Graphical abstract
Chemical compounds studied in this article: Acetic acid PubChem CID 176; Propionic acidPubChem CID 1032; Butyric acid PubChem CID 264; Ethanol PubChem CID 702; Urolithin A PubChem CID 5,488,186; Urolithin BPubChem CID 5,380,406; Maysin PubChem CID 70,698,181; Apimaysin PubChem CID 194,566; Pyrogallol PubChem CID 1057; Gallic acid PubChem CID 370
Keywords: Tejuino, In vitro colonic fermentation, Gut metabolites, SCFAs, Volatile compounds, Phenolic compounds
Highlights
-
•
26 phenolic compounds were identified in Tejuino-IF highlighting pyrogallol and urolithins.
-
•
Tejuino indigestible fraction (IF) in vitro colonic fermentation produces gut metabolites.
-
•
Butyric acid production was higher than propionic during colonic fermentation of Tejuino-IF.
-
•
Production of volatile compounds can influence the increase antioxidant capacity.
-
•
PCA was performed to correlate volatile metabolites of fermentation, pH, PC and AOX.
Abstract
Tejuino, is a Mexican fermented beverage prepared by germination-fermentation or nixtamalization-fermentation (artisanal and commercial mode respectively) of maize. The aim of this study was to evaluate the gut metabolites, volatile, and phenolic compounds (PC) produced by the indigestible fraction (IF) of Tejuino during an in vitro colonic fermentation. Twenty-six PC in the IF were identified; the hydroxycinnamic acids (30–40 %) were the most abundant. In the IF of Tejuino pyrogallol, and urolithins were identified. Some of the representative PC of maize as maysin derivatives (apimaysin and 3-methoxymaysin) (flavonoids). The quantification of acetic and butyric acid become notable after 6 h of the colonic fermentation of IF of Tejuino. Ninety-seven volatile compounds were found, and the PCA shows the predominant compounds as short chain fatty acids, esters of organic acids and indole derivatives. These results suggest that Tejuino could be an important source of metabolites with high biological value.
1. Introduction
Tejuino is an indigenous Mexican beverage made from fermented maize (Zea mays), consumed mainly in this country’s western and northwest states (Rubio-Castillo et al., 2021a). The ethnic groups use germinated and nixtamalized maize for its preparation; it is millet, cooked, and processed by spontaneous fermentation (Rubio-Castillo et al., 2021b). The artisanal process is carried out by soaking the maize to germinate the seeds in the dark (to avoid the rancid taste of the final product), pre-fermentation, cooking, filtering out, and a final fermentation during 7 days. In contrast, the commercial Tejuino is made with masa (nixtamalized and milling maize) followed by another cooking and a short fermentation (12–24 h). Nixtamalization is a thermal process consisting of cooking of maize grains in alkaline water or calcium solution, with the primary objective of softening the pericarp and endosperm, facilitating the milling (Bello-Pérez et al., 2015).
The fermentation process in Tejuino starts spontaneously with available carbohydrates and microorganisms in the maize or masa containers (Rubio-Castillo et al., 2021a). This process produces several nutritional improvements, including faster amino acid release, decreasing grain tannin levels, increasing iron bioavailability, free calcium levels, nicotinic acid, and niacin availability, and increasing dietary fiber and protein content (Bello-Pérez et al., 2015). Furthermore, several bioactive compounds, such as cinnamic and ferulic acids are released during nixtamalization (Díaz-Ruiz et al., 2003). During maize germination, starch, dietary fiber, and protein are hydrolyzed by endogenous enzymes, and some phenolic compounds (PC) in the grain are improved, such as vanillic acid, syringic acid, protocatechuic, caffeic, ρ-coumaric, ferulic and, sinapic acids; flavonoids, as catechin and epicatechin, kaempferol and quercetin-3-O-galactoside (Hiran et al., 2016). The composition of artisanal and commercial Tejuino reported is, 87–92 % of moisture, 3–27 % of protein, 77–79 % of carbohydrates, and 4–9 % of fat (Rubio-Castillo et al., 2021b). Some of the constituents of Tejuino, as resistant starch, dietary fiber, and PC, are not digestible by human gastrointestinal enzymes, these compounds are known as indigestible fraction (IF).
IF can reach the large intestine and act as a substrate for colonic microbiota. Colonic fermentation of complex carbohydrates produces metabolites such as short-chain fatty acids (SCFAs), and non-bioavailable PC are biotransformed into bioactive compounds that modify the intestinal microbiota and its functions. In the colonic fermentation of IF and PC, gut bacteria produce compounds that can modulate the composition of the intestinal microbiota, stimulating the growth of beneficial bacteria and inhibiting pathogens (Zamora-Gasga et al., 2017). Today it is insufficient to evaluate only the chemical-proximal characterization in food. The development of various in vitro digestion systems has become a trend since it allows us to emulate, as closely as possible in an economical and accessible way, what can happen in the body. Even more, the relevance and importance that colonic fermentation has in the individual has made us turn to look and consider the metabolites that the microbiota is capable of bioconvert at the moment of ingesting food, without neglecting that the communication that exists between metabolites with the brain is an area that expands more every day since the relationship between these metabolites and even mood is not trivial (Cárdenas-Castro et al., 2019).
Therefore, gut microbiota's changes in gut metabolite production and PC biotransformation during colonic fermentation of the IF from Tejuino have not been reported. The present study aimed to evaluate the gut metabolites (SCFAs, PC, and volatile compounds) produced during the in vitro colonic fermentation of the IF of two types of Tejuino: commercial (nixtamalized) and artisanal (germinated maize).
2. Materials and methods
2.1. Preparation of the Tejuino samples
Two samples of commercial Tejuino were purchased in Tepic, Nayarit, Mexico, one batch in the Tecnologico zone was code as “TT”, the other batch was obtained from Leon St was code as “TL”. The artisanal Tejuino was obtained from Wixárika ethnic group in La Yesca, Nayarit, Mexico was codified as “AR”. All fresh samples were (500 mL each) were frozen (−80 °C) and freeze-dried (FreeZone6, Labconco, Kansas City, USA). Samples were milled (NB-101B, Nutribullet, Los Angeles, CA, USA), sieved (mesh size of 500 μm) and, stored at − 20 °C until analysis.
2.2. Indigestible fraction (IF) isolation and in vitro colonic fermentation of IF of Tejuino
A digestion procedure that mimics the physiological simulation on the upper digestive tract (stomach and small intestine) was utilized for isolate the total IF (TIF) (Zamora-Gasga et al., 2017). TIF was collected, freeze-dried, milled (NB-101B, Nutribullet, Los Angeles, CA, USA), and sieved (mesh size of 500-μm), and were storage at − 20 °C. The TIF isolated from Tejuino samples were subjected to an in vitro colonic fermentation process in disposable tubes prepared with pre-conditioned nutritive medium under strict anaerobic conditions using a gas mixture (10:10:80, H2: CO2: N2) in sterile basal medium adjusted to pH 7 at 37 °C.
A mixture of fresh fecal samples was used (before 2 h after defecation) from four healthy volunteer adults (2 men and 2 women; between 25 and 30 years old) who declared no gastrointestinal diseases and no intake of antibiotics at least 3 months before the beginning of the study. A 1:10 (w/v) dilution of the fecal samples with phosphate buffer (0.1 mol/L, pH 7) was prepared and homogenized in a digital high-speed homogenizer system (IKA-Ultra-Turrax, T18, USA; 1 min, 6000 rpm). The resulting fecal suspension (1 mL) was distributed in disposable tubes (containing 9 mL nutritive medium), and 0.1 g of the isolated TIF from each Tejuino sample was added. In parallel, two different controls were incubated under the same conditions: a) raffinose (50 mg R0514, Sigma Aldrich, MO, USA) was used as a fermentable sugar reference, and b) the culture media with fecal suspension without substrate was used as a negative control. All incubations were performed by triplicate, and the corresponding tubes from samples and controls were analyzed at each fermentation time for pH changes, total soluble polyphenols (TSP) and antioxidant capacity (AOX) by 1,1-diphenyl-2-picryl hydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays, each analysis is described below. The tubes obtained at each time of fermentation were centrifuged (Hermle Z 323 K, Wehingen, Germany) to 3500g, 15 min at 4 °C, and supernatants were always kept at − 20 °C until analysis.
Total soluble polyphenols (TSP) were quantified from supernatants 0.5 mL (n = 3) using a microplate reader (Biotek Synergy, HT, USA). Absorbance was determined at 750 nm according to the methodology modified by Alvarez-Parrilla et al. (2011) and results were expressed as a mmol gallic acid equivalent (mmol GAE/g TIF isolated).
Antioxidant capacity (AOX) was evaluated according to Alvarez-Parrilla et al. (2011) for DPPH and FRAP assays. Results were expressed as a mmol Trolox (6-hydroxy-2.5.7.8-tetramethylchromane-2-carboxylic) equivalent (mmol TE/g TIF isolated). Also, pH was measured using a microprocessor pHmeter (Hanna Instruments, pH 211, RI, USA).
2.3. Metabolite profile analysis
2.3.1. Identification and quantification of PC by HPLC-DAD-ESI-MS
For the identification of PC from the IF of Tejuino and bioconversion products of the in vitro colonic fermentation, the supernatants were injected (10 μL, flow 0.4 mL/min) into an HPLC (Agilent Technologies, 1260 series, Santa Clara, CA, USA) equipped with a UV–vis detector (DAD) and an Agilent Quadrupol 6120 mass detector, in m/z 100–1000 scan mode, the data will be analyzed in the OpenLab CDS program, (ChemStation Editing Software, Agilent Technologies, Santa Clara, CA, USA). The characterization of these PC was performed considering the retention time (Rt) in the DAD and the mass spectrometry signal directly compared with standards; calibration curves were prepared for the quantification of the compounds (Cárdenas-Castro et al., 2021); when the standard was not available, the calibration curve corresponding to its phenolic precursor was used to tentatively quantify the compound. The results were presented by µmol/L.
2.3.2. Metabolites identified by headspace solid-phase micro-extraction HS-SPME-GC/MS
Supernatants from colonic fermentation were identified by gas chromatography-mass spectrometry (GC–MS) using a headspace solid-phase micro-extraction (HS-SPME); 500 μL were placed into a 20-mL vial sealed with a magnetic cap with a ply-tetra-fluoro-ethylene (PTFE)/silicon septum; a solid phase microextraction (SPME Carboxen®/DVB/PDMS/Stableflex™, SU57348U, Agilent Technologies, Santa Clara, CA, USA) fiber assembly was used. Extractions conditions were as follows: extraction temperature 45 °C, incubation time 5 min, speed agitator 250 rpm and 120 min extraction time; a desorption temperature of 250 °C for 10 min was used (Zamora-Gasga et al., 2017). The samples were analyzed by gas chromatography and mass spectrometry detection (GS–MS) (Agilent Technologies, 7890A GC, Santa Clara, CA, USA) equipped with an Agilent 5975C VL mass selective detector and a multi-purpose autosampler MPS2 XL (Gerstel). The GC separation of samples was carried out on a HP-INNOwax capillary column (60 m × 250 μm × 0.25 μm; Agilent, Santa Clara, CA, USA) using Helium as the carrier gas at a flow rate of 1.5 mL/min. The GC injector was held at a temperature of 250 °C and the mass spectrometry source and quadrupole were maintained at 230 °C and 150 °C, respectively. The injector was used in the splitless mode. Oven temperature started at 40 °C for 5 min and was programmed at 5 °C /min from 40 to 200 °C, held at 200 °C for 2 min, then programmed at 20 °C/min from 40 to 230 °C, and held at 230 °C for 15 min. Quantification of the samples was obtained through calibration curves of acetic, propionic, and butyric acids. Data analysis was performed using MSD ChemStation software (Agilent G1701EAversion E.20.00.493).
2.4. Statistical analysis
Data were expressed as the mean ± standard deviation (n = 3). Comparisons between samples was evaluated using the STATISTICA 10 software (StatSoft, OK, USA) with one-way ANOVA; the fermentation analysis data (Sample × Time) by two-way ANOVA; individual means of all data were compared using the Fisher LSD test to probe significant differences. Significance was assessed at 95 % level of confidence. Principal Components Analysis (PCA) and non-metric multidimensional scaling (nMDS) of fermentation metabolites was performed based on the mean values of three replicates. PCA were calculated without rotation and the number of extracted factors was based on eigenvalues > 1.0 and explaining variance (%) > 60 %.
3. Results and discussion
3.1. Tentative identification and semi-quantification of PC in the IF of Tejuino
In the Supplementary Material (Table S1) is shown a total of thirty-one compounds identified by HPLC-DAD-MS that includes the characterization of the IF, PC from the fermentation it shows Rt, and molecular formula of each identified compound. In this sense, twenty-six different PC were tentative identified and semi-quantified in the IF of the Tejuino samples, being hydroxycinnamic, hydroxyphenyl, benzoic, ellagic, other phenolic acids, and flavonoids (Table 1). The most abundant group were hydroxycinnamic acids (30–41 %), followed by pyrogallol a phenolic acid (20–33.7 %). Ellagic acids are between 10 and 19 % of abundance, within the flavonoids, the quercetin in the TL sample can be ascribable to the variety of maize. Hydroxybenzoic acids continue in a range of 7.54–10 %, hydroxy propionic and hydroxyphenyl acetic acids groups being the least abundant. AR was the sample with the largest amount of total abundance follow by TL sample and TT. This, can be due to the fermentation time of artisanal Tejuino (7 days) (Rubio-Castillo et al., 2021a).
Table 1.
Tentative phenolic compounds content (µmol/L) at T0 before colonic fermentation in the indigestible fraction (IF) of artisanal (AR) and commercial (TT and TL) Tejuino samples.1.
|
Tejuino samples |
|||||
|---|---|---|---|---|---|
| Phenolic compound | Rt(min) | AR | TT | TL | |
| Hydroxycinnamic acids | |||||
| 1 | Caffeoylquinic acid | 3.8 | LoQ | 16.63 ± 4b | 10.61 ± 1.6a |
| 2 | Vanillic acid | 14.02 | 83.1 ± 3a | 105.13 ± 0.4c | 96.77 ± 1.6b |
| 3 | p-coumaric acid | 17.18 | ND | ND | 1.28 ± 0.1 |
| 4 | Ferulic acid | 17.7 | 209.2 ± 20.6b | ND | 6.17 ± 6.0a |
| 5 | p-coumaroylquinic acid | 19.65 | ND | ND | 145.57 ± 25.5 |
| Total (µmol/L) (%) | 292.3 ± 33.4b | 121.7 ± 6.1a | 260.4 ± 49.2b | ||
| (41.52 %) | (30.43 %) | (38.12 %) | |||
| Hydroxyphenylpropionic acids | |||||
| 6 | 3-(4-hydroxyphenyl)propionic acid | 15.64 | 31.3 ± 0.2c | 28.89 ± 0.5b | 19.22 ± 0.8a |
| 7 | 3-(3-hydroxyphenyl)propionic acid | 17.3 | 1.4 ± 0.9a | 0.85 ± 0.1a | LoQ |
| Total (µmol/L) (%) | 32.7 ± 1c | 29.74 ± 0.8b | 19.22 ± 0.8a | ||
| (4.64 %) | (7.43 %) | (2.81 %) | |||
| Hydroxyphenylacetic acid | |||||
| 8 | 3-hydroxyphenylacetic acid | 15.9 | LoQ | 0.81 ± 0.1 | LoQ |
| Total (µmol/L) (%) | (0 %) | (0.2 %) | (0 %) | ||
| Hydroxybenzoic acid and related compounds | |||||
| 9 | Syringic acid | 4.4 | 41.4 ± 3a | 30.17 ± 4.a | 53.02 ± 19a |
| 10 | Gallic acid | 5.79 | 12.2 ± 2 | ND | ND |
| 11 | 3-hydroxybenzoic acid | 13.06 | 18.2 ± 2 | ND | ND |
| Total (µmol /L) (%) | 71.8 ± 1b | 30.17 ± 4a | 53.02 ± 19b | ||
| (10.2 %) | (7.54 %) | (7.76 %) | |||
| Flavonoids | |||||
| 12 | Methoxymaysin | 11.51 | 0.2 ± 0.1a | 0.14 ± 0.1a | ND |
| 13 | Quercetina-3-O-galactoside | 14.41 | ND | 2.12 ± 0.3a | 3.1 ± 0.1b |
| 14 | Maysin | 15.08 | 0.9 ± 0.1a | 0.77 ± 0.1a | 1.88 ± 0.1b |
| 15 | Maysin derivative | 15.37 | 8.4 ± 1.8b | LoQ | 1.1 ± 0.1a |
| 16 | Apimaysin | 16.32 | 1.6 ± 0.1b | 1.49 ± 0.2b | 0.46 ± 0.3a |
| 17 | Quercetin-3-p-sambubioside | 16.36 | 0.5 ± 0.3 | ND | ND |
| 18 | Maysin derivative | 19.04 | 12.2 ± 0.3b | 4.77 ± 0.1a | 5.41 ± 0.2a |
| 19 | Quercetin | 20.33 | ND | ND | 114.81 ± 11.1 |
| 20 | 3-Methoxymaysin | 21.3 | 2.0 ± 0.9a | 5.39 ± 0.3b | 6.34 ± 0.3c |
| Total (µmol /L) (%) | 25.8 ± 5.1b | 14.68 ± 1.4a | 133.1 ± 17.3c | ||
| (3.66 %) | (3.69 %) | (19.48 %) | |||
| Ellagic acids and related compounds | |||||
| 21 | Isourolithin A | 9.92 | 128.8 ± 13.3c | 49.5 ± 5.8a | 58.4 ± 2.5b |
| 22 | Ellagic acid | 17.47 | 3.6 ± 3.0a | 4.3 ± 0.3a | 5.4 ± 5.0a |
| 23 | Urolithin A | 20.09 | 2.9 ± 0.1ª | 6.2 ± 1.7b | ND |
| 24 | Urolithin C | 20.58 | 2.5 ± 0.6a | 3.8 ± 0.7b | 4.4 ± 0.5b |
| 25 | Urolithin B | 22.44 | LoQ | 4.1 ± 0.2ª | 5.1 ± 0.6b |
| Total (µmol /L) (%) | 137.8 ± 13.5c | 67.9 ± 4.1a | 73.3 ± 5.1b | ||
| (19.57 %) | (16.97 %) | (10.73 %) | |||
| Other phenolics acids | |||||
| 26 | Pyrogallol | 4.58 | 143.6 ± 0.3b | 134.98 ± 3.1a | 144.1 ± 0.4b |
| Total (µmol/L) (%) | (20.4 %) | (33.74 %) | (21.1 %) | ||
| Total soluble polyphenols (µmol/L) (%) | 704 ± 18.6b | 400.1 ± 5.5c | 683.1 ± 14.7a | ||
| (100 %) | (100 %) | (100 %) | |||
Values represent means ± SD (n = 3). Different lowercase letters in the same row indicate significant difference (p ≤ 0.05). ND: not detected; LoQ: Limit of Quantification. The commercial samples were obtained in the city of Tepic Nayarit, Mexico. TT, Tejuino obtained from Tecnológico zone; TL, Tejuino obtained from Leon Street. AR, Tejuino obtained from la Yesca, Nayarit, México, prepared by Wixárika ethnic group.
In the AR sample, ferulic acid was the most abundant, followed by pyrogallol; in TT sample pyrogallol was the major compound follow by vanillic acid; and in TL sample p-coumaroylquinic acid was the most abundant compound follow by pyrogallol, and quercetin.
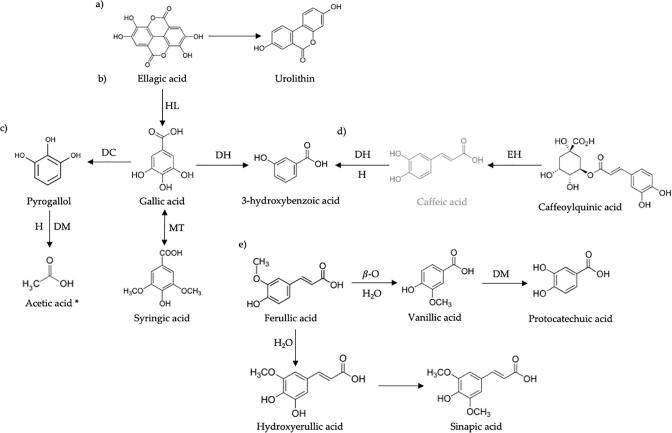
Pyrogallol may be derived from conversion of gallic acid by its decarboxylation (Fig. 1c); Pereira-Caro et al., (2017) reports that these compounds (identified in this study) were the result of the bacteria metabolism in a food fermentation. Mitra et al. (2021) suggest that pyrogallol could be used has anticancer treatment for colorectal cancer, this compound can be able to modulate they related genes. In the case of quercetin, ferulic and sinapic acids are reported for yellow maize, used to prepare Tejuino. PC identified in the IF of Tejuino showed significant differences (p < 0.05) into samples, and some compounds were identified only once, as p-coumaric acid and p-coumaroylquinic acid in TT, gallic and syringic acids in AR (Table 1, Supplementary Material). However, vanillic acid 3-(4-hydroxyphenyl) propionic, syringic, and ellagic acids, were found in all the samples. Vanillic acid can regulate changes in the gastrointestinal tract by favouring the growth of beneficial bacteria as Lactiplantibacillus and Enterococcus, the presence of these bacteria have been reported in Tejuino (Rubio-Castillo et al. 2021a). Phenolic acids and their microbial metabolites affect intestinal bacteria composition and their metabolic activity (Iqbal et al., 2020), gallic acid supplementation can affect the morphology of the gut and enhance the absorption of nutrients; hydroxycinnamic acids like chlorogenic, p-coumaric and caffeoylquinic acids affected some pathogen microorganisms (i.e., Staphylococcus aureus).
Fig. 1.
Propose biotransformation route of principal phenolics compounds during in vitro colonic fermentation of indigestible fraction of Tejuino. a) route of urolithins; b) route of hydroxybenzoic acids; c) route of pyrogallol; d) route of hydroxycinnamic acids; and e) route of protocatechuic acid. HL: hydrolysis; DC: decarboxylation; MT: methylation; DH: dehydration; H: hydrogenation; EH: ester hydrolysis; β-O: β-oxidation; H2O: hydration; DM: demethylation (Tomás-Barberán et al., 2016, Del juncal-Guzmán et al., 2021, Pereira-Caro et al., 2017; Esteban-Torres et al., 2018; Balaj et al., 2022; Zeng et al., 2016). *SCFA.
Furthermore, within maysin, apimaysin and 3-methoxymaysin (flavonoids) are some of representative compounds of maize identified in this study. Flavonoids as maysin and chlorogenic acid were presents in some tissues of maize plant, presents defense mechanisms, seed coat development, and other functions (Jin et al., 2017). Maysin was correlate highly positively with the antioxidant capacity of corn silks; gallic acid is a component of maize grain and was reported to have antioxidant, antitumor, anti-carcinogenic, and anti-mutagenic effects in animal cells (Sourani et al., 2016) and carcinomas cell line. The presence of urolithins in the IF of Tejuino may be due to the natural fermentation process by bioconversion of the ellagic acid (Tomás-Barberán et al., 2016) (Fig. 1a). This may be due to bacterial metabolism in food fermentations, as Tejuino natural fermentation, urolithins can be absorbed in small intestine and proximal colon (Espín et al., 2013).
3.2. Metabolites production by in vitro colonic fermentation
3.2.1. Changes in pH, TSP and AOX during in vitro colonic fermentation of TIF of Tejuino
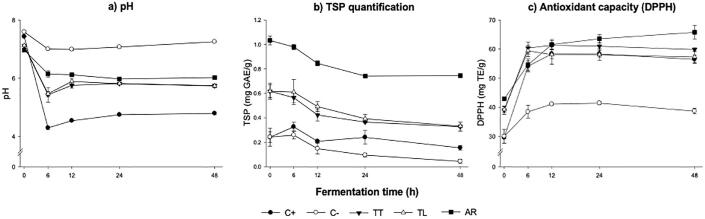
Intestinal pH provides information about the changes during the fermentation process. Fig. 2a shows the pH values of the in vitro colonic fermentation in controls and TIF of Tejuino samples. At 6 h there was a decrease in pH in all samples and controls, and a gradual increase was observed at 12, 24 and 48 h after fermentation, except for AR, which remains in the same range. The pH decreasing in TIF from Tejuino samples occurs between 1 and 1.5 units at 6 h, while the positive control (raffinose) presents a decrease of 2.5 units. Negative control showed the highest pH values (pH 7.3 at 48 h of fermentation) at all times (p < 0.05). In contrast, the positive control showed the lowest pH value at all points (pH 4.8 at 48 h after fermentation). The AR pH was maintained at 6 throughout the fermentation (p > 0.05) and the commercial samples TT and TL showed a 5.7 pH at 48 h. Low colonic pH has been used as a marker of the beneficial effects (Zamora-Gasga et al. 2018) and, can influence the production of gut metabolites (such as propionic and butyric acids) that can bind to GPR43 proteins that play a role in the regulation of human body energy homeostasis and intestinal immunity (Markowiak-Kopeć & Śliżewska, 2020).
Fig. 2.
Changes in pH, total soluble polyphenolics and AOX in total indigestible fraction (TIF) of Tejuino samples (artisanal AR, and commercial TT and TL) and controls (blank and raffinose) during in vitro colonic fermentation. a) pH; b) total soluble phenolics (TSP) quantification; c) antioxidant activity (DPPH). Positive control (-l-), negative control (-¡-), indigestible fraction of Tejuino samples: TT (-q-), TL (-r-), and AR (-n-). Values are mean ± standard deviation (n = 3).
The changes that occurred in the content of TSP are shown in Fig. 2b as mg GAE/g. The negative control (0.24 to 0.04 mg GAE/g) and positive control (0.24 to 0.16 mg GAE/g) showed lower TSP content (p < 0.05). The TT and TL samples showed values of 0.62 and 0.33 mg GAE/g, at 6 and 48 h, respectively, and significant decreases were found in relation to time (p < 0.05). AR sample showed the highest value, its content was from 0.75 to 1.03 mg GAE/g at 6 and 48 h, respectively. The amount of TSP decreases due to the action of pH; some PC have been shown to be affected by microbial metabolism, the glycosylated part of PC can serve as a substrate for the growth of microorganisms in colonic fermentation (Plessas, 2022).
The AOX values are shown in the Fig. 2c, where highest values were observed at 12 h of fermentation (except TL sample) and decreased over time in all samples, except for AR that maintained the increase and showed higher AOX (p < 0.05) compared to the others (65.81 mg TE/g at 4 h). TT and TL samples had a similar AOX values during all fermentation (39–60 mg TE/g). Despite the decrease in the content of TSP (Fig. 1b), the DPPH antioxidant capacity increases. Cárdenas-Castro et al. (2021) attributed this behavior to the set of all volatile compounds produced during the colonic fermentation of IF, explained that the production of gut metabolites (as SCFAs) by the action of the microbiota showed increase of the AOX, besides, the production of volatile compounds in the colonic fermentation can exert effect mainly as free radicals neutralizers (Zamora-Gasga et al., 2017). The FRAP assay was not detectable. This may be because there is no chelating activity in the samples (data not shown); this behavior suggests that the gut metabolites in the fermentation process exhibit different antioxidant properties. In addition, the antioxidant compounds can affect some other components of the sample.
3.2.2. Identification and semi-quantification of PC from in vitro colonic fermentation of IF of Tejuino
PC precursors and metabolites derived from in vitro microbial colonic fermentation of IF of Tejuino are summarized in Table 2, where seven phenolic groups were identified: hydroxycinnamic, hydroxyphenylpropionic, hydroxyphenylacetic, hydroxybenzoic, ellagic, others phenolic acids and flavonoids. At the moment, there is not studies of colonic fermentation of traditional beverages maize-made.
Table 2.
Quantification of phenolic compounds (PC) in fermentation extracts of indigestible fraction (IF) isolated from Tejuino samples (AR: artisanal, and TT and TL: commercial) and controls during in vitro colonic fermentation (6, 12, 24 and 48 h) (µmol L−1).1
| RT (min) |
Fermentation time | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | |||||||
| Phenolic compound | AR | TT | TL | AR | TT | TL | ||
| Hydroxycinnamic acid and related compounds | ||||||||
| 1 | Caffeoylquinic acid | 3.8 | 3.5 ± 2.6a | 7.2 ± 0.31b | 2.03 ± 0.72a | 1.5 ± 0.02a | 2.3 ± 0.3a | 3.0 ± 0.2a |
| 2 | Vanillic acid | 14.02 | LoQ | 342.8 ± 24.7d | 451.11 ± 94.58e | ND | ND | 1.7 ± 0.5a |
| 3 | p-coumaric acid | 17.18 | 0.5 ± 0.1a | 2.7 ± 0.4 a | 2.39 ± 2.31a | 1.2 ± 0.11a | 1.5 ± 1.2a | 1.0 ± 0.4a |
| 4 | Sinapic acid | 17.54 | 43.0 ± 9.2a | 41.7 ± 2.5a | 68.52 ± 10.45b | 79.6 ± 0.97bc | 68.2 ± 4.3b | 72.5 ± 4.8b |
| 5 | Ferulic acid | 17.7 | ND | ND | LoQ | ND | 2.1 ± 0.6a | LoQ |
| 6 | 2-hydroxycinnamic acid | 18.9 | 7.7 ± 1.0b | 6.6 ± 1.8b | ND | 4.0 ± 0.1a | 10.2 ± 2.0c | 6.9 ± 1.8b |
| 7 | p-coumaroylquinic acid | 19.65 | 13.9 ± 1.5c | 9.2 ± 0.8b | 5 ± 0.02a | 11.23 ± 1.6bc | 18.4 ± 0.9e | 16.1 ± 1.5d |
| Total (µmol L−1) (%) | 68.6 ± 4.0a (14.41 %) | 410.2 ± 19.7h (53.03 %) | 529.05 ± 82.56i (54.95 %) | 97.53 ± 0.66c (31 %) | 102.7 ± 0.5d (30.87 %) | 101.2 ± 1.2d (30.75 %) | ||
| Hydroxyphenylpropionic acid and related compounds | ||||||||
| 8 | Methoxy-hydroxyphenylpropionic acid | 4.18 | 184.5 ± 0.1ef | 152.9 ± 6.0de | 184.45 ± 29.07ef | 127.2 ± 2.9cd | 123.36 ± 13.47bcd | 116.67 ± 16.8bc |
| 9 | 3-(4-Hydroxyphenyl)propionic acid | 15.64 | ND | 13.1 ± 1.1bc | 8.53 ± 7.48ab | ND | LoQ | 3.1 ± 0.8a |
| 10 | 3-(3-Hydroxyphenyl)propionic acid | 17.3 | ND | ND | 0.49 ± 0.43a | ND | ND | 1.33 ± 0.4a |
| Total (µmol L−1) (%) | 184.5 ± 0.1d (38.76 %) | 166 ± 4.9c (21.46 %) | 193.47 ± 21.16de (20.1 %) | 127.2 ± 2.9b (40.43 %) | 123.36 ± 13.4ab (37.08 %) | 121.1 ± 15.6ab (36.8 %) | ||
| Hydroxyphenylacetic acid | ||||||||
| 11 | 3-hydroxyphenylacetic acid | 15.9 | 42.4 ± 34.7 | ND | ND | ND | ND | ND |
| Total (µmol L−1) (%) | (8.91 %) | (0 %) | (0 %) | (0 %) | (0 %) | (0 %) | ||
| Hydroxybenzoic acid and related compounds | ||||||||
| 12 | Syringic acid | 4.4 | 13.7 ± 1.5ab | 22.2 ± 5.1abc | 35.8 ± 8.3bcde | 10.7 ± 1.8a | ND | 36.4 ± 22.23bcde |
| 13 | Gallic acid | 5.79 | 12.1 ± 0.2a | 12.3 ± 3.2a | 10.8 ± 3.7a | ND | 15.4 ± 2.5a | 15.4 ± 4.7a |
| 14 | Protocatechuic acid | 12.58 | ND | 1.4 ± 0.6 a | 1.7 ± 1.2 a | ND | ND | ND |
| 15 | 3-hydroxybenzoic acid | 13.06 | 19.5 ± 17.8a | ND | ND | ND | ND | ND |
| Total (µmol L−1) (%) | 45.3 ± 16.5de (9.52 %) | 35.9 ± 1.3d (4.64 %) | 48.3 ± 3.4e(5.02 %) | 10.7 ± 1.8b (3.4 %) | 15.4 ± 2.5c (4.63 %) | 41.1 ± 17.53de (12.49 %) | ||
| Flavonoids | ||||||||
| 16 | Gallocatechin | 4.46 | 32.1 ± 6.1b | 6.41 ± 8.8a | 14.31 ± 4.0ab | 12.9 ± 9.5ab | 11.0 ± 1.1ab | 7.0 ± 1.1a |
| 17 | Methoxymaysin | 11.51 | ND | 1.24 ± 0.1 | ND | ND | ND | ND |
| 18 | Quercetina-3-O-galactoside | 14.41 | 18.3 ± 3.1f | 12.88 ± 2.8d | 15.75 ± 2.9def | 15.3 ± 0.8def | 13.8 ± 1.1de | 13.3 ± 2.0de |
| 19 | Maysin derivative | 15.37 | 24.3 ± 3.0e | 20.49 ± 1.0bcd | 21.54 ± 0.8cde | 18.9 ± 2.0abc | 19.6 ± 0.5abcd | 17.6 ± 1.0ab |
| 20 | Apimaysin | 16.32 | 5.3 ± 1.6ab | 5.54 ± 2.0ab | 5.59 ± 1.1b | 4.8 ± 2.0ab | 5.2 ± 0.4b | 6.1 ± 0.3b |
| 21 | Quercetin-3-p-sambubioside | 16.36 | 4.2 ± 0.4d | 10.04 ± 6.5defg | 7.65 ± 0.3f | 5.3 ± 1.0d | 8.0 ± 0.7g | 3.4 ± 0.1c |
| 22 | Maysin derivative | 19.04 | 9.3 ± 0.1g | 7.94 ± 0.9f | 5.29 ± 0.2cd | 5.4 ± 0.2cd | 5.67 ± 0.1d | 5.9 ± 0.3d |
| 23 | Quercetin | 20.33 | 0.4 ± 0.1a | ND | 1.18 ± 0.2b | ND | ND | ND |
| 24 | 3-Methoxymaysin | 21.3 | LoQ | ND | ND | 0.59 ± 0.3a | ND | ND |
| Total (µmol L−1) (%) | 93.9 ± 2.2g (19.72 %) | 64.54 ± 3.3e (8.34 %) | 71.31 ± 1.7f (7.41 %) | 63.19 ± 5.2e (20.09 %) | 63.27 ± 0.7e (19.02 %) | 53.3 ± 0.2d (16.2 %) | ||
| Ellagic acid and related compounds | ||||||||
| 25 | Isourolithin A | 9.92 | 27.3 ± 1.1e | 8.23 ± 0.5b | 20.24 ± 10.5bcde | 7.48 ± 5.7ab | 6.1 ± 0.4a | LoQ |
| 26 | Ellagic acid | 17.47 | 4.0 ± 1.1abc | 6.68 ± 1.4bcd | 1.3 ± 0.3a | 6.4 ± 0.5bcd | 16.4 ± 0.9e | 8.0 ± 2.4cd |
| 27 | Urolithin A | 20.09 | LoQ | 3.45 ± 0.2a | 3 ± 2.8.0a | ND | ND | ND |
| 28 | Urolithin C | 20.58 | ND | ND | ND | ND | ND | ND |
| 29 | Urolithin B | 22.44 | LoQ | 5.85 ± 5.2abcd | 1.4 ± 1.1a | 2.1 ± 0.6ab | 5.5 ± 3.7abcd | 4.4 ± 1.7ab |
| Total (µmol L−1) (%) | 31.31 ± 0.01d (6.57 %) | 24.21 ± 4.1b (3.13 %) | 25.94 ± 6.9bc (2.69 %) | 15.98 ± 4.6ab (5.08 %) | 28 ± 2.4cb (8.42 %) | 12.4 ± 0.7a (3.77 %) | ||
| Other phenolic acids | ||||||||
| 30 | Pyrogallol | 4.58 | 9.96 ± 0.51a | 72.69 ± 10.58c | 94.63 ± 31.87c | ND | ND | ND |
| Total (µmol L−1) (%) | (2.1 %) | (9.4 %) | (9.83 %) | (0 %) | (0 %) | (0 %) | ||
| Total soluble polyphenols (%) | 476.0 ± 6.26d (100 %) | 773.54 ± 4.48f (100 %) | 962.7 ± 17.53g (100 %) | 314.6 ± 10.24b (100 %) | 332.73 ± 14.5bc (100 %) | 329.1 ± 2.23bc (100 %) | ||
| Phenolic compound | RT (min) |
Fermentation time |
||||||
| 24h |
48h |
|||||||
| AR | TT | TL | AR | TT | TL | |||
| Hydroxycinnamic acid and related compounds | ||||||||
| 1 | Caffeoylquinic acid | 3.8 | ND | 3.8 ± 1.6a | 2.3 ± 0.1a | 3.69 ± 0.4a | 2.6 ± 0.1a | LoQ |
| 2 | Vanillic acid | 14.02 | ND | ND | 15.7 ± 0.6ab | ND | ND | 7.8 ± 7.5ab |
| 3 | p-coumaric acid | 17.18 | ND | ND | ND | 2.13 ± 0.1 a | ND | 2.4 ± 0.5a |
| 4 | Sinapic acid | 17.54 | 145.8 ± 3.2e | 138.4 ± 8.5e | 148.5 ± 12.7e | 91.81 ± 0.7cd | 92.8 ± 2.1cd | 75.7 ± 11.1bc |
| 5 | Ferulic acid | 17.7 | LoQ | 18.6 ± 1.4a | 11.0 ± 1.5a | ND | ND | ND |
| 6 | 2-hydroxycinnamic acid | 18.9 | 14.2 ± 3.1d | 9.9 ± 6.0c | 19.2 ± 2.2e | ND | ND | ND |
| 7 | p-coumaroylquinic acid | 19.65 | 6 ± 1.8a | 11.0 ± 1.0bc | 9.1 ± 1.4bc | ND | ND | 4.2 ± 1.4a |
| Total (µmol/L) (%) |
166 ± 2.4e (34.7 %) |
181.7 ± 1.2f (37.98 %) |
205.8 ± 8.1g (36.16 %) |
97.63 ± 0.4c (29.21 %) |
95.4 ± 2bc (35.37 %) |
90.1 ± 2.67b (32.03 %) |
||
| Hydroxyphenylpropionic acid and related compounds | ||||||||
| 8 | Methoxy-hydroxyphenylpropionic acid | 4.18 | 224.5 ± 4.4h | 192.8 ± 12.9fg | 189.5 ± 2.9f | 118.2 ± 5.2bc | 93.4 ± 17.4ab | 82.8 ± 21.3a |
| 9 | 3-(4-Hydroxyphenyl)propionic acid | 15.64 | ND | ND | ND | ND | ND | ND |
| 10 | 3-(3-Hydroxyphenyl)propionic acid | 17.3 | ND | ND | ND | ND | ND | ND |
| Total (µmol/L) (%) |
224.5 ± 4.4f (46.9 %) |
192.8 ± 12.9de (40.3 %) |
189.5 ± 2.9e (33.29 %) |
118.2 ± 5.2ab (35.36 %) |
93.4 ± 17.4a (34.63 %) |
82.8 ± 21.3a (29.43 %) |
||
| Hydroxyphenylacetic acid | ||||||||
| 11 | 3-hydroxyphenylacetic acid | 15.9 | ND | ND | ND | ND | ND | ND |
| Total (µmol/L) (%) | (0 %) | (0 %) | (0 %) | (0 %) | (0 %) | (0 %) | ||
| Hydroxybenzoic acid and related compounds | ||||||||
| 12 | Syringic acid | 4.4 | ND | 15.8 ± 9.2ab | 54.1 ± 1.1d | 22.4 ± 16.6abc | LoQ | 17.6 ± 3.3ab |
| 13 | Gallic acid | 5.79 | ND | ND | ND | ND | ND | 9.3 ± 0.4a |
| 14 | Protocatechuic acid | 12.58 | 14.5 ± 1.5b | ND | ND | ND | 2.8 ± 2.6a | 1.1 ± 0.5a |
| 15 | 3-hydroxybenzoic acid | 13.06 | ND | ND | 50.4 ± 9.5a | ND | ND | ND |
| Total (µmol/L) (%) |
14.5 ± 1.5c (3.03 %) |
15.8 ± 9.2bc (3.3 %) |
104.5 ± 8.4f (18.36 %) |
22.4 ± 16.6bc (6.7 %) |
2.8 ± 2.6a (1.04 %) |
28 ± 2.4c (9.95 %) |
||
| Flavonoids | ||||||||
| 16 | Gallocatechin | 4.46 | ND | 20.3 ± 6.8ab | ND | 7.3 ± 3.9a | ND | 11.4 ± 2.7ab |
| 17 | Methoxymaysin | 11.51 | ND | ND | ND | ND | ND | ND |
| 18 | Quercetina-3-O-galactoside | 14.41 | 5.3 ± 1.1a | 7.1 ± 0.3b | 9.2 ± 0.1c | 15.2 ± 1.0def | 16.4 ± 0.9ef | 16.4 ± 0.9ef |
| 19 | Maysin derivative | 15.37 | 16.9 ± 0.1a | 17.6 ± 1.8ab | 19.7 ± 0.5abcd | 19.2 ± 1.6abc | LoQ | 18.1 ± 0.6ab |
| 20 | Apimaysin | 16.32 | 3.9 ± 0.4a | 6.3 ± 0.7b | 5.3 ± 0.3b | 6.4 ± 0.6b | 5.7 ± 0.1ab | 6.1 ± 0.4b |
| 21 | Quercetin-3-p-sambubioside | 16.36 | 2.3 ± 0.8a | LoQ | 3.1 ± 0.1b | 7.2 ± 1.1e | 7.1 ± 0.1e | 7.5 ± 1.8def |
| 22 | Maysin derivative | 19.04 | 7.1 ± 0.2e | 6.0 ± 0.6d | 5.6 ± 0.1d | 3.4 ± 0.2b | 2.9 ± 0.2b | 1.4 ± 0.6a |
| 23 | Quercetin | 20.33 | 4.1 ± 1.5cd | 2.8 ± 1.8bcd | LoQ | 3.8 ± 0.3c | 3.0 ± 0.3c | 0.5 ± 0.1a |
| 24 | 3-Methoxymaysin | 21.3 | ND | ND | ND | ND | ND | ND |
| Total (µmol/L) (%) |
39.6 ± 0.8b (8.3 %) |
60.1 ± 5.8e (12.56 %) |
42.9 ± 0.1c (7.54 %) |
62.5 ± 1.5e (18.7 %) |
35.1 ± 0.4a (13.01 %) |
61.4 ± 1.3e (21.83 %) |
||
| Ellagic acid and related compounds | ||||||||
| 25 | Isourolithin A | 9.92 | 10.2 ± 1.7bc | 11.7 ± 3.4bc | 8.3 ± 2.3b | 18.3 ± 0.5d | 16.5 ± 6.9cd | 16.4 ± 11.8abcde |
| 26 | Ellagic acid | 17.47 | ND | 3.2 ± 0.4ab | 7.5 ± 0.1bcd | 9.7 ± 0.9d | 7.7 ± 2.7cd | LoQ |
| 27 | Urolithin A | 20.09 | ND | 5.1 ± 2.8ab | 6.2 ± 0.1ab | ND | 3.3 ± 1.4a | 2.6 ± 0.3a |
| 28 | Urolithin C | 20.58 | ND | ND | ND | ND | 2.1 ± 1.2a | ND |
| 29 | Urolithin B | 22.44 | 10.4 ± 0.3e | 8.0 ± 0.1bcd | 4.5 ± 2.6abc | 5.5 ± 2.6abcd | ND | ND |
| Total (µmol/L) (%) |
20.6 ± 1.0b (4.3 %) |
28 ± 0.1d (5.85 %) |
26.5 ± 0.5c (4.66 %) |
33.5 ± 1.2e (10.02 %) |
29.6 ± 1.6d (10.98 %) |
19 ± 11.5ab (6.75 %) |
||
| Other phenolic acids | ||||||||
| 30 | Pyrogallol | 4.58 | 13.3 ± 1.8b | ND | ND | ND | 13.4 ± 0.1b | ND |
| Total (µmol/L) (%) | (2.77 %) | (0 %) | (0 %) | (0 %) | (4.97 %) | (0 %) | ||
| Total soluble polyphenols (%) | 478.5 ± 2.1d (100 %) |
478.4 ± 15.77d (100 %) |
569.2 ± 3.8e (100 %) |
334.23 ± 13.7bc (100 %) |
269.7 ± 14.7a (100 %) |
281.3 ± 8.77a (100 %) |
||
1Values represent means ± SD (n = 3). Different lowercase letters in the same row indicate significant difference (p ≤ 0.05) between all samples and all times of fermentation. ND: not detected; LoQ: Limit of Quantification. The commercial samples were obtained in the city of Tepic Nayarit, Mexico. TT, Tejuino obtained from Tecnológico zone; TL, Tejuino obtained from Leon Street. AR, Tejuino obtained from La Yesca, Nayarit, México, prepared by Wixárika ethnic group.
1Values represent means ± SD (n = 3). Different lowercase letters in the same row indicate significant difference (p ≤ 0.05) between all samples and all times of fermentation. ND: not detected; LoQ: Limit of Quantification. The commercial samples were obtained in the city of Tepic Nayarit, Mexico. TT, Tejuino obtained from Tecnológico zone; TL, Tejuino obtained from Leon Street. AR, Tejuino obtained from La Yesca, Nayarit, México, prepared by Wixárika ethnic group.
Thirty different compounds were identified in the HPLC-DAD-MS in AR, TT and TL samples; metoxy-hydroxyphenyl propionic acid was the compound with highest total abundance (identify in all samples in all fermentation times), followed by isourolithin A, sinapic acid, vanillic acid, gallocatechin and pyrogallol. At 6 h of in vitro colonic fermentation, hydroxycinnamic acid and related compounds was the group the major abundances (42–54.9 %) follow by hydroxyphenylpropionic acids (20–25.9 %), flavonoids (7.4–13 %) and other phenolic acids as pyrogallol (1–9.8 %). Ellagic, hydroxybenzoic, and hydroxyphenylacetic acids was the compound with least abundance. Pereira-Caro et al., (2017) mentions that PC associated with dietary fiber can be released for the enzymatic action of bacteria and other fermenting microorganisms such as moulds and yeasts; and some of these compounds can be biotransformed into other bioactives, such as gallic acid to pyrogallol (Fig. 2). The bioavailability of polyphenols is also increased due their transformation into absorbable metabolites. Particularly, Lactobacilli bacteria are capable of metabolizing polyphenols producing energy for use by cells and simpler compounds that can interfere with metabolic activities of gut bacteria (Iqbal et al., 2020).
Furthermore, ellagitannins and ellagic acid are metabolized by the gut microbiota to produce urolithins that could be responsible for the health effects attributed to ellagitannin-containing food products (García-Villalba et al., 2016). The compounds profile was different in the samples throughout the fermentation time (p < 0.05). Pyrogallol was quantified in the characterization of the Tejuino IF (Table 1). During the spontaneous fermentation of the maize, can be possible that the gallic acid present was bioconverted by native bacteria to pyrogallol but during the colonic fermentation this pyrogallol was demethylated to acetic acid (Fig. 3c) (Zeng et al., 2017) this could explain the decrease in the production of pyrogallol (48 h) (Table 2). In this sense, phenolic acids and their microbial metabolites affect intestinal bacteria composition and their metabolic activity; i.e., gallic acid prevents the formation of biofilm as it reduced biofilms formation activity of E. Coli, P. aeruginosa, S. aureus, and L. monocytogenes by 70 % (Iqbal et al., 2020).
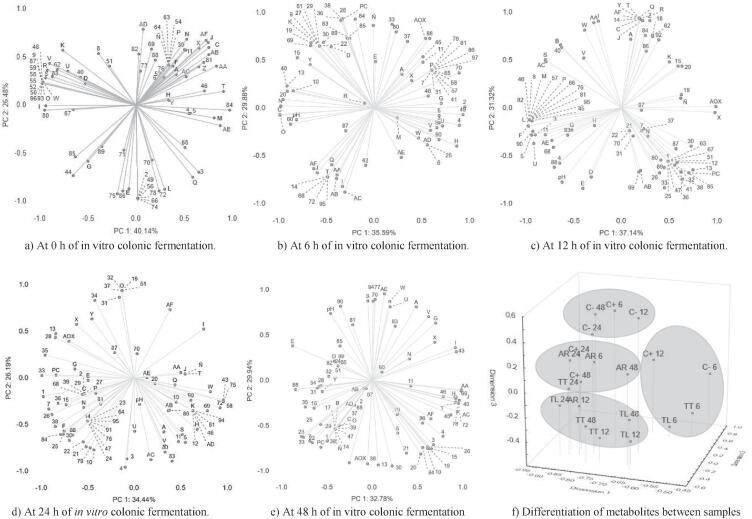
Fig. 3.
Principal component analysis (PCA) of first two factor scores and factor loadings of phenolics compounds (A-AF), volatile metabolites (1–97) of all samples (artisanal AR, and commercial TT, TL) of Tejuino indigestible fraction (IF), controls (positive C+, and negative C-), and pH, phenolic compounds and AOX, of in vitro colonic fermentation: a) at 6 h of fermentation; b) at 12 h of fermentation; c) at 24 h of fermentation and d) at 48 h of fermentation. e) differentiation of gut metabolites using non-metric multidimensional scaling ordination between IF of Tejuino samples (AR artisanal sample and commercial sample TT and TL) and controls (C+, and C-) during different fermentation time (6, 12, 24 and 48 h).
Caffeoylquinic acid was detected in all samples of TIF of Tejuino (Supplementary Material, Table S1), but, in general, its content was decreased over fermentation time. However, AR contains 1.73 µmol/L at initial time of fermentation and 3.69 µmol/L at 48 h of fermentation; TL sample showed 2.6 µmol/L at the end of the fermentation; this decrease may be due to microbial metabolism, in which caffeoylquinic acid may undergo ester hydrolysis and decarboxylation, producing 3-hydroxybenzoic acid by action of Firmicutes, Bacteroidetes or Actinobacterium (Balaj et al., 2022), as shows Fig. 1d. This compound was not previously reported for maize or maize-product fermentation.
Even though sinapic acid has been reported in maize composition, but was not found in the characterization of the IF of Tejuino (Table 1) may be due to the natural spontaneous fermentation of Tejuino. During the colonic fermentation the sinapic acid can be produced by the bioconversion of the ferulic acid (Fig. 1e) (Kulik et al., 2017). Nevertheless, have been related to the metabolism of Roseburia (Yang et al., 2019) thus, this increase may be due to the presence of this fermentative microorganism. Furthermore, Yang et al. (2019) report that sinapic acid supplementation in rats, impacted the intestinal microbiome improving the proportion the butyric acid producer as Blautia and Dorea, and inhibiting the growth of bacterial species associated with diseases and inflammation such Bacteroides and Desulfovibrionaceae.
The abundance of vanillic acid at 6 h of fermentation was higher than the other times for TT, and TL samples. However, a decrease of this acid was observed during the fermentation time; 12, 24 & 48 h); this can be due to gut microbiota during colonic fermentation. Álvarez-Rodríguez et al., 2003 explained that the microbial biotransformation of vanillic acid to succinyl-CoA + acetyl-CoA, by action of Streptomyces strains, Bacillus megaterium, Rhodotolula rubra, and Nocardia sp. Furthermore, vanillic acid can be produced by microbial biotransformation of ferulic acid, 3-(4-dihydroxyphenyl)-propionic acid could be other product (Pathak et al., 2018). Nonetheless, it can also be biotransformed to protocatechuic acid across of demethylation (Fig. 1) explaining the decrease of this compound; that has been reported as a maize component, and was found in the characterization of the Tejuino IF (Table 1). Caffeic acid can be biotransformed to 3-hydroxyphenylpropionic acid and benzoic acid; both these metabolites are also generated from chlorogenic acid by metabolism of Lactobacillus and Bifidobacterium; besides, hydroxycinnamic acids as chlorogenic acid; p-coumaric acid affected the metabolism of pathogen microorganisms as Escherichia coli, Bacillus cereus, and Staphylococcus aureus (Borges et al., 2013). Polyphenols provide protection to epithelial cells and prevent inflammation in the intestines resulting in improved gut barrier function (Iqbal et al., 2020).
Some of the flavonoids found was maysin derivatives (apimaysin, methoxymaysin), although maysin was identified only in IF (Table 1; Supplementary Material Table S1); maysin derivatives has not been recently reported for fermented foods made from maize; there is very little information about this compound and its possible functionalities. However, it is been reported that maysin derivatives could have a neuroprotector effect; showed effectiveness in preventing the typical toxic events, i.e., oxidative stress and imbalance of intracellular calcium homeostasis (Leri et al., 2020). Oral administration of maysin in mice fed a high-fat diet decreased weight gain and epididymal fat weight, it also reduced serum levels of TG, total cholesterol, LDL cholesterol, and glucose; these results suggest for the first time that maysin exerts an anti-obesity effect in vitro and in vivo; recent studies have reported that maysin exerts anticancer, neuroprotective, and immunomodulating activities (Lee et al., 2017).
Ellagic tannins from foods are first hydrolyzed to ellagic acid in stomach and small intestine then converted into urolithins with high bioavailability by the gut microbiota (genera Gordonibacter and Ellagibacter). Urolithin has beneficial biological effects, it can improve cholesterol metabolism, inhibit graft tumor growth, induce adipocyte browning, and others (Zhang et al., 2022).
PC possess probiotic action and support growth of selective bacteria by acting as a source of nutritional supply; the microbial derived metabolites and primary PC can affect microbial composition of the gut and signalling pathways; provide protection to epithelial cells and prevent inflammation in the intestine resulting in improved gut barrier functions (Iqbal et al., 2020).
3.2.3. Scfas production during in vitro colonic fermentation of IF of Tejuino
The fermentation of the IF of Tejuino produced SCFAs at the beginning of the fermentation, the characterization of the volatile compounds did no show the presence of SCFAs (time 0). At 6 h of fermentation TL mainly produces acetic acid (44.03 mmol/L) and butyric acid (43.43 mmol /L), shows a molar relation of 46:08:46 (acetic, propionic and butyric acid, respectively) (Table 3). SCFAs production by TL was decreased at 12 h of fermentation but increase at 24 h and kept at 48 h (22.97 mmol/L of acetic acid, and 28.8 mmol/L of butyric acid) with a molar relation of 41:08:51. TT sample present molar relation different to TL at 6 h (41:08:51) and 48 h (33:06:61), the production of SCFAs was lower at 6 h of fermentation (10.13 mmol/L of acetic acid and 12.35 mmol/L of propionic acid) than 48 h (15.55 and 29.29 mmol/L of acetic and butyric acid, respectively). AR sample only produces butyric acid (3.89 mmol/L) at 6 h of fermentation; at 24 h SCFAs was not detected, at 12 h only 7.87 mmol/L of butyric acid was measured, and at 48 h present a molar relation of 36:04:60 of SCFAs production (acetic, propionic, and butyric acid, respectively). TL was the sample with the higher SCFAs production mainly acetic and butyric acids. Total molar ratio of TT (35:06:59), TL (44:08:48) and AR (28:04:68) highlights that propionic acid was produced in lower amounts in all samples and controls. Resistant starch produces significatively higher amounts of butyric acid, serves as an energy source for colonic epithelial cells, supplying approximately 60–70 % of their total energy requirements (Zamora-Gasga et al., 2018). Fermentation rate is important to determinate the site of fermentation, can be controlled by changing transfer time to fermentation site. However, most dietary fiber and particularly soluble fiber, are rapidly fermentable in the proximal colon are responsible of the changes in SCFAs concentrations from 6 h could occur in the distal colon, where approximately 40 % of SCFAs concentrations are lower than those in the proximal colon, since most of the saccharolytic bacterial fermentation takes place in the proximal colon (Wang et al., 2019a).
Table 3.
Short chain fatty acids (SCFAs, μmol L−1) production at 6, 12, 24 and 48 h of in vitro colonic fermentation of controls (C−, C + ) and indigestible fraction (IF) isolated from Tejuino samples (artisanal AR and commercial TT, TL).1.
| SCFA / fermentation time | C− | C+ | IF of Tejuino samples |
||
|---|---|---|---|---|---|
| AR | TT | TL | |||
| Acetic acid | |||||
| 6 h | 29.72 ± 23.16ab | ND | ND | 10.13 ± 0.8ab | 44.03 ± 32.24b |
| 12 h | ND | 7.40 ± 2.03a | ND | 7.79 ± 2.74a | 10.91 ± 11.73ª |
| 24 h | 9.87 ± 7.17a | 8.52 ± 5.61a | ND | 18.08 ± 2.72a | 20.2 ± 16.01a |
| 48 h | ND | 21.43 ± 7.99a | 17.51 ± 3.28a | 15.55 ± 5.56a | 22.97 ± 10.46a |
| Propionic acid | |||||
| 6 h | 6.22 ± 5.22a | ND | ND | 2.03 ± 0.48a | 7.85 ± 4.72a |
| 12 h | ND | 2.22 ± 0.84a | ND | ND | 1.53 ± 0.18a |
| 24 h | ND | 3.12 ± 1.87a | ND | 4.07 ± 1.39a | 3.7 ± 278a |
| 48 h | ND | 6.49 ± 1.9b | 2.71 ± 2.45a | 3.08 ± 0.71a | 4.21 ± 1.97ab |
| Butyric acid | |||||
| 6 h | 14.33 ± 10.47a | ND | 3.89 ± 2.56a | 12.35 ± 0.97a | 43.43 ± 21.33b |
| 12 h | ND | 11.93 ± 5.54a | ND | 12.49 ± 4.63a | 4.69 ± 2.94a |
| 24 h | 14.8 ± 22.44a | 26.34 ± 12.45a | 7.87 ± 5.2a | 31.62 ± 1.39a | 31.62 ± 23.46a |
| 48 h | 1.83 ± 0.49a | 53.79 ± 13.00c | 29.75 ± 18.63ab | 29.29 ± 10.18b | 28.8 ± 10.88b |
| Molar ratio (acetic: propionic: butyric) | |||||
| 6 h | 59:12:29 | 00:00:00 | 00:00:100 | 41:08:51 | 46:08:46 |
| 12 h | 00:00:00 | 35:10:55 | 00:00:00 | 38:00:59 | 64:09:27 |
| 24 h | 40:00:60 | 22:08:70 | 00:00:100 | 34:07:59 | 36:07:57 |
| 48 h | 00:00:100 | 26:08:66 | 36:04:60 | 33:06:61 | 41:08:51 |
| Total molar ratio | 52:08:40 | 27:08:65 | 28:04:68 | 35:06:59 | 44:08:48 |
1Values are reported in μmol L−1 produced per 100 mg substrate as mean ± standard deviation (n = 3); different lowercase letters indicate significant differences in same rows among substrates for a time (p ≤ 0.05). C-: negative control. C+: positive control. ND: not detected. The commercial samples were obtained in the city of Tepic Nayarit, Mexico. TT, Tejuino obtained from Tecnológico zone; TL, Tejuino obtained from Leon Street. AR, Tejuino obtained from La Yesca, Nayarit, México, prepared by Wixárika ethnic group.
Dietary fiber can regulate the yield and molar ratios of SCFAs metabolites; SCFAs are considered to have beneficial effects on the human colon. They can act as an energy source, affecting colonic mucosal growth, promoting sodium and water absorption, and mitigating diarrhea (Walton et al., 2013).
Generally, acetic and butyric acids are produces mainly by fermentation of aldehydes (e.g., glucose, galactose, mannose and xylose), whereas propionic acid is produced mainly by ketone (e.g., fructose, arabinose and tagatose) fermentation (Hu et al., 2013). Propionic acid has been found to be positively correlated with Bacteroides, whereas, butyric acid production is positively correlated with Ruminococcaceae and Faecallibacterium as well as Roseburia, and Coprococcus (Yang et al., 2013). The chemical and physical structure of dietary fiber are the major factors determining SCFAs profiles during in vitro fermentation with human faecal inoculum (Wang et al., 2019b). A study showed that dietary supplementation with foods rich in dietary fiber increased the abundance of saccharolytic bacteria, including Bifidobacteria, Lactobacillus, Enterococcus, and Ruminococcus, and increased acetic and butyric acids in the proximal colon (Wang et al., 2019a).
3.2.4. PCA of volatile and PC of in vitro colonic fermentation of TIF of Tejuino
A total of 97 different volatile compounds and 31 PC were detected (Fig. 3) at 0, 6, 12, 24 and 48 h from in vitro colonic fermentation of IF of Tejuino. PCA was performed to correlate the volatile metabolites of fermentation, pH, PC and AOX. Two Principal Components were obtained (eigenvalues > 1) depending of the fermentation time that explained ∼ 66 % at initial time (0 h) (Fig. 3a); ∼65 % at 6 h (Fig. 3b); ∼68 % at 12 h (Fig. 3c); ∼60 % at 24 h (Fig. 3d), and ∼ 62 % at 48 h (Fig. 3e) of the total variance in the metabolite production. Loading scatter plots at each fermentation time are shown in Fig. 3.
At initial time of the fermentation PC1 explain the 40.14 % of the variance (Fig. 3a), positive axis was influenced by the high abundance of urolithin A (ID: AA), maysin (ID: T), 3-hydroxyphenilacetic acid (ID: M), benzene 1,3-bis(1,1-dimethylethyl) (ID: 84) and urolithin B (ID: AE). The negative axis was influenced by a high amount of phenolic compounds as protocatechuic acid (ID: O), methoxymaysin (ID: R), quercetin-3-O-galactoside (ID: S), apimaysin (ID: V) and quercetin-3-p-sambubioside; besides volatile compounds as dodecane, 3-methyl (ID: 50), eicosane, 2-methyl (ID: 52), heptadecane, 8-methyl (ID: 55), hexadecane, 3-methyl (ID: 58), hexadecane, 7,9-dimethyl (ID: 59), and indole (ID: 87). The PC2 positive axis explain the 26.48 % of the variance, was influenced only by urolithin C (ID: AD); and the negative axis was influenced by two phenolic compounds, 4-hydroxyphenilacetic acid (ID: L) and sinapic acid (ID: E); and the follows volatile compounds, 1-dodecanol (ID: 2), dodecane,2,6,10-trimethyl (ID: 49), heptane, 5-ethyl-2,2,3-trimethyl (ID: 56), n-hexane (ID: 60), heptanal (ID: 72), nonanal (ID: 74), octanal (ID: 75), 2-octanone (ID: 78), and butylated hydroxytoluene (ID: 86). The phenolic compounds found in the initial time correspond to the those of maize (i.e., maysin, methoxymaysin) and derivatives, they are described in Table 1 (Section 3.1), the volatile compounds can be the result of the spontaneous fermentation of Tejuino as explained by Rubio-Castillo et al., (2021a), where the volatile compounds that characterized the aroma of this beverage are described.
At 6 h of the fermentation PC1 explain the 35.59 % of the variance (Fig. 3b), positive axis was influenced by the high abundance of benzaldehyde 3,4-dimethyl (ID:70), 1-dodecanol (ID: 2), 1-hexanol (ID: 4), dodecane-2,6,10-trimethyl-ester (ID: 49) and p-coumaroylquinic acid (ID: H); the negative axis was influenced by a low abundance of ethanol (ID: 10), acetic acid (ID: 13), butanoic acid (ID: 15), cyclodecane (ID: 40), n-hexane (ID: 60), protocatechuic acid (ID: O), and 3-hydroxybenzoic acid (ID: P), besides, the pH. The PC2 explain the 29.88 % of the variance. PC2 positive axis was influenced by the increase in abundance of butanoic acid ethyl ester (ID: 26), butanoic acid ethyl ester (ID: 28), hexanoic acid ethyl ester (ID: 33), pentanoic acid ethyl ester (ID: 36), propanoic acid ethyl ester (ID: 38), and gallic acid (ID:N). PC2 negative axis was influenced by the decrease of abundance of methoxy-hydroxyphenylpropionic acid (ID: I), isourolithin A (ID: AA), ellagic acid (ID: AB) and urolithin A (ID: AC).
At 12 h of fermentation the PC1 explains the 37.14 % of the variance (Fig. 3c), and the positive axis was influenced by the increase of the abundance of acetic acid (ID: 13), ethyl acetate (ID: 30), and maysin derivative (ID: X); and the PC; the negative axis was influenced by the decrease in the abundance of 1-butanol (ID: 1), 1-hexanol 2-ethyl (ID: 5), fenchol (ID: 11), isopropyl alcohol (ID: 12), heptadecane (ID: 53), o-xylene (ID: 82), phenol (ID: 90), quercetin-3-O-galactoside (ID: S), maysin derivative (ID: U), urolithin A (ID: AC), and urolithin B (ID: AE). The PC2 explain the 31.32 % of the variance, and the positive axis was influenced by the increase abundance of methoxy-hydroxyphenylpropionic acid (ID: I), 3–4-dihydroxyphenylpropionic acid (ID: J), gallocatechin (ID: Q), quercetin-3-p-sambubioside (ID: W), 3-methoxymaysin (ID: Z), and isourolithin A (ID: AA); the negative axis was influenced by the decrease of abundance butanoic acid ethyl ester (ID: 26), butanoic acid methyl ester (ID: 28), hexanoic acid ethyl ester (ID: 33), pentanoic acid ethyl ester (ID: 36), propanoic acid ethyl ester (ID: 38), sinapic acid (ID: E), ferulic acid ID: D); besides, the pH and phenolic compounds. The profile of abundance of organic acids of 6–12 h changing, Fig. 3a shows increase of acetic, propionic, and butyric acids; however, Rios-Covian et al. (2015) reports that Faecaellibacterium can increase butyric acid levels due its metabolism, and had consumption capacity acetic acid produced by Bifidobacterium. On the other hand, phenol, fenchol and others amino acids metabolism products can be producers by Bacteroides (e.g., Clostridium and Eubacterium) and they can modulate the secretion of GLP-1 that could be involved in the development of neural functions (Russell et al., 2011). At 24 h of fermentation the PC1 explains the 34.44 % of the variance (Fig. 3d) and positive axis was influenced for the increase of abundance of volatile compounds as benzaldehyde 3,4-dimethyl (ID: 69), heptanal (ID: 72), cyclopropane nonyl (ID: 43), and the follows phenolic compounds, as methoxy-hydrophenilpropionic acid (ID: I), and quercetin-3-p-sambubioside (ID: W); the negative axis was influenced by 1-butanol (ID: 1), 1-pentanol (ID: 7), acetic acid (ID: 13), butanoic acid ethyl ester (ID: 26), butanoic acid methyl ester (ID: 28), hexanoic acid ethyl ester (ID: 33), methyl valerate (ID: 35), pentanoic acid ethyl ester (ID: 36), and propanoic acid ethyl ester (ID: 38), also, the PC and the AOX. The PC2 explains the 26.19 % of the variance and the positive axis was influenced for the increase of abundance of heptanoic acid ethyl ester ID: 31), hexanoic acid methyl ester (ID: 34), and pyrogallol (ID: AF); the negative axis was influenced for the decrease of abundance of 1-heptanol (ID: 3), 1-hexanol (ID: 4), 1-pentanol (ID: 7), bicycloheptan-2-ol (ID: 9), ethanol (ID: 10), propanoic acid 2-methyl (ID: 21), acetone (ID: 79), d-limonene (ID: 80), 2,4-di-tert-butylpheno1 (ID: 83), and urolithin A (ID: AC), apimaysin (ID: V). Increase of alcohols, organic acids and esters at 24 h of in vitro colonic fermentation, and instead, the decrease of concentration or abundance of organic acids could be associated whit the presence of microorganisms as Gemmiger, Lachnoclostridium, and Roseburia (Sáyago-Ayerdi et al., 2019), these genera have presented inversely correlation with atherosclerotic lesion development in a genetically diverse mouse population. At 48 h of fermentation the PC1 explains the 32.78 % of the variance (Fig. 3e) and the positive axis was influenced for the increase of abundance of 1-hexanol (ID: 4), cyclopropane nonyl (ID: 43), decane 3,6-dimethyl (ID: 45), decane 3,7-dimethyl (ID: 46), benzaldehyde 3,4-dimethyl (ID: 69), p-coumaloylquinic acid (ID: H), maysin derivative (ID: AA), quercetin-3-p-sambubioside (ID: AC); the negative axis was influenced for the decrease of abundance of 1-pentanol (ID: 7), heptanoic acid (ID: 16), acetic acid hexyl ester (ID: 22), butanoic acid 2-methylpropyl ester (ID: 23), hexanoic acid ethyl ester (ID: 33), methyl valerate (ID: 35), p-cresol (ID: 88), and sinapic acid (ID: E). The PC2 explains 29.94 % of the variance and the positive axis was influenced for the increase of abundance of phenol (ID; 90), methylamine, N,N-dimethyl (ID: 94), caffeoylquinic acid (ID: A), quercetina-3-O-galactoside (ID: S), maysin derivative (ID: U), apimaysin (ID: V), quercetin-3-p-sambubioside (ID: W), and urolithin B (ID: AE), and the influence by the pH; the negative axis was influenced for the decrease of abundance of acetic acid (ID: 13), propanoic acid (ID: 20), propanoic acid 2-methyl (ID: 21), ethyl acetate (ID: 30), pentanoic acid ethyl ester (ID: 36) and the PC and AOX. The beneficial properties of polyphenols are attributed to the formation of their biologically active metabolites and their ability to modulate changes in gut microbial populations (Iqbal et al., 2020). Furthermore, it was reported that maysin affects different cell types' cell viabilities, with incredibly less cytotoxicity on pre-adipocyte cells than on cancer cells (Lee et al., 2017). Medium chain fatty acids (MCFAs) where found in this study, there is a few reports on these metabolites. However, MCFAs (pentanoic, hexanoic and heptanoic acid) were identified as the most discriminatory metabolites between healthy controls and patients with inflammatory bowel disease; MCFAs have been shown to activate the peroxisome proliferator activated receptor (PPAR)-γ protein that regulates fatty acid and storage and glucose metabolism (De Preter et al., 2015).
Faecal metabolic fingerprint of fermentation is different at 0, 6, 12, 24 and 48 h of fermentation of TIF of Tejuino; this can be associated with the biotransformation of PC during fermentation (Fig. 2) by gut metabolism. Fatty acid esters were the most abundant metabolites produced during in vitro colonic fermentation of the samples. These compounds were identified in patients with chronic diseases of the gastrointestinal tract (Walton et al., 2013). The chemical characteristics (e.g., monosaccharide and linkage compositions, molecular size and the arrangements of the sugars) and physical form dietary fibers are critical factors determining fermentation rate, SCFAs profile and bacterial growth (Wang et al., 2019b). A three-dimensional non-metric multidimensional scaling plot with colonic fermentation metabolites and fermentation times together had the stress of 0.05, and four clusters with metabolites profiles were formed (Fig. 3f). TT and TL samples, and the negative control showed similarity between them at 6 h, together with the positive control at 12 h. The negative controls at 12, 24 and 48 h shower greater similarity with the positive control at 6 h. AR at 6, 24 and 48 h presented similarity with the positive control at 24 and 48 h, and TT sample at 24 h. Finally, AR sample at 12 h presented similitude with the TT sample at 12, 24 and 48 h, and with TL sample at 12, 24, and 48 h. Within each of these groups, the distance between the sample and the fermentation times suggests that the metabolic profile depend on the residence time in the colon.
4. Conclusions
The novel aspect in this study was the evaluation of the effects of colonic fermentation of the human faecal microbiota on the production of volatile compounds, SCFAs, and PC from the IF of Tejuino beverage. Thirty-three TSP were identified in this study (mainly hydroxycinnamic acids and flavonoids); and ninety-seven volatile compounds of the fermented IF Tejuino; the production of SCFAs (mainly acetic and butyric acids) occurs after 6 h of colonic fermentation; these metabolites could have potential biological applications as growth promoters of beneficial microorganisms. The results obtained suggest that the IF from Tejuino could be an important source of metabolites with high biological value.
Funding
A.E.R.-C. and S.G.S.-A. acknowledge TecNM for the financial support 10368.21-P and CONACYT for the project FOP02-2021–04-316948 and the net ALSUB-CYTED 118RT0543.
CRediT authorship contribution statement
Ángel Eduardo Rubio-Castillo: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing – original draft. Víctor M. Zamora-Gasga: Methodology, Software, Formal analysis, Investigation, Writing – review & editing. Jorge A. Sánchez-Burgos: Methodology, Software, Formal analysis, Writing – review & editing. Víctor M. Ruiz-Valdiviezo: . Efigenia Montalvo-González: Investigation. Rita M. Velázquez-Estrada: Investigation. Aarón F. González-Córdova: Investigation, Supervision, Project administration. Sonia G. Sáyago-Ayerdi: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the National Council for Science and Technology (CONACyT) of Mexico for the graduate scholarship of author Rubio-Castillo (Registration number: 296712). Acknowledge TecNM for the financial support 10368.21-P and CONACYT for the project TecNM/ITTepic-CONACyT FOP02-2021-04-316948 and the net ALSUB-CYTED 118RT0543.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2022.100150.
Contributor Information
Aarón F. González-Córdova, Email: aaronglz@ciad.mx.
Sonia G. Sáyago-Ayerdi, Email: ssayago@ittepic.edu.mx.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alvarez-Parrilla E., de la Rosa L.A., Amarowicz R., Shahidi F. Antioxidant activity of fresh and processed Jalapeno and Serrano peppers. Journal of Agricultural and Food Chemistry. 2011;59(1):163–173. doi: 10.1021/jf103434u. [DOI] [PubMed] [Google Scholar]
- Álvarez-Rodríguez M.L., Belloch C., Villa M., Uruburu F., Larriba G., Coque J.-J.-R. Degradation of vanillic acid and production of guaiacol by microorganisms isolated from cork samples. FEMS Microbiology Letters. 2003;220(1):49–55. doi: 10.1016/S0378-1097(03)00053-3. [DOI] [PubMed] [Google Scholar]
- Balaj G., Tamanai-Shacoori Z., Olivier-Jimenez D., Sauvager A., Faustin M., Bousarghin L., David-Le Gall S., Guyot S., Nebija D., Tomasi S., Abasq M.L. An insight into an intriguing oxidative biotransformation pathway of 5-O-caffeoylquinic acid by a gut bacterium. Food & Function. 2022;13:6195–6204. doi: 10.1039/d1fo04304h. [DOI] [PubMed] [Google Scholar]
- Bello-Pérez L.A., Flores-Silva P.C., Camelo-Méndez G.A., Paredes-López O., De Figueroa-Cárdenas J.D. Effect of the nixtamalization process on the dietary fiber content, starch digestibility, and antioxidant capacity of blue maize tortilla. Cereal Chemistry. 2015;92(3):265–270. doi: 10.1094/CCHEM-06-14-0139-R. [DOI] [Google Scholar]
- Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Castro A.P., Zamora-Gasga V.M., Alvarez-Parrilla E., Ruíz-Valdiviezo V.M., Venema K., Sáyago-Ayerdi S.G. In vitro gastrointestinal digestion and colonic fermentation of tomato (Solanum lycopersicum L.) and husk tomato (Physalis ixocarpa Brot.): Phenolic compounds released and bioconverted by gut microbiota. Food Chemistry. 2021;360 doi: 10.1016/j.foodchem.2021.130051. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Castro A.P., Bianchi F., Tallarico-Adorno M.A., Montalvo-González E., Sáyago-Ayerdi S.G., Sivieri K. In vitro colonic fermentation of Mexican “taco” from corn-tortilla and black beans in a Simulator of Human Microbial Ecosystem (SHIME®) system. Food Research International. 2019;118:81–88. doi: 10.1016/j.foodres.2018.05.072. [DOI] [PubMed] [Google Scholar]
- De Preter V., Machiels K., Joossens M., Arijs I., Matthys C., Vermeire S., Rutgeerts P., Verbeke K. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut. 2015;64(3):447–458. doi: 10.1136/gutjnl-2013-306423. [DOI] [PubMed] [Google Scholar]
- Del juncal-Guzmán, D., Hernández-Maldonado, L. M., Sánchez-Burgos, J. A., González-Aguilar, G. A., Ruiz-Valdiviezo, V. M., Tovar, J., & Sáyago-Ayerdi, S. G. In vitro gastrointestinal digestion and colonic fermentation of phenolic compounds in UV-C irradiated pineapple (Ananas comosus) snack-bars Lwt 138 2021 110636 10.1016/j.lwt.2020.110636.
- Díaz-Ruiz G., Guyot J., Ruiz-Teran F., Morlon-Guyot J., Wacher C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: a functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003;69(8):4367–4374. doi: 10.1128/AEM.69.8.4367-4374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín J.C., Larrosa M., García-Conesa M.T., Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Villalba R., Espín J.C., Tomás-Barberán F.A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. Journal of Chromatography A. 2016;1428:162–175. doi: 10.1016/j.chroma.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Hiran P., Kerdchoechuen O., Laohakunjit N. Combined effects of fermentation and germination on nutritional compositions, functional properties and volatiles of maize seeds. Journal of Cereal Science. 2016;71:207–216. doi: 10.1016/j.jcs.2016.09.001. [DOI] [Google Scholar]
- Hu J.-L., Nie S.-P., Li C., Xie M.-Y. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocolloids. 2013;33(2):384–392. doi: 10.1016/j.foodhyd.2013.04.006. [DOI] [Google Scholar]
- Iqbal Y., Cottrell J.J., Suleria H.A.R., Dunshea F.R. Gut microbiota-polyphenol interactions in chicken: A review. Animals. 2020;10(8):1391. doi: 10.3390/ani10081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Zhang X., Zhao M., Deng M., Du Y., Zhou Y., Wang S., Tohge T., Fernie A.R., Willmitzer L. Integrated genomics-based mapping reveals the genetics underlying maize flavonoid biosynthesis. BMC Plant Biology. 2017;17(1):1–17. doi: 10.1186/s12870-017-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik T., Stuper-Szablewska K., Bilska K., Buśko M., Ostrowska-Kołodziejczak A., Załuski D., Perkowski J. Sinapic acid affects phenolic and trichothecene profiles of F. culmorum and F. graminearum sensu stricto. Toxins. 2017;9(9):264. doi: 10.3390/toxins9090264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.W., Seo J.Y., Kim S.-L., Lee J., Choi J.W., Park Y.I. Corn silk maysin ameliorates obesity in vitro and in vivo via suppression of lipogenesis, differentiation, and function of adipocytes. Biomedicine & Pharmacotherapy. 2017;93:267–275. doi: 10.1016/j.biopha.2017.06.039. [DOI] [PubMed] [Google Scholar]
- Leri M., Vasarri M., Palazzi L., Barletta E., Nielsen E., Bucciantini M., Degl’Innocenti,, D. Maysin plays a protective role against α-Synuclein oligomers cytotoxicity by triggering autophagy activation. Food and Chemical Toxicology. 2020;144 doi: 10.1016/j.fct.2020.111626. [DOI] [PubMed] [Google Scholar]
- Markowiak-Kopeć P., Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D., Dey A., Biswas I., Das Mohapatra P.K. Bioactive compounds as a potential inhibitor of colorectal cancer; an insilico study of Gallic acid and Pyrogallol. Annals of Colorectal Research. 2021;9(1):32–39. doi: 10.30476/ACRR.2021.89642.1080. [DOI] [Google Scholar]
- Pathak, S., Kesavan, P., Banerjee, A., Banerjee, A., Celep, G. S., Bissi, L., & Marotta, F. (2018). Metabolism of dietary polyphenols by human gut microbiota and their health benefits. In Polyphenols: Mechanisms of action in human health and disease (pp. 347–359). Elsevier. https://doi.org/10.1016/B978-0-12-813006-3.00025-8.
- Pereira-Caro G., Moreno-Rojas J.M., Brindani N., Del Rio D., Lean M.E.J., Hara Y., Crozier A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. Journal of Agricultural and Food Chemistry. 2017;65(26):5365–5374. doi: 10.1021/acs.jafc.7b01707. [DOI] [PubMed] [Google Scholar]
- Plessas S. Advancements in the use of fermented fruit juices by lactic acid bacteria as functional foods: Prospects and challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum application. Fermentation. 2022;8(1):6. doi: 10.3390/fermentation8010006. [DOI] [Google Scholar]
- Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., de Los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiology Letters. 2015;362(21):fnv176. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- Rubio-Castillo, Á. E., Méndez-Romero, J. I., Reyes-Díaz, R., Santiago-López, L., Vallejo-Cordoba, B., Hernández-Mendoza, A., Sáyago-Ayerdi, S. G., & González-Córdova, A. F. (2021)a. Tejuino, a Traditional Fermented Beverage: Composition, Safety Quality, and Microbial Identification. Foods, 10(10), 2446. https://doi.org/10.3390/foods10102446. [DOI] [PMC free article] [PubMed]
- Rubio-Castillo A.E., Santiago-Lopez L., Vallejo-Cordoba B., Hernandez-Mendoza A., Sayago-Ayerdi S.G., Gonzalez-Cordova A.F. Traditional non-distilled fermented beverages from Mexico to based on maize: An approach to Tejuino beverage. International Journal of Gastronomy and Food Science. 2021;23 doi: 10.1016/j.ijgfs.2020.100283. [DOI] [Google Scholar]
- Russell D.A., Ross R.P., Fitzgerald G.F., Stanton C. Metabolic activities and probiotic potential of bifidobacteria. International Journal of Food Microbiology. 2011;149(1):88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Sáyago-Ayerdi S.G., Zamora-Gasga V.M., Venema K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2) Food Research International. 2019;118:89–95. doi: 10.1016/j.foodres.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Sourani, Z., Pourgheysari, B., Beshkar, P., Shirzad, H., & Shirzad, M. (2016). Gallic acid inhibits proliferation and induces apoptosis in lymphoblastic leukemia cell line (C121). Iranian Journal of Medical Sciences, 41(6), 525. PMCID: PMC5106568. [PMC free article] [PubMed]
- Tomás-Barberán F.A., Selma M.V., Espín J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Current Opinion in Clinical Nutrition and Metabolic Care. 2016;19(6):471–476. doi: 10.1097/MCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- Walton C., Fowler D.P., Turner C., Jia W., Whitehead R.N., Griffiths L., Dawson C., Waring R.H., Ramsden D.B., Cole J.A. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflammatory Bowel Diseases. 2013;19(10):2069–2078. doi: 10.1097/MIB.0b013e31829a91f6. [DOI] [PubMed] [Google Scholar]
- Wang G., Yu Y., Wang Y., Wang J., Guan R., Sun Y., Shi F., Gao J., Fu X. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. Journal of Cellular Physiology. 2019;234(10):17023–17049. doi: 10.1002/jcp.28436. [DOI] [PubMed] [Google Scholar]
- Wang M., Wichienchot S., He X., Fu X., Huang Q., Zhang B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends in Food Science & Technology. 2019;88:1–9. doi: 10.1016/j.tifs.2019.03.005. [DOI] [Google Scholar]
- Yang J., Martínez I., Walter J., Keshavarzian A., Rose D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Yang C., Deng Q., Xu J., Wang X., Hu C., Tang H., Huang F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Research International. 2019;116:1202–1211. doi: 10.1016/j.foodres.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Zeng X., Collins M.A., Borole A.P., Pavlostathis S.G. The extent of fermentative transformation of phenolic compounds in the bioanode controls exoelectrogenic activity in a microbial electrolysis cell. Water Research. 2017;109:299–309. doi: 10.1016/j.watres.2016.11.057. [DOI] [PubMed] [Google Scholar]
- Zamora-Gasga V.M., Álvarez-Vidal C., Montalvo-González E., Loarca-Piña G., Vázquez-Landaverde P.A., Bello-Pérez L.A., Tovar J., Sáyago-Ayerdi S.G. Gut metabolites associated with pH and antioxidant capacity during in vitro colonic fermentation of Mexican corn products. Cereal Chemistry. 2018;95(3):399–410. doi: 10.1002/cche.10039. [DOI] [Google Scholar]
- Zamora-Gasga V.M., Montalvo-González E., Loarca-Piña G., Vázquez-Landaverde P.A., Tovar J., Sáyago-Ayerdi S.G. Microbial metabolites profile during in vitro human colonic fermentation of breakfast menus consumed by Mexican school children. Food Research International. 2017;97:7–14. doi: 10.1016/j.foodres.2017.03.038. [DOI] [PubMed] [Google Scholar]
- Zhang M., Cui S., Mao B., Zhang Q., Zhao J., Zhang H.…Chen W. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Critical Reviews in Food Science and Nutrition. 2022;1–23 doi: 10.1080/10408398.2022.2036693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.