectively eliminated inoculation-related mortality from the germfree mouse model of V. cholerae infection.
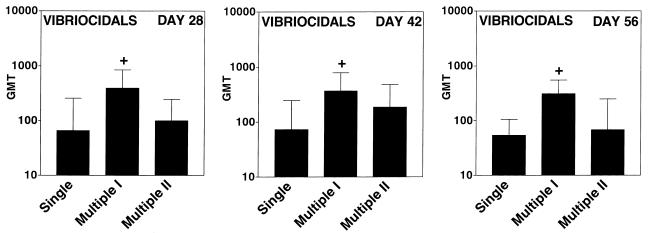
Vibriocidal antibodies were measured on serum samples collected on days 28, 42, and 56 (Fig. 1). Vibriocidal antibodies are a measure of immune responses against V. cholerae organisms themselves and reflect the ability of V. cholerae strains to colonize the intestine. In our experiment, all groups of mice developed vibriocidal antibody responses as expected. Interestingly, the vibriocidal antibody responses did not increase over time with booster inoculations in any group of animals, perhaps reflecting the short (1- to 2-day) presence of V. cholerae organisms in the intestines of mice after a day 14 or later inoculation. The vibriocidal antibody response was, however, higher in animals that received multiple primary inoculation I then in animals that received the single primary inoculation (Fig. 1). This increase presumably related to the higher number of V. cholerae organisms in the intestines of animals receiving multiple oral inoculations during the first week of vaccination. Interestingly, the addition of a day 14 booster did not increase the vibriocidal antibody response in animals that received a primary multiple inoculation series; indeed, it was associated with a lower response. Compared to the response in mice that received multiple inoculation I, the vibriocidal antibody response in mice that received multiple inoculation II (with a day 14 booster) was lower on day 28 (P ≤ 0.05) and day 56 (P ≤ 0.05).
FIG. 1.
Geometric mean titers (GMT) of vibriocidal antibody responses on day 28, 42, and 56 following oral inoculation of mice with V. cholerae vaccine strains by using various inoculation schedules (the fewest mice were evaluated on day 56): single inoculation (on day 56, the number of evaluated mice was 16), multiple inoculation I (on day 56, the number of evaluated mice was 15), and multiple inoculation II (on day 56, the number of evaluated mice was 9). Mice received either control strain Peru2ΔglnA(pKEK71-NotI) or vaccine strain Peru2ΔglnA(pTIC5). Within each inoculation group, no difference was detected between mice that received Peru2ΔglnA(pTIC5) or Peru2ΔglnA(pKEK71-NotI) (data not shown), and, therefore, mean vibriocidal-antibody titers are grouped by inoculation schedule. Error bars depict standard errors of the mean for each group. +, P ≤ 0.01 compared to animals receiving the single primary inoculation.
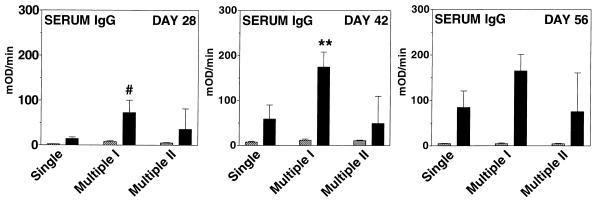
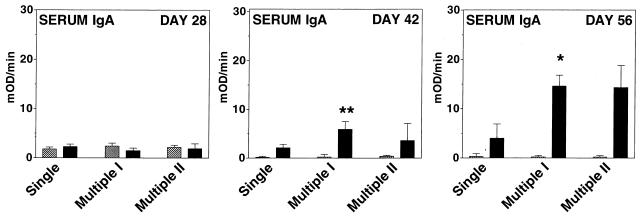
Specific anti-CtxB antibody responses were measured in serum samples collected on days 28, 42, and 56, and anti-CtxB antibody responses were measured in bile on day 56. The most prominent anti-CtxB IgG responses were seen in mice that received multiple inoculation I (Fig. 2). Compared to the response in mice that received a single primary inoculation, mice that received multiple inoculation I had a statistically significant serum anti-CtxB IgG antibody response on day 28 (P ≤ 0.001) and day 42 (P ≤ 0.02). Similarly, compared to the single-inoculation group, more prominent anti-CtxB serum IgA responses were seen in mice that received multiple inoculation I or II (Fig. 3); compared to the single-inoculation cohort, mice that received multiple inoculation I had a statistically significant serum anti-CtxB IgA antibody response on day 42 (P ≤ 0.02) and day 56 (P ≤ 0.05).
FIG. 2.
Serum IgG anti-CtxB ELISA results for day 28, 42, and 56 samples from mice inoculated with V. cholerae control strain Peru2ΔglnA(pKEK71-NotI) (hatched columns) or vaccine strain Peru2ΔglnA(pTIC5) (solid columns) by using various inoculation schedules as for Fig. 1 (the fewest mice were evaluated on day 56). On day 56, the number of evaluated control mice that had been inoculated with Peru2ΔglnA(pKEK71-NotI) in the single primary inoculation group was 7, in multiple primary inoculation group I it was 4, and in multiple primary inoculation group II it was 4. On day 56, the number of evaluated mice that had been inoculated with vaccine strain Peru2ΔglnA(pTIC5) in the single primary inoculation group was 9, in multiple primary inoculation group I it was 11, and in multiple primary inoculation group II it was 5. The geometric mean plus the standard error of the mean is reported for each group. mOD, milli-optical density units. #, P ≤ 0.001; ∗∗, P ≤ 0.02, compared to animals receiving vaccine strain Peru2ΔglnA(pTIC5) with the single primary inoculation schedule.
FIG. 3.
Serum IgA anti-CtxB ELISA results for day 28, 42, and 56 samples from mice inoculated with V. cholerae control strain Peru2ΔglnA(pKEK71-NotI) (hatched columns) or vaccine strain Peru2ΔglnA(pTIC5) (solid columns) using various inoculation schedules as for Fig. 1. For the number of mice in each group, see the legend to Fig. 2. The geometric mean plus the standard error of the mean is reported for each group. mOD, milli-optical density units. ∗∗, P ≤ 0.02, and ∗, P ≤ 0.05, compared to animals receiving vaccine strain Peru2ΔglnA(pTIC5) with the single primary inoculation schedule.
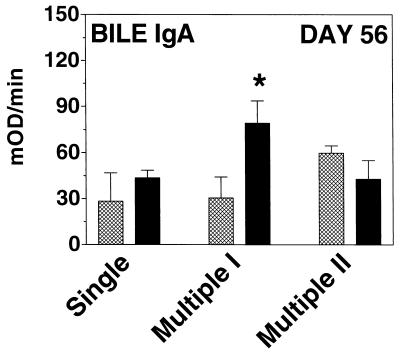
On day 56, a statistically significant difference was detectable in the anti-CtxB IgA responses in bile among the various groups of animals that received vaccine strain Peru2ΔglnA(pTIC5) (Fig. 4). The most prominent anti-CtxB IgA response in bile was detected in mice that received the vaccine strain in multiple inoculation schedule I; compared to the response in mice that received the single primary inoculation, mice that received multiple inoculation I had a statistically significant anti-CtxB IgA antibody response in bile (P ≤ 0.05).
FIG. 4.
Bile IgA anti-CtxB ELISA results on day 56 samples from mice inoculated with V. cholerae control strain Peru2ΔglnA(pKEK71-NotI) (hatched columns) or vaccine strain Peru2ΔglnA(pTIC5) (solid columns) by using various inoculation schedules as for Fig. 1. For the number of mice in each group, see the legend to Fig. 2. The geometric mean plus the standard error of the mean is reported for each group. mOD, milli-optical density units. ∗, P ≤ 0.05 compared to animals receiving vaccine strain Peru2ΔglnA(pTIC5) with the single primary inoculation schedule.
In summary, using the germfree mouse model of V. cholerae infection, we compared a number of oral inoculation schedules. We found that systemic and mucosal immune responses were more prominent in animals that received a primary inoculation series of four oral inoculations administered on alternate days (days 0, 2, 4, and 6) than in animals that received a single primary inoculation. This improved immunogenicity is probably related to the higher numbers of organisms in the intestinal tracts of animals that receive a primary vaccination series consisting of multiple inoculations. Additionally, building on our previous finding that a single booster oral inoculation of V. cholerae in mice increases immune responses, we found that additional booster inoculations are associated with immune responses that continue to increase over time. We detected the most prominent specific immune responses in samples collected on day 56, the last day samples were collected in this study. Interestingly, this increase was observed only for immune responses directed against an expressed antigen; vibriocidal immune responses did not increase after day 28. We also found that immune responses in mice that received booster inoculations of vaccine strains on days 14, 28, and 42 were less prominent than those in mice that received booster inoculations on days 28 and 42. Why the addition of a day 14 booster decreased immunogenicity in multiple-inoculation group II animals is currently unclear.
Based on these data, we have modified our germfree mouse model as follows: we orally inoculate mice with a primary series of V. cholerae vaccine and vector strains on days 0, 2, 4, and 6; we administer booster inoculations on days 28 and 42; and we sample immunological responses in animals on day 56. We currently administer oral inocula of 125 μl. These modifications allow improved preliminary evaluation of vaccine and vector strains of V. cholerae in animals. Additional modifications to the model are currently being explored.
Acknowledgments
This work was supported by Public Health Service grants KO8 AI01332 (to E.T.R.) and AI40725 (to S.B.C.), both from the National Institutes of Allergy and Infectious Diseases.
We are extremely grateful to Samuel L. Stanley, Jr., Tonghai Zhang, and Lynne Foster for their assistance with SREHP-12–CtxB, Karl E. Klose for assistance with ΔglnA strains, Sims K. Kochi and Kevin P. Killeen for pKEK71-NotI, and John J. Mekalanos for V. cholerae Peru2.
REFERENCES
- 1.Butterton J R, Boyko S A, Calderwood S B. Use of the Vibrio cholerae irgA gene as a locus for insertion and expression of heterologous antigens in cholera vaccine strains. Vaccine. 1993;11:1327–1335. doi: 10.1016/0264-410x(93)90103-5. [DOI] [PubMed] [Google Scholar]
- 2.Butterton J R, Ryan E T, Acheson D W, Calderwood S B. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterton J R, Ryan E T, Shahin R A, Calderwood S B. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun. 1996;64:4373–4377. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cray W C J, Tokunaga E, Pierce N F. Successful colonization and immunization of adult rabbits by oral inoculation with Vibrio cholerae O1. Infect Immun. 1983;41:735–741. doi: 10.1128/iai.41.2.735-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreccio C, Levine M M, Rodriguez H, Contreras R. Comparative efficacy of two, three, or four doses of Ty21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J Infect Dis. 1989;159:766–769. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M B, DiRita V J, Calderwood S B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;50:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.John, M., T. I. Crean, S. B. Calderwood, and E. T. Ryan. In vitro and in vivo analyses of constitutive and in vivo-induced promoters in attenuated vaccine and vector strians of Vibrio cholerae. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 7.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 8.Levine M M, Black C, Ferreccio C, Clements M L, Lanata C, Rooney J, Germanier R. The efficacy of attenuated Salmonella typhi oral vaccine strain Ty21a evaluated in controlled field trials. In: Holmgren J, Lindberg A, Molby R, editors. Development of vaccines and drugs against diarrhea. 11th Nobel Conference, Stockholm, 1985. 1986. pp. 90–101. Studentlitteratur, Lund, Sweden. [Google Scholar]
- 9.Levine M M, Black R E, Clements M L, Cisneros L, Nalin D R, Young C R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 10.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 11.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 12.Pearson G D, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson L A, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S H. Animal models in cholera research. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera. Washington, D.C.: ASM Press; 1994. pp. 203–226. [Google Scholar]
- 15.Ryan E T, Butterton J R, Smith R N, Carroll P A, Crean T I, Calderwood S B. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect Immun. 1997;65:2941–2949. doi: 10.1128/iai.65.7.2941-2949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan E T, Butterton J R, Zhang T, Baker M A, Stanley S L, Jr, Calderwood S B. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect Immun. 1997;65:3118–3125. doi: 10.1128/iai.65.8.3118-3125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan E T, Crean T I, John M, Butterton J R, Clements J D, Calderwood S B. In vivo expression and immunoadjuvancy of a mutant of heat-labile enterotoxin of Escherichia coli in vaccine and vector strains of Vibrio cholerae. Infect Immun. 1999;67:1694–1701. doi: 10.1128/iai.67.4.1694-1701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan E T, Crean T I, Kochi S K, John M, Luciano A A, Killeen K P, Klose K E, Calderwood S B. Development of a ΔglnA balanced lethal plasmid system for expression of heterologous antigens by attenuated vaccine vector strains of Vibrio cholerae. Infect Immun. 2000;68:221–226. doi: 10.1128/iai.68.1.221-226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 20.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]